Abstract
Following organ injury, morphogenic epithelial responses can vary depending on local cell density. In the present study, the role of cell confluence in determining the responsiveness of renal epithelial cells to the dedifferentiating morphogenic signals of hepatocyte growth factor (HGF) was examined. Increasing confluence resulted in a greater tendency of cells to organize into epithelial tubes and a significant decrease in migratory responsiveness to HGF. Analysis of downstream signaling revealed that the HGF receptor c-Met was equally activated in confluent and nonconfluent cells following HGF stimulation but that phosphoinositide 3-kinase-dependent activation of Akt and Rac were selectively diminished in confluent cells. In nonconfluent cells treated with HGF, the high level of Akt activation resulted in inhibitory phosphorylation of glycogen synthase kinase 3β (GSK-3β) and increased β-catenin nuclear signaling. In contrast, confluent cells, in which HGF-stimulated Akt activation was diminished, displayed less inhibitory phosphorylation of GSK-3β and less nuclear signaling by β-catenin. Overexpression of β-catenin (SA), which cannot be phosphorylated by GSK-3β and targeted for ubiquitination, significantly increased migration in fully confluent cells. Thus, cells maintained at high confluence selectively downregulate signaling events such as Rac activation and β-catenin-dependent transcription that would otherwise promote cell dedifferentiation and migration.
Studies performed with animals subjected to ischemic acute renal failure demonstrate that cells capable of surviving the initial injury transiently dedifferentiate and migrate into the region of the denuded tubular basement membrane, where they proliferate and eventually reconstitute a functional tubular epithelium (3). This repair involves initial process extension and flattening by the cells at the free edge of the necrotic injury, followed by migration as the cells spread to cover the denuded tubular basement membrane. It is believed that this initial phase of epithelial dedifferentiation is stimulated by the actions of several growth factors, including hepatocyte growth factor (HGF), which is upregulated both locally and systemically in multiple animal models of acute renal injury (16, 23).
In contrast to the responses of cells at the edge of the wound, epithelial cells in nearby regions of the tubule where normal cell confluence is maintained fail to undergo morphogenic dedifferentiation despite the presence of these same growth factor stimuli. It is likely that the ability to regulate morphogenic responses to HGF is important since dedifferentiation, migration, and proliferation by these fully confluent cells would result in disruption of normal regions of the tubule epithelium, potentially leading to adverse outcomes, such as cyst formation and invasion through the basement membrane. In fact, the failure to inhibit morphogenic responses to local growth factors may account for the recent observations that epithelial cells may undergo transformation into a fibroblast phenotype and thus promote organ fibrosis (17).
The differential responses of subconfluent versus confluent cells to growth factor stimulation could be due to a number of factors, including gradients of the stimulatory factors around the site of the injury, confluence-dependent regulation of receptor expression/activation, and confluence-dependent regulation of downstream intracellular signaling pathways. Of these, confluence-dependent inhibition of epidermal growth factor (EGF) receptor activation has previously been demonstrated in mammary epithelial cells (39). However, to our knowledge, this has not been examined for the HGF receptor c-Met.
HGF, a mesenchymal-derived heparin binding growth factor also known as scatter factor (2, 42), binds to c-Met expressed on both epithelial and endothelial cells and activates multiple cellular responses, including cell proliferation, migration, and tubulogenesis (6). The Met receptor is upregulated and activated extensively in the kidney following acute ischemic injury (32). Met is a member of the tyrosine kinase receptor superfamily and, following HGF ligand binding, undergoes dimerization and autophosphorylation on several tyrosine residues in the cytoplasmic domain of the receptor. These in turn mediate recruitment of the adaptor protein Grb2 and the scaffolding protein Gab1 and subsequent activation of multiple downstream signaling pathways, including the extracellular signal-regulated kinase (ERK)-mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI 3-K) pathways (11, 41). Our laboratory as well as others have demonstrated the critical importance of these two pathways in regulating epithelial cell scattering, migration, and tubule formation (13-15, 18, 21, 27).
In prior studies of the role of HGF signaling in epithelial morphogenesis, we demonstrated that the focal adhesion protein paxillin can serve as a scaffold for HGF-stimulated ERK activation at focal adhesions, thereby stimulating paxillin-Fak association and subsequent PI 3-K-dependent Rac activation (14, 15). These signaling events are believed to be required for lamellipodial extension and focal adhesion turnover (35, 40). During the course of these experiments, we noted that the phenotypic responses of our epithelial cells to HGF were lost at high confluence and that under these conditions, paxillin-Fak association was no longer regulated by HGF.
These results suggested that HGF signaling is differentially regulated under conditions of high confluence and led to the present studies aimed at defining the mechanism of this response. In these recent experiments, we show that renal epithelial cells plated at high density demonstrate significantly less migration through Transwell filters than nonconfluent cells. While Met receptor expression and phosphorylation are equivalent under both conditions, there is a significant diminution in the activation of Akt (a downstream target of the PI 3-K) in confluent cells compared to that in nonconfluent cells. This results in a decrease in Akt-dependent inhibition of glycogen synthase kinase 3β (GSK-3β) and thus loss of β-catenin nuclear signaling. Overexpression of a nonubiquitinatable form of β-catenin in confluent cells partially reverses the inhibition of migration through Transwell filters. Thus, our results indicate that high cell confluence can specifically downregulate signaling pathways that promote cell dedifferentiation following HGF stimulation.
MATERIALS AND METHODS
Cell culture and reagents.
Mouse inner medullary collecting duct (mIMCD-3) epithelial cells were maintained using standard cell culture techniques in Dulbecco's modified Eagle's medium-F12 medium containing 10% fetal bovine serum. Anti-Akt, anti-phospho Akt, anti-phospho β-catenin, anti-GSK-3β, and anti-phospho GSK-3β antibodies were obtained from Cell Signaling, anti-Gab-1, anti-Fak, anti-c-Met, and anti-phospho c-Met antibodies from Santa Cruz Biotechnology, and and anti-p85 and anti-phosphotyrosine from Upstate Biotechnology. The phospho Akt antibody is directed against serine 473, the phospho β-catenin antibody against serines 33 and 37 and threonine 41, the phospho GSK-3β antibody against serine 9 (inhibitory), and the phospho c-Met antibody against tyrosines 1234 and 1235. Akt inhibitor IV and GSK-3β inhibitor IX (37) were purchased from Calbiochem. LY294002 was purchased from Promega. The pCGN-GSK-3βδ9 mutant and pCGN-GSK-3βWT were obtained as generous gifts from Akira Kikuchi. All other reagents used were obtained from Sigma Chemical Company unless otherwise mentioned.
Cell density.
Cells were dissociated in the absence of trypsin by using cell dissociation buffer (Gibco), spun down and resuspended in phosphate-buffered saline (PBS), and counted by a hemocytometer. In 10-cm dishes, 4 × 105 cells were plated for nonconfluent samples and 4 × 106 cells were plated for confluent samples. For the 24-well plates used in the Transwell assay, 2.5 × 104 cells were plated for sparsely nonconfluent, 2 × 105 for nonconfluent, and 7.5 × 105 for fully confluent samples.
Immunoprecipitation and Western analysis.
Cells were serum starved for 24 h, followed by HGF stimulation (40 ng/ml) for the indicated time. Cells were lysed in radioimmunoprecipitation assay buffer (0.16 M NaCl, 20 mM Trizma base, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM sodium fluoride, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin, and 1 μg/ml leupeptin), insoluble material was removed by centrifugation, and the supernatant protein content was determined using the Bradford assay.
For coimmunoprecipitation experiments, 1 mg of cell lysate was immunoprecipitated with the appropriate antibody overnight, collected by adding protein G-Sepharose (1:1 slurry in PBS; Sigma) for monoclonal antibodies and protein A-Sepharose for polyclonal antibodies, and washed three times with 500 μl of ice-cold radioimmunoprecipitation assay buffer. Associated proteins were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electrophoretically transferred to Immobilon-P membranes (Millipore), immunoblotted with the appropriate antibody, and visualized by enhanced chemiluminescence (Amersham Biosciences, Inc.). Quantitation of coimmunoprecipitating proteins was performed using NIH Image software.
Migration assays.
Cells were dissociated using cell dissociation buffer (Gibco), spun down and resuspended in PBS, and counted by a hemocytometer. The indicated numbers of cells were allowed to attach to the Transwell membranes in Dulbecco's modified Eagle's medium-F12 medium containing 10% fetal bovine serum. After 6 h, the bottoms of the membranes were wiped to remove migrated cells and the remaining cells were placed in serum-free medium with or without HGF (40 ng/ml) added to the lower chamber. After 14 h, nonmigrated cells were removed from the upper surface of the membrane and the migrated cells were fixed and stained using a Hema 3 stain set (Fisher Diagnostics). Migrated cells were counted in nine contiguous fields of 0.1 mm2 in triplicates representing one experiment. The entire experiment was repeated on five separate occasions. For some experiments, cells were transiently cotransfected with either 5 μg CS2.β-catenin(SA) and 1 μg pLNCX-ires-eGFP or pLNCX-ires-eGFP alone in a 10-cm tissue culture dish using Lipofectamine 2000 (Invitrogen), followed by dissociation and plating of the indicated number of cells as described above in a Transwell filter. CS2.β-catenin(SA) is a pCDNA3.1 expression plasmid encoding β-catenin in which the five serine residues at the amino terminus have been mutated to alanine (20). In these experiments, only green fluorescent cells were counted, and therefore, the total numbers of migrating cells were significantly lower than those in experiments in which all cells were counted. To calculate the percentage of migrated fluorescent cells, the total number of plated cells was multiplied by 0.29 (the average transfection efficiency of the IMCD cells) to determine the number of transfected cells plated.
Luciferase assay.
Cells were transiently transfected in separate dishes with either 5 μg TOPFLASH or 5 μg FOPFLASH plus 2.5 μg Renilla (Dual-Luciferase reporter assay system; Promega). After 24 h, cells were trypsinized and 20,000 cells/well were plated on 96-well plates and 24-well plates to produce confluent and nonconfluent densities, respectively, serum starved overnight, and stimulated in the presence or absence of HGF for 1 and 3 h. For the inhibitor studies, cells were pretreated with either Akt IV inhibitor (10 μM) or GSK IX inhibitor (1 μM) for 1 hour prior to HGF stimulation. For the GSK expression studies, 5 μg of the pCGN-GSK-3βδ9 mutant or 5 μg pCGN-GSK-3βWT was cotransfected with TOPFLASH and Renilla as described above. As a positive control, 5 μg CS2.β-catenin(SA) was cotransfected with TOPFLASH and Renilla. Luciferase activity in cell lysates was determined with a luminometer, and transfection efficiency was normalized using Renilla expression as per the manufacturer's instructions. Expression of FOPFLASH revealed low basal levels of luciferase activity that did not change in confluent or nonconfluent cells treated in the presence or absence of HGF (data not shown).
Rac activation assay.
GTP-Rac was detected by performing a glutathione S-transferase-linked PAK binding domain (PBD) pull-down assay as per the manufacturer's instructions (Upstate Biotechnology). Lysates from nonconfluent and confluent IMCD cells were stimulated in the presence or absence of HGF, incubated with 20 μg of the PAK-1-PBD agarose for 60 min at 4°C, and washed three times with lysis buffer, and samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with anti-Rac antibody. Whole-cell lysates were immunoblotted separately to confirm equality of starting material.
RESULTS AND DISCUSSION
High cell density inhibits morphogenic responses to HGF.
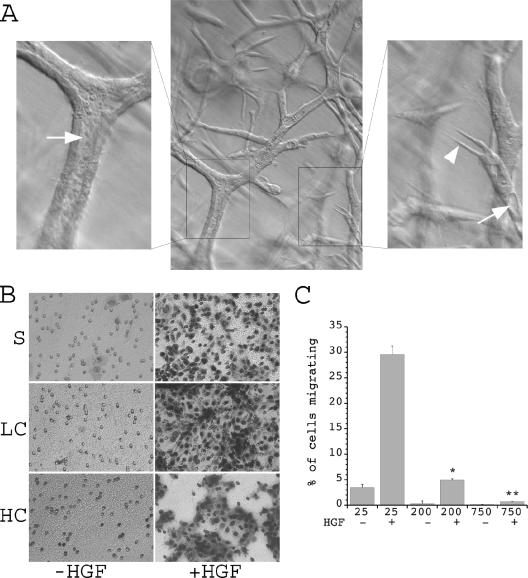
As described above, fully confluent cells in noninjured segments of the renal tubule do not appear to dedifferentiate and migrate in response to injury, even though expression of HGF and c-Met are increased under these conditions (16, 23, 32). These in vivo observations are supported by examination of in vitro tubulogenesis in a three-dimensional matrix, during which morphogenic responses to a uniform concentration of HGF are found to vary in the same culture dish depending on local cell density. In these experiments, cells are initially seeded in a collagen-Matrigel mixture at very low density. When exposed to HGF, these cells undergo marked morphogenic responses, including process extension and formation of elongated cell cords (18). However, as the cells continue to proliferate, those that reach the highest cell density in the center of these cords organize into a classical tubule with a monolayer of compact epithelial cell-appearing cells surrounding a lumen, even though HGF is continuously present (Fig. 1A). In contrast, cells at lower density near the tips of the forming tubules continue to exhibit a dedifferentiated phenotype with process extension and elongated morphology.
FIG. 1.
Phenotypic effects of cell confluence. (A) mIMCD-3 cells grown in a three-dimensional matrix of collagen and Matrigel for 10 days form multicellular branching tubes. Cells at the tips of growing tubules receive the least cell-cell contact and exhibit a dedifferentiated phenotype with process formation (right panel, arrowhead), while more proximal cells that receive fewer cell-matrix and more cell-cell contacts demonstrate a more differentiated phenotype with nascent lumen formation (right panel, arrow). Cells that have reached the highest density organize into a tight monolayer with a clearly defined central lumen (left panel, arrow). (B) Stable mIMCD-3 cells were plated on Transwell plates under sparse (S), low confluence (LC), or high confluence (HC) conditions, allowed to attach for 6 h, and then treated in the presence or absence of HGF, and cells that had migrated to the bottom of the Transwell in 14 h were photographed (magnification, ×20). Cell migration was scored positive if the nuclei were visible. (C) Quantification of the percentage of migrating cells from five separate experiments for each condition. Values along the x axis indicate the number of cells (in thousands) per well.  , P < 0.01 versus cells at 25,000 cells/well plus HGF;
, P < 0.01 versus cells at 25,000 cells/well plus HGF; 
 , P < 0.01 versus cells at 25,000 or 200,000 cells/well plus HGF.
, P < 0.01 versus cells at 25,000 or 200,000 cells/well plus HGF.
Since local factors such as basement membrane deposition could influence the alteration in morphogenic responses seen in this model, we also utilized a Transwell assay of individual cell migration as an independent mechanism to quantify the effects of cell density on morphogenic responses to HGF. Cells were plated at various densities to yield either a sparse monolayer (25,000 cells/well on a 24-well plate, in which case almost all cells are individual), a nonconfluent monolayer (200,000 cells/well, in which >90% of the cells have at least one free edge not in contact with another cell), or a fully confluent monolayer (750,000 cells/well, resulting in a densely packed monolayer). IMCD cells were seeded on the Transwell filters at these densities for 6 h to permit the formation of cell-cell junctions (12). HGF was then added to the lower chamber, and the cells were allowed to migrate towards the chemotactic stimulus for 14 h. Staining for cells that had migrated across the Transwell membrane revealed that the absolute numbers of migrating cells at baseline were very low and were similar in all three groups. Under the two subconfluent conditions, migration in response to HGF was markedly increased compared to that at baseline. When the number of cells that migrated was compared to the total number plated, a progressive decrease in the percentage of cells capable of responding to the HGF chemotactic stimulus when plated under increasing degrees of confluence was defined (Fig. 1B and C). In addition, in highly confluent cells, the absolute number of cells that migrated in response to HGF was significantly less than that in subconfluent cells, even though a greater number of cells was present on the upper surface of the membrane (see Fig. S1A in the supplemental material). Combined, these results demonstrate that cells at high levels of confluence downregulate morphogenic responses to HGF.
Expression and phosphorylation of c-Met is unchanged in confluent and nonconfluent cells.
Previous studies have demonstrated that the localization of the EGF receptor is altered in fully confluent cells, thus diminishing EGF-dependent receptor activation (39). We therefore examined the possibility that the decreased morphogenic responses to HGF in highly confluent cells occur due to a decrease in Met expression and/or activation. HGF-induced dimerization of Met results in autophosphorylation of the activation loop at Tyr 1234 and Tyr 1235, with resultant activation of the tyrosine kinase domain (30, 41). To determine the effect of cell confluence on this process of receptor activation, IMCD cells were plated at either 4 × 105 cells/10-cm dish (nonconfluent, >90% of cells with at least one free edge) or 4 × 106 cells/10-cm dish (confluent) (Fig. 2A), followed by stimulation with HGF and immunoblotting with an activation state-specific antibody that recognizes the dually phosphorylated Met receptor (anti-pMet). These experiments demonstrated that total receptor amount did not decrease in highly confluent cells and that the levels of phosphorylation at the activation site after HGF stimulation were not significantly different in the two groups (Fig. 2B and C). Furthermore, immunoblotting of the receptor with a nonspecific antiphosphotyrosine antibody demonstrated that total receptor phosphorylation levels were also not detectably different under nonconfluent and confluent conditions (Fig. 2D). These results suggest that c-Met remains at the surface and is available for normal ligand-dependent phosphorylation in fully confluent cells, although the selective loss of phosphorylation at a single tyrosine residue outside the activation loop cannot be ruled out using this approach.
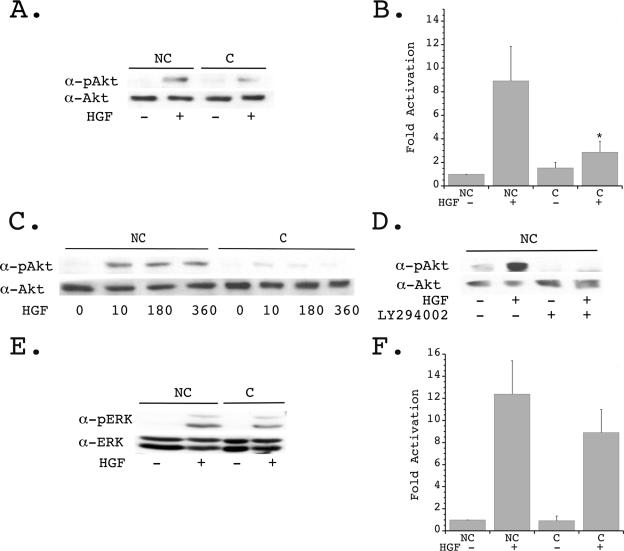
FIG. 2.
Confluence does not alter Met expression or activation. (A) Representative Hoffman contrast images of confluent and nonconfluent mIMCD-3 cells are shown prior to HGF stimulation. (B) Quiescent confluent and nonconfluent mIMCD-3 cells serum starved for 24 h were stimulated in the presence or absence of HGF (40 ng/ml) for 10 min and lysed, and proteins were immunoblotted with anti (α)-pMet antibody (upper panel) and α-Met antibody (lower panel). (C) Densitometric quantification of five independent experiments was performed as described for panel B. (D) Quiescent confluent and nonconfluent mIMCD-3 cells were stimulated in the presence or absence of HGF, and lysates were immunoprecipitated with α-Met and immunoblotted with α-pTyr (upper panel) and α-Met (lower panel).
Akt activation is downregulated in confluent cells.
Previous studies in our laboratory and others have shown that HGF-stimulated cell migration requires activation of several intracellular signaling pathways, including the PI 3-K and MAPK pathways (9, 15, 19). HGF-stimulated activation of the downstream proteins Akt (for the PI 3-K pathway) and ERK (for the MAPK pathway) was therefore examined under nonconfluent and confluent conditions using activation state-specific antibodies. In nonconfluent cells, HGF stimulated an eightfold increase in Akt phosphorylation, whereas fully confluent cells demonstrated only a threefold increase in activation of Akt (Fig. 3A and B). A time course of phosphorylation revealed that Akt remained in an activated state for up to 6 h following HGF stimulation in nonconfluent cells, whereas the modest increase seen in fully confluent cells progressively declined over this time period (Fig. 3C). To determine whether the enhanced Akt activation seen in nonconfluent cells was indeed downstream of the PI 3-K, cells were pretreated with the PI 3-K inhibitor LY294002. Under these conditions, basal and HGF-stimulated Akt phosphorylation was markedly diminished (Fig. 3D). In contrast to the substantial decrease in PI 3-K pathway activation, HGF-stimulated phosphorylation of ERK was decreased approximately 1.5-fold in fully confluent cells and did not reach statistical significance (Fig. 3E and F).
FIG. 3.
Regulation of Akt activation by cell confluence. (A) Quiescent confluent (C) and nonconfluent (NC) mIMCD-3 cells plated for 24 h were stimulated in the presence or absence of HGF (40 ng/ml) for 10 min and immunoblotted with anti (α)-pAkt (upper panel) and α-Akt (lower panel). (B) Densitometric quantification of four independent experiments was performed as described for panel A.  , P < 0.01 versus nonconfluent cells plus HGF. (C) Quiescent nonconfluent and confluent cells were treated with HGF for the indicated times (in minutes), and cell lysates were immunoblotted with α-pAkt (upper panel) and α-Akt (lower panel). (D) Quiescent nonconfluent cells were pretreated for 20 min in the presence or absence of 10 mM LY294002, then stimulated in the presence or absence of HGF for 10 min, and immunoblotted with α-pAkt (upper panel) and α-Akt (lower panel). (E) Quiescent confluent and nonconfluent mIMCD-3 cells plated for 24 h were stimulated in the presence or absence of HGF (40 ng/ml) for 10 min and immunoblotted with α-pERK (upper panel) and α-ERK (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.
, P < 0.01 versus nonconfluent cells plus HGF. (C) Quiescent nonconfluent and confluent cells were treated with HGF for the indicated times (in minutes), and cell lysates were immunoblotted with α-pAkt (upper panel) and α-Akt (lower panel). (D) Quiescent nonconfluent cells were pretreated for 20 min in the presence or absence of 10 mM LY294002, then stimulated in the presence or absence of HGF for 10 min, and immunoblotted with α-pAkt (upper panel) and α-Akt (lower panel). (E) Quiescent confluent and nonconfluent mIMCD-3 cells plated for 24 h were stimulated in the presence or absence of HGF (40 ng/ml) for 10 min and immunoblotted with α-pERK (upper panel) and α-ERK (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.
Confluent cells negatively regulate Fak/p85 signaling.
Following HGF stimulation, PI 3-K activation has been shown to be mediated by recruitment and phosphorylation of Gab1 by the activated Met receptor, creating docking sites for the p85 subunit of the PI 3-K (22, 41). To determine whether this interaction is downregulated in fully confluent cells, the coimmunoprecipitation of p85 and Gab1 was examined. These experiments revealed a 4.9-fold increase in Gab1-p85 association following HGF treatment in nonconfluent cells, with a modest, but not statistically significant, decrease in this interaction in fully confluent cells (Fig. 4A and B).
FIG. 4.
Confluence regulates Fak/p85 signaling following HGF stimulation. (A) Quiescent confluent (C) and nonconfluent (NC) mIMCD-3 cells were stimulated in the presence or absence of HGF, and lysates were immunoprecipitated (IP) with anti (α)-Gab1 and immunoblotted with α-p85 (upper panel) and α-Gab1 (lower panel). (B) Densitometric quantification of three independent experiments was performed as described for panel A. (C) Quiescent confluent and nonconfluent mIMCD-3 cells were stimulated in the presence or absence of HGF, and lysates were immunoprecipitated with α-Fak and immunoblotted with α-p85 (upper panel) and α-Fak (lower panel). (D) Densitometric quantification of three independent experiments was performed as described for panel C.  , P < 0.01 versus nonconfluent cells plus HGF. (E) Quiescent confluent and nonconfluent mIMCD-3 cells were treated in the presence or absence of HGF, followed by PBD pull-down of GTP-Rac and immunoblotting with α-Rac (upper panel). Whole-cell lysates (WCL) were immunoblotted with α-Rac (detecting both GDP-Rac and GTP-Rac) to determine equality of starting material (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.
, P < 0.01 versus nonconfluent cells plus HGF. (E) Quiescent confluent and nonconfluent mIMCD-3 cells were treated in the presence or absence of HGF, followed by PBD pull-down of GTP-Rac and immunoblotting with α-Rac (upper panel). Whole-cell lysates (WCL) were immunoblotted with α-Rac (detecting both GDP-Rac and GTP-Rac) to determine equality of starting material (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.
In addition to PI 3-K activation via association with phosphorylated Gab1, previous studies have shown that Fak activation can mediate p85 recruitment to focal adhesions and subsequent PI 3-K activation (33, 34). In agreement with this, our recent studies have demonstrated that a significant component of whole-cell Akt activation following HGF stimulation is mediated by recruitment of p85 to a Fak-paxillin protein complex at the cell membrane (14). To determine whether this pathway for PI 3-K activation is regulated in a cell confluence-dependent fashion, the association of Fak and p85 was examined in HGF-stimulated nonconfluent and confluent cells. As previously described, HGF stimulation of nonconfluent cells results in a 4.5-fold increase in coimmunoprecipitation of Fak and p85 (Fig. 4C and D). However, in fully confluent cells, HGF treatment failed to increase Fak-p85 coimmunoprecipitation (Fig. 4C and D). Interestingly, basal association of p85 with Fak is modestly increased in fully confluent cells (Fig. 4C, compare lanes 1 and 3), consistent with a slight increase in basal Akt activation in these cells (Fig. 3B). These data demonstrate that PI 3-K activation and resultant Akt phosphorylation are regulated in a confluence-dependent manner, apparently mediated by changes in the recruitment and/or activation of the PI 3-K at sites of cell-matrix interaction.
Activation of the PI 3-K pathway has been demonstrated to stimulate Rac activation at the leading edge of cells following HGF treatment and thus is an important mediator of the early phases of cell shape change during migration, such as actin cytoskeletal rearrangement and lamellipodial formation (14, 35). To determine whether Rac activation is regulated by cell confluence, the amount of GTP-loaded Rac was quantitated in subconfluent and confluent cells with or without treatment with HGF. Interestingly, basal levels of GTP-Rac were modestly increased in highly confluent cells compared to those in subconfluent cells (Fig. 4E and F). This increase is consistent with the observations of Noren and coworkers regarding confluent MDCK cells and has been proposed to be mediated by calcium-dependent cadherin interactions and to be important for normal assembly of adherens junctions (26).
Following stimulation with HGF, GTP-Rac levels were increased 5.5-fold in nonconfluent cells but were essentially unchanged from baseline in fully confluent cells (Fig. 4E and F). Thus, downregulation of HGF-stimulated Rac activation in highly confluent cells is likely to prevent the activation of actin cytoskeletal rearrangement that is necessary for morphogenic cell dedifferentiation and migration.
Akt-dependent phosphorylation of GSK-3β at the inhibitory site is upregulated in confluent cells.
In addition to Rac-dependent changes in the actin cytoskeleton, the morphogenic responses to HGF during epithelial dedifferentiation require the cell to modify cell-cell interactions (during scattering, for example). In renal epithelial cells, cell-cell interactions are dependent on the presence of cadherin-based adherens junctions. These junctions are stabilized by intercellular interactions between the extracellular domains of cadherins, such as E-cadherin and Ksp-cadherin, which mediate the formation of intracellular protein complexes comprised of the carboxy-terminal cadherin domain and the cytosolic proteins α-catenin, β-catenin, and plakoglobin (γ-catenin). In addition to its role in stabilizing adherens junctions, β-catenin is also capable of translocating to the nucleus, where it can bind to the TCF/LEF transcription complex and activate the transcription of multiple genes involved in cell proliferation and dedifferentiation, including fibronectin, matrilysin, CD44, c-myc, and cyclin D1 genes (4).
Due to the role of nuclear β-catenin in promoting cell dedifferentiation, the free cytosolic levels are tightly controlled in highly differentiated cells by targeting of cytosolic β-catenin for ubiquination and degradation via the proteosomal degradatory pathway. This degradatory targeting is mediated by phosphorylation of β-catenin via GSK-3β, a subunit of the adenomatosis polyposis coli protein complex. Mutations in the members of this complex that disrupt β-catenin phosphorylation and degradation result in the development of epithelial cancers due to uncontrolled cell proliferation, dedifferentiation, and invasion into the surrounding matrix (5). The proper regulation of interactions between β-catenin and GSK-3β also appears to be critical for the dedifferentiation responses that are required for HGF-stimulated MDCK cell cysts to extend processes and form tubules. Pollack and coworkers found that cysts comprised of MDCK cells expressing N-terminal β-catenin mutants form proliferating aggregates of cells rather than undergo nascent tubule formation when stimulated with HGF (29). In these cell aggregates, there was excessive accumulation of β-catenin in cytosolic adenomatosis polyposis coli protein clusters, suggesting that HGF-stimulated β-catenin nuclear signaling was interrupted.
The mechanisms by which GSK-3β can be regulated are complex and not fully understood. Several studies have suggested that GSK-3β can be inhibited by Akt-dependent phosphorylation at serine 9 (7, 38). Phosphorylation at this site prevents GSK-3β from phosphorylating β-catenin and thus promotes β-catenin nuclear translocation and activation of its transcriptional activity (8, 36). In contrast, GSK-3β regulation downstream of the Wnt signaling pathway involves the frizzled-disheveled proteins and occurs independent of phosphorylation at serine 9 (10, 24). The finding that HGF-stimulated cell morphogenesis is inhibited in highly confluent cells and that the PI 3-K/Akt pathway appears to be downregulated under these conditions led us to examine the hypothesis that Akt-dependent inhibition of GSK-3β is an important activator of β-catenin nuclear signaling in nonconfluent cells and plays a significant role in determining the ability of HGF to promote cell dedifferentiation.
In nonconfluent cells stimulated with HGF, there is approximately a twofold increase in phosphorylation of GSK-3β at the inhibitory site on serine 9 (Fig. 5A and B). Phosphorylation at this site was sustained for at least 6 h (Fig. 5C), paralleling the activation of Akt. Consistent with prior studies demonstrating that inhibitory phosphorylation of GSK-3β may be mediated by Akt, the PI 3-K inhibitor LY294002 and Akt IV inhibitor (specific for Akt) markedly diminished HGF-stimulated phosphorylation of GSK-3β at serine 9 in nonconfluent cells (Fig. 5D). In contrast, fully confluent cells in which HGF-stimulated Akt activation is downregulated demonstrate only a minimal increase in phosphorylation of GSK-3β at serine 9 after treatment with HGF (Fig. 5A to C), suggesting that GSK-3β activity may be inhibited following HGF stimulation in nonconfluent cells but not in confluent cells.
FIG. 5.
GSK-3β phosphorylation and activity are regulated by cell confluence. (A) Quiescent confluent (C) and nonconfluent (NC) mIMCD-3 cells plated for 24 h were stimulated in the presence or absence of HGF (40 ng/ml) for 10 min and immunoblotted with anti (α)-pGSK-3β (upper panel) and α-GSK-3β (lower panel). (B) Densitometric quantification of four independent experiments was performed as described for panel A.  , P < 0.01 versus nonconfluent cells plus HGF. (C) Quiescent nonconfluent and confluent cells were treated with HGF for the indicated times (minutes), and lysates were immunoblotted with α-pGSK-3β (upper panel) and α-GSK-3β (lower panel). (D) Quiescent nonconfluent cells were pretreated for 20 min with either 10 μM LY294002 or 10 μM Akt IV inhibitor (AktI) and then treated in the presence or absence of HGF for 10 min, and cell lysates were immunoblotted with α-pGSK-3β (upper panel) and α-GSK-3β (lower panel). (E) Quiescent confluent and nonconfluent mIMCD-3 cells were stimulated with HGF for the indicated times and immunoblotted with α-pβ-catenin (upper panel) and α-β-catenin (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.
, P < 0.01 versus nonconfluent cells plus HGF. (C) Quiescent nonconfluent and confluent cells were treated with HGF for the indicated times (minutes), and lysates were immunoblotted with α-pGSK-3β (upper panel) and α-GSK-3β (lower panel). (D) Quiescent nonconfluent cells were pretreated for 20 min with either 10 μM LY294002 or 10 μM Akt IV inhibitor (AktI) and then treated in the presence or absence of HGF for 10 min, and cell lysates were immunoblotted with α-pGSK-3β (upper panel) and α-GSK-3β (lower panel). (E) Quiescent confluent and nonconfluent mIMCD-3 cells were stimulated with HGF for the indicated times and immunoblotted with α-pβ-catenin (upper panel) and α-β-catenin (lower panel). (F) Densitometric quantification of four independent experiments was performed as described for panel E.  , P < 0.01 versus nonconfluent cells in the absence of HGF;
, P < 0.01 versus nonconfluent cells in the absence of HGF; 
 , P < 0.01 versus nonconfluent cells in the presence of HGF for 360 min.
, P < 0.01 versus nonconfluent cells in the presence of HGF for 360 min.
HGF has been shown to induce the translocation of β-catenin out of adherens junctions and into the cytosol (31). Active GSK-3β can phosphorylate free cytosolic β-catenin at Ser 33, Ser 37, and Thr 41, thus targeting the protein for ubiquination and degradation via the proteosomal pathway (1, 28). To determine whether HGF regulates this degradatory process in a cell confluence-dependent fashion, the level of β-catenin phosphorylation at these sites was examined in HGF-stimulated confluent and nonconfluent cells. In nonconfluent cells, basal levels of β-catenin phosphorylation were relatively high, with a progressive decrease in phosphorylation beginning 10 min after HGF stimulation (Fig. 5E and F). This loss of phosphorylation was sustained for at least 6 h, consistent with the timing of Akt activation (Fig. 4) and GSK-3β inhibition (Fig. 5C). Conversely, in confluent cells, there was a significantly lower level of basal phosphorylation of β-catenin, with a progressive increase during the 6 h following HGF treatment (Fig. 5E and F). The higher basal phosphorylation of β-catenin in nonconfluent cells presumably reflects the increased amount of free β-catenin in these cells available for phosphorylation by GSK-3β due to the small number of adherens junctions present to sequester β-catenin at the membrane. Total cellular levels of β-catenin were not substantially changed under either cell condition, suggesting that the level of cytosolic β-catenin available for phosphorylation and degradation is minor compared to total β-catenin levels in the cell (see Fig. S1B in the supplemental material).
Confluence-dependent regulation of β-catenin nuclear signaling is mediated by Akt and GSK-3β.
These results suggest that the HGF-stimulated release of β-catenin from cell-cell junctions in nonconfluent cells is likely to result in increased nuclear translocation and activation of transcription since GSK-3β-dependent β-catenin degradation is inhibited. In contrast, the increase in GSK-3β-dependent phosphorylation of β-catenin seen in HGF-stimulated fully confluent cells would be predicted to prevent a rise in free cytosolic β-catenin and thus abrogate the nuclear signaling pathway. To examine this possibility, β-catenin transcriptional activity was quantitated via a luciferase assay using the reporter construct TOPFLASH. Transient transfection of TOPFLASH into IMCD cells was performed, followed by seeding of cells under nonconfluent or confluent conditions. HGF stimulation of nonconfluent cells resulted in a 2.2- to 3-fold increase in β-catenin transcriptional activity, whereas no increase was detected in fully confluent cells (Fig. 6A to D). Cotransfection of β-catenin (SA) and TOPFLASH served as a positive control and resulted in a 2.6-fold activation independent of HGF and a 4.2-fold activation in the presence of HGF (see Fig. S1C in the supplemental material). These data demonstrate that in nonconfluent cells, there is an HGF-dependent increase in the transcriptional activity of β-catenin that is not seen in confluent cells.
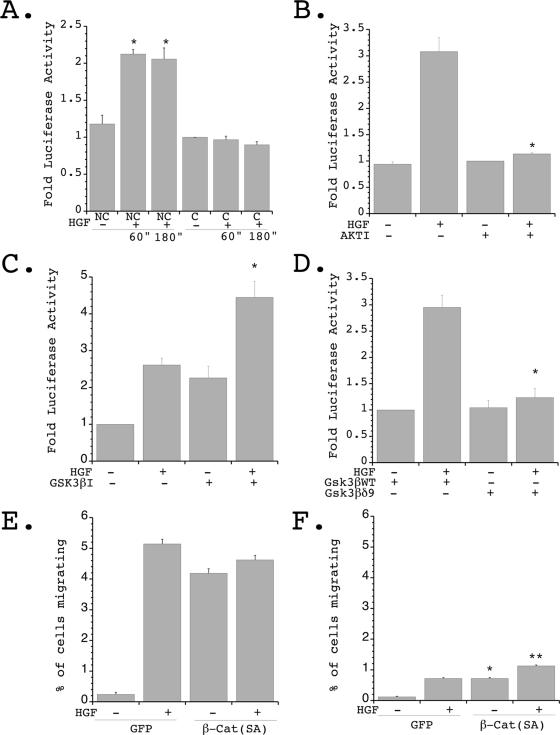
FIG. 6.
Inhibition of GSK-3β by Akt regulates HGF-stimulated β-catenin nuclear signaling and cell migration. (A) Following transient transfection with the β-catenin reporter plasmid TOPFLASH, 20,000 cells/well were plated in either 96-well or 24-well plates to create confluent (C) and nonconfluent (NC) conditions and stimulated in the presence or absence of HGF for the indicated times, and luciferase values were determined and normalized to transfection efficiency. Seven independent experiments were performed in triplicate wells.  , P < 0.01 versus nonconfluent cells at time zero and versus confluent cells in the presence of HGF for 60 or 180 min. (B) Cells were transfected as described for panel A and plated under nonconfluent conditions in the presence or absence of Akt IV inhibitor (AKTI) (10 μM) for 1 hour, followed by stimulation in the presence or absence of HGF (40 ng/ml) for 1 hour, and luciferase values were determined and normalized to transfection efficiency. Four independent experiments were performed in triplicate wells.
, P < 0.01 versus nonconfluent cells at time zero and versus confluent cells in the presence of HGF for 60 or 180 min. (B) Cells were transfected as described for panel A and plated under nonconfluent conditions in the presence or absence of Akt IV inhibitor (AKTI) (10 μM) for 1 hour, followed by stimulation in the presence or absence of HGF (40 ng/ml) for 1 hour, and luciferase values were determined and normalized to transfection efficiency. Four independent experiments were performed in triplicate wells.  , P < 0.01 versus HGF stimulated in the absence of the Akt inhibitor. (C) Cells were transfected as described for panel A and plated under nonconfluent conditions in the presence or absence of GSK-3β IX inhibitor (GSK3βI) (1 μM) for 1 hour, followed by stimulation in the presence or absence of HGF (40 ng/ml) for 1 hour, and luciferase values were determined and normalized to transfection efficiency. Three independent experiments were performed in triplicate wells.
, P < 0.01 versus HGF stimulated in the absence of the Akt inhibitor. (C) Cells were transfected as described for panel A and plated under nonconfluent conditions in the presence or absence of GSK-3β IX inhibitor (GSK3βI) (1 μM) for 1 hour, followed by stimulation in the presence or absence of HGF (40 ng/ml) for 1 hour, and luciferase values were determined and normalized to transfection efficiency. Three independent experiments were performed in triplicate wells.  , P < 0.05 versus HGF stimulated in the absence of the GSK-3β inhibitor. (D) Cells were transiently transfected with TOPFLASH and either p-CGN-GSK-3βδ9 or p-CGN-GSK-3βWT, followed by plating under nonconfluent conditions and stimulation in the presence or absence of HGF for 1 hour. Luciferase values were determined and normalized to transfection efficiency. Four independent experiments were performed in triplicate wells.
, P < 0.05 versus HGF stimulated in the absence of the GSK-3β inhibitor. (D) Cells were transiently transfected with TOPFLASH and either p-CGN-GSK-3βδ9 or p-CGN-GSK-3βWT, followed by plating under nonconfluent conditions and stimulation in the presence or absence of HGF for 1 hour. Luciferase values were determined and normalized to transfection efficiency. Four independent experiments were performed in triplicate wells.  , P < 0.01 versus HGF stimulated in the presence of wild-type (WT) GSK-3β. (E) mIMCD-3 cells were transiently transfected with β-catenin (SA) [β-Cat (SA)] and eGFP (GFP) or eGFP alone and plated under nonconfluent conditions in the presence or absence of stimulation with HGF. The percentage of migrating GFP-positive cells was quantitated. Five independent experiments were performed. (F) Cells treated as described for panel E were plated under confluent conditions in the presence or absence of HGF, and the percentage of migrating GFP-positive cells was quantitated. Five independent experiments were performed.
, P < 0.01 versus HGF stimulated in the presence of wild-type (WT) GSK-3β. (E) mIMCD-3 cells were transiently transfected with β-catenin (SA) [β-Cat (SA)] and eGFP (GFP) or eGFP alone and plated under nonconfluent conditions in the presence or absence of stimulation with HGF. The percentage of migrating GFP-positive cells was quantitated. Five independent experiments were performed. (F) Cells treated as described for panel E were plated under confluent conditions in the presence or absence of HGF, and the percentage of migrating GFP-positive cells was quantitated. Five independent experiments were performed.  , P < 0.01 versus confluent cells expressing eGFP alone in the absence of HGF;
, P < 0.01 versus confluent cells expressing eGFP alone in the absence of HGF; 
 , P < 0.01 versus confluent cells expressing eGFP alone in the presence of HGF.
, P < 0.01 versus confluent cells expressing eGFP alone in the presence of HGF.
To determine whether the confluence-dependent regulation of β-catenin nuclear signaling was dependent on the PI 3-K/Akt/GSK-3β pathway, TOPFLASH luciferase assays were performed with nonconfluent cells exposed to pathway inhibitors. Pretreatment of nonconfluent IMCD cells with the Akt IV inhibitor resulted in complete inhibition of the HGF-stimulated TOPFLASH luciferase activity (Fig. 6B). In contrast, inhibition of GSK-3β in nonconfluent cells led to an increase in β-catenin nuclear signaling both at baseline and following HGF stimulation (Fig. 6C). Finally, expression of a GSK-3β mutant that lacks the first nine amino acids and therefore cannot be phosphorylated and inhibited by Akt (36) resulted in a complete loss of HGF-stimulated β-catenin nuclear signaling (Fig. 6D). Taken together, these results support the hypothesis that the HGF-stimulated increase in β-catenin signaling seen in nonconfluent cells requires PI 3-K/Akt-dependent phosphorylation and inhibition of GSK-3β.
Constitutively active β-catenin increases migration in confluent cells.
The above results demonstrate that downregulation of HGF-stimulated Akt activation in fully confluent cells can lead to sustained GSK-3β activation and thus prevent β-catenin-mediated nuclear signaling. To determine whether this pathway is important for preventing HGF-stimulated cell morphogenesis in highly confluent cells, the ability of β-catenin (SA) (a mutant that cannot be phosphorylated by GSK-3β) to restore HGF-stimulated migration of fully confluent cells was examined. β-Catenin (SA) has been demonstrated to be resistant to proteolysis by the ubiquitin-proteasome system and to therefore increase transcription of nuclear β-catenin targets (see Fig. S1C in the supplemental material) (20, 25). IMCD cells were transiently cotransfected with either enhanced green fluorescent protein (eGFP) and β-catenin (SA) or eGFP alone and plated under either nonconfluent or confluent conditions on 24-well Transwell plates in the presence or absence of HGF stimulation. Cells were allowed to migrate across the membrane for 12 h, and eGFP-expressing cells were quantified by fluorescence microscopy.
In these experiments, expression of β-catenin (SA) increased basal migratory rates of both nonconfluent and confluent cells (Fig. 6E and F). Following HGF stimulation, there was a 1.6-fold increase in cell migration in the confluent cells expressing β-catenin (SA) (Fig. 6F), with the total number of migrating cells approaching that seen in nonconfluent cells (see Fig. S4 in the supplemental material). These results demonstrate that expression of a β-catenin mutant which cannot be phosphorylated by GSK-3β can stimulate increased levels of cell migration even in fully confluent cells and suggest that GSK-3β-dependent degradation of β-catenin normally plays an important role in preventing HGF-stimulated cell dedifferentiation and migration in fully confluent epithelial monolayers. Of note, since 3.75 times more cells were present on the upper surface of the Transwell membrane under confluent conditions than under nonconfluent conditions, complete rescue of the migration defect in confluent cells would have been predicted to result in the migration of approximately 110 cells/mm2. Thus, while expression of constitutively active β-catenin can increase HGF-stimulated migration of fully confluent cells, these results suggest that other factors play a significant role in regulating this process as well.
Cumulatively, these results address the in vivo and in vitro observations that growth factor stimuli that can induce morphogenic changes in nonconfluent cells bordering an area of injury fail to induce these same changes in fully confluent cells in nearby tubules. This is likely to be a critical control mechanism to allow the repair of one tubule section without disruption of the architecture or function of nearby uninjured tubule sections. While previous studies with the EGF receptor have suggested that prevention of receptor activation is the major mechanism of confluence-dependent downregulation of that pathway, the present studies of the c-Met receptor demonstrate that the receptor is activated but that there is selective downregulation of prodedifferentiation/promigratory signaling pathways in fully confluent cells. It is interesting to note that the fraction of PI 3-K/Akt activation mediated by c-Met-Gab1 signaling is not interrupted in these cells, suggesting that specific sites of PI 3-K activation at the membrane are likely to regulate select downstream signaling events. Thus, it is possible that PI 3-K/Akt activation mediated by Fak is downregulated in confluent cells to prevent cell dedifferentiation while PI 3-K/Akt activation via Gab1 is preserved and could play a role in preventing the apoptotic response seen following acute renal injury.
Supplementary Material
Acknowledgments
This work was supported by an NIH grant to L.G.C. (DK065109) and an AHA grant to S.I. (0575043N).
Footnotes
Published ahead of print on 9 October 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16:3797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birchmeier, C., W. Birchmeier, E. Gherardi, and G. F. Vande Woude. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4:915-925. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre, J. V., and A. Zuk. 2004. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 66:480-485. [DOI] [PubMed] [Google Scholar]
- 4.Brabletz, T., A. Jung, and T. Kirchner. 2002. Beta-catenin and the morphogenesis of colorectal cancer. Virchows Arch. 441:1-11. [DOI] [PubMed] [Google Scholar]
- 5.Brabletz, T., A. Jung, S. Reu, M. Porzner, F. Hlubek, L. A. Kunz-Schughart, R. Knuechel, and T. Kirchner. 2001. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA 98:10356-10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantley, L. G., E. J. Barros, M. Gandhi, M. Rauchman, and S. K. Nigam. 1994. Regulation of mitogenesis, motogenesis, and tubulogenesis by hepatocyte growth factor in renal collecting duct cells. Am. J. Physiol. 267:F271-F280. [DOI] [PubMed] [Google Scholar]
- 7.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 8.Dash, P. R., G. S. Whitley, L. J. Ayling, A. P. Johnstone, and J. E. Cartwright. 2005. Trophoblast apoptosis is inhibited by hepatocyte growth factor through the Akt and beta-catenin mediated up-regulation of inducible nitric oxide synthase. Cell. Signal. 17:571-580. [DOI] [PubMed] [Google Scholar]
- 9.Derman, M. P., M. J. Cunha, E. J. Barros, S. K. Nigam, and L. G. Cantley. 1995. HGF-mediated chemotaxis and tubulogenesis require activation of the phosphatidylinositol 3-kinase. Am. J. Physiol. 268:F1211-F1217. [DOI] [PubMed] [Google Scholar]
- 10.Ding, V. W., R.-H. Chen, and F. McCormick. 2000. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J. Biol. Chem. 275:32475-32481. [DOI] [PubMed] [Google Scholar]
- 11.Fixman, E. D., M. Holgado-Madruga, L. Nguyen, D. M. Kamikura, T. M. Fournier, A. J. Wong, and M. Park. 1997. Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins, Cbl and Gab1. J. Biol. Chem. 272:20167-20172. [DOI] [PubMed] [Google Scholar]
- 12.Gumbiner, B., B. Stevenson, and A. Grimaldi. 1988. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 107:1575-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellman, N. E., A. J. Greco, K. K. Rogers, C. Kanchagar, D. F. Balkovetz, and J. H. Lipschutz. 2005. Activated extracellular signal-regulated kinases are necessary and sufficient to initiate tubulogenesis in renal tubular MDCK strain I cell cysts. Am. J. Physiol. Renal Physiol. 289:F777-F785. [DOI] [PubMed] [Google Scholar]
- 14.Ishibe, S., D. Joly, Z. X. Liu, and L. G. Cantley. 2004. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol. Cell 16:257-267. [DOI] [PubMed] [Google Scholar]
- 15.Ishibe, S., D. Joly, X. Zhu, and L. G. Cantley. 2003. Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol. Cell 12:1275-1285. [DOI] [PubMed] [Google Scholar]
- 16.Joannidis, M., K. Spokes, T. Nakamura, D. Faletto, and L. G. Cantley. 1994. Regional expression of hepatocyte growth factor/c-met in experimental renal hypertrophy and hyperplasia. Am. J. Physiol. 267:F231-F236. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri, R., and E. G. Neilson. 2003. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 112:1776-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karihaloo, A., C. Nickel, and L. G. Cantley. 2005. Signals which build a tubule. Nephron Exp. Nephrol. 100:e40-e45. [DOI] [PubMed] [Google Scholar]
- 19.Karihaloo, A., D. A. O'Rourke, C. Nickel, K. Spokes, and L. G. Cantley. 2001. Differential MAPK pathways utilized for HGF- and EGF-dependent renal epithelial morphogenesis. J. Biol. Chem. 276:9166-9173. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa, M., S. Hatakeyama, M. Shirane, M. Matsumoto, N. Ishida, K. Hattori, I. Nakamichi, A. Kikuchi, and K. Nakayama. 1999. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 18:2401-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koyama, N., M. Kashimata, H. Sakashita, H. Sakagami, and E. W. Gresik. 2003. EGF-stimulated signaling by means of PI3K, PLCgamma1, and PKC isozymes regulates branching morphogenesis of the fetal mouse submandibular gland. Dev. Dyn. 227:216-226. [DOI] [PubMed] [Google Scholar]
- 22.Laffargue, M., P. Raynal, A. Yart, C. Peres, R. Wetzker, S. Roche, B. Payrastre, and H. Chap. 1999. An epidermal growth factor receptor/Gab1 signaling pathway is required for activation of phosphoinositide 3-kinase by lysophosphatidic acid. J. Biol. Chem. 274:32835-32841. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Y., E. M. Tolbert, L. Lin, M. A. Thursby, A. M. Sun, T. Nakamura, and L. D. Dworkin. 1999. Up-regulation of hepatocyte growth factor receptor: an amplification and targeting mechanism for hepatocyte growth factor action in acute renal failure. Kidney Int. 55:442-453. [DOI] [PubMed] [Google Scholar]
- 24.McManus, E. J., K. Sakamoto, L. J. Armit, L. Ronaldson, N. Shpiro, R. Marquez, and D. R. Alessi. 2005. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 24:1571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natsume, H., S. Sasaki, M. Kitagawa, Y. Kashiwabara, A. Matsushita, K. Nakano, K. Nishiyama, K. Nagayama, H. Misawa, H. Masuda, and H. Nakamura. 2003. Beta-catenin/Tcf-1-mediated transactivation of cyclin D1 promoter is negatively regulated by thyroid hormone. Biochem. Biophys. Res. Commun. 309:408-413. [DOI] [PubMed] [Google Scholar]
- 26.Noren, N. K., C. M. Niessen, B. M. Gumbiner, and K. Burridge. 2001. Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276:33305-33308. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien, L. E., K. Tang, E. S. Kats, A. Schutz-Geschwender, J. H. Lipschutz, and K. E. Mostov. 2004. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev. Cell 7:21-32. [DOI] [PubMed] [Google Scholar]
- 28.Orford, K., C. Crockett, J. P. Jensen, A. M. Weissman, and S. W. Byers. 1997. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J. Biol. Chem. 272:24735-24738. [DOI] [PubMed] [Google Scholar]
- 29.Pollack, A. L., A. I. Barth, Y. Altschuler, W. J. Nelson, and K. E. Mostov. 1997. Dynamics of beta-catenin interactions with APC protein regulate epithelial tubulogenesis. J. Cell Biol. 137:1651-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponzetto, C., Z. Zhen, E. Audero, F. Maina, A. Bardelli, M. L. Basile, S. Giordano, R. Narsimhan, and P. Comoglio. 1996. Specific uncoupling of GRB2 from the Met receptor. Differential effects on transformation and motility. J. Biol. Chem. 271:14119-14123. [DOI] [PubMed] [Google Scholar]
- 31.Potempa, S., and A. J. Ridley. 1998. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol. Biol. Cell 9:2185-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabkin, R., F. Fervenza, T. Tsao, R. Sibley, M. Friedlaender, F. Hsu, C. Lassman, M. Hausmann, P. Huie, and R. H. Schwall. 2001. Hepatocyte growth factor receptor in acute tubular necrosis. J. Am. Soc. Nephrol. 12:531-540. [DOI] [PubMed] [Google Scholar]
- 33.Reddy, M. A., N. V. Prasadarao, C. A. Wass, and K. S. Kim. 2000. Phosphatidylinositol 3-kinase activation and interaction with focal adhesion kinase in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J. Biol. Chem. 275:36769-36774. [DOI] [PubMed] [Google Scholar]
- 34.Reiske, H. R., S. C. Kao, L. A. Cary, J. L. Guan, J. F. Lai, and H. C. Chen. 1999. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J. Biol. Chem. 274:12361-12366. [DOI] [PubMed] [Google Scholar]
- 35.Royal, I., N. Lamarche-Vane, L. Lamorte, K. Kaibuchi, and M. Park. 2000. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol. Biol. Cell 11:1709-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salas, T. R., S. A. Reddy, J. L. Clifford, R. J. Davis, A. Kikuchi, S. M. Lippman, and D. G. Menter. 2003. Alleviating the suppression of glycogen synthase kinase-3beta by Akt leads to the phosphorylation of cAMP-response element-binding protein and its transactivation in intact cell nuclei. J. Biol. Chem. 278:41338-41346. [DOI] [PubMed] [Google Scholar]
- 37.Shao, J., C. Jung, C. Liu, and H. Sheng. 2005. Prostaglandin E2 stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J. Biol. Chem. 280:26565-26572. [DOI] [PubMed] [Google Scholar]
- 38.Shaw, M., P. Cohen, and D. R. Alessi. 1997. Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett. 416:307-311. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi, K., and K. Suzuki. 1996. Density-dependent inhibition of growth involves prevention of EGF receptor activation by E-cadherin-mediated cell-cell adhesion. Exp. Cell Res. 226:214-222. [DOI] [PubMed] [Google Scholar]
- 40.Webb, D. J., K. Donais, L. A. Whitmore, S. M. Thomas, C. E. Turner, J. T. Parsons, and A. F. Horwitz. 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6:154-161. [DOI] [PubMed] [Google Scholar]
- 41.Weidner, K. M., S. Di Cesare, M. Sachs, V. Brinkmann, J. Behrens, and W. Birchmeier. 1996. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384:173-176. [DOI] [PubMed] [Google Scholar]
- 42.Weidner, K. M., G. Hartmann, M. Sachs, and W. Birchmeier. 1993. Properties and functions of scatter factor/hepatocyte growth factor and its receptor c-Met. Am. J. Respir. Cell Mol. Biol. 8:229-237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.