Abstract
Previous studies have demonstrated that peptides corresponding to a six-amino-acid NEMO-binding domain from the C terminus of IκB kinase alpha (IKKα) and IKKβ can disrupt the IKK complex and block NF-κB activation. We have now mapped and characterized the corresponding amino-terminal IKK-binding domain (IBD) of NEMO. Peptides corresponding to the IBD were efficiently recruited to the IKK complex but displayed only a weak inhibitory potential on cytokine-induced NF-κB activity. This is most likely due to the formation of sodium dodecyl sulfate- and urea-resistant NEMO dimers through a dimerization domain at the amino terminus of NEMO that overlaps with the region responsible for binding to IKKs. Mutational analysis revealed different α-helical subdomains within an amino-terminal coiled-coil region are important for NEMO dimerization and IKKβ binding. Furthermore, NEMO dimerization is required for the tumor necrosis factor alpha-induced NF-κB activation, even when interaction with the IKKs is unaffected. Hence, our data provide novel insights into the role of the amino terminus of NEMO for the architecture of the IKK complex and its activation.
The NF-κB family of transcription factors is involved in the regulation of a wide variety of physiological and pathological processes. Besides its crucial role in the regulation of inflammatory, innate, and adaptive immune responses, NF-κB is also involved in development and cell survival (15). Inactive NF-κB is retained in the cytoplasm by its interaction with cytoplasmic inhibitory proteins, known as IκBs. Upon stimulation of cells with a wide variety of different stimuli, the IκB proteins are phosphorylated, ubiquitinated, and subsequently degraded via the proteasomal pathway (10, 11). The released NF-κB then translocates into the nucleus and binds to its cognate DNA recognition sites. The phosphorylation of the IκB proteins is mediated by a multisubunit kinase complex composed of two catalytic subunits, IκB kinase alpha (IKKα) and IKKβ, which form either homo- or heterodimers, as well as a noncatalytic regulatory subunit, NEMO (28, 40, 49, 52). The two catalytic kinase subunits, IKKα and IKKβ, are highly homologous, but they perform distinct biological functions. IKKβ is the predominant kinase activated upon stimulation with proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) or interleukin-1β (IL-1β), whereas the functions of IKKα are more diverse (16, 23, 24). For example, IKKα, in conjunction with the serine/threonine-kinase NIK, regulates the alternative NF-κB-signaling pathway, which is responsible for signal-induced processing of the precursor protein NF-κB2/p100 into its mature form, p52 (6, 7, 29, 30, 34, 39). This alternative pathway is activated upon stimulation with a subset of NF-κB agonists, such as CD40, lymphotoxin β, or lipopolysaccharide. The NEMO regulatory subunit plays a critical role in the assembly of the IKK complex and is thought to link the IKK complex to upstream activators by mechanisms that remain to be defined. Furthermore, different regions of NEMO exert distinct functions. For example, the amino terminus is necessary for interaction with the IKKs, the human T-cell leukemia virus type 1 Tax protein, or protein phosphatases (13, 21, 36, 51), whereas the carboxy terminus of NEMO is required for binding to ubiquitin or the deubiquitinase CYLD or for the oligomerization of NEMO (3, 9, 36, 37, 38, 44, 47, 51). Despite the lack of a precise model that can explain activation of the IKK complex, it has become progressively more clear that posttranslational modifications of NEMO play a critical role in this process. Ubiquitination, sumoylation, and phosphorylation of NEMO have all been reported. Whereas the function of NEMO ubiquitination and NEMO sumoylation is better understood (4, 17, 20, 46, 53), the role of NEMO phosphorylation remains largely unclear (5, 33, 43). However, a recent study demonstrated a crucial role for NEMO serine 85 phosphorylation for NF-κB activation by genotoxic stress (48).
The crucial role for oligomerization of NEMO in the assembly and function of the IKK complex is reflected in the fact that the IKK complex can be activated by enforced oligomerization of NEMO (32). However, conflicting data have been reported regarding the oligomerization status of endogenous NEMO. Whereas Agou et al. reported that NEMO forms a trimer in vivo and in vitro, other authors have reported a tetrameric organization (2, 3, 44). The tetrameric organization involves both the amino- as well as the carboxy-terminal regions of NEMO. In contrast, only the carboxy-terminal, second coiled-coil domain and the leucine zipper domain are thought to be necessary and sufficient for the formation of the NEMO trimers. In addition, lipopolysaccharide-induced NF-κB activity is attenuated by cell-permeable peptides spanning either the leucine zipper or the second coiled-coil domain of NEMO (1). Another cell-permeable peptide known to impair the signal-induced NF-κB activity is the NEMO-binding domain peptide, which corresponds to six amino acids (aa) in the carboxy terminus of IKKα and IKKβ (26). In contrast to this short and well-defined NEMO-binding domain, its counterpart, the IKK-binding domain (IBD) in NEMO, remains poorly characterized.
In this study, we have further characterized the IBD in greater detail. We demonstrate here that the IBD not only mediates the binding to the IKKs but also contributes to the oligomerization of NEMO. A coiled-coil domain spanning amino acids 47 to 80 within the IBD and consisting of three α-helical subdomains is crucial for both dimerization as well as binding to IKKs. However, by using various internal NEMO deletion mutants, we have been able to separate the domains required for IKK interaction and dimerization. We further demonstrate that this amino-terminal dimerization domain in NEMO is required for robust, inducible NF-κB activation. Therefore, our studies reveal that the amino terminus of NEMO contains important functional domains that not only mediate interaction with IKKs but also regulate IKK activation by promoting oligomerization of NEMO.
MATERIALS AND METHODS
Cell culture, transient transfection, retroviral infection, and reagents.
Human 293 cells, human HeLa cells (obtained from the American Type Culture Collection), and NEMO-deficient murine embryonic fibroblasts (MEFs) were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum, penicillin (50 U/ml), and streptomycin (50 μg/ml). Jurkat T cells and the NEMO-deficient Jurkat T cells were cultured in RPMI medium supplemented with 10% fetal bovine serum, penicillin (50 U/ml), and streptomycin (50 μg/ml). For transient transfections of 2 × 105 293 cells, the CaPO4 transfection method was used according to standard protocols. Transient transfection of MEFs was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol, and Jurkat T cells were transfected by electroporation (250 V, 975 μF). The retroviral transfection of the NEMO-deficient murine embryonic fibroblasts was performed as described elsewhere (8). Human recombinant TNF-α was purchased from R&D systems (Minneapolis, MN), and recombinant murine TNF-α was from Sigma.
Plasmids and antibodies.
Expression vectors encoding FLAG-tagged human full-length NEMO, NEMO ΔαH (aa 81 to 419), NEMO1-197, and NEMO179-419 have been described elsewhere (27). Expression vectors for FLAG-tagged NEMO1-120 (IBD1), NEMO40-120 (IBD2), NEMO60-120 (IBD3), and NEMO1-100 were generated by introduction of PCR-amplified DNA fragments into the EcoRI and XbaI sites of pFLAG-CMV2. NEMOdel1 to NEMOdel7 mutants were generated by PCR using joining primers. All PCR conditions and sequences of the mutagenesis primers are available upon request. The retroviral vectors for wild-type NEMO and the NEMOdel5 and NEMOdel7 mutants were constructed by inserting an EcoRI-BamHI fragment into the pEGZ vector. The expression vector for FLAG-tagged wild-type IKKβ has been described elsewhere (26). The 3xκB firefly luciferase reporter plasmid and the renilla luciferase reporter plasmid were described previously (25). Specific antibodies recognizing the FLAG epitope, β-tubulin, or the Xpress epitope were purchased from Sigma and Invitrogen, respectively. Antibodies specific for IKKα, IKKβ, or NEMO were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA).
Immunoprecipitation, immunoblotting, and quantification.
The immunoprecipitation and immunoblotting procedures were performed as described previously (25). In brief, 500 to 1,000 μg of protein extracts was mixed with 25 μl of bovine serum albumin-blocked FLAG antibody (M2, Sigma) linked to agarose beads. The samples were incubated for 1 to 12 h at 4°C with agitation. After incubation, the precipitates were washed extensively in TNT buffer. The resulting immunopurified proteins were used for immunoblotting experiments. In the case of the OneStrep pull-down analysis, 25 μl of Streptactin beads (QIAGEN) was used instead of the FLAG-conjugated agarose beads. For the immunoblotting analysis, either the immunopurified protein complexes, or, as indicated, 50 to 100 μg of a protein extract was loaded on a standard sodium dodecyl sulfate (SDS)-polyacrylamide gel (PAA). SDS-polyacrylamide gel electrophoresis (PAGE) and the transfer to nitrocellulose (Schleicher & Schuell) or nylon membranes (Immobilon polyvinylidene difluoride membrane; Millipore) were performed using standard protocols. The membrane was blocked with 5% milk powder in TBS plus Tween 20 prior to the incubation with the primary antibody (1:1,000 in TBS plus Tween 20), subsequently washed three times for 5 min each, and incubated in a TBS-Tween 20 solution containing either horseradish peroxidase-conjugated or IRDye700/800-conjugated secondary antibody (1:5,000). The detection was performed using either ECL substrates from Amersham Biosciences or the Odyssey infrared scanning system (LICOR). Quantification of NEMO-IKK interaction of NEMO dimerization was performed from three independent experiments using either the Odyssey software 1.2 or the NIH image 1.62 program.
Gel filtration.
For the gel filtration experiments, whole-cell extracts from 4 × 108 HeLa or 293 cells were clarified by centrifugation at 100,000 × g for 1 h at 4°C. The resulting S100 extract was fractionated either on a Superose 6 or a Superdex 75 column using the Akta fast-performance liquid chromatography system (GE Healthcare). Thirty microliters of the 0.5-ml (Superose 6) or 0.25-ml (Superdex 75) fractions was analyzed by immunoblot as described above.
Luciferase reporter assay.
For the reporter gene assays, 293 cells were transiently transfected as described above. In general, we used 200 ng of the NF-κB-dependent reporter construct along with 30 ng of a renilla luciferase reporter construct under the control of the human β-actin promoter, which leads to constitutive expression of the renilla luciferase. 293 cells (1 × 105) were transfected in 1 well of a 24-well plate. Equal DNA concentration in each experiment (1.2 μg/well) was maintained by adding the appropriate empty vector to the DNA mixture. The cells were lysed after 24 h with TNT, and the firefly and the renilla luciferase activities were measured according to the protocol for the dual-luciferase system (Promega). The resulting firefly luciferase values were normalized by the values of the renilla luciferase. The experiments were done in parallel and were repeated at least three times.
RESULTS
Identification of the IKK-binding domain (IBD) of NEMO.
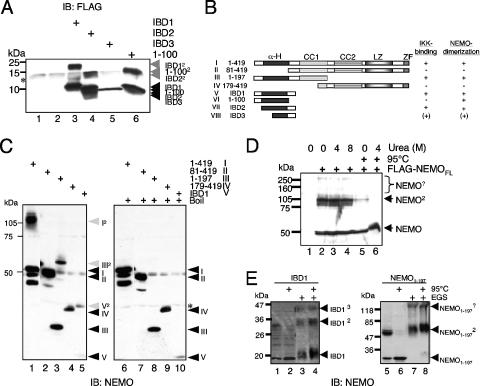
Various reports have demonstrated that the domain of NEMO necessary and sufficient for the interaction with IKKs is located in the amino-terminal portion of NEMO (13, 18, 26, 44, 51). To better map this domain and to determine whether there is a specificity in binding to IKKα versus IKKβ, we constructed different amino-terminal NEMO deletion mutants spanning the first 120 amino acids (IBD1), the region from amino acid 40 to 120 (IBD2), the region from aa 60 to 120 (IBD3), and the first 100 amino acids of NEMO (NEMO1-100) (Fig. 1A). In coimmunoprecipitation experiments with ectopically expressed NEMO deletion mutants, we observed an interaction of Xpress-tagged IKKβ with IBD1, IBD2, and IBD3 (Fig. 1B, lanes 8 to 10, lower part). In contrast, the Xpress-tagged IKKα interacted with IBD1 and IBD2 but was unable to bind to IBD3 (Fig. 1B, lanes 3 to 6, upper part), demonstrating different binding requirements for IKKα and IKKβ. In addition, both IKKα as well as IKKβ were unable to bind to the aa 1 to 100 fragment of NEMO (Fig. 1C, lanes 3 and 6). Next, we analyzed whether these NEMO peptides were able to interact with the endogenous IKK complex by transiently overexpressing IBD1, IBD2, and NEMO1-100 in 293 cells and performing anti-FLAG immunoprecipitations. As expected, IBD1 and IBD2, but not NEMO1-100, interacted with the endogenous IKKβ both in unstimulated as well as TNF-α-stimulated cells (Fig. 1D, lanes 9 to 16).
FIG. 1.
IKK-binding domains of NEMO are weak inhibitors of TNF-α-induced NF-κB activity. (A) A schematic representation of the NEMO peptides used in the analysis. (B) 293 cells were transiently transfected with expression vectors encoding either Xpress-tagged IKKα (lanes 1 to 5) or IKKβ (lanes 6 to 10) alone or with expression vectors for FLAG-tagged IBD1 (aa 1 to 120), IBD2 (aa 40 to 120) or IBD3 (aa 60 to 120). Forty-eight hours posttransfection, whole-cell extracts were prepared and the IBD:IKK complexes were precipitated using anti-FLAG beads. The resulting samples were subjected to an immunoblot (IB) analysis using anti-Xpress and anti-FLAG antibodies as indicated. A fraction of the extracts (10%) was subjected to immunoblot analysis with the indicated antibodies to check for the expression of the various ectopically expressed proteins. (C) A similar coimmunoprecipitation experiment was performed using F-IBD1 and NEMO1-100 in conjunction with Xpress-tagged IKKα or IKKβ. (D) 293 cells were transiently transfected with 3 μg of expression vectors encoding IBD1, IBD2, or NEMO1-100. Whole-cell extracts were prepared 48 h posttransfection and were used in immunoprecipitation experiments with anti-FLAG beads. The resulting immunoprecipitates were subjected to an immunoblot analysis using either the anti-IKKα/β antibody or the FLAG antibody (left part). As a control, a fraction of the whole-cell extracts was subjected to an anti-FLAG or an anti-IKKα/β immunoblot. The anti-IKKα/β antibody preferentially recognizes the IKKβ subunit. (E) 293 cells were transiently transfected with an NF-κB-dependent firefly luciferase reporter construct (200 ng) together with a renilla luciferase construct (30 ng) without or along with the indicated amounts of the different expression vectors for IBD1, IBD2, IBD3, and NEMO1-100. Twenty-four hours posttransfection, the cells were stimulated with TNF-α for four additional hours. Subsequently the cells were harvested, whole-cell extracts were prepared, and the firefly as well as the renilla luciferase activity was measured. The experiment was performed three times in parallel, and the mean value is shown. (F) 293 cells were transiently transfected with 0 or 3 μg F-IBD1 vector prior to immunoprecipitation experiments with anti-IKKα (upper part) or anti-IKKβ antibodies (lower part). The resulting immunoprecipitates (IP) were subjected to immunoblot analysis with the indicated antibodies. NRS, normal rabbit serum.
Overexpression of the IBD fragments does not disrupt the IKK complex or block NF-κB activation.
We have recently demonstrated that a peptide composed of six amino acids, corresponding to the carboxy-terminal NEMO-binding-domain (NBD) of the IKKs, potently inhibits the canonical NF-κB-signaling pathway by disrupting the IKK complex (26). We were therefore interested in determining whether peptides corresponding to the IKK-binding domain (IBD) in NEMO might also interfere with the inducible activation of NF-κB activity. However, when cotransfected with an NF-κB-dependent reporter gene construct, neither the different IBD peptides nor the NEMO1-100 fragment was able to impair the TNF-α-induced NF-κB activity in 293 cells (Fig. 1E). In addition, overexpression of IBD1 did not alter the interaction of endogenous NEMO and IKKα or IKKβ (Fig. 1F). Thus, although the IBD peptides were able to interact with the endogenous IKK complex, they are unable to disrupt the interaction of the IKKs with NEMO or impair the signal-induced NF-κB activity.
The amino terminus of NEMO forms an SDS-resistant dimer.
The inability of the different IBD fragments to effectively disrupt the interaction of IKKs with NEMO and to impair TNF-α-induced NF-κB activation suggested that the IBD segments differ in some basic way in comparison to the NBDs. This led us to more carefully examine the behavior of the IBDs. One notable property of the IBDs became apparent upon examination of immunoblots of gels where the different exogenously expressed IBD peptides or the aa 1 to 100 peptides were analyzed. In all cases, we observed additional bands that were exactly double the predicted molecular weight of IBD1, IBD2, and NEMO1-100. This suggested that the region around the NEMO IBD might form a stable dimer that is surprisingly robust and even resistant to fractionation on SDS-PAGE (Fig. 2A). NEMO has already been reported to form structures of higher molecular order based on interactions at the carboxy-terminal second coiled-coil and leucine zipper domains (2, 36, 44, 51), and the N-terminal IBD might provide an additional dimerization surface.
FIG. 2.
NEMO forms SDS-resistant dimers. (A) Whole-cell extracts from 293 cells transiently transfected with expression vectors for IBD1 (lane 3), IBD2 (lane 4), IBD3 (lane 5), or NEMO1-100 (lane 6) were subjected to an anti-NEMO immunoblot analysis. (B) Schematic representation of the different NEMO fragments used. (C) Anti-NEMO immunoblot of whole-cell extracts from exogenously expressed full-length NEMO (I in lanes 1 and 6), NEMO81-419 (II in lanes 2 and 7), NEMO1-197 (III in lanes 3 and 8), NEMO179-419 (IV in lanes 4 and 9), or IBD1 (V in lanes 5 and 10). The samples were either left untreated (left part) or boiled for 5 min (right part) prior to the separation by SDS-PAGE. The monomeric NEMO fragments are indicated by black, and the dimeric forms are indicated by gray arrows. *, unspecific bands. (D) Whole-cell extracts from 293 cells transiently transfected with an expression vector for FLAG-NEMO were either left untreated or were incubated with increasing concentrations of urea. Selected samples were heated (95°C) additionally. NEMO2, NEMO-containing high-molecular-weight protein complexes. (E) Ectopic expressed IBD1 or NEMO1-197 was either left untreated (lanes 1 to 2 and 5 to 6) or were subjected to a cross-linking procedure using 25 mM EGS (ethylene glycol bis[succinimidylsuccinate]) prior to an anti-NEMO immunoblot. An additional heating step was included as indicated. IB, immunoblot.
To further study the potential N-terminal dimerization domain in NEMO that overlaps with the IBD domain, we ectopically expressed full-length NEMO, a NEMO mutant lacking the first 80 amino acids (aa 81 to 419), an amino-terminal NEMO mutant (aa 1 to 197), a carboxy-terminal NEMO mutant (aa 179 to 419), and IBD1 (aa 1 to 120), designated I to V, and analyzed the transfected cell lysates by SDS-PAGE followed by immunoblot analysis for NEMO (see Fig. 2B for a schematic representation of the deletion mutants used). Once again, additional bands corresponding to dimeric forms of the expressed proteins were observed in the case of full-length NEMO (I; Fig. 2C, lane 1), the amino-terminal mutant (II; Fig. 2C, lane 3), and IBD1 (V; Fig. 2C, lane 5). However, the carboxy-terminal NEMO mutant (IV; Fig. 2C, lane 4) or the NEMO mutant lacking the first 80 amino acids (II; Fig. 2C, lane 2) did not yield dimeric forms, suggesting that the amino terminus of NEMO is necessary and sufficient for the observed dimerization. As we used 0.2% SDS in both the PAA gel and the loading buffer, the possibility remained that the NEMO dimerization was mediated by a covalent interaction. However, when we introduced an additional heating step (95°C, 5 min), the higher molecular weight signals disappeared, thus proving a noncovalent yet SDS-resistant nature of this NEMO-NEMO interaction (Fig. 2C, compare lanes 1, 3, and 5 with lanes 6, 8, and 10). The strength of this NEMO-NEMO interaction was further demonstrated by the finding that even the presence of 8 M urea in the sample buffer did not affect the NEMO dimerization (Fig. 2D). To determine the oligomerization status of the amino terminus of NEMO in vivo, we performed cross-linking experiments using ectopically expressed NEMO1-197 and the IBD1 peptide. As shown in Fig. 2E, the majority of the NEMO amino terminus and IBD1 is in a dimeric state. However, we also observed additional bands corresponding to oligomers of higher order. In addition, the distribution of ectopically expressed IBD1 in a size exclusion experiment using a Superdex 75 column also supported the notion that N-terminal NEMO fragments form oligomers of higher order in vivo, possibly dimers and trimers (see Fig. S1 in the supplemental material). However, although the IBD can form oligomers of higher molecular order, the predominant oligomeric form is a dimer. This dimerization appears to be mediated through a short domain in the amino terminus of NEMO, spanning amino acids 40 to 80, and generates a stable, SDS-resistant NEMO-NEMO interaction. Interestingly, this domain lies within the region involved in the formation of the IKK-NEMO interaction and may help explain the inability of the IBD fragments to disrupt interaction of NEMO with IKKs (26).
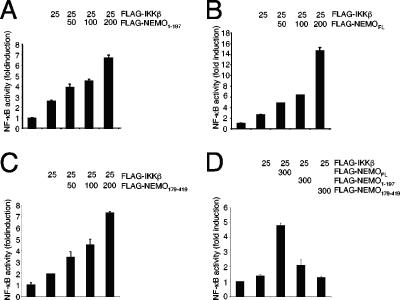
The amino-terminal NEMO dimerization is sufficient for the proximity-induced IKK activation.
Oligomerization of NEMO is believed to be an important step in signal-induced activation of the IKK complex (1, 2). Furthermore, the activity of IKKα and IKKβ can be augmented by an enforced oligomerization of these kinases which can be induced by addition of NEMO (32). Since this “proximity-induced IKK-activation” by NEMO requires the binding of NEMO to the IKK subunits and the oligomerization of NEMO, we asked whether an amino-terminal mutant of NEMO, containing the first 197 amino acids of NEMO, is able to enhance the activity of IKK. Indeed, in luciferase reporter assays with an NF-κB-dependent reporter construct, we observed a significant increase in the activity of IKKβ by the addition of the amino-terminal NEMO fragment (Fig. 3A). However, the increase in the IKKβ-mediated NF-κB activity was less pronounced compared to the results of a similar experiment using the full-length version of NEMO (Fig. 3B). Interestingly, the addition of a carboxy-terminal NEMO fragment, spanning the region between amino acids 179 and 419 and thus containing the previously characterized minimal oligomerization domain (3, 44), also augmented the IKKβ-mediated NF-κB activity (Fig. 3C). These findings suggest that both the N- and C-terminal portions of NEMO might participate in activation of the IKK complex, following their recruitment to the endogenous IKK complex, by acting as a “bridge” between two or more endogenous NEMO proteins. Such a model is supported by the finding that neither of the NEMO fragments, in contrast to full-length NEMO, are able to enhance the IKKβ-mediated NF-κB activation in NEMO-deficient MEFs (Fig. 3D).
FIG. 3.
Amino-terminal NEMO dimerization is sufficient for the proximity-induced IKK activation. 293 cells were transiently transfected with a luciferase reporter construct controlled by a multimerized NF-κB-binding site in conjunction with a β-actin promoter-controlled renilla luciferase reporter construct, without or in combination with a small amount (25 ng) of an IKKβ-encoding expression vector. In addition, increasing amounts of an expression vector coding for (A) the amino terminus of NEMO (amino acids 1 to 197), (B) full-length NEMO, or (C) the carboxy terminus of NEMO (amino acids 179 to 419) were added. After 24 h, the cells were lysed in TNT and the activity of the firefly as well as the renilla luciferase was determined. A mean value of the corrected relative luciferase activity of two parallel experiments is shown. This experiment was performed three times with a similar result. (D) NEMO-deficient MEFs were transiently transfected with the indicated amounts of expression vectors for FLAG-tagged IKKβ, full-length NEMO, NEMO1-197, or NEMO179-419 in conjunction with the NF-κB-dependent firefly luciferase reporter construct and the β-actin promoter-dependent renilla luciferase reporter construct using the Lipofectamine 2000 reagent (Invitrogen).
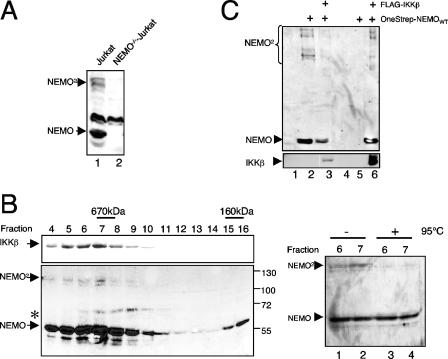
SDS-resistant NEMO dimers are recruited to the IKK complex.
To determine whether endogenous NEMO also forms SDS-resistant dimers, we compared whole-cell extracts from wild-type and NEMO-deficient Jurkat T cells in an anti-NEMO immunoblot. As shown in Fig. 4A, two NEMO-specific signals of 55 and 110 kDa were detected in wild-type Jurkat cell extracts but not in NEMO-deficient Jurkat cells, strongly suggesting the existence of endogenous NEMO dimers. The oligomerization potential of NEMO has been demonstrated to be critical for IKK activation (2, 32), and therefore we asked whether NEMO dimers are recruited to the IKK complex. For this we fractionated HeLa S100 extracts on a Superose 6 column and tested the resulting fractions for the appearance of NEMO dimers in an immunoblot analysis (Fig. 4B). The fractionation revealed two NEMO peaks, one in a high-molecular-mass range between 600 and 900 kDa (fractions 4 to 10), and a second peak at approximately 160 kDa (fractions 15 and 16). Interestingly, the dimeric NEMO-specific signal at 110 kDa was present only in the high-molecular-mass peak, which tracks with the previously reported heterotrimeric IKK complex (IKKα/IKKβ/NEMO), as indicated by the comigration of IKKβ (Fig. 4B, left part, upper panel). Since the higher molecular mass signal was reduced for untreated compared to boiled samples of fractions 6 and 7 in a control experiment, we concluded that the 110-kDa NEMO signal represents the endogenous NEMO dimer (Fig. 4B, right part). To formally prove that the IKKs, especially IKKβ, interact with the NEMO dimers, we performed a coimmunoprecipitation experiment with ectopically expressed FLAG-IKKβ in combination with OneStrep-tagged NEMO. As shown in Fig. 4C, IKKβ interacts with both NEMO dimers as well as NEMO monomers (Fig. 4C, lane 6). Based on these findings, we concluded that the NEMO dimer is an integral part of the heterotrimeric IKK complex.
FIG. 4.
Endogenous NEMO dimers are recruited to the IKK complex. (A) Dimer formation of endogenous NEMO. Fifty micrograms of whole-cell extract from wild-type Jurkat T cells (lane 1) or a NEMO-deficient Jurkat mutant (lane 2) was subjected to an anti-NEMO immunoblot analysis. (B) NEMO dimers comigrate with the IKK complex. S100 extracts from 4 × 108 HeLa cells were fractionated on a Superose 6 column, and 6% of the indicated fractions was subjected to an anti-IKKβ (upper panel) or an anti-NEMO (lower panel) immunoblot analysis. The asterisk marks a nonspecific signal. As a control, 6% of the indicated fractions were either subjected to an additional heating step (right part, lanes 3 and 4) or was left untreated (lanes 1 and 2) prior to an anti-NEMO immunoblot analysis. (C) IKKβ interacts with NEMO monomers and dimers. 293 HEK cells were transiently transfected with 2 μg of expression vectors for FLAG-IKKβ and OneStrep-NEMO, as indicated, and the resulting whole-cell extracts were used for an anti-FLAG immunoprecipitation and subsequent anti-NEMO (upper part) or anti-IKKβ (lower part) immunoblot analysis.
NEMO dimerization remains unaffected by stimulation with different NF-κB agonists.
Recent studies showed conflicting data concerning potential signal-induced alteration of the IKK complex, especially regarding signal-dependent augmentation of NEMO oligomerization (32, 44). Furthermore, the ability of the amino terminus of NEMO to activate NF-κB (Fig. 3A) led us to wonder whether signal-induced increases in SDS-resistant NEMO dimerization might be involved in the activation process of the IKK complex. To test this possibility, we analyzed the NEMO dimerization in Jurkat T cells upon TNF-α stimulation (Fig. 5A) or upon stimulation with phorbol myristate acetate (PMA) (Fig. 5B) for various times. As a control we included extracts from a NEMO-deficient Jurkat mutant cell line in our study (Fig. 5A and B, lane 1). As shown in Fig. 5, neither TNF-α nor PMA stimulation led to marked alteration in the amount of SDS-resistant NEMO dimers. Thus, although we cannot exclude the possibility that the dimer-monomer ratio of a small fraction of NEMO proteins is altered upon cell stimulation, the amino-terminal NEMO dimerization is not dramatically altered during activation of the IKK complex.
FIG. 5.
NEMO dimer formation remains unaltered upon cell stimulation. Jurkat T cells were stimulated with TNF-α (A) or with PMA (B) for the indicated times, and whole-cell extracts were prepared. The resulting extracts were subjected to an anti-NEMO (upper panel) or an anti-IκBα immunoblot analysis (lower panel). As a control for the specificity of the NEMO signals, whole-cell extracts from NEMO-deficient Jurkat cells were included in the analysis (A and B, lanes 1). IB, immunoblot.
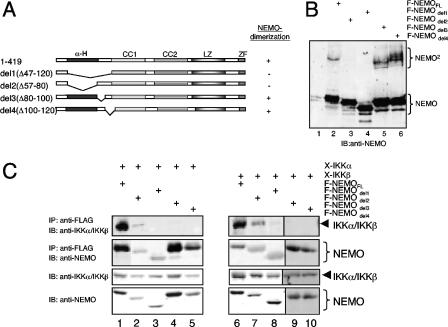
The domains in NEMO necessary for IKK interaction or NEMO dimerization are overlapping but not identical.
To dissect the relationship between the NEMO oligomerization and the interaction of NEMO with the IKKs, we generated internal NEMO deletion mutants, lacking either the entire IKK-binding domain (del1; aa 47 to 120), only the α-helical coiled-coil-domain (del2; aa 47 to 80), the amino acids 80 to 100 (del3), or the amino acids 101 to 120 (del4). As expected, deletion of either the entire IBD (del1) or the α-helical domain (del2), but not amino acids 80 to 100 (del3) or 101 to 120 (del4), strongly reduced NEMO dimerization (for quantification, see Fig. S2C in the supplemental material). In contrast, del1, del3, and del4 showed no IKKα or IKKβ interaction when tested in coimmunoprecipitation experiments, while deletion del2 still allowed a weak residual IKK interaction (Fig. 6C). Taken together, these data demonstrate different requirements for the IKK-NEMO interaction and the NEMO dimerization; however, as expected, the coiled-coil domain (amino acids 47 to 80) plays an important role for both protein-protein interactions.
FIG. 6.
Different structural requirements for IKK binding and NEMO dimerization. (A) Schematic representation of the different deletion mutants of NEMO used. (B and C) 293 cells were either transiently transfected with expression vectors for the different FLAG-tagged NEMO variants alone (B) or in combination with expression vectors for Xpress-tagged IKKα or IKKβ (C). The resulting whole-cell extracts were subjected directly to an anti-NEMO immunoblot analysis (B) or to an anti-FLAG immunoprecipitation prior to anti-IKKα (C, left part), anti-IKKβ (C, right part), or anti-NEMO (C, both parts) immunoblot analysis. IB, immunoblot; IP, immunoprecipitate.
The α-helical coiled-coil domain in the amino terminus of NEMO, crucial for NEMO dimerization and IKK interaction, is composed of three amphiphatic α-helical subdomains, designated α1 (aa 47 to 56), α2 (aa 60 to 70), and α3 (aa 72 to 81). To further separate regions that are important for dimerization versus IKK interaction, we generated additional internal NEMO deletion mutants that lacked either subdomain α1 (del5; aa 47 to 56), subdomain α2 (del6; aa 57 to 69), or subdomain α3 (del7; aa 70 to 79). Once again, we tested the different deletion mutants for their dimerization potential and their ability to interact with the IKK subunits. Although all of the mutants tested were able to form dimers, we observed a significant decrease in dimer formation by the del5 and del6 mutants (Fig. 7B; see also Fig. S2C in the supplemental material). In contrast, only the del7 mutant showed a decrease in the interaction with IKKα, and it showed a more severe decrease with IKKβ (Fig. 7C and D; a quantification of three independent experiments is given in Fig. S2A and S2B in the supplemental material). Therefore, these results suggest that while the α1 subdomain plays a crucial role for the NEMO dimerization, the α3 subdomain is more important for interaction with IKKs.
FIG. 7.
Reduced NEMO dimer formation and IKKβ binding by deletion of specific subdomains of the α-helical NEMO coiled-coil domain. (A) Schematic representation of the different NEMO variants used. (B) The NEMO dimer formation was monitored by an anti-NEMO immunoblot after transient transfection of 293 HEK cells with the indicated FLAG-tagged NEMO variants. (C) For the NEMO-IKKα interaction study, 2 μg of an Xpress-IKKα expression vector was either transfected alone or in combination with 1 μg of the indicated FLAG-NEMO variants. After 48 h, whole-cell extracts were prepared and an anti-FLAG immunoprecipitation experiment was performed. The resulting protein complexes were subjected to a Western blot analysis using the indicated antibodies. To control the expression of the different proteins, 10% of the lysate was used directly for an immunoblot. (D) An experiment similar to that described for panel C was performed after cotransfection of Xpress-IKKβ instead of IKKα. IB, immunoblot; IP, immunoprecipitate.
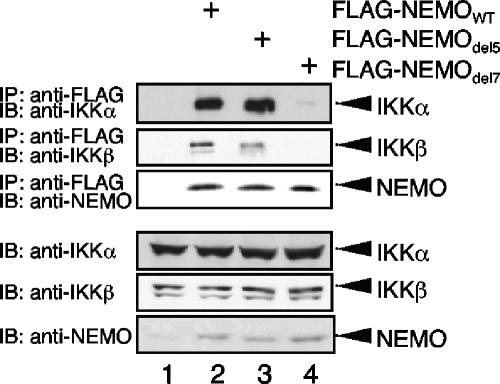
We finally tested whether the del5 mutant and del7 mutant displayed similar differences in interaction with endogenous IKKs, as seen with overexpressed IKKs in Fig. 7C and D. We transiently reconstituted NEMO-deficient Jurkat T cells with either wild-type NEMO or del5 and del7 mutants of NEMO. The del5 and wild-type NEMO interacted with IKKα or IKKβ with a comparable efficiency; however, the interaction of del7 with both IKK subunits IKKα and IKKβ was strongly reduced (Fig. 8).
FIG. 8.
Interaction of NEMOdel5 and NEMOdel7 with endogenous IKK subunits. Whole-cell extracts from NEMO-deficient Jurkat T cells transiently transfected with the indicated FLAG-NEMO constructs were subjected to an anti-FLAG immunoprecipitation and subsequently anti-IKKα, anti-IKKβ, and anti-NEMO immunoblot analysis (upper part). As a control for the equal expression of the different IKK subunits, a fraction of the whole-cell extracts was subjected to immunoblot analysis with the indicated antibodies (lower part). IB, immunoblot; IP, immunoprecipitate.
The amino-terminal NEMO dimerization is required for TNF-α-induced NF-κB activity.
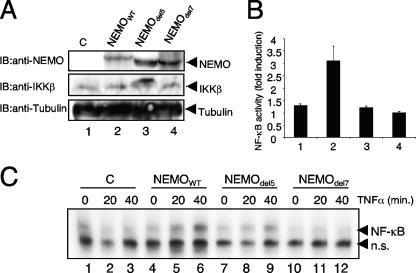
A NEMO mutant with a disturbed leucine zipper in the carboxy-terminal minimal oligomerization domain is unable to support the signal-induced NF-κB activation (2). Since this carboxy-terminal oligomerization is a crucial structural feature of NEMO, we wanted to test whether the amino-terminal dimerization domain might also play a functional role in activation of the IKK complex. We therefore stably reconstituted NEMO-deficient murine embryonic fibroblasts (MEFs) with wild-type and del5 and del7 mutants of NEMO. As shown in Fig. 9, the retroviral infection led to an equal expression of the NEMO variants in the different stable cell lines (Fig. 9A, upper panel). However, upon stimulation with TNF-α, MEFs reconstituted with del5 mutant of NEMO displayed dramatically reduced NF-κB activation, both in NF-κB-dependent reporter gene analysis (Fig. 9B) and in electrophoretic mobility shift assay analysis (Fig. 9C, compare lanes 6 and 9). As expected, the TNF-α-induced NF-κB activity in del7 mutant NEMO-reconstituted cells was more dramatically impaired in comparison to cells reconstituted with wild-type NEMO (Fig. 9B and C, lane 12 versus 9). As the del5 mutant binds to IKKs with efficiency equal to that of the wild type, this result strongly suggests that the reduced NF-κB activation observed in MEFs reconstituted with the del5 mutant is due to the inability of this mutant NEMO to dimerize. Therefore, these results demonstrate that dimerization of NEMO through its amino-terminal dimerization domain is required for robust, inducible NF-κB activation.
FIG. 9.
Function analysis of the amino-terminal NEMO dimerization in vivo. (A) Anti-NEMO (upper panel), anti-IKKβ (middle panel), or anti-tubulin (lower panel) immunoblot analysis of 70 μg whole-cell extracts from the different retroviral transduced NEMO-deficient MEFs. (B) The different NEMO cell lines were transiently transfected with 400 ng of an NF-κB-dependent luciferase reporter construct and 30 ng of a β-actin-dependent renilla luciferase reporter. Eighteen hours posttransfection, the cells were either left untreated or stimulated with recombinant murine TNF-α (30 ng/ml) for five additional hours prior to the luciferase measurement. The ratio of the normalized values for stimulated versus unstimulated samples is depicted as fold induction. (C) NF-κB-specific electrophoretic mobility shift assay experiment with 10 μg nuclear proteins from the different untreated or TNF-α-stimulated NEMO cell lines. n.s., nonspecific signal. IB, immunoblot; C, control.
DISCUSSION
The transcription factor NF-κB is linked to various diseases, including cancer, arthritis, and asthma (12, 14, 31, 41, 42, 50). Thus, NF-κB represents a promising target for drug development. Peptides that inhibit the NF-κB activation pathway have recently been used successfully to dissect the role of NF-κB in mouse models of disease, as well as in different biochemical studies (19, 26, 45). Several peptides, including the NEMO-binding domain (NBD) peptide, the BA-CC2 peptide, and the BA-LZ peptide (1) affect the activity of the IKK complex by interfering with the formation of the IKK complex, either by disturbing the IKK-NEMO interaction (NBD peptide) or by interfering with the NEMO oligomerization (BA-CC2 and BA-LZ peptides), thereby emphasizing the importance of the structural integrity of the IKK complex for its function. In contrast, short amino-terminal NEMO fragments, similar to the IKK-binding domain (IBD) of NEMO, do not efficiently impair the activation of the IKK complex, although the different IBD peptides tested in this report interacted efficiently with both endogenously and exogenously expressed IKKs (Fig. 1). These observations are consistent with previously published studies showing that overexpression of the amino terminus of NEMO either has no or at best a mild inhibitory effect on signal-induced NF-κB activity (18, 44, 51). One reason for this discrepancy might be the presence of a highly stable dimerization domain at the N terminus of NEMO. Although only a weak amino-terminal NEMO-NEMO interaction was reported previously by Tegethoff et al. (44), we found that the amino-terminal NEMO-NEMO interaction can withstand harsh conditions, including 0.2% SDS or 8 M urea, thus promoting formation of stable NEMO oligomers. Noncovalent, but SDS-resistant, protein interactions have been demonstrated previously, for example, for the neuronal isoform of nitric oxide synthase (22) or the BH3-only-containing protein BNIP3 (35). The amino terminus of NEMO therefore not only recruits the IKKs to the IKK complex but also confers structural stability to the IKK complex through the formation of stable NEMO-NEMO interactions. Thus, the peptides corresponding to the amino terminus of NEMO bind to the IKK subunits; however, they do not disrupt the IKK complex, as they probably act as a “bridge” connecting the NEMO subunit to IKK subunits. This notion is supported by the enhanced IKKβ-mediated NF-κB activity by cotransfection of the amino-terminal half of NEMO (Fig. 3) and the augmentation of TNF-α-induced NF-κB activity observed upon cotransfection of IBD-encoding vectors (Fig. 1E). According to the “proximity activation model,” the addition of NEMO augments the activity of IKKα or IKKβ by promoting oligomerization of the IKK subunits and inducing a transautophosphorylation at the activation loops of the IKK subunits (32). Previous studies had suggested that the amino terminus of NEMO was required to recruit the IKKs, while the carboxy terminus mediated the NEMO-NEMO interaction (36, 51). However, our results suggest that the amino terminus can contribute to both the NEMO dimerization and the IKK interaction. While this amino-terminal dimerization is necessary for signal-induced activation of IKK, it is not sufficient, since activation of IKKβ in NEMO-deficient MEFs was only achieved by addition of full-length NEMO but not by the isolated amino or carboxy termini, suggesting that this process requires the formation of an intact IKK complex through interactions including both the amino and the carboxy termini.
Another important point is the relationship between NEMO dimerization and NEMO-IKK interaction, particularly the question of whether dimerization of NEMO is necessary for interaction with IKK subunits. We found that the NEMO domains necessary for the interaction with both IKKs (IBD2) and for the formation of SDS-resistant dimers overlap but are not identical. First, deletion of the entire IBD (aa 47 to 120) abolished the IKK interaction of NEMO and strongly reduced the NEMO dimerization. Second, a NEMO mutant lacking the α-helix (aa 47 to 80) also showed a severely reduced NEMO dimerization and NEMO-IKK interaction, yet the formation of both protein-protein interactions mediated by the amino terminus of NEMO is still possible to a small extent. In contrast, deleting either amino acids 80 to 100 or 100 to 120 of NEMO had no severe negative effect on the NEMO dimerization but abolished the IKK-NEMO interaction. In addition, the NEMOdel5 mutant displays a reduced dimerization but binds efficiently to IKKα as well as to IKKβ. Taken together, our data suggest that the NEMO dimerization is not sufficient for the NEMO-IKK interaction. However, the possibility remains that the amino-terminal dimerization facilitates efficient IKK-NEMO interaction, since the residual NEMO dimerization observed with the NEMOdel5 mutant might be sufficient for the NEMO-IKK interaction. Clearly, the reduction in the TNF-α-induced NF-κB activation observed with NEMOdel5-stable cells is not due to a dramatic reduction of NEMO-IKK interaction.
Previous publications suggested significant differences between the IKKα-NEMO and IKKβ-NEMO interactions (18, 26, 27, 44). The IKKα-NEMO interaction was not only shown to be weaker than the IKKβ-NEMO interaction, the IKKα-interaction also required a longer part of NEMO (aa 50 to 120) than the IKKβ interaction (aa 65 to 120). In contrast to these data, we demonstrate here, by using various internal NEMO deletion mutants, that the structural requirements for the NEMO interaction with IKKα and IKKβ are nearly similar (see Fig. S2 in the supplemental data), although we observed a significantly stronger reduction of the NEMO-IKKβ interaction by deleting the α3-subhelical domain (aa 70 to 79) compared with the IKKα-NEMO interaction in cotransfection experiments. However, both IKK-NEMO interactions were severely affected in a transient complementation experiment using NEMO-deficient Jurkat T cells. This difference could be explained by a more subtle NEMO interaction of endogenous IKK proteins or by the necessity of a high number of IKK-NEMO interactions for the formation of a functional IKK complex.
Taken together, we provide novel insights into the architecture of the IKK complex by the characterization of a robust, SDS-resistant NEMO dimerization as a required structural feature. Furthermore, we provide evidence that the α-helix (aa 47 to 80) is not absolutely required for the NEMO-IKK interaction, and the core IKK-binding domain of NEMO encompasses the amino acids 80 to 120. However, deleting the α-helix (NEMOdel2) had a dramatic effect on the IKK-NEMO interaction. However, it remains unclear whether this is due to a separate IKK interaction of the α-helix or to structural alteration of the entire amino terminus of NEMO leading to an inefficient NEMO-IKK interaction. The NEMO dimerization might also explain the discrepancies between the existing models regarding the NEMO oligomerization. As both current models demonstrate a trimerization potential of the carboxy-terminal half of NEMO (aa 197 to 419), another NEMO moiety might bind due to the amino-terminal dimerization and thus lead to the tetramer formation observed by Tegethoff et al (44). Finally, since the activity of the IKK complex is affected by modulating the IKK-NEMO interaction due to the phosphorylation of a serine residue in the NBD of IKKβ, it will be interesting in future studies to analyze whether the reported phosphorylation in the amino terminus of NEMO modulates the structure of the IKK complex as well.
Supplementary Material
Acknowledgments
This work was supported by a grant from NIH (R37-AI33443). Ralf Marienfeld was supported by an Emmy-Noether-Fellowship (MA 2367/1-1) by the Deutsche Forschungsgemeinschaft.
NEMO-deficient Jurkat T cells were a kind gift of S. C. Sun (Pennsylvania State University), and NEMO-deficient murine embryonic fibroblasts were kindly provided by Michael Karin (University of California). We thank Thomas Wirth (University Ulm) for reagents and critical discussion of this work.
Footnotes
Published ahead of print on 25 September 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Agou, F., G. Courtois, J. Chiaravalli, F. Baleux, Y. M. Coic, F. Traincard, A. Israel, and M. Veron. 2004. Inhibition of NF-kappa B activation by peptides targeting NEMO oligomerization. J. Biol. Chem. 279:54248-54257. [DOI] [PubMed] [Google Scholar]
- 2.Agou, F., F. Traincard, E. Vinolo, G. Courtois, S. Yamaoka, A. Israel, and M. Veron. 2004. The trimerization domain of NEMO is composed of the interacting C-terminal CC2 and LZ coiled-coil subdomains. J. Biol. Chem. 279:27861-27869. [DOI] [PubMed] [Google Scholar]
- 3.Agou, F., F. Ye, S. Goffinont, G. Courtois, S. Yamaoka, A. Israel, and M. Veron. 2002. NEMO trimerizes through its coiled-coil C-terminal domain. J. Biol. Chem. 277:17464-17475. [DOI] [PubMed] [Google Scholar]
- 4.Brummelkamp, T. R., S. M. Nijman, A. M. Dirac, and R. Bernards. 2003. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature 424:797-801. [DOI] [PubMed] [Google Scholar]
- 5.Carter, R. S., K. N. Pennington, B. J. Ungurait, and D. W. Ballard. 2003. In vivo identification of inducible phosphoacceptors in the IKKgamma/NEMO subunit of human IkappaB kinase. J. Biol. Chem. 278:19642-19648. [DOI] [PubMed] [Google Scholar]
- 6.Claudio, E., K. Brown, S. Park, H. Wang, and U. Siebenlist. 2002. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat. Immunol. 3:958-965. [DOI] [PubMed] [Google Scholar]
- 7.Coope, H. J., P. G. Atkinson, B. Huhse, M. Belich, J. Janzen, M. J. Holman, G. G. Klaus, L. H. Johnston, and S. C. Ley. 2002. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J. 21:5375-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denk, A., M. Goebeler, S. Schmid, I. Berberich, O. Ritz, D. Lindemann, S. Ludwig, and T. Wirth. 2001. Activation of NF-kappa B via the Ikappa B kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. J. Biol. Chem. 276:28451-28458. [DOI] [PubMed] [Google Scholar]
- 9.Ea, C. K., L. Deng, Z. P. Xia, G. Pineda, and Z. J. Chen. 2006. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22:245-257. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-kappaB puzzle. Cell 109(Suppl.):S81-S96. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 12.Han, Z., D. L. Boyle, A. M. Manning, and G. S. Firestein. 1998. AP-1 and NF-kappaB regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity 28:197-208. [DOI] [PubMed] [Google Scholar]
- 13.Harhaj, E. W., and S. C. Sun. 1999. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 274:22911-22914. [DOI] [PubMed] [Google Scholar]
- 14.Hart, L. A., V. L. Krishnan, I. M. Adcock, P. J. Barnes, and K. F. Chung. 1998. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am. J. Respir. Crit. Care Med. 158:1585-1592. [DOI] [PubMed] [Google Scholar]
- 15.Hayden, M. S., and S. Ghosh. 2004. Signaling to NF-kappaB. Genes Dev. 18:2195-2224. [DOI] [PubMed] [Google Scholar]
- 16.Hu, Y., V. Baud, M. Delhase, P. Zhang, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science 284:316-320. [DOI] [PubMed] [Google Scholar]
- 17.Huang, T. T., S. M. Wuerzberger-Davis, Z. H. Wu, and S. Miyamoto. 2003. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell 115:565-576. [DOI] [PubMed] [Google Scholar]
- 18.Iha, H., K. V. Kibler, V. R. Yedavalli, J. M. Peloponese, K. Haller, A. Miyazato, T. Kasai, and K. T. Jeang. 2003. Segregation of NF-kappaB activation through NEMO/IKKgamma by Tax and TNFalpha: implications for stimulus-specific interruption of oncogenic signaling. Oncogene 22:8912-8923. [DOI] [PubMed] [Google Scholar]
- 19.Jimi, E., K. Aoki, H. Saito, F. D'Acquisto, M. J. May, I. Nakamura, T. Sudo, T. Kojima, F. Okamoto, H. Fukushima, K. Okabe, K. Ohya, and S. Ghosh. 2004. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat. Med. 10:617-624. [DOI] [PubMed] [Google Scholar]
- 20.Kovalenko, A., C. Chable-Bessia, G. Cantarella, A. Israel, D. Wallach, and G. Courtois. 2003. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 424:801-805. [DOI] [PubMed] [Google Scholar]
- 21.Kray, A. E., R. S. Carter, K. N. Pennington, R. J. Gomez, L. E. Sanders, J. M. Llanes, W. N. Khan, D. W. Ballard, and B. E. Wadzinski. 2005. Positive regulation of IkappaB kinase signaling by protein serine/threonine phosphatase 2A. J. Biol. Chem. 280:35974-35982. [DOI] [PubMed] [Google Scholar]
- 22.Lajoix, A. D., M. Pugniere, F. Roquet, J. C. Mani, S. Dietz, N. Linck, F. Faurie, G. Ribes, P. Petit, and R. Gross. 2004. Changes in the dimeric state of neuronal nitric oxide synthase affect the kinetics of secretagogue-induced insulin response. Diabetes 53:1467-1474. [DOI] [PubMed] [Google Scholar]
- 23.Li, Q., D. Van Antwerp, F. Mercurio, K. F. Lee, and I. M. Verma. 1999. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science 284:321-325. [DOI] [PubMed] [Google Scholar]
- 24.Li, Z. W., W. Chu, Y. Hu, M. Delhase, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J. Exp. Med. 189:1839-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marienfeld, R., M. J. May, I. Berberich, E. Serfling, S. Ghosh, and M. Neumann. 2003. RelB forms transcriptionally inactive complexes with RelA/p65. J. Biol. Chem. 278:19852-19860. [DOI] [PubMed] [Google Scholar]
- 26.May, M. J., F. D'Acquisto, L. A. Madge, J. Glockner, J. S. Pober, and S. Ghosh. 2000. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science 289:1550-1554. [DOI] [PubMed] [Google Scholar]
- 27.May, M. J., R. B. Marienfeld, and S. Ghosh. 2002. Characterization of the Ikappa B-kinase NEMO binding domain. J. Biol. Chem. 277:45992-46000. [DOI] [PubMed] [Google Scholar]
- 28.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 29.Mordmuller, B., D. Krappmann, M. Esen, E. Wegener, and C. Scheidereit. 2003. Lymphotoxin and lipopolysaccharide induce NF-kappaB-p52 generation by a co-translational mechanism. EMBO Rep. 4:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller, J. R., and U. Siebenlist. 2003. Lymphotoxin beta receptor induces sequential activation of distinct NF-kappa B factors via separate signaling pathways. J. Biol. Chem. 278:12006-12012. [DOI] [PubMed] [Google Scholar]
- 31.Panta, G. R., S. Kaur, L. G. Cavin, M. L. Cortes, F. Mercurio, L. Lothstein, T. W. Sweatman, M. Israel, and M. Arsura. 2004. ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-κB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Mol. Cell. Biol. 24:1823-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poyet, J. L., S. M. Srinivasula, J. H. Lin, T. Fernandes-Alnemri, S. Yamaoka, P. N. Tsichlis, and E. S. Alnemri. 2000. Activation of the Ikappa B kinases by RIP via IKKgamma/NEMO-mediated oligomerization. J. Biol. Chem. 275:37966-37977. [DOI] [PubMed] [Google Scholar]
- 33.Prajapati, S., and R. B. Gaynor. 2002. Regulation of Ikappa B kinase (IKK)gamma/NEMO function by IKKbeta-mediated phosphorylation. J. Biol. Chem. 277:24331-24339. [DOI] [PubMed] [Google Scholar]
- 34.Ramakrishnan, P., W. Wang, and D. Wallach. 2004. Receptor-specific signaling for both the alternative and the canonical NF-kappaB activation pathways by NF-kappaB-inducing kinase. Immunity 21:477-489. [DOI] [PubMed] [Google Scholar]
- 35.Ray, R., G. Chen, C. Vande Velde, J. Cizeau, J. H. Park, J. C. Reed, R. D. Gietz, and A. H. Greenberg. 2000. BNIP3 heterodimerizes with Bcl-2/Bcl-X(L) and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. J. Biol. Chem. 275:1439-1448. [DOI] [PubMed] [Google Scholar]
- 36.Rothwarf, D. M., E. Zandi, G. Natoli, and M. Karin. 1998. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature 395:297-300. [DOI] [PubMed] [Google Scholar]
- 37.Saito, K., T. Kigawa, S. Koshiba, K. Sato, Y. Matsuo, A. Sakamoto, T. Takagi, M. Shirouzu, T. Yabuki, E. Nunokawa, E. Seki, T. Matsuda, M. Aoki, Y. Miyata, N. Hirakawa, M. Inoue, T. Terada, T. Nagase, R. Kikuno, M. Nakayama, O. Ohara, A. Tanaka, and S. Yokoyama. 2004. The CAP-Gly domain of CYLD associates with the proline-rich sequence in NEMO/IKKgamma. Structure 12:1719-1728. [DOI] [PubMed] [Google Scholar]
- 38.Schomer-Miller, B., T. Higashimoto, Y. K. Lee, and E. Zandi. 2006. Regulation of IkappaB kinase (IKK) complex by IKKgamma-dependent phosphorylation of the T-loop and C terminus of IKKbeta. J. Biol. Chem. 281:15268-15276. [DOI] [PubMed] [Google Scholar]
- 39.Senftleben, U., Y. Cao, G. Xiao, F. R. Greten, G. Krahn, G. Bonizzi, Y. Chen, Y. Hu, A. Fong, S. C. Sun, and M. Karin. 2001. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293:1495-1499. [DOI] [PubMed] [Google Scholar]
- 40.Sigala, J. L., V. Bottero, D. B. Young, A. Shevchenko, F. Mercurio, and I. M. Verma. 2004. Activation of transcription factor NF-kappaB requires ELKS, an IkappaB kinase regulatory subunit. Science 304:1963-1967. [DOI] [PubMed] [Google Scholar]
- 41.Suhasini, M., C. D. Reddy, E. P. Reddy, J. A. DiDonato, and R. B. Pilz. 1997. cAMP-induced NF-kappaB (p50/relB) binding to a c-myb intronic enhancer correlates with c-myb up-regulation and inhibition of erythroleukemia cell differentiation. Oncogene 15:1859-1870. [DOI] [PubMed] [Google Scholar]
- 42.Tak, P. P., D. M. Gerlag, K. R. Aupperle, D. A. van de Geest, M. Overbeek, B. L. Bennett, D. L. Boyle, A. M. Manning, and G. S. Firestein. 2001. Inhibitor of nuclear factor kappaB kinase beta is a key regulator of synovial inflammation. Arthritis Rheum. 44:1897-1907. [DOI] [PubMed] [Google Scholar]
- 43.Tarassishin, L., and M. S. Horwitz. 2001. Sites on FIP-3 (NEMO/ IKKgamma) essential for its phosphorylation and NF-kappaB modulating activity. Biochem. Biophys. Res. Commun. 285:555-560. [DOI] [PubMed] [Google Scholar]
- 44.Tegethoff, S., J. Behlke, and C. Scheidereit. 2003. Tetrameric oligomerization of IκB kinase gamma (IKKγ) is obligatory for IKK complex activity and NF-κB activation. Mol. Cell. Biol. 23:2029-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas, R. P., B. J. Farrow, S. Kim, M. J. May, M. R. Hellmich, and B. M. Evers. 2002. Selective targeting of the nuclear factor-kappaB pathway enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated pancreatic cancer cell death. Surgery 132:127-134. [DOI] [PubMed] [Google Scholar]
- 46.Trompouki, E., E. Hatzivassiliou, T. Tsichritzis, H. Farmer, A. Ashworth, and G. Mosialos. 2003. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 424:793-796. [DOI] [PubMed] [Google Scholar]
- 47.Wu, C. J., D. B. Conze, T. Li, S. M. Srinivasula, and J. D. Ashwell. 2006. NEMO is a sensor of Lys 63-linked polyubiquitination and functions in NF-kappaB activation. Nat. Cell Biol. 8:398-406. [DOI] [PubMed] [Google Scholar]
- 48.Wu, Z. H., Y. Shi, R. S. Tibbetts, and S. Miyamoto. 2006. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science 311:1141-1146. [DOI] [PubMed] [Google Scholar]
- 49.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 50.Yang, L., L. Cohn, D. H. Zhang, R. Homer, A. Ray, and P. Ray. 1998. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J. Exp. Med. 188:1739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye, J., X. Xie, L. Tarassishin, and M. S. Horwitz. 2000. Regulation of the NF-kappaB activation pathway by isolated domains of FIP3/IKKgamma, a component of the IkappaB-alpha kinase complex. J. Biol. Chem. 275:9882-9889. [DOI] [PubMed] [Google Scholar]
- 52.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]
- 53.Zhou, H., I. Wertz, K. O'Rourke, M. Ultsch, S. Seshagiri, M. Eby, W. Xiao, and V. M. Dixit. 2003. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature 427:167-171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.