Abstract
PLS3 (phospholipid scramblase-3) is a new member of the family of phospholipid scramblases and transports CL (cardiolipin) from the inner to the outer mitochondrial membrane. In the present paper we examined whether changing the levels of functional PLS3 in HeLa cells altered de novo CL biosynthesis and its resynthesis. HeLa cells overexpressing PLS3 or expressing a disrupted PLS3 (F258V) or control were incubated with [1,3-3H]glycerol and radioactivity incorporated into CL was determined. CL biosynthesis from [1,3-3H]glycerol was increased 1.8-fold in PLS3 cells and 2.1-fold in F258V cells compared with control. This was due to a 64% (P<0.05) and 2.6-fold (P<0.05) elevation in CL synthase activity in PLS3 and F258V cells respectively, compared with control, and not due to changes in phosphatidylglycerolphosphate synthase activity. The increase in CL synthase activity in these cells was due to an increase in its mRNA expression. In contrast, resynthesis of CL from [1-14C]linoleic acid was reduced 52% (P<0.05) in PLS3 and 45% (P<0.05) in F258V cells compared with control and this was due to a reduction in mitochondrial monolysocardiolipin acyltransferase activity. Although protein levels of mitochondrial monolysocardiolipin acyltransferase were unaltered, activity and mRNA expression of endoplasmic reticulum monolysocardiolipin acyltransferase was upregulated in PLS3 and F258V cells compared with controls. These data indicate that the CL resynthesis in HeLa cells is sensitive to the mitochondrial localization of CL and not the level of the reacylating enzymes. Alterations in functional PLS3 levels in PLS3 or F258V cells did not affect the mitochondrial decarboxylation of phosphatidylserine to phosphatidylethanolamine indicating that the biosynthetic changes to CL were specific for this mitochondrial phospholipid. We hypothesize that the cardiolipin resynthesis machinery in the cell ‘senses’ altered levels of CL on mitochondrial membranes and that de novo CL biosynthesis is up-regulated in HeLa cells as a compensatory mechanism in response to altered movement of mitochondrial CL. The results identify PLS3 as a novel regulator of CL de novo biosynthesis and its resynthesis.
Keywords: cardiolipin, lysocardiolipin, mitochondrion, monolysocardiolipin acyltransferase, phospholipid scramblase-3, phospholipid synthesis, phospholipid resynthesis
Abbreviations: ALCAT1, acyl-CoA:lysocardiolipin acyltransferase 1; CDP-DG, cytidine-5′-diphosphate-1,2-diacylglycerol; CDS, CDP-DG synthetase; CL, cardiolipin; CLS, CL synthase; DMEM, Dulbecco's modified Eagle's medium; ER, endoplasmic reticulum; FBS, fetal bovine serum; hCLS, human CLS; MLCL, monolysocardiolipin; MLCL AT, MLCL acyltransferase; PE, phosphatidylethanolamine; PG, phosphatidylglycerol: PGPS phosphatidylglycerolphosphate synthase; PLA2, phospholipase A2; PLS, phospholipid scramblase; PS, phosphatidylserine; RT, reverse transcriptase
INTRODUCTION
Phospholipids are important structural and functional components of the biological membrane and define compartmentalization of organelles and the protective barrier, the cell membrane, which surrounds cells [1]. The major polyglycerophospholipid in mammalian tissues is bis-(1,2-diacyl-sn-glycero-3-phospho)-1′,3′-sn-glycerol or CL (cardiolipin) [2]. CL is responsible for modulation of the activity of a number of mitochondrial membrane enzymes involved in the generation of ATP [for reviews see 3–5]. Alteration in the content and the molecular species composition of CL will alter oxygen consumption in mammalian mitochondria [6,7]. For example, in rat heart subjected to ischaemia and reperfusion, the reduction in complex III activity is coupled with a reduction in CL [8]. CL interaction with mitochondrial proteins is specific since substitution with other phospholipids does not fully reconstitute their activity. Indeed, it has been suggested that CL may be the ‘glue’ that holds the mitochondrial respiratory complexes together [9]. The role CL plays in apoptosis has been extensively reviewed [10,11]. We have previously demonstrated that addition of the pro-apoptotic factor TNF-α (tumour necrosis factor-α) to H9c2 cells stimulated mitochondrial PLA2 (phospholipase A2) activity towards mitochondrial phospholipids [12]. MLCL (monolysocardiolipin) accumulates during Fas-mediated apoptosis as a byproduct of CL degradation by PLA2 and enhances t-Bid binding to membranes [13]. Based upon these and several other studies, it appears that CL metabolism may play a central role in the pathway leading to cellular apoptosis. Moreover, reduction in CL levels and accumulation of MLCL is a key observation in Barth Syndrome, an X-linked genetic disease [reviewed in 14]. Thus maintenance of the appropriate content, localization and movement of CL within mitochondria is essential for proper mammalian cell function.
CL de novo biosynthesis occurs in the inner mitochondrial membrane via the CDP-DG (cytidine-5′-diphosphate-1,2-diacylglycerol) pathway [reviewed in 4,5]. Initially, phosphatidic acid is converted to CDP-DG by CDS (CDP-DG synthetase). CDP-DG then condenses with sn-glycerol-3-phosphate to form PG (phosphatidylglycerol) catalysed by PGPS (phosphatidylglycerolphosphate synthase) and PGPP (phosphatidylglycerolphosphate phosphatase). PG is then converted to CL by condensation with CDP-DG catalysed by CLS (CL synthase). The newly formed CL is then resynthesized by a deacylation/reacylation pathway involving PLA2 and either a mitochondrial or ER (endoplasmic reticulum) MLCL AT (MLCL acyltransferase) or by a CL transacylase to enrich the content of linoleic acid in CL [4].
PLS (phospholipid scramblases) are membrane-bound enzymes responsible for bi-directional movement of phospholipids [15]. Four family members have been identified. PLS1 is localized to the plasma membrane and PLS2 is localized predominantly in the nucleus [16]. We have previously identified a new member of the family PLS3 and demonstrated that it was localized to the mitochondria [17]. One function of PLS3 is to transport CL from the mitochondrial inner membrane to the outer membrane. The enzyme is activated by phosphorylation by PKCδ (protein kinase C δ) and is dependent on the binding of calcium [18]. Disruption of its conserved calcium-binding motif results in an inactive mutant F258V [17]. Cells transfected with F258V exhibited reduced proliferative capacity and decreased mitochondrial mass with less cytochrome C and CL content, poor mitochondrial respiration, reduced oxygen consumption and intracellular ATP. Electron microscopic examination revealed that the mitochondria in F258V-expressing cells have densely packed cristae and are fewer in number but larger than those in control cells. The abnormal mitochondrial metabolism and structure in F258V-expressing HeLa cells were associated with decreased sensitivity to UV- and tBid-induced apoptosis and diminished translocation of CL to the mitochondrial outer membrane. In contrast, wild-type PLS3-transfected HeLa cells exhibited increased mitochondrial mass, enhanced respiration, increased sensitivity to apoptosis and enhanced CL translocation. It is unknown how altered transport of CL between the inner and outer mitochondrial membranes changes CL biosynthesis. In the present paper we show that de novo biosynthesis of CL from glycerol is unregulated and CL resynthesis from linoleic acid is decreased in growing HeLa cells when the function of PLS3 is modulated.
MATERIALS AND METHODS
Materials
[14C]Glycerol-3-phosphate, [5-3H]CTP, [1,3-3H]glycerol, [1-14C]linoleic acid and [3H]serine were obtained from either Dupont or Amersham. [14C]PG was synthesized from [14C]glycerol-3-phosphate [19]. DMEM (Dulbecco's modified Eagle's medium) and FBS (fetal bovine serum) were products of Canadian Life Technologies (Gibco). Lipid standards were obtained from Serdary Research Laboratories. TLC plates (silica gel G, 0.25 mm thickness) were obtained from Fisher Scientific. Ecolite scintillant was obtained from ICN Biochemicals. HeLa cells were obtained from American Type Culture Collection. HeLa cells overexpressing PLS3 or expressing a disrupted PLS3 (F258V) were obtained as described in [17]. A Western blotting analysis system was used for protein expression studies and was obtained from Amersham Pharmacia Biotech. Kodak X-OMAT film was obtained from Eastman Kodak. The Qiagen OneStep RT (reverse transcriptase)-PCR kit was used for PCR studies. All other chemicals were certified ACS grade or better and obtained from Sigma or Fisher Scientific.
Culture, radiolabelling and harvesting of HeLa cells
HeLa cells were grown in DMEM containing 10% FBS until 70% confluence. Cells were then incubated for up to 8 h with DMEM in the absence or presence of 0.1 mM [1,3-3H]glycerol (10 μCi/dish) or 0.1 mM [1-14C]linoleic acid (bound to albumin with a 1:1 molar ratio; 1 μCi/dish) or for 1 h with [3H]serine (5 μCi/dish). In the [3H]serine experiments, cells were incubated in the absence or presence of 0.2 μM myriocin. The medium was removed and the cells washed twice with ice-cold PBS and then harvested for lipid extraction from the dish with 2 ml methanol/water (1:1 v/v). CL was separated from other phospholipids by two-dimensional TLC and radioactivity incorporated into CL was determined as described in [19].
Determination of enzyme activities
HeLa cells were incubated for 3 days with DMEM containing 10% FBS with a change of medium once after 24 h. Subsequently, the cells were washed twice with ice-cold PBS and harvested with 2 ml lysis buffer [10 mM Tris/HCl, (pH 7.4) and 0.25 M sucrose]. Cells were homogenized with 30 strokes of a Dounce A homogenizer. The homogenate was centrifuged at 1000 g for 5 min and the supernatant centrifuged at 10000 g for 15 min. The pellet was resuspended in 0.5 ml homogenization buffer and used for the assay of mitochondrial enzyme activities. The supernatant was centrifuged at 100000 g for 60 min. The pellet was resuspended in 0.5 ml homogenization buffer and used for the assay of ER ALCAT1 (acyl-CoA:lysocardiolipin acyltransferase 1) enzyme activity. CDS, PGPS, CLS and mitochondrial MLCL AT enzyme activities were determined as described in [19,20]. In some experiments purified pig liver mitochondrial MLCL AT was incubated in the absence or presence of 0.1–100 μM CL. CL transacylase activity was determined as described in [21]. Mitochondrial PLA2 activity was determined as described in [22].
RT-PCR analysis
The cDNA for ALCAT1 was amplified with a pair of specific primers synthesized by Invitrogen™ Life Technologies. Total RNAs from vector control, PLS3 and F258V HeLa cells were isolated using Trizol reagent according to the manufacturer's instructions. The RNA pellets were suspended in autoclaved, double-distilled water and quantified by absorbance at 260 nm using the 260/280 nm ratio as an index of purity. The integrity of the RNA was confirmed by denaturing agarose gel electrophoresis of the isolated RNA. The first strand cDNA from 1 μg of total RNA was synthesized by employing 150 units of moloney murine leukaemia virus RT, 25 pmol of random hexamer primer, 20 units of ribonuclease inhibitor, 1 mM dithiothreitol and 10 pmol each of the four deoxynucleotides, in a total volume of 15 μl. The reaction mixture was incubated at 37 °C for 1 h and terminated by boiling the sample at 95 °C for 5 min. An aliquot of the resultant cDNA preparation was used directly for each amplification reaction. The primers for mammalian ALCAT1 have been previously described in [23]. PCR was performed in 20 μl reaction mixtures containing 8 pmol of primer, 8 pmol of each dNTP and 0.4 units of Taq DNA polymerase. The mixture was overlaid with 30 μl of mineral oil to prevent evaporation and was incubated in a PerkinElmer DNA Thermal Cycler under the following conditions: denaturation at 94 °C for 30 s; annealing at 58 °C for 30 s; and extension at 72 °C for 1.5 min; repeated for 26 cycles. Under the RT-PCR conditions employed, the level of the PCR product was dependent upon the amount of templates employed in the reaction (results not shown). The amplified RT-PCR product was analysed by agarose gel (1.5%) electrophoresis in 1×TAE buffer (40 mM Tris acetate and 2 mM sodium EDTA) and visualized by staining with 0.5 μg ethidium bromide. The primers for human β-actin: forward 5′-GTG GGG CGC CCC AGG CAC CA-3′; reverse 5′-CTC CTT AAT GTC ACG CAC GAT TTG-3′. The PCR product length was 540 bp. The PCR conditions were denaturation at 94 °C for 1 min; annealing at 55 °C for 1 min; and extension at 72 °C for 30 s; repeated for 25 cycles. The cDNA for CLS was amplified with a pair of specific primers synthesized by Invitrogen™ Life Technologies. Human CLS (hCLS1) primers have been previously described in [24]; hCLS1 forward primer: 5′-TTT GTT GGA TGG ATT TAT TGC TC-3′ and hCLS1 reverse primer: 5′-TGT TCG TGG TGT TGG AAG AG-3′. The PCR conditions were denaturation at 94 °C for 30 s; annealing at 60 °C for 30 s; and extension at 72 °C for 45 s; repeated for 25 cycles. Under the RT-PCR conditions employed, the level of the PCR product was dependent upon the amount of templates employed in the reaction (results not shown). The amplified RT-PCR product was analysed by agarose gel (1.2%) electrophoresis as described above. To dissect whether the mechanism of mRNA changes is caused by an increase in its rate of synthesis, a decrease in its rate of degradation, or a combination of these two processes, we studied mRNA stability using actinomycin D as an inhibitor of RNA synthesis. The mRNA levels for ALCAT1, hCLS1 and β-actin were determined by RT-PCR at 4 h intervals following the actinomycin treatment. No apparent changes in mRNA degradation were observed within a 24 h period, indicating PLS3 disruption did not cause any change in the degradation of the mRNA of each enzyme. The relative intensities of the bands were analysed by scanning the film, and subsequently determined by Scion Image software.
Electrophoresis and Western blot analysis
HeLa cells were incubated for 3 days with DMEM containing 10% FBS with a change of medium once after 24 h. The cells were harvested, homogenized and cellular fractions prepared as described above. A 25 μg aliquot of the cellular fraction was subjected to SDS/PAGE (7.5% gels) with molecular-mass standards using a BioRad Mini-Protean® II Dual Slab Cell electrophoresis unit. Proteins were transferred from the gel onto PVDF membranes by incubation for 90 min at 15 volts using a BioRad Trans-Blot SD Semi-Dry Transfer Cell. Expression of mitochondrial MLCL AT was examined by incubating the PVDF membrane with the anti-MLCL AT antibody [20] (1:1000 dilution) dissolved in Tris-buffered saline containing 0.1% Tween 20 and 2% (w/v) non-fat dried milk powder overnight at 4 °C. Subsequently, the membrane was washed and incubated with a peroxidase labelled anti-rabbit secondary antibody (1:5000) for 5–30 min at room temperature (23 °C). Expression of β-actin was examined by incubating the PVDF membrane with the antibody as previously described [25]. Protein bands in the membrane were visualized by enhanced chemiluminescence. The relative intensities of the bands were analysed by scanning the film, and subsequently determined by Scion Image software.
Other determinations
The fatty acid composition of CL was determined by gas chromatographic analysis as previously described [26]. The protein concentration was determined as described in [27]. The student's t test was used for determination of statistical significance. The level of significance was defined as P<0.05.
RESULTS
CL de novo biosynthesis from [1,3-3H]-glycerol is elevated by alterations in PLS3 function
It is unknown how movement of CL between inner and outer mitochondrial membranes affects CL biosynthesis. Thus we examined how overexpression or disruption of transport of CL between the inner and outer mitochondrial membrane by PLS3 alters de novo biosynthesis of CL. Vector (control) HeLa cells, HeLa cells overexpressing PLS3 or HeLa cells expressing the F258V mutant were incubated for 8 h with [1,3-3H]glycerol and the radioactivity incorporated into CL determined. As seen in Figure 1(A), [1,3-3H]glycerol incorporated into CL was 1.8-fold (P<0.05) and 2.1-fold (P<0.05) higher in PLS3 and F258V cells compared with control cells. [1,3-3H]Glycerol incorporated into PG was unaltered (Figure 1B). [1,3-3H]Glycerol incorporated into phosphatidic acid and CDP-DG was low and near background levels (results not shown). Total uptake of [1,3-3H]glycerol at 8 h of incubation was 2.1×105, 2.2×105 and 2.1×105 dpm/mg protein in control, PLS3 and F258V cells respectively. Thus total uptake of [1,3-3H]glycerol was similar among all three cell lines. These data indicated that de novo CL biosynthesis from glycerol was increased by either overexpression or disruption of PLS3 levels in HeLa cells.
Figure 1. Incorporation of [1,3-3H]glycerol into CL and PG in control, PLS3 and F258V cells.
Vector (control) HeLa cells, HeLa cells overexpressing PLS3 (PLS3) or HeLa cells expressing the PLS3 mutant (F258V) were incubated for 8 h with [1,3-3H]glycerol and the radioactivity incorporated into CL (A) and PG (B) was determined as described in the Materials and methods section. Values represent the mean±S.D. of three experiments. *P<0.05.
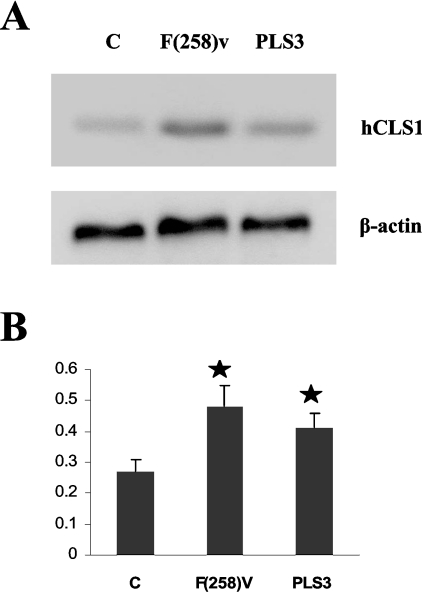
The reason for the increase in [1,3-3H]glycerol incorporation into CL was then examined. The activities of the de novo CL biosynthetic enzymes were determined in mitochondrial fractions prepared from control, PLS3 or F258V cells. CDS and PGPS enzyme activities were unaltered in PLS3 and F258V cells compared with control (Table 1). In contrast, CLS activities were elevated 1.6-fold (P<0.05) and 2.6-fold (P<0.05) in PLS3 and F258V cells respectively, compared with control. Thus the increase in [1,3-3H]glycerol incorporation into CL in PLS3 and F258V cells was due to an increase in CLS activity, indicating an increased de novo biosynthesis of CL in these cells. Total mRNA was prepared from control, PLS3 or F258V cells and the relative levels of CLS mRNA were determined. The CLS mRNA were elevated 1.8-fold in F258V and 1.5-fold in PLS3 cells compared with controls, confirming that the expression of CLS was unregulated (Figure 2). Thus altering PLS3 function in HeLa cells stimulates de novo CL biosynthesis via an increase in CLS mRNA expression and its activity.
Table 1. Activities of the de novo CL biosynthetic and resynthesis enzymes in control, PLS3 and F258V cells.
Activities of the de novo CL biosynthetic and resynthesis enzymes in control HeLa cells, HeLa cells overexpressing PLS3 (PLS3) or HeLa cells expressing PLS3 mutant (F258V). Activities were determined as described in the Materials and methods section. Values represent the means±S.D. of three experiments. *P<0.05.
| Activity (pmol/min per mg of protein) | |||
|---|---|---|---|
| Enzyme | Control | PLS3 | F258V |
| CDS | 20±4 | 21±12 | 18±4 |
| PGPS | 448±16 | 495±42 | 494±64 |
| CLS | 1.4±0.2 | 2.3±0.5* | 3.7±0.8* |
| PLA2 | 191±34 | 180±40 | 200±25 |
| Mitochondrial MLCL AT | 181±38 | 95±16* | 80±3* |
| ALCAT1 | 52±8 | 94±5* | 85±6* |
Figure 2. Expression of hCLS1 mRNA in control, PLS3 and F258V cells.
Total mRNA was isolated from HeLa cells (control), HeLa cells overexpressing PLS3 (PLS3) or HeLa cells expressing the PLS3 mutant (F258V) and the relative levels of CLS mRNA determined as described in the Materials and methods section. (A) Lane 1, control; lane 2, PLS, lane 3, F258V. Top panel, hCLS1; bottom panel, β-actin. A representative gel is presented. (B) Ratio of the relative intensity of hCLS1 to β-actin. The relative intensities of the bands were analysed by scanning the film, and were subsequently determined by Scion Image software. Values represent the means±S.D. of three experiments. *P<0.05.
CL resynthesis is reduced by alterations in PLS3
Since newly synthesized CL may be rapidly remodelled in mammalian cells [reviewed in 4], we examined whether modulation of CL transport between mitochondrial inner and outer membranes alters the resynthesis of CL from MLCL. Control or PLS3 or F258V cells were incubated for 8 h with [1-14C]linoleic acid and the radioactivity incorporated into CL was determined. As seen in Figure 3, [1-14C]linoleic acid incorporation into CL was reduced by 51% (P<0.05) in PLS3 cells and 45% (P<0.05) in F258V cells compared with controls. The total uptake of [1-14C]linoleic acid was 4.9×106 dpm/mg protein and 4.8×106 dpm/mg protein in PLS3 and F258V cells respectively, and was unaltered compared with control cells (5.0×106 dpm/mg). These data indicated that CL resynthesis from linoleic acid was decreased by modulation of PLS3.
Figure 3. [1-14C]linoleic acid incorporation into CL in control, PLS3 and F258V cells.
HeLa cells (control), HeLa cells overexpressing PLS3 (PLS3) or HeLa cells expressing PLS3 mutant (F258V) were incubated with 0.1 mM [1-14C]linoleic acid for 8 h and the radioactivity incorporated into CL determined as described in the Materials and methods section. Values (expressed as dpm/mg protein) represent the means±S.D. of three separate experiments. *P<0.05.
We next investigated the mechanism for the reduction of [1-14C]-linoleic acid incorporation into CL. PLA2 activities were unaltered in PLS3 and F258V cells compared with controls indicating that the reduction in [1-14C]linoleic acid incorporated into CL was not due to an increase in a PLA2-mediated hydrolysis of CL (Table 1). CL transacylase activity was low (<1.0 pmol/min per mg protein in these cells) and we could not detect differences between control, PLS3 and F258V cells. In contrast, mitochondrial MLCL AT activities were reduced by 48% (P<0.05) and 55% (P<0.05) in PLS3 or F258V cells respectively, compared with controls (Table 1). Thus the reduction in mitochondrial MLCL AT activities in these cells would explain the reduced incorporation of [1-14C]linoleic acid into CL. Previously we purified a mitochondrial MLCL AT to homogeneity from pig liver mitochondria and generated a polyclonal antibody to the protein [20]. Western blot analysis using the anti-MLCL AT antibody was performed in control, PLS3 or F258V cells. As seen in Figure 4A the protein level of mitochondrial MLCL AT was unaltered in PLS3 or F258V cells compared with controls. Thus the reduction in MLCL AT activities in these cells was not due to a reduction in protein expression of mitochondrial MLCL AT. We then tested an ER form of MLCL AT (ALCAT1) [23]. ER ALCAT1 activities were elevated 48% (P<0.05) in PLS3 cells and 55% (P<0.05) in F258V cells compared with controls (Table 1). Total mRNA was prepared from control, PLS3 or F258V cells and the levels of ALCAT1 mRNA were determined. The mRNA level of ALCAT1 was elevated in both PLS3 and F258V cells compared with controls (Figure 4), indicating that ER ALCAT1 was unregulated in response to a reduced resynthesis of CL in these cells and/or an elevated CL biosynthesis.
Figure 4. Western blot of mitochondrial MLCL AT and expression of ALCAT1 mRNA in control, PLS3 and F258V cells.
(A) Cellular protein fractions were prepared and Western blot analysis of mitochondrial MLCL AT in control HeLa cells (lane 1), F258V cells (lane 2) or PLS3 cells (lane 3) was performed using an anti-MLCL AT antibody and an anti-β-actin antibody as described in the Materials and methods section. A representative gel is presented. The relative intensities of the bands were analysed by scanning the film and are shown on the right. (B) Total mRNA was isolated from control HeLa cells (lane 1), F258V cells (lane 2) or PLS3 cells (lane 3) and the relative levels of ALCAT1 mRNA determined as described in the Materials and methods section. A representative gel is presented. The relative intensities of the bands were analysed by scanning the film and are depicted on the right. Values (expressed as dpm/mg protein) represent the means±S.D. of three separate experiments. *P<0.05.
Previously we demonstrated that the majority of mitochondrial MLCL AT activity was localized to the outer mitochondrial membrane [28]. Since the amount of CL on the outer membrane is elevated in PLS3 cells compared with controls [17], we examined whether CL could regulate mitochondrial MLCL AT enzyme activity. Purified pig liver mitochondrial MLCL AT was incubated in the absence or presence of 0.1–100 μM CL and enzyme activity was determined. As seen in Table 2 mitochondrial MLCL AT activity was inhibited by increasing the amount of CL in the incubation mixture. The inhibition of enzyme activity was similar to that observed with other exogenous phospholipids [29]. Thus it was possible that the increase in CL content on the outer membrane in PLS3 cells may have led to the reduction in mitochondrial MLCL AT activity in these cells. In F258V cells total CL levels were reduced by almost 50% compared with controls [17]. Thus it was possible that a change in the phospholipid composition of mitochondrial membranes mediated by a reduction in CL levels resulted in a decrease in mitochondrial MLCL AT activity. Finally, we examined the fatty acid composition of CL in these cell lines. The major fatty acid species observed in HeLa cell CL were 14:0 (6%), 16:0 (55%), 18:0 (9%), 18:1 (7%), 18:2 (7%) and others (16%), and these species were unaltered between control, PLS3 and F258V cells. Thus the observed reduction in mitochondrial MLCL AT activity was not due to an alteration in the fatty acid composition of mitochondrial CL in PLS3 or F258V cells.
Table 2. Mitochondrial MLCL AT activity in the presence of various concentrations of CL.
Purified pig liver mitochondrial MLCL AT was incubated with 0–100 μM CL and enzyme activity determined. Data represents the mean of two separate preparations assayed in duplicate. Results between samples did not differ by more than 15%.
| CL added (μM) | MLCL AT activity (pmol/min per mg of protein) | |
|---|---|---|
| No addition | 1667 | |
| 0.1 | 1295 | |
| 1.0 | 1093 | |
| 10 | 1024 | |
| 100 | 786 |
Phosphatidylserine (PS) and phosphatidylethanolamine (PE) synthesis from serine is not affected by alterations in PLS3
The PLS3 and F258V cells have dramatic alterations in mitochondrial morphology and function [17]. To determine whether the effects of PLS3 on de novo synthesis of mitochondrial phospholipids were specific to CL, we examined the biosynthesis of PE from PS, a phospholipid biosynthetic process exclusive to mitochondria. Control, PLS3 or F258V cells were incubated for 1 h with [3H]serine in the absence or presence of the serine palmitoyltransferase inhibitor myriocin and the radioactivity incorporated into PS and PE was determined. Myriocin was added to inhibit [3H]PE synthesis from sphingoid base catabolism [30]. Total [3H]serine incorporation into PS and PE was similar among all three cell lines in the absence or presence of myriocin (Table 3). Thus overexpression or disruption of functional PLS3 activity in HeLa cells does not alter de novo PS biosynthesis from serine nor the mitochondrial decarboxylation of PS to PE.
Table 3. Synthesis of PS and PE from [3H]serine in control, PLS3 and F258V cells.
HeLa cells, HeLa cells overexpressing PLS3 (PLS3) or HeLa cells expressing PLS3 mutant (F258V) were incubated with [3H]serine in the absence or presence of myriocin for 1 h and the radioactivity incorporated into phospholipids was determined as described in the Materials and methods section. Values represent the mean of two experiments and are expressed as a percentage of the total uptake of [3H]serine. Results between samples did not differ by more than 15%.
| Uptake of [3H]serine (%) | ||||||
|---|---|---|---|---|---|---|
| Minus myriocin | Plus myriocin | |||||
| Phospholipid | Control | PLS3 | F258V | Control | PLS3 | F258V |
| PS | 12 | 10 | 13 | 11 | 13 | 13 |
| PE | 6 | 5 | 6 | 5 | 6 | 5 |
DISCUSSION
The objective of this study was to examine whether modulation of PLS3, which transports CL between the mitochondrial inner and outer membranes, in HeLa cells altered de novo CL biosynthesis and its resynthesis. We found that modulation of PLS3 activity resulted in an increased de novo CL biosynthesis from [1,3-3H]glycerol and a decreased CL resynthesis from [1-14C]linoleic acid. The reason for the increased CL synthesis from glycerol was due to increased CLS activity and its mRNA expression in cells with altered PLS3. The reason for the decreased CL resynthesis from linoleic acid was due to a decrease in mitochondrial MLCL AT enzyme activity in cells with altered PLS3.
PLS3 transports CL between mitochondrial inner and outer membranes [17]. HeLa cells expressing either PLS3 or the F258V mutant exhibited increased de novo biosynthesis of CL from [1,3-3H]glycerol due to an increase in CLS activity and its mRNA expression. The alteration in de novo CL biosynthesis appeared specific for CL since the mitochondrial decarboxylation of PS to PE was not affected in HeLa cells overexpressing a functional PLS3 or in cells expressing a disrupted PLS3 (F258V). CL de novo biosynthesis occurs on the matrix side of the inner mitochondrial membrane in mammalian cells [31,32]. Total CL levels were reduced in F258V cells by almost 50% compared with control or PLS3 cells [17]. In addition, the amount of CL on the outer membrane was elevated 22% in PLS3 cells compared with control or F258V cells. Based on our data, we hypothesize that the cell may ‘sense’ altered levels of CL on mitochondrial membranes and that alteration of CL biosynthesis secondary to the movement of CL between mitochondrial inner and outer membranes may be a compensatory response. When PLS3 is overexpressed, the de novo biosynthesis of CL may be increased to replace the CL that is removed from the inner membrane. When the function of PLS3 is suppressed by its mutant, the de novo biosynthesis of CL may also be increased to provide CL to the outer mitochondrial membrane. Hence, PLS3 is identified as a novel regulator of de novo CL biosynthesis.
The observed reduction in CL resynthesis from [1-14C]linoleic acid in PLS3 or F258V cells is more intriguing. In both PLS3 and F258V HeLa cell lines the reduction in [1-14C]linoleic acid incorporation into CL was not due to an alteration in the activities of mitochondrial PLA2 nor an alteration in the fatty acid composition of CL. In contrast, mitochondrial MLCL AT enzyme activity was reduced in both PLS3 and F258V cells compared with control which would explain the reduction in [1-14C]linoleic acid incorporation into CL. The majority of mitochondrial MLCL AT activity is localized to the outer mitochondrial membrane [28]. Protein expression of mitochondrial MLCL AT was unaltered in PLS3 or F258V cells compared with controls, indicating that the observed reduction in resynthesis in these cells was not due to a reduction in the protein levels of this enzyme within the mitochondria. Thus it is possible that the mitochondrial MLCL AT may be sensitive to changes in the level and location of CL within the mitochondria. When purified pig liver mitochondria were incubated with increasing amounts of CL, MLCL AT activity was inhibited. Thus it is possible that the increased CL content on the mitochondrial outer membrane may lead to the reduction in mitochondrial MLCL AT activity observed in PLS3 cells. In contrast, F258V cells have a lower total amount of CL but not other phospholipids [17]. Previously we have demonstrated that alteration in the phospholipid composition of isolated rat heart mitochondria, mediated by the addition of exogenous phosphatidylcholine or PE to crude mitochondrial fractions, resulted in a reduction in mitochondrial MLCL AT activity [32]. Thus it is possible that a change in the phospholipid composition of mitochondrial membranes mediated by a reduction in CL level results in a decrease in mitochondrial MLCL AT activity in F258V cells.
In contrast to mitochondrial MLCL AT, MLCL AT activity in the ER and mRNA expression of ALCAT1 were elevated in PLS3 and F258V cells compared with controls. This upregulation of ER MLCL AT activity and ALCAT1 mRNA expression could serve as a compensatory mechanism for the reduction in CL resynthesis from [1-14C]linoleic acid in cells expressing altered functional levels of PLS3 in an effort to restore CL resynthesis to normal levels. MLCL is generated during Fas-mediated apoptosis of U937 cells and altered movement of CL and its metabolites from mitochondria to other cellular membranes has been proposed [33]. In addition, zones of contact between mitochondria and ER have been documented in mammalian cells [34]. Thus ALCAT1 could potentially serve as an extra-mitochondrial MLCL AT involved in the regulation of CL resynthesis outside mitochondria and on the outer mitochondrial membrane. Moreover, since CL movement is altered in PLS3 or F258V cells, it is possible that its deacylated intermediates may not be readily accessible to the CL resynthesis machinery within the cell. In any event, PLS3 is now identified as a novel regulator of CL de novo biosynthesis and its resynthesis in HeLa cells.
Acknowledgments
The authors wish to thank Dr Michael Schlame (Department of Anesthesiology, New York University School of Medicine, New York, U.S.A.) for helpful discussions. This work was supported by operating grants from the Canadian Institutes of Health Research and Heart and Stroke Foundation of Manitoba (G.M.H.), the National Institutes of Health, U.S.A. [(R.M.L.) and (K.F.)]. G.M.H. is a Canada Research Chair in Molecular Cardiolipin Metabolism.
References
- 1.White D. A. The phospholipid composition of mammalian tissues. In: Ansell G. B., Hawthorne J. N., Dawson R. M. C., editors. Form and Function of Phospholipids. Amsterdam: Elsevier; 1973. pp. 441–482. [Google Scholar]
- 2.Hostetler K. Y. Polyglycerolphospholipids. In: Hawthorne J. N., Ansell G. B., editors. Phospholipids. Amsterdam: Elsevier; 1982. pp. 215–242. [Google Scholar]
- 3.Hoch F. L. Cardiolipins and biomembrane function. Biochim. Biophys. Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- 4.Hatch G. M. Cell biology of cardiac mitochondrial phospholipids. Biochem Cell Biol. 2004;82:99–112. doi: 10.1139/o03-074. [DOI] [PubMed] [Google Scholar]
- 5.Schlame M., Rua D., Greenberg M. L. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 6.Yamaoka S., Urade R., Kito M. Cardiolipin molecular species in rat heart mitochondria are sensitive to essential fatty acid-deficient dietary lipids. J. Nutr. 1990;120:415–421. doi: 10.1093/jn/120.5.415. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsuka T., Nishijima M., Suzuki K., Akamatsu Y. Mitochondrial dysfunction of a Chinese hamster ovary cell mutant deficient in cardiolipin. J. Biol. Chem. 1993;268:22914–22919. [PubMed] [Google Scholar]
- 8.Petrosillo G., Ruggiero F. M., Di Venosa N., Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and mitochondria. FASEB J. 2003;17:714–716. doi: 10.1096/fj.02-0729fje. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M., Mileykovskaya E., Dowhan W. Glueing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- 10.McMillin J. B., Dowhan W. Cardiolipin and apoptosis. Biochim. Biophys. Acta. 2002;1585:97–107. doi: 10.1016/s1388-1981(02)00329-3. [DOI] [PubMed] [Google Scholar]
- 11.Esposti M. D. Lipids, cardiolipin and apoptosis: a greasy license to kill. Cell Death Differ. 2002;9:234–236. doi: 10.1038/sj.cdd.4400997. [DOI] [PubMed] [Google Scholar]
- 12.Xu F. Y., Kelly S. L., Hatch G. M. N-acetylsphingosine stimulates phosphatidylglycerolphosphate synthase activity in H9c2 cardiac cells. Biochem. J. 1999;337:483–490. [PMC free article] [PubMed] [Google Scholar]
- 13.Esposti M. D., Cristea I. M., Gaskell S. J., Nakao Y., Dive C. Proapoptotic Bid binds to monolysocardiolipin, a new molecular collection between mitochondrial membranes and cell death. Cell Death Differ. 2003;10:1300–1309. doi: 10.1038/sj.cdd.4401306. [DOI] [PubMed] [Google Scholar]
- 14.Hauff K., Hatch G. M. Cardiolipin metabolism in Barth Syndrome. Prog. Lipid Res. 2006;45:91–101. doi: 10.1016/j.plipres.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Bevers E. M., Comfurius P., Dekkers D. W., Zwaal L. F. Lipid translocation across the plasma membrane of mammalian cells. Biochim. Biophys. Acta. 1999;1439:317–330. doi: 10.1016/s1388-1981(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 16.Yu A., McMaster C. R., Byers D. M., Ridgway N. D., Cooke H. W. Stimulation of phosphatidylserine biosynthesis and facilitation of UV-induced apoptosis in Chinese hamster ovary cells overexpressing phospholipid scramblase 1. J. Biol. Chem. 2003;278:9706–9714. doi: 10.1074/jbc.M204614200. [DOI] [PubMed] [Google Scholar]
- 17.Liu J., Dai Q., Freeman A., Chen J., Grossman D., Lee R. M. Phospholipid scramblase 3 controls mitochondrial structure, function and apoptotic response. Mol. Cancer Res. 2003;1:892–902. [PubMed] [Google Scholar]
- 18.Liu J., Chen J., Dai Q., Lee R. M. Phospholipid scramblase 3 is the mitochondria target of protein kinase C delta-induced apoptosis. Cancer Res. 2003;63:1153–1163. [PubMed] [Google Scholar]
- 19.Hatch G. M. Cardiolipin biosynthesis in the isolated heart. Biochem. J. 1994;297:201–208. doi: 10.1042/bj2970201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor W. A., Hatch G. M. Purification and characterization of monolysocardiolipin acyltransferase from pig liver mitochondria. J. Biol. Chem. 2003;278:12716–12721. doi: 10.1074/jbc.M210329200. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y., Kelley R. I., Blank T. J. J., Schlame M. Remodeling of cardiolipin by phospholipid transacylation. J. Biol. Chem. 2003;278:51380–51385. doi: 10.1074/jbc.M307382200. [DOI] [PubMed] [Google Scholar]
- 22.Cao S. G., Angel A., Hatch G. M. Decrease in cardiac phosphatidylglycerol in streptozotocin-induced diabetic rats does not affect cardiolipin biosynthesis: evidence for distinct pools of phosphatidylglycerol in the heart. Biochem J. 1995;306:759–764. doi: 10.1042/bj3060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao J., Liu Y., Lockwood J., Burn P., Shi Y. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J. Biol. Chem. 2004;279:31727–31734. doi: 10.1074/jbc.M402930200. [DOI] [PubMed] [Google Scholar]
- 24.Lu B., Xu F., Jiang Y. J., Choy P. C., Hatch G. M., Grunfeld C., Feingold K. R. Cloning and characterization of a gene encoding human cardiolipin synthase (hCLS1) J. Lipid Res. 2006;47:1140–1145. doi: 10.1194/jlr.C600004-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Webster J., Jiang J., Lu B., Xu F., Taylor W., Mymin D., Zhang M., Minuk G., Hatch G. M. On the mechanism of the increase in cardiolipin biosynthesis and resynthesis in hepatocytes during rat liver regeneration. Biochem. J. 2005;386:137–143. doi: 10.1042/BJ20040655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma B. J., Taylor W. A., Dolinsky V. W., Hatch G. M. Acylation of monolysocardiolipin in rat heart. J. Lipid Res. 1999;40:1837–1845. [PubMed] [Google Scholar]
- 27.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Ma B. J., Hatch G. M. Monolysocardiolipin acyltransferase activity: effect of insulin and localization of activity. Proc. West. Pharmacol. Soc. 2000;43:39–41. [PubMed] [Google Scholar]
- 29.Ma B. J., Taylor W. A., Dolinsky V. W., Hatch G. M. Acylation of monolysocardiolipin in rat heart. J. Lipid Res. 1999;40:1837–1845. [PubMed] [Google Scholar]
- 30.Smith E. R., Merrill A. H., Jr Differential roles of de novo sphingolipid biosynthesis and turnover in the ‘burst’ of free sphingosine and sphinganine, and their 1-phosphates and N-acyl-derivatives, that occurs upon changing the medium of cells in culture. J. Biol. Chem. 1995;270:18749–18758. doi: 10.1074/jbc.270.32.18749. [DOI] [PubMed] [Google Scholar]
- 31.Hostetler K. Y., van den Bosch H. Subcellular and submitochondrial localization of the biosynthesis of cardiolipin and related phospholipids in rat liver. Biochim. Biophys. Acta. 1972;260:380–386. doi: 10.1016/0005-2760(72)90052-5. [DOI] [PubMed] [Google Scholar]
- 32.Schlame M., Haldar D. Cardiolipin is synthesized on the matrix side of the inner membrane in rat liver mitochondria. J. Biol. Chem. 1993;268:74–79. [PubMed] [Google Scholar]
- 33.Sorice M., Cirella A., Cristea I. M., Garofalo T., Di Renzo L., Alessandri C., Valesini G., Esposti M. D. Cardiolipin and its metabolites move from mitochondria to other cellular membranes during death receptor-mediated apoptosis. Cell Death Differ. 2004;11:1133–1145. doi: 10.1038/sj.cdd.4401457. [DOI] [PubMed] [Google Scholar]
- 34.Vance J. E. Molecular and cell biology of phosphatidylserine and phosphatidylethanolamine metabolism. Prog. Nucleic Acid Res. Mol. Biol. 2003;75:69–111. doi: 10.1016/s0079-6603(03)75003-x. [DOI] [PubMed] [Google Scholar]