Abstract
Sixteen strains of Escherichia coli isolated between January and June 2005 in a hospital in Algiers carry the ISEcp1 element and the TEM and either CTX-M-3 (n = 3) or CTX-M-15 (n = 13) β-lactamases. Fourteen of the isolates are multidrug resistant. Five isolates from the neonatal ward were indistinguishable by pulsed-field gel electrophoresis.
CTX-M-type enzymes are the extended-spectrum β-lactamases (ESBL) most commonly produced by Enterobacteriaceae (4), and more than 55 CTX-M-type β-lactamases have been described (http://www.lahey.org/studies/webt.htm). Despite the prevalence of ESBL in Enterobacteriaceae, data from Algeria are scarce (although the prevalence has been reported to be 20 to 45%) (18). We investigated the phenotypic and genetic profiles of clinical Escherichia coli ESBL producers isolated in an Algerian hospital.
(This work was presented at the 16th European Congress of Clinical Microbiology and Infectious Diseases, abstract P509, 2006.)
Between January and June 2005, 279 nonduplicate E. coli strains were recovered consecutively from patients at the Mustapha Pacha Hospital (1,800 beds) of Algiers, Algeria, and routinely analyzed in the hospital's microbiology laboratory. All strains were identified with an API 20E System (bioMérieux, Marcy l'Étoile, France). Antimicrobial susceptibility was determined by disk diffusion according to the CLSI guidelines (16), and 22 (7.9%) of the strains were resistant to extended-spectrum cephalosporins. Only 16 of these 22 were available for this study; related specimens, patient age, and ward of hospitalization are specified in Table 1. A double disk diffusion test (11) and Etest ESBL strips (AB Biodisk, Solna, Sweden), with cefotaxime and ceftazidime plus clavulanate, confirmed that all were ESBL producers.
TABLE 1.
Distribution, clinical features, and phenotypic and genotypic characteristics of 16 ESBL-producing E. coli strainsa
| Strain | Patient age | Specimen source | Hospital ward | pIs | β-Lactamases produced | MIC (μg/ml) ofb:
|
Other resistance marker(s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMC | AZT | CTX | CAZ | CAZ/CA | FOX | IMP | |||||||
| 50 | 23 yr | Wound | Orthopedic surgery | 5.4, 8.0 | TEM-1, AmpC, CTX-M-3 | ≤4/2 | 16 | >32 | 1 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, AN, CHL, SXT |
| 53 | 47 yr | Ascitic fluid | Gastroenterology | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | 8/4 | >16 | >32 | >16 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, AN, OFX, SXT |
| 95 | 4 yr | Urine | Pediatric | 5.4, 8.0 | TEM-1, AmpC, CTX-M-3 | ≤4/2 | 16 | >32 | ≤0.5 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, AN, SXT |
| 97 | 32 yr | Wound | Digestive surgery | 5.4, 8.0 | TEM-1, AmpC, CTX-M-3 | 8/4 | >16 | >32 | 1 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, SXT |
| 102 | 3 yr | Urine | Pediatric | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | >16/8 | >16 | >32 | >16 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, AN, CHL, SXT |
| 108 | 20 days | Urine | Pediatric | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | ≤4/2 | >16 | >32 | >16 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, SXT |
| 109 | 24 yr | Wound | Gastroenterology | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | ≤4/2 | >16 | >32 | 16 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB |
| 131 | 2 days | CSF | Neonatal | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | 8/4 | >16 | >32 | >16 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, SXT |
| 143 | 86 yr | Urine | Outpatient | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | 8/4 | >16 | >32 | >16 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, AN, CHL, OFX, SXT |
| 163 | 8 days | Blood | Neonatal | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | 8/4 | >16 | >32 | >16 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, SXT |
| 165 | 12 yr | Urine | Pediatric | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | >16/8 | >16 | >32 | >16 | >2 | >16 | ≤0.5 | GM, TOB, SXT |
| 168 | 14 yr | Blood | Pediatric | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | ≤4/2 | >16 | >32 | >16 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, SXT |
| 171 | 16 days | CSF | Neonatal | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | ≤4/2 | >16 | >32 | >16 | ≤0.5 | ≤4 | ≤0.5 | GM, SXT |
| 192 | 9 yr | Blood | Pediatric | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | ≤4/2 | >16 | >32 | >16 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, SXT |
| 229 | 3 days | Blood | Neonatal | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | ≤4/2 | >16 | >32 | >16 | ≤0.5 | ≤4 | ≤0.5 | GM |
| 254 | 8 yr | Wound | Pediatric surgery | 5.4, 8.9 | TEM-1, AmpC, CTX-M-15 | ≤4/2 | >16 | >32 | >16 | ≤0.5 | ≤4 | ≤0.5 | GM, TOB, AN, OFX, SXT |
CSF, cerebrospinal fluid; AMC, amoxicillin-clavulanic acid; AZT, aztreonam; CTX, cefotaxime; CAZ, ceftazidime; CAZ/CA, ceftazidime-clavulanic acid; FOX, cefoxitin; IMP, imipenem; GM, gentamicin; TOB, tobramycin; AN, amikacin; CHL, chloramphenicol; SXT, trimethoprim-sulfamethoxazole; and OFX, ofloxacin.
When two values separated by a slash are given for the MIC, the first value is of the antibiotic alone and the second value is of the antibiotic in the presence of clavulanate.
All 16 isolates were positive for blaTEM- and blaCTX-M-related genes and negative for blaOXA and blaSHV genes as assessed by PCR using previously described specific primers (13), and all isolates carried the ubiquitous ampC gene (13). Isoelectric focusing confirmed that all strains expressed both TEM-derived (pI of 5.4) and CTX-M-derived (pI of 8.0 and 8.9) enzymes (Table 1). The presence of an ISEcp1 element upstream from blaCTX-M genes and the absence of IS26 and IS903 elements were shown by PCR experiments (8). ExoSAP IT (USB Corporation, Cleveland, Ohio) was used for purification of PCR products, which were sequenced with an ABI3100 automatic sequencer (Applied Biosystems, Warrington, United Kingdom). Thirteen isolates carried the blaCTX-M-15 gene and three the blaCTX-M-3 gene; 16 isolates carried a blaTEM-1B-type gene.
MICs of antibiotics were determined by broth microdilution (MicroScan panel Sólo 1S; Dade Behring, West Sacramento, California): 100% of strains were resistant to gentamicin, 31% to amikacin, 88% to cotrimoxazole, and 19% to ciprofloxacin; 88% of strains were multidrug resistant (Table 1). The 13 isolates carrying both TEM and CTX-M-15 enzymes were more resistant to ceftazidime (with MICs >16 μg/ml) than the 3 CTX-M-3-plus-TEM producers (with MICs ≤0.5 to 1 μg/ml). CTX-M-15, which harbors the Asp240→Gly substitution, confers higher levels of resistance to ceftazidime than its parental enzyme CTX-M-3 (17).
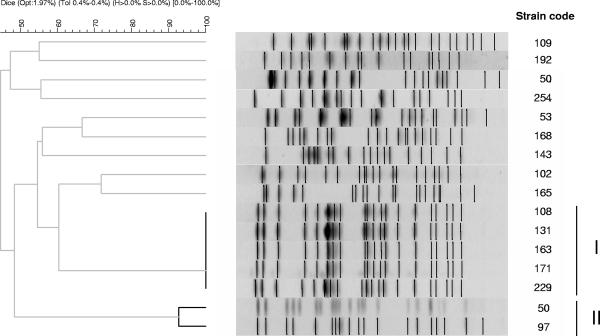
The diversity of the isolates was investigated by a protocol for pulsed-field gel electrophoresis (PFGE) modified from that previously described (6), using XbaI-digested genomic DNA as suggested for E. coli (7) (Fig. 1). PFGE was performed on a CHEF MAPPER PFGE apparatus (Bio-Rad, Hercules, California) using a run time of 24 h, with initial and final switch times of 0.1 s and 36 s, respectively. Strain INSRA5754 and the Lambda ladder (Biolabs, Beverly, MA) were used as markers for intragel normalization and intergel comparison. The PFGE profiles of five isolates producing CTX-M-15 β-lactamase from the neonatal ward were indistinguishable (100% similarity; cluster I). This suggests the spread of an epidemic clone. Two other clones producing CTX-M-3 β-lactamase were closely related (with >90% similarity; cluster II). The PFGE profiles of the other isolates were heterogeneous.
FIG. 1.
Genetic relatedness of the 16 E. coli strains as assessed by PFGE. A band position tolerance of 0.4% was used in PFGE pattern analysis with the Dice band-based similarity coefficient. Strain code numbers are shown on the right. Clones in clusters I and II (indicated by vertical bands on the right) contain isolates of >80% similarity. Clones in cluster I were 100% identical, and those in cluster II had >90% similarity.
ESBL-positive Enterobacteriaceae are frequently isolated in hospitals in Algeria, and the overall frequency of ESBL producers at the Mustapha Pacha hospital from January to June 2005 was 20.4% (n = 217 of the 1,066 Enterobacteriaceae isolates): 22 of 279 (7.9%) E. coli isolates, 131 of 259 (50.6%) Klebsiella sp. isolates, 8 of 131 (6.1%) Proteus sp. isolates, 35 of 90 (38.9%) Enterobacter sp. isolates, 13 of 48 (27.1%) Serratia sp. isolates, 2 of 19 (10.5%) Morganella morganii isolates, 4 of 18 (22.2%) Citrobacter sp. isolates, and 2 of 14 (14.3%) Salmonella sp. isolates. CTX-M-15 has been described in Asia, Europe, and recently Africa (2, 9, 10, 12, 19) in both nosocomial and community-acquired E. coli isolates (14, 21). Several studies in African countries report a high prevalence of ESBL-producing Enterobacteriaceae (3, 10, 18, 19). There have been reports of ESBL producers in North Africa: TEM-3 in S. enterica serovar Typhimurium in Morocco (1), CTX-M-27 in S. enterica serovar Livingstone in Tunisia (5), and CTX-M-3 in S. enterica serovar Senftenberg in Algeria (15). The frequency of Enterobacteriaceae producing ESBL in Algeria has not been reported.
The production of similar TEM and CTX-M-type enzymes in various genetically related strains and in isolates from different wards of the hospital suggests horizontal transfer of the corresponding genes. Five CTX-M-15-producing isolates were genetically indistinguishable; they were isolated from patients in the neonatal ward, except for isolate 108, which was from a patient hospitalized elsewhere for 20 days but who had previously been in this ward. Three of the patients in the neonatal ward were preterm (with 31 to 34 weeks): the patient infected with strain 171 had nosocomial meningitis, and the patients with isolates 131 and 229 had probably acquired the infection by transmission from the mother. The two cases of meningitis were cured, but the case of bacteremia was fatal.
Invasive infections due to E. coli isolates that produce ESBL are a major problem in neonates, because the choice of drug is restricted. The widespread use of cefotaxime and ceftriaxone has been suggested to have favored the emergence of CTX-M enzymes (20). However, treatment of infections with ESBL-producing strains in this hospital usually does not involve those antibiotics for meningitis. Therefore, this hospital may have experienced the spread of an epidemic clone not directly due to antibiotic selection pressure but with ISEcp1 insertion sequences, involved in the mobilization of CTX-M-enzymes, contributing to the process. Dissemination of community clones in the hospital environment is also a possibility.
To our knowledge, this is the first report of CTX-M enzymes in E. coli from Algeria. We show that CTX-M-15 is widespread among E. coli isolates which are multidrug resistant, substantially restricting therapeutic alternatives. Implementation of a strict hospital infection control policy associated with efforts to promote judicious use of antibiotics is needed. Continuous monitoring of ESBL-producing Enterobacteriaceae in the community and the hospital setting is also required.
Footnotes
Published ahead of print on 20 September 2006.
REFERENCES
- 1.AitMhand, R., A. Soukri, N. Moustaoui, H. Amarouch, N. ElAdaghri, D. Sirot, and M. Benbachir. 2002. Plasmid-mediated TEM-3 extended-spectrum β-lactamase production in Salmonella typhimurium in Casablanca. J. Antimicrob. Chemother. 49:169-172. [DOI] [PubMed] [Google Scholar]
- 2.Baraniak, A., J. Fiett, W. Hryniewics, P. Nordman, and M. Gniadowski. 2002. Ceftazidime hydrolysing CTX-M-15 extended-spectrum-β-lactamase (ESBL) in Poland. J. Antimicrob. Chemother. 50:393-396. [DOI] [PubMed] [Google Scholar]
- 3.Blomberg, B., R. Jureen, K. P. Manji, B. S. Tamim, D. S. M. Mwakagile, W. K. Urassa, M. Fataki, V. Msangi, M. G. Tellevik, S. Y. Maselle, and N. Langeland. 2005. High rate of fatal cases of pediatric septicemia caused by gram-negative bacteria with extended-spectrum β-lactamases in Dar es Salaam, Tanzania. J. Clin. Microbiol. 43:745-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet, R. 2004. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouallègue-Godet, O., Y. Bensalem, L. Fabre, M. Demartin, P. A. D. Grimont, R. Mzoughi, and F. X. Weill. 2005. Nosocomial outbreak caused by Salmonella enterica serotype Livingstone producing CTX-M-27 extended-spectrum β-lactamase in a neonatal unit in Sousse, Tunisia. J. Clin. Microbiol. 43:1037-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caniça, M., R. Dias, M. V. Vaz-Pato, and C. Carvalho. 2003. Two major Spanish clones of penicillin-resistant Streptococcus pneumoniae in Portuguese isolates of clinical origin. J. Antimicrob. Chemother. 51:409-414. [DOI] [PubMed] [Google Scholar]
- 7.Davis, M. A., D. D. Hancock, T. E. Besser, and D. R. Call. 2003. Evaluation of pulsed-field gel electrophoresis as a tool for determining the degree of genetic relatedness between strains of Escherichia coli O157:H7. J. Clin. Microbiol. 41:1843-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckert, C., V. Gautier, M. Saladin-Allard, N. Hidri, C. Verdet, Z. Ould-Hocine, G. Barnaud, F. Delisle, A. Rossier, T. Lambert, A. Philippon, and G. Arlet. 2004. Dissemination of CTX-M-type β-lactamases among clinical isolates of Enterobacteriaceae in Paris, France. Antimicrob. Agents Chemother. 48:1249-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelstein, M., M. Pimkin, I. Palagin, I. Edelstein, and L. Stratchounski. 2003. Prevalence and molecular epidemiology of CTX-M extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob. Agents Chemother. 47:3724-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangoue-Pieboji, J., V. Miriagou, S. Vourli, E. Tzelepi, P. Ngassam, and L. S. Tzouvelekis. 2005. Emergence of CTX-M-15-producing enterobacteria in Cameroon and characterization of a blaCTX-M-15-carrying element. Antimicrob. Agents Chemother. 49:441-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 12.Karim, A., L. Poirel, S. Nagarajan, and P. Nordmann. 2001. Plasmid-mediated extended-spectrum β-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol. Lett. 201:237-241. [DOI] [PubMed] [Google Scholar]
- 13.Mendonça, N., D. Louro, A. P. Castro, J. Diogo, and M. Caniça. 2006. CTX-M-15, OXA-30 and TEM-1-producing Escherichia coli in two Portuguese regions. J. Antimicrob. Chemother. 57:1014-1016. [DOI] [PubMed] [Google Scholar]
- 14.Moubareck, C., Z. Daoud, N. L. Hakimé, M. Hamzé, N. Mangeney, H. Matta, J. E. Mokhbat, R. Rohban, D. K. Sakis, and F. Doucet-Populaire. 2005. Countrywide spread of community- and hospital-acquired extended-spectrum β-lactamase (CTX-M-15)-producing Enterobacteriaceae in Lebanon. J. Clin. Microbiol. 43:3309-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naas, T., A. Lezzar, C. Bentchouala, F. Smati, J. M. Scheftel, H. Monteil, and P. Nordman. 2005. Mutidrug-resistant Salmonella enterica serotype Senftenberg isolates producing CTX-M β-lactamases from Constantine, Algeria. J. Antimicrob. Chemother. 20:439-440. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2004. Performance standards for antimicrobial susceptibility testing: 14th informational supplement. M100-S14. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Poirel, L., M. Gniadkowski, and P. Nordmann. 2002. Biochemical analysis of ceftazidime-hydrolyzing extended-spectrum β-lactamase CTX-M-15 and its structurally related β-lactamase CTX-M-3. J. Antimicrob. Chemother. 50:1031-1034. [DOI] [PubMed] [Google Scholar]
- 18.Ramdani-Bouguessa, N., F. Slimani, A. El Ksouri, and R. Denine. 2001. Antimicrobial susceptibilities of Enterobacteriaceae, Pseudomonas spp and Acinetobacter spp isolated from an Algerian hospital, abstract P 1345. 11th European Congress of Clinical Microbiology and Infectious Diseases.
- 19.Soge, O., A. M. Queenan, K. K. Ojo, B. A. Adeniyi, and M. C. Roberts. 2006. CTX-M-15 extended-spectrum β-lactamase from Nigerian Klebsiella pneumoniae. J. Antimicrob. Chemother. 57:24-30. [DOI] [PubMed] [Google Scholar]
- 20.Wang, H., S. Kelkar, W. Wu, M. Chen, and J. P. Quinn. 2003. Clinical isolates of Enterobacteriaceae producing extended-spectrum β-lactamases: prevalence of CTX-M-3 at a hospital in China. Antimicrob. Agents Chemother. 47:790-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodford, N., M. E. Ward, M. E. Kaufmann, J. Turton, E. J. Fagan, D. James, A. P. Johnson, R. Pike, M. Warner, T. Cheasty, A. Pearson, S. Harry, J. B. Leach, A. Loughrey, J. A. Lowes, R. E. Warren, and D. M. Livermore. 2004. Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum β-lactamases in the UK. J. Antimicrob. Chemother. 54:735-743. [DOI] [PubMed] [Google Scholar]