Abstract
Natural hybridization and backcrossing between Aegilops cylindrica and Triticum aestivum can lead to introgression of wheat DNA into the wild species. Hybrids between Ae. cylindrica and wheat lines bearing herbicide resistance (bar), reporter (gus), fungal disease resistance (kp4), and increased insect tolerance (gna) transgenes were produced by pollination of emasculated Ae. cylindrica plants. F1 hybrids were backcrossed to Ae. cylindrica under open-pollination conditions, and first backcrosses were selfed using pollen bags. Female fertility of F1 ranged from 0.03 to 0.6%. Eighteen percent of the sown BC1s germinated and flowered. Chromosome numbers ranged from 30 to 84 and several of the plants bore wheat-specific sequence-characterized amplified regions (SCARs) and the bar gene. Self fertility in two BC1 plants was 0.16 and 5.21%, and the others were completely self-sterile. Among 19 BC1S1 individuals one plant was transgenic, had 43 chromosomes, contained the bar gene, and survived glufosinate treatments. The other BC1S1 plants had between 28 and 31 chromosomes, and several of them carried SCARs specific to wheat A and D genomes. Fertility of these plants was higher under open-pollination conditions than by selfing and did not necessarily correlate with even or euploid chromosome number. Some individuals having supernumerary wheat chromosomes recovered full fertility.
AEGILOPS cylindrica Host is a widespread Mediterranean, western Asiatic, and even circumboreal element, present as an adventive in northern Italy, France, Switzerland, and other countries of western, northern, and eastern Europe (Van Slageren 1994). Probably introduced in the United States at the end of the 19th century, Ae. cylindrica is now widespread there and is considered as a troublesome weed in wheat fields (Donald and Ogg 1991; Van Slageren 1994). Indeed, Ae. cylindrica is one of the species in the genus showing the most pronounced tendency to weediness (Van Slageren 1994). Increased weediness due to transgene introgression into wild populations of Ae. cylindrica, for example in the case of herbicide resistance genes, is a major concern about the cultivation of genetically modified wheat (Hegde and Waines 2004).
Allotetraploid Ae. cylindrica (2n = 4x = 28, DDCC genome) and allohexaploid bread wheat (Triticum aestivum L., 2n = 6x = 42, BBAADD genome) (Waines and Barnhart 1992) form natural hybrids where they grow in sympatry (Rajhathy 1960; Van Slageren 1994; Morrison et al. 2002a). Morphologically, F1 hybrids are completely uniform and appear to be intermediate between the parental species, although some parental characters, like glume stiffness and hairiness, are dominantly expressed in the hybrids (Belea 1968). This uniformity contrasts with the morphological variation of the offspring of the hybrids [backcross (BC)1] for spike shape, leaf shape, and growth habit, characters that cannot be used to identify the pollen parent (Snyder et al. 2000).
Wheat and Ae. cylindrica share the D genome (Kimber and Zhao 1983), issued from their common diploid ancestor Ae. tauschii Coss. First-generation hybrids always have 35 chromosomes (Rajhathy 1960). At meiosis in pentaploid hybrids (2n = 5x = 35, DDCBA genome), the D genome chromosomes of wheat pair with those of Ae. cylindrica while A, B, and C genome chromosomes remain as univalents (Zemetra et al. 1998). The highly disrupted meiosis in F1 hybrids results in complete male sterility and extremely reduced female fertility, determined as the percentage of florets bearing seeds (Zemetra et al. 1998; Wang et al. 2001).
In field-grown T. aestivum × Ae. cylindrica F1 hybrids, female fertility was 2.2% when backcrossed with Ae. cylindrica (Zemetra et al. 1998), whereas in another study the same cross resulted in 0.87% female fertility (Wang et al. 2001). Average percentage of seed set in T. aestivum × Ae. cylindrica F1 hybrids, when surrounded by different proportions of the parental species, was 2.3 and 3.8% in 2 different years while self fertility of BC1s averaged 0.3 and 0.06% in 2 years (Snyder et al. 2000). In Oregon, female fertility of hybrids collected in the field was 1% but the recurrent parent was not established (Morrison et al. 2002a).
Female fertility in BC1s when pollinated by Ae. cylindrica was 4.4%, and at each successive backcross to Ae. cylindrica mean fertility increases, the second-generation backcross (BC2) already having 6.9% self fertility and 18% female fertility, whereas in the second backcross selfed twice (BC2S2) generation, self fertility is restored to 78.9% (Wang et al. 2001). Fertility in these backcrosses varied quite substantially among individuals, often ranging from 0% seed set to a high proportion of fertile florets.
The first backcross generation of hybrids, bearing always 35 chromosomes (Rajhathy 1960), is variable in chromosome numbers. In BC1 plants issued from T. aestivum × Ae. cylindrica crosses, with Ae. cylindrica as the recurrent parent, chromosome numbers determined upon 15 plants ranged from 30 to 49 chromosomes (Zemetra et al. 1998), and in another study the same type of BC1 plants had 34 to 49 chromosomes (based on 10 individuals) and an average of 12 C genome chromosomes (issued from Ae. cylindrica) (Wang et al. 2002).
Experimental research in the United States involved essentially T. aestivum × Ae. cylindrica hybrids and their backcross derivatives (reviewed in Hegde and Waines 2004). However, it is supposed that when spontaneous hybridization takes place, the wild plant generally acts as the female parent, leading to unidirectional gene flow (Ladizinsky 1985). Ae. cylindrica × T. aestivum hybridization would be the preferential way for crop-to-wild gene flow to occur. BC1 plants where Ae. cylindrica was the female parent in F1 hybridization and the male in the backcross had only 28 to 29 chromosomes, an Ae. cylindrica-like morphology, and restored fertility (Guadagnuolo et al. 2001).
By the use of genomic in situ hybridization (GISH) technology, it was demonstrated that introgression of wheat DNA from the A or the B genomes into Ae. cylindrica was possible starting from F1 hybrids where wheat acted as the female parent; BC2S2 plants had up to three extra, nonhomologous A or B genome chromosomes (Wang et al. 2000). In an introgressive series where wheat acted as the male progenitor, DNA markers specific to wheat A and D genome chromosomes were detected in BC1S1 plants having a euploid Ae. cylindrica chromosome number (2n = 28) or up to three additional chromosomes (Schoenenberger et al. 2005). The analysis of the pattern of retention of D genome chromosomes in T. aestivum × Ae. cylindrica backcross derivatives, using wheat D genome-specific microsatellite markers, has shown that most alleles of the D genome of wheat are neither preferentially inherited nor lost and may be retained in an Ae. cylindrica population (Kroiss et al. 2004).
Wheat genes may be expressed in Ae. cylindrica when introgressed. Thus, imidazolinone-resistant wheat having the resistance gene on chromosome 6D (Anderson et al. 2004) was used to produce T. aestivum × Ae. cylindrica hybrids. In BC1 produced by crossing F1 hybrids to either of the progenitors, six of the seven recovered BC1 plants were resistant to imazamox treatment (Seefeldt et al. 1998). Moreover, it was shown in a reduced sample of experimentally produced Ae. cylindrica × T. aestivum hybrids and BC1s with Ae. cylindrica as the pollen parent that a herbicide resistance trait conferred by the bar gene was inherited to the BC1s only if located on the D genome of the transformed wheat progenitors, whereas if it was located on the B genome none of the BC1s were resistant against glufosinate (Lin 2001).
The aim of this work was to simulate a natural introgressive series from transgenic wheat to Ae. cylindrica with the wild species as the female parent and to assess, at each generation, parameters that are essential to risk assessment like fertility, survival, chromosome constitution, introgression of wheat-specific sequence-characterized amplified regions (SCARs), and transgenes and their expression.
MATERIALS AND METHODS
Plant material:
Transgenic Ae. cylindrica Host × T. aestivum L. hybrids, first backcross generations (BC1) with Ae. cylindrica as the recurrent parent, and selfed offspring of the first backcrosses (BC1S1) were generated in a greenhouse. Hybrids produced by crosses with near-isogenic wheat varieties (wild type, WT) were grown in outdoor beds. Numbering of the individuals follows the genealogy of the plants, and F1 hybrids producing viable seeds were numbered from 1 to 13, as well as their BC1 offspring (e.g., F1-1 is the mother plant of BC1-1, etc.). All plants were potted in 9-cm square pots, except for the BC1, which were planted in pots of 20-cm diameter, to recover bigger plants. All Ae. cylindrica plants originated from seeds collected from distinct individuals in a natural population in Sierre, Switzerland (Guadagnuolo et al. 2001). All plants were sown in autumn, except for the spring wheat varieties used as pollen donors. Several transgenic spring wheat lines and their near-isogenic counterparts were used as pollen donors for hybridizations (Table 1).
TABLE 1.
Wheat lines used for hybridizations, number of hybrids produced and sown, germination rate, survival rate to flowering, and number of spikes produced
| Pollen donor variety/ transformation event | Transgene | Chromosome location | Hybrid seeds produced | Hybrids sown | Germination rate (%) | Survival to flower (%) | Mean no. of spikes/individual |
|---|---|---|---|---|---|---|---|
| Greina 16a | kp4 bar | — | 248 | 150 | 80 | 71.3 | 2.7 |
| Golin 5a | kp4 bar | — | 353 | 150 | 88 | 80 | 4.3 |
| Bobwhite 1b | gus bar | 2A | 3 | 0 | — | ||
| Bobwhite 3b | gus bar | 6B | 28 | 0 | — | ||
| Bobwhite 5b | bar | 1D | 27 | 21 | 90.5 | 90.5 | 3.1 |
| Bobwhite 7b | gus bar | 2B | 13 | 9 | 88.8 | 88.8 | 2.8 |
| Bobwhite 8b | gus bar | 6A | 38 | 24 | 83.3 | 83.3 | 2.7 |
| Bobwhite 92c | gna | — | 14 | 0 | — | ||
| Bobwhite 99c | gna | — | 90 | 22 | 86.4 | 86.4 | 2.5 |
| Bobwhite 150c | gna | — | 12 | 0 | — | ||
| Greina (wild type) | — | 89 | 48 | 93.8 | 83.3 | 8.8 | |
| Golin (wild type) | — | 56 | 27 | 100 | 100 | 6.2 | |
| Bobwhite (wild type) | — | 88 | 0 | — |
Cytogenetic analysis:
Chromosomes were counted at mitosis in root tip meristems, and the number of observed satellitiferous chromosomes was noted. Root tips were pretreated in a saturated water solution of α-bromonaphtalene for 180 min at room temperature. Meioses were observed from pollen mother cells in anthers. Root tips and immature anthers were fixed for at least 1 week in absolute ethanol and glacial acetic acid (3:1) was added with acetocarmine and traces of iron acetate. Before staining, root tips were treated in a water solution containing 5% pectinase and 2% cellulase (w/v) for softening of the tissue. After fixation, root tips or anthers were stained in acetocarmine (1%) added with traces of iron acetate for 1 hr and then heated gently for 2 min over a flame. The material was then stored in 45% acetic acid, squashed, and observed at 1000× magnification under a light microscope.
Greenhouse experiments:
Ae. cylindrica plants were emasculated, and spikes were covered with pollen-proof bags, pollinated with wheat pollen 2–5 days after, and bagged again during maturation of the seeds. Transgenic F1 hybrids were grown in a greenhouse surrounded by a dense planting of Ae. cylindrica. Ventilators were set around the artificial plot during flowering for maximizing pollen flow from Ae. cylindrica to the hybrids. The BC1 seeds produced were all sown in a greenhouse. To evaluate self fertility all spikes were bagged. The BC1S1s were sown in pots for further analysis, up to 15 spikes per plant were covered with pollen bags to evaluate self fertility, and the remaining spikes were exposed to pollen of a surrounding Ae. cylindrica population of at least 2000 plants to evaluate fertility under open-pollination conditions.
Fertility assessment:
Hybrids between wheat and Ae. cylindrica are completely male sterile (Wang et al. 2001), and only female fertility was assessed. Female fertility was calculated as follows: number of flowers that produced a seed/total number of flowers × 100. Similarly, self fertility of the BC1 was indicated as the number of flowers that produced a seed/total number of flowers × 100. Spikelets are axes bearing an indeterminate number of alternate flowers, but only the basal two to three (rarely four) were completely developed; the apical ones were always aborted. To estimate the number of flowers produced, we divided hybrid spikes into two regions: the basal part, where spikelets have three completely developed flowers, and the upper part, where the spikelets contain two well-formed flowers. Basal spikelets were bigger in size, with the lemmas of the third- and higher-order flower well protruding from the glumes, whereas apical spikelets were smaller and more compact. To determine the fertility of BC1S1 plants all flowers were counted individually. Differences between self- and open-pollination fertilities of BC1S1 were assessed with the Wilcoxon ranked signed test, using SPSS 11 (SPSS, Chicago).
DNA extraction and PCR:
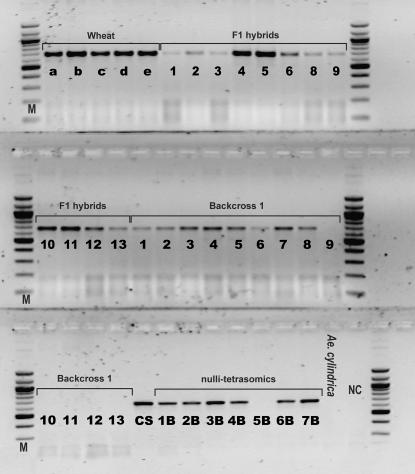
Genomic DNA was extracted from a bulk of five individuals of each transgenic line and their near-isogenic wheat lines and from individual hybrids, BC1s, BC1S1s, and Ae. cylindrica of the mother population, following an SDS–Na-acetate protocol (Savova-Bianchi 1996). The bar gene conferring resistance to phosphinothricin herbicides and the wheat-specific DNA marker IB10 (Schoenenberger et al. 2005) were amplified in 132 Golin 5 hybrids and 120 Greina 16 hybrids produced. Furthermore, we performed PCRs using the different wheat-specific SCAR markers DP9, D1P9, and GB10 and the bar gene in all individuals of the introgressive series that produced an offspring; these latter reactions were repeated at least twice. Amplification conditions of the bar gene followed Takumi and Shimada (1996) whereas PCR protocols and chromosome assignment of the SCAR markers are described in Schoenenberger et al. (2005). Wheat cv. Chinese Spring (CS) and its nulli-tetrasomic (NT) aneuploids for chromosome assignment of introgressed markers (Figure 2) were obtained from Beat Keller of the Institute of Plant Biology of the University of Zürich, Switzerland.
Figure 2.—
Amplification of the GB10 SCAR marker (662 bp) in wheat (a, Golin 5; b, Greina 16; c, Bobwhite WT; d, Bobwhite 5; e, Bobwhite 8), F1 hybrids, BC1, CS-NT lines, and Ae. cylindrica. NC, negative control; M, 100-bp ladder.
DNA sequencing:
Both strands of the bar gene, amplified in the transgenic wheat pollen donor plants as well as in a BC1S1 individual, were sequenced as described by Chassot et al. (2001) in an ABI 310 automated sequencer (Applied Biosystems, Foster City, CA), with a modified cycle sequencing temperature profile: 96° for 10 sec, 55° for 5 sec, and 60° for 4 min. Recovered sequences were compared with GenBank's nr database sequences to verify homology with the bar gene.
Herbicide treatment:
To test expression of the bar gene, single marked leaves of BC1S1 plants were sprayed with 150 mg/liter Basta (Bayer CropScience). Two weeks after application, herbicide susceptibility was assessed by scoring necrosed leaves. The assay was repeated twice on distinct leaves.
RESULTS
F1 hybrids:
Manual Ae. cylindrica × T. aestivum hybridizations yielded a total of 1059 F1 hybrid seeds, and 451 of these were sown for the production of BC1 (Table 1). Hybrids issued from some of the crosses were not sown due to the low number of produced seeds. Overall germination rate of the hybrids was 86.5%, and 79.8% of the sown individuals reached maturity (i.e., produced at least one spike). Mortality of the plants was mainly due to frost during the winter and to some rare dwarf plants which died before maturity. Being planted in pots, the hybrids produced only small amounts of tillers and spikes (Table 1). Nontransgenic hybrids were grown outside and produced more tillers, while the transgenic ones were grown indoors for legal reasons.
Ae. cylindrica × T. aestivum hybrids always had 35 chromosomes, corresponding to the expected pentaploid value (Table 2, Figure 1). PCRs of the wheat-specific DNA fragment IB10 and the bar gene in all 252 Golin 5 and Greina 16 hybrids demonstrated the hybrid nature and the presence of the transgene in the manually produced plants. Only 3 individuals of 132 Golin 5 hybrids lacked the IB10 fragment and 4 missed the bar gene, whereas both fragments were detected in all Greina 16 hybrids (data not shown). Lack of amplification was attributed to the bad quality of the DNA extract rather than to loss of the fragments. Furthermore, PCRs of wheat-specific SCAR markers performed on all individuals of the introgressive series that produced an offspring showed that all hybrids bearing viable seed possess the specific wheat markers (Table 2, Figure 2). The bar gene was amplified in all hybrids except one (F1-13), issued by a cross with Bobwhite 5 (Figure 3). Mean female fertility of the hybrids was 0.2% and varied between 0.03 and 0.6%. In other words, we found one BC1 seed each 2–18 hybrid individuals, depending on the wheat variety implicated in the Ae. cylindrica × T. aestivum hybridization (Table 3).
TABLE 2.
Chromosome number, presence of satellitiferous chromosomes, wheat genome-specific SCARs (DP9, GB10, and D1P9), and a transgene (bar) and its expression in the introgressive series
| Individual | Chromosome number 2n = | No. counts | Satellitiferous chromosomes | DP9 (6A) | GB10 (5B) | D1P9 (6D) | bar gene | Basta test |
|---|---|---|---|---|---|---|---|---|
| Ae. cylindrica | 28 | 3 | 1 (1C) | − | − | − | − | + |
| GM-T. aestivum | 42 (literature) | 2 (1B and 6B) | + | + | + | + | ||
| F1 | ||||||||
| 1 | 35 | 2 | + | + | + | + | ||
| 2 | 35 | 3 | 1 | + | + | + | + | |
| 3 | + | + | + | + | ||||
| 4 | 35 | 3 | + | + | + | + | ||
| 5 | 35 | 2 | + | + | + | + | ||
| 6 | 35 | 2 | + | + | + | + | ||
| 8 | 35 | 2 | + | + | + | + | ||
| 9 | 35 | 2 | 1 | + | + | + | + | |
| 10 | + | + | + | + | ||||
| 11 | + | + | + | + | ||||
| 12 | 35 | 2 | 1 | + | + | + | + | |
| 13 | 35 | 2 | + | + | + | − | ||
| Go5-12 | 35 | 4 | ||||||
| Go5-136 | 35 | 2 | ||||||
| Go5-85 | 35 | 1 | ||||||
| Go5-21 | 35 | 1 | ||||||
| Go5-127 | 35 | 1 | ||||||
| Go5-128 | 35 | 1 | ||||||
| Go5-48 | 35 | 2 | ||||||
| BW 5-5 | 35 | 2 | ||||||
| Gr-21 | 35 | 2 | 1 | |||||
| BC1 | ||||||||
| 1 | 49 | 4 | 2 | + | + | + | − | |
| 2 | 49 | 3 | 2 | + | + | − | − | |
| 3 | 36 | 5 | + | + | − | + | ||
| 4 | 43–44 | 1 | + | + | + | + | ||
| 5 | 49 | 2 | + | + | + | + | ||
| 6 | 44 | 3 | + | + | + | − | ||
| 7 | 84 | 1 | + | + | + | + | ||
| 8 | 41 | 5 | 2 | + | + | − | + | |
| 9 | 40 | 5 | + | − | − | + | ||
| 10 | 38 | 4 | + | − | + | + | ||
| 11 | 41 | 3 | 1 | + | − | + | + | |
| 12 | 34 | 3 | + | − | + | − | ||
| 13 | 30 | 4 | ≥1 | + | − | + | − | |
| BC1S1 | ||||||||
| 11-1 | 43 | 7 | + | + | + | − | ||
| 13-1 | 28 | 3 | − | − | − | + | ||
| 13-2 | 29 + t | 4 | + | + | − | + | ||
| 13-3 | 29 | 4 | − | + | − | + | ||
| 13-4 | 29 | 3 | − | − | − | + | ||
| 13-5 | 28 | 3 | − | + | − | + | ||
| 13-6 | − | − | − | − | + | |||
| 13-7 | 30 | 3 | − | + | − | + | ||
| 13-8 | 29 | 6 | + | + | − | + | ||
| 13-9 | 31 | 4 | + | + | − | + | ||
| 13-10 | 28 | 1 | − | − | − | + | ||
| 13-11 | 28 | 5 | 2 | + | + | − | + | |
| 13-12 | 28 | 4 | − | + | − | + | ||
| 13-13 | 30 | 4 | − | + | − | + | ||
| 13-14 | 29 | 3 | + | + | − | + | ||
| 13-15 | 28 | 5 | − | + | − | + | ||
| 13-16 | 28 | 4 | − | + | − | + | ||
| 13-17 | 28 | 3 | − | − | − | + | ||
| 13-18 | 30 | 3 | − | + | − | + |
+, present/sensitive; −, absent/resistant; t, telocentric; Go5, Golin 5; Gr, Greina WT; BW5, Bobwhite 5. A part of the information relative to the BC1S1-13 family was already published in Schoenenberger et al. (2005).
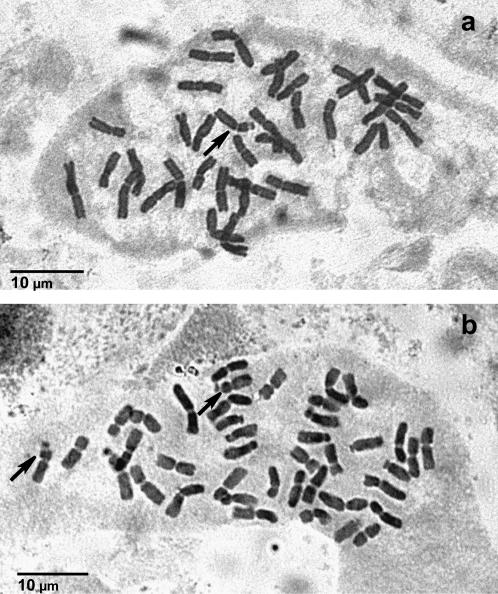
Figure 1.—
Metaphases in root tip cells of (a) an Ae. cylindrica × T. aestivum F1 hybrid with 2n = 35 chromosomes and (b) BC1-11 with 2n = 41 chromosomes. Arrows indicate satellitiferous Ae. cylindrica chromosome 1C.
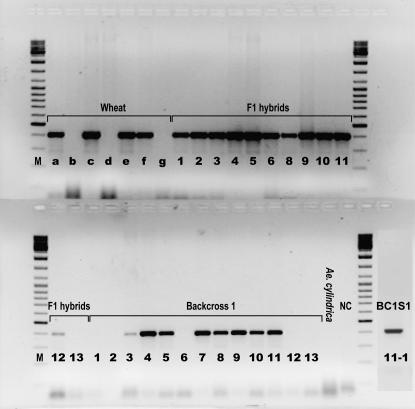
Figure 3.—
Amplification of the bar gene (402 bp) in wheat (a, Golin 5; b, Golin WT; c, Greina 16; d, Greina WT; e, Bobwhite 8; f, Bobwhite 5; g, Bobwhite WT), F1 hybrids, BC1, BC1S1, and Ae. cylindrica. NC, negative control; M, 100-bp ladder.
TABLE 3.
Fertility of Aegilops cylindrica × Triticum aestivum hybrids, BC1 and BC1S1
| Progenitor | Flowers | Seeds/ individuals | Female fertility (%) | |
|---|---|---|---|---|
| F1 | ||||
| Golin 5 | 18,197 | 0.33 | 0.22 | |
| Golin WT | 4,712 | 0.11 | 0.06 | |
| Greina 16 | 8,495 | 0.056 | 0.07 | |
| Greina WT | 11,383 | 0.075 | 0.03 | |
| Bobwhite 5 | 1,798 | 0.53 | 0.6 | |
| Bobwhite 7 | 806 | 0.25 | 0.2 | |
| Bobwhite 8 | 1,825 | 0.25 | 0.3 | |
| Bobwhite 99 | 1,634 | 0.26 | 0.3 | |
| Progenitor | Flowers | Seeds | Self fertility (%) | |
| BC1 | ||||
| 1 | Golin 5 | 913 | 0 | 0 |
| 2 | Golin 5 | 780 | 0 | 0 |
| 3 | Golin 5 | 1,756 | 0 | 0 |
| 4 | Golin 5 | 858 | 0 | 0 |
| 5 | Golin 5 | 987 | 0 | 0 |
| 6 | Golin 5 | 667 | 0 | 0 |
| 7 | Golin 5 | 82 | 0 | 0 |
| 8 | Greina 16 | 911 | 0 | 0 |
| 9 | Bobwhite 8 | 1,051 | 0 | 0 |
| 10 | Bobwhite 8 | 907 | 0 | 0 |
| 11 | Bobwhite 8 | 1,234 | 2 | 0.16 |
| 12 | Bobwhite 5 | 624 | 0 | 0 |
| 13 | Bobwhite 5 | 768 | 40 | 5.21 |
| Flowers
|
Seeds
|
Fertility (%)
|
|||||
|---|---|---|---|---|---|---|---|
| BC1 parent | Self | Open | Self | Open | Self | Open | |
| BC1S1 | |||||||
| 11-1 | 11 | 459 | 489 | 0 | 0 | 0 | 0 |
| 13-1 | 13 | 94 | 87 | 1 | 6 | 1.1 | 6.9 |
| 13-2 | 13 | 14 | 84 | 7 | 60 | 50 | 71.4 |
| 13-3 | 13 | — | 160 | — | 26 | — | 16.3 |
| 13-4 | 13 | 286 | 104 | 0 | 16 | 0 | 15.4 |
| 13-5 | 13 | 245 | 130 | 42 | 79 | 17.1 | 60.8 |
| 13-6 | 13 | 230 | 106 | 192 | 93 | 83.5 | 87.7 |
| 13-7 | 13 | 298 | 121 | 0 | 18 | 0 | 14.9 |
| 13-8 | 13 | 164 | 94 | 17 | 32 | 10.4 | 34 |
| 13-9 | 13 | 14 | 97 | 3 | 30 | 21.4 | 30.9 |
| 13-10 | 13 | — | 148 | — | 45 | — | 30.4 |
| 13-11 | 13 | 130 | 140 | 0 | 12 | 0 | 8.6 |
| 13-12 | 13 | — | 125 | — | 103 | — | 82.4 |
| 13-13 | 13 | — | 114 | — | 65 | — | 57 |
| 13-14 | 13 | 28 | 111 | 1 | 80 | 3.6 | 72.1 |
| 13-15 | 13 | 4 | 91 | 1 | 63 | 25 | 69.2 |
| 13-16 | 13 | 186 | 71 | 124 | 57 | 66.7 | 80.3 |
| 13-17 | 13 | 176 | 92 | 116 | 69 | 65.9 | 75 |
| 13-18 | 13 | — | 98 | — | 75 | — | 76.5 |
BC1:
In total, 74 BC1 seeds were recovered, 68 of them issued from hybrids with genetically modified (GM) wheat and 6 from hybrids with conventional wheat. Seventy-three of the BC1 seeds were sown (Greina 16, 6 seeds; Greina WT, 3 seeds; Golin 5, 40 seeds; Golin WT, 3 seeds; Bobwhite 5, 9 seeds; Bobwhite 7, 2 seeds; Bobwhite 8, 5 seeds; and Bobwhite 99, 5 seeds). Thirteen BC1 plants germinated and flowered (17.8% survival). Chromosome numbers ranged from 30 to 84 chromosomes (Table 2, Figure 1). Male meioses in the BC1 were mostly irregular, with a high proportion of univalents at metaphase I (Table 4). We observed several bivalents and some trivalents; in one cell of a BC1 individual we observed a tetravalent. Pollen grains were mostly collapsed and irregular in size and considered to be sterile. Only in some individuals we found a proportion of well-formed turgescent pollen grains, whose presence correlated with some self fertility of the plant. Amplifications of wheat genome-specific SCAR markers showed the presence of wheat DNA from A, B, and D genomes in several of the BC1 plants (Table 2). The bar gene was maintained in 8 of 13 BC1 plants (Figure 3). Most of the BC1s were completely self sterile. However, two BC1 plants (BC1-11 and BC1-13) produced an offspring, and self fertility was 0.16 and 5.21%, respectively (Table 3). BC1 plants displayed a high morphologic variability, traits ranging from Ae. cylindrica-like to F1 hybrid-like. Growth habit ranged from squarrose (Ae. cylindrica-like) to erect (wheat-like), leaf width ranged from 4 to 13 mm, and spike morphology ranged from Ae. cylindrica-like to F1-hybrid-like (data not shown). Spike emergence was not synchronous; there was a delay of 22 days between the first plant to flower and the last ones. One plant (BC1-7) grew extremely slowly and flowered 45 days after the first.
TABLE 4.
Meiosis in BC1
| Meiotic configuration (approximate)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| BC1 no. | 2n | I | II | III | IV | Micronuclei | Collapsed pollen | Regularity of pollen diameter |
| 1 | 49 | − | − | − | ||||
| 2 | 49 | + | + | − | = | = | −, some very big | |
| 3 | 36 | − | + | − | + | − | ||
| 4 | 43–44 | + | −− | |||||
| 5 | 49 | 49 in some cells | = | − | + | + | − | |
| 6 | 44 | + | = | − | − | = | ||
| 7 | 84 | + | + | −− | ||||
| 8 | 41 | + | −, in some cells + | = | − | − | ||
| 9 | 40 | − | + | = | = | − | ||
| 10 | 38 | 12 | 13 | − | − | = | − | |
| 11 | 41 | = | = | = | = | − | ||
| 12 | 34 | = | = | − | 1 | − | − | − |
| 13 | 30 | − | + | − | − | = | ||
−, few; =, some; +, many. Regularity of pollen diameter: −−, very irregular; −, irregular; =, quite regular.
BC1S1:
A total of 42 BC1S1 seeds were recovered from two BC1 plants (Table 3) and 22 of them were sown (2 seeds from individual BC1-11 and 20 from BC1-13). One BC1S1-11 and 18 BC1S1-13s germinated and grew to maturity. BC1S1-11-1 had 43 chromosomes while chromosome numbers of the BC1S1-13 family ranged from 28 to 31 (Table 2). Wheat-specific SCAR markers DP9 and D1P9 were detected in several of the BC1S1 individuals whereas wheat chromosome 5B-specific SCAR GB10 was missing already at the BC1 generation (Figure 2). The bar gene was absent in individual BC1-13 and in its entire offspring, the BC1S1-13 family. However, bar was present in individual BC1-11 and in its only surviving descendant, BC1S1-11-1 (Figure 3). Presence of the bar gene correlated with resistance to Basta treatment, whereas all plants missing the bar gene were susceptible to the herbicide application. Sequencing of the amplification product in individual BC1S1-11-1 and in its wheat progenitor Bobwhite 8 revealed 100% identity of the sequenced 402-bp fragment between the two. Database comparison of the obtained sequence in GenBank's nr databases, using the basic local alignment search tool (BLAST), revealed 100% sequence homology with the Streptomyces hygroscopicus bar gene for phosphinothricin acetyl transferase (bases 117–518) (White et al. 1990). BC1S1 plants displayed a high range of fertility under self- and open-pollination conditions. Spikes in open pollination with pollen from Ae. cylindrica produced significantly (P = 0.001) more seeds than those in the self-pollination treatment. Fertility among the 19 BC1S1 individuals analyzed varied considerably; it ranged from 0% (self and open pollinated) to almost fully fertile (Table 3). Interestingly, fertility does not always correlate with even or euploid Ae. cylindrica chromosome number, some individuals having 28 chromosomes being much more sterile than others having 29 or even 31 chromosomes.
DISCUSSION
Germination and survival:
The germination rate of F1 hybrids is comparable with the germination rate of pure Ae. cylindrica, which was often found to be ∼90% in our past experiences (data not shown). However, germination of BC1 seeds was much lower. In fact, most of the seeds sown were shriveled and did not germinate, as already observed by Rajhathy (1960) and Snyder et al. (2000) on reciprocal crosses. At the next generation (BC1S1), germination was again similar to that of pure Ae. cylindrica. The negative fitness effect of hybridization on germinability at the BC1 generation seems to be overcome at the BC1S1 generation.
Cytology:
First-generation hybrids always have 35 chromosomes and a DDCBA genome, whereas chromosome constitutions of BC1 plants vary considerably. Chromosome numbers of female gametes of F1 hybrids have not been established so far, but if we assume that the chromosomes of the A, B, and C genomes, which are present as haploid sets (Zemetra et al. 1998), are randomly distributed during meiosis to the megaspores or lost, a female gamete of a hybrid should hypothetically have 17–18 chromosomes and a BC1 plant 31–32 chromosomes. However, a female gamete of a hybrid that can be successfully fertilized by a male gamete of Ae. cylindrica seems to have usually >17–18 chromosomes. In fact, we observed that several BC1 plants had a high chromosome number, and assuming that Ae. cylindrica contributed with gametes of 14 chromosomes to the constitution of the BC1s, it seems that viable female gametes of F1 hybrids, whose chromosome number is often between 20 and 35, maintain a high number of wheat chromosomes. BC1s having as many as 49 chromosomes were observed several times on reciprocal crosses (Zemetra et al. 1998; Wang et al. 2002; this study, Table 3). The most plausible explanation would be the fecundation of an unreduced female gamete of an F1 hybrid (n = 5x = 35) with a normal Ae. cylindrica male gamete, resulting in heptaploid individuals with DDDCCBA genomes. Moreover, BC1-7 was probably produced by the fecundation of a gamete of the F1 having 70 chromosomes (n = 10x), resulting in a dodecaploid individual having the putative DDDDDCCCBBAA genome. David et al. (2004) observed similarly that fertile Ae. ovata × T. turgidum durum hybrids always produced an offspring having an amphiploid chromosome number or close to it. Hybrid sterility was thus overcome by producing unreduced gametes. Moreover, the ability to produce unreduced gametes depended on the Ae. ovata progenitor population. In Ae. cylindrica × T. aestivum hybrids too, a high chromosome number of the BC1 indicates that fertility is restored by the production of unreduced gametes. However, we never observed self-fertile hybrids and we never counted 2n = 70 (amphiploid value) in a descendant of a hybrid but we cannot exclude a priori that decaploid Ae. cylindrica × T. aestivum amphiploids can be produced. However, the three BC1 individuals resulting from unreduced female gametes (i.e., those possessing 49 chromosomes) were completely self sterile and BC1-7 grew extremely slowly, probably because of the excessive chromosome load. Under open-pollination conditions, BC1 plants produced from Ae. cylindrica × T. aestivum and backcrosses had 28–29 chromosomes and restored (but not quantified) fertility (Guadagnuolo et al. 2001). These contrasting results may be explained by cold temperatures registered when meiosis occurred in the F1 hybrids in spring 2003, inducing meiotic abnormalities leading to enhanced frequency of unreduced gamete formation. On the other hand, we cannot exclude a genotype effect of the wheat progenitor on unreduced gamete formation.
Meiotic irregularity in BC1s is not surprising if we consider that a maximum of 14 bivalents may be formed in these individuals represented by the C and D genome chromosomes. The A and B genome chromosomes would form univalents, with the exception of individuals produced by 10x gametes, where all chromosomes are doubled. In individuals originating from unreduced gametes, chromosomes of the D genome may form multivalents. Wheat A and B genome chromosomes as well as Ae. cylindrica C genome chromosomes may be implicated in homeologous pairing, resulting in translocated chromosome segments. Moreover, the formation of micronuclei by univalent chromosomes lost in the cytoplasm during anaphase was observed in all analyzed meioses, and these micronuclei probably contain wheat A and B genome chromosomes and are eliminated.
BC1S1 individuals with 28 chromosomes, that is, a euploid chromosome number, may have a complete DDCC genome and fully restored fertility (e.g., BC1S1-13-17). These individuals might have some wheat D genome DNA left (e.g., BC1S1-13-16). On the other hand, plants with 28 chromosomes may have alien substitution chromosomes, resulting in reduced (self) fertility (e.g., BC1S1-13-1) or intergenomic translocations between wheat and Ae. cylindrica chromosome segments (see also Schoenenberger et al. 2005). Plants with 29–31 chromosomes may contain wheat chromosomes present as monosomic additions or as homolog pairs, which seems plausible particularly in individuals showing a relatively high self fertility (e.g., BC1S1-13-2 and -13-9).
Satellitiferous chromosomes are often easy to recognize and could serve as cytological markers to identify introgressed chromosomes. Wheat chromosomes 1B and 6B have secondary constrictions (Gill et al. 1991), whereas in Ae. cylindrica a pair of satellites attached to the shorter arm of a heterobrachial chromosome is easily recognized (Priadcencu et al. 1967) and identified as chromosome 1C (Linc et al. 1999). Chromosome 1C was clearly recognizable, present in one copy in the F1 hybrid and as a pair in the BC1 (Table 2, Figure 1). Unfortunately, wheat chromosomes 1B and 6B were not clearly distinguishable by their secondary constrictions in the F1 hybrids and descendants, and we could not tell if they introgressed into Ae. cylindrica.
Crop-to-wild gene flow may occur through recombination in homologous chromosome pairs, translocation, or chromosome retention (Wang et al. 2000; Schoenenberger et al. 2005). Additional retained chromosomes could be stable over generations if present in homologous pairs, which may occur once male fertility is restored, and aneuploid Ae. cylindrica populations may be founded. A fourth possible mechanism for gene flow to occur is the formation of amphiploids via unreduced gametes, enhancing the fertility of the otherwise mostly sterile hybrids (David et al. 2004). Chromosome retention in introgressed aneuploids may lead to production of a new, fully fertile Aegilops taxon, containing several additional chromosome pairs, from the A and B genomes of wheat, if a certain reproductive isolation is achieved. In fact, hybridization can lead to the formation of new taxa if intersterility between a neopolyploid or a chromosomically rearranged homoploid and its parental species is achieved (Abbott 1992; Ellstrand and Schierenbeck 2000). On the other hand these polyploids can serve as a bridge for gene flow between the parental taxa (Petit et al. 1999).
SCAR and bar gene introgression:
Segregation was observed in the BC1 generation for markers D1P9 and GB10. Surprisingly, marker GB10 was maintained in all BC1s having a wheat progenitor of the varieties Golin and Greina, whereas no BC1 maintained this SCAR if Bobwhite was the progenitor. Marker DP9 was maintained in all BC1s although it is specific to the A genome of wheat. Apparently different wheat chromosomes or chromosome regions have different abilities to be maintained as backcrossing takes place. Furthermore, there is a varietal effect of the wheat progenitor on segregation of its chromosomes or chromosome fractions in an Ae. cylindrica background. Similarly, in wheat intervarietal linkage maps, segregation distortion in favor of one of the parental varieties was often observed (e.g., Cadalen et al. 1997; Paillard et al. 2003). D genome marker D1P9 segregated at a higher than expected ratio of 1:1 in the BC1. In fact, in 13 BC1 plants, 9 had the wheat-specific marker D1P9. Deviation from expected segregation of microsatellite markers specific to the D genome of wheat was observed in T. aestivum × Ae. cylindrica BC1s having Ae. cylindrica as the recurrent parent; interestingly, deviation was always due to a higher than expected ratio of wheat alleles (Kroiss et al. 2004). However, these alleles were not located on chromosome 6D as in our case, and the wheat varieties used were not the same.
BC1 individual 13 had both SCARs DP9 and D1P9 in a hemizygous state. Its selfed offspring segregated for marker D1P9; in fact, in 18 plants 13 contained the marker (3:1 ratio). Marker DP9 is on an A genome chromosome, it is present in a hemizygous state in all BC1s, and it was passed to the BC1-13 family as an added monosome, as a substitution chromosome, as a homologous pair, or as a translocated segment (Schoenenberger et al. 2005).
Individual BC1S1-11 was resistant to glufosinate, showing that a wheat transgene can be expressed in an Ae. cylindrica background; moreover, this is the first generation where it could be present in a homozygous state.
Fertility:
BC1 plants can be self fertile and produce seeds. It is therefore not required to have two additional crosses after hybridization to recover partially self-fertile plants as stated by Zemetra et al. (1998). In fact, Snyder et al. (2000) obtained partially self-fertile BC1s to either parental species and seed set averaged 0.06 and 0.3% in 2 years; BC1 individuals having Ae. cylindrica as the recurrent parent could have as much as 46% of the florets bearing seeds by selfing. Unfortunately, chromosome numbers of these plants are unknown, but could be close to those of euploid Ae. cylindrica. Production of the BC2 generation has been defined as being critical from the standpoint of gene flow, because it could serve as a pollen donor to Ae. cylindrica and it could propagate even in the absence of the progenitor species if self fertility is restored (Wang et al. 2001). We show that in some cases, particularly in plants having chromosome numbers close to those of euploid Ae. cylindrica, gene flow may need less backcrossing generations to occur. BC1 plants having 28 or 29 chromosomes were found to be fertile under open-pollination conditions in the presence of pure Ae. cylindrica in a previous study (Guadagnuolo et al. 2001). It cannot be excluded that a proportion of the offspring of these plants were produced by selfing.
BC1S1 plants having 70–80% of their florets bearing seed may be considered as fully fertile if compared with pure Ae. cylindrica.
Fertility rates of the hybrids and germination of BC1 seed are low. Nevertheless, under severe selection pressure as in the case of herbicide-resistant GM-wheat cultures invaded by Ae. cylindrica, resistances in the wild species due to gene flow would provide a high selective advantage to introgressed hybrids and successive generations. As genes located on the three genomes of wheat may introgress at least up to BC1S1, the insertion of the transgene on the A or the B genome instead of the D genome is not a sufficient containment measure.
Evidence of past introgression:
Research on introgression between wheat and Ae. cylindrica has focused exclusively on present mechanisms of introgression, while no literature documents past gene flow. Nevertheless, Caldwell et al. (2004) investigated single-nucleotide polymorphisms (SNPs) in a coding region of the D genome in Ae. tauschii, Ae. cylindrica, and wheat. The authors detected 12 different haplotypes, 11 in Ae. tauschii, 2 in Ae. cylindrica, and 2 in wheat. They concluded that different haplotypes of the diploid D genome ancestor Ae. tauschii contributed to the formation of allotetraploid Ae. cylindrica. Surprisingly, haplotype 1, present in all three species, was the most frequent haplotype in wheat (98.5%) and the least frequent in Ae. cylindrica (4.3% of 70 accessions). This pattern is typical from gene flow and suggests that the presence of haplotype 1 in both species could be the consequence of introgression rather than of common ancestry. If true, this study would be the first report on past gene flow from wheat to Ae. cylindrica and would assess that introgression that was demonstrated in our study occurred spontaneously in the past.
Acknowledgments
We are grateful to Christof Sautter of the Institute of Plant Sciences, Federal Institute of Technology Zürich, Switzerland, for providing seeds of the wheat varieties Greina and Golin and for his comments on the manuscript; to Eva Stoger of the Institute for Biology of the Rheinisch-Westfälische Technische Hochschule, Aachen, Germany, for providing seeds of the wheat variety Bobwhite; to Sarah Mamie and Xavier Berney for technical assistance; and to the team of the Botanical Garden of the University and the City of Neuchâtel for taking care of the plants. This project was funded by the National Centre of Competence in Research Plant Survival, a research program of the Swiss National Science Foundation.
References
- Abbott, R. J., 1992. Plant invasions, interspecific hybridization and the evolution of new plant taxa. Trends Ecol. Evol. 7: 401–405. [DOI] [PubMed] [Google Scholar]
- Abranches, R., A. P. Santos, E. Wegel, S. Williams, A. Castilho et al., 2000. Widely separated multiple transgene integration sites in wheat chromosomes are brought together at interphase. Plant J. 24: 713–723. [DOI] [PubMed] [Google Scholar]
- Anderson, J. A., L. Matthiesen and J. Hegstad, 2004. Resistance to an imidazolinone herbicide is conferred by a gene on chromosome 6DL in the wheat line cv. 9804. Weed Sci. 52: 83–90. [Google Scholar]
- Belea, A., 1968. Examination of F1 Hybrids of Aegilops Cylindrica Host × Triticum aestivum L. Acta Agron. Hung. 17: 151–160. [Google Scholar]
- Cadalen, T., C. Boeuf, S. Bernard and M. Bernard, 1997. An intervarietal molecular marker map in Triticum aestivum L em Thell and comparison with a map from a wide cross. Theor. Appl. Genet. 94: 367–377. [Google Scholar]
- Caldwell, K. S., J. Dvorak, E. S. Lagudah, E. Akhunov, M. C. Luo et al., 2004. Sequence polymorphism in polyploid wheat and their D-genome diploid ancestor. Genetics 167: 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassot, P., S. Nemomissa, Y. M. Yuan and P. Küpfer, 2001. High paraphyly of Swertia L. (Gentianaceae) in the Gentianella- lineage as revealed by nuclear and chloroplast DNA sequence variation. Plant Syst. Evol. 229: 1–21. [Google Scholar]
- Clausen, M., R. Kräuter, G. Schachermayr, I. Potrykus and C. Sautter, 2000. Antifungal activity of a virally encoded gene in transgenic wheat. Nat. Biotechnol. 18: 446–449. [DOI] [PubMed] [Google Scholar]
- David, J. L., E. Benavente, C. Brès-Patry, J.-C. Dusautoir and M. Echaide, 2004. Are neopolyploids a likely route for a transgene walk to the wild? The Aegilops ovata × Triticum turgidum durum case. Biol. J. Linn. Soc. 82: 503–510. [Google Scholar]
- Donald, W. W., and A. G. Ogg, 1991. Biology and control of jointed goatgrass (Aegilops cylindrica), a review. Weed Technol. 5: 3–17. [Google Scholar]
- Ellstrand, N. C., and K. A. Schierenbeck, 2000. Hybridization as a stimulus for the evolution of invasiveness in plants? Proc. Natl. Acad. Sci. USA 97: 7043–7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, B. S., B. Friebe and T. R. Endo, 1991. Standard karyotype and nomenclature system for description of chromosome bands and structural-aberrations in wheat (Triticum aestivum). Genome 34: 830–839. [Google Scholar]
- Guadagnuolo, R., D. Savova-Bianchi and F. Felber, 2001. Gene flow from wheat (Triticum aestivum L.) to jointed goatgrass (Aegilops cylindrica Host.), as revealed by RAPD and microsatellite markers. Theor. Appl. Genet. 103: 1–8. [Google Scholar]
- Hegde, S. G., and J. G. Waines, 2004. Hybridization and introgression between bread wheat and wild and weedy relatives in North America. Crop Sci. 44: 1145–1155. [Google Scholar]
- Kimber, G., and Y. H. Zhao, 1983. The D genome of the Triticeae. Can. J. Genet. Cytol. 25: 581–589. [Google Scholar]
- Kroiss, L. J., P. Tempalli, J. L. Hansen, M. I. Vales, O. Riera-Lizarazu et al., 2004. Marker-assessed retention of wheat chromatin in wheat (Triticum aestivum) by jointed goatgrass (Aegilops cylindrica) backcross derivatives. Crop Sci. 44: 1429–1433. [Google Scholar]
- Ladizinsky, G., 1985. Founder effect in crop-plant evolution. Econ. Bot. 39: 191–199. [Google Scholar]
- Lin, Y., 2001. Risk assessment of bar gene transfer from B and D genomes of transformed wheat (Triticum aestivum) lines to jointed goatgrass (Aegilops cylindrica). J. Anhui Agric. Uni. 28: 115–118. [Google Scholar]
- Linc, G., B. R. Friebe, R. G. Kynast, M. Molnar-Lang, B. Köszegi et al., 1999. Molecular cytogenetic analysis of Aegilops cylindrica Host. Genome 42: 497–503. [PubMed] [Google Scholar]
- Morrison, L. A., L. Crémieux and C. A. Mallory-Smith, 2002. a Infestations of jointed goatgrass (Aegilops cylindrica) and its hybrids in Oregon wheat fields. Weed Sci. 50: 737–747. [Google Scholar]
- Paillard, S., T. Schnurbusch, M. Winzeler, M. Messmer, P. Sourdille et al., 2003. An integrative genetic linkage map of winter wheat (Triticum aestivum L.). Theor. Appl. Genet. 107: 1235–1242. [DOI] [PubMed] [Google Scholar]
- Petit, C., F. Bretagnolle and F. Felber, 1999. Evolutionary consequences of diploid-polyploid hybrid zones in wild species. Trends Ecol. Evol. 14: 306–311. [DOI] [PubMed] [Google Scholar]
- Priadcencu, A., C. Miclea and L. Moisescu, 1967. The local form of the species of Aegilops cylindrica Host. and its genetic importance. Rev. Roum. Biol. Ser. Bot. 12: 421–425. [Google Scholar]
- Rajhathy, T., 1960. Continuous spontaneous crosses between Aegilops cylindrica and Triticum aestivum. Wheat Inf. Serv. 11: 20. [Google Scholar]
- Savova-Bianchi, D., 1996. Evaluation of gene flow between crops and related weeds: risk assessment for releasing transgenic barley (Hordeum vulgare L.) and Alfalfa (Medicago sativa L.) in Switzerland. Ph.D. Thesis, University of Neuchâtel, Neuchâtel, Switzerland.
- Schoenenberger, N., F. Felber, D. Savova-Bianchi and R. Guadagnuolo, 2005. Introgression of wheat DNA markers from A, B and D genomes in early generation progeny of Aegilops cylindrica Host x Triticum aestivum L. hybrids. Theor. Appl. Genet. 111: 1338–1346. [DOI] [PubMed] [Google Scholar]
- Seefeldt, S. S., R. Zemetra, F. L. Young and S. S. Jones, 1998. Production of herbicide resistant jointed goatgrass (Aegilops cylindrica) x wheat (Triticum aestivum) hybrids in the field by natural hybridization. Weed Sci. 46: 632–634. [Google Scholar]
- Snyder, J. R., C. A. Mallory-Smith, S. Balter, J. L. Hansen and R. S. Zemetra, 2000. Seed production on Triticum aestivum by Aegilops cylindrica hybrids in the field. Weed Sci. 48: 588–593. [Google Scholar]
- Stoger, E., S. Williams, P. Christou, R. E. Down and J. A. Gatehouse, 1999. Expression of the insecticidal lectin from snowdrop (Galanthus nivalis agglutinin; GNA) in transgenic wheat plants: effects on predation by the grain aphid Sitobion avenae. Mol. Breeding 5: 65–73. [Google Scholar]
- Takumi, S., and T. Shimada, 1996. Production of transgenic wheat through particle bombardment of scutellar tissues: frequency is influenced by culture duration. J. Plant Physiol. 149: 418–423. [Google Scholar]
- Van Slageren, M. W., 1994. Wild Wheats: A Monograph of Aegilops L. and Amblyopyrum (Jaub. & Spach) Eig (Poaceae). Wageningen Agricultural University Press, Wageningen, The Netherlands/ICARDA, Aleppo, Syria.
- Waines, J. G., and D. Barnhart, 1992. Biosystematic research in Aegilops and Triticum. Hereditas 116: 207–212. [Google Scholar]
- Wang, Z., R. S. Zemetra, J. Hansen and C. A. Mallory-Smith, 2001. The fertility of wheat x jointed goatgrass hybrid and its backcross progenies. Weed Sci. 49: 340–345. [Google Scholar]
- Wang, Z. N., A. Hang, J. Hansen, C. Burton, C. A. Mallory-Smith et al., 2000. Visualization of A- and B-genome chromosomes in wheat (Triticum aestivum L.) x jointed goatgrass (Aegilops cylindrica Host) backcross progenies. Genome 43: 1038–1044. [DOI] [PubMed] [Google Scholar]
- Wang, Z. N., R. S. Zemetra, J. Hansen, A. Hang, C. A. Mallory-Smith et al., 2002. Determination of the paternity of wheat (Triticum aestivum L) x jointed goatgrass (Aegilops cylindrica host) BC1 plants by using genomic in situ hybridization (GISH) technique. Crop Sci. 42: 939–943. [Google Scholar]
- White, J., S.-Y. P. Chang, M. J. Bibb and M. J. Bibb, 1990. A cassette containing the bar gene of Streptomyces hygroscopicus: a selectable marker for plant transformation. Nucleic Acids Res. 18: 1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemetra, R. S., J. Hansen and C. A. Mallory-Smith, 1998. Potential for gene transfer between wheat (Triticum aestivum) and jointed goatgrass (Aegilops cylindrica). Weed Sci. 46: 313–317. [Google Scholar]