Abstract
The Clinical and Laboratory Standards Institute (CLSI; formerly National Committee for Clinical Laboratory Standards, or NCCLS) M38-A standard for the susceptibility testing of filamentous fungi does not specifically address the testing of dermatophytes. In 2003, a multicenter study investigated the reproducibility of the microdilution method developed at the Center for Medical Mycology, Cleveland, Ohio, for testing the susceptibility of dermatophytes. Data from that study supported the introduction of this method for testing dermatophytes in the future version of the CLSI M38-A standard. In order for the method to be accepted by CLSI, appropriate quality control isolates needed to be identified. To that end, an interlaboratory study, involving the original six laboratories plus two additional sites, was conducted to evaluate potential candidates for quality control isolates. These candidate strains included five Trichophyton rubrum strains known to have elevated MICs to terbinafine and five Trichophyton mentagrophytes strains. Antifungal agents tested included ciclopirox, fluconazole, griseofulvin, itraconazole, posaconazole, terbinafine, and voriconazole. Based on the data generated, two quality control isolates, one T. rubrum isolate and one T. mentagrophytes isolate, were identified and submitted to the American Type Culture Collection (ATCC) for inclusion as reference strains. Ranges encompassing 95.2 to 97.9% of all data points for all seven drugs were established.
The Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) reference method for broth dilution antifungal susceptibility testing of filamentous fungi (M38-A) (2) does not address the antifungal susceptibility of the dermatophyte Trichophyton, Microsporum, and Epidermophyton species, in which conidium formation is sometimes problematic. As part of the CLSI subcommittee's efforts to standardize antifungal susceptibility testing, an interlaboratory study was conducted to determine the reproducibility of the MIC testing method of common dermatophyte strains using the microdilution method developed at the Center for Medical Mycology (4, 5). Results from that study indicate excellent reproducibility of MIC data for dermatophyte isolates (3). As with the standardization of yeast and filamentous mold susceptibility testing, studies were needed to establish quality control (QC) guidelines for this new methodology (6). The antifungal drugs used to treat dermatophytoses differ from those used against yeasts and other filamentous molds. For instance, ciclopirox, griseofulvin, and terbinafine must be included in any dermatophyte susceptibility panel. Further, the MICs of the azole class of antifungals, including fluconazole, itraconazole, posaconazole, and voriconazole, are characteristically low against dermatophytes. Therefore, appropriate QC dermatophyte strains needed to be identified as guidelines for susceptibility testing with these additional drugs.
The purpose of this multicenter study was to identify QC strains for dermatophyte susceptibility testing and to establish MIC ranges for a panel of antifungal drugs against these strains.
MATERIALS AND METHODS
Study participants.
Eight laboratories at the following institutions participated in this interlaboratory study: Center for Medical Mycology, University Hospitals/Case Western Reserve University, Cleveland, Ohio; Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia; Department of Health, State of New York, Albany, New York; VCU Medical Center, Richmond, Virginia; Department of Pathology, University of Iowa College of Medicine, Iowa City, Iowa; National Centre for Mycology, University of Alberta Hospital, Edmonton, Alberta, Canada; Laboratory Service, University of Texas Health Science Center, Audie L. Murphy Memorial Veterans Hospital, San Antonio, Texas; and Pediatric Oncology Branch, National Cancer Institute, Bethesda, Maryland.
Isolates.
A total of 10 different dermatophyte strains were tested against the seven antifungals tested in the initial interlaboratory study to validate the method. The organisms were taken from the culture collection at the Center for Medical Mycology and included five strains of Trichophyton rubrum shown to have elevated MICs to terbinafine and five strains of Trichophyton mentagrophytes. These isolates had originally been subcultured onto potato dextrose agar (PDA) slants, incubated at 30°C until luxuriant, and frozen at −80°C. Stored isolates were thawed and subcultured onto potato dextrose agar plates, from which sets of new slants were prepared and shipped.
MIC panels.
MIC trays of antifungal dilutions were prepared by TREK Diagnostics, Westlake, OH, in three different lots of RPMI and supplied frozen to the sites, along with corresponding lots of RPMI broth. The antifungal ranges were as follows: ciclopirox (Dermik Laboratories), 0.06 to 32.0 μg/ml; fluconazole (Pfizer Inc.), 0.125 to 64 μg/ml; griseofulvin (Sigma Chemical Co.), 0.015 to 8.0 μg/ml; itraconazole (Ortho-McNeil, Inc.), 0.001 to 0.5 μg/ml; posaconazole (Schering-Plough), 0.004 to 2.0 μg/ml; terbinafine (Novartis Pharma), 0.002 to 1.0 μg/ml; and voriconazole (Pfizer Inc.), 0.001 to 0.5 μg/ml.
Susceptibility method.
The method used to determine the susceptibility of these dermatophytes to the antifungals was based on the publications of Norris et al. (5) and Jessup et al. (4). T. mentagrophytes isolates were subcultured onto PDA and incubated at 30°C for 4 to 5 days or until good conidiation was produced.
T. rubrum isolates were subcultured onto cereal agar (100 g Del Monte baby oatmeal cereal, 15 g granulated agar, and 0.03 g gentamicin per liter) instead of PDA in order to induce conidium production. For T. rubrum strains, turbidity produced by transference of oatmeal agar precluded the use of McFarland standards to standardize the inoculum. Therefore, cell counts using a hemacytometer were needed in order to standardize inocula. A suspension of conidia in sterile saline was made by gently swabbing the colony surface with a sterile swab. The suspension was allowed to settle for 5 to 10 min to remove heavier particles, and conidia were counted using a hemacytometer. Dilutions of this conidial suspension were then prepared in 10 ml of each lot (three lots were evaluated) of RPMI 1640, adjusted to a final concentration of 1 × 103 to 3 × 103 CFU/ml. Microtiter plates were removed from the freezer and allowed to thaw. Each drug concentration well and growth control well was inoculated with 100 μl of cell suspension using a multichannel pipette. The final volume in each well was 200 μl. Microtiter plates were incubated at 35°C for 4 days or until good growth (confluent hyphal growth covering the bottom of the well) was obtained in the growth control well (some strains required incubation of up to 7 days to obtain good growth). Plates were examined visually for 50% and 80% growth inhibition compared to the growth control. MIC results at both inhibition endpoints were recorded in μg/ml.
Data analysis.
Each strain was tested 10 times in each RPMI lot and in each laboratory, resulting in 240 MICs per isolate per antifungal. All reported results were included in the data analysis, and results were considered in agreement if they fell within 2 dilutions. The method of analysis was that specified by the CLSI M23-A2 document (1), which sets a proposed QC range to be the modal MIC plus or minus one log2 dilution. This will result in a 3-dilution range for approximately 50% of the drug-strain combinations. If the 3-dilution range does not encompass ≥95% of the data points, then the proposed range is expanded by 1 dilution in an effort to incorporate >95% of the observed results. If the MIC distribution is bimodal, then a range that is one log2 dilution lower than the first mode and one log2 dilution higher than the second mode is proposed. In all cases, the proposed range must encompass ≥95% of the observed data.
RESULTS AND DISCUSSION
Eight laboratories tested 10 candidate QC strains, including five T. rubrum and five T. mentagrophytes strains. Sites were asked to record endpoints of 50% and 80% inhibition compared to growth control. While no significant differences were noted from different lot numbers of RPMI, 50% inhibition endpoints were widely inconsistent. In contrast, reproducible results were obtained from 80% inhibition endpoints. There exists an inherent 1-dilution variation in MIC microdilution testing, and a 2-dilution difference meets the generally accepted criteria for reproducibility (1).
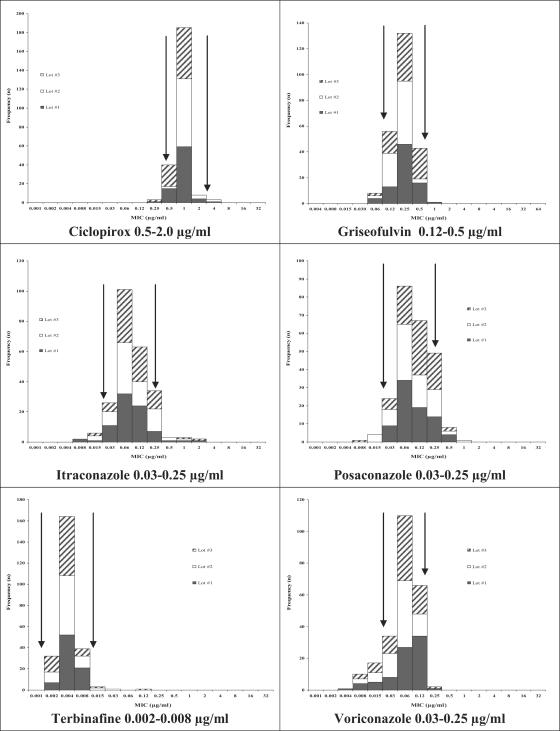
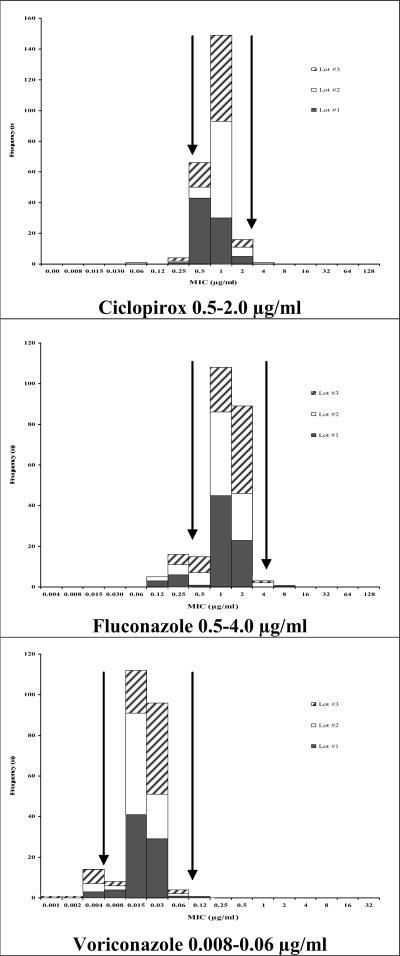
There was excellent interlaboratory agreement with all antifungals against the T. mentagrophytes MRL1957 strain, with the exception of fluconazole. Interlaboratory agreement for fluconazole against this T. mentagrophytes strain was <95%, and therefore no range was established. Interlaboratory agreement was achieved with three antifungals against the T. rubrum MRL666 strain. Although this isolate is known to have an elevated MIC to terbinafine (>1 μg/ml), the testing range for terbinafine needs to be well below that in order to determine the MIC endpoints of the vast majority of dermatophyte isolates. Therefore, to avoid “open-ended” QC ranges, no terbinafine MIC range was recommended for this isolate. Further, interlaboratory agreement for griseofulvin, itraconazole, and posaconazole against the T. rubrum strain was <95%, and no reference ranges were established. The ranges and interlaboratory agreement for all seven antifungal agents against the selected reference strains are listed in Table 1.
TABLE 1.
Antifungal MIC ranges for QC strains
| Organism | Antifungal agent | MIC range (μg/ml)a | Mode (μg/ml) | % of MICs within range |
|---|---|---|---|---|
| T. mentagrophytes | Ciclopirox | 0.5-2.0 | 1.0 | 97.5 |
| MRL1957 (ATCC | Griseofulvin | 0.12-0.5 | 0.25 | 96.3 |
| pending) reference strain | Itraconazole | 0.03-0.25 | 0.06 | 96.2 |
| Posaconazole | 0.03-0.25 | 0.06 | 95.2 | |
| Terbinafine | 0.002-0.008 | 0.004 | 97.9 | |
| Voriconazole | 0.03-0.25 | 0.06 | 95.2 | |
| T. rubrum MRL666 (ATCC | Ciclopirox | 0.5-2.0 | 1.0 | 97.5 |
| pending) reference strain | Fluconazole | 0.5-4.0 | 1.0 | 95.2 |
| Voriconazole | 0.008-0.06 | 0.015 | 96.1 |
These ranges are based on >95% inclusion of all data points.
Although it was not possible to establish a QC range for each drug-isolate combination, it is important to establish a range for each of these antifungals against at least one strain, as this panel of drugs is widely used in the treatment of dermatophytoses. Based on the highest interlaboratory agreement among the candidate strains tested, the CLSI committee selected two strains, T. mentagrophytes MRL1957 and T. rubrum MRL666, as reference isolates. These isolates have been submitted to the American Type Culture Collection (ATCC) for inclusion as reference strains. Figures 1 and 2 illustrate the distribution of MICs for these isolates.
FIG. 1.
Proposed antifungal MIC ranges for T. mentagrophytes MRL1957, as determined in three different lots of RPMI 1640. Arrows indicate suggested ranges.
FIG. 2.
Proposed antifungal MIC ranges for T. rubrum MRL666, as determined in three different lots of RPMI 1640. Arrows indicate suggested ranges.
Based upon the results of these two studies, this method has been adopted as an amendment to the CLSI M38-A standard for the testing of dermatophytes. Correlation between in vitro dermatophyte MICs and clinical outcomes remains to be determined.
Acknowledgments
The Center for Medical Mycology thanks Dermik Laboratories, Pfizer Inc., and Schering-Plough Research Institute for their generous support of this study.
We also thank Steven D. Brown, Clinical Microbiology Institute, Wilsonville, OR, for collating the data.
Footnotes
Published ahead of print on 18 October 2006.
REFERENCES
- 1.CLSI/NCCLS. 2001. Development of in vitro susceptibility testing criteria and quality control parameters: approved guideline, 2nd ed. NCCLS document M23-A2. NCCLS, Wayne, Pa.
- 2.CLSI/NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard. NCCLS document M38-A. NCCLS, Wayne, Pa.
- 3.Ghannoum, M. A., V. Chaturvedi, A. Espinel-Ingroff, M. A. Pfaller, M. G. Rinaldi, W. Lee-Yang, and D. W. Warnock. 2004. Intra- and interlaboratory study of a method for testing the antifungal susceptibilities of dermatophytes. J. Clin. Microbiol. 42:2977-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessup, C. J., J. Warner, N. Isham, I. Hasan, and M. A. Ghannoum. 2000. Antifungal susceptibility testing of dermatophytes: establishing a medium for inducing conidial growth and evaluation of susceptibility of clinical isolates. J. Clin. Microbiol. 38:314-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris, H. A., B. E. Elewski, and M. A. Ghannoum. 2000. Optimal growth conditions for the determination of the antifungal susceptibility of three species of dermatophytes with the use of a microdilution method. J. Am. Acad. Dermatol. 40:S9-S13. [DOI] [PubMed] [Google Scholar]
- 6.Pfaller, M. A., M. Bale, B. Buschelman, M. Lancaster, A. Espinel-Ingroff, J. H. Rex, M. G. Rinaldi, C. R. Cooper, and M. R. McGinnis. 1995. Quality control guidelines for National Committee for Clinical Laboratory Standards recommended broth macrodilution testing of amphotericin B, fluconazole, and flucytosine. J. Clin. Microbiol. 33:1104-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]