Abstract
The transmembrane proteins Delta and Notch act as ligand and receptor in a conserved signaling pathway required for a variety of cell fate specification events in many organisms. Binding of Delta to Notch results in a proteolytic cascade that releases the Notch intracellular domain, allowing it to participate in transcriptional activation in the nucleus. Recent research has implicated the endocytic and ubiquitylation machinery as essential components of Delta–Notch signaling. Our analysis of chimeric and missense Delta variants has delineated a number of structural requirements for Delta trafficking, receptor binding, and signaling. We find that while the Delta N-terminal domain is necessary and sufficient for binding to Notch, the integrity of the epidermal-growth-factor-like repeat (ELR) 2 is also required for Notch binding. Screening of 117 Delta mutant lines for proteins that exhibit aberrant subcellular trafficking has led to the identification of 18 Delta alleles (DlTD alleles) that encode “trafficking-defective” Delta proteins. We find, unexpectedly, that many DlTD alleles contain missense mutations in ELRs within the Delta extracellular domain. Finally, we find that two DlTD alleles contain lysine missense mutations within the Delta intracellular domain (DeltaICD) that may identify residues important for DeltaICD mono-ubiquitylation and subsequent Delta endocytosis and signaling.
THE Notch signaling pathway affects cell fate decisions throughout development in a vast array of organisms. Notch (N) was originally classified as a neurogenic gene on the basis of the observation that homozygosity and hemizygosity for N loss-of-function mutations result in hypertrophy of neural tissue at the expense of epidermal tissue in developing embryos (Poulson 1937). The Notch pathway has since been shown to play key roles in cell specification within many tissues, including the eye, wing, and notum (Artavanis-Tsakonas et al. 1999; Portin 2002), and ongoing research continues to broaden the understood spectrum of Notch activity. Dysfunction of this pathway has been implicated in several human diseases, including lymphocytic leukemia, spondylocostal dysostosis, and Alagille syndrome (Gridley 2003; Weng et al. 2004).
In Drosophila, the extracellular domains of the Notch transmembrane ligands Delta and Serrate bind to the Notch extracellular domain (NotchECD) in a manner that is dependent on NotchECD ELRs 11 and 12 (Rebay et al. 1991). A subsequent proteolytic cascade results in the release and translocation of the Notch intracellular domain (NotchICD) to the nucleus, where it forms a complex with the Suppressor of Hairless [Su(H)] protein and regulates transcription of target genes, including those of the Enhancer of split-Complex (Greenwald 1998; Artavanis-Tsakonas et al. 1999; Baron 2003; Kadesch 2004). Regulation of the Notch pathway is varied and complex. Some proteins regulate signaling positively (Deltex) or negatively (Numb) by binding to the NotchICD. Others, such as Hairless, impede signaling by binding directly to Su(H); while yet another regulatory mechanism involves Fringe-dependent glycosylation of the NotchECD (Artavanis-Tsakonas et al. 1999; Schweisguth 2004; Le Borgne et al. 2005a).
A number of research findings have recently implicated components of the subcellular trafficking machinery in Delta signal activation (Le Borgne et al. 2005a; Chitnis 2006; Le Borgne 2006). Delta signal activation appears to depend on ubiquitylation of Delta by the E3 ubiquitin ligases Neuralized (Neur) or Mind bomb1 (Mib1), both of which can stimulate Delta endocytosis and signaling (Deblandre et al. 2001; Lai et al. 2001; Pavlopoulos et al. 2001; Lai et al. 2005; Le Borgne et al. 2005b). Proteins encoded by the Bearded family of genes (e.g., Tom, Brd-C) have recently been shown to bind Neur and inhibit Delta endocytosis (Bardin and Schweisguth 2006; De Renzis et al. 2006). Epsin, an adapter for clathrin-mediated endocytosis, can activate Delta signaling in Drosophila and Caenorhabditis elegans and also appears to regulate ligand endocytosis, possibly by targeting Delta to a recycling endosomal compartment (Overstreet et al. 2004; Tian et al. 2004; Wang and Struhl 2004, 2005). Other clathrin-coat components such as the clathrin heavy chain and α-adaptin have also been implicated in Notch signaling on the basis of genetic interactions (Cadavid et al. 2000; K. M. Klueg and M. A. T. Muskavitch, unpublished observation). Recent work suggests that the recycling endosome-associated protein Rab11, as well as the Rab11-binding proteins Sec15 and Nuclear fallout (Nuf), are necessary for Delta trafficking, and has reinforced the idea that Delta must be transported through recycling endosomes as a prerequisite for Notch activation (Emery et al. 2005; Jafar-Nejad et al. 2005). In what may be a second signaling-related endocytic event, Delta endocytosis and uptake of the NotchECD into signal-sending cells has also been correlated with active Delta–Notch signaling (Parks et al. 2000). This trans-endocytosis of the NotchECD is dependent on the vesicle-scission enzyme dynamin (Parks et al. 2000), a protein essential for Notch signaling in many contexts (Poodry 1990). Interestingly, dynamin activity is required in both signal-sending and signal-receiving cells for active Notch signaling (Seugnet et al. 1997a).
Delta protein is detected exclusively in intracellular endocytic vesicles in many Drosophila tissues at specific times during development (Krämer and Phistry 1996; Huppert et al. 1997; Krämer and Phistry 1999; Parks et al. 2000). In the developing retina, wild-type Delta protein is solely detected in vesicles at all stages and in all cell types (Parks et al. 1995). We have previously described Delta (Dl) alleles that encode proteins that accumulate aberrantly on retinal cell surfaces and constitute loss-of-function alleles (Parks et al. 1995, 2000), consistent with the notion that Delta internalization is critical for function.
In this report, we describe the functional analysis of a number of Delta variants, including missense variants constructed on the basis of lesions found among a set of Dl loss-of-function alleles encoding proteins that aberrantly localize to cell surfaces [called DeltaTD (trafficking defective) proteins or DlTD alleles]. We have assessed the ability of subsets of these variants to bind to Notch, to undergo endocytosis, and to generate Notch-dependent signals in vivo. We provide evidence that specific Delta amino-terminal (N-terminal) sequences, ELRs, and intracellular domain residues play specific roles in Delta–Notch signaling. We demonstrate that the Delta N-terminal domain, including the Delta-Serrate-Lag-2 (DSL) domain, and Delta ELR 2 are required for Notch binding and signaling in vivo; that sequences within the Delta N-terminal domain, other than the DSL domain, are required for Delta–Notch binding and homotypic Delta binding; that mutations in multiple DeltaELRs are implicated in Delta endocytosis and Notch signaling in vivo; and that alterations in lysine residues within the DeltaICD, potential sites for ubiquitylation, are correlated with loss of Delta endocytosis and signaling in vivo.
MATERIALS AND METHODS
Sequences used for alignments:
The following sequences were used: Drosophila melanogaster Delta, NP_732412.1; Homo sapiens Delta-like 1, NP_005609.2; Mus musculus Delta-like 1, NP_031891.2; Gallus gallus Delta-like 1, NP_990304.1; Danio rerio DeltaA, NP_571029.1; D. melanogaster Serrate, NP_524527.3; H. sapiens Jagged 1, NP_000205.1.
Drosophila strains:
Oregon-R, BER-1, ss e4 ro, and E(spl)D tx are maintained in our laboratory. The stock ru h th st cu sr es ca (ru cu ca) was obtained from the Bloomington Drosophila Stock Center. Stocks of e4 tx, Canton-S and y w NAx9B2/y w f:= were obtained from S. Artavanis-Tsakonas (Massachusetts General Hospital Cancer Center, Boston). Dl trafficking-defective (DlTD) alleles were maintained over TM6C, cu Sb e Tb ca and include DlBE21 and DlBX9 from a mutant screen using E(spl)D tx (Alton et al. 1989); DlBE30, DlBE32, DlBX43, DlCE3, DlCE6, DlCE7, DlCE9, DlCE15, and DlCE16 from a mutant screen using ss e4 ro (Alton et al. 1989); DlRF (Xu et al. 1990; Parody and Muskavitch 1993) from parental strain y w NAx9B2/y w f:=; Dl9Q76 (received from M. Mortin, National Institutes of Health, Bethesda, MD; isolated by Tearle and Nusslein-Volhard 1987) from a mutant screen using ru cu ca; Dl48.7 (from S. Artavanis-Tsakonas) isolated from a screen using e4 tx; Dl90/TM2 and Dl195/TM2 (from S. Artavanis-Tsakonas) from parental strain Canton-S; and Dl87h/TM6C (parent unknown; obtained from W. J. Welshons, Iowa State University, Ames, IA).
The 1348-Gal4 (Huppert et al. 1997), 31-1-Gal4 (Brand and Perrimon 1993), and dpp-Gal4 drivers (Staehling-Hampton et al. 1994) were employed to induce ectopic expression in combination with responders including UAS-DeltaWT-1 (Jacobsen et al. 1998), UAS-DeltaWT-2 (designated UAS-Delta in Seugnet et al. 1997b), or UAS-DeltaVariants (see below).
Molecular cloning and sequence analysis:
Constructs used to direct expression of DeltaWT (pMTDl1) and Notch (pMTNMg) have been described (Fehon et al. 1990). In the descriptions below, Delta nucleotides correspond to those of the Dl1 cDNA (Kopczynski et al. 1988; EMBL accession no. Y00222). pDl1 refers to the Dl1 cDNA cloned into pBluescript+ (Kopczynski et al. 1988). The metallothionein promoter vector pMT (pRmHA-3) is described in Bunch et al. (1988).
Delta deletion and insertion mutants:
DeltaΔaa32–198:
pDl1 was digested to completion with SalI and partially with ScaI and the 2.6-kb vector-containing fragment was isolated. pDl1 was digested to completion with SalI and partially with NaeI and the 2.2-kb fragment coding for the Delta carboxyl-terminus was isolated. The two fragments were ligated, and the resulting insert was transferred into pMT following excision with EcoRI. This construct contains Dl1 nucleotides 1–235/734–2892.
DeltaΔaa192–331:
pDl1 was digested to completion with BglII and partially with BamHI and the termini of the 5.4-kb vector-containing fragment were filled using Klenow DNA polymerase. The resulting blunt-ended fragment was ligated and transferred to pMT following EcoRI excision of the resulting insert. This construct contains Dl1 nucleotides 1–713/1134–2892.
DeltaΔELR1–3:
Base pairs 236–830 of Delta were PCR amplified from pDl1 using the primers 5′-ACTTCAGCAACGATCACGGG-3′ and 5′-TTGGGTATGTGACAGTAATCG-3′. The 0.6-kb product was treated with T4 DNA polymerase. pDl1 was digested to completion with BglII and partially with ScaI, and the termini of the 4.9-kb vector-containing fragment were filled using Klenow DNA polymerase. The 0.6- and 4.9-kb fragments were ligated together and transformed into bacteria. The 1.9-kb BamHI–SalI Delta-coding fragment from this construct was then substituted for the 2.4-kb BamHI–SalI fragment in pMTDl1. This construct contains Dl1 nucleotides 1–830/1134–2892.
DeltaΔELR4–5:
pDl1 was digested to completion with BglII and partially with PstI. The 5.6-kb vector-containing fragment was isolated and ligated in the presence of the oligonucleotide 5′-GATCTGCA-3′. The resulting insert was transferred into pMT following excision with EcoRI. This construct contains Dl1 nucleotides 1–1137/1405–2892.
DeltaΔICD:
pDl1 was digested partially with DdeI, and the 5.8-kb fragment was isolated. Termini were filled with Klenow DNA polymerase and religated in the presence of the oligonucleotide 5′-TTAAGTTAACTTAA-3′. The resulting insert was transferred into pMT following excision with EcoRI. This construct contains Dl1 nucleotides 1–2021/TTAAGTTAACTTAA/2227–2892.
DeltaStu:
pMTDl1 was digested with StuI, and the resulting 5.8-kb fragment was isolated. The fragment was then religated in the presence of the oligonucleotide 5′-GGAAGATCTTCC-3′. A clone containing multiple inserts was isolated (pDeltaStuA). This clone was digested to completion with BglII, and the resulting 0.6- and 5.2-kb fragments were ligated. This construct contains Dl1 nucleotides 1–535/GGAAGATCTTCC/536–2892.
DeltaNae:
pMTDl1 was digested with NaeI, and the resulting 5.8-kb fragment was isolated. The fragment was then religated in the presence of the oligonucleotide 5′-GGAAGATCTTCC-3′. A clone containing multiple inserts was isolated (pDeltaNaeA). This clone was digested to completion with BglII, and the resulting 0.6- and 5.2-kb fragments were ligated. This construct contains Dl1 nucleotides 1–733/GGAAGATCTTCC/734–2892.
Delta-neuroglian chimeras:
These were generated using pRMHa3-104 (gift of A. J. Bieber, Mayo Clinic College of Medicine, Rochester, MN), which consists of a cDNA encoding the long form of neuroglian (1B7A-250) inserted into pMT (Hortsch et al. 1990).
DeltaNG1:
pRmHa3-104 was digested with BglII and EcoRI, and the 4.9-kb vector-containing fragment was isolated. pDeltaNaeA was digested with BglII and EcoRI, and the 0.7-kb fragment encoding the Delta N terminus was isolated. These two fragments were then ligated. This construct contains Dl1 nucleotides 1–733/GGAA/neuroglian nucleotides 2889–3955.
DeltaNG2:
pRmHa3-104 was digested with BglII and EcoRI, and the 4.9-kb vector-containing fragment was isolated. pDeltaΔELR1–3 was digested with EcoRI and BglII, and the 0.8-kb fragment encoding the Delta N terminus was isolated. These two fragments were then ligated. This construct contains Dl1 nucleotides 1–830/neuroglian nucleotides 2889–3955.
DeltaNG3:
pRmHa3-104 was digested with BglII and EcoRI, and the 4.9-kb vector-containing fragment was isolated. pDl1 was digested with EcoRI and BglII, and the 1.1-kb fragment encoding the Delta N terminus was isolated. These two fragments were then ligated. This construct contains Dl1 nucleotides 1–1133/neuroglian nucleotides 2889–3955.
DeltaNG4:
pRmHa3-104 was digested with BglII and EcoRI, and the 4.9-kb vector-containing fragment was isolated. pDeltaΔaa32–198 was digested with EcoRI and BglII, and the 0.6-kb fragment encoding the Delta N terminus was isolated. These two fragments were then ligated. This construct contains Dl1 nucleotides 1–235/734–1133/neuroglian nucleotides 2889–3955.
Delta trafficking-defective mutants:
A StyI RFLP within the Dl locus was identified for the DlTD strains and their parents, as described in Parks et al. (2000). Genomic DNA from flies carrying a DlTD allele over a wild-type allele with the opposing StyI RFLP [either BER-1 or E(spl)D tx females] was isolated for each of the DlTD alleles. The sixth exon of each DlTD allele was cloned and sequenced as in Parks et al. (2000).
The following lesions from these alleles were then introduced singly into a full-length Dl cDNAs in pMT or pUAST (see below): C288Y (from Dl195), C301Y (from DlCE9), C301S (from DlBE21), N340S (from DlCE6), and C553Y (from DlCE16).
Cell lines and aggregation protocols:
The S2 Drosophila cell line (Schneider 1972) was grown and transfected as described in Fehon et al. (1990), except that BSS (140 mm NaCl, 0.75 mm Na2HPO4, 25 mm BES, pH 6.95) was sometimes used in place of HBS (140 mm NaCl, 0.75 mm Na2HPO4, 25 mm HEPES, pH 7.1). Aggregations were performed either in 25-ml flasks with 3 × 107 transfected cells in a volume of 3 ml [1.5 × 107 each of pMTDl1 (or variants) and pMTNMg-transfected cells for heterotypic aggregations] or in the wells of 12-well microtiter plates with 1 × 107 transfected cells in a volume of 1 ml [0.5 × 107 each of pMTDl1 (or variants) and pMTNMg-transfected cells for heterotypic aggregations]. The resulting cell mixtures were fixed and stained as previously described (Fehon et al. 1990). A minimum of 200 cells that express Delta and/or Notch, as appropriate, were counted for each replicate of each aggregation, unless otherwise noted. For aggregations involving Delta variants C288Y, C301Y, and C301S, S2 cells were electroporated as described (Klueg and Muskavitch 1999) and staining for Delta and Notch was performed as in Klueg and Muskavitch (1998) (antibodies used were GP581 and C458.2H; see below). A total of 100 cell units were examined in each experimental replicate (a “cell unit” is described as one or more stained cells).
Germline transformation and crosses:
Several of the lesions described above were introduced into a full-length Delta cDNA under the control of yeast UAS sequences in the pUAST vector (Brand and Perrimon 1993), resulting in P[UAS-DeltaΔICD], P[UAS-DeltaStu], P[UAS-DeltaNae], P[UAS-DeltaC288Y], P[UAS-DeltaC301Y], P[UAS-DeltaNG2], P[UAS-DeltaNG3], P[UAS-DeltaN340S], and P[UAS-DeltaC553Y]. Germline transformation and subsequent crosses were carried out as described in Jacobsen et al. (1998). All crosses of UAS responder lines to Gal4 driver lines were performed at 25° in a w1118 background.
Immunohistochemistry and antibodies used:
Retinal and third instar wing tissues were treated as described in Parks et al. (1997) using a monoclonal antibody to Delta ELRs 4–5 [C594.9B (MAb9B, also known as MAb202; Diederich et al. 1994)] used at 1:5000 dilution, as well as a monoclonal antibody to the intracellular domain of the long form of Drosophila neuroglian [BP104; from A. J. Bieber, Mayo Clinic College of Medicine] used at a dilution of 1:10. The screen for DlTD alleles was performed using mouse polyclonal antiserum to the Delta extracellular domain (M5; Kooh et al. 1993), used at 1:2000 dilution. S2 cell staining was performed using guinea pig polyclonal antiserum to Delta ELRs 4–9 (GP581; Huppert et al. 1997) and monoclonal antibody ascites to the NotchECD [C458.2H (MAbC458; Diederich et al. 1994)], used at a dilution of 1:5000, and the neuroglian antibody mentioned above used at 1:10 dilution. Micrographs were taken using either a Sony DXC-960MD video camera or a Zeiss Axiocam mounted on a Zeiss Axioskop microscope.
Phenotypic assessment of transgenic adults:
Independent lines carrying each UAS-DeltaVariant transposon were crossed to the dpp-Gal4 driver, and third instar wing/notal discs were assayed for Delta expression as described above. For each construct, three lines expressing Delta protein at levels comparable to or greater than that observed for UAS-DeltaWT-1 were crossed to each of the drivers 1348-Gal4, 31-1-Gal4, and dpp-Gal4, except in the case of UAS-DeltaNG2 for which only two expressing lines exist. Adult progeny were scored for gain-of-function or loss-of-function phenotypes as described in Table 2 footnotes. Results were compared to those for UAS-DeltaWT-1 and UAS-DeltaWT-2 in heterozygous combination with each of the above drivers. For one of the three responder lines crossed, the adult phenotypes of at least 50 critical class females were scored and supporting numbers were collected from two other responder lines. In cases in which the Delta variant appeared to have no effect, 100 critical class females were scored. For responder lines heterozygous with 31-1-Gal4, notal macrochaetae were scored for bristle loss/double sockets as described in Jacobsen et al. (1998). If notal macrochaetae were unaffected, the more sensitive leg bristles were then assessed for gain-of-function or loss-of-function phenotypes.
TABLE 2.
Overexpression phenotypes of wild-type Delta and Delta variants
| Delta variant | Binds Notcha | 1348-Gal4b | 31-1-Gal4c | dpp-Gal4d |
|---|---|---|---|---|
| DeltaWT | + | Wild type | Wild type | Wild type |
| DeltaΔICD | + | DN | LOF | DN |
| DeltaStu | − | LOF | LOF | LOF |
| DeltaNae | − | LOF | LOF | LOF |
| DeltaC288Y | − | LOF | LOF | LOF |
| DeltaC301Y | ± | LOF | LOF | LOF |
| DeltaNG2 | + | LOF | LOF | LOF |
| DeltaNG3 | + | DN | LOF | DN |
Based on ability to support aggregation with Notch cells in S2 cell aggregation assays (see Table 1).
Wild-type variants (activated Notch signaling) cause vein loss; loss-of-function (LOF) variants have no effect; variants with dominant-negative (DN) effects (loss of Notch signaling) cause vein thickening.
Wild-type variants cause bristle shaft-to-socket transformations; LOF variants have no effect.
Wild-type variants cause ectopic wing margins, deformed wings and legs, and extra bristles; LOF variants have no effect; DN variants show thickening of wing vein 3 and severe wing notching.
RESULTS
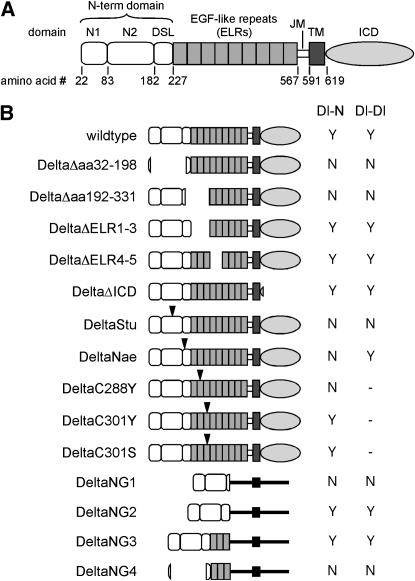
The extracellular domain of the Delta protein (DeltaECD), like that of Serrate, consists of an N-terminal segment that includes the DSL domain (Tax et al. 1994; Fleming 1998), named after several Notch ligands that share extensive homology, followed by a tandem array of a motif related to epidermal growth factor (i.e., EGF-like repeats or “ELRs”), and a juxtamembrane segment (JM). DeltaECD sequences are followed by a single transmembrane domain (TM) and a novel intracellular domain (DeltaICD; Figure 1A).
Figure 1.—
Delta and Delta constructs. (A) Delta protein schematic. The N terminus is to the left. Delta domains include: signal peptide (not shown) from aa 6 to ∼21 (see Figure 2 legend); N1 from aa 22 to 82; N2 from aa 83 to 181; DSL from aa 182 to 226; nine ELRs from aa 227 to 566; JM from aa 567 to 590; TM from aa 591 to 618; and ICD from aa 619 to 833. (B) Delta constructs used in this report. See materials and methods for detailed descriptions. Arrowheads indicate sites of insertions or missense mutations. The “Dl-N” column indicates whether the DeltaVariant can (Y) or cannot (N) mediate interactions between Delta- and Notch-expressing S2 cells (see also Table 1). The “Dl-Dl” column indicates whether DeltaVariant can (Y) or cannot (N) mediate interactions among among Delta-expressing S2 cells (see also Table 1), “—” indicates that the variant was not tested.
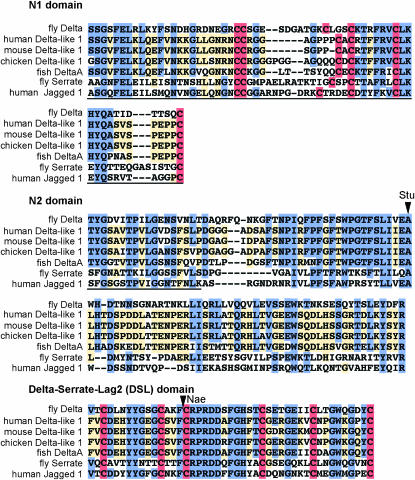
In addition to the homology already noted for the DSL domain (Tax et al. 1994; Fleming 1998; the fly DSL domain is 65% identical and 76% similar to the human Delta-like 1 DSL), there is also significant homology among these ligands in the region N-terminal to the DSL domain (Figure 2). A PFAM domain designated “MNNL” (N-terminal Notch ligand) corresponding to fly Delta aa 22–100 has been identified through analysis of 24 “seed” Notch ligands (Finn et al. 2006; see Figure 2). We find that significant homology among fly and vertebrate Delta, Serrate, and Jagged proteins extends from fly Delta aa 22 to 181, and we have divided this region into two domains on the basis of respective cysteine content. We designate “N1” as the most N-terminal domain, from Delta aa 22 to 82, on the basis of the presence of six cysteine residues that could form three disulfide bonds and locally constrain this segment of the protein. These cysteines are conserved among vertebrate Delta orthologs, as well as in the other Drosophila Notch ligand, Serrate, and its human ortholog, Jagged 1. Fly Delta N1 is 44% identical and 54% similar to the N1 of human Delta-like 1. In between N1 and the DSL domain is a cysteine-free stretch of amino acids from aa 83 to 181 that we designate “N2.” Fly N2 is 45% identical and 60% similar to human Delta-like 1 N2.
Figure 2.—
Alignment of the N-terminal domains of fly and vertebrate DSL proteins. Sequences N-terminal to the EGF-like repeats of each protein were aligned using VectorNTI (Invitrogen, San Diego) and divided into three domains (N1, N2, and DSL) on the basis of cysteine content (see text). Identical residues shared by fly Delta and at least two other DSL proteins are shaded in blue. Identical residues shared by three or more proteins (not including fly Delta) are shaded in yellow. Cysteines are shaded in red. The MNNL domain (aa 22–100) predicted by PFAM is underlined (see text). We have started N1 at fly Delta aa 22, although we note that SignalP predicts cleavage of the signal peptide between aa 22 and 23. Sites of two insertional mutants [DeltaStu (Stu) and DeltaNae (Nae)] used in this study are indicated by arrowheads.
The fly DeltaECD contains 9 ELRs, whereas fly Serrate contains 14 ELRs as well as an additional cysteine-rich domain just N-terminal to the transmembrane domain (Fleming 1998). ELRs have been implicated in intermolecular interactions involving a number of proteins (Appella et al. 1988; Davis 1990), including Notch (Rebay et al. 1991; Fleming 1998).
The importance of the various Delta domains for protein function in vivo has been implied by several previous studies. For example, a cysteine-to-tyrosine substitution within the DSL domain has been correlated with a strong loss-of-function allele of lag2, the C. elegans homolog of Delta (Henderson et al. 1994). In Drosophila Delta, two glycine-to-arginine substitutions, one in ELR 3 and the other in ELR 9, correlate with dominant modifiers of the N mutation split (Lieber et al. 1992), and a missense mutation within ELR 5 of the human Delta homolog Dll3 has been implicated in spondylocostal dysostosis, a group of vertebral malsegmentation syndromes (Bulman et al. 2000). Recently, Mahoney et al. (2006) have described several Dl loss-of-function alleles that contain cysteine missense mutations in ELRs 4, 6, 7, 8, and 9.
The N terminus of the DeltaECD is necessary and sufficient for heterotypic interaction with Notch:
We utilized a cell aggregation assay (Fehon et al. 1990) to define DeltaECD sequences required for interaction with Notch. We previously found that Delta–Notch-dependent aggregation exhibits only a slight dependence on the Delta DNA input level over a 100-fold range in this assay (Klueg and Muskavitch 1999). Given these data and our observations that each of the Delta variants exhibited substantial surface accumulation (data not shown), we infer that the inability of a given Delta variant to support heterotypic aggregation most probably reflects a functional deficit exhibited by that variant.
We assessed a number of Delta deletion mutants to identify sequences necessary for interaction with wild-type Notch. Variants containing deletions of the DeltaICD (DeltaΔICD) or various ELRs (DeltaΔELR1–3 and DeltaΔELR4–5) retain the ability to promote Delta–Notch aggregation (Figure 1B; Table 1), indicating that these domains are not necessary for Delta binding to Notch in cultured cells (see also Sakamoto et al. 2002). In contrast, deletions that eliminate the majority of the Delta N-terminal domain (DeltaΔ32–198) or impinge on the carboxy-terminal (C-terminal) part of this region (DeltaΔ192–331) can no longer support Delta–Notch aggregation (Figure 1B; Table 1), implying that the N-terminal domain of Delta is necessary for binding to Notch.
TABLE 1.
Aggregation mediated by wild-type and variant Delta proteins
| Heterotypic aggregationa
|
Homotypic aggregation: | ||
|---|---|---|---|
| % Notch cells in aggregates | % Delta cells in aggregates | % Delta cells in aggregatesb | |
| Wild type | 26 (11)c | 33 (12)c | 27 (10)c |
| DeltaΔaa32–198 | 0 | 0 | 0 |
| DeltaΔaa192–331 | 0.6 (0.6) | 0.4 (0.4) | 0 |
| DeltaΔELR1–3 | 15 (3)d | 25 (11)d | 26 (14)d |
| DeltaΔELR4–5 | 19 (2) | 17 (2) | 13 (2) |
| DeltaΔICD | 18 (2) | 22 (1) | 18 (3) |
| DeltaStu | 0 | 0 | 0 |
| DeltaNae | 0 | 25 (5) | 27 (7) |
| C288Y | 0e | 0e | ND |
| C301Y | 7 (2)f | 6 (1)f | ND |
| C301S | 12 (5)f | 7 (3)f | ND |
| DeltaNG1 | 0 | 0 | 0 |
| DeltaNG2 | 23 (6) | 13 (1) | 4 (1)d |
| DeltaNG3 | 13 (1) | 16 (1) | 27 (17) |
| DeltaNG4 | 0 | 0 | 0.5 (0.3) |
ND, not determined.
Mean percentage of Delta or Notch cells in aggregates of four or more cells. Standard error is indicated in parentheses. N = 3 replicates unless otherwise noted.
Mean percentage of Delta cells in aggregates of four or more cells. Standard error is indicated in parentheses. N = 3 replicates unless otherwise noted.
N = 5 replicates.
N = 4 replicates.
Experiments carried out independently with DeltaWT. Averages for DeltaWT: 20% (4) of Delta cells and 17% (4) of Notch cells were in aggregates.
Experiments carried out independently with DeltaWT. Averages for DeltaWT: 17% (3) of Delta cells and 19% (2) of Notch cells were in aggregates.
We then investigated the effects of disrupting various Delta domains with short, in-frame linker insertions or missense mutations. Insertion of the tetrapeptide KIFR between R198 and P199 results in a protein unable to bind Notch (DeltaNae; Figure 1B; Table 1). This insertion lies within the DSL domain and its impact implies that integrity of the DSL domain is necessary for Delta–Notch binding. Interestingly, replacement of the highly conserved Delta residue A132 with the pentapeptide GKIFP (DeltaStu) also results in loss of Notch binding (Figure 1B; Table 1). This insertion lies N-terminal to the DSL domain in the domain that we designate “N2” (see above), strongly suggesting that the N2 domain is also required for Delta–Notch binding.
To determine whether the Delta sequences identified above as necessary for Delta–Notch binding are also sufficient for binding, we generated and assayed chimeric proteins in which different portions of Delta are fused to a segment of Drosophila Neuroglian (Figure 1B; Table 1; Bieber et al. 1989). Given that Drosophila Neuroglian is a homotypic adhesion molecule (Hortsch et al. 1995), we utilized a segment of the protein that is not sufficient for Neuroglian self-association in aggregation assays (Hortsch et al. 1995, 1998). We find that a segment of the Delta N terminus comprised of the first 230 amino acids (DeltaNG2) is sufficient to mediate interactions with Notch. Removal of most of the DSL domain from this construct (DeltaNG1) eliminates the ability of this chimera to bind to Notch. This requirement for the Delta N-terminal domain is confirmed by a comparison of DeltaNG3 and NG4 chimeras. The ability of DeltaNG3 (which contains a complete N-terminal domain) to bind Notch is abolished by removal of amino acids 32–198 (DeltaNG4).
These data suggest that the N-terminal domain of Delta is necessary and sufficient for binding to Notch. However, we have other evidence implying that the integrity of sequences outside this domain can impinge on the ability of Delta to bind to Notch. A cysteine missense mutation in ELR 2 (DeltaC288Y) of full-length Delta completely eliminates Delta–Notch binding in this assay. In addition, missense mutations in ELR 3 (DeltaC301Y and DeltaC301S) diminish, but do not abolish, the ability of Delta to bind to Notch (Figure 1B; Table 1). These data suggest that the integrity of ELR 2 is required for Delta–Notch binding and that the integrity of ELR 3, while not required, can affect the ability of Delta to bind to Notch. The fact that a point mutation in ELR 3 diminishes Notch binding, while deletion of ELR 3 has no detectable effects, highlights a caveat associated with generating protein structure–function inferences based solely on the analysis of deletion constructs.
The N terminus of the DeltaECD is necessary and sufficient for homotypic interactions:
Previous aggregation studies revealed that Delta is capable of participating in homotypic interactions (Fehon et al. 1990). Analysis of the same set of Delta deletion and insertion variants and Delta-neuroglian chimeras for sequences required for Delta–Delta interactions yielded results almost identical to those for the Delta–Notch interaction (Figure 1B; Table 1). Deletion mutants reveal that the DeltaICD and ELRs 1–5 are not necessary for Delta–Delta binding (i.e., DeltaΔICD, DeltaΔELR1–3, DeltaΔELR4–5). In contrast, N-terminal deletions (DeltaΔaa32–198 and DeltaΔaa192–331) eliminate the ability of Delta to aggregate homotypically, implying that the N-terminal domain of Delta is also necessary for Delta–Delta binding. This is further substantiated by the loss of Delta–Delta binding exhibited by the DeltaStu variant. Analysis of Delta-neuroglian chimeras indicates that the N-terminal domain not only is necessary but also is sufficient for Delta homotypic interactions (i.e., DeltaNG1, 2, 3, and 4). Interestingly, a linker insertion into the DSL domain (DeltaNae) that eliminates Delta–Notch binding does not affect the ability of Delta to interact homotypically. This indicates that Delta–Notch binding is structurally distinguishable from Delta–Delta binding.
Structural requirements for Delta subcellular trafficking:
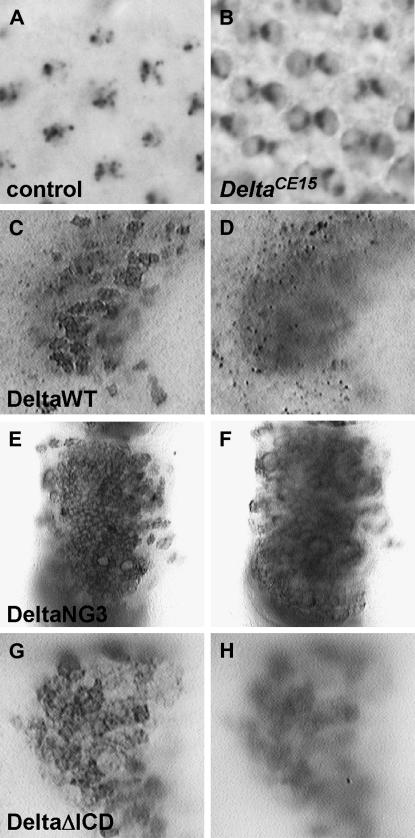
In the retina, the Delta protein is exclusively detected in endocytic vesicles during most developmental stages (Figure 3A; Parks et al. 1995). We have previously shown that three loss-of-function alleles (DlRF, DlCE9, and DlBE21) encode proteins that accumulate to high levels on cell surfaces (Parks et al. 1995, 2000). To further understand the relationship between Delta function and structure, particularly within the context of Delta trafficking, we used the developing retina to screen 117 heterozygous Dl mutant stocks for subcellular mislocalization. Pupae heterozygous for a given Dl mutation were aged for 48 hr after puparium formation (APF) at 18°, and pupal retinas were stained for Delta protein. A total of 18 trafficking-defective DlTD alleles, which encode proteins that accumulate aberrantly on retinal cell surfaces, were identified in this screen (e.g., Figure 3B). All 18 alleles are loss-of-function mutations, ranging from severe to relatively mild in character (Alton et al. 1989; Parks and Muskavitch 1993; data not shown).
Figure 3.—
Subcellular localization of DeltaTD proteins. (A and B) Endogenous Delta is found in endocytic vesicles within cone cells in a wild-type 24-hr APF retina (A). In contrast, Delta encoded by the DlCE15 allele localizes to cone cell surfaces in a 24-hr APF DlCE15//TM6C retina (B). (C–H) UAS-DeltaVariants were driven within the anterior–posterior (A–P) boundary of third instar larval wing discs by dpp-Gal4. (C and D) DeltaWT under the control of dpp-Gal4 localizes to cell surfaces (C) in higher focal planes and vesicles (D) in lower focal planes. (E and F). DeltaNG3 under the control of dpp-Gal4 localizes to cell surfaces (E) in higher focal planes. Few or no vesicles are seen in lower focal planes (F). (G and H) DeltaΔICD under the control of dpp-Gal4 has increased accumulation on cell surfaces (G) in higher focal planes. Although not evident in this micrograph (H), vesicles can be seen in lower focal planes in some preparations.
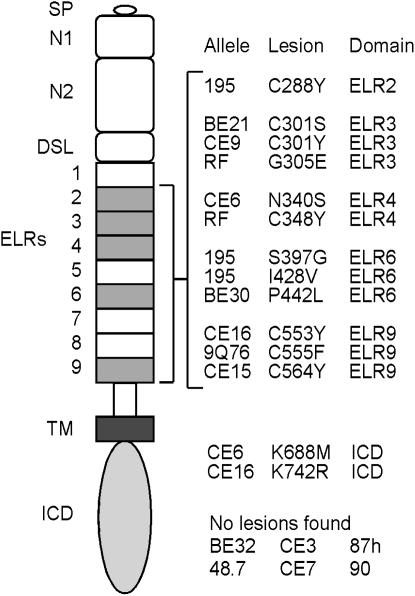
The sequence of the sixth exon of Delta codes for >70% of the protein, including eight of the nine ELRs, the transmembrane domain, and the entire intracellular domain. Sequence analysis of the sixth exon for 15 DlTD alleles and their six respective wild-type parental alleles reveals that nine alleles carry a total of 14 point mutations that result in amino acid substitutions (Figure 4). No mutations were found for six DlTD alleles, suggesting that these defects are caused by mutations elsewhere in the Delta coding sequence.
Figure 4.—
Schematic of missense mutations associated with the DlTD alleles. Domains are indicated (see text). Shading indicates ELRs that contain mutations. Locations of amino acid residue changes found within DlTD alleles are listed in columns to the right of the Delta schematic. No amino acid changes were detected in the six DlTD alleles listed at the bottom. This implies that the lesions associated with these alleles map within the first five exons of the Dl gene. SP, signal peptide; see Figure 1 legend for other abbreviations.
Of the 14 correlated mutations, 12 map within the ELRs of the DeltaECD (Figure 4). Only 2 correlated mutations were identified within the DeltaICD, each found within alleles that also carry DeltaECD substitutions. Intriguingly, 7 of the 12 trafficking-defective mutations within Delta ELRs are cysteine missense mutations, 5 of which are cysteine-to-tyrosine substitutions. One allele (Dl195) carries a cysteine substitution in ELR 2, and another allele (DlRF) contains a substitution in ELR 4. The alleles DlCE9 and DlBE21 each carry substitutions within ELR 3 (at the same residue), and cysteine substitutions within ELR 9 are found within the alleles DlCE16, Dl9Q76, and DlCE15.
We previously reported that dpp-Gal4-driven DeltaWT appears on cell surfaces and in numerous vesicles along the compartment boundary in third instar wing discs (Figure 3, C and D; Parks et al. 2000). We have also shown that the lesion associated with DlCE9 (C301Y) causes dpp-Gal4-driven Delta to be sequestered primarily on cell surfaces in this same region (Parks et al. 2000). From these data, we concluded that a single point mutation in the DeltaECD can result in severe trafficking defects and is probably causative for the aberrant cell-surface accumulation that we detect for the endogenously expressed Delta trafficking-defective protein. In addition to DeltaC301Y, both DeltaNG2 and DeltaNG3 proteins are sequestered primarily, if not exclusively, on cell surfaces when driven by dpp-Gal4 (data not shown; Figure 3, E and F). These data further suggest that the DeltaECD plays a role in governing trafficking and that additional Delta sequences C-terminal to ELR 3 are required for proper Delta trafficking. The missense mutations correlated with the DlTD alleles suggest that several of the ELRs, in particular ELR 3 and ELR 9, may play important roles in proper Delta trafficking and function.
We and others have shown that the DeltaICD is necessary for Delta signaling and trafficking (Figure 3, G and H; Chitnis et al. 1995; Sun and Artavanis-Tsakonas 1996; Dorsky et al. 1997; Henrique et al. 1997; Huppert et al. 1997; Culi et al. 2001). Interestingly, two DlTD alleles contain lysine missense mutations in the DeltaICD (DlCE6, K688M; DlCE16, K742R; Figure 4). Each allele also includes an ELR mutation (N340S and C553Y, respectively). Both N340S and C553Y have been isolated and tested in a variety of assays. Neither mutation has effects on Delta–Notch aggregation or on Notch trans-endocytosis in cell culture (data not shown). N340S does not affect Delta subcellular localization in our dpp-Gal4 trafficking assay (see above) or Delta signaling in animals as assayed below (data not shown). C553Y does appear to impede Delta trafficking in the dpp-Gal4 assay, but with an expressivity lower than that associated with C301Y; it also affects Delta signaling in some, but not all, contexts (see below). This suggests that the N340S lesion does not contribute significantly to the trafficking and signaling defects associated with DlCE6 in animals, and that C553Y, while responsible for some aspects of these phenotypes, may not account for all the loss-of-function effects associated with DlCE16. The DeltaICD contains 12 lysines that could act as substrates for mono-ubiquitylation, although 3 of these map within a putative stop-transfer sequence. Mono-ubiquitylation of the DeltaICD by Neur or Mib1 is thought to be an important signal for Delta endocytosis (Le Borgne et al. 2005a; Le Borgne 2006). We suggest that K688 and K742 may be ubiquitylated, either sequentially or simultaneously, and function as signals that regulate Delta endocytosis. These two DeltaICD mutations are the first to shed light on possible requirements for specific residues within the DeltaICD for trafficking and function.
Structural requirements for Delta signaling:
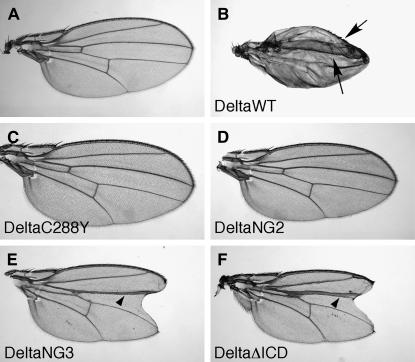
Ectopic Delta expression can activate ectopic Notch signaling in several contexts to yield gain-of-function phenotypes (Table 2). In animals raised at 25°, expression of DeltaWT under the control of a wing-blade intervein driver (1348-Gal4) produces vein loss in adult wings (Huppert et al. 1997), expression under the control of dpp-Gal4 yields misshapen wings and ectopic wing margins (Figure 5B; Parks et al. 2000), and expression driven by 31-1-Gal4 results in a variety of effects including notal macrochaeta and leg bristle shaft-to-socket transformations (Jacobsen et al. 1998). It has been previously reported that DeltaC301Y under the control of the 1348-Gal4 and dpp-Gal4 drivers fails to activate ectopic Notch signaling in either context (Parks et al. 2000). We assayed the effects of ectopic expression of DeltaC301Y under the control of the 31-1-Gal4 driver. In addition, several other Delta variants were assayed using all three drivers described above and compared to DeltaWT (Figure 5; Table 2). Each line was also examined for protein expression in larval wing discs of animals heterozygous for the Delta variant and the dpp-Gal4 driver (data not shown), and only lines with expression levels similar to or higher than those observed in UAS-DeltaWT/dpp-Gal4 animals were used for comparison.
Figure 5.—
Overexpression phenotypes of wild-type and mutant forms of Delta. UAS-controlled DeltaVariants were overexpressed along the A–P boundary of the wing using dpp-Gal4. (A) Wild-type wing. (B) Ectopic expression of DeltaWT by dpp-Gal4 results in small misshapen wings and ectopic wing margins (arrows point to margins). (C and D) Ectopic expression of DeltaC288Y (C) or DeltaNG2 (D) has no effect on wing development. (E and F) Ectopic expression of either DeltaNG3 (E) or DeltaΔICD (F) results in thickening of the third wing vein along the A–P border (arrowheads) and wing notching, both of which are Notch loss-of-signaling phenotypes.
DeltaC288Y, DeltaStu, and DeltaNae fail to activate ectopic Notch signaling in all contexts tested (Figure 5C; data not shown) as does DeltaC301Y (Parks et al. 2000). These results are not unexpected because none of these variants, unlike DeltaC301Y, retain the ability to bind Notch. These results imply that proper Delta signaling is therefore dependent on the structural integrity of both ELR 2 and ELR 3, and on the integrity of the N-terminal domain. However, this region is not sufficient to mediate Notch signaling, as shown by the expression of the proteins DeltaNG2 and DeltaNG3. The DeltaNG2 chimera fails to activate ectopic Notch signaling (Figure 5D), and ectopically expressed DeltaNG3 produces Notch pathway loss-of-function phenotypes (Figure 5E), indicating that it dominantly disrupts signaling in some way (see below). These data suggest that additional Delta sequences C-terminal to ELR 3 are also necessary for Delta function in vivo. In addition, although the C553Y lesion supports wild-type levels of Notch binding and trans-endocytosis in cell culture and can activate ectopic Notch signaling when expressed under dpp-Gal4 control (data not shown), preliminary data indicate that it fails to activate ectopic signaling when expressed under 31-1-Gal4 or 1348-Gal4 control (data not shown). This suggests that the C553Y lesion can contribute to DlTD loss-of-function phenotypes in some contexts and is consistent with requirements for C-terminal ELRs in Delta function.
It has been previously demonstrated by us and others that Delta variants lacking the intracellular domain act in a dominant-negative fashion in multiple organisms (Table 2; Figure 5F; Chitnis et al. 1995; Sun and Artavanis-Tsakonas 1996; Dorsky et al. 1997; Henrique et al. 1997; Huppert et al. 1997; Culi et al. 2001). The fact that DeltaNG3 also acts in a dominant-negative fashion (Table 2; Figure 5E) indicates that the Delta N-terminal domain plus the first three ELRs are sufficient for this effect. In contrast, DeltaNG2, which binds Notch comparably to DeltaNG3 (Table 1) and is also missing the DeltaICD, fails to either activate Notch or act in a dominant-negative fashion (Table 2; Figure 5D). This suggests that the dominant negativity exhibited by DeltaNG3 is dependent on ELRs 1–3. When DeltaΔICD is expressed under the control of the heat-shock promoter starting 6 hr APF, the number of microchaetae approximately doubles in comparison to controls (data not shown). This effect is lost when DeltaΔICD proteins also carrying either Nae or Stu insertions (which cannot bind to Notch) are expressed in the same manner (data not shown). This indicates that the ability of DeltaΔICD to cause dominant-negative microchaeta multiplication is lost concomitant with loss of the ability to bind Notch, suggesting that dominant-negative effects of these and similar variants from other species are also dependent on binding to Notch.
DISCUSSION
Requirements for the Delta N-terminal region in Delta–Notch binding and signaling:
Alignment of the N-terminal domains of fly and vertebrate Delta and Serrate/Jagged reveals striking conservation. The sequences sufficient for interaction with Notch (see below) can be grouped into three regions: the DSL domain and two putative domains that we designate “N1” and “N2.” These regions differ in their respective cysteine content. The N1 and DSL domains each contain six cysteines, while N2 contains none. The even number of cysteines within the N1 and DSL domains allows for the possibility that disulfide bonding may occur to generate specific substructures for these domains.
We have utilized cell aggregation assays to define a region within the Drosophila DeltaECD that is both necessary and sufficient for Delta–Notch interactions in cultured cells. Functional analyses of a combination of constructs reveal that the Delta N-terminal region (Delta aa 1–230), which encompasses the N1, N2, and DSL domains, is sufficient for Delta–Notch binding in cultured cells (e.g., DeltaNG2). This implies that these Delta sequences are sufficient to mediate the Delta–Notch binding dependent on ELRs 11 and 12 within the NotchECD (Rebay et al. 1991). However, the loss-of-function character of DeltaNG2 and the dominant-negative character of DeltaNG3, both of which bind to Notch at wild-type levels, imply that simple presentation of this Delta N-terminal region on the cell surface is not sufficient to mediate wild-type Delta–Notch signaling. The failure of DeltaNG3 to undergo endocytosis and to signal is consistent with a variety of findings implying that Delta endocytosis is required for Delta signaling (Le Borgne et al. 2005a; Chitnis 2006; Le Borgne 2006).
A number of lines of evidence imply that N-terminal sequences upstream of the DSL domain are critical for the function of Delta and other DSL family members. In Drosophila, we find that an insertional mutation within the Delta N2 domain (i.e., the DeltaStu variant) eliminates ligand binding to Notch and signaling in vivo. Fleming (1998) has previously reported that aggregation between Serrate- and Notch-expressing cells depends on the Serrate DSL domain and sequences N-terminal to this domain. More recently, Trang et al. (2004) report that a missense mutation in the Serrate N2 domain, R176C, results in a hypomorphic protein. They suggest that this mutation may cause aberrant cysteine pairing, which would be consistent with the existence of cysteine-dependent secondary structure within either the N1 or the DSL domain, or both. In C. elegans, Henderson et al. (1997) have shown that LAG-2 proteins missing either the DSL domain or the region N-terminal to the DSL domain cannot rescue lag-2 mutants, while full-length LAG-2 can. They also found that whereas LAG-2 variants missing the DSL domain were incapable of causing any phenotypes when expressed in a wild-type background, variants missing the region N-terminal to the DSL domain caused dominant-negative phenotypes. Additional evidence for the functional importance of the region N-terminal to the DSL domain comes from human Jagged-1 mutations associated with Alagille syndrome (Warthen et al. 2006 and references therein). These mutations are found throughout the Jagged-1 N-terminal region, and some alter highly conserved amino acids within N1 and N2. The effects of these mutations are largely unknown, although L37S (in the N1 domain) and R184H (in the N2 domain) are thought to cause retention in the ER and loss of glycosylation (Lu et al. 2003), suggesting that the N terminus may be important for Jagged-1 transport through the export pathway. All of these data support the premise that the sequence conservation within domains N2 and N1 reflects structural requirements for DSL family member subcellular trafficking and Delta–Notch interaction.
The broad organization of the Delta N terminus is analogous to that of the extracellular domain of the vertebrate EGF receptor (EGFR) in which sequences believed to interact with EGF (i.e., domain III) are flanked by two cysteine-rich domains (domains II and IV) (Lax et al. 1988). Domains II and IV are thought to interact with each other in the absence of ligand (Ferguson 2004). In the presence of ligand, this interaction is disrupted, and domain II is freed to interact with domain II from another EGFR molecule, which then drives receptor dimerization. It is unknown whether the cysteines within the Delta N1 or DSL domains undergo pair bonding or whether these two specific domains interact in cis or in trans. However, it is tempting to speculate that intramolecular interactions among these cysteines and intermolecular interactions between the N-terminal non-ELR portions of two Delta molecules (i.e., the N2 domain) may play a role in Delta function and Notch signaling.
Structural requirements for, and possible implications of, Delta–Delta binding:
We have previously shown that cultured cells expressing wild-type Delta exhibit homotypic aggregation (Fehon et al. 1990). We demonstrate here that the Delta N-terminal region plus ELRs 1–3 (DeltaNG3) are sufficient for these interactions. In addition, we present data indicating that ELRs 1–5 are dispensable for this interaction (DeltaΔELR1–3 and DeltaΔELR4–5) and that the N-terminal domain alone can support homotypic interactions (DeltaNG2), albeit at greatly reduced levels. The functional relevance of Delta–Delta interactions in trans is currently unknown. Trans interactions between Delta proteins may help to regulate the amount of Delta available for interactions with Notch. Alternatively, Delta and Notch are thought to associate both in cis and in trans (Fehon et al. 1990; de Celis and Bray 1997; Micchelli et al. 1997; Jacobsen et al. 1998; Sakamoto et al. 2002), and the ability of Delta to self-associate in trans may reflect the ability of Delta to form multimers in cis. Multimerization of Delta in cis may help regulate amounts of Delta available for Notch binding or, more excitingly, could be a prerequisite for Notch binding.
The structural requirements for Delta–Notch and Delta–Delta binding in cultured cells differ in only one regard. DeltaNae, which contains an insertion in the DSL domain and cannot bind to Notch, retains the ability to interact with itself. If Delta–Delta interactions can occur in cis, the requirement for an intact DSL domain for Delta–Notch but not for Delta–Delta binding would be consistent with a requirement for Delta multimerization in cis prior to binding to Notch. Any disruption of Delta–Delta binding (e.g., DeltaStu) would be expected to disrupt Delta–Notch interactions; however, disruptions of Delta–Notch binding (e.g., DeltaNae) would not necessarily be expected to alter the ability of Delta to bind to itself.
Requirements for Delta ELRs in Delta–Notch binding, Delta trafficking, Notch trans-endocytosis, and signaling:
We report here the identification of 18 Drosophila Dl alleles that encode trafficking-defective Delta proteins on the basis of an immunohistochemical analysis of Delta subcellular localization in retinal cone cells. We had originally hypothesized that the molecular lesions associated with trafficking-defective phenotypes would localize to the DeltaICD, because loss of the DeltaICD antagonizes Notch signaling (Chitnis et al. 1995; Sun and Artavanis-Tsakonas 1996; Dorsky et al. 1997; Henrique et al. 1997; Huppert et al. 1997; Culi et al. 2001; this report) and causes abnormal accumulation of Delta on the cell surface. Contrary to our prediction, a majority of lesions associated with these trafficking-defective alleles are found within the DeltaECD ELRs.
We find that alteration of a single cysteine within Delta ELR 2 (i.e., C288Y) prevents Delta–Notch binding in cultured cells and impedes signaling in vivo. Phylogenetic analysis fails to support a close relationship among the majority of C. elegans and Drosophila or vertebrate ELRs from DSL family members (Lissemore and Starmer 1999), suggesting that the DSL family ELR array has evolved structurally and functionally. However, this same analysis indicates that ELR 2 is the only ELR that has been conserved among Drosophila and vertebrate DSL family members, suggesting that ELR 2 plays an important role in DSL ligand function and Notch signaling. Our results provide the first experimental evidence demonstrating that ELR 2 is necessary for Delta–Notch binding and signaling in Drosophila and are consistent with results indicating that ELR 1 and 2 of mouse Jagged 1 are required for high-affinity Notch binding by an N-terminal fragment of this DSL family member (Shimizu et al. 1999).
Previously, we reported that Delta variants containing C301Y and C301S mutations exhibit a reduced ability to mediate trans-endocytosis of Notch in cultured cells and that DeltaC301Y is unable to traffic properly in developing wing discs or to mediate signaling in the three contexts examined (Parks et al. 2000; this report). These observations suggest that the integrity of Delta ELR 3 is necessary for endocytosis of Delta, trans-endocytosis of Notch, and Notch signaling. While single cysteine changes in ELR 3 do not completely abolish Delta–Notch binding, the percentage of cells in aggregates is reduced for C301Y and C301S variants, indicating that the integrity of ELR 3 is necessary for wild-type levels of Notch binding. This is reminiscent of observations that a point mutation associated with Dlsup5, a G305N change in Drosophila ELR 3 (originally published as ELR 4), affects ligand–receptor interactions in cultured cells and affects signaling in vivo (Lieber et al. 1992). Delta proteins containing G305N can mediate aggregation with Notch-expressing cells, but cannot compete effectively with cells that express wild-type Delta, suggesting that they bind Notch with lower affinity.
It is possible that Delta ELRs 2 and 3 participate directly in Delta–Notch binding; however, this appears unlikely, given that the DeltaΔELR1–3 protein exhibits wild-type levels of Notch binding. We propose instead that cysteine missense changes in ELRs 2 and 3 result in local disruptions of intrarepeat disulfide bonding, which, in turn, lead to conformational changes elsewhere within the mutated Delta protein that abolish or reduce the ability of full-length Delta to bind to Notch.
The ELR missense mutations in ELRs 4, 6, and 9 correlated with DlTD alleles suggest that ELRs C-terminal to ELR 3 may also be necessary for correct trafficking. The inability of DeltaNG3 to undergo substantial endocytosis when overexpressed in wing discs is consistent with this inference. However, we have also shown that DeltaNG3 can support Notch trans-endocytosis in cultured cells (Parks et al. 2000), suggesting that, at least in tissue culture, the Delta N-terminal region plus ELRs 1–3 is sufficient to support Delta endocytosis and Notch trans-endocytosis when fused with a heterologous transmembrane domain and intracellular domain.
In light of these findings, we suggest three mechanisms by which Delta ELRs could affect Delta endocytosis. Model A: Delta ELRs mediate a conformational change that is transduced to the DeltaICD following Notch binding and affects essential interactions between the DeltaICD and endocytic machinery; altering key ELRs disrupts this transduction. This model seems unlikely as we should have found a greater proportion of DeltaICD lesions associated with the trafficking-defective alleles if this were the case. Model B: The binding of Delta to Notch triggers downstream signaling events, including the endocytosis of Delta–Notch complexes (Parks et al. 2000), and mutations in specific ELRs disrupt binding and therefore endocytosis. This model also appears unlikely, given that DeltaC288Y, DeltaNae, and DeltaStu can undergo endocytosis despite the fact that they cannot bind Notch (data not shown). Model C: ELRs are required for docking with components required for Delta endocytosis that extend into the extracellular compartment. Several components of the endocytic machinery have been implicated in Notch signaling, including dynamin, clathrin, α-adaptin, and epsin (see Introduction). Although most components of the endocytic machinery are cytoplasmic, some components of the clathrin coat, like synaptotagmin, are integral membrane proteins. It is possible that the DeltaECD interacts with transmembrane or extracellular proteins, which act as adaptors or regulators of endocytosis in a manner that depends on the structural integrity of one or more ELRs within the DeltaECD. Elucidation of the mechanism(s) by which ELRs regulate Delta endocytosis and Notch trans-endocytosis will require further experimentation.
Potential roles for DeltaECD glycosylation in Delta signaling:
Of the 13 lesions identified within DeltaECD, 7 are cysteine missense mutations. These mutations are predicted to disrupt disulfide bonding within ELRs. In addition, many of these mutations lie within potential O-fucosylation and O-glucosylation sites (Bruckner et al. 2000; Moloney et al. 2000). ELRs 3, 4, 6, 7, 8, and 9 contain consensus sites for modification by O-fucosylation, and ELR 9 contains a potential site for modification by O-glucosylation, as well as a potential O-glucosylation site that overlaps with an O-fucosylation site. The DlTD lesions in ELRs 3, 4, 6, and 9 all fall within potential O-fucosylation sites. O-fucose glycans are an unusual form of glycosylation associated with EGF-like motifs and have been shown to be necessary for Notch function (reviewed in Haltiwanger and Stanley 2002; Haines and Irvine 2003). Previous analyses have shown that Delta is glycosylated (P. J. Kooh and M. A. T. Muskavitch, unpublished observations), but it remains to be determined experimentally which, if any, of the Delta ELRs are targets for glycosylation in vivo.
Requirements for the DeltaICD in Delta trafficking, Notch trans-endocytosis, and signaling:
Delta proteins that lack the DeltaICD act in a dominant-negative fashion in several contexts, including the eye and wing in Drosophila, as well as in zebrafish and Xenopus (see above). These findings are consistent with the hypothesis that ubiquitylation, endocytosis, and productive receptor binding by DSL family members depend on the ligand intracellular domain. We demonstrate here that the N-terminal domain plus ELRs 1–3 within DeltaNG3 are sufficient to cause analogous dominant-negative effects and that removal of ELRs 1–3 from this construct to yield DeltaNG2 results in loss of dominant negativity, suggesting that these ELRs may contribute to DeltaΔICD dominant-negative effects in Drosophila. In addition, the ability of DeltaΔICD to cause dominant-negative microchaeta multiplication is lost when we introduce mutations that abolish the ability of the construct to bind to Notch, suggesting that dominant-negative effects of DeltaΔICD also depend on binding to Notch in Drosophila.
It is possible that DeltaΔICD binds to either Notch or wild-type Delta and sequesters enough of one or the other to prevent wild-type signaling. This model is supported by data suggesting that the ability to bind to Notch is necessary for dominant-negative effects of DeltaΔICD. However, it seems likely this sequestration model is too simple. Removal of ELRs 1–3 does not affect the ability of DeltaΔ1–3 to bind to Notch, yet abolishes the dominant-negative effects of DeltaNG3. Similarly, the DeltaNae mutation (which allows Delta–Delta interactions, but not Delta–Notch interactions) also abolishes the dominant-negative effects of DeltaΔICD. We favor the hypothesis that the Delta ELRs are necessary for regulating Delta endocytosis via binding to one or more components of the endocytic machinery. If such components are limiting, Delta could bind those components and Notch and effectively sequester Notch in inactive complexes that cannot be endocytosed due to the loss of the DeltaICD as a substrate for ubiquitylation. Release of either the limiting component or Notch would prevent formation of these complexes. This model is consistent with current data and implies that Delta endocytosis must follow Notch binding during the process of Notch activation.
We show here that Delta variants lacking the DeltaICD can still bind to Notch (like DeltaWT), but cannot activate the pathway when overexpressed (unlike DeltaWT), indicating that the DeltaICD is required for normal Delta function. Replacement of the DeltaICD with a ubiquitin moiety restores function (Itoh et al. 2003; Wang and Struhl 2004), suggesting that the primary purpose of the DeltaICD is to provide target sequences for ubiquitylation. The implication that any intracellular domain structure sufficient to undergo ubiquitylation is sufficient for Delta signal generation is consistent with the lack of homology among metazoan DSL family member intracellular domains.
We have found that of the 12 DlTD alleles carrying mutations within exon 6, only 2 have lesions within the DeltaICD. Intriguingly, both of these lesions affect lysine residues (K688M and K742R) that could be targets for ubiquitylation (Haglund et al. 2003). These mutations suggest that these lysine residues, especially K688, may be required for correct Delta trafficking and raise the possibility that Delta ubiquitylation could be required for multiple steps during Notch signaling. Delta ubiquitylation is apparently required for transit through a recycling endosomal compartment prior to signaling (Wang and Struhl 2004; Emery et al. 2005; Jafar-Nejad et al. 2005; Wang and Struhl 2005). In addition, Delta-dependent NotchECD trans-endocytosis has been correlated with Notch signaling (Parks et al. 2000), and this endocytic event could depend on Delta ubiquitylation at a site distinct from that required for Delta transit to the recycling endosome. Delta signaling could depend on sequential ubiquitylation of K688 and K742 for these successive processes. Alternatively, normal Delta-dependent signaling could require simultaneous multi-ubiquitylation (Haglund et al. 2003) of the DeltaICD at two or more sites, including these two residues.
In summary, we have made progress in unraveling the complexities of the relationship between the structure of Delta, arguably the best-understood DSL family member and Notch ligand, and its function in Notch signaling. The DSL domain, additional N-terminal sequences, and ELRs within the DeltaECD are implicated in ligand–receptor binding, endocytosis, and signaling in vivo. Our findings highlight the importance of the Delta DSL domain for receptor binding and signaling, but reveal that sequences N-terminal to the DSL domain are also critical for these ligand functions. We have discovered, quite unexpectedly, that ELRs within the DeltaECD can affect receptor binding and signaling and provide evidence supporting the hypothesis that components of the endocytic machinery that extend into the extracellular compartment contribute to Delta endocytosis and Delta–Notch signaling. Mapping of trafficking-defective allele-associated mutations to the DeltaICD may suggest that multiple Delta ubiquitylation events are required for Delta endocytosis and for activation of the Notch receptor by Delta. Extension of this analysis will provide deeper insights into the structural requirements for Delta function in Notch signaling and the many regulatory mechanisms that modulate Delta–Notch signaling in metazoa.
Acknowledgments
We thank Spyros Artavanis-Tsakonas, Mark Mortin, and William J. Welshons for sharing fly stocks and Allan J. Bieber for supplying neuroglian constructs and antibody. We also thank Marisa Osswalt for helpful edits and advice. This work was supported by grant NP-707A from the American Cancer Society, grant GM33291 from the National Institutes of Health, and funds from the DeLuca Professorship awarded to M.A.T.M., and by postdoctoral fellowship PF-4328 from the American Cancer Society to K.M.K.
References
- Alton, A. K. K., C. C. Fechtel, S. B. Kopczynski, S. B. Shepard, P. J. Kooh et al., 1989. Molecular genetics of Delta, a locus required for ectodermal differentiation in Drosophila. Dev. Genet. 10: 261–272. [DOI] [PubMed] [Google Scholar]
- Appella, E., I. T. Weber and F. Blasi, 1988. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett. 231: 1–4. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas, S., M. D. Rand and R. J. Lake, 1999. Notch signalling: cell fate control and signal integration in development. Science 284: 770–775. [DOI] [PubMed] [Google Scholar]
- Bardin, A. J., and F. Schweisguth, 2006. Bearded family members inhibit neuralized-mediated endocytosis and signaling activity of delta in Drosophila. Dev. Cell 10: 245–255. [DOI] [PubMed] [Google Scholar]
- Baron, M., 2003. An overview of the Notch signalling pathway. Semin. Cell Dev. Biol. 14: 113–119. [DOI] [PubMed] [Google Scholar]
- Bieber, A. J., P. M. Snow, M. Hortsch, N. H. Patel, J. R. Jacobs et al., 1989. Drosophila neuroglian: a member of the immunogobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell 59: 447–460. [DOI] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Bruckner, K., L. Perez, H. Clausen and S. Cohen, 2000. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature 406: 411–415. [DOI] [PubMed] [Google Scholar]
- Bulman, M. P., K. Kusumi, T. M. Frayling, C. McKeown, C. Garrett et al., 2000. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat. Genet. 24: 438–441. [DOI] [PubMed] [Google Scholar]
- Bunch, T. A., Y. Grinblat and L. S. B. Goldstein, 1988. Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids 16: 1043–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadavid, A. L., A. Ginzel and J. A. Fischer, 2000. The function of the Drosophila fat facets deubiquitinating enzyme in limiting photoreceptor cell number is intimately associated with endocytosis. Development 127: 1727–1736. [DOI] [PubMed] [Google Scholar]
- Chitnis, A., 2006. Why is delta endocytosis required for effective activation of notch? Dev. Dyn. 235: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis, A., D. Henrique, J. Lewis, D. Ish-Horowicz and C. Kintner, 1995. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature 375: 761–766. [DOI] [PubMed] [Google Scholar]
- Culi, J., E. Martin-Blanco and J. Modolell, 2001. The EGF receptor and N signalling pathways act antagonistically in Drosophila mesothorax bristle patterning. Development 128: 299–308. [DOI] [PubMed] [Google Scholar]
- Davis, C. G., 1990. The many faces of epidermal growth factor repeats. New Biol. 2: 410–419. [PubMed] [Google Scholar]
- Deblandre, G. A., E. C. Lai and C. Kintner, 2001. Xenopus neuralized is a ubiquitin ligase that interacts with XDelta1 and regulates Notch signaling. Dev. Cell 1: 795–806. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., and S. Bray, 1997. Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development 124: 3241–3251. [DOI] [PubMed] [Google Scholar]
- De Renzis, S., J. Yu, R. Zinzen and E. Wieschaus, 2006. Dorsal-ventral pattern of delta trafficking is established by a snail-tom-neuralized pathway. Dev. Cell 10: 257–264. [DOI] [PubMed] [Google Scholar]
- Diederich, R. J., K. Matsuno, H. Hing and S. Artavanis-Tsakonas, 1994. Cytosolic interaction between deltex and Notch ankyrin repeats implicates deltex in the Notch signalling pathway. Development 120: 473–481. [DOI] [PubMed] [Google Scholar]
- Dorsky, R. I., W. S. Chang, D. H. Rapaport and W. A. Harris, 1997. Regulation of neuronal diversity in the Xenopus retina by Delta signalling. Nature 385: 67–70. [DOI] [PubMed] [Google Scholar]
- Emery, G., A. Hutterer, D. Berdnik, B. Mayer, F. Wirtz-Peitz et al., 2005. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell 122: 763–773. [DOI] [PubMed] [Google Scholar]
- Fehon, R. G., P. J. Kooh, I. Rebay, C. L. Regan, T. Xu et al., 1990. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF homologous genes in Drosophila. Cell 61: 523–534. [DOI] [PubMed] [Google Scholar]
- Ferguson, K. M., 2004. Active and inactive conformations of the epidermal growth factor receptor. Biochem. Soc. Trans. 32: 742–745. [DOI] [PubMed] [Google Scholar]
- Finn, R. D., J. Mistry, B. Schuster-Bockler, S. Griffiths-Jones, V. Hollich et al., 2006. Pfam: clans, web tools and services. Nucleic Acids Res. 34: D247–D251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming, R. J., 1998. Structural conservation of Notch receptors and ligands. Semin. Cell Dev. Biol. 9: 599–607. [DOI] [PubMed] [Google Scholar]
- Greenwald, I., 1998. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 124: 1751–1762. [DOI] [PubMed] [Google Scholar]
- Gridley, T., 2003. Notch signaling and inherited disease syndromes. Hum. Mol. Genet. 12 (Spec No. 1): R9–R13. [DOI] [PubMed] [Google Scholar]
- Haglund, K., P. P. Di Fiore and I. Dikic, 2003. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem. Sci. 28: 598–603. [DOI] [PubMed] [Google Scholar]
- Haines, N., and K. D. Irvine, 2003. Glycosylation regulates Notch signalling. Nat. Rev. Mol. Cell Biol. 4: 786–797. [DOI] [PubMed] [Google Scholar]
- Haltiwanger, R. S., and P. Stanley, 2002. Modulation of receptor signaling by glycosylation: fringe is an O-fucose-beta1,3-N-acetylglucosaminyltransferase. Biochim. Biophys. Acta 1573: 328–335. [DOI] [PubMed] [Google Scholar]
- Henderson, S. T., D. Gao, E. J. Lambie and J. Kimble, 1994. lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C.elegans. Development 120: 2913–2924. [DOI] [PubMed] [Google Scholar]
- Henderson, S. T., D. Gao, S. Christenson and J. Kimble, 1997. Functional domains of LAG-2, a putative signaling ligand for LIN-12 and GLP-1 receptors in Caenorhaditis elegans. Mol. Biol. Cell 8: 1751–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique, D., E. Hirsinger, J. Adam, I. Le Roux, O. Pourquie et al., 1997. Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr. Biol. 7: 661–670. [DOI] [PubMed] [Google Scholar]
- Hortsch, M., A. J. Bieber, N. H. Patel and C. S. Goodman, 1990. Differential splicing generates a nervous system-specific form of Drosophila neuroglian. Neuron 4: 697–709. [DOI] [PubMed] [Google Scholar]
- Hortsch, M., Y. E. Wang, Y. Marikar and A. J. Bieber, 1995. The cytoplasmic domain of the Drosophila cell adhesion molecule neuroglian is not essential for its homophilic adhesive properties in S2 cells. J. Cell Biol. 270: 18809–18817. [DOI] [PubMed] [Google Scholar]
- Hortsch, M., D. Homer, J. D. Malhotra, S. Chang, J. Frankel et al., 1998. Structural requirements for outside-in and inside-out signaling by Drosophila neuroglian, a member of the L1 family of cell adhesion molecules. J. Cell Biol. 142: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert, S. S., T. L. Jacobsen and M. A. T. Muskavitch, 1997. Feedback regulation is central to Delta-Notch signalling required for Drosophila wing vein morphogenesis. Development 124: 3283–3291. [DOI] [PubMed] [Google Scholar]
- Itoh, M., C. H. Kim, G. Palardy, T. Oda, Y. J. Jiang et al., 2003. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 4: 67–82. [DOI] [PubMed] [Google Scholar]
- Jacobsen, T. L., K. Brennan, A. M. Arias and M. A. T. Muskavitch, 1998. Cis-interactions between Delta and Notch modulate neurogenic signalling in Drosophila. Development 125: 4531–4540. [DOI] [PubMed] [Google Scholar]
- Jafar-Nejad, H., H. K. Andrews, M. Acar, V. Bayat, F. Wirtz-Peitz et al., 2005. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev. Cell 9: 351–363. [DOI] [PubMed] [Google Scholar]
- Kadesch, T., 2004. Notch signaling: the demise of elegant simplicity. Curr. Opin. Genet. Dev. 14: 506–512. [DOI] [PubMed] [Google Scholar]
- Klueg, K. M., and M. A. T. Muskavitch, 1998. Ligand-receptor interactions and trans-endocytosis of Delta, Serrate and Notch, members of the Notch signalling pathway in Drosophila. Mol. Biol. Cell 9: 198A. [DOI] [PubMed] [Google Scholar]
- Klueg, K. M., and M. A. T. Muskavitch, 1999. Ligand-receptor interactions and trans-endocytosis of Delta, Serrate and Notch: members of the Notch signalling pathway in Drosophila. J. Cell Sci. 112: 3289–3297. [DOI] [PubMed] [Google Scholar]
- Kooh, P. J., R. G. Fehon and M. A. Muskavitch, 1993. Implications of dynamic patterns of Delta and Notch expression for cellular interactions during Drosophila development. Development 117: 493–507. [DOI] [PubMed] [Google Scholar]
- Kopczynski, C. C., A. K. Alton, K. Fechtel, P. J. Kooh and M. A. Muskavitch, 1988. Delta, a Drosophila neurogenic gene, is transcriptionally complex and encodes a protein related to blood coagulation factors and epidermal growth factors of vertebrates. Genes Dev. 2: 1723–1735. [DOI] [PubMed] [Google Scholar]
- Krämer, H., and M. Phistry, 1996. Mutations in the Drosophila hook gene inhibit endocytosis of the Boss transmembrane ligand into multivesicular bodies. J. Cell Biol. 133: 1205–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer, H., and M. Phistry, 1999. Genetic analysis of hook, a gene required for endocytic trafficking in Drosophila. Genetics 151: 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, E. C., G. A. Deblandre, C. Kintner and G. M. Rubin, 2001. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev. Cell 1: 783–794. [DOI] [PubMed] [Google Scholar]
- Lai, E. C., F. Roegiers, X. Qin, Y. N. Jan and G. M. Rubin, 2005. The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development 132: 2319–2332. [DOI] [PubMed] [Google Scholar]
- Lax, I., W. H. Burgess, F. Bellot, A. Ullrich, J. Schlessinger et al., 1988. Localization of a major receptor-binding domain for epidermal growth factor by affinity labeling. Mol. Cell. Biol. 8: 1831–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne, R., 2006. Regulation of Notch signalling by endocytosis and endosomal sorting. Curr. Opin. Cell Biol. 18: 213–222. [DOI] [PubMed] [Google Scholar]
- Le Borgne, R., A. Bardin and F. Schweisguth, 2005. a The roles of receptor and ligand endocytosis in regulating Notch signaling. Development 132: 1751–1762. [DOI] [PubMed] [Google Scholar]
- Le Borgne, R., S. Remaud, S. Hamel and F. Schweisguth, 2005. b Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol. 3: e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber, T., C. Wesley, E. Alcamo, B. Hassel, J. Krane et al., 1992. Single amino acid substitutions in EGF-like elements of Notch and Delta modify Drosophila development and affect cell adhesion in vitro. Neuron 9: 847–859. [DOI] [PubMed] [Google Scholar]
- Lissemore, J. L., and W. T. Starmer, 1999. Phylogenetic analysis of vertebrate and invertebrate Delta/Serrate/LAG-2 (DSL) proteins. Mol. Phylogenet. Evol. 11: 308–319. [DOI] [PubMed] [Google Scholar]
- Lu, F., J. J. Morrissette and N. B. Spinner, 2003. Conditional JAG1 mutation shows the developing heart is more sensitive than developing liver to JAG1 dosage. Am. J. Hum. Genet. 72: 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney, M. B., A. L. Parks, D. A. Ruddy, S. Y. Tiong, H. Esengil et al., 2006. Presenilin-based genetic screens in Drosophila melanogaster identify novel Notch pathway modifiers. Genetics 172: 2309–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli, C. A., E. J. Rulifson and S. S. Blair, 1997. The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development 124: 1485–1495. [DOI] [PubMed] [Google Scholar]
- Moloney, D. J., V. M. Panin, S. H. Johnston, J. Chen, L. Shao et al., 2000. Fringe is a glycosyltransferase that modifies Notch. Nature 406: 369–375. [DOI] [PubMed] [Google Scholar]
- Overstreet, E., E. Fitch and J. A. Fischer, 2004. Fat facets and liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development 131: 5355–5366. [DOI] [PubMed] [Google Scholar]
- Parks, A. L., and M. A. T. Muskavitch, 1993. Delta function is required for bristle organ determination and morphogenesis in Drosophila. Dev. Biol. 157: 484–496. [DOI] [PubMed] [Google Scholar]
- Parks, A. L., F. R. Turner and M. A. T. Muskavitch, 1995. Relationships between complex Delta expression and the specification of retinal cell fates during Drosophila eye development. Mech. Dev. 50: 201–216. [DOI] [PubMed] [Google Scholar]
- Parks, A. L., S. S. Huppert and M. A. T. Muskavitch, 1997. The dynamics of neurogenic signalling underlying bristle development in Drosophila melanogaster. Mech. Dev. 63: 61–74. [DOI] [PubMed] [Google Scholar]
- Parks, A. L., K. M. Klueg, J. R. Stout and M. A. Muskavitch, 2000. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development 127: 1373–1385. [DOI] [PubMed] [Google Scholar]
- Parody, T. R., and M. A. T. Muskavitch, 1993. The pleiotropic function of Delta during postembryonic development of Drosophila melanogaster. Genetics 135: 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlopoulos, E., C. Pitsouli, K. M. Klueg, M. A. Muskavitch, N. K. Moschonas et al., 2001. Neuralized encodes a peripheral membrane protein involved in Delta signaling and endocytosis. Dev. Cell 1: 807–816. [DOI] [PubMed] [Google Scholar]
- Poodry, C. A., 1990. shibire, a neurogenic mutant of Drosophila. Dev. Biol. 138: 464–472. [DOI] [PubMed] [Google Scholar]
- Portin, P., 2002. General outlines of the molecular genetics of the Notch signalling pathway in Drosophila melanogaster: a review. Hereditas 136: 89–96. [DOI] [PubMed] [Google Scholar]
- Poulson, D. F., 1937. Chromosomal deficiencies and the embryonic development of Drosophila melanogaster. Proc. Natl. Acad. Sci.USA 23: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebay, I., R. J. Fleming, R. G. Fehon, L. F. Cherbas, P. T. Cherbas et al., 1991. Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell 67: 687–699. [DOI] [PubMed] [Google Scholar]
- Sakamoto, K., O. O'Hara, M. Takagi, S. Takeda and K. Katsube, 2002. Intracellular cell-autonomous association of Notch and its ligands: a novel mechanism of Notch signal modification. Dev. Biol. 241: 313–326. [DOI] [PubMed] [Google Scholar]
- Schneider, I., 1972. Cell lines derived from late embryonic stages of Drosophila melanogaster. J. Embryol. Exp. Morphol. 27: 353–365. [PubMed] [Google Scholar]
- Schweisguth, F., 2004. Notch signaling activity. Curr. Biol. 14: R129–R138. [PubMed] [Google Scholar]
- Seugnet, L., P. Simpson and M. Haenlin, 1997. a Requirement for dynamin during Notch signalling in Drosophila neurogenesis. Dev. Biol. 192: 585–598. [DOI] [PubMed] [Google Scholar]
- Seugnet, L., P. Simpson and M. Haenlin, 1997. b Transcriptional regulation of Notch and Delta: requirement for neuroblast segregation in Drosophila. Development 124: 2015–2025. [DOI] [PubMed] [Google Scholar]
- Shimizu, K., S. Chiba, K. Kumano, N. Hosoya, T. Takahashi et al., 1999. Mouse jagged1 physically interacts with notch2 and other notch receptors. Assessment by quantitative methods. J. Biol. Chem. 274: 32961–32969. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton, K., P. D. Jackson, M. J. Clark, A. H. Brand and F. M. Hoffmann, 1994. Specificity of bone morphogenetic protein-related factors: cell fate and gene expression changes in Drosophila embryos induced by decapentaplegic but not 60A. Cell Growth Differ. 5: 585–593. [PubMed] [Google Scholar]
- Sun, X., and S. Artavanis-Tsakonas, 1996. The intracellular deletions of DELTA and SERRATE define dominant negative forms of the Drosophila Notch ligands. Development 122: 2465–2474. [DOI] [PubMed] [Google Scholar]
- Tax, F., J. J. Yeargers and J. H. Thomas, 1994. Sequence of C. elegans lag-2 reveals a cell-signalling domain shared with Delta and Serrate of Drosophila. Nature 368: 150–154. [DOI] [PubMed] [Google Scholar]
- Tearle, R. G., and C. Nusslein-Volhard, 1987. Tubingen mutants and stock list. Dros. Inf. Serv. 66: 209–269.
- Tian, X., D. Hansen, T. Schedl and J. B. Skeath, 2004. Epsin potentiates Notch pathway activity in Drosophila and C. elegans. Development 131: 5807–5815. [DOI] [PubMed] [Google Scholar]
- Trang, T. T., V. Tannous, Y. Gu, J. Mosher and R. J. Fleming, 2004. The Ser(+r83k) mutation is a second site mutation of SerD affecting the N-terminus of serrate. Genesis 39: 42–51. [DOI] [PubMed] [Google Scholar]
- Wang, W., and G. Struhl, 2004. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development 131: 5367–5380. [DOI] [PubMed] [Google Scholar]
- Wang, W., and G. Struhl, 2005. Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development 132: 2883–2894. [DOI] [PubMed] [Google Scholar]
- Warthen, D. M., E. C. Moore, B. M. Kamath, J. J. Morrissette, P. Sanchez et al., 2006. Jagged1 (JAG1) mutations in Alagille syndrome: increasing the mutation detection rate. Hum. Mutat. 27: 436–443. [DOI] [PubMed] [Google Scholar]
- Weng, A. P., A. A. Ferrando, W. Lee, J. P. Morris, IV, L. B. Silverman et al., 2004. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306: 269–271. [DOI] [PubMed] [Google Scholar]
- Xu, T., I. Rebay, R. J. Fleming, T. N. Scottdale and S. Artavanis-Tsakonas, 1990. The Notch locus and the genetic circuitry involved in early Drosophila neurogenesis. Genes Dev. 4: 464–475. [DOI] [PubMed] [Google Scholar]