Abstract
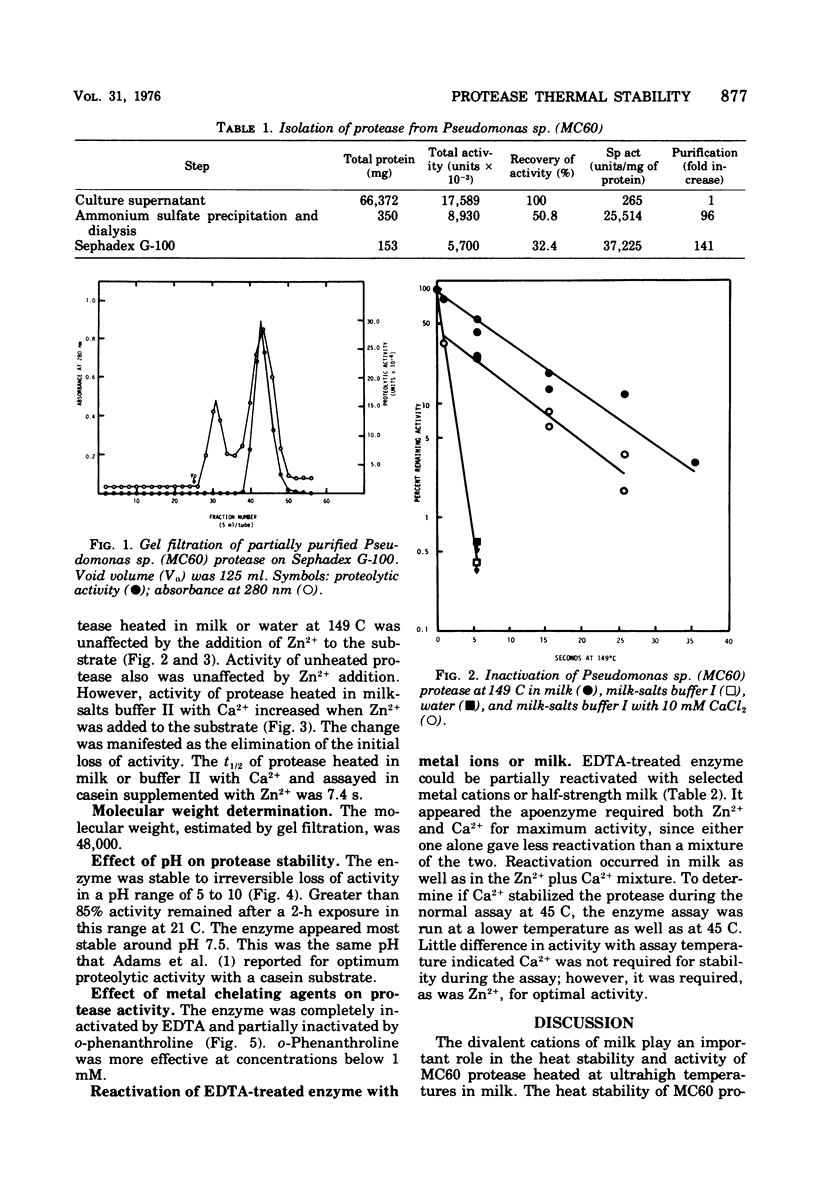
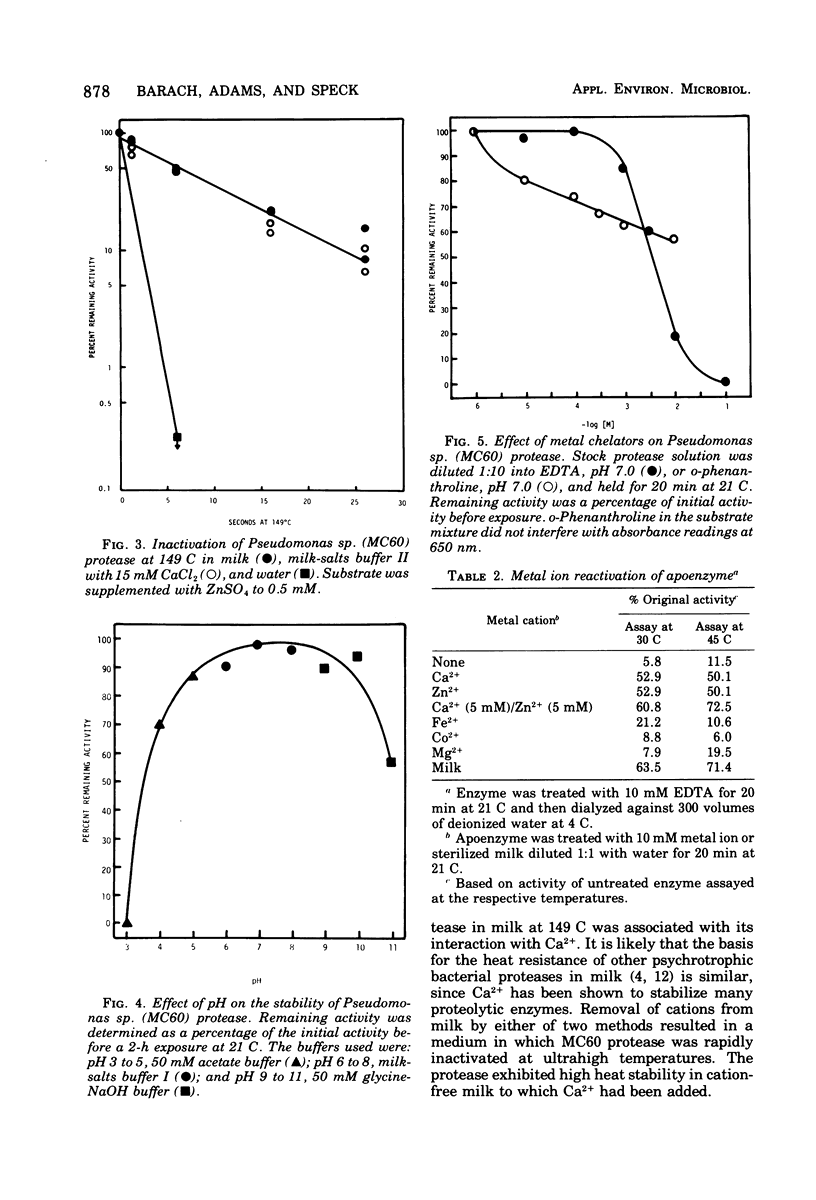
The heat-stable extracellular protease of Pseudomonas sp. (isolate MC60) was investigated. Heat resistance of the enzyme in milk at sterilization temperature was dependent on the presence of Ca2+. The half-life of the enzyme at ultrahigh temperature (149 C) in skim milk or milk-salts buffer with Ca2+ was approximately 7.0 s. Treatment of milk with chelators completely removed the heatstabilizing effect of milk. The enzyme was partially purified by ammonium sulfate precipitation and column chromatography on Sephadex G-100. At 21 C the enzyme retained greater than 85% activity after exposure to pH values between 5 and 10. Enzyme activity was reduced by metal chelating agents. Both Ca2+ and Zn2+ were required for optimal enzyme activity. Molecular weight was estimated at 48,000 by gel filtration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. M., Barach J. T., Speck M. L. Heat resistant proteases produced in milk by psychrotrophic bacteria of dairy origin. J Dairy Sci. 1975 Jun;58(6):828–834. doi: 10.3168/jds.s0022-0302(75)84645-5. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boethling R. S. Purification and properties of a serine protease from Pseudomonas matophilia. J Bacteriol. 1975 Mar;121(3):933–941. doi: 10.1128/jb.121.3.933-941.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN J. E., VALLEE B. L. Metallocarboxypeptidases. J Biol Chem. 1960 Feb;235:390–395. [PubMed] [Google Scholar]
- Feder J., Garrett L. R., Wildi B. S. Studies on the role of calcium in thermolysin. Biochemistry. 1971 Nov 23;10(24):4552–4556. doi: 10.1021/bi00800a032. [DOI] [PubMed] [Google Scholar]
- Friedberg F. Effects of metal binding on protein structure. Q Rev Biophys. 1974 Feb;7(1):1–33. doi: 10.1017/s0033583500001335. [DOI] [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H. PSEUDOMONAS AERUGINOSA PEPTIDE PEPTIDOHYDROLASE. 3. SOME CHARACTERS AS A CA2+-METALLOENZYME. Biochim Biophys Acta. 1964 Nov 22;92:351–360. [PubMed] [Google Scholar]
- Mayerhofer H. J., Marshall R. T., White C. H., Lu M. Characterization of a heat-stable protease of Pseudomonas fluorescens P26. Appl Microbiol. 1973 Jan;25(1):44–48. doi: 10.1128/am.25.1.44-48.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Mizusawa K., Yoshida F. Purification and properties of an extracellular proteinase of psychrophilic Escherichia freundii. Eur J Biochem. 1974 May 2;44(1):87–96. doi: 10.1111/j.1432-1033.1974.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Porzio M. A., Pearson A. M. Isolation of an extracellular neutral proteinase from Pseudomonas fragi. Biochim Biophys Acta. 1975 Mar 28;384(1):235–241. doi: 10.1016/0005-2744(75)90112-6. [DOI] [PubMed] [Google Scholar]
- Siegel S., Awad W. M., Jr The proteolytic enzymes of the K-1 strain of Streptomyces griseus obtained from a commercial preparation (Pronase). IV. Structure-function studies of the two smallest serine endopeptidases; stabilization by glycerol during reaction with acetic anhydride. J Biol Chem. 1973 May 10;248(9):3233–3240. [PubMed] [Google Scholar]



