Abstract
Genetic studies in budding yeast have provided many fundamental insights into the specialized cell division of meiosis, including the identification of evolutionarily conserved meiosis-specific genes and an understanding of the molecular basis for recombination. Biochemical studies have lagged behind, however, due to the difficulty in obtaining highly synchronized populations of yeast cells. A chemical genetic approach was used to create a novel conditional allele of the highly conserved protein kinase Cdc7 (cdc7-as3) that enables cells to be synchronized immediately prior to recombination. When Cdc7-as3 is inactivated by addition of inhibitor to sporulation medium, cells undergo a delayed premeiotic S phase, then arrest in prophase before double-strand break (DSB) formation. The arrest is easily reversed by removal of the inhibitor, after which cells rapidly and synchronously proceed through recombination and meiosis I. Using the synchrony resulting from the cdc7-as3 system, DSB-dependent phosphorylation of the meiosis-specific chromosomal core protein, Hop1, was shown to occur after DSBs. The cdc7-as3 mutant therefore provides a valuable tool not only for understanding the role of Cdc7 in meiosis, but also for facilitating biochemical and cytological studies of recombination.
GENETIC studies in fungi have been highly enlightening with regard to understanding the chromosome mechanics of meiosis. For example, analysis of the patterns of gene conversion events and reciprocal exchanges, in conjunction with gap repair experiments in vegetative cells, led to the development of the double-strand-break (DSB) repair model for recombination. Subsequent studies have shown not only that this model is fundamentally correct for yeast, but also that many aspects of meiotic recombination are conserved throughout evolution (Orr-Weaver et al. 1981; Szostak et al. 1983; Hollingsworth and Brill 2004) The budding yeast Saccharomyces cerevisiae has been a particularly useful model organism for identifying and characterizing genes important for meiosis. Many meiosis-specific recombination genes originally identified in S. cerevisiae, such as SPO11, DMC1, MSH4, and MSH5 and chromosomal core genes such as HOP1, are present in other species and play similar roles in meiosis (Villeneuve and Hillers 2001).
Whereas the genetics of meiosis in budding yeast is highly sophisticated, biochemical studies have lagged behind, in large part due to the difficulty of obtaining highly synchronized cultures of meiotic cells. A major step forward in this direction occurred with the development of the fast-sporulating SK1 strain as a tool for meiotic studies (e.g., Padmore et al. 1991; Hunter and Kleckner 2001). However, while the degree of synchrony in SK1 meiotic cultures is higher than that observed in commonly used, slow-sporulating yeast strains, it is still not sufficient to precisely delineate one meiotic stage from another. To accomplish this, strains that can be arrested at various points during meiosis must be created and then released to produce synchronous populations of cells, analogous to α-factor arrest in vegetative cells. This article describes a method for synchronizing cells at a critical transition point in meiosis, the onset of meiotic recombination.
Cdc7 is a highly conserved, serine/threonine protein kinase that has been studied extensively for its essential role in the initiation of DNA replication in vegetative cells (Sclafani 2000; Masai and Arai 2002). Cdc7 encodes the catalytic subunit of the kinase, but for the kinase to be active, it must form a complex with a regulatory protein called Dbf4. At the restrictive temperature, temperature-sensitive (ts) alleles of CDC7 exhibit a unique arrest point in meiosis—after premeiotic S but before recombination and synapsis (Schild and Byers 1978). This arrest can be reversed by lowering the temperature, raising the possibility that Cdc7 inactivation could be used to synchronize cells immediately prior to recombination. Working with ts mutants in meiosis is problematic, however, in that wild-type strains fail to undergo meiosis at high temperature. As a result, semipermissive temperatures, which often result in leaky phenotypes (e.g., Shuster and Byers 1989; Forsburg and Hodson 2000), must be used. An alternative approach for inactivating Cdc7 is therefore necessary. A conditional system using a tetracycline-repressible allele of DBF4 has recently been published (Valentin et al. 2005). However, in this system there is a significant lag time between the loss of DBF4 expression and degradation of the Dbf4 protein and so a clean arrest phenotype prior to recombination was not observed.
In the past few years a chemical genetic approach has been developed to create conditional versions of protein kinases (Bishop et al. 2001). This approach involves enlarging the ATP-binding pocket of the kinase of interest by mutation, thereby allowing specific inactivation of the kinase by the addition of purine analogs to the culture medium. An analog-sensitive (as) version of CDC7, cdc7-as3, was created and analyzed for its meiotic phenotypes. In the presence of inhibitor, cdc7-as3 strongly resembles cdc7ts mutants at the restrictive temperature. DNA replication, while delayed, is completed and cells arrest in prophase. No recombination is observed due to a failure to make DSBs. Removal of inhibitor after DNA replication results in a highly synchronized population of cells that rapidly undergoes DSB formation and repair. Therefore the cdc7-as3 mutant not only provides an excellent tool for understanding the role of Cdc7 in meiosis, but also may be useful for scientists studying different aspects of recombination in budding yeast.
MATERIALS AND METHODS
Plasmids:
cdc7-as alleles were created by site-directed mutagenesis of the CDC7-myc gene carried in the URA3 integrating plasmid pJO36 (QuikChange kit, Stratagene, La Jolla, CA) (Oshiro et al. 1999). The cdc7-as1 allele contains the L120G mutation, cdc7-as2 contains L120A, and cdc7-as3 contains L120A V181A.
Strains:
All diploids were derived from the SK1 background and have the following genotype: MATa leu2Δ∷hisG his4-x/MATα leu2-K HIS4 ARG4/arg4-Nsp lys2/lys2 hoΔ∷LYS2/hoΔ∷LYS2 ura3/ura3. Variations between diploids depends upon which CDC7 allele is present: NH457F, CDC7-myc; NH450F, cdc7-myc-as1; NH451F, cdc7-myc-as2; and NH452F, cdc7-myc-as3. NH663 and NH664 are isogenic with NH457F and NH452F, respectively, and are homozygous for sae2Δ∷URA3.
Construction of cdc7-as diploids was done as follows: SK1 diploids containing CDC7-myc and cdc7-as-myc were constructed by two-step gene replacement (Rothstein 1991). Because initially it was unknown whether the cdc7-as mutants would cause a phenotype in the presence of an inhibitor, the strain construction was designed to integrate the cdc7-as plasmids adjacent to a deletion of CDC7 so that only pop-out events that left the cdc7-as allele in the chromosome were able to produce viable FOAr colonies. First, one copy of CDC7 was substituted with the kanMX4 gene in the diploid NH144 using polymerase chain reaction (Longtine et al. 1998). Second, pJO36 and its mutant derivatives were digested with EcoRI, which cuts in sequences downstream of the CDC7 open reading frame, and transformed into the CDC7/cdc7Δ heterozygote. Transformants in which the plasmid integrated adjacent to cdc7Δ∷kanMX were identified by sporulating the diploids, dissecting tetrads, and picking spore colonies that were both Ura+ and G418r. Finding such colonies demonstrated that the cdc7-as mutants were able to complement the vegetative growth defect of cdc7Δ. The spore colonies were grown in YPAD (2% Bacto-peptone, 1% yeast extract, 0.002% adenine) and plated onto medium containing 5-fluoroorotic acid to select haploid strains that had lost the cdc7Δ allele, leaving only cdc7-as behind. Haploids of opposite mating type were then crossed to make homozygous cdc7-as diploids. SAE2/COM1 was substituted with sae2Δ∷URA3 using pME1220 (Woltering et al. 2000) in the haploid parents of NH457F and NH452F to make NH663 and NH664, respectively.
Inhibitors:
Stocks of 10 mm PP1 [4-amino-1-tert-butyl-3-(p-methylyphenyl)pyrazolo [3,4-d]pyrimidine] and 1-NM-PP1 [4-amino-1-(tert-butyl)-3-(1′-naphthylmethyl)pyrazolo [3, 4-d] pyrimidine] were synthesized at the University of California at San Franscisco as described in Bishop et al. (1999).
Sporulation assay:
Diploids were grown in YPA (2% Bacto-peptone, 1% yeast extract, 2% potassium acetate) to a density of 3 × 107 cells/ml and transferred to 2% potassium acetate. Five 1-ml aliquots of cells were placed into test tubes and the appropriate amount of inhibitor was added. The cells were incubated on a roller at 30° for 24 hr. Sporulation was assayed by phase-contrast light microscopy. Cells exhibiting both immature and mature asci were counted as having sporulated. A total of 200 cells were examined for each time point.
Time courses:
Cells were sporulated in 2% potassium acetate at 30° as described in de los Santos and Hollingsworth (1999). Flow cytometry was performed by fixing 3 ml of sporulating cells with 70% ethanol overnight at 4°. All solutions contained 5 mm EDTA. The cells were washed once in 50 mm Tris–HCl, pH 8, resuspended in 0.5 ml of the same solution containing 1 mg/ml freshly prepared RNAse (Sigma, St. Louis), and incubated at 37° for at least 2 hr. After pelleting, the cells were resuspended in 200 μl of pepsin solution (0.5% HCl, 5 mg/ml pepsin) (Sigma) and incubated for 60 min at 37°. The pepsin was neutralized by the addition of 1 ml 50 mm Tris–HCl, pH 8. The cells were pelleted and resuspended in 0.5 ml 50 mm Tris–HCl, pH 7.5. Fifty microliters of cells were diluted into 1 ml 50 mm Tris–HCl containing 1 μm Sytox Green (Molecular Probes, Eugene, OR). Samples were sonicated briefly prior to being analyzed. DNA content analysis was performed at the Flow Cytometry Core Facility (SUNY, Stony Brook, NY). DSBs were examined at the naturally occurring YCR048w hotspot as described in Woltering et al. (2000). DSB formation was quantitated using a Molecular Dynamics Phosphoimager and ImageQuant 1.11 software. Hop1 immunoprecipitation and detection were performed as described in de los Santos and Hollingsworth (1999). Meiotic progression was monitored by fixing the cells in 3.7% formaldehyde and staining them with 4′,6-diamidino-2-phenylinodole (DAPI). Binucleate cells have completed meioisis I (MI) and tetranucleate cells have completed meiosis II (MII). All time courses were repeated at least three times, except for the sae2Δ experiment that was done twice.
Washout protocol:
To wash out the PP1, 100 ml of sporulating culture was divided between two 50-ml conical tubes and cells were pelleted by a 3-min spin in a table top centrifuge. Each pellet was then resuspended in 50 ml of 2% potassium acetate. This procedure was repeated for a total of six spins. After the final spin, the cells were pooled and resuspended in 80 ml of 2% potassium and returned to the 30° shaker.
RESULTS
Mutation of the ATP-binding pocket of CDC7 creates as alleles:
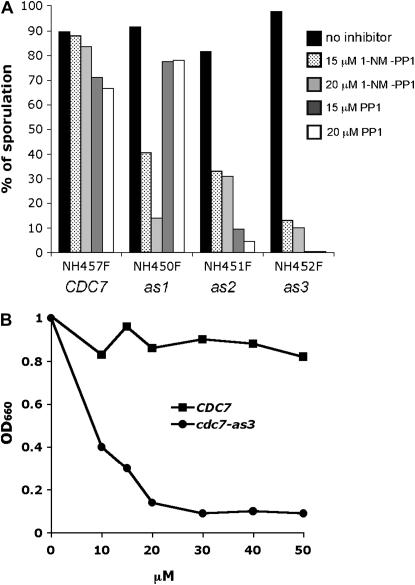
Mutations that increase the size of the ATP-binding pocket of Cdc7 were introduced by substituting leucine at position 120 with either glycine (L120G) or alanine (L120A). In addition, a L120A V181A double-mutant allele was generated on the basis of the rationale proposed by Weiss et al. (2000) that large amino acids present immediately upstream of the conserved DFG motif in the kinase may interfere with binding of the inhibitor, PP1, and its derivatives. To monitor meiosis and sporulation, the cdc7-as alleles were introduced into the efficiently sporulating SK1 strain background as described in materials and methods. Diploids containing CDC7-myc, cdc7-L120G-myc, cdc7-L120A-myc, and cdc7-L120A V181A-myc (hereafter referred to as CDC7, cdc7-as1, cdc7-as2, and cdc7-as3, respectively) were grown to log phase in rich acetate medium and then transferred to sporulation (Spo) medium to induce meiosis. Immediately after transfer to Spo medium, different amounts of the purine analogs, 1-NM-PP1 or PP1, were added to 1-ml aliquots of cells and the cells were incubated on a roller at 30° for 24 hr. Sporulation was then assessed by phase-contrast light microscopy. 1-NM-PP1 had very little effect on sporulation in the wild-type diploid, even when the concentration was 20 μm. In contrast, all three cdc7-as diploids showed a large decrease in sporulation in the presence of 1-NM-PP1 (Figure 1A). However, even at 20 μm, ∼10% asci were observed; therefore, the inhibition of cdc7-as by 1-NM-PP1 is incomplete. Addition of 15 μm PP1 to the CDC7 diploid reduced sporulation to 69%, indicating that there is a slight general effect of the inhibitor on sporulation (Figure 1A). This effect does not appear to extend to meiosis, as the spores are highly viable (91.3%, 26 asci dissected). The Cdc7-as kinases were differentially sensitive to PP1, depending upon which amino acid was substituted for leucine at position 120. While cdc7-as1 was resistant to 20 μm PP1, in the cdc7-as2 strain sporulation was reduced to 4.5% (Figure 1A). The sensitivity of the L120A substitution was increased by the presence of V181A as cdc7-as3 produced <1% sporulation with 15 μm PP1 (Figure 1A).
Figure 1.—
Sporulation and vegetative growth in different cdc7-as diploids with various inhibitors. (A) SK1 diploids containing CDC7 (NH457F), cdc7-as1 (NH450F), cdc7-as2 (NH451F), and cdc7-as3 (NH452F) were sporulated in the presence of the indicated amount of either 1-NM-PP1 or PP1 for 24 hr at 30°. Sporulation was monitored by phase-contrast light microscopy. A total of 200 cells were counted for each condition. (B) Overnight cultures of either NH457F (CDC7) or NH452F (cdc7-as3) were diluted to 105 cells/ml and PP1 was added to aliquots of cells as indicated. After incubation at 30° for 24 hr, growth was measured by determining the absorbance at 660 nm using a spectrophometer.
A second test for sensitivity to PP1 assayed vegetative growth in the presence or absence of inhibitor. CDC7 and cdc7-as3 diploids were grown to stationary phase overnight in YPD and then diluted to a density of 105 cells/ml. Different concentrations of PP1 were added to 1-ml aliquots of cells that were then returned to the roller and incubated overnight at 30°. Growth was measured by monitoring the optical density at 660 nm. In the absence of inhibitor, both the CDC7 and the cdc7-as3 diploids grew to an OD of 1.0 after 24 hr (Figure 1B). For the wild-type diploid, concentrations up to 50 μm PP1 had little effect. In contrast, growth of the cdc7-as3 strain was strongly suppressed by addition of PP1, with the maximum decrease occurring above 20 μm PP1 (Figure 1B). We conclude that, in the SK1 background, PP1 has little to no effect on growth, meiosis, and sporulation of wild-type cells. It should be noted, however, that a wild-type strain with a different genetic background is highly sensitive to PP1 and that the cdc7-as3 mutant in this background exhibited a different pattern of sensitivity, with 1-NM-PP1 being most effective (Ira et al. 2004). Therefore the type and amount of inhibitor used to inactivate cdc7-as3 should be empirically determined for each strain background. In SK1, PP1 inhibition of cdc7-as3 blocks vegetative growth and exhibits a very tight meiotic arrest with 15 μm PP1. We therefore characterized the role of Cdc7 kinase activity in meiosis using the cdc7-as3 allele and the PP1 inhibitor.
Cdc7 kinase activity promotes efficient premeiotic S and functions in prophase to allow recombination and meiotic progression:
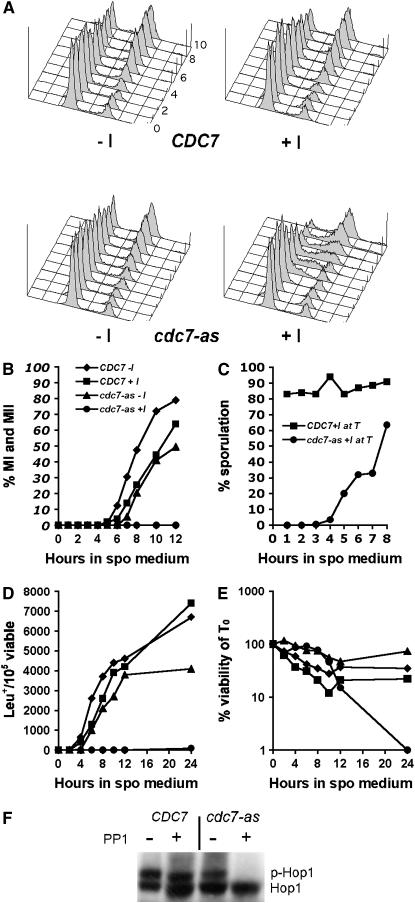
The requirement for Cdc7 kinase activity during meiosis was examined using time courses of CDC7 and cdc7-as3 diploids in which 15 μm PP1 was added upon transfer to Spo medium. Flow cytometry was used to monitor DNA replication. DNA content profiles for the CDC7 diploid with and without PP1 were very similar, with DNA replication starting between 3 and 4 hr after transfer to Spo medium and finishing around 6 hr (Figure 2A). For the cdc7-as3 diploid without inhibitor, the onset of premeiotic DNA replication was delayed ∼1 hr and was not completed until 8 hr. Addition of PP1 to cdc7-as3 cells resulted in a further delay in cells completing S phase, indicated by a significant number of cells between the 2N and 4N peaks (Figure 2A). By 10 hr, however, the bulk of the DNA was replicated.
Figure 2.—
Meiotic time courses of CDC7 and cdc7-as3 diploids in the presence or absence of PP1. CDC7 (NH457F) and cdc7-as3 (NH452F) diploids were transferred to sporulation medium at which time 15 μm PP1 was added to half of each culture and incubated at 30° (+I, with PP1; −I, without PP1). (A) Flow cytometry of cells to monitor DNA content. Time points were taken at 1-hr intervals, starting with 0. The 9-hr time point is not shown. (B) Meiotic progression measured by DAPI staining of nuclei. Bi- and tetra-nucleate cells have completed MI and MII, respectively. (C) Execution-point analysis. A total of 15 μm PP1 was added at the indicated time point to an aliquot of cells without PP1 and sporulation was then assessed after a total of 24 hr. (D) Meiotic intragenic recombination at the leu2 locus was measured by the production of Leu+ prototrophs normalized to the number of viable cells. (E) Cell viability was measured by determining the number of cells able to grow on rich medium. (F) Hop1 phosphorylation detected by immunoblot analysis at the 6-hr time point. Unphosphorylated Hop1 and phosphorylated Hop1 are indicated by Hop1 and p-Hop1, respectively.
Addition of PP1 to the CDC7 diploid caused a 1-hr delay in the onset of the first meiotic division (Figure 2B). A similar delay was observed in the cdc7-as3 diploid without inhibitor, indicating that the cdc7-as3 allele is not completely wild type. Chromosome behavior appears to be normal, however, as all three diploids (CDC7 ± PP1; cdc7-as3 −PP1) exhibited >91% spore viability. In contrast, addition of PP1 to the cdc7-as3 diploid at time 0 (T0) completely blocked progression out of prophase (Figure 2B). Detection of several meiosis-specific proteins and microarray analysis demonstrated that the cells were arrested after entry into meiosis (see below; data not shown).
The sporulation defect was used to determine the timing requirement for Cdc7 kinase activity by execution-point analysis. Inactivation of Cdc7-as prior to its time of action should cause cells to arrest, while addition of PP1 after Cdc7-as has phosphorylated its target proteins should have no effect on sporulation. During the time course, 1-ml aliquots of cdc7-as3 cells were taken at each time point and PP1 was added to a final concentration of 15 μm. The cells were then returned to the incubator until 24 hr, at which time sporulation was monitored. The cdc7-as3 cells started to become resistant to PP1 after 4 hr, nearly 2 hr before the first meiotic division (Figure 2C). Therefore the requirement for Cdc7 kinase activity during meiosis is limited to prophase.
Intragenic recombination was monitored in CDC7 and cdc7-as3 diploids by the formation of Leu+ prototrophs. The kinetics of prototroph formation was nearly identical in the CDC7 diploid plus and minus inhibitor (Figure 2D). In the absence of inhibitor, the cdc7-as3 mutant exhibited a 1-hr delay in the onset of recombination, with a slight decrease in the total number of Leu+ prototrophs. In contrast, no intragenic recombination was detected in the cdc7-as diploid with PP1 (Figure 2D).
Cdc7 kinase activity is required for DSB formation:
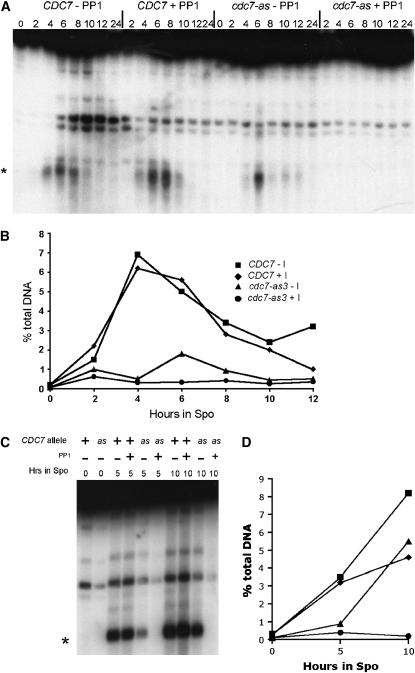
During meiosis, recombination is initiated by the introduction of DSBs by the highly conserved, topoisomerase-like protein, Spo11 (Keeney 2001). To determine whether the cdc7-as3 recombination defect is due to a lack of DSBs, DSB formation was monitored at the naturally occurring YCR048w hotspot (Wu and Lichten 1994). Quantitation of DSBs revealed that the kinetics and degree of DSB formation were similar in the CDC7 diploid both in the presence and the absence of PP1 (Figure 3, A and B). DSBs were detectable but delayed and reduced in the cdc7-as strain without inhibitor. In contrast, no DSBs were observed in the cdc7-as3 strain to which PP1 was added (Figure 3, A and C).
Figure 3.—
DSBs in CDC7 and cdc7-as3 diploids sporulated in the presence or absence of PP1. The indicated diploids were transferred to sporulation medium and 15 μm PP1 was added to half of each culture and the cells were incubated at 30°. (A) DSBs monitored at the YCR048w hotspot (Wu and Lichten 1994) for the time course shown in Figure 2. Asterisks in A and C indicate the most prominent DSB fragments. (B) Quantitation of DSBs from A. (C) DSBs in CDC7 sae2Δ (NH663) and cdc7-as3 sae2Δ (NH664) diploids. (D) Quantitation of DSBs from C.
The failure to detect DSBs when Cdc7-as3 is inhibited could be due either to a defect in DSB formation or, alternatively, to a defect in DSB processing such that the breaks are rapidly degraded or repaired. If the latter case is true, then preventing DSB resection should increase the number of DSBs detectable by Southern blot analysis. DSBs were therefore examined in strains deleted for SAE2/COM1, a gene required for resection of DSBs (McKee and Kleckner 1997; Prinz et al. 1997). CDC7 sae2Δ and cdc7-as3 sae2Δ diploids were sporulated in the presence or absence of 15 μm PP1 and time points were taken at 0, 5, and 10 hr. Consistent with the result from the SAE2 diploid, DSBs accumulated more slowly in the cdc7-as mutant without inhibitor, perhaps because of the delay in DNA replication (Figure 3, C and D). After 10 hr, however, the level of DSBs in the cdc7-as3 sae2Δ strain was equivalent to the CDC7 sae2Δ diploid. No DSBs were observed in the cdc7-as3 sae2Δ diploid with PP1, suggesting that the failure to detect DSBs is due to a failure of initiation, rather than to rapid degradation and/or repair (Figure 3, C and D).
Cdc7 kinase activity is required for phosphorylation of Hop1:
HOP1 and RED1 encode meiosis-specific phospho-proteins that localize to chromosome cores and are required for wild-type levels of DSBs (Smith and Roeder 1997; Wan et al. 2004; Niu et al. 2005). Red1 and Hop1 protein mobilities were analyzed in the time course shown in Figure 2. Addition of PP1 to cdc7-as3 had no effect on Red1 phosphorylation (data not shown). In contrast, inactivation of Cdc7-as3 eliminated phospho-Hop1 (Figure 2F). Hop1 is therefore a potential substrate of Cdc7. Alternatively, the lack of Hop1 phosphorylation could be an indirect consequence of Cdc7 inactivation, due to the fact that DSBs are required for generating phospho-Hop1 (Niu et al. 2005).
The cdc7-as3 arrest is reversible and provides a useful system for inducing Spo11-mediated DSBs:
Schild and Byers (1978) previously showed that the meiotic arrest caused by temperature-sensitive cdc7 mutants can be reversed by lowering the temperature. To determine whether the arrest caused by chemical inactivation of Cdc7-as3 is reversible, 1-ml aliquots were taken of CDC7 and cdc7-as3 cells to which inhibitor had been added at T0 in the time course shown in Figure 2. The inhibitor was removed by several washes before being resuspended in Spo medium and returned to the incubator. After 24 hr, sporulation was monitored. When the inhibitor was removed after 1 hr, 68% of the cdc7-as3 cells sporulated, equivalent to the 70% observed for CDC7 cells without PP1. Therefore, PP1 can be removed by our washing protocol. The same high level of sporulation (73%) was observed when PP1 was washed out of cdc7-as3 cells after 8 hr and the spores were 95.2% viable. Therefore the meiotic arrest conferred by the blockage of Cdc7 kinase activity is reversible with no obvious meiotic defects up to 8 hr. However, when PP1 was washed out of the cdc7-as3 cells after 24 hr, sporulation decreased to 5%, coinciding with a 100-fold decrease in viability (Figure 2E). It may be that after several hours at the cdc7 arrest, irreversible damage to the DNA occurs, which triggers a second checkpoint that blocks sporulation as well as decreases cell viability.
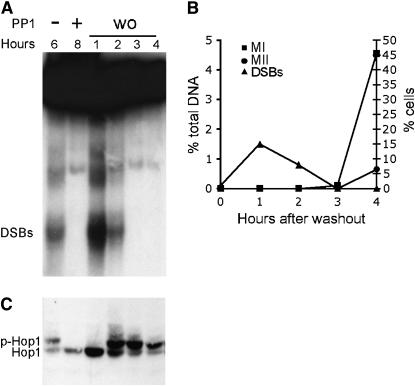
The reversible nature of the cdc7-as3 arrest raises the possibility that this system could provide an efficient means for inducing Spo11-mediated DSBs in a synchronized population of cells. This idea was tested by taking cdc7-as3 cells after 8 hr in Spo medium with PP1, washing out the inhibitor, and monitoring DSB formation and meiotic progression. DSBs appeared within 1 hr after washout of the inhibitor and were repaired within 3 hr (Figure 4A). The length of time between the appearance of DSBs and their repair was significantly shorter in the washout situation compared to cdc7-as3 diploid without inhibitor (Figure 3). This result supports the idea that the lag in DNA repair observed in cdc7-as3 strains is likely due to poorer synchrony rather than to a defect in recombination per se. After washout, the DSBs disappear even more rapidly than in the wild-type situation (CDC7 without PP1, Figure 3A), where DSBs did not disappear until 8 hr after they were first detected. This result demonstrates that the cdc7-as3 washout method confers better synchrony than can be obtained by our normal sporulation protocol. Further evidence that the cells are synchronized is the observation that between 3 and 4 hr after washout, nearly 50% of the cells proceeded through the first meiotic division (Figure 4B). Chromosome behavior subsequent to the washout is apparently normal, as the cells exhibited 87% sporulation and 97% spore viability.
Figure 4.—
DSBs, Hop1 phosphorylation, and meiotic progression after release of the cdc7-as3 meiotic arrest. The cdc7-as3 diploid, NH452F, was transferred to Spo medium at 30° and 15 μm PP1 was added to half of the culture. After 8 hr, the inhibitor was washed out (wo) and the cells returned to 30°. (A) DSBs at the YCR048w hotspot. (B) Quantitation of DSBs in A. (C) Meiotic progression measured by DAPI staining of nuclei. (D) Hop1 was immunoprecipitated and detected on immunoblots using α-Hop1 antibodies. p-Hop1 indicates phosphorylated Hop1. Hours indicates the number of hours in Spo medium or the number of hours after washout.
Hop1 phosphorylation occurs after DSB formation:
As a proof of principle, the cdc7-as3 system was used to determine whether DSB formation and Hop1 phosphorylation are temporally distinct events. DSB formation is required for Hop1 phosphorylation, and this phosphorylation is proposed to be important in suppressing sister-chromatid repair during meiosis (Niu et al. 2005). The question is whether DSBs and Hop1 phosphorylation occur at the same or different times. Sporulation of a cdc7-as3 diploid in the absence of PP1 for 6 hr results in both DSBs and phosphorylated Hop1 (Figure 4, A and C). When Cdc7-as3 is inhibited by PP1 for 8 hr in Spo medium, no phosphorylated Hop1 is detected. Although DSBs were formed within 1 hr after removal of PP1, phosphorylated Hop1 was not detected for another hour (Figure 4, A and C). Therefore Hop1 phosphorylation occurs at a discrete period of time after DSB formation.
DISCUSSION
We have used a chemical genetic approach to create a conditional allele of CDC7, an essential kinase that has been extensively studied with regard to its role in the initiation of DNA replication in vegetative cells. There are several advantages to the cdc7-as3 approach. Kinase activity is specifically inactivated by addition of small-molecule inhibitors directly to the sporulation medium. Therefore sporulation can occur at temperatures that are most conducive to meiosis, unlike experiments that use temperature- or cold-sensitive mutants. Mutant phenotypes can be directly attributed to the inability of the kinase to phosphorylate a target protein. Furthermore, there is very little, if any, lag time between addition of inhibitor and inactivation of the kinase, in contrast to the DBF4 expression shutoff approach, in which it takes 3 hr for the Dbf4 protein to disappear (Valentin et al. 2005). Finally, the inhibitor is easily removed by washing the cells, making it possible to reverse the cdc7-as3 arrest and to synchronize cells prior to recombination.
The cdc7-as3 allele is not completely wild type, as it exhibits some meiotic phenotypes even in the absence of inhibitor. Premeiotic S is delayed, as is DSB formation, perhaps because the two processes are normally coupled in meiosis (see below). DSBs are reduced in cdc7-as3 diploids without inhibitor, in part perhaps because of a greater degree of asynchrony compared to wild type. When DSBs are prevented from repairing by sae2, cdc7-as3 diploids accumulate wild-type levels of breaks, arguing that initiation is not affected by the as3 mutation. Furthermore, the spore viability of cdc7-as3 is as high as wild type. Therefore if there is a reduction in breaks, the reduction is above the threshold required for effective chromosome segregation (Henderson and Keeney 2004).
The phenotypes of the cdc7-as3 mutant in the presence of inhibitor are highly similar to those observed by Schild and Byers (1978) for cdc7ts diploids. In both cases, cells arrest after premeiotic S. Given that CDC7 is essential for DNA replication in vegetative cells, the finding that cdc7ts diploids undergo premeiotic S was unexpected (Schild and Byers 1978; Hollingsworth and Sclafani 1993). One explanation for this paradox is that CDC7 is required for premeiotic DNA synthesis but not all of the temperature-sensitive kinase is inactivated at the restrictive temperature. This situation was previously observed with ts mutants of CDC28, which arrest in pachytene after premeiotic S and recombination (Shuster and Byers 1989). Using a chemically inhibitable form of the kinase, cdc28-as1, Benjamin et al. (2003) showed that Cdc28 functions at two discrete steps during meiosis: first, in the initiation of DNA replication and, second, in the pachytene-to-MI transition. Blocking DNA replication with cdc28-as1 required 10 times more inhibitor than the pachytene arrest, supporting the idea that DNA replication is less sensitive to reduced Cdc28 kinase levels than later meiotic events (Benjamin et al. 2003).
These studies show that despite high concentrations of PP1, premeiotic DNA synthesis still occurs in cdc7-as3 diploids. Monitoring DNA replication over time using flow cytometry demonstrated, however, that S phase during meiosis is prolonged in the absence of Cdc7 kinase activity, indicating that Cdc7 is necessary to promote efficient premeiotic DNA replication. Our work agrees with a recent study by Valentin et al. (2005) who found that shutting off transcription of DBF4 prior to the onset of meiosis greatly delays premeiotic S. Whether Cdc7 is absolutely required for premeiotic DNA synthesis is still unresolved. The replication that occurs in both the cdc7-as3 and the DBF4 shutoff experiments could be due to a failure to completely eliminate Cdc7 kinase activity, either by incomplete inhibition of the kinase or by residual amounts of Dbf4 protein. Whether it is essential for DNA replication during meiosis or not, Cdc7 clearly plays a positive role in this process.
cdc7 mutants are defective in recombination. This work shows that this defect is due to a failure to make DSBs. Although other mutants such as spo11 that fail to make DSBs are proficient in meiotic progression, cdc7 mutants arrest in prophase (Malone et al. 2004). Therefore CDC7 plays a role not only in DSB formation but also in enabling the onset of the first meiotic division. The cdc7-as3 system provides an excellent tool for understanding the multiple roles that Cdc7 kinase activity plays during meiosis.
In addition to being useful for elucidating Cdc7 function itself, the reversible nature of the cdc7-as3 arrest provides a valuable tool for studying various aspects of meiotic recombination. When inhibitor is removed, Spo11-induced DSBs rapidly appear, indicating that cells are arrested at a step in meiosis immediately prior to DSB formation. The spore viability of cells treated in this way is high, indicating that there are no gross meiotic defects that arise from this protocol. There may, of course, be subtle differences from wild-type meiosis. For example, in budding yeast, initiation of recombination is coupled to DNA replication (Borde et al. 2000). This fact was elegantly demonstrated by experiments in which all of the origins of replication were deleted from one arm of chromosome III. Replication on this arm was delayed as expected, given that the fork had to travel from a single origin along the length of the arm. DSBs were correspondingly delayed, suggesting that breaks do not happen until after the replication fork has passed. An interesting question, therefore, is what effect uncoupling replication from recombination has on the distribution of DSBs in the cdc7-as3 cells. Understanding the answer to this question may help in understanding how the processes are coupled in the first place.
Being able to synchronously induce Spo11-mediated DSBs in budding yeast has a variety of potential applications. For example, this system allows one to monitor the localization and/or modification state of proteins before and after DSB formation. Using the synchrony conferred by cdc7-as3, we have shown that not only is Hop1 phosphorylation dependent upon DSBs, but also there is a lag between break formation and phosphorylation. This finding suggests that the kinase responsible for phosphorylating Hop1 is not part of the DSB machinery but may instead need to localize to DSBs before it can phosphorylate Hop1. An advantage with this approach is that the cells are synchronized before and after DSB formation, and it is exactly the same culture of cells that is being monitored in the two different states. Having a highly synchronized population may also enable the detection of transient recombination intermediates using assays such as two-dimensional gel analysis.
Acknowledgments
We thank Bruce Futcher, Janet Leatherwood, Michael Lichten, Aaron Neiman, Bob Scalfani, Rolf Sternglanz, and David Stuart for helpful discussions and Aaron Neiman for comments on the manuscript. We are grateful to Bob Sclafani for providing the CDC7-myc plasmid. This work was supported by National Institutes of Health grants GM50717 to N.M.H. and AI44009 to K.M.S.
References
- Benjamin, K. R., C. Zhang, K. M. Shokat and I. Herskowitz, 2003. Control of landmark events in meiosis by the CDK Cdc28 and the meiosis-specific kinase Ime2. Genes Dev. 17: 1524–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, A. C., C.-Y. Kung, K. Shah, L. Witucki, K. M. Shokat et al., 1999. Generation of monospecific nanomolar tyrosine kinase inhibitors via a chemical genetic approach. J. Am. Chem. Soc. 121: 627–631. [Google Scholar]
- Bishop, A. C., O. Buzko and K. M. Shokat, 2001. Magic bullets for protein kinases. Trends Cell Biol. 11: 167–172. [DOI] [PubMed] [Google Scholar]
- Borde, V., A. S. Goldman and M. Lichten, 2000. Direct coupling between meiotic DNA replication and recombination initiation. Science 290: 806–809. [DOI] [PubMed] [Google Scholar]
- de los Santos, T., and N. M. Hollingsworth, 1999. Red1p: a MEK1-dependent phosphoprotein that physically interacts with Hop1p during meiosis in yeast. J. Biol. Chem. 274: 1783–1790. [DOI] [PubMed] [Google Scholar]
- Forsburg, S. L., and J. A. Hodson, 2000. Mitotic replication initiation proteins are not required for pre-meiotic S phase. Nat. Genet. 25: 263–268. [DOI] [PubMed] [Google Scholar]
- Henderson, K. A. and S. Keeney, 2004. Tying synaptonemal complex initiation to the formation and programmed repair of DNA double-strand breaks. Proc. Natl. Acad. Sci. USA 101: 4519–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth, R. E., Jr., and R. A. Sclafani, 1993. Yeast pre-meiotic DNA replication utilizes mitotic origin ARS1 independently of CDC7 function. Chromosoma 102: 415–420. [DOI] [PubMed] [Google Scholar]
- Hollingsworth, N. M., and S. J. Brill, 2004. The Mus81 solution to resolution: generating meiotic crossovers without Holliday junctions. Genes Dev. 18: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, N., and N. Kleckner, 2001. The single-end invasion: an asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell 106: 59–70. [DOI] [PubMed] [Google Scholar]
- Ira, G., A. Pellicioli, A. Balijja, X. Wang, S. Fiorani et al., 2004. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431: 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney, S., 2001. Mechanism and control of meiotic recombination initiation. Curr. Top. Dev. Biol. 52: 1–53. [DOI] [PubMed] [Google Scholar]
- Longtine, M. S., A. McKenzie, III, D. J. Demarini, N. G. Shah, A. Wach et al., 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Malone, R. E., S. J. Haring, K. E. Foreman, M. L. Pansegrau, S. M. Smith et al., 2004. The signal from the initiation of meiotic recombination to the first division of meiosis. Eukaryot. Cell 3: 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai, H., and K. Arai, 2002. Cdc7 kinase complex: a key regulator in the initiation of DNA replication. J. Cell. Physiol. 190: 287–296. [DOI] [PubMed] [Google Scholar]
- McKee, A. H. Z., and N. Kleckner, 1997. A general method for identifying recessive diploid-specific mutations in Saccharomyces cerevisiae, its application to the isolation of mutants blocked at intermediate stages of meiotic prophase and characterization of a new gene SAE2. Genetics 146: 797–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, H., L. Wan, B. Baumgartner, D. Schaefer, J. Loidl et al., 2005. Partner choice during meiosis is regulated by Hop1-promoted dimerization of Mek1. Mol. Biol. Cell 16: 5804–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver, T. L., J. W. Szostak, and R. J. Rothstein 1981. Yeast transformation: a model system for the study of recombination. Proc. Natl. Acad. Sci. USA 78: 6354–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro, G., J. C. Owens, Y. Shellman, R. A. Sclafani and J. J. Li, 1999. Cell cycle control of Cdc7p kinase activity through regulation of Dbf4p stability. Mol. Cell. Biol. 19: 4888–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmore, R., L. Cao and N. R. Kleckner, 1991. Temporal comparison of recombination and synaptonemal complex formation during meiosis in Saccharomyces cerevisiae. Cell 66: 1239–1256. [DOI] [PubMed] [Google Scholar]
- Prinz, S., A. Amon and F. Klein, 1997. Isolation of COM1, a new gene required to complete meiotic double-strand break-induced recombination in Saccharomyces cerevisiae. Genetics 146: 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein, R., 1991. Targeting, disruption, replacement and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 194: 281–301. [DOI] [PubMed] [Google Scholar]
- Schild, D., and B. Byers, 1978. Meiotic effects of DNA-defective cell division cycle mutations of Saccharomyces cerevisiae. Chromosoma 70: 109–130. [DOI] [PubMed] [Google Scholar]
- Sclafani, R. A., 2000. Cdc7p-Dbf4p becomes famous in the cell cycle. J. Cell Sci. 113(Pt. 12): 2111–2117. [DOI] [PubMed] [Google Scholar]
- Shuster, E. O., and B. Byers, 1989. Pachytene arrest and other meiotic effects of the start mutations in Saccharomyces cerevisiae. Genetics 123: 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. V., and G. S. Roeder, 1997. The yeast Red1 protein localizes to the cores of meiotic chromosomes. J. Cell Biol. 136: 957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak, J. W., T. L. Orr-Weaver, R. J. Rothstein and F. W. Stahl, 1983. The double strand-break model for recombination. Cell 33: 25–35. [DOI] [PubMed] [Google Scholar]
- Valentin, G., E. Schwob and F. Della Seta, 2005. Dual role of the CDC7-regulatory protein DBF4 during yeast meiosis. J. Biol. Chem. 281: 2929–2934. [DOI] [PubMed] [Google Scholar]
- Villeneuve, A. M., and K. J. Hillers, 2001. Whence meiosis? Cell 106: 647–650. [DOI] [PubMed] [Google Scholar]
- Weiss, E. L., A. C. Bishop, K. M. Shokat and D. G. Drubin, 2000. Chemical genetic analysis of the budding-yeast p21-activated kinase Cla4p. Nat. Cell Biol. 2: 677–685. [DOI] [PubMed] [Google Scholar]
- Wan, L., T. de los Santos, C. Zhang, K. Shokat and N. M. Hollingsworth, 2004. Mek1 kinase activity functions downstream of RED1 in the regulation of meiotic DSB repair in budding yeast. Mol. Biol. Cell 15: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering, D., B. Baumgartner, S. Bagchi, B. Larkin, J. Loidl et al., 2000. Meiotic segregation, synapsis, and recombination checkpoint functions require physical interaction between the chromosomal proteins Red1p and Hop1p. Mol. Cell. Biol. 20: 6646–6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, T.-C., and M. Lichten, 1994. Meiosis-induced double-strand break sites determined by yeast chromatin structure. Science 263: 515–518. [DOI] [PubMed] [Google Scholar]