Abstract
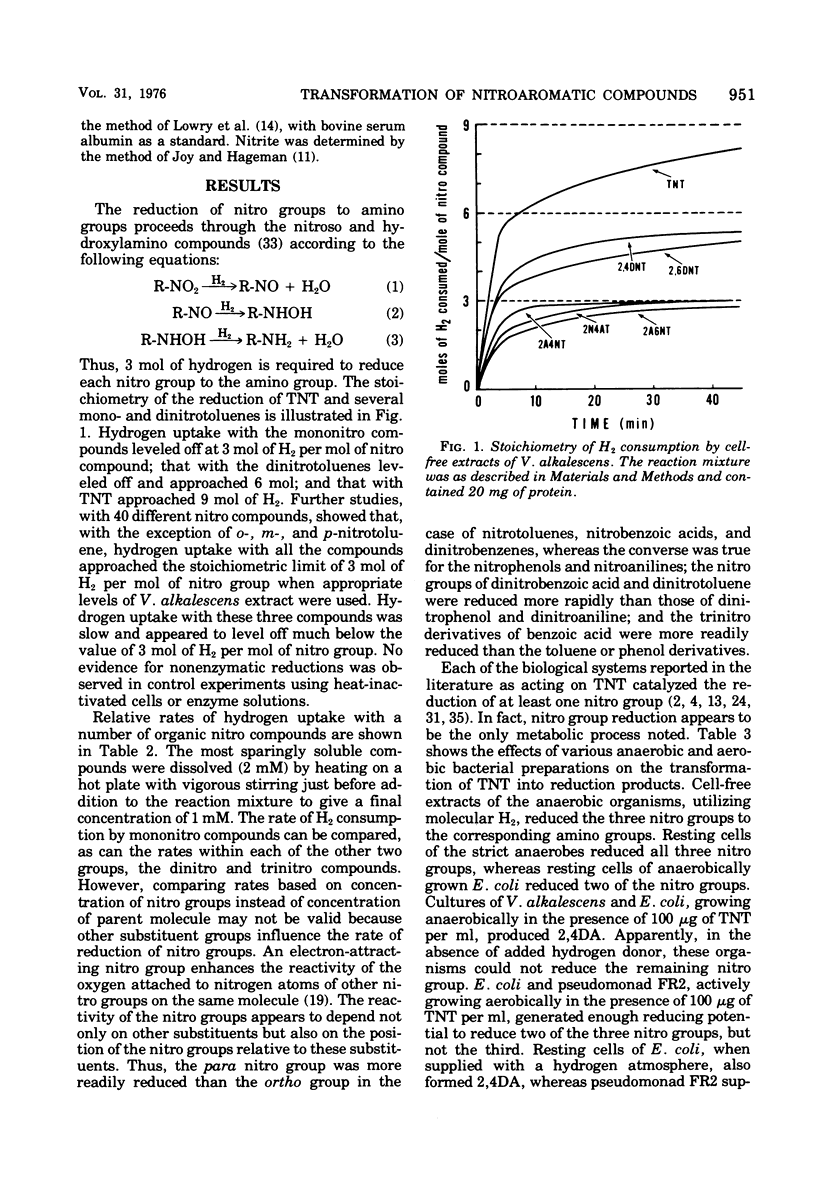
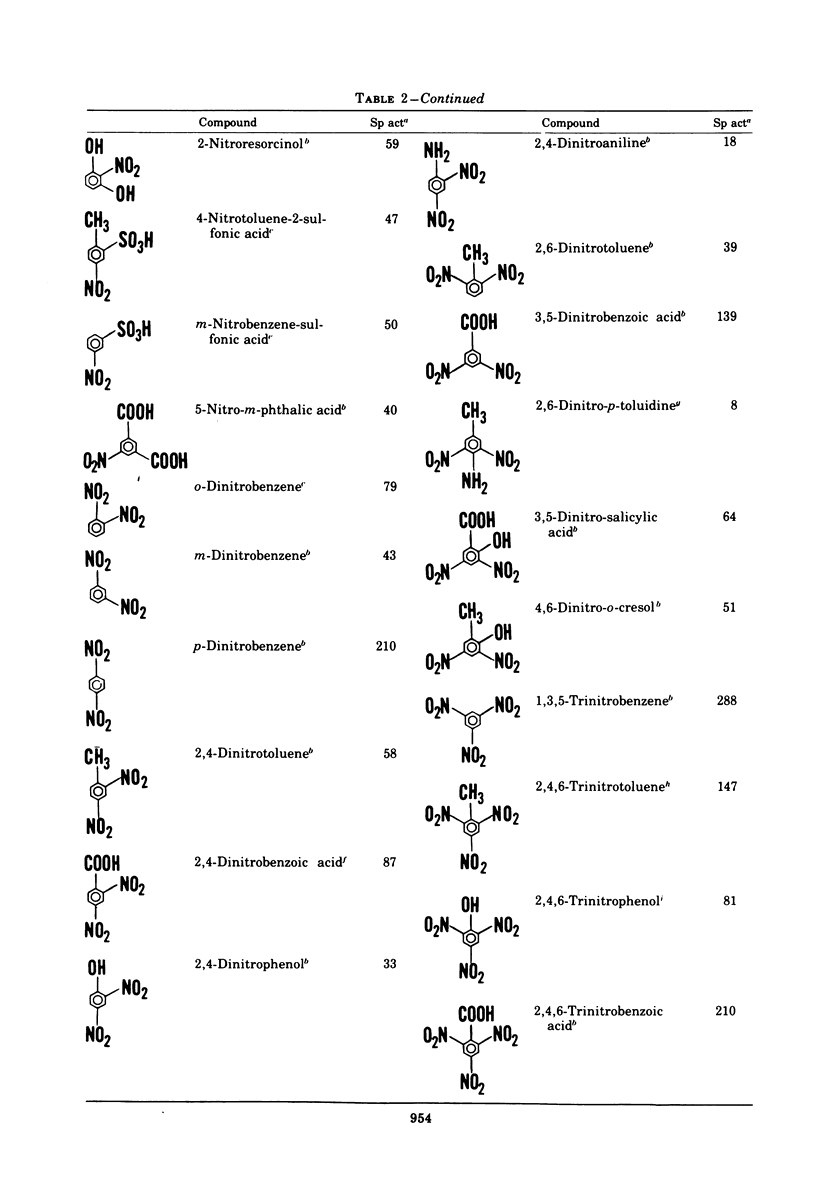
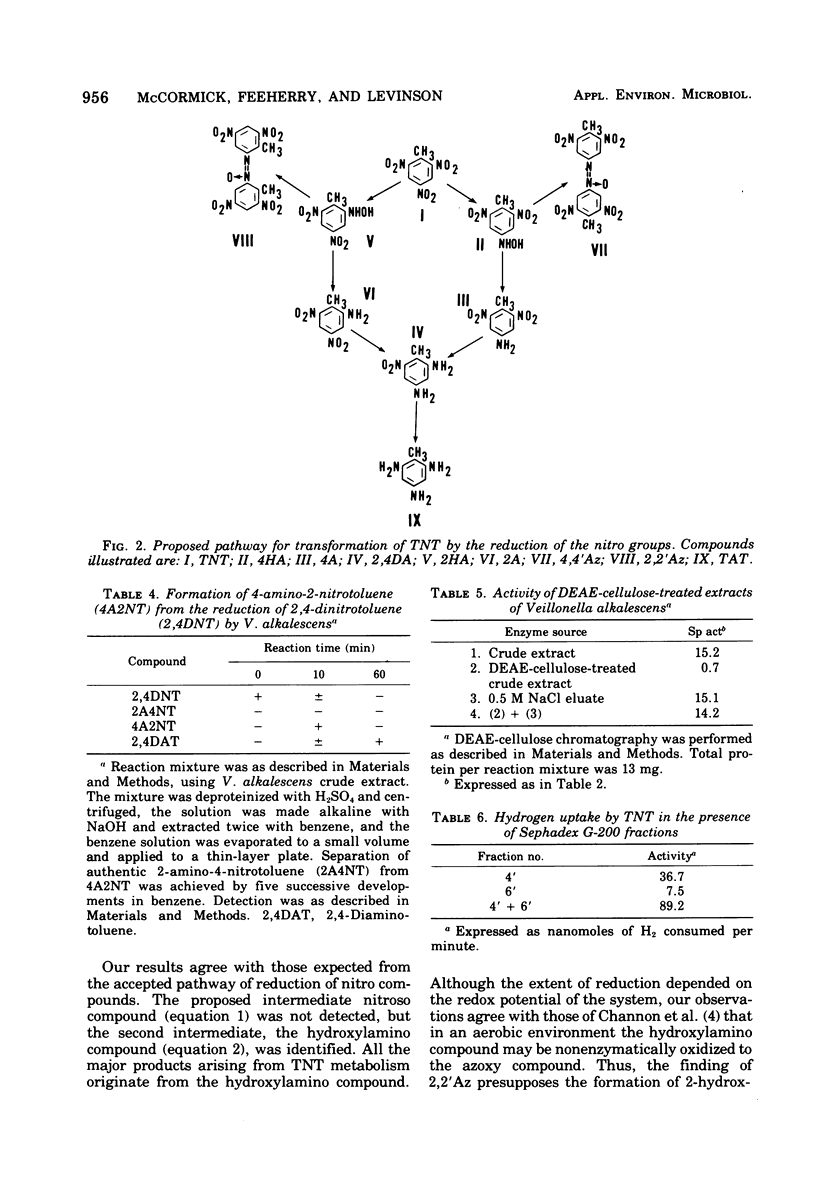
A variety of nitroaromatic compounds, including 2,4,6-trinitrotoluene (TNT), were reduced by hydrogen in the presence of enzyme preparations from Veillonella alkalescens. Consistent with the proposed reduction pathway, R-NO2 H2 leads to R-NO H2 leads to R-NHOH H2 leads to R-NH2, 3 mol of H2 was utilized per mol of nitro group. The rates of reduction of 40 mono-, di-, and trinitroaromatic compounds by V. alkalescens extract were determined. The reactivity of the nitro groups depended on other substituents and on the position of the nitro groups relative to these substituents. In the case of the nitrotoluenes, the para-nitro group was the most readily reduced, the 4-nitro position of 2,4-dinitrotulene being reduced first. The pattern of reduction of TNT (disappearance of TNT and reduction products formed) depended on the type of preparation (cell-free extract, resting cells, or growing culture), on the species, and on the atmosphere (air or H2). The "nitro-reductase" activity of V. alkalescens extracts was associated with protein fractions, one having some ferredoxin-like properties and the other possessing hydrogenase activity. Efforts to eliminate hydrogenase from the reaction have thus far been unsuccessful. The question of whether ferredoxin acts as a nonspecific reductase for nitroaromatic compounds remains unresolved.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARTWRIGHT N. J., CAIN R. B. Bacterial degradation of the nitrobenzoic acids. Biochem J. 1959 Feb;71(2):248–261. doi: 10.1042/bj0710248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon H. J., Mills G. T., Williams R. T. The metabolism of 2:4:6-trinitrotoluene (alpha-T.N.T.). Biochem J. 1944;38(1):70–85. doi: 10.1042/bj0380070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dagley S. Microbial degradation of organic compounds in the biosphere. Am Sci. 1975 Nov-Dec;63(6):681–689. [PubMed] [Google Scholar]
- Joy K. W., Hageman R. H. The purification and properties of nitrite reductase from higher plants, and its dependence on ferredoxin. Biochem J. 1966 Jul;100(1):263–273. doi: 10.1042/bj1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCormick N. G., Ordal E. J., Whiteley H. R. DEGRADATION OF PYRUVATE BY MICROCOCCUS LACTILYTICUS I. : General Properties of the Formate-Exchange Reaction. J Bacteriol. 1962 Apr;83(4):887–898. doi: 10.1128/jb.83.4.887-898.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick N. G., Ordal E. J., Whiteley H. R. DEGRADATION OF PYRUVATE BY MICROCOCCUS LACTILYTICUS II. : Studies of Cofactors in the Formate-Exchange Reaction. J Bacteriol. 1962 Apr;83(4):899–906. doi: 10.1128/jb.83.4.899-906.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nay M. W., Jr, Randall C. W., King P. H. Biological treatability of trinitrotoluene manufacturing wastewater. J Water Pollut Control Fed. 1974 Mar;46(3):485–497. [PubMed] [Google Scholar]
- PECK H. D., Jr Evidence for oxidative phosphorylation during the reduction of sulfate with hydrogen by Desulfovibrio desulfuricans. J Biol Chem. 1960 Sep;235:2734–2738. [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. A new procedure for assay of bacterial hydrogenases. J Bacteriol. 1956 Jan;71(1):70–80. doi: 10.1128/jb.71.1.70-80.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz J. Preparation and properties of clostridial ferredoxins. Methods Enzymol. 1972;24:431–446. doi: 10.1016/0076-6879(72)24089-7. [DOI] [PubMed] [Google Scholar]
- SAZ A. K., SLIE R. B. The inhibition of organiz nitro reductase by aureomycin in cell-free extracts. II. Cofactor requirements for the nitro reductase enzyme complex. Arch Biochem Biophys. 1954 Jul;51(1):5–16. doi: 10.1016/0003-9861(54)90447-6. [DOI] [PubMed] [Google Scholar]
- SIMPSON J. R., EVANS W. C. The metabolism of nitrophenols by certain bacteria. Biochem J. 1953 Jul 17;55(320TH):xxiv–xxiv. [PubMed] [Google Scholar]
- VILLANUEVA J. R. THE PURIFICATION OF A NITRO-REDUCTASE OF NOCARDIA V. J Biol Chem. 1964 Mar;239:773–776. [PubMed] [Google Scholar]
- Won W. D., Heckly R. J., Glover D. J., Hoffsommer J. C. Metabolic disposition of 2,4,6-trinitrotoluene. Appl Microbiol. 1974 Mar;27(3):513–516. doi: 10.1128/am.27.3.513-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Valentine R. C. Ferredoxins and flavodoxins of bacteria. Annu Rev Microbiol. 1972;26:139–162. doi: 10.1146/annurev.mi.26.100172.001035. [DOI] [PubMed] [Google Scholar]


