Abstract
Background:
This study examines the interactive effects of acute stress and nicotine-associated contextual cues on locomotor activity and activity-dependent gene expression in subregions of the prefrontal cortex.
Methods:
Locomotor activity of rats was measured in a context associated with either low-dose nicotine or saline administration with or without 5 minutes of pre-exposure to ferrets, a nonphysical stressor. After 45 minutes in the test environment, plasma corticosterone levels and mRNA levels of the immediate-early genes Arc, NGFI-B, and c-Fos in prefrontal and primary motor cortical subregions were measured.
Results:
Stress alone increased plasma corticosterone and prefrontal cortex gene expression. Low-dose nicotine cues had no effect on corticosterone levels nor did they elicit conditioned motor activation, and they caused minor elevations in gene expression. Stress and low-dose nicotine cues, however, interacted to elicit conditioned motor activation and further increases in early response gene expression in prefrontal but not in the primary motor cortical subregions.
Conclusions:
Stress interacts with nicotine-associated cues to uncover locomotor arousal, a state associated with prefrontal neuronal activation and immediate early gene expression. Thus, in nicotine-experienced individuals, stress may be an important determinant of subjective reactivity and prefrontal cortex activation that occurs in response to nicotine-associated cues.
Keywords: Addiction, relapse, reinstatement, craving, cigarette, imaging
Though their primary molecular targets may differ, nicotine and other drugs of abuse can elicit similar changes in susceptible persons that may result in addiction. Plastic changes within the mesocorticolimbic system are thought to underlie this phenomenon (Hyman 2005). One hallmark of addiction is the difficulty in remaining abstinent from drug use, especially when confronted with stressors or cues associated with taking addictive drugs (Weiss 2005). How stress and conditioned cues are related with regard to their influence on relapse is not well understood. A more mechanistic understanding of their interaction is necessary for the development of effective abstinence maintenance strategies.
Both stress and drug cues are known to individually precipitate reinstatement of drug-seeking behavior, including nicotine-seeking behavior, in rats (Buczek et al 1999; Caggiula et al 2001; Shaham et al 2003). In the case of alcohol, acute stress and cues associated with prior alcohol drinking can have additive, pharmacologically dissociable, effects on the reinstatement of alcohol-seeking behavior in rats (Liu and Weiss 2002). These findings suggest that stress and drug cues can influence behaviors related to drug use through distinct but interrelated neurochemical pathways. Based on these observations, it is possible that stress and nicotine-related cues are capable of interacting to influence locomotor arousal and nicotine-seeking behavior.
With regard to the brain regions subserving such potential interactions, the prefrontal cortex is a likely candidate. The prefrontal cortex is known to be exquisitely sensitive to stress (McEwen 2004) and is often activated by drug-associated cues in imaging and gene expression studies (Schiltz et al 2005; Schroeder et al 2000, 2001; Schroeder and Kelley 2002; Wilson et al 2004). In addition, activity in the prefrontal cortex is necessary for both stress- and cue-induced reinstatement of drug-seeking behavior (Fuchs et al 2004; Kalivas and Volkow 2005; McFarland et al 2004). Therefore, the prefrontal cortex is a site that may mediate the interactive effects of stress and drug-associated cues on behavior.
Measurement of early response gene induction provides an indirect measure of neuronal activation and as such can help to elucidate neural circuits activated by stress or drug cues. Messenger RNA (mRNA) levels of the AP-1 transcription factor c-Fos are used as an early measure of altered neuronal metabolic activity (Finkbeiner and Greenberg 1998; Herdegen and Leah 1998). Like c-fos, detection of the immediate early gene arc can be used to indirectly assess levels of neuronal activity (Guzowski et al 2001; Ramirez-Amaya et al 2005). Unlike the transcription factor c-Fos, Arc mRNA and protein are targeted to activate dendrites in an N-methyl-d-aspartate (NMDA) receptor-dependent manner and Arc protein presumably exerts effects on postsynaptic electrophysiological properties through its interaction with the endocytic machinery trafficking alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors (Fujimoto et al 2004; Steward and Worley 2001). Nerve growth factor-induced clone B (NGFI-B), an orphan nuclear receptor, belongs to a different family of transcription factors than c-Fos and is expressed in close association with dopaminergic targets in an activity-dependent manner (Liu and Baker 1999; Zetter-strom et al 1996). Increases in ngfi-b expression in response to cocaine are modulated by the glucocorticoid receptor suggesting that stress and cue-elicited dopamine may interact to influence this gene’s expression (Martens et al 2005; St-Hilaire et al 2003). Thus, in situ hybridization (ISH) analysis of these particular genes may allow detection of any putative interactive effects of stress and nicotine cues on neuronal activity.
Cues associated with nicotine administration are capable of eliciting conditioned locomotor activation (Bevins et al 2001; Bevins and Palmatier 2003; Palmatier and Bevins 2002; Walter and Kuschinsky 1989). Lower doses of nicotine fail to elicit this conditioned behavior (Bevins and Palmatier 2003). We gave rats a low-dose regimen of nicotine in distinct environmental contexts and on the test day, stressed half of the rats by exposure to a ferret prior to reintroduction into either a nicotine- or saline-paired context. Brief periods of ferret exposure have been shown to elicit increased corticosterone levels and anxiety-like behaviors including decreased open arm entries on the elevated plus maze in rodents (Adamec 2001; Plata-Salaman et al 2000). If stress and nicotine-associated cues are capable of interacting, we hypothesized that we should see differences in conditioned locomotor activation to nicotine cues between the stressed and unstressed groups. In addition, ISH for the immediate early genes Arc, NGFI-B, and c-Fos were carried out in the prefrontal cortex to address the possibility that both stress and nicotine cues can interact to influence activity within this region.
Methods and Materials
Subjects
The 16 male Sprague-Dawley rats (250-300 g) used in this study were housed in pairs in clear plastic cages in an animal colony. Food and water were available at all times except during training and testing. Lighting in the animal colony was on a 12-hour light/dark cycle with lights on 0700 to 1900 hours. Rats were handled daily for 3 days prior to the beginning of training. All animal care was in strict accordance with Institutional Animal Care and Use Committee guidelines.
Behavioral Paradigm
The experimental paradigm is illustrated in Figure 1A. Each rat received a daily, low-dose nicotine injection (.14 mg base/kg, subcutaneous [SC], pH 7.0, Sigma) in one of two environments with distinct sensory cues (context A or B) and a counterbalanced saline injection in the other environment for 14 days. Polycarbonate cages (19 × 8 × 10 inch, San Diego Instruments, San Diego, California) in context A contained wire mesh bottoms over aspen chips. Cages in context B were of the same material and dimensions but had a plain plastic floor and were scented with vanilla extract and in a different room than context A. Therefore, contexts A and B differed in olfactory, somatosensory, and visual cues. Locomotor activity was measured over the 14-day training phase by a photobeam activity system (San Diego Instruments, San Diego, California). Three days after this training procedure, all rats were re-exposed to context A (where half of the rats had previously received saline and the other half had received nicotine) after a mock injection including all the handling cues but no needle stick. Half of each of these groups was exposed to 5 minutes of predator stress immediately prior to the mock injections and placement into context A. Predator stress consisted of noncontact exposure to a ferret, an ethologically relevant, psychological stressor (Plata-Salaman et al 2000). Specifically, rats in the stressed groups were placed individually into protective wire cages (7.75 × 6 × 5.5 inch) that were then placed into a larger cage located within the room in which the ferrets were housed. A ferret was introduced into the larger cage and was allowed to explore the protective wire cage for 5 minutes. The protective wire cage allowed the rat to see, hear, and smell the ferret without permitting any physical contact between the animals. Individual ferrets were only given access to two rats in a day to avoid habituation of their response to the rats. After 45 minutes in context A, where locomotor activity was again measured, rats were anesthetized with halothane and rapidly decapitated for tissue collection.
Figure 1.

Schematic diagrams of the experimental paradigm and the brain regions analyzed for gene expression. (A)The experimental paradigm consisted of a 14-day training period in which all rats received one of two daily injections in two distinct environments (see Methods and Materials). Loco-motor activity was measured in context A over the training phase of the study. On the test day, 3 days following the final day of training, rats were reintroduced to context A with or without 5 minutes of ferret exposure immediately prior to reentry into context A. Locomotor activity was measured in context A for 45 minutes, after which time rats were sacrificed and trunk blood and brains were taken for plasma corticosterone assay and in situ hybridization analysis of immediate early gene expression in the pre-frontal and the primary motor cortices. (S.C. = saline cues, N.C. = nicotine cues). (B)The brain regions analyzed for activity-dependent gene expression via in situ hybridization included the prelimbic cortex (PreL), infralimbic cortex (InfrL), ventral orbital prefrontal cortex (VO), lateral orbital prefrontal cortex (LO), and the primary motor cortex (M1). Numbers represent distance from bregma in millimeters. S.C., saline cues; N.C., nicotine cues; PreL, prelimbic cortex; InfrL, infralimbic cortex; VO, ventral orbital prefrontal cortex; LO, lateral orbital prefrontal cortex; M1, primary motor cortex. (Adapted with permission from Paxinos and Watson 1998).
Tissue Isolation and Corticosterone Assay
After decapitation, approximately 10 mL of trunk blood was collected in 15-mL conical tubes containing 100 μL of 100 mmol/L ethylenediaminetetraacetic acid (EDTA) (Sigma). Tubes were kept on ice until centrifugation at 1700g at 4°C for 15 minutes. Plasma was then stored at -80°C until assayed. A rat corticosterone EIA kit (ICN Diagnostics, Costa Mesa, California) was used to quantify plasma corticosterone levels. Intra-assay variability was <10%.
Heads were cooled for 30 seconds in -70°C isopentane. Brains were rapidly dissected from cooled heads and frozen in -40°C isopentane. Frozen brains were kept at -80°C until they were used. Cryosections (20 μm) at the level of the prefrontal cortex from each of the brains were thaw-mounted onto Superfrost slides (Fischer Scientific), warmed at 57°C for 1 minute, and stored at -80°C for ISH.
In Situ Hybridization
Sections on slides were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 2 hours at 4°C. Slides were then washed for 5 minutes in 2X sodium chloride-sodium citrate (SSC) three times. Slides were incubated in .2 μg/mL Proteinase K (Qiagen, Hilden, GmbH, Germany) in .1 M Tris base (Fischer Scientific) and 50 mmol/L EDTA, pH 8.1, for 10 minutes at 37°C. Slides were washed in 2X SSC at room temperature for 2 minutes. Slides were incubated in .1 M triethanolamine (TEA) at room temperature with rapid stirring, and acetic anhydride was added to a final concentration of .25% (vol/vol) for 10 minutes. Slides were then washed in 2X SSC for 5 minutes. Finally, sections were dehydrated in an ascending ethanol series and air-dried for 15 minutes.
Templates for riboprobes were amplified from a rat brain complementary DNA (cDNA) library in the cases of Arc and NGFI-B or a BamHI linearized pBluescript-II-SK plasmid containing c-Fos sequence (kindly provided by T. Curran) in the case of c-Fos. The following primers were used to generate the templates using standard polymerase chain reaction (PCR) conditions: Arc, forward primer 5′-CCCCAGGAAGCTGATGGCTACGAC-3 (693-714), reverse primer 5 =-CAGAGATGCATAATACGACTCACTATAGGGAGA-GAGTGTCAGCCCCAGCTCAATCAAG-3 (1472-1494) and NGFI-B, forward primer 5′-GGTGTATGGCTGCTACCCTGG-3 ′ =(428-448), reverse primer 5′-CAGAGATGCATAATACGACTCACTATAGGGAG-AGAGTCCAAATGTGCTCGAATGAGG-3 (1208-1229). Numbers in parentheses after the primer sequence ′ represent the base number as defined by the gene sequence in the Unigene database. The underlined sequence corresponds to the T7 polymerase recognition site. Arc, NGFI-B, and c-Fos mRNAs peak about 30 minutes after behavioral stimulation (Chan et al 1993; Guzowski et al 2001). An in vitro transcription reaction using amplified template was used to generate 35S-labeled antisense probes. The in vitro transcription was carried out in 1X Transcription Optimized Buffer, 10 mmol/L dithiothreitol (DTT), 1 U/μL RNasin, .375 mmol/L adenosine 5-triphosphate (ATP), cytidine 5′-triphosphate (CTP), guanosine ′ 5-triphosphate (GTP), 1 U/μL T7 RNA polymerase (all Promega, ′ Madison, Wisconsin), 3.5 μCi/μL[α-35S]UTP (PerkinElmer), and 100 ng template DNA and incubated for 2 hours at 37°C. RQ1 RNase-free DNase (Promega) was added at a concentration of .15 U/mL and incubated for an additional 15 minutes at 37°C. The labeled probes were purified using ProbeQuant G-50 Micro columns (Amersham Biosciences, Piscataway, New Jersey). Radioactivity of labeled probe was determined via scintillation counting and riboprobe was diluted in hybridization solution (3X SSC, 10% dextran sulphate, 1X Denhardt’s solution, .2 mg/mL transfer ribonucleic acid [tRNA], 50 mmol/L NaPO4 buffer, and freshly added DTT to a final concentration of 50 mmol/L) to ∼106 cpm/100 μL. One hundred microliters of the hybridization solution at 55°C with labeled probe was applied to each slide. Slides were then coverslipped and incubated at 55°C in a hybridization chamber saturated with 75% formamide for 16 hours.
After hybridization, coverslips were removed and slides were washed in 2X SSC with 2 mmol/L DTT at room temperature for 10 minutes, three times. Slides were incubated in 1.5 U/mL RNase A (Qiagen) in RNase buffer (10 mmol/L Tris/HCl and .5 M NaCl, pH 8.0) at 37°C for 1 hour followed by washes in 1X SSC with 1 mmol/L DTT at room temperature for 5 minutes, .5X SSC with 1 mmol/L DTT at room temperature for 5 minutes, and .1X SSC with 2 mmol/L DTT at 70°C for 1 hour. The sections were then dehydrated in an ascending series of ethanol and then air-dried. Sections were exposed to a phosphorimager screen for 4 to 6 days. Screens were scanned on a Storm phosphorimager (GE Healthcare) and quantification of signal in particular brain regions (Figure 1B) was performed using ImageQuant software (GE Healthcare). Values for each hemisphere were averaged together to arrive at a single value for each region and each rat. Slides were then covered with NTB2 emulsion (Eastman Kodak Co., Rochester, New York) and exposed for 28 to 40 days for analysis of silver grain distribution. After development, slides were counterstained with Nissl stain and dehydrated through a graded series of ethanol and xylene. A coverslip was then applied. Images were taken with a Leica DC 300F digital camera linked to Image Pro-Plus software (JKN Electronics, Bellingham, Massachusetts) on a PC through a Leica DMRX microscope.
Statistical Analyses
Behavioral data from the activity cages were analyzed using StatView software (SAS Institute, Cary, North Carolina). For the 14-day nicotine treatment, a two-factor, between-within analysis of variance (ANOVA) was performed with treatment as the between-subjects factor and days as the within-subjects factor. For the corticosterone data and behavioral data on the test day, a two-factor ANOVA was performed with stress and cues as the between-subjects factors. Two kinds of statistical analysis were carried out on the ISH data. To determine regional effects, a three-factor, between-within ANOVA was carried out with stress and cues as between-subjects factors and brain region as the within-subjects factor. If overall interactions indicated significance, a post hoc, two-factor ANOVA was performed with stress and cues as the between-subjects factors for each individual brain region.
Results
Behavioral and Corticosterone Data
The nicotine treatment resulted in increased motor activity during conditioning. During the 14-day treatment period, nicotine (.14 mg/kg, SC) had a mild but significant stimulatory effect on locomotor activity in context A [F(1,14) = 26.087, p = .0002] (Figure 2A). There was a significant treatment × day interaction for locomotor activity over the 14-day training period, suggesting that behavioral activation following repeated nicotine administration became progressively greater [F(13,182) = 9.335, p < .0001] (Figure 2A).
Figure 2.
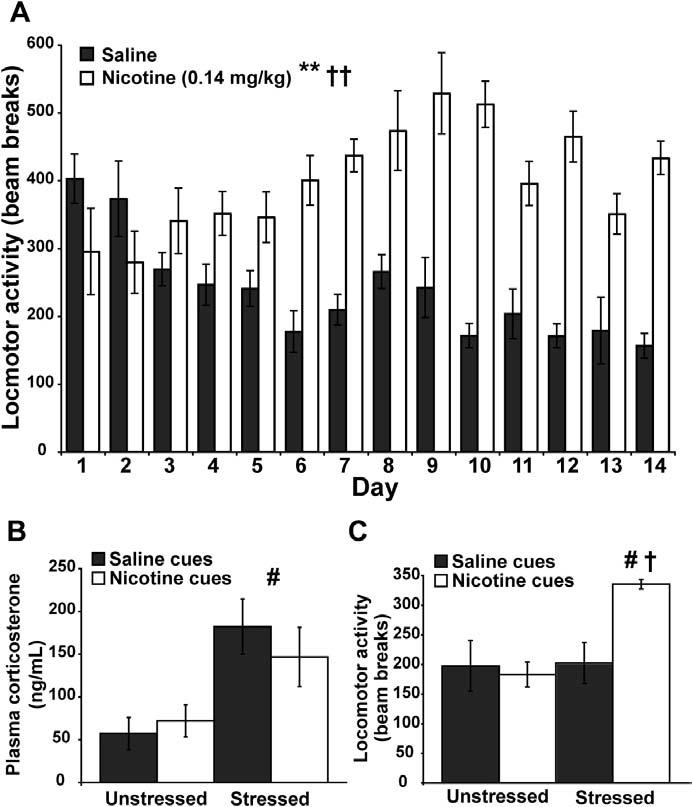
Behavioral and corticosterone data. (A)Daily locomotor activity in context A over the 14-day training period. Nicotine significantly potentiated locomotor activity over the training period (n = 8 per group, **p < .001). There was also a significant treatment × day interaction (††p < .01). (B)Plasma corticosterone levels on the test day. While cues did not have an effect on plasma corticosterone levels, rats exposed to predator stress exhibited significantly higher levels of plasma corticosterone than unstressed rats (n = 4 per group, #p < .05). (C)Locomotor activity data on the test day. There was a main effect of stress, but not of cues, on locomotor activity on the test day (n = 4 per group, #p < .05). There was also a significant interaction between stress and cues on locomotor activity, which was evident by increased motor activity in the group of rats stressed and exposed to nicotine cues (n = 4 per group, †p < .05).
Ferret stress activated the hypothalamic-pituitary-adrenal axis as measured via plasma corticosterone levels. On the test day, exposure to ferrets induced a significant increase in plasma corticosterone in the stressed groups compared with the unstressed groups [F(1,12) = 13.593, p = .0031], but no difference in plasma corticosterone was detected between the saline cues groups and the nicotine cues groups [F(1,12) = .147, p = .7083] (Figure 2B). No stress × cues interaction was observed in plasma corticosterone levels [F(1,12) = .866, p = .3705].
While there was no effect of nicotine cues alone on motor activity, rats that were stressed and exposed to nicotine cues on the test day exhibited conditioned locomotor arousal. There was a significant main effect of stress on locomotor activity on the test day [F(1,12) = 6.941, p = .0218]. Cues were without a main effect on test-day locomotor activity [F(1,12) = 3.954, p = .0700] (Figure 2C). Most notably, there was a significant stress × cues interaction on test-day locomotor activity [F(1,12) = 6.127, p = .0292].
In Situ Hybridization Data
Whereas low-dose nicotine cues alone exhibited a small effect on gene expression, low-dose nicotine cues in the presence of stress greatly increased the expression of the immediate early genes analyzed in the prefrontal cortex. Further, some general effects of stress and nicotine cues were noted. Overall, an increase in the expression of arc, ngfi-b, and c-fos was evident in the prefrontal cortex following predator stress. Similarly, nicotine cues increased expression of all of the immediate-early genes in the prefrontal cortex. Most notably, stress and nicotine cues interacted to further increase arc and c-fos expression in the prefrontal cortex. These results demonstrate that stress and nicotine cues can interact to specifically augment activity-regulated gene expression in prefrontal cortical subregions.
The arc ISH results can be found in Figure 3. A repeated measures ANOVA revealed significant main effects for arc expression in the regions analyzed by stress [F(1,12) = 157.116, p < .0001] and cues [F(1,12) = 13.174, p = .0035]. In addition, there was a significant stress × cue interaction for overall arc expression [F(1,12) = 6.463, p = .0258]. Post hoc two-factor ANOVAs within each region revealed significant differences in gene expression (Figure 3). There was a main effect of stress on arc expression in all of the areas analyzed. There was also a main effect of cues on arc expression in all of the regions analyzed except for the primary motor cortex. In addition, significant stress × cue interactions in prefrontal cortical subregions but not in the primary motor cortex existed for arc expression.
Figure 3.

Expression of arc in the rat brain in response to stress and nicotine cues. (A)Representative psuedocolor phosphorescence autoradiograms of coronal, right hemisphere sections from each of the experimental groups hybridized with a probe against arc. In each panel, the upper left pair of sections is from the unstressed/saline cues group, the upper right pair are from the unstressed/nicotine cues group, the lower left pair are from the stressed/saline cues group, and the lower right pair are from the stressed/nicotine cues group. Directly below each autoradiogram is a brightfield photomicrograph illustrating silver grain accumulation over an individual, large, lightly Nissl stained nuclei from the ventral orbital cortex (VO) and prelimbic cortex (PreL) taken from autoradiographic emulsions for each of the groups. This pattern of silver grain accumulation is consistent with a neuronal expression pattern for arc and was consistent across the prefrontal subregions analyzed. (B)Graphical representation of densitometric analysis for the prelimbic (PreL), infralimbic (InfrL), ventral orbital (VO), lateral orbital (LO), and primary motor (M1) cortices in each of the experimental groups for arc expression (n = 4 per group). There was a main effect of stress on arc expression in the PreL [F(1,12) = 118.611, p < .0001], InfrL [F(1,12) = 104.596, p < .0001], VO [F(1,12) = 235.464, p < .0001], LO [F(1,12) = 320.311, p < .0001], and the M1 [F(1,12) = 8.791, p = .0118] (#p < .05). There was also a main effect of cues on arc expression in the PreL [F(1,12) = 7.349, p = .0189], InfrL [F(1,12) = 8.725, p = .0121],VO[F(1,12)=16.157,p=.0017],andLO[F(1,12)=28.662,p=.0002],but not in the M1 [F(1,12) = 3.657, p = .0800] (*p < .05). In addition, stress and cues interacted to potentiate arc expression in the PreL [F(1,12) = 6.125, p = .0292], InfrL [F(1,12) = 8.335, p = .0136], VO [F(1,12) = 5.898, p = .0318], and LO [F(1,12) = 10.556, p = .0070] but not in the M1 [F(1,12) = .033, p = .8592] (†p < .05). VO, ventral orbital prefrontal cortex; PreL, prelimbic cortex; InfrL, infralimbic cortex; LO, lateral orbital prefrontal cortex; M1, primary motor cortex.
The ngfi-b ISH results are shown in Figure 4. A repeated measures ANOVA revealed significant treatment effects for ngfi-b expression in the regions analyzed for stress [F(1,12) = 100.782, p < .0001] and cues [F(1,12) = 14.348, p = .0026]. No interaction between stress and cues on ngfi-b expression was detected [F(1,12) = 2.760, p = .1225]. Post hoc two-factor ANOVAs within each region revealed significant differences in ngfi-b expression (Figure 4). There were main effects of stress on ngfi-b expression in the prefrontal cortical subregions analyzed but not in the primary cortex. There was a main effect of cues on ngfi-b expression in all of the regions analyzed.
Figure 4.

Expression of ngfi-b in the rat brain in response to stress and nicotine cues. (A)Representative psuedocolor phosphorescence autoradio-grams of coronal, right hemisphere sections from each of the experimental groups hybridized with a probe against ngfi-b (same layout as Figure 3). Brightfield photomicrographs illustrate silver grain accumulation over individual, large, lightly Nissl stained nuclei from the VO and PreL taken from autoradiographic emulsions for each of the groups. This pattern of silver grain accumulation is consistent with a neuronal expression pattern for ngfi-b and was consistent across the prefrontal subregions analyzed. (B)Graphical representation of densitometric analysis of ngfi-b expression in brain regions (n = 4 per group). There was a main effect of stress on ngfi-b expression in the PreL [F(1,12) = 84.448, p < .0001], InfrL [F(1,12) = 54.142, p < .0001], VO [F(1,12) = 108.905, p < .0001], and the LO [F(1,12) = 55.016, p < .0001] but not in the M1 [F(1,12) = 4.136, p = .0647] (#p < .05). There was also a main effect of cues on ngfi-b expression in the PreL [F(1,12) = 14.481, p = .0025], the InfrL [F(1,12) = 11.976, p = .0047], the VO [F(1,12) = 8.9466, p = .0113], the LO [F(1,12) = 7.128, p = .0204], and the M1 [F(1,12) = 12.112, p = .0045] (*p < .05).VO,ventralorbitalprefrontalcortex;PreL,prelimbiccortex;InfrL,infralimbic cortex; LO, lateral orbital prefrontal cortex; M1, primary motor cortex.
The c-fos ISH results are shown in Figure 5. A repeated measures ANOVA revealed significant main effects for c-fos expression in the regions analyzed by stress [F(1,12) = 285.038, p < .0001] and cues [F(1,12) = 19.564, p = .0008]. There was also a significant stress × cue interaction for c-fos expression in the regions analyzed [F(1,12) = 9.966, p = .0083]. Post hoc two-factor ANOVAs within each region revealed significant differences in c-fos expression (Figure 5). There was a main effect of stress in all of the prefrontal cortical subregions analyzed but not in the primary motor cortex. There was a significant effect of cues on c-fos expression in the prefrontal subregions analyzed but not in the primary motor cortex. We observed a significant stress × interaction on c-fos expression in the prefrontal cortical subregions but not in the primary motor cortex.
Figure 5.
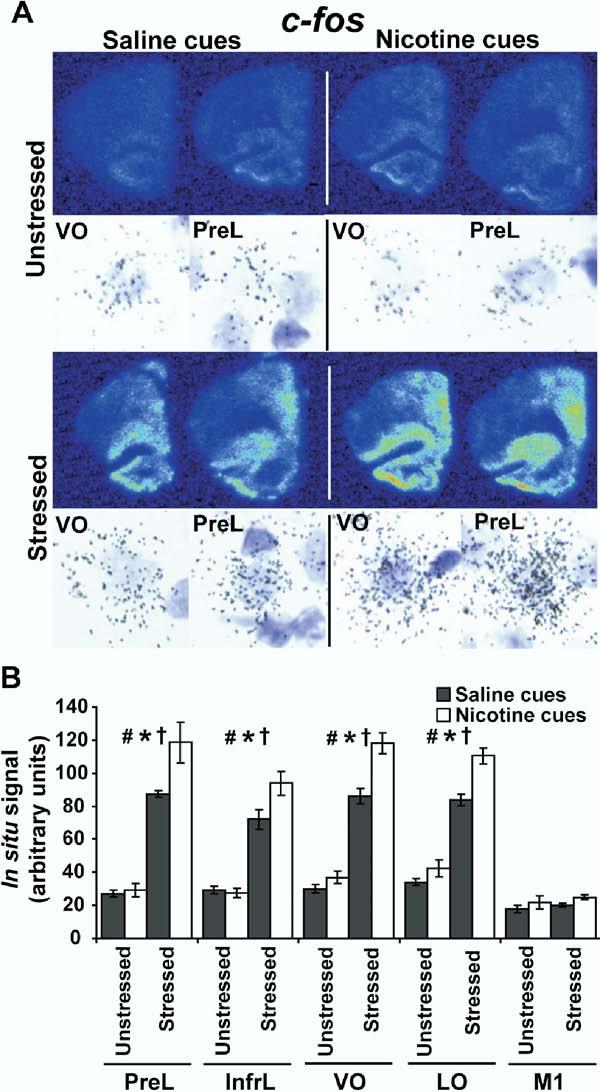
Expression of c-fos in the rat brain in response to stress and nicotine cues. (A)Representative psuedocolor phosphorescence autoradiograms of coronal, right hemisphere sections from each of the experimental groups hybridized with a probe against c-fos (same layout as Figure 3). Brightfield photomicrographs illustrate silver grain accumulation over individual, large, lightly Nissl stained nuclei from the VO and PreL taken from autoradiographic emulsions for each of the groups. This pattern of silver grain accumulation is consistent with a neuronal expression pattern for c-fos and was consistent across the prefrontal subregions analyzed. (B)Graphical representation of densitometric analysis for the PreL, InfrL, VO, LO, and M1 cortices in each of the experimental groups for c-fos expression (n = 4 per group). There was a main effect of stress on c-fos expression in PreL [F(1,12) = 127.873, p < .0001], InfrL [F(1,12) = 117.443, p < .0001], VO [F(1,12) = 241.081, p < .0001], and LO [F(1,12) = 209.024, p < .0001] but not M1 cortices [F(1,12) = 1.219, p = .2912] (#p < .05). There was also a main effect of cues on c-fos expression in the PreL [F(1,12) = 6.286, p = .0276], InfrL [F(1,12) = 4.037, p = .0675], VO [F(1,12) = 19.326, p = .0009], and LO cortices [F(1,12) = 18.377, p = .0011] but not the M1 cortex [F(1,12) = 3.150, p= .1013]) (*p < .05). In addition, stress and cues interacted to potentiate c-fos expression in the PreL [F(1,12) = 4.859, p = .0478], InfrL [F(1,12) = 5.362, p = .0391], VO [F(1,12) = 8.251, p = .0140], and LO cortices [F(1,12) = 5.082, p= .0437] but not in the M1 cortex [F(1,12) = .029, p = .8669] (†p< .05). VO, ventral orbital prefrontal cortex; PreL, prelimbic cortex; InfrL, infralimbic cortex; LO, lateral orbital prefrontal cortex; M1, primary motor cortex.
Discussion
The primary observation in this study is that the acute stress level of nicotine-experienced rats can unveil locomotor arousal to nicotine-associated cues. Further, stress can influence the degree of prefrontal cortical neuronal activation that occurs in response to these cues. Ferret exposure, an ethologically relevant stressor, was capable of eliciting a stress response in rats as evidenced by increased plasma corticosterone levels. The stressor also increased immediate early gene expression in the prefrontal cortex. Nicotine cues elicited a minor increase in the expression of the analyzed genes in the prefrontal cortex. Stress and nicotine cues, however, interacted to greatly augment the expression of these genes in prefrontal subregions. This interactive effect was not observed in the primary motor cortex. These considerations have important implications for behavioral and functional neuroimaging studies of drug-cue reactivity and for the underlying mechanisms subserving both stress- and cue-induced relapse.
Conditioned Locomotor Arousal
In this study, rats conditioned with .14 mg/kg of nicotine over 14 days fail to exhibit conditioned locomotor activation in response to cues associated with the nicotine treatment unless they were stressed prior to exposure to these cues. The absence of conditioned locomotor arousal is apparent despite the activating effects of this nicotine treatment regimen on locomotor activity (Figure 2A). Rats conditioned with doses of nicotine greater than the dose used in this study exhibit heightened conditioned locomotor activity to nicotine cues in the absence of stress (Bevins and Palmatier 2003; Palmatier and Bevins 2002; Schiltz et al 2005; Schroeder et al 2001; Walter and Kuschinsky 1989). Human studies corroborate these findings in that heavy smokers have been shown to be more reactive to cigarette cues than light smokers (Stritzke et al 2004). These observations suggest that a smaller total nicotine dose and/or differences in the rate or frequency of nicotine administration may be responsible for the lack of conditioned behavior to low-dose nicotine cues in both rodents and humans.
Both appetitive and aversive nicotine conditioned responses have been reported for a variety of nicotine doses. Lower doses of nicotine, covering the dose used in this study, are capable of inducing a conditioned place preference, while higher doses of nicotine are capable of eliciting a conditioned place aversion; although, a higher degree of variability has been observed in these experiments compared with conditioned place preference with more classical psychostimulants (Calcagnetti and Schechter 1994; Fudala and Iwamoto 1986; Fudala et al 1985; Jorenby et al 1990; Le Foll and Goldberg 2005; Shoaib et al 1994). Unconditioned as well as conditioned anxiety to nicotine at .1 mg/kg nicotine dose have been reported in the social interaction test and elevated plus maze (Irvine et al 2001a, 2001b; Tucci et al 2003). We have previously reported conditioned locomotor hyperactivity in response to a context associated with nicotine or morphine but also yohimbine, an anxiogenic α2 antagonist (Schiltz et al 2005; Schroeder et al 2000, 2001, 2003; Schroeder and Kelley 2002). These considerations raise the question of whether, in the present experiment, stress is unveiling an appetitive or aversive motivational state in response to the low-dose nicotine cues. We prefer the interpretation that stress increases the salience of the appetitive aspects of the conditioned context in our experiment, thereby increasing the magnitude of the conditioned response to these cues in a manner consistent with an incentive sensitization model of nicotine conditioning. This interpretation seems more likely, since rats tend to develop tolerance to the anxiogenic effects of low doses of nicotine and because stress can elicit reinstatement of nicotine-seeking behavior (Buczek et al 1999; Irvine et al 2001a, 2001b). Additionally, smokers exposed to anxiety-provoking or attention-demanding stimuli increase their smoke intake (Frith 1971; McKennell 1970; Rose et al 1983). Nevertheless, regardless of the valence of the conditioned response, it is clear that stress, together with cues, is capable of inducing conditioned behavioral arousal.
Dopamine may play a role in the conditioned locomotor response observed here. Systemic administration of a D1 receptor antagonist or a D3 receptor-selective partial agonist blocks nicotine-conditioned locomotor activation (Bevins et al 2001; Le Foll and Goldberg 2005). Pharmacological manipulations of the dopamine system in human cigarette smokers have also been shown to modulate cigarette cue reactivity (Brody et al 2004; Mahler and de Wit 2005). These results might be expected given that prefrontal dopamine transmission plays a general role in guiding behavior elicited by external stimuli. Both ventral tegmental area (VTA) dopaminergic and prefrontal cortical neurons are responsive to reward-predicting stimuli (Leon and Shadlen 1999; Pratt and Mizumori 2001; Roesch and Olson 2003; Tobler et al 2005; Tremblay and Schultz 1999). In addition, extracellular levels of dopamine increase in the prefrontal cortex in response to reward cues and stressors including predator odor (Bassareo et al 2002; Bassareo and Di Chiara 1997; Merali et al 2004; Morrow et al 2000). The mesoprefrontal dopamine system is therefore poised to integrate responses to both stress and nicotine-paired cues.
These observations suggest a model for the stress-elicited conditioned locomotor activation. The rats in the unstressed/nicotine cues group may have had negligible dopamine release in the prefrontal cortex, since cues that do not acquire incentive properties fail to elicit prefrontal cortex dopamine release (Bassareo and Di Chiara 1997). We hypothesize that ferret exposure elicits dopamine efflux in the prefrontal cortex of rats in the stressed/nicotine cues group. Application of dopamine or VTA stimulation increases the excitability of the prefrontal cortex (Henze et al 2000; Onn and Wang 2005; Penit-Soria et al 1987; Shi et al 1997; Yang and Seamans 1996). If stress induces increased prefrontal dopamine, additional mesoprefrontal and glutamatergic input from structures responsive to the low-dose nicotine associated cues (possibly the hippocampus and basolateral amygdala) would then be capable of generating further increased activity in this region. Sufficient prefrontal glutamatergic input to the nucleus accumbens has been demonstrated to be necessary for a variety of drug-cue induced behaviors and is necessary for both stress- and cue-induced reinstatement of drug-seeking behavior (Kalivas and Volkow 2005). The proposed model would account for both the stress-unveiled behavior and gene expression (see below).
Stress has previously been shown to impair memory consolidation. Acute exposure to predator odor impairs working memory, a result consistent with alterations in mesoprefontal dopamine activity (Morrow et al 2000). Additional work has shown that acute predator stress impairs long-term hippocampal-dependent spatial memory and this impairment is associated with altered gene expression in the hippocampus and the prefrontal cortex (Diamond et al 1999; Sandi et al 2005; Woodson et al 2003). The ability of predator stress to facilitate recall of and responsiveness to a previously conditioned context is another novel finding of this study.
Gene Expression
Predator stress was capable of increasing expression of arc, ngfi-b, and c-fos in prefrontal cortical subregions and only arc in the primary motor cortex to a much smaller degree. To our knowledge, this is the first report that describes an increase in the expression of these genes in the prefrontal cortex of rats (with a low-dose nicotine treatment background) exposed to a predator. Further, low-dose nicotine cues were only capable of minimally increasing the expression of these genes in the prefrontal cortex and only ngfi-b in the primary motor cortex. Moreover, stress and nicotine cues interacted to boost the expression of arc and c-fos in the prefrontal cortex but not in the primary motor cortex (Figures 3 and 5). Therefore, conditioned locomotor arousal and increased prefrontal gene expression are state-dependent. Unstressed rats do not exhibit locomotor arousal or augmented prefrontal cortex activation in response to nicotine cues.
Our observations do not preclude the possibility that other neurotransmitters-including other monoamines, acetylcholine, and neuropeptides-may play a role in the stress-unveiled, conditioned locomotor hyperactivity (Merali et al 2004). The gene expression patterns, however, are indicative of increased glutamatergic transmission. Expression of arc, c-fos, and ngfi-b is influenced by neuromodulatory systems, but their upregulation seems to be dependent on ionotropic glutamate receptor, especially NMDA receptor, stimulation (Jacobs et al 1994; Pei et al 2004; Shen and Gundlach 2000; Steward and Worley 2001). The observed gene expression fits nicely with our model, since, as mentioned above, prefrontal dopamine enhances NMDA-mediated excitability (Wang and O’Donnell 2001).
Conclusions
Psychological states influence whether drug cue reactivity occurs. Reactivity to cigarette cues is stronger if a smoker believes that he or she will soon be allowed to smoke (Droungas et al 1995; Wertz and Sayette 2001). The prefrontal cortical activation to drug-associated cues is also highly influenced by perceived drug use availability (Wilson et al 2004). The present study is the first demonstrating that the stress or anxiety level of nicotine-experienced individuals is capable of unveiling a latent, conditioned behavioral response to low-dose nicotine cues. The associated stress-unveiled, activity-dependent gene expression in the prefrontal cortex in response to these cues suggests that the prefrontal cortex is involved with either altering the perceived value of and/or the behavioral response to conditioned stimuli as a function of stress levels. The prefrontal cortex is well positioned to influence these processes given its role in executive function and guiding adaptive behavior (Kalivas and Nakamura 1999; Kalivas and Volkow 2005). Furthermore, these results demonstrate that behavioral correlates of neuroadaptations caused by an addictive drug may not be observed unless the drug-experienced subject exhibits a minimum level of stress. In other words, failing to elicit conditioned responses to drug-paired cues may be due to a retrieval deficit and not the absence of associative learning during the progression from a drug-naïve state to a drug-experienced state. Acute psychological stress would then be not only capable of predisposing abstinent, heavier smokers to relapse but also predisposing abstinent, lighter smokers to exhibit increased cue reactivity, which, in turn, may further increase the probability of cigarette use and progression toward a more cue-reactive state. The current findings suggest that this process may occur via enhanced activity within the prefrontal cortex.
Acknowledgments
We thank Dr. Kim Jochman and Chris Nizzi for expert assistance with the predator exposure stress and corticosterone EIA procedures, respectively.
Footnotes
This work was supported by National Institutes of Health grants DA016503 (CAS), DA04788 (AEK), and DA13780 (CFL).
References
- Adamec R. Does long term potentiation in periacqueductal gray (PAG) mediate lasting changes in rodent anxiety-like behavior (ALB) produced by predator stress?-Effects of low frequency stimulation (LFS) of PAG on place preference and changes in ALB produced by predator stress. Behav Brain Res. 2001;120:111–135. doi: 10.1016/s0166-4328(00)00366-1. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci. 2002;22:4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci. 1997;17:851–861. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: Dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Nicotine-conditioned locomotor sensitization in rats: Assessment of the US-preexposure effect. Behav Brain Res. 2003;143:65–74. doi: 10.1016/s0166-4328(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, et al. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: A preliminary study. Psychiatry Res. 2004;130:269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Nicotine place preference using the biased method of conditioning. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:925–933. doi: 10.1016/0278-5846(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Chan RK, Brown ER, Ericsson A, Kovacs KJ, Sawchenko PE. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci. 1993;13:5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict Behav. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Greenberg ME. Ca2+ channel-regulated neuronal gene expression. J Neurobiol. 1998;37:171–189. [PubMed] [Google Scholar]
- Frith CD. Smoking behaviour and its relation to the smoker’s immediate experience. Br J Soc Clin Psychol. 1971;10:73–78. doi: 10.1111/j.2044-8260.1971.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Further studies on nicotine-induced conditioned place preference in the rat. Pharmacol Biochem Behav. 1986;25:1041–1049. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22:237–241. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Tanaka H, Kumamaru E, Okamura K, Miki N. Arc interacts with microtubules/microtubule-associated protein 2 and attenuates microtubule-associated protein 2 immunoreactivity in the dendrites. J Neurosci Res. 2004;76:51–63. doi: 10.1002/jnr.20056. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: A comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol. 2000;84:2799–2809. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: Control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: A disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Development of tolerance to nicotine’s anxiogenic effect in the social interaction test. Brain Res. 2001a;894:95–100. doi: 10.1016/s0006-8993(01)01984-9. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Tolerance to nicotine’s effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacol Biochem Behav. 2001b;68:319–325. doi: 10.1016/s0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Jacobs O, Van Bree L, Mailleux P, Zhang F, Schiffmann SN, Halleux P, et al. Homolateral cerebrocortical increase of immediate early gene and neurotransmitter messenger RNAs after minimal cortical lesion: Blockade by N-methyl-D-aspartate antagonist. Neuroscience. 1994;59:827–836. doi: 10.1016/0306-4522(94)90287-9. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Steinpreis RE, Sherman JE, Baker TB. Aversion instead of preference learning indicated by nicotine place conditioning in rats. Psychopharmacology (Berl) 1990;101:533–538. doi: 10.1007/BF02244233. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9:223–227. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron. 1999;24:415–425. doi: 10.1016/s0896-6273(00)80854-5. [DOI] [PubMed] [Google Scholar]
- Liu N, Baker H. Activity-dependent Nurr1 and NGFI-B gene expression in adult mouse olfactory bulb. Neuroreport. 1999;10:747–751. doi: 10.1097/00001756-199903170-00016. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: Exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Effects of haloperidol on reactions to smoking cues in humans. Behav Pharmacol. 2005;16:123–126. doi: 10.1097/00008877-200503000-00008. [DOI] [PubMed] [Google Scholar]
- Martens C, Bilodeau S, Maira M, Gauthier Y, Drouin J. Protein-protein interactions and transcriptional antagonism between the subfamily of NGFI-B/Nur77 orphan nuclear receptors and glucocorticoid receptor. Mol Endocrinol. 2005;19:885–897. doi: 10.1210/me.2004-0333. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKennell AC. Smoking motivation factors. Br J Soc Clin Psychol. 1970;9:8–22. doi: 10.1111/j.2044-8260.1970.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Anisman H. Anticipatory cues differentially provoke in vivo peptidergic and monoaminergic release at the medial prefrontal cortex. Neuropsychopharmacology. 2004;29:1409–1418. doi: 10.1038/sj.npp.1300441. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Elsworth JD. TMT, a predator odor, elevates mesoprefrontal dopamine metabolic activity and disrupts short-term working memory in the rat. Brain Res Bull. 2000;52:519–523. doi: 10.1016/s0361-9230(00)00290-2. [DOI] [PubMed] [Google Scholar]
- Onn SP, Wang XB. Differential modulation of anterior cingulate cortical activity by afferents from ventral tegmental area and mediodorsal thalamus. Eur J Neurosci. 2005;21:2975–2992. doi: 10.1111/j.1460-9568.2005.04122.x. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Examination of GABAergic and dopaminergic compounds in the acquisition of nicotine-conditioned hyperactivity in rats. Neuropsychobiology. 2002;45:87–94. doi: 10.1159/000048682. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Pei Q, Tordera R, Sprakes M, Sharp T. Glutamate receptor activation is involved in 5-HT2 agonist-induced Arc gene expression in the rat cortex. Neuropharmacology. 2004;46:331–339. doi: 10.1016/j.neuropharm.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Penit-Soria J, Audinat E, Crepel F. Excitation of rat prefrontal cortical neurons by dopamine: An in vitro electrophysiological study. Brain Res. 1987;425:263–274. doi: 10.1016/0006-8993(87)90509-9. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Bedard T, et al. Neither acute nor chronic exposure to a naturalistic (predator) stressor influences the interleukin-1beta system, tumor necrosis factor-alpha, transforming growth factor-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Bull. 2000;51:187–193. doi: 10.1016/s0361-9230(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Mizumori SJ. Neurons in rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behav Brain Res. 2001;123:165–183. doi: 10.1016/s0166-4328(01)00204-2. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced arc mRNA and protein expression: Evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. J Neurophysiol. 2003;90:1766–1789. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME. Cigarette smoking during anxiety-provoking and monotonous tasks. Addict Behav. 1983;8:353–359. doi: 10.1016/0306-4603(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Sandi C, Woodson JC, Haynes VF, Park CR, Touyarot K, Lopez-Fernandez MA, et al. Acute stress-induced impairment of spatial memory is associated with decreased expression of neural cell adhesion molecule in the hippocampus and prefrontal cortex. Biol Psychiatry. 2005;57:856–864. doi: 10.1016/j.biopsych.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Schiltz CA, Kelley AE, Landry CF. Contextual cues associated with nicotine administration increase arc mRNA expression in corticolimbic areas of the rat brain. Eur J Neurosci. 2005;21:1703–1711. doi: 10.1111/j.1460-9568.2005.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105:535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Holahan MR, Landry CF, Kelley AE. Morphine-associated environmental cues elicit conditioned gene expression. Synapse. 2000;37:146–158. doi: 10.1002/1098-2396(200008)37:2<146::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Kelley AE. Conditioned Fos expression following morphine-paired contextual cue exposure is environment specific. Behav Neurosci. 2002;116:727–732. doi: 10.1037//0735-7044.116.4.727. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Schiltz CA, Kelley AE. Neural activation profile elicited by cues associated with the anxiogenic drug yohimbine differs from that observed for reward-paired cues. Neuropsychopharmacology. 2003;28:14–21. doi: 10.1038/sj.npp.1300007. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shen PJ, Gundlach AL. Differential modulatory effects of alpha- and beta-adrenoceptor agonists and antagonists on cortical immediate-early gene expression following focal cerebrocortical lesion-induced spreading depression. Brain Res Mol Brain Res. 2000;83:133–144. doi: 10.1016/s0169-328x(00)00216-3. [DOI] [PubMed] [Google Scholar]
- Shi WX, Zheng P, Liang XF, Bunney BS. Characterization of dopamineinduced depolarization of prefrontal cortical neurons. Synapse. 1997;26:415–422. doi: 10.1002/(SICI)1098-2396(199708)26:4<415::AID-SYN9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP, Kumar RC. Nicotine-induced place preferences following prior nicotine exposure in rats. Psychopharmacology (Berl) 1994;113:445–452. doi: 10.1007/BF02245221. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- St-Hilaire M, Tremblay PO, Levesque D, Barden N, Rouillard C. Effects of cocaine on c-fos and NGFI-B mRNA expression in transgenic mice underexpressing glucocorticoid receptors. Neuropsychopharmacology. 2003;28:478–489. doi: 10.1038/sj.npp.1300067. [DOI] [PubMed] [Google Scholar]
- Stritzke WG, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: Advances in reliability, specificity, and validity. Psychol Addict Behav. 2004;18:148–159. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Tucci S, Cheeta S, Seth P, File SE. Corticotropin releasing factor antagonist, alpha-helical CRF(9-41), reverses nicotine-induced conditioned, but not unconditioned, anxiety. Psychopharmacology (Berl) 2003;167:251–256. doi: 10.1007/s00213-003-1403-4. [DOI] [PubMed] [Google Scholar]
- Walter S, Kuschinsky K. Conditioning of nicotine effects on motility and behaviour in rats. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:208–213. doi: 10.1007/BF00165145. [DOI] [PubMed] [Google Scholar]
- Wang J, O’Donnell P. D(1) dopamine receptors potentiate NMDA-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb Cortex. 2001;11:452–462. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity of self-reported urge. Exp Clin Psychopharmacol. 2001;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: A neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson JC, Macintosh D, Fleshner M, Diamond DM. Emotion-induced amnesia in rats: Working memory-specific impairment, cortico-sterone-memory correlation, and fear versus arousal effects on memory. Learn Mem. 2003;10:326–336. doi: 10.1101/lm.62903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V-VI rat prefrontal cortex neurons in vitro: Modulation of dendritic-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterstrom RH, Williams R, Perlmann T, Olson L. Cellular expression of the immediate early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Brain Res Mol Brain Res. 1996;41:111–120. doi: 10.1016/0169-328x(96)00074-5. [DOI] [PubMed] [Google Scholar]


