Abstract
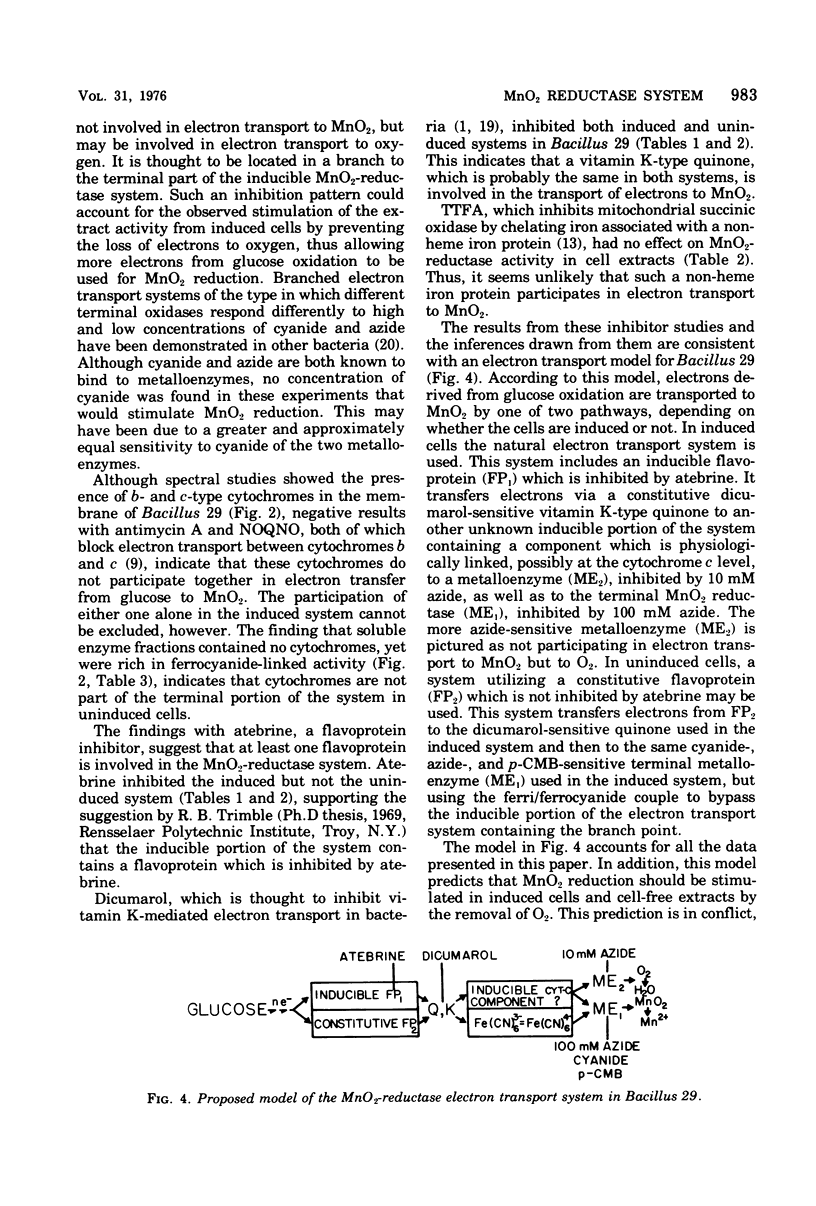
The response of MnO2 reduction by uninduced and induced whole cells and cell extracts of Bacillus 29 to several electron transport inhibitors was compared. MnO2 reduction with glucose by uninduced whole cells and cell extracts was strongly inhibited at 0.1 mM dicumarol, 100 mM azide, and 8 mM cyanide but not by atebrine or carbon monoxide, suggesting the involvement of a vitamin K--type quinone and a metalloenzyme in the electron transport chain. MnO2 reduction with ferrocyanide by uninduced cell extracts was inhibited by 5 mM cyanide and 100 mM azide but not by atebrine, dicumarol, or carbon monoxide, suggesting that the metalloenzyme was associated with the terminal oxidase activity. MnO2 reduction with glucose by induced whole cells and cell extracts, was inhibited by 1 mM atebrine, 0.1 mM dicumarol, and 10 mM cyanide but not by antimycin A, 2n-nonyl-4-hydroxyguinoline-N-oxide) (NOQNO), 4,4,4-trifluoro-1-(2-thienyl),1,3-butanedione, or carbon monoxide. Induced cell extract was also inhibited by 100 mM azide, but stimulated by 1 mM and 10 mM azide. Induced whole cells were stimulated by 10 mM and 100 mM azide. These results suggested that electron transport from glucose to MnO2 in induced cells involved such components as flavoprotein, a vitamin K-type quinone, and metalloenzyme. The stimulatory effect of azide on induced cells was explained on the basis of a branching in the terminal part of the electron transport chain, one branch involving a metalloenzyme for the reduction of MnO2 and the other involving a metalloenzyme for the reduction of oxygen. The latter was assumed to be the more azide sensitive. Spectral studies showed the presence of a-, b-, and c-type cytochromes in membrane but not in soluble fractions. Of these cytochromes, only the c type may be involved in electron transport of MnO2, owing to the lack of inhibition by antimycin A or 2n-nonyl-4-hydroxyquinoline-N-oxide. The terminal MnO2 reductase appears to be loosely attached to the cell membrane of Bacillus 29 because of cell fractionation it is found associated with both particulate and soluble fractions. Electron photomicrographs of bacilli attached to synthetic Fe-Mn oxide revealed an intimate contact of the cell walls with the oxide particles.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASANO A., BRODIE A. F. OXIDATIVE PHOSPHORYLATION IN FRACTIONATED BACTERIAL SYSTEMS. XIV. RESPIRATORY CHAINS OF MYCOBACTERIUM PHLEI. J Biol Chem. 1964 Dec;239:4280–4291. [PubMed] [Google Scholar]
- Ehrlich H. L. Bacteriology of Manganese Nodules: I. Bacterial Action on Manganese in Nodule Enrichments. Appl Microbiol. 1963 Jan;11(1):15–19. doi: 10.1128/am.11.1.15-19.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. L. Bacteriology of manganese nodules. V. Effect of hydrostatic pressure on bacterial oxidation of MnII and reduction of MnO2. Appl Microbiol. 1971 Feb;21(2):306–310. [PMC free article] [PubMed] [Google Scholar]
- Ghiorse W. C., Ehrlich H. L. Effects of seawater cations and temperature on manganese dioxide-reductase activity in a marine Bacillus. Appl Microbiol. 1974 Nov;28(5):785–792. doi: 10.1128/am.28.5.785-792.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Reaveley D. A., Rogers H. J. Some enzymic activities and chemical properties of the mesosomes and cytoplasmic membranes of Bacillus licheniformis 6346. Biochem J. 1969 Jun;113(1):67–79. doi: 10.1042/bj1130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Ehrlich H. L. Bacteriology of manganese nodules. IV. Induction of an MnO2-reductase system in a marine bacillus. Appl Microbiol. 1970 Jun;19(6):966–972. doi: 10.1128/am.19.6.966-972.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Ehrlich H. L. Bacteriology of manganese nodules: III. Reduction of MnO(2) by two strains of nodule bacteria. Appl Microbiol. 1968 May;16(5):695–702. doi: 10.1128/am.16.5.695-702.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
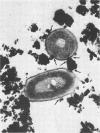
- White D. C., Sinclair P. R. Branched electron-transport systems in bacteria. Adv Microb Physiol. 1971;5:173–211. doi: 10.1016/s0065-2911(08)60407-5. [DOI] [PubMed] [Google Scholar]