Abstract
Target cell entry of murine leukaemia virus vectors proceeds via primary attachment, independent of the viral envelope protein and subsequent envelope–receptor interaction. Although much attention has been paid to modifying the latter for target cell specificity, the initial binding interaction has been overlooked, despite its opposing involvement both in providing the virus available for receptor binding and in depleting free virus. As a first step towards modifying primary attachment, both to provide specificity and to enhance vector availability, we sought to determine the nature of this interaction. Following an initial screen of GAGs (glycosaminoglycans) for their ability to inhibit virus binding and transduction, we have shown that production of virus from cells in which GAG sulfation is inhibited, or treatment of virus with heparinase III, reduces both particle attachment and infection. Detection in purified virus preparations of a neo-epitope generated by heparinase III confirmed the presence of virus-associated HSPG [HS (heparan sulfate) proteoglycan], acquired from the producer cell. We propose that host-acquired cell-surface HSPG (potentially including syndecan-2) provides a means of virus attachment to target cells that precedes specific receptor interaction and membrane fusion. Inhibition of HS biosynthesis may provide a sufficiently reduced background of primary binding such that novel mechanisms of attachment, ideally with appropriate target cell specificity, can be introduced.
Keywords: glycosaminoglycan, heparan sulfate proteoglycan, murine leukaemia virus, retroviral vector, virus attachment
Abbreviations: CA, capsid protein; C4S, chondroitin 4-sulfate; C6S, chondroitin 6-sulfate; CS, chondroitin sulfate; DMEM, Dulbecco's modified Eagle's medium; DS, dermatan sulfate; FCS, foetal calf serum; GAG, glycosaminoglycan; GPI, glycosylphosphatidylinositol; HS, heparan sulfate; HSPG, HS proteoglycan; MLV, murine leukaemia virus; PI-PLC, phosphatidylinositol-phospholipase C; SU, surface component of viral envelope protein; TBS, Tris-buffered saline
INTRODUCTION
The major limitations to in vivo gene delivery with retroviral vectors, aside from recent safety concerns associated with proviral integration, are efficiency and specificity. Transduction with MLV (murine leukaemia virus) vectors is routinely enhanced in vitro by the inclusion of polycations, such as polybrene, to overcome the electrostatic effects of apposing negatively charged virus and target cell membranes; cationic agents such as liposomes not only enhance virus binding in this way but can modify the efficiency of membrane fusion consequent on virus envelope–receptor interaction. However, such approaches will be of limited value in vivo: ideally, especially for applications requiring systemic administration, the retroviral particles would be endowed with intrinsic means of rapid, specific association with the intended target cells without significant interaction with non-target cells.
Strategies to achieve such specificity have focused on modification of the SU (surface component of the viral envelope protein), by addition of targeting moieties (with or without replacement of the original receptor-binding domain), although most have been hampered by the difficulties of retaining fusogenic capacity in the resulting chimaeric protein [1]. Such studies have laboured under the premise that the crucial interaction for the normal route of infection is that of the virus envelope with its cellular receptor, leading to membrane fusion and release of the viral core to the cytoplasm. However, although MLV infectivity is indeed dependent on a cognate envelope–receptor interaction for entry, viral particles initially adsorb on cells independently of their envelope protein [2]. Since adsorption is equivalent with/without envelope protein [2,3], direct binding to the receptor (Pit-2 in the case of the commonly used amphotropic pseudotype) must be minimal; rather, it is the surface-adsorbed virus that is available for receptor interaction. In vitro, agents such as polybrene enhance transduction by non-specifically increasing the level of receptor-independent virus adsorption and thus the pool available for receptor binding [4]. However, understanding the nature of the initial association in the absence of such agents is crucial to developing novel binding specificities suitable for in vivo application; previous studies, concentrating on modifying envelope protein properties, have failed to address primary attachment as an issue that can negate such efforts at retargeting [5]. In consequence, such envelopes can be considered to coat viruses for which fusion, rather than binding, is conditional upon target recognition, and the intended strategy will only become evident when combined with ablation of envelope/receptor-independent binding. Better still would be replacement of the latter with a novel means of primary attachment specific to the intended target cell.
A second reason for seeking to modify the nature of the initial virus association with cellular membranes is that widespread adsorption of current vectors is a formidable problem for their efficient delivery via systemic administration: rapid depletion of the available circulating virus pool means that much of the injected dose fails to reach the target site. Thus, in addition to providing targeting, controlling the specificity of primary attachment will increase virus circulating times and therefore the accumulation of vector at the target site.
In the present study, we have investigated the role of GAGs (glycosaminoglycans) in virus attachment to the cell surface and subsequent transduction by amphotropic MLV retroviral vectors (MLV-A). We show that sulfated GAG, specifically HS (heparan sulfate) acquired from the producer cells, is a component of the virus particles that is involved in infection of TE671 cells.
EXPERIMENTAL
Cell culture and virus production
Human rhabdomyosarcoma TE671 and producer cells were grown in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) FCS (foetal calf serum). Serum-free culture supernatants containing MLV-A for transducing the β-galactosidase gene were obtained after overnight incubation of TELCeB6/AF producer cells [6] at 37 °C in OptiMEM (Invitrogen, Paisley, U.K.). Supernatants were filtered (pore size 0.45 μm) and frozen at −80 °C before use. MLV-A titre on TE671 cells was 1–3×107 infectious units/ml in the presence of 8 μg/ml polybrene.
Viral transduction assay
TE671 cells were plated at 2.5×104 cells/well in 24-well plates for 18 h before infection by exposure to retrovirus for 40 min at 37 °C. Before use, retrovirus preparations were diluted 1:10 in OptiMEM (to prevent saturation), of which 50 μl in a total volume of 250 μl was used per well. For some experiments, target cells were pre-exposed to soluble GAGs (see below). Limiting the exposure period to 40 min, after which virus was removed and replaced with DMEM/FCS, ensured that the readout was a reflection of the initial kinetics of transduction [7]. At 48 h after viral infection, cells were histochemically stained for β-galactosidase expression by washing with PBS, fixing with 0.5% glutaraldehyde in PBS for 15 min at room temperature (22 °C), washing again and incubating in PBS containing 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% Igepal CA-630, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide and 0.1% X-Gal (5-bromo-4-chloroindol-3-yl β-D-galactopyranoside) for 4 h at 37 °C. The total number of cells and the number of blue cells were counted in multiple microscopic fields of view and all experiments were conducted in triplicate. The degree of transduction was determined as the average number of transduction events per cell (typically 0.05–0.1 for untreated controls), as described previously [7]. The statistical significance of differences between groups was evaluated for 95 and 99% confidence by one-way ANOVA, using pairwise comparison versus untreated control and Dunnett's error protection (Analyse-it Software, Leeds, U.K.).
Virus binding assays
Cells were detached by treatment with versene and resuspended in OptiMEM at 2×106 cells/ml, from which 50 μl aliquots were placed in polypropylene tubes. For some experiments, target cells were pre-exposed to soluble GAGs (see below). Virus (200 μl) was added and the mixture was incubated for 40 min at 37 °C. Controls were cells or virus alone. The cells were washed by the addition of 10 ml of PBS and pelleted by centrifugation at 300 g for 5 min; they were resuspended in 5 ml of PBS, pelleted again, resuspended in 1 ml of PBS and transferred to an Eppendorf tube. The cell pellet was dissolved in 30 μl of SDS/PAGE sample buffer containing 5% 2-mercaptoethanol. All experiments were conducted in triplicate so far; replicates were combined before separating proteins by SDS/PAGE. Detection of MLV CA (capsid protein) was achieved by Western blotting, as described below. Aliquots of the input virus were included as a guide to the efficiency of binding; moreover, the proportionality of CA signal to virus concentration enabled assessment of quantitative differences in virus binding, as reported previously [3]. Quantification of reduced virus binding due to experimental intervention was confirmed by repeat Western blot analysis of the experimental sample alongside 2-fold serial dilutions of the control sample. Additionally, the results reported below were experimentally reproducible.
Treatment of cells with soluble GAGs
All GAGs were purchased from Sigma (Poole, Dorset, U.K.): CS (chondroitin sulfate) A [C4S (chondroitin 4-sulfate)] from bovine trachea (Sigma catalogue number C-9819), CS B [DS (dermatan sulfate)] from porcine intestinal mucosa (C-3788), CS C [C6S (chondroitin 6-sulfate)] from shark cartilage (C-4384), HS from bovine kidney (H-7640), heparin from bovine intestinal mucosa (H-0777), or hyaluronic acid from bovine vitreous humor (H-7630); stock solutions were prepared at 5 mg/ml in PBS. Target cells in a 24-well plate for infection (see above) were incubated for 1 h at 37 °C in 200 μl of OptiMEM containing 50 μg/ml GAG. Virus was then added (50 μl/well, resulting in a final GAG concentration of 40 μg/ml), as described above. In a parallel experiment, the GAG solution was replaced with OptiMEM, after washing the cells, before addition of virus. Suspension cells for virus binding assay were similarly pre-exposed to 200 μg/ml GAG, before addition of virus (200 μl per 50 μl of cell suspension, resulting in a final GAG concentration of 40 μg/ml), as described above.
Inhibition of sulfation
Target cells were incubated at 37 °C for a total of 4 days in DMEM/FCS supplemented with 35 mM sodium chlorate to desulfate proteins and proteoglycans [8]; they were plated at 2.5×104 cells/well in 24-well plates 18 h before addition of virus. Similarly, virus producer cells were incubated in DMEM/FCS containing sodium chlorate for 4 days at 37 °C before harvesting virus for infection of untreated target cells. Sodium chlorate is a potent inhibitor of all cellular sulfation reactions but has no effect on protein synthesis or other post-translational modifications [9]; nevertheless, it was important to control for possible effects on virus production, for which reason Western blot analysis of the viral envelope glycoprotein (SU) and capsid (CA), as described below, was used to monitor virus harvested from treated producer cells.
Digestion of GAGs
TE671 cells (5×105 cells) were detached using versene and washed twice in PBS and 0.1% BSA. To digest HS, cells were resuspended in PBS-ABC (PBS containing 0.5 mM MgCl2 and 0.9 mM CaCl2) and 0.1% BSA and treated with heparinase III (up to 10 units/ml; Sigma H-8891) for 1 h at 37 °C. Cell pellets were washed twice in PBS and then resuspended in 600 μl of OptiMEM before infection with 150 μl retrovirus (1:10 diluted) for 40 min at 37 °C. Cells were then washed and resuspended in 3 ml of DMEM/FCS and 3×1 ml aliquots were plated into 24-well plates. Virus-associated HS was digested with heparinase III (up to 20 units/ml) for 4 h at 37 °C by combining equal volumes of virus in OptiMEM and enzyme in PBS-ABC and 0.1% BSA. Digested virus was subsequently diluted 1:5 (equivalent to 1:10 dilution of the original stock) for use in the transduction assay, or used without dilution for assay of virus binding. Chondroitinase ABC (up to 10 units/ml; Sigma C-3667) was similarly used to digest C4S, C6S, DS and (less efficiently) hyaluronic acid. PI-PLC (phosphatidylinositol-phospholipase C; up to 4 units/ml; Sigma P-8804) was used at 30 °C to digest GPI (glycosylphosphatidylinositol)-anchored proteoglycans. The preparation was then diluted to give a final 1:10 dilution of virus before addition (50 μl) to the cells for infection for 40 min at 37 °C. The cells were histochemically stained for β-galactosidase expression 48 h after viral infection, as described above. It was important to exclude potential protease exposure during GAG digestion, for which reason Western blot analysis of SU and CA, as described below, was used to monitor treated virus: being on the surface of the virus particle (in contrast with CA, protected within the viral core), SU is an indicator of exposure to protease.
Analysis of cell-surface GAG by flow cytometry
Cells were detached using versene, treated to digest specific cell-surface GAGs as indicated, washed and resuspended in PBS. Aliquots (2×105 cells) were pelleted, resuspended in 50 μl of primary antibody and incubated on ice for 30 min. Primary antibodies used were murine monoclonal antibodies 10E4 (Calbiochem, San Diego, CA, U.S.A.) and 3G10 (Seikagaku Corporation; purchased from AMS Biotechnology Europe, Abingdon, Oxfordshire, U.K.) diluted 1:100 for detection of HS, CS-56 (Sigma) diluted 1:100 for detection of CS and CBL471 (Cymbus Bioscience, Southampton, U.K.) diluted 1:20 for detection of human CD55. Following washing, cells were similarly incubated on ice in 50 μl of 1:10 diluted FITC-conjugated goat anti-mouse F(ab′)2 secondary antibody (Dako, Ely, Cambridgeshire, U.K.). Staining was analysed by FACS, using propidium iodide to exclude non-viable cells.
Virus purification and concentration
Harvested virus (40 ml) was purified by low-speed centrifugation in 4×50 ml polypropylene tubes at 2500 g for 16 h at 4 °C. Most of the supernatant was removed and the remaining 500 μl in each tube was combined. OptiMEM (3 ml) was then added to the preparation; this constituted purified virus. For evaluating virus-associated HSPG (HS proteoglycan) by Western blotting, 400 μl of purified virus was treated with heparinase III, pelleted by ultracentrifugation at 30000 rev./min for 1 h at 4 °C using a Beckman SW41 Ti rotor and dissolved in 50 μl of SDS/PAGE sample buffer containing 5% 2-mercaptoethanol.
Preparation of cell lysates and cellular membrane proteins
Cells (2×106) were detached and treated with 10 units/ml heparinase III as above, after which they were pelleted, resuspended in 100 μl of 1% Triton X-100, 10 mM Hepes, 3.5 mM MgCl2 and 1 mM PMSF and incubated on ice for 30 min. Nuclei were pelleted by centrifugation for 15 min in a microfuge and the supernatant (lysate) was combined with an equal volume of 2×SDS/PAGE sample buffer containing 5% 2-mercaptoethanol. To prepare cellular microsomal membranes, 2×108 TE671 cells were detached with versene, washed, resuspended in 2 ml of PBS-ABC, 0.1% BSA with 10 units/ml heparinase III and incubated for 2 h at 37 °C; the cells were subsequently pelleted and lysed in 4 ml of hypo-osmotic buffer (10 mM Tris/HCl, pH 7.4, 2 mM MgCl2 and 1 mM CaCl2) on ice for 30 min. Cellular debris was pelleted and subjected to repeat hypo-osmotic lysis, after which the supernatants from both rounds were combined. Membrane fragments were pelleted by ultracentrifugation at 35000 rev./min for 1 h at 4 °C using a Beckman SW55 Ti rotor, resuspended in 1 ml of 10 mM Tris/HCl (pH 7.4) and combined with an equal volume of 2×SDS/PAGE sample buffer containing 5% 2-mercaptoethanol.
Western blot analysis
Electrophoresis of samples was performed under reducing conditions using SDS/12% polyacrylamide gels before electrophoretic transfer to nitrocellulose (Hybond ECL®; Amersham Biosciences). Membranes were blocked for 16 h in 5% (w/v) non-fat milk powder in TBS (Tris-buffered saline) and then exposed to primary antibody at room temperature for 1 h in TBS, 5% milk and 0.1% Tween 20. MLV CA and SU proteins were detected using goat anti-Rauscher leukaemia virus p30 at 1:2000 dilution and anti-Rauscher leukaemia virus gp70 (glycoprotein 70) at 1:1000 dilution respectively (Quality Biotech, Camden, NJ, U.S.A.). Proteins from which HS had been removed by heparinase III digestion were detected using murine monoclonal antibody 3G10 (Seikagaku Corporation) at 1:1000 dilution. Syndecans were detected using rabbit polyclonal antibodies for human syndecan-2 (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) at 1:200 dilution and for human syndecan-4 (Zymed, San Francisco, CA, U.S.A.) at 1:100 dilution. Membranes were washed and incubated for 1 h at room temperature in the appropriate peroxidase-conjugated rabbit anti-goat, rabbit anti-mouse or swine anti-rabbit secondary antibody (Dako) at 1:2000 dilution, followed by ECL® (enhanced chemiluminescence) detection (Amersham Biosciences) using a Kodak Biomax Light film.
RESULTS
Amphotropic-pseudotyped retroviral vector was obtained from a producer cell line developed for clinical suitability (i.e. production of high-titre, helper-free, serum-resistant recombinant retrovirus) [6] and assayed for the involvement of GAGs in attachment to adherent human TE671 target cells. The primary assay used was of transduction (by expression of β-galactosidase) – for which virus attachment is a prerequisite – owing to the greater ability, compared with direct assay of binding, to quantify the effect of the various treatments; findings were corroborated by assay of virus binding. The initial transduction rate was determined by limiting exposure of target cells to 40 min since the degree of transduction (i.e. the number of transduction events/number of cells exposed) has linear kinetics through this period for a range of virus concentrations and degrees of binding [3,7]. Moreover, transduction rate correlates with binding [3]. Detection of cell-associated viral CA was subsequently used to verify that effects following GAG inhibition/modification were indeed due to altered binding.
Inhibition of transduction and virus binding with soluble GAGs
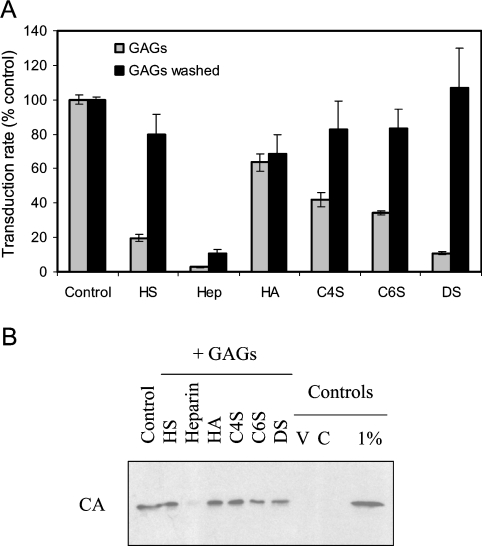
As a starting point to investigate the potential role of GAG chains in mediating virus attachment, target cells were incubated with soluble GAGs before exposure to virus. At 40 μg/ml, heparin was the most potent inhibitor of transduction of TE671 cells by MLV-A (>10-fold), followed by HS and DS (Figure 1A); similar results were obtained when virus was incubated with GAG before adding to cells (results not shown). Inhibition due to HS and DS was reduced at lower concentrations of GAG but heparin remained just as potent at 5 μg/ml (results not shown). Since MLV-A infectivity can be inhibited by anions [10], a potential complication of interpreting such studies is that the soluble GAGs are anionic, although their differential inhibition suggests biochemical specificity. Furthermore, when cells were exposed to GAGs and washed before addition of virus, significant inhibition was seen for heparin only (Figure 1A), indicating a specific, stable cell surface–GAG interaction.
Figure 1. Inhibition of MLV-A attachment by soluble GAGs.
(A) An indirect assay of the effect of GAGs on attachment was provided by determining the effect on the initial transduction rate (by assessing transduction resulting from 40 min exposure to virus). Virus stocks were diluted 1:10 to prevent saturation, resulting in transduction efficiencies of up to 10%. Cells were incubated with GAGs, which were either left in the medium during virus exposure (light grey bars; ‘GAGs’) or washed off the cells before addition of virus (dark grey bars; ‘GAGs washed’). In the former case, all GAGs resulted in significant inhibition (P<0.01, one-way ANOVA compared with control), most markedly for heparin. For washed cells, only prior exposure to heparin resulted in significant inhibition (P<0.01); there was no significant effect due to exposure to other GAGs (P>0.05). Results shown are means±S.E.M. values (n=6). (B) Determination of cell-associated CA by Western blotting was used as a direct assay of the effect of GAG exposure on virus binding. Negative controls for the binding assay were virus alone (V) and cells without virus exposure (C). Levels of virus binding for all GAGs except heparin resulted in a signal comparable with that due to loading 1% of the virus to which the cells were exposed (1% control lane). Hep, heparin; HA, hyaluronic acid.
Virus binding to cells in the presence of soluble GAGs was also directly evaluated. The nature of the assay, based on determining cell-associated viral CA that is integral to the virus particle, necessitated the use of suspension target cells and a greater concentration of virus, under which conditions binding was strongly inhibited by heparin only (Figure 1B).
These results suggest the involvement of a GAG of the heparin/HS structure in mediating virus binding to cells. Heparin and HS have the same basic disaccharide repeat but differ in the degree of sulfation: although HS is less modified overall than heparin, it can contain heparin-like subdomains since the sulfation density is heterogeneous [11]. For this reason, and given the possible differences between human HS and the use of soluble bovine HS in these experiments, it is not possible to distinguish between the involvement of heparin or HS. However, since heparin is the highly sulfated form found in mast cells, it can be concluded that the GAG involved is HS, and that the interacting domain is such that heparin can compete more effectively than bovine kidney HS.
Inhibition of GAG sulfation
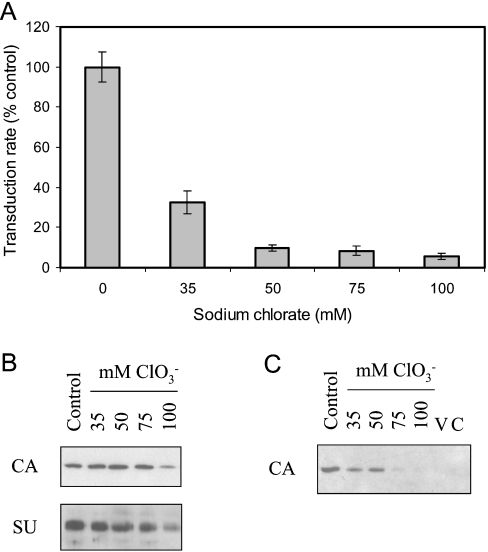
Sodium chlorate (NaClO3) was used to inhibit GAG sulfation, by inhibition of ATP-sulfurylase, an enzyme required for the synthesis of the sulfate donor, 3′-phosphoadenosyl-5′-phosphosulfate [8,9]. Incubation of target cells in 35 mM NaClO3 for 4 days had no effect on transduction. In contrast, addition to producer cells before and throughout virus preparation consistently reduced the initial transduction rate (Figure 2A), without discernible effect on virus particle number (i.e. CA content) or SU envelope protein incorporation (Figure 2B). The addition of NaClO3 to control virus had no direct effect on transduction. Although 35 mM NaClO3 is typical for inhibition of the sulfurylase [8], the effect of using higher concentrations was also evaluated: there was a dose-dependent reduction in transduction rate, without impact on virus production, to 10-fold at 50 mM/75 mM NaClO3 (Figures 2A and 2B).
Figure 2. Inhibition of sulfation during MLV-A production reduces subsequent attachment.
(A) Transduction rate assay of the effect of incubating producer cells in the indicated concentrations of sodium chlorate (NaClO3). All concentrations resulted in significant inhibition (P<0.01, one-way ANOVA compared with control); use of 50 mM NaClO3 was significantly different compared with use of 35 mM NaClO3 (P<0.01), but there was no additional benefit for use of higher concentrations (P>0.05). Results shown are means±S.E.M. values (n=3). (B) Virus preparations during inhibition of sulfation that were used in (A) were assayed by Western blotting for CA to control for the possible effect of NaClO3 on virus particle number, and for SU to control for possible reduction in envelope incorporation (and thus reduction in transduction rate independent of attachment). Virus particle number (and consequently SU content) was reduced only at the highest concentration of NaClO3, itself a reflection of reduced producer cell density at this concentration; thus the maximal reduction in transduction rate seen in (A) resulted from use of NaClO3 concentrations for which there was no reduction in virus production or envelope incorporation. (C) Determination of cell-associated viral CA by Western blotting was used as a direct assay of the effect of NaClO3 treatment of producer cells on binding of the viruses used in (A). Negative controls for the binding assay were virus alone (V) and cells without virus exposure (C).
The effect of treatment on virus binding paralleled that on transduction: viruses prepared from NaClO3-treated producer cells with equivalent particle numbers showed a dose-dependent reduction in binding (Figure 2C). The magnitude of the effect on binding was estimated to be approx. 5-fold by comparison with the signal intensities of dilutions of cell-associated control virus; this is similar to the effect on transduction, given the experimental differences between the assays. These results are consistent with the presence in virus preparations of a producer cell-derived sulfated component that promotes cell attachment and hence transduction. In principle, such a component could be intrinsic to the virus particle or an unassociated factor in the medium conditioned by the producer cells. However, since exogenous sulfated GAGs are inhibitory, the reduced sulfation of such molecules endogenous to the conditioned medium would be unlikely to reduce binding and transduction, suggesting that the necessary component is virus-associated.
Removal of GAG by enzyme treatment
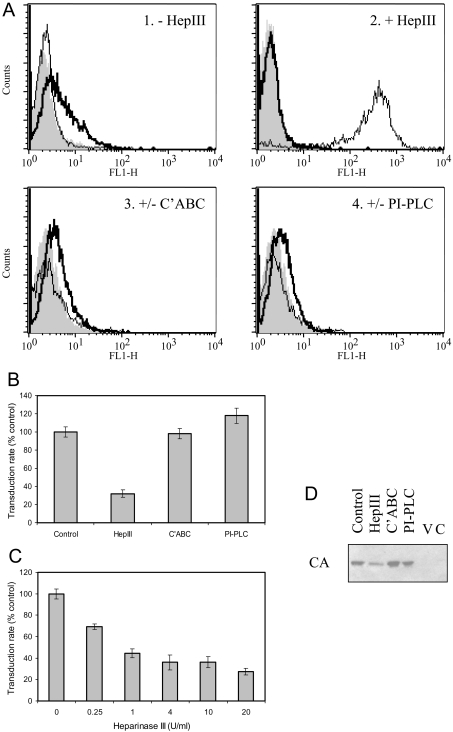
Target cells and virus preparations were independently treated with heparinase III or chondroitinase ABC to remove HS or CS/DS respectively. Because enzyme digestion tended to detach adherent cells, the target cells were used in suspension for this experiment and attached after virus exposure. The efficacy and specificity of cell-surface HS and CS removal were validated by FACS (Figure 3A). HS expression was assessed using two monoclonal antibodies: 10E4 staining was lost upon treatment with heparinase III, while strong 3G10 staining of the whole cell population was apparent only after digestion (Figure 3A). The latter antibody detects a neo-epitope (desaturated uronate) generated by removal of HS with heparinase III, while 10E4 is specific for a particular HS domain that may not be represented in all HSPGs [12].
Figure 3. Effect of enzymatic digestion of GAGs.
(A) TE671 cells were analysed by FACS for cell-surface HS, without/with digestion for 1 h at 37 °C with 10 units/ml heparinase III (panels 1 and 2: ‘−HepIII’ and ‘+HepIII’ respectively), using monoclonal antibodies 10E4 (thick line) or 3G10 (thin line); data are overlaid on those resulting from use of the secondary antibody alone (solid grey profile). 10E4 staining for HS was eradicated by heparinase III (HepIII) digestion, while 3G10 staining for the neo-epitope generated upon digestion was dependent on such treatment. Similarly, detection of CS (panel 3: ‘+/−C'ABC’) in control cells (thick line) was eradicated by treatment with 10 units/ml chondroitinase ABC for 1 h at 37 °C (thin line), and detection of CD55 (panel 4: ‘+/−PI-PLC’) in control cells (thick line) was eradicated by treatment with 4 units/ml PI-PLC for 1 h at 30 °C (thin line). Enzyme treatment was specific for the appropriate target; additionally, 4-fold serial dilutions of the amount of enzyme used indicated that CS removal was equally effective at 0.6 unit/ml C'ABC, whereas residual HS and CD55 were detected following digestion with 2.5 units/ml heparinase III and 1 unit/ml PI-PLC respectively (results not shown). (B) Transduction rate assay of the effect of incubating virus for 4 h at 37 °C without enzyme (control) or with 4 units/ml heparinase III or chondroitinase ABC (C'ABC), or for 4 h at 30 °C with 4 units/ml PI-PLC. Treatment with heparinase III resulted in significant reduction (P<0.01, one-way ANOVA compared with control), whereas C'ABC and PI-PLC were ineffective (P>0.05). Results shown are means±S.E.M. values (n=3). (C) The effect on the transduction rate of treating virus with heparinase III was dose-dependent on enzyme concentration. All concentrations resulted in significant inhibition (P<0.01, one-way ANOVA compared with control), but there was no additional benefit for use of concentrations above 1 unit/ml (P>0.05). Results shown are means±S.E.M. values (n=3). (D) Determination of cell-associated viral CA by Western blotting was used as a direct assay of the effect of enzyme treatment of virus as in (B) on subsequent binding. Negative controls for the binding assay were virus alone (V) and cells without virus exposure (C).
Target cell digestion was ineffective with respect to transduction (results not shown). In contrast, incubation of virus preparations with heparinase III before addition to adherent target cells consistently resulted in reduced transduction (Figure 3B). Although the presence of enzyme at reduced concentration could in principle affect target cell surface GAG during infection, the lack of effect of digesting cells before virus exposure allows the result to be attributed to an effect on the virus. Chondroitinase ABC treatment of virus was ineffective, ruling out the involvement of C4S, C6S or DS (Figure 3B). These results, indicating the involvement of HS in the virus preparation, support the above conclusion that a sulfated component is necessary and are consistent with the potent inhibition by heparin.
To reach this conclusion it was important to exclude potential protease exposure during enzymatic treatment for GAG removal, since this would confound interpretation. Because treatment exposes viral surface proteins but not core proteins, digested virus was examined by Western blot for CA and SU abundance compared with untreated controls; reduced SU abundance, or its degradation, would be indicative of exposure of the viral particle surface to protease. Both CA and SU abundances were invariant upon digestion, with or without prior virus purification (see below), confirming the absence of exposure to protease during treatment for GAG removal.
To distinguish between the potential involvement of HS attached to transmembrane and GPI-tethered proteoglycan, virus was treated with PI-PLC to digest the latter; although the conditions used effectively digested cell-surface CD55 (a GPI-linked protein; Figure 3A), there was no effect on transduction of treating virus (Figure 3B).
The effect of heparinase III digestion of virus was dose-dependent but maximal for ≥1 unit/ml, resulting in 3-fold reduction in transduction (Figure 3C). Impaired transduction was matched by a comparable reduction in virus binding for treatment with heparinase III, but not chondroitinase ABC or PI-PLC (Figure 3D). Consistent with the above, these experiments indicate the involvement of a non-GPI-linked HSPG in mediating MLV-A attachment to TE671 cells.
Virus purification
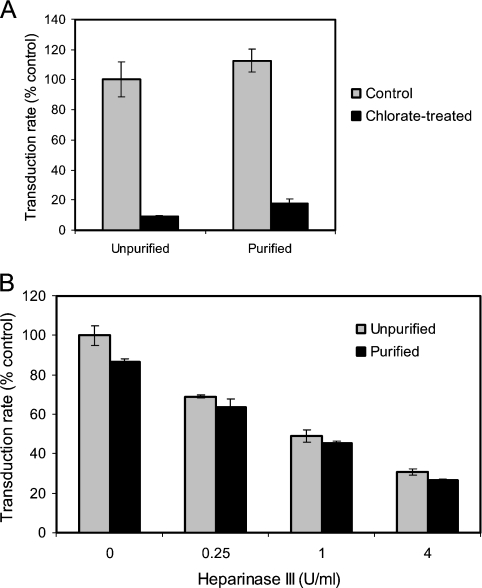
Infectivity using crude preparations of virus, as in the experiments described above, has been reported in some instances to be affected by soluble components, including shed SU and CS proteoglycan [13]. Although TE671-conditioned medium does not have non-specific inhibitory activity and TE671-derived producers are relatively free of soluble SU [14], it was important to establish that the experiments implicating HSPG in attachment were unaffected by soluble components of the producer cell conditioned medium containing the virus. MLV-A was purified by low-speed centrifugation to concentrate viral particles; the method is sufficiently gentle to prevent the particle aggregation and loss of SU associated with ultracentrifugation and avoids concentrating soluble SU or non-viral proteins [15]. This resulted in recovery of 50% of the viral particles, as judged both by infectious titre and relative CA content. For comparison with unpurified preparations, the purified virus was diluted to give an equivalent particle number on this basis.
Virus purified following production from cells treated with 35 mM NaClO3 showed reduced transduction, equivalent to that seen without purification (Figure 4A). Similarly, heparinase III digestion of purified virus reduced transduction to the same degree as virus without purification (Figure 4B). As described for the experiments above in Figures 2 and 3, reduced transduction was paralleled by virus binding (results not shown). These results confirm that the producer-acquired HSPG involved in viral attachment is particle-associated.
Figure 4. Inhibition of sulfation and heparinase III digestion using purified virus preparations.
(A) Transduction rate assay of the effect of incubating producer cells in 35 mM sodium chlorate (NaClO3) with or without subsequent low-speed centrifugation for purification of virus particles from soluble factors in the conditioned medium. Purified virus was first diluted to normalize CA content compared with the unpurified preparation. The initial transduction rate is expressed relative to that of the unpurified control virus. There was no significant difference in the effect of NaClO3 with/without purification (P>0.05, one-way ANOVA pairwise). Results shown are means±S.E.M. values (n=3). (B) Transduction rate assay of the effect of digesting virus, with or without prior purification, for 4 h at 37 °C with the indicated concentrations of heparinase III. Purified virus was first diluted to normalize CA content compared with the unpurified preparation. The initial transduction rate is expressed relative to that of the unpurified control virus. There was no significant difference in the effect of digestion at any concentration (P>0.05, one-way ANOVA pairwise). Results shown are means±S.E.M. values (n=3).
Detection of virus-associated and cell-surface HSPG
As shown above, HS detection on TE671 cells using antibody 10E4 was considerably weaker than that using 3G10 after heparinase III digestion (Figure 3A); identical results were obtained for the TE671-derived virus producer cells. In contrast, but consistent with the variable presence of the 10E4 epitope, the cell line MDA-MB157 showed strong staining with 10E4 in addition to strong post-digestion staining with 3G10 (results not shown), from which it can be concluded that TE671 cells have abundant surface HS that contains relatively little of the 10E4 epitope. Since creation of the uronate neo-epitope is the more definitive indication of the presence of HSPGs, heparinase III digestion and Western blot analysis using antibody 3G10 was used to assess virus-associated HSPG.
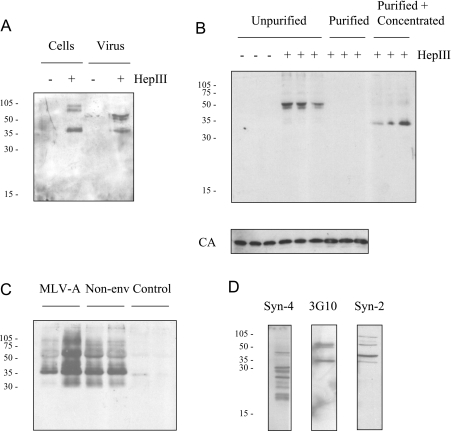
Western blot analysis of producer cell lysates with 3G10 stained a band of 35 kDa and a doublet of higher molecular mass, dependent on heparinase III digestion; virus (i.e. cell supernatants) contained heparinase III-dependent 3G10-positive 35 kDa and doublet 50–60 kDa bands, the first of which was equivalent to the species in the cell lysate (Figure 5A). Additional digestion with chondroitinase ABC had no effect (results not shown). The same bands were obtained respectively for lysates of treated TE671 cells and their digested conditioned medium, the latter indicating an abundance of soluble HSPGs in the supernatant. Consistent with this, most of the 3G10-reactive material was retained in the centrifugation supernatant of the virus preparations purified for the experiment described above (Figure 5B). Because the sensitivity to digestion of these purified viruses suggested that some HSPG must have co-purified, heparinase III-digested virus was further concentrated by ultracentrifugation before Western blotting; predominantly, the 35 kDa band was detected (Figure 5B). In subsequent experiments the higher-molecular-mass material was variably present, in addition to the greatly enriched 35 kDa species (Figure 5C); identical treatment of TE671 conditioned medium did not detect this band, which was, however, present for preparations of virus lacking the viral envelope protein.
Figure 5. Western blot analysis of virus-associated and cell-surface HSPGs.
(A) Total cell lysates of producer cells and virus, each with or without heparinase III (HepIII)-digestion, were analysed by Western blotting with antibody 3G10. Numbers to the left indicate the positions of molecular mass markers (kDa). (B) Three independent virus preparations with (‘Purified’) or without (‘Unpurified’) prior purification by low-speed centrifugation were digested as indicated and analysed by Western blotting using 3G10. Purified virus was first diluted to normalize CA content compared with the unpurified preparation (lower panel). Digested, purified virus was also further concentrated by ultracentrifugation (‘Purified+Concentrated’). (C) Virus in duplicate preparations from MLV-A producer cells and those lacking the envelope protein (‘Non-env’) was purified, digested with heparinase III and further concentrated before Western blot analysis with 3G10. Medium conditioned by TE671 cells (‘Control’) was similarly processed. (D) Cellular membrane proteins from heparinase III-treated TE671 cells were analysed by Western blotting for syndecan-2 (Syn-2), syndecan-4 (Syn-4) and 3G10.
Reasoning that syndecan-2 and syndecan-4 [16,17] were likely candidates for host-acquired HSPG mediating virus attachment consistent with the foregoing data, microsomal (predominantly plasma) membrane preparations from heparinase III-digested TE671 cells were analysed by Western blotting with antibodies for the core protein of each of these in parallel with 3G10; moreover, blots used with the syndecan antibodies were stripped and re-used with 3G10. The syndecan antibodies each detected several bands, the smallest of which corresponded to the core protein size; multiple higher-molecular-mass species are likely to be due to heterogeneity of GAG modification and heparinase III susceptibility, and to SDS-resistant dimerization. There was no congruence between the bands detected by the syndecan-4 antibody and those detected by 3G10; in contrast, there was overlap of the bands detected with the 3G10 and syndecan-2 antibodies, although the latter detected additional unique bands (Figure 5D).
DISCUSSION
Recombinant MLV initially binds to target cells independently of its specific envelope protein [2,3]. Our experiments indicate that the major mechanism of such primary attachment involves particle-associated HS and that proteoglycans containing such GAG chains are detectable in highly purified virus preparations. This conclusion is supported by a variety of experiments that are consistent in the effects of GAG manipulation in both direct virus binding and transduction assays. Notably, inhibition of GAG sulfation and heparinase III digestion were as effective on purified virus as they were on crude producer cell virus harvests. An important aspect of these experiments is that virus exposure of the target cells was sufficiently short such that the read-out provided a reflection of the initial kinetics of the assay; this is of greater relevance to in vivo application than is the consequence of the extended exposure that is often employed in vitro.
Inhibition of sulfation resulted in up to 10-fold reduction of binding/transduction without effect on virus particle number or envelope incorporation, whereas maximal heparinase III digestion was somewhat less effective. The likely explanation for this difference is that, whereas sulfation of the whole GAG chain will be inhibited, digestion removes only a portion of each chain. HS consists of alternating hexosamine and uronic acid monosaccharide units but there is considerable heterogeneity, including the nature of each unit (D-glucosamine or N-acetyl-D-glucosamine; D-glucuronic acid or D-iduronic acid) and their modifications (notably sulfation at several potential positions) [11]. Heparinase III is specific for hexosamine–glucuronic acid linkages, so digestion may leave significant (although shorter) HS chains intact. Importantly, sulfation is critical for molecular interaction with HS [11,12,18].
HS heterogeneity also underlies the antibody data obtained by FACS and Western blotting: while all 10E4 epitopes are heparinase III-sensitive, not all generate 3G10 epitopes upon digestion of the hexosamine–glucuronic acid linkage, depending on the nature of the adjacent hexosamine; similarly, not all heparinase III-sensitive linkages that do generate 3G10 epitopes originate from 10E4-reactive sites (additionally dependent on the sulfation status). Thus 10E4 and 3G10 stainings are not superimposable, although there is some overlap [12]. An important implication is that 3G10 does not detect all digested HSPGs, dependent on the exact nature of the non-reducing end (i.e. the heparinase III substrate site closest to the protein core). This was evident from the lack of reactivity in our experiments of 3G10 with multiple species detected by antibodies to the syndecan-2/4 core proteins. The presence of the 3G10-positive 35 kDa band in the purified, heparinase III-treated virus preparations can thus be taken as indicative of the virus containing HSPG, but this species is not necessarily responsible for virus attachment. Virus incorporation of HSPGs was equivalent in the absence of the envelope protein, indicating that acquisition is passive at the time of budding from the producer cell plasma membrane. Despite there being a relative abundance of soluble HSPGs detected in virus preparations compared with those incorporated into the virus particles, the equivalence of virus behaviour following purification indicates that the soluble material does not affect infectivity; this suggests that the HS motif that mediates virus attachment is specifically present in the acquired HSPG.
Virus attachment by means of low-affinity capture leading to high-affinity interaction and entry has many precedents [19]. Cell-surface HS has been implicated in many cases as assisting primary attachment, for viruses from diverse taxonomic groups; for example, specific HS monosaccharide sequences are important mediators of herpesvirus binding by viral glycoproteins B and C or HIV binding by the envelope glycoprotein gp120, in each case preceding interaction with protein entry receptors [20]. HS involvement as the virus-associated component of the interaction, as the experiments reported herein indicate to be the case for MLV, is a novel finding. Previously, however, Lei et al. [21] have reported that HS associated with MLV mediates binding to fibronectin; fragments of fibronectin containing both the HS-binding domain and one (or both) integrin-binding domain(s) have been used as molecular bridges to enhance transduction of non-adherent cells [22], particularly for ex vivo gene delivery to bone marrow stem cells [23,24] but additionally for epidermal stem cells [25]. Lei et al. [21] showed that HS was not important as a target cell component for fibronectin-assisted transduction and that immobilization of MLV from both murine and human packaging cells, regardless of envelope protein expression, was sensitive to treatment of virus with heparinase III but not chondroitinase ABC; in the absence of fibronectin, transduction of NIH 3T3 target cells was dependent on inclusion of polybrene and so the potential involvement of viral HS interaction with target cells was not explored [21]. Our data regarding a direct interaction of viral HS with TE671 cells are consistent with this report, indicating parallels between HSPG-mediated fibronectin immobilization and cellular capture of MLV.
In contrast with the above, a report by Walker et al. [26] described MLV binding to heparin and suggested this as the mechanism of primary attachment to cells. However, their use of extended (72 h) exposure of cells to virus complicates interpretation of their transduction experiments and the suggestion is contradicted by their own data that showed equivalent binding (independent of envelope) to proteoglycan-deficient and wild-type cells, both of which were inhibitable by heparin; in contrast, these data are consistent with the conclusion of our studies that virus-associated HS mediates binding to cells. Importantly, our data derive from a short (40 min) exposure of target cells, during which the transduction kinetics are linear, enabling conclusions to be drawn concerning the major means of primary attachment of relevance to practical application of MLV vectors, without complication by slow mechanisms that are of no such significance. Another important feature is that our experiments avoided the use of agents such as polybrene, inclusion of which complicates the conclusion of some studies [27] given that polybrene promotes a dominant electrostatic interaction [4,21] that is itself dependent on cell-surface GAG [27] and that polybrene/GAG/MLV complexation enhances transfer of virus to the cell surface [28] (N. Kureishy and C. D. Porter, unpublished work); again, such agents are not relevant to in vivo application of MLV vectors, despite their utility in vitro.
Many viruses acquire host proteins within or on the virion surface [29]; HIV has been widely studied in this regard [30], and it has been found that incorporation of biologically active HLA-DR (human leucocyte antigen DR) [31] and ICAM-1 (intercellular adhesion molecule 1) [32] into the HIV envelope can assist infectivity. MLV has been shown to acquire specific heterologous proteins upon budding from the host cell plasma membrane [33]; in an experimental system, incorporation of such proteins was less efficient than that of the homologous envelope protein and reflected surface abundance [34]. There are therefore precedents for proposing viral incorporation of host proteins, in this case HSPG(s), that are responsible for cellular attachment. Given that binding is independent of envelope protein expression, there can be no envelope dependence for incorporation of such factors. The HSPGs remain unidentified, although our data exclude GPI-linked glypicans; on the basis of some congruence with 3G10 staining, syndecan-2 is a candidate [16]. The cellular ‘receptors’ similarly remain unknown.
It is probably simplistic to imagine that primary attachment of MLV involves a single molecular interaction, but that described above appears to be the major one. Consequently, MLV-A entry, dependent on the SU–Pit-2 interaction for membrane fusion, can be understood to proceed via a series of steps involving (i) cell-free motion of virus particles, leading to (ii) primary attachment via associated HSPG to HS-binding cell-surface components as a means of virus concentration on the two-dimensional cell membrane to enhance (iii) cognate receptor interaction. The first step represents a significant limitation in vitro/ex vivo, for which Brownian particle motion in the medium provides an adequate model [35,36]; it has been addressed in many ways, e.g. the use of cations [4,7], centrifugation during virus exposure [37], CaPO4 co-precipitation [38], flow-through transduction [39] and the use of fibronectin fragments (see above) – all of which seek to promote juxtaposition of the virus and target cell. The opposite problem pertains for in vivo application requiring systemic administration, for which rapid depletion due to virus proximity to, and adsorption on, non-target cell surfaces (mostly unproductive for infection) limits availability and therefore efficient delivery to distant sites.
The identification of virally associated HS as a natural mediator of primary attachment is important for controlling this key step in the infectious process. Since there may be multiple HSPGs contributing the HS responsible for virus attachment, their identification and eventual elimination represent a difficult challenge and inhibition might best be approached by blocking HS biosynthesis during virus production, either by inhibition of sulfation (as in the experiments above) or mutation/knock-down of critical synthetic enzymes, such as EXT1 (exostosin 1) [40]. Controlling primary attachment is key both to maintaining virus availability and to efficient capture once the target site is reached: replacement of the promiscuous mechanism with one specific for the target cell will be of greater importance for achieving specific delivery than will modification of the final step in the infectious process. Indeed, introduction of sufficient specificity at the level of primary attachment will obviate complex envelope engineering, and the natural receptor interaction can be retained for efficient fusion. In conclusion, we have shown that MLV-associated HS mediates cellular capture and propose that modification of this interaction will be essential for practical application by maintaining vector availability and promoting specific attachment.
Acknowledgments
This work was funded by the Medical Research Council.
References
- 1.Lavillette D., Russell S. J., Cosset F. L. Retargeting gene delivery using surface-engineered retroviral vector particles. Curr. Opin. Biotechnol. 2001;12:461–466. doi: 10.1016/s0958-1669(00)00246-9. [DOI] [PubMed] [Google Scholar]
- 2.Pizzato M., Marlow S. A., Blair E. D., Takeuchi Y. Initial binding of murine leukemia virus particles to cells does not require specific Env–receptor interaction. J. Virol. 1999;73:8599–8611. doi: 10.1128/jvi.73.10.8599-8611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter C. D. Rescue of retroviral envelope fusion deficiencies by cationic liposomes. J. Gene Med. 2002;4:622–633. doi: 10.1002/jgm.310. [DOI] [PubMed] [Google Scholar]
- 4.Davis H. E., Morgan J. R., Yarmush M. L. Polybrene increases retrovirus gene transfer efficiency by enhancing receptor-independent virus adsorption on target cell membranes. Biophys. Chem. 2002;97:159–172. doi: 10.1016/s0301-4622(02)00057-1. [DOI] [PubMed] [Google Scholar]
- 5.Pizzato M., Blair E. D., Fling M., Kopf J., Tomassetti A., Weiss R. A., Takeuchi Y. Evidence for nonspecific adsorption of targeted retrovirus vector particles to cells. Gene Ther. 2001;8:1088–1096. doi: 10.1038/sj.gt.3301494. [DOI] [PubMed] [Google Scholar]
- 6.Cosset F. L., Takeuchi Y., Battini J. L., Weiss R. A., Collins M. K. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter C. D., Lukacs K. V., Box G., Takeuchi Y., Collins M. K. Cationic liposomes enhance the rate of transduction by a recombinant retroviral vector in vitro and in vivo. J. Virol. 1998;72:4832–4840. doi: 10.1128/jvi.72.6.4832-4840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mislick K. A., Baldeschwieler J. D. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12349–12354. doi: 10.1073/pnas.93.22.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeuerle P. A., Huttner W. B. Chlorate: a potent inhibitor of protein sulfation in intact cells. Biochem. Biophys. Res. Commun. 1986;141:870–877. doi: 10.1016/s0006-291x(86)80253-4. [DOI] [PubMed] [Google Scholar]
- 10.Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969;38:414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- 11.Rabenstein D. L. Heparin and heparan sulfate: structure and function. Nat. Prod. Rep. 2002;19:312–331. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- 12.David G., Bai X. M., Van der Schueren B., Cassiman J. J., Van den Berghe H. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J. Cell Biol. 1992;119:961–975. doi: 10.1083/jcb.119.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Doux J. M., Morgan J. R., Snow R. G., Yarmush M. L. Proteoglycans secreted by packaging cell lines inhibit retrovirus infection. J. Virol. 1996;70:6468–6473. doi: 10.1128/jvi.70.9.6468-6473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slingsby J. H., Baban D., Sutton J., Esapa M., Price T., Kingsman S. M., Kingsman A. J., Slade A. Analysis of 4070A envelope levels in retroviral preparations and effect on target cell transduction efficiency. Hum. Gene Ther. 2000;11:1439–1451. doi: 10.1089/10430340050057512. [DOI] [PubMed] [Google Scholar]
- 15.Bowles N. E., Eisensmith R. C., Mohuiddin R., Pyron M., Woo S. L. A simple and efficient method for the concentration and purification of recombinant retrovirus for increased hepatocyte transduction in vivo. Hum. Gene Ther. 1996;7:1735–1742. doi: 10.1089/hum.1996.7.14-1735. [DOI] [PubMed] [Google Scholar]
- 16.Couchman J. R. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat. Rev. Mol. Cell Biol. 2003;4:926–937. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- 17.Oh E. S., Couchman J. R. Syndecans-2 and -4: close cousins, but not identical twins. Mol. Cell. 2004;17:181–187. [PubMed] [Google Scholar]
- 18.Esko J. D., Selleck S. B. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 19.Haywood A. M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J. Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J., Thorp S. C. Cell surface heparan sulfate and its roles in assisting viral infections. Med. Res. Rev. 2002;22:1–25. doi: 10.1002/med.1026. [DOI] [PubMed] [Google Scholar]
- 21.Lei P., Bajaj B., Andreadis S. T. Retrovirus-associated heparan sulfate mediates immobilization and gene transfer on recombinant fibronectin. J. Virol. 2002;76:8722–8728. doi: 10.1128/JVI.76.17.8722-8728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajaj B., Lei P., Andreadis S. T. High efficiencies of gene transfer with immobilized recombinant retrovirus: kinetics and optimization. Biotechnol. Prog. 2001;17:587–596. doi: 10.1021/bp010039n. [DOI] [PubMed] [Google Scholar]
- 23.Moritz T., Patel V. P., Williams D. A. Bone marrow extracellular matrix molecules improve gene transfer into human hematopoietic cells via retroviral vectors. J. Clin. Invest. 1994;93:1451–1457. doi: 10.1172/JCI117122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanenberg H., Xiao X. L., Dilloo D., Hashino K., Kato I., Williams D. A. Co-localization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat. Med. 1996;2:876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 25.Bajaj B. G., Lei P., Andreadis S. T. Efficient gene transfer to human epidermal keratinocytes on fibronectin: in vitro evidence for transduction of epidermal stem cells. Mol. Ther. 2005;11:969–979. doi: 10.1016/j.ymthe.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Walker S. J., Pizzato M., Takeuchi Y., Devereux S. Heparin binds to murine leukemia virus and inhibits Env-independent attachment and infection. J. Virol. 2002;76:6909–6918. doi: 10.1128/JVI.76.14.6909-6918.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guibinga G. H., Miyanohara A., Esko J. D., Friedmann T. Cell surface heparan sulfate is a receptor for attachment of envelope protein-free retrovirus-like particles and VSV-G pseudotyped MLV-derived retrovirus vectors to target cells. Mol. Ther. 2002;5:538–546. doi: 10.1006/mthe.2002.0578. [DOI] [PubMed] [Google Scholar]
- 28.Landazuri N., Le Doux J. M. Complexation of retroviruses with charged polymers enhances gene transfer by increasing the rate that viruses are delivered to cells. J. Gene Med. 2004;6:1304–1319. doi: 10.1002/jgm.618. [DOI] [PubMed] [Google Scholar]
- 29.Cantin R., Methot S., Tremblay M. J. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J. Virol. 2005;79:6577–6587. doi: 10.1128/JVI.79.11.6577-6587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tremblay M. J., Fortin J. F., Cantin R. The acquisition of host-encoded proteins by nascent HIV-1. Immunol. Today. 1998;19:346–351. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 31.Cantin R., Fortin J. F., Lamontagne G., Tremblay M. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J. Virol. 1997;71:1922–1930. doi: 10.1128/jvi.71.3.1922-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fortin J. F., Cantin R., Lamontagne G., Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calafat J., Janssen H., Demant P., Hilgers J., Zavada J. Specific selection of host cell glycoproteins during assembly of murine leukaemia virus and vesicular stomatitis virus: presence of Thy-1 glycoprotein and absence of H-2, Pgp-1 and T-200 glycoproteins on the envelopes of these virus particles. J. Gen. Virol. 1983;64:1241–1253. doi: 10.1099/0022-1317-64-6-1241. [DOI] [PubMed] [Google Scholar]
- 34.Suomalainen M., Garoff H. Incorporation of homologous and heterologous proteins into the envelope of Moloney murine leukemia virus. J. Virol. 1994;68:4879–4889. doi: 10.1128/jvi.68.8.4879-4889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chuck A. S., Clarke M. F., Palsson B. O. Retroviral infection is limited by Brownian motion. Hum. Gene Ther. 1996;7:1527–1534. doi: 10.1089/hum.1996.7.13-1527. [DOI] [PubMed] [Google Scholar]
- 36.Palsson B., Andreadis S. The physico-chemical factors that govern retrovirus-mediated gene transfer. Exp. Hematol. 1997;25:94–102. [PubMed] [Google Scholar]
- 37.Swaney W. P., Sorgi F. L., Bahnson A. B., Barranger J. A. The effect of cationic liposome pretreatment and centrifugation on retrovirus-mediated gene transfer. Gene Ther. 1997;4:1379–1386. doi: 10.1038/sj.gt.3300529. [DOI] [PubMed] [Google Scholar]
- 38.Morling F. J., Russell S. J. Enhanced transduction efficiency of retroviral vectors coprecipitated with calcium phosphate. Gene Ther. 1995;2:504–508. [PubMed] [Google Scholar]
- 39.Chuck A. S., Palsson B. O. Consistent and high rates of gene transfer can be obtained using flow-through transduction over a wide range of retroviral titers. Hum. Gene Ther. 1996;7:743–750. doi: 10.1089/hum.1996.7.6-743. [DOI] [PubMed] [Google Scholar]
- 40.McCormick C., Duncan G., Goutsos K. T., Tufaro F. The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc. Natl. Acad. Sci. U.S.A. 2000;97:668–673. doi: 10.1073/pnas.97.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]