Abstract
Reactivation of latent varicella-zoster virus (VZV), presenting as localized zoster or as disseminated infection, is a common and potentially serious complication in hematopoietic stem cell transplantation (HSCT) recipients. We retrospectively studied anti-VZV immunoglobulin G titers by the immune adherence hemagglutination method after HSCT and also studied VZV DNA by real-time PCR during clinical VZV reactivation using cryopreserved serum samples. No significant difference was found between anti-VZV titers in 13 patients with VZV infection (localized zoster in 11 patients and disseminated zoster in 2 patients) and in 13 subjects without VZV infection at each time point after HSCT. Preexisting anti-VZV titers of disseminated zoster cases tended to be lower than those of localized zoster cases (P = 0.10). Serum VZV DNA copy numbers at the onset of disseminated zoster cases tended to be higher than those of localized zoster cases (P = 0.09). A strong inverse correlation was found between preexisting anti-VZV titer and serum VZV DNA at onset (r = −0.90, P = 0.006). In HSCT recipients, preexisting antibody does not prevent the development of VZV reactivation but may contribute to decreased viral load at onset, resulting in a mild clinical course.
Reactivation of varicella-zoster virus (VZV) is a common event in patients undergoing hematopoietic stem cell transplantation (HSCT) (1, 4, 6, 9). In post-HSCT recipients, VZV reactivation can occur frequently as localized zoster and sometimes as disseminated cutaneous lesions resembling varicella with or without visceral involvement, which results in a high mortality rate (1, 4, 6, 9). Previous studies revealed that VZV-specific memory T cells play a crucial role in suppressing reactivation (1, 8). Although all recipients experience a T-cell immunodeficient period after HSCT, the clinical severity of VZV infection after HSCT varies from self-limiting cases to fatal disseminated cases. The risk factors that determine clinical severity have not been elucidated. Despite current use of varicella-zoster immunoglobulin (VZIG), the role of humoral immunity against VZV infection has been poorly evaluated. The aim of this study was to determine the relationship between sustained anti-VZV antibody titers and the clinical manifestation of the VZV reactivation in HSCT recipients.
MATERIALS AND METHODS
Patients.
Patients who had undergone HSCT in Hokkaido University Hospital during the period from February 1995 to March 2004 were enrolled as subjects of the present study. Thirteen patients with VZV infection after HSCT were enrolled. Eleven patients (allogeneic [allo], seven; autologous [auto], four) suffered localized zoster. Two patients (both allo) suffered disseminated zoster with visceral involvement (one with acute abdomen and one with interstitial pneumonia). Thirteen patients who had been monitored for at least 3 years after HSCT without clinical VZV infection were studied as control subjects. Patient characteristics are presented in Table 1. The backgrounds of the two groups, including age, male/female ratio, autologous/allogeneic HSCT, total body irradiation regimen, and graft-versus-host disease (GVHD), are almost the same. In an allo-HSCT setting, acyclovir was administered orally at a dose of 1,000 mg/day from day −7 to day 35 to prevent herpes simplex virus infection. Ten grams of immunoglobulin was administered intravenously on day 0 and every other week until day 100 for prophylaxis of opportunistic viral infection in patients who had undergone allo-HSCT. In an auto-HSCT setting, acyclovir was administered orally at a dose of 1,000 mg/day from day −7 to engraftment, and 2.5 g of immunoglobulin was administered on days −7, 0, 7, and 14. We retrospectively studied the transition of anti-VZV-immunoglobulin G titer by the immune adherence hemagglutination (IAHA) method (before and at 3, 6, 12, and 24 months after HSCT) and VZV DNA by real-time PCR during clinical VZV infection (onset and paired serum) using cryopreserved serum samples.
TABLE 1.
Patient characteristics
| Characteristic | Value(s) for patient group
|
P | |
|---|---|---|---|
| VZV infection positive | VZV infection negative | ||
| No. of patients | 13 | 13 | |
| Male/female ratio | 7/6 | 7/6 | |
| Age (yr) | 0.23 | ||
| Range | 21-65 | 16-53 | |
| Median | 35 | 36 | |
| Auto-HSCT/allo-HSCT patient ratio | 3/10 | 1/12 | 0.30 |
| TBIa regimen | +9; −4 | +9; −4 | |
| Presence of acute GVHD (0-I/II-IV) | 3/7 in 10 allo-HSCT | 8/4 in 12 allo-HSCT | 0.10 |
| Presence of chronic GVHD | +8; −2 in 10 allo-HSCT | +6; −6 in 12 allo-HSCT | 0.16 |
TBI, total body irradiation.
Diagnosis of clinical VZV infection.
VZV infection was defined by the appearance of typical cutaneous vesicular lesions or the detection of VZV antigen. Localized zoster was defined as the presence of vesicular lesions in a dermatomal distribution. Disseminated zoster was defined as a generalized vesicular eruption that is identical to that of varicella. Visceral dissemination was defined as clinical evidence of internal organ involvement in the absence of other identified pathogens that might have accounted for the clinical syndrome.
IAHA assay.
IAHA analysis was performed as described previously (5).
Real-time PCR assay.
VZV DNA extraction from serum was performed by using a QIAamp DNA blood minikit (QIAGEN GmbH, Hilden, Germany). The primer pair VZV28-F (5′-CAGATTATCCGACATGCAGTCAA-3′) and VZV28-R (5′-ACCGGCAAGTCGCCAAT-3′) and the probe VZV28-T (5′-CAACGTCGCTTAACG-3′) for real-time PCR were designed in the DNA polymerase gene (gene 28) of VZV by using Primer Express software (Perkin-Elmer, Norwalk, CT). The reaction mixtures (50 μl) contained 2× TaqMan universal PCR master mix (Perkin-Elmer), 15 μl of DNA, the primers (each at 900 nM), and the TaqMan MGB probe (200 nM). After 2 min at 50°C, the AmpliTaq Gold DNA polymerase was activated at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and at 60°C for 1 min. All reactions were carried out six times with a 7500 ABI Prism sequence detector (Applied Biosystems, Foster City, CA). A real-time PCR standard, pCR2.1/VZV28 plasmid, was constructed by cloning a PCR product containing the VZV gene 28 region to the TA cloning vector pCR2.1 (Invitrogen, Carlsbad, CA). The pCR2.1/VZV28 plasmid was diluted to 5 × 107, 5 × 106, 5 × 105, 5 × 104, 5 × 103, 5 × 102, or 5 × 101 copies in 15 μl, and these dilutions were used to generate a standard curve.
Statistical analysis.
Comparisons between different groups of patients or clinical data were made by using the Fisher exact test for categorical data and the t test or Mann-Whitney U test for numerical variables. Correlation between two values was analyzed by Pearson correlation test. VZV DNA was analyzed after log transformation. The lower limit of the assay was used in the case of negative PCR results.
RESULTS
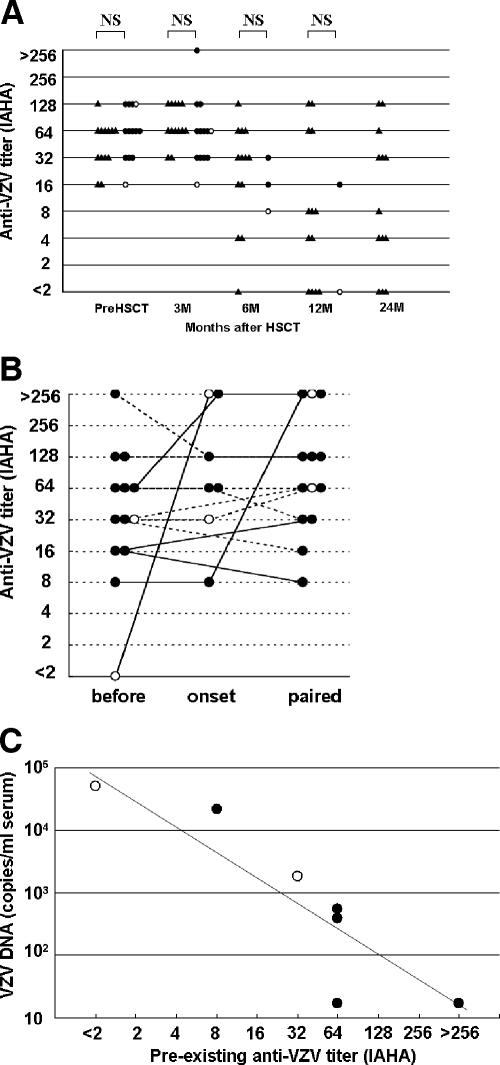
VZV infection occurred within 1 year after HSCT in 11 of the 13 patients. The onset of VZV infection varied day 56 to day 595 (median, day 155) after HSCT. All VZV infections occurred after cessation of prophylaxis with acyclovir. In 6 of the 10 allo-HSCT cases, VZV infection occurred during use of an immunosuppressant. All patients were treated with acyclovir. There were no deaths from VZV infection. All patients were seropositive for VZV before HSCT (Fig. 1A). None of the patients had a known history of VZV exposure before VZV infection. All VZV infections were considered to be a reactivation of innate VZV. One patient with VZV reactivation, the most severe case, had become seronegative before the development of disseminated zoster and interstitial pneumonia. VZV reactivation in the other 12 cases occurred during the persistent existence of anti-VZV. There was no statistically significant difference between the anti-VZV titers in the 13 patients with VZV reactivation and those in the 13 controls at each time point after HSCT (Fig. 1A). The last results before clinical VZV infection (preexisting anti-VZV) varied widely from <2-fold to >256-fold (median, 32-fold). Preexisting anti-VZV titers of disseminated zoster cases tended to be lower than those of localized zoster cases (P = 0.10). Increase in titer (IAHA, >4-fold) after VZV infection was not observed in any of the eight recipients with VZV infection within the first 6 months after HSCT but was observed in three of the five recipients with VZV infection after 6 months (P = 0.04) (Fig. 1B). Seven available samples that had been obtained from two patients with disseminated zoster and five patients with localized zoster at onset of VZV infection (stored on day 1 of skin eruption before administration of acyclovir) were examined by real-time PCR. VZV DNA was detected in all disseminated zoster cases and in three of the five localized zoster cases, whereas VZV was undetectable in all paired sera (2 to 9 weeks after onset [data not shown]). VZV DNA copy numbers at onset of disseminated zoster cases tended to be higher than those of localized zoster cases (P = 0.09). The preexisting anti-VZV titer and the serum VZV DNA level were inversely correlated (r = −0.90, P = 0.006) (Fig. 1C).
FIG. 1.
(A) Transition of anti-VZV titer after HSCT (control, ▴; localized zoster, •; disseminated zoster, ○). In cases of VZV infection, only the results before VZV onset were plotted. (B) Transition of anti-VZV before, at onset of and after VZV infection in 13 cases of VZV reactivation (localized zoster, •; disseminated zoster, ○). VZV infection within the first 6 months after HSCT (continuous line) and VZV infection at more than 6 months after HSCT (dashed line) are also indicated. (C) Correlation of preexisting anti-VZV titer and serum VZV DNA (localized zoster, •; disseminated zoster, ○). Preexisting anti-VZV titer and serum VZV DNA were inversely correlated (determined by Pearson correlation test; r = −0.90, P = 0.006).
DISCUSSION
Reactivation of latent VZV, presenting as localized zoster or as disseminated infection, is a common and potentially serious complication in HSCT recipients. Previous studies revealed that 23 to 60% of patients can be expected to develop VZV infection after HSCT (1, 4, 6, 9). Analyses of risk factors such as allogeneic versus autologous transplant, GVHD, underlying disease, and pre-bone marrow transplant irradiation have not revealed definitive associations (1, 4). Although the importance of cell-mediated immunity in VZV infection has been recognized (1, 8), there has been little investigation of the role of humoral immunity.
Passive immunization in the form of VZIG has been clinically used mainly for prophylaxis after exposure and sometimes for treatment of VZV infection. The Center for Disease Control guideline recommends immediate VZIG administration after VZV exposure for HSCT recipients (3). Anti-VZV can interfere with the initial phases of VZV replication and reduce attack rate (10). Even if infection occurs, the severity of the cutaneous disease and the risk for dissemination are modified by VZIG prophylaxis, indicating that specific antibodies present at the beginning of the incubation period can restrict the pathogenic potential of the virus (2). In contrast, passive antibodies administered after the onset of illness do not alter the severity of varicella (2).
In the case of primary VZV infection, no one has preexisting anti-VZV, and the disease is always disseminated. In this situation, early detection of high anti-VZV titers does not predict milder infection (2). In contrast, in the case of VZV reactivation, loss of specific cell-mediated immunity is observed in all cases, but titers of preexisting anti-VZV vary. Mazur et al. reported that the absence or low level of circulating innate anti-VZV was a significant risk factor for the dissemination of virus in herpes zoster (7). Based on these findings, we speculated a protective role of preexisting anti-VZV titer against VZV reactivation after HSCT.
Although preexisting anti-VZV could not prevent development of VZV reactivation in our series, a strong inverse correlation was found between preexisting anti-VZV and serum VZV DNA at the onset of VZV infection. Our data suggest that a low level of preexisting anti-VZV results in a severe and disseminated clinical course in HSCT recipients. We speculated that circulating anti-VZV cannot prevent the cell-to-cell spread of VZV, which leads to development of VZV infection, but that it has a neutralizing effect against viremia, which determines the severity of VZV disease.
Our results suggest that monitoring the anti-VZV titer has little diagnostic value, especially within 6 months after HSCT, because an increase in anti-VZV titer (IAHA, >4-fold) in paired sera after VZV infection was not observed in this period. In two of the three cases with active humoral immune response, anti-VZV was already increased at clinical onset. Anti-VZV titer at clinical onset was not correlated with clinical severity, and this may be the reason why previous studies failed to show a role of humoral immunity by analyzing anti-VZV at clinical onset. Active humoral immune response was dependent not on severity of clinical presentation (localized or disseminated disease) but on the timing of the onset after HSCT. This observation is consistent with a previous finding that reconstitution of cytotoxic T lymphocytes was also dependent on the time after HSCT (1).
In conclusion, preexisting antibody does not prevent the development of VZV reactivation but may contribute to decreased viral load at onset, resulting in a mild clinical course in HSCT recipients. These findings should be confirmed in a larger series, along with an evaluation of cellular immunity.
Acknowledgments
We thank Michiaki Takahashi and the staff of The Research Foundation for Microbial Diseases of Osaka University (Osaka, Japan) for their technical support and advice in the analysis of the serum samples.
Footnotes
Published ahead of print on 11 October 2006.
REFERENCES
- 1.Arvin, A. M. 2000. Varicella-zoster virus: pathogenesis, immunity, and clinical management in hematopoietic cell transplant recipients. Biol. Blood Marrow Transplant. 6:219-230. [DOI] [PubMed] [Google Scholar]
- 2.Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2767. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 3.Centers for Disease Control and Prevention. 2004. CDC guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Morbid. Mortal. Wkly. Rep. 49:RR-10. [PubMed] [Google Scholar]
- 4.Han, C. S., W. Miller, R. Haake, and D. Weisdorf. 1994. Varicella-zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 13:277-283. [PubMed] [Google Scholar]
- 5.Kalter, Z. G., S. Steinberg, and A. A. Gershon. 1997. Immune adherence hemagglutination: further observations on demonstration of antibody to varicella-zoster virus. J. Infect. Dis. 135:1010-1013. [DOI] [PubMed] [Google Scholar]
- 6.Leung, T. F., K. W. Chik, C. K. Li, M. M. K. Shing, P. K. S. Chan, V. Lee, and P. M. P. Yuen. 2000. Incidence, risk factors and outcome of varicella-zoster virus infection in children after hematopoietic stem cell transplantation. Bone Marrow Transplant. 25:167-172. [DOI] [PubMed] [Google Scholar]
- 7.Mazur, M. H., R. J. Whitley, and R. Dolin. 1979. Serum antibody levels as risk factors in the dissemination of herpes zoster. Arch. Intern. Med. 139:1341-1345. [PubMed] [Google Scholar]
- 8.Takahashi, M. 2004. Effectiveness of live varicella vaccine. Expert Opin. Biol. Ther. 4:199-216. [DOI] [PubMed] [Google Scholar]
- 9.Wacker, P., O. Hartmann, E. Benhamou, E. Salloum, and J. lemerle. 1989. Varicella-zoster virus infections after autologous bone marrow transplantation in children. Bone Marrow Transplant. 4:191-194. [PubMed] [Google Scholar]
- 10.Zaia, J. A., M. J. Levin, S. R. Preblud, et al. 1983. Evaluation of varicella-zoster immune globulin: protection of immunosuppressed children after household exposure to varicella. J. Infect. Dis. 149:737-743. [DOI] [PubMed] [Google Scholar]