Abstract
Besides SPAM1 (sperm adhesion molecule 1; formerly named PH-20), further hyaluronidase-like proteins, HYAL5 (hyaluronoglucosaminidase 5) and HYALP1 (hyaluronoglucosaminidase pseudogene 1) are also expressed in murine testicular tissue. As they share a high degree of sequence similarity with known hyaluronidases, all three polypeptides could potentially exhibit hyaluronidase activity, a function that is beneficial for spermatozoa in order to penetrate the hyaluronan-rich cumulus, which surrounds the oocyte. Recently, it was reported that SPAM1-deficient mice are fertile and spermatozoa derived from mutant mice still exhibit hyaluronidase activity [Baba, Kashiwabara, Honda, Yamagata, Wu, Ikawa, Okabe and Baba (2002) J. Biol. Chem. 277, 30310–30314]. We have now recombinantly expressed mouse SPAM1, HYAL5 and HYALP1 in Xenopus laevis oocytes and determined their respective expression pattern in testis. Transcripts of all three genes are expressed in seminiferous tubules in regions where maturing spermatogenic cells reside. SPAM1 and HYAL5 but not HYALP1 proteins exhibit hyaluronidase activity at neutral pH. The two active hyaluronidases are both bound to the cell surface via a glycosylphosphatidylinositol anchor. Furthermore, structural characteristics are discussed that are necessary for hyaluronidases in order to exhibit hyaluronan cleavage.
Keywords: extracellular matrix, fertilization, glycosaminoglycan, hyaluronan, hyaluronidase, sperm adhesion molecule 1 (SPAM1)
Abbreviations: ECM, extracellular matrix; GAG, glycosaminoglycan; GPI, glycosylphosphatidylinositol; HYAL, hyaluronoglucosaminidase; HYALP1, hyaluronoglucosaminidase pseudogene 1; PLC, phospholipase C; RT, reverse transcriptase; SPAM1, sperm adhesion molecule 1
INTRODUCTION
Hyaluronan, a polymer consisting of repeating β-1,4-linked disaccharide units of N-acetyl-D-glucosamine and D-glucuronic acid, is a common GAG (glycosaminoglycan) present in the ECM (extracellular matrix) of connective tissues [1]. Hyaluronan is implicated in many physiological processes, including cell migration, proliferation and differentiation. Hyaluronidases, enzymes that hydrolyse hyaluronan to oligosaccharides, have been identified in mammalian, insect and bacterial species [2,3].
Hyaluronan accumulates between cumulus cells [4] together with inter-α trypsin inhibitor [5] as well as a variety of other matrix proteins [6–8]. The jelly-like shell protects the oocyte and facilitates its extrusion at ovulation [9]. Furthermore, the swollen cumulus is more easily captured by the fimbria of the oviduct and thus supports oocyte transportation to the site of fertilization [10]. The tight matrix however is difficult to perforate by mechanical means. Spermatozoa hyaluronidase activity positively correlates with fertilization rate, because spermatozoa that possess high motility and fertilization efficiency penetrate highly viscous solutions of hyaluronan at a higher rate [11,12]. Therefore the motility and spermatozoa hyaluronidase activity are thought to facilitate path finding through the ECM of cumulus cells, since no proteases have yet been implicated in this process [13]. A widely conserved mammalian spermatozoa surface protein, PH-20 [14], also called SPAM1 (sperm adhesion molecule 1) [15,16], is well known for its hyaluronidase activity [17,18]. This GPI (glycosylphosphatidylinositol)-anchored spermatozoa hyaluronidase has multiple roles in fertilization [19]. SPAM1 and sHYAL2 (soluble form of hyaluronoglucosaminidase 2) exhibit activity at both neutral and acidic pH [20], whereas for other hyaluronidases, enzymatic activity appears to be restricted to either acidic pH, e.g. HYAL1 [21], or neutral pH as known for Xenopus XKH1 [22].
Unexpectedly, mice carrying a null mutation in the Spam1 gene are fertile [23]. In vitro fertilization assays showed that mouse spermatozoa, lacking SPAM1, possess a reduced ability to disperse cumulus cells from the cumulus mass, resulting in delayed fertilization solely at the early stages after insemination. In this particular context, spermatozoa extracts derived from mutant mice contain at least one further hyaluronidase, HYAL5, which is active in solutions of both neutral pH and extracellular ionic composition [24].
In the mouse genome, there are seven hyaluronidase-like sequences, six of them clustered in groups of three at two chromosomal sites, at chromosome 9 F1–F2 (Hyal1, Hyal2 and Hyal3) and at chromosome 6 A2 (Hyal4, Spam1/PH-20 and Hyalp1) [23,25,26]. The orthologous human genes are localized on syntenic regions, on chromosomes 3p21.3 and 7q31.3. HYALP1 is an expressed pseudogene in humans [27]. In mouse, Hyalp1 shows more characteristics of a gene that codes for a transcript that can be translated into a polypeptide chain similar to Spam1. The seventh mouse hyaluronidase-like gene, tentatively called Hyal5, is localized in close proximity to the cluster at chromosomal region 6 A2. So far, no further hyaluronidase-like gene has been discovered in the human genome at this position. Both genes, Hyalp1 and Hyal5, encode open reading frames for a polypeptide highly similar to the biochemically characterized hyaluronidases [26]. Moreover, these three genes are strongly expressed in testis. So far, none of these murine gene products has been recombinantly expressed and assayed for enzymatic activity. Hence, HYAL5, HYALP1 or both could actually be functionally involved in fertilization in the mouse. We therefore expressed these proteins in frog oocytes and studied their enzymatic properties with respect to hyaluronan degradation.
MATERIALS AND METHODS
Frog surgery, oocytes preparation, cDNA construction and cRNA injection
A small lobe of ovary was surgically removed from an anaesthetized adult Xenopus laevis female. The ovarian tissue was rinsed in O-R2 buffer (82.5 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM Na2HPO4 and 5 mM Hepes, pH 7.8) and individual oocytes were defolliculated manually. The coding regions of Spam1, Hyal5 and Hyalp1 were cloned into pT7TS (a gift from P. A. Krieg, University of Arizona, Tucson, AZ, U.S.A.), suitable for generating in vitro-transcribed mRNA. Hyal5 cDNA (clone ID IMAGp998B198849.1) was purchased from the German Resource Centre Berlin (RZPD). The protein-coding regions of Spam1 and Hyalp1 were amplified by PCR using cDNA derived from mouse testis as a template. To amplify Spam1, the following primers were used, 5′-GGTGATCAATGGGAGAGTTGAGATTTAAGCACCTC-3′ and 5′-CCGCTAGCCTAAGGAGTACTGACTAGTGTCTTCCG-3′; and to amplify Hyalp1 the following primers were used, 5′-TCGAGATATCATGTTTATCCAGTGGGTGACACAG-3′ and 5′-GGACTAGTTTAGAAAATGCTTGAAATCAAAGA-3′.
Approx. 50 ng of capped cRNA was injected into oocytes, using a Nanoliter injector (World Precision Instruments), that were then cultured in O-R2 containing 100 units/ml penicillin and 100 μg/ml streptomycin at 16 °C for up to 2 days. GPI-linked proteins were removed from the plasma membranes using 50 milliunits of Bacillus cereus PLC (phospholipase C; Molecular Probes) in 30 μl of O-R2 buffer (pH 7.0) containing 4 mM CaCl2 and 50 μg/ml BSA per oocyte at ambient temperature for 1 h.
Expression analysis
For expression analysis, RNA was extracted as described previously [28]. Mice were dissected. Specimens were carefully rinsed in PBS and snap frozen in liquid nitrogen. Cells or pieces of tissue were homogenized in 4.2 M guanidinium thiocyanate and 100 mM Hepes (pH 7.5). The resulting solution was extracted with phenol/chloroform/methylbutanol (25:50:1) and the aqueous phase was supplemented with 10% (v/v) 2 M sodium acetate (pH 4.4) and 20% (v/v) water and extracted with phenol/chloroform (3:1; v/v). The RNA within the aqueous phase was precipitated by addition of an equal volume of propan-2-ol. The RNA pellet was rinsed with 80% (v/v) ethanol, dried and dissolved in 6 M urea and 100 mM Hepes (pH 7.5). As a final purification step the RNA was precipitated by 50% (v/v) 9 M LiCl. Total RNA (5 μg) derived from various tissues and organs was separated by 1.2% agarose gel electrophoresis and subsequently transferred to Zetaprobe nylon membrane (Bio-Rad). After UV-cross-linking, blots were hybridized with 33P-labelled cDNA probes. RT (reverse transcriptase)-PCR assays were carried out using specific primer pairs for β-actin, Hyal5 and Hyalp1 on template cDNAs derived from a range of C57BL/6J mouse tissues, and from a murine Sertoli cell line, TM4 [29]. PCR was performed in 25 μl reaction volumes, containing 1 μM primer and 2.5 units of Pfu DNA polymerase in reaction buffer with MgSO4 (as recommended by the manufacturer; Fermentas) for 30 and 40 cycles at 95 °C for 1 min, 58 °C for 1 min and 72 °C for 1 min. Subsequently, the products were analysed on a 2% agarose gel. Quantitative RT–PCR assays were performed using a LightCycler instrument and LightCycler 3.5 software (Roche). The reactions (15 μl) were carried out using the LC-FastStart DNA Master SYBR Green I kit (Roche), 2 μM primer and 3 mM MgCl2. After the activation of the enzyme at 95 °C for 7 min, 45 amplification cycles were performed at 95 °C for 15 s, 58 °C for 8 s and 72 °C for 12 s. The following oligonucleotide primers were used in order to amplify cytoplasmic β-actin: forward 5′-GGCTGTATTCCCCTCCATCG-3′, reverse 5′-CCAGTTGGTAACAATGCCATGT-3′; Hyal5: forward 5′-AAAGACCCTCGAAATCCAC-3′, reverse 5′-CCACATACCATTCCAGAGG-3′; and Hyalp1: forward 5′-TGTCACTTTGTGGAGATCGGA-3′, reverse 5′-GCCAACGTGAGAGTAACATTCA-3′. These primer pairs yielded amplification products of 154 bp for β-actin, 144 bp for Hyal5 and 222 bp for Hyalp1. The relative abundance of Hyal5 and Hyalp1 transcripts was calculated relative to β-actin, and the values were normalized to the expression level in testis.
In situ hybridization was performed as described previously [30]. Briefly, testicular tissues from C57BL/6J mice were fixed in PBS containing 4% (v/v) paraformaldehyde. The tissue was embedded in Paraplast Plus (Sigma), sectioned to a thickness of 7 μm and mounted on poly(L-lysine)-coated microscope slides. After treatment with proteinase K and prehybridization, sections were incubated at 50 °C with digoxigenin-labelled RNA probes in hybridization buffer containing 50% (v/v) formamide, 1×SSC (0.15 M NaCl and 0.015 M sodium citrate), 1×Denhardt's solution (0.02% Ficoll 400, 0.02% polyvinylpyrrolidone and 0.02% BSA), 5 mM EDTA, 50 μg/ml yeast RNA, 0.2% Tween 20, 0.5% CHAPS and 10 μg/ml heparin. Thereafter, sections were washed with hybridization buffer and the probes were detected using anti-digoxigenin alkaline phosphatase Fab conjugate and BM Purple as a substrate (Roche).
Hyaluronidase activity measurement
Enzyme activity was measured as described previously [31]. Briefly, enzymes were mixed with 5-aminofluorescein-labelled hyaluronan and incubated at different pH values (100 mM sodium phosphate buffer containing 50 μg/ml BSA and 0.1% Triton X-100 first adjusted with citric acid to pH 3.5 and then titrated to the appropriate pH with sodium hydroxide). The reaction mixture was separated by 1% agarose gel electrophoresis. The gel was subsequently blotted on to a positively charged nylon membrane (Biodyne PLUS, 0.45 μm; Pall Life Sciences) and the hapten was detected after incubation with anti-fluorescein alkaline phosphatase Fab conjugate (Roche) by means of enhanced chemiluminescence technology (Amersham Biosciences).
Molecular modelling
Tertiary structural models for the hyaluronidase domains of SPAM1, HYAL5 and HYALP1 were generated applying homology modelling of SWISS-MODEL [32] using its alignment interface together with mouse hyaluronidase-like protein sequences of SPAM1 (accession number AAP49832), HYAL5 (accession number BAC55071) and HYALP1 (accession number BAB30316) pre-aligned with the aid of ClustalW and the X-ray structure of bee hyaluronidase (PDB accession code: 1fcv). Images were generated with the aid of DeepView/Swiss-PdbViewer version 3.7 [33].
RESULTS
Structural features
SPAM1, HYAL5 and HYALP1 were found to share conserved elements with hyaluronidase from honeybee. Besides the first highly conserved N-terminal domain, all mammalian hyaluronidase-like proteins have a second domain at the C-terminal part of the amino acid sequence. For SPAM1-type hyaluronidases, processing and GPI anchor linkage are needed at the C-terminus in order to bind the enzyme to the outer surface of the plasma membrane [34]. Bioinformatic prediction of whether one of the three different mouse proteins would contain a consensus pattern for GPI linkage yielded inconclusive results [35,36]. Therefore it did not become clear from these theoretical considerations which one of the three hyaluronidase-like genes would potentially code for a hyaluronan-degrading enzyme, and thus is functionally involved in fertilization in mouse.
Expression pattern
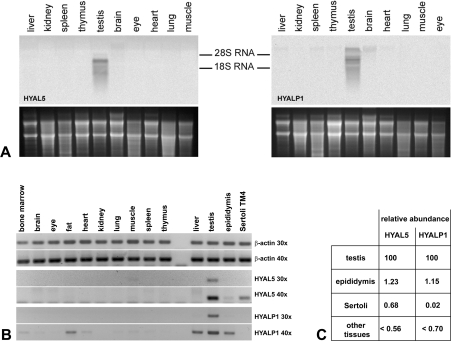
Using Northern blots, Hyal5 as well as Hyalp1 were found to be expressed in mouse testis (Figure 1A). Like Spam1, transcripts of the other two genes are barely detectable within other tissues (Figure 1B). Applying quantitative RT-PCR techniques, trace amounts of both Hyal5 and Hyalp1 could be detected in epididymis. This may well be due to spermatozoa present in the highly convoluted epididymal duct. In the TM4 Sertoli cell line [29,37], only Hyal5 transcripts could be detected with the absence of Hyalp1 transcript. A small number of Hyalp1 transcripts as compared with the amount found in testis was also detected in liver, bone marrow, brain, fat, skeletal muscle, lung and spleen as well as in epididymis (Figures 1B and 1C). As shown previously, SPAM1 and HYAL5 proteins appear to be present on acrosome intact spermatozoa [23]. Furthermore, HYALP1 has been found not only in testis and caudal spermatozoa but also in spermatozoa-free epididymis [24].
Figure 1. Expression analysis.
Hyal5 and Hyalp1 mRNA expression was determined in various tissues using Northern-blot analyses (A), RT-PCR (B) and quantitative PCR analysis (C). As a loading control, an image of the ethidium bromide-stained agarose gel before transfer to the nylon membrane for hybridization with α-33P-labelled probe is shown in the lower part of (A). For relative quantification after PCR (B and C), template cDNAs were diluted with regard to β-actin mRNA content. Amplification was for either 30 or 40 cycles, as indicated. After evaluation of quantitative PCR results, the transcript levels were further normalized to the respective mRNA content of Hyal5 or Hyalp1 in testis (C).
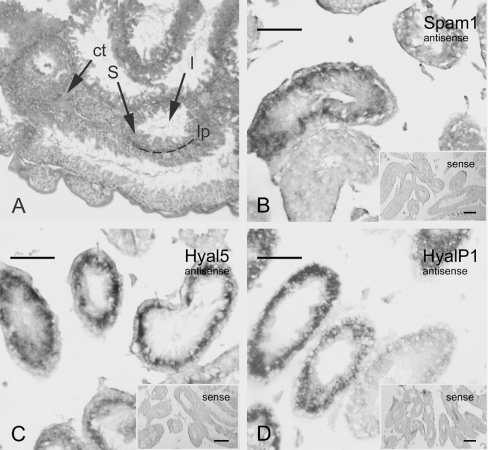
The precise distribution of Spam1, Hyal5 and Hyalp1 transcripts in testicular tissue was determined by in situ hybridization (Figure 2). All three hyaluronidase-like transcripts show an equivalent expression pattern of a ring-shaped area within seminiferous tubules (Figures 2B, 2C and 2D). Some of the sectioned tubules show weak to no signals for Spam1, Hyal5 and Hyalp1 expression. For controls, hybridizations with sense probes were performed in parallel. Low background and no specific signals were observed (Figures 2B, 2C and 2D; inlaid micrographs). In sub-human species, a particular cellular association consisting of spermatogenic cells of a distinct stage occupies a relatively long segment along the length of the tubule. High expression of Hyal5 or Hyalp1 transcripts in Sertoli cells appears unlikely since only minor amounts were detected in cultured TM4 cells. We therefore deduced from this expression pattern that transcripts of mouse hyaluronidase-like genes are accumulating in spermatogenic cells, most probably in pachytene and/or leptotene spermatocytes (stage I, II, V and/or VI). No expression was found near or at the basal lamina, where type A and B spermatogonia, preleptotene spermatocytes of stage III and IV reside. Due to the expression pattern, we conclude that all three polypeptides, SPAM1, HYAL5 and/or HYALP1 protein, are present in spermatids and could thus also bind to mature spermatozoa.
Figure 2. Transcript localization in testicular tissue.
(A) Histological section of murine testis stained with haematoxylin/eosin. Indicated is connective tissue between testicular tubules (ct), the lumen of a tubule (l), the cellular layer containing maturing spermatogenic cells (S) and the lamina propria (lp), the outer layer of tubules. (B–D) mRNA distribution of Hyal5, Hyalp1 and Spam1 in testicular tissue was determined by in situ hybridization on histological sections either with antisense or sense probes (inlaid micrographs). Digoxigenin-labelled antisense RNA probes were detected with the aid of anti-digoxigenin Fab alkaline phosphatase complex and BM Purple (Roche) as substrate. Scale bars=100 μm.
Enzymatic activity
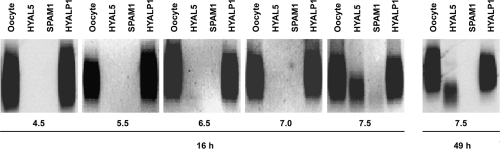
Since mouse HYAL5, HYALP1 and SPAM1 have not been recombinantly expressed to examine their hyaluronidase activities, we translated in vitro-transcribed cRNA of all three candidates in intact X. laevis oocytes. Cell lysates were tested for hyaluronidase activity using a sensitive assay [31]. For HYAL5 and SPAM1, hyaluronidase activity was found in a broad pH range under both acid and neutral conditions. Residual activity could still be measured at pH 7.5 (Figure 3). In contrast with other hyaluronidases such as HYAL2, which primarily generates intermediate length reaction products of approx. 20 kDa in size, both HYAL5 and SPAM1 were found to degrade hyaluronan to short fragments. No hydrolysis was detectable after reaction of HYAL5- and SPAM1-expressing ooyctes with GAGs such as chondroitin sulfate A, B and C or heparan sulfate as substrates (results not shown). For recombinant HYALP1, no hydrolysing activity with respect to hyaluronan was detectable although Hyalp1 and Hyal5 cRNA were equally well translated in vitro in the presence of rabbit reticulocyte lysate (Figure 4). When cell-free expression systems together with microsomal preparations were used for the translation of cRNAs that encode well-characterized hyaluronidases, in no case were enzymatically active protein products obtained. Hence, we extensively tested HYALP1-expressing oocytes or corresponding culture supernatants, in order to detect the presence of trace amounts of hyaluronan-degrading activity. Besides SPAM1 and HYAL5, we observed that the positive controls, human PH-20, HYAL1, honeybee hyaluronidase, Xenopus XKH1 [22] and XHyal2 [38], were enzymatically active when expressed in Xenopus oocytes. Yet no hyaluronan-hydrolysing activity was detectable after Hyalp1 cRNA injection either in cells or in the culture supernatant. Furthermore, chondroitin sulfate A and C as well as heparan sulfate were not depolymerized by HYALP1-expressing oocytes (results not shown).
Figure 3. Hyaluronidase activity of HYAL5- and HYALP1-expressing X. laevis oocytes.
Oocytes injected with cRNA encoding Spam1, Hyal5 or Hyalp1 and uninjected controls (oocyte) were cultured in 200 μl of O-R2 buffer for 24 h. Homogenized oocytes expressing hyaluronidase-like proteins were incubated with 20 ng of 5-aminofluorescein-labelled hyaluronan for either 16 or 49 h respectively. Hyaluronidase activity was tested at different pH values as indicated.
Figure 4. In vitro translation of Hyal5 and Hyalp1 cRNAs.
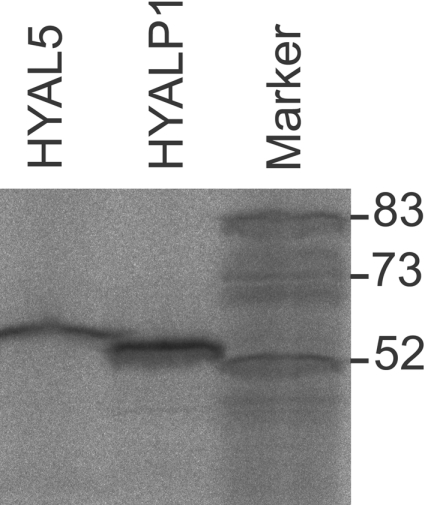
After protein synthesis with the aid of a cell-free reticulocyte system (Novagen), 35S-labelled protein products were separated by SDS/PAGE and detected by means of fluorography and phosphoimaging.
Next, frog oocytes expressing HYALP1, HYAL5 and SPAM1 were incubated with PLC in order to test whether the enzymes had been linked via GPI anchors to the cell surface. In the cases of HYAL5 and SPAM1, enzyme activity was found in the supernatant of PLC-treated oocytes (Figure 5). HYAL2, a hyaluronidase that is normally bound to the plasma membrane via a GPI anchor and inactive at neutral pH [39], was however found to exhibit weak enzymatic activity also at neutral pH, when recombinantly expressed in its soluble form, sHYAL2 [20]. In the case of HYALP1-expressing oocytes, no hyaluronan-degrading activity was detectable in the supernatant. Notably, the rat homologue of SPAM1, 2B1 glycoprotein (PH-20), is also not rendered free from the cell surface by PLC treatment [40]. We therefore have to admit that HYALP1 could be linked to the membrane and only exerts enzymatic activity after being released by a yet unknown process. In conclusion, HYAL5 and SPAM1, after entering the secretory pathway, are sorted and eventually bind through a GPI anchor to the plasma membrane as active hyaluronidases.
Figure 5. Subcellular localization of HYAL5, HYALP1 and SPAM1.
Hyal5, HyalP1 and Spam1 cRNAs respectively were translated in intact Xenopus oocytes. Oocytes were incubated for 2 days in O-R2. Subsequently, healthy oocytes were subjected to PLC treatment (indicated by +) or phospholipase buffer without enzyme (−). The supernatant of these reactions was mixed with fluorescein-labelled hyaluronan and soluble hyaluronidase activity was determined using standard conditions at pH 6.5.
DISCUSSION
In testicular extracts, hyaluronidase activity has been recognized previously by biochemical means [41]. Molecular cloning of the hyaluronidase present in bee venom [17] revealed a strong similarity with a known protein that is localized on the posterior head of human spermatozoa, first characterized by means of a monoclonal antibody, and was therefore called PH-20 [14]. PH-20 from humans exhibits strong and robust hyaluronidase activity at neutral pH [18,42,43]. As recently described, point mutations not only in Spam1, but also in other hyaluronidase-like genes such as Hyal5 and HyalP1, correlate with spermatozoa dysfunction [26]. As pointed out above, no further gene that potentially would encode hyaluronidase-like proteins other than HYAL1–4 and PH-20/SPAM1 has been discovered in the human genome to date. Besides mouse, Hyal5 is also present in rat (accession number BC091219). This suggests that further gene duplication has occurred in rodents [26]. Yet, mature spermatozoa of SPAM1-deficient mice still exhibit hyaluronidase activity. It could be used to fertilize eggs [23] and is able to disperse cumulus cells from the cumulus mass [24]. In light of our results, we assume that this is due to functionally active HYAL5 present on the surface of Spam1−/− spermatozoa. We furthermore conclude, after having shown that SPAM1 and HYAL5 are both functionally active hyaluronidases that both enzymes participate in spermatozoa fertilization of the egg.
The biological role for HYALP1 remains to be defined. SPAM1, HYAL5 and HYALP1 (39–64% identical; 64–81% similar) also share identical residues and a high degree of similarity with other well-characterized hyaluronidases such as human PH-20 or bee hyaluronidase. The most remarkable structural feature of hyaluronidase is a large groove [44] (Figure 6). The amino acid residues of the groove that were found to come in close proximity to the substrate are arranged in well-conserved sequence blocks, and we thus consider them functionally relevant for proper positioning of the sugar polymer. In particular, we propose that four more amino acid moieties (indicated by the symbols shown in Figure 6A) that are located in close proximity to the active centre control the positioning of the substrate at the active centre. Two amino acid residues were found conserved in all three proteins (open and closed circles in Figure 6A). The other two are different in HYALP (labelled with a square and a triangle in Figure 6A). Molecular modelling revealed a close relationship between the geometries of the active centres of bee hyaluronidase, SPAM1 and HYAL5. However, major spatial differences were evident in the structure of HYALP1, in particular amino acid moieties that appear to be important for the proper positioning of the substrate with regard to the acidic amino acid moieties within the active centre (Figure 6B). The active sites of these enzymes consist of aspartate and glutamate residues interspaced by one amino acid residue [45,46]. Notably, HYAL5 and SPAM1, yet also HYALP1, show this particular feature. Since both active centre residues are present in HYALP1, we assume that the protein, although hydrolysis of hyaluronan was undetectable, might exhibit some yet unknown enzymatic activities. For the substrate binding groove of HYALP1 that showed a high resemblance to the structures of bee hyaluronidase and the structural models of other known hyaluronidases, binding of hyaluronan or other GAGs could indeed be possible. Human PH-20 exhibits many more functions besides hyaluronan degradation [19]. Whether HYALP1 is functionally involved in metabolism of GAG or plays some role during spermatozoa maturation will be investigated in future experiments.
Figure 6. Structural models.
(A) Mouse hyaluronidase-like protein sequences, SPAM1 (accession number AAP49832), HYAL5 (accession number BAC55071) and HYALP1 (accession number BAB30316), were pre-aligned using ClustalW and the resulting alignment was finally adjusted manually. Amino acid residues identical in all three sequences were box-shaded. (B) On the basis of the X-ray structure of bee hyaluronidase (PDB accession 1FCV), three-dimensional structural models for SPAM1, HYAL5 and HYALP1 were generated (upper row). Residues that were marked with ○, ●, ■ and ▲ in (A) were found to be located opposite and in close proximity to the active centre (ac) within the tapering substrate-binding cleft. These appear to interact directly with the substrate. The three-dimensional models were slabbed along the line drawn in the frontal view of the bee hyaluronidase (bee hyase) structure. Surfaces were drawn in light grey; interior parts of the molecules in dark colour. Side projections of the active centre reveal variant geometries of the active centres of active hyaluronidases (bee hyaluronidase, HYAL5 and SPAM1) and inactive HYALP1.
Acknowledgments
S.R. is a DOC fellow of the Austrian Academy of Sciences. G.L. is an APART fellow of the Austrian Academy of Sciences and was further supported by the Jubilee Fund of the Austrian National Bank as well as by the Austrian Science Fund, project S9309-B09. We are grateful to Günther Kreil for fruitful discussions and a careful reading of this paper.
References
- 1.Laurent T. C., Laurent U. B, Fraser J. R. The structure and function of hyaluronan: an overview. Immunol. Cell Biol. 1996;74:A1–A7. doi: 10.1038/icb.1996.32. [DOI] [PubMed] [Google Scholar]
- 2.Kreil G. Hyaluronidases – a group of neglected enzymes. Protein Sci. 1995;4:1666–1669. doi: 10.1002/pro.5560040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern R. Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology. 2003;13:105R–115R. doi: 10.1093/glycob/cwg112. [DOI] [PubMed] [Google Scholar]
- 4.Salustri A., Yanagishita M., Underhill C. B., Laurent T. C., Hascall V. C. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev. Biol. 1992;151:541–551. doi: 10.1016/0012-1606(92)90192-j. [DOI] [PubMed] [Google Scholar]
- 5.Chen L., Zhang H., Powers R. W., Russell P. T., Larsen W. J. Covalent linkage between proteins of the inter-alpha-inhibitor family and hyaluronic acid is mediated by a factor produced by granulosa cells. J. Biol. Chem. 1996;271:19409–19414. doi: 10.1074/jbc.271.32.19409. [DOI] [PubMed] [Google Scholar]
- 6.Salustri A., Camaioni A., Di Giacomo M., Fulop C., Hascall V. C. Hyaluronan and proteoglycans in ovarian follicles. Hum. Reprod. Update. 1999;5:293–301. doi: 10.1093/humupd/5.4.293. [DOI] [PubMed] [Google Scholar]
- 7.Camaioni A., Salustri A., Yanagishita M., Hascall V. C. Proteoglycans and proteins in the extracellular matrix of mouse cumulus cell–oocyte complexes. Arch. Biochem. Biophys. 1996;325:190–198. doi: 10.1006/abbi.1996.0024. [DOI] [PubMed] [Google Scholar]
- 8.Richards J. S. Ovulation: new factors that prepare the oocyte for fertilization. Mol. Cell. Endocrinol. 2005;234:75–79. doi: 10.1016/j.mce.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Richards J. S. Delivery of the oocyte from the follicle to the oviduct: a time of vulnerability. Ernst Schering Res. Found. Workshop. 2002:43–62. doi: 10.1007/978-3-662-04960-0_4. [DOI] [PubMed] [Google Scholar]
- 10.Talbot P., Geiske C., Knoll M. Oocyte pickup by the mammalian oviduct. Mol. Biol. Cell. 1999;10:5–8. doi: 10.1091/mbc.10.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aitken R. J., Bowie H., Buckingham D., Harkiss D., Richardson D. W., West K. M. Sperm penetration into a hyaluronic acid polymer as a means of monitoring functional competence. J. Androl. 1992;13:44–54. [PubMed] [Google Scholar]
- 12.Mortimer D., Mortimer S. T., Shu M. A., Swart R. A simplified approach to sperm-cervical mucus interaction testing using a hyaluronate migration test. Hum. Reprod. 1990;5:835–841. doi: 10.1093/oxfordjournals.humrep.a137194. [DOI] [PubMed] [Google Scholar]
- 13.Primakoff P., Myles D. G. Penetration, adhesion, and fusion in mammalian sperm–egg interaction. Science. 2002;296:2183–2185. doi: 10.1126/science.1072029. [DOI] [PubMed] [Google Scholar]
- 14.Myles D. G., Primakoff P. Localized surface antigens of guinea pig sperm migrate to new regions prior to fertilization. J. Cell Biol. 1984;99:1634–1641. doi: 10.1083/jcb.99.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones M. H., Davey P. M., Aplin H., Affara N. A. Expression analysis, genomic structure, and mapping to 7q31 of the human sperm adhesion molecule gene SPAM1. Genomics. 1995;29:796–800. doi: 10.1006/geno.1995.9931. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y., Martin-Deleon P. A. The murine Spam1 gene: RNA expression pattern and lower steady-state levels associated with the Rb(6.16) translocation. Mol. Reprod. Dev. 1997;46:252–257. doi: 10.1002/(SICI)1098-2795(199703)46:3<252::AID-MRD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 17.Gmachl M., Kreil G. Bee venom hyaluronidase is homologous to a membrane protein of mammalian sperm. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3569–3573. doi: 10.1073/pnas.90.8.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gmachl M., Sagan S., Ketter S., Kreil G. The human sperm protein PH-20 has hyaluronidase activity. FEBS Lett. 1993;336:545–548. doi: 10.1016/0014-5793(93)80873-s. [DOI] [PubMed] [Google Scholar]
- 19.Cherr G. N., Yudin A. I., Overstreet J. W. The dual functions of GPI-anchored PH-20: hyaluronidase and intracellular signaling. Matrix Biol. 2001;20:515–525. doi: 10.1016/s0945-053x(01)00171-8. [DOI] [PubMed] [Google Scholar]
- 20.Vigdorovich V., Strong R. K., Miller A. D. Expression and characterization of a soluble, active form of the jaagsiekte sheep retrovirus receptor, Hyal2. J. Virol. 2005;79:79–86. doi: 10.1128/JVI.79.1.79-86.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frost G. I., Csoka T. B., Wong T., Stern R. Purification, cloning, and expression of human plasma hyaluronidase. Biochem. Biophys. Res. Commun. 1997;236:10–15. doi: 10.1006/bbrc.1997.6773. [DOI] [PubMed] [Google Scholar]
- 22.Reitinger S., Müllegger J., Lepperdinger G. Xenopus kidney hyaluronidase-1 (XKH1), a novel type of membrane-bound hyaluronidase solely degrades hyaluronan at neutral pH(1) FEBS Lett. 2001;505:213–216. doi: 10.1016/s0014-5793(01)02813-7. [DOI] [PubMed] [Google Scholar]
- 23.Baba D., Kashiwabara S., Honda A., Yamagata K., Wu Q., Ikawa M., Okabe M., Baba T. Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J. Biol. Chem. 2002;277:30310–30314. doi: 10.1074/jbc.M204596200. [DOI] [PubMed] [Google Scholar]
- 24.Kim E., Baba D., Kimura M., Yamashita M., Kashiwabara S. I., Baba T. Identification of a hyaluronidase, Hyal5, involved in penetration of mouse sperm through cumulus mass. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18028–18033. doi: 10.1073/pnas.0506825102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Csoka A. B., Frost G. I., Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H., Shertok S., Miller K., Taylor L., Martin-Deleon P. A. Sperm dysfunction in the Rb(6.16)- and Rb(6.15)-bearing mice revisited: involvement of Hyalp1 and Hyal5. Mol. Reprod. Dev. 2005;72:404–410. doi: 10.1002/mrd.20360. [DOI] [PubMed] [Google Scholar]
- 27.Csoka A. B., Scherer S. W., Stern R. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics. 1999;60:356–361. doi: 10.1006/geno.1999.5876. [DOI] [PubMed] [Google Scholar]
- 28.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 29.Mather J. P. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol. Reprod. 1980;23:243–252. doi: 10.1095/biolreprod23.1.243. [DOI] [PubMed] [Google Scholar]
- 30.Tontsch S., Lepperdinger G., Artner I., Bauer H. Automated in Situ Hybridization. Boca Raton, FL: CRC Press; 2005. [Google Scholar]
- 31.Müllegger J., Reitinger S., Lepperdinger G. Hapten-labeled hyaluronan, a substrate to monitor hyaluronidase activity by enhanced chemiluminescence-assisted detection on filter blots. Anal. Biochem. 2001;293:291–293. doi: 10.1006/abio.2001.5125. [DOI] [PubMed] [Google Scholar]
- 32.Schwede T., Kopp J., Guex N., Peitsch M. C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guex N., Peitsch M. C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 34.Phelps B. M., Primakoff P., Koppel D. E., Low M. G., Myles D. G. Restricted lateral diffusion of PH-20, a PI-anchored sperm membrane protein. Science. 1988;240:1780–1782. doi: 10.1126/science.3381102. [DOI] [PubMed] [Google Scholar]
- 35.Kronegg J., Buloz D. Detection/prediction of GPI cleavage site (GPI-anchor) in a protein (DGPI) 1999 URL: http://129.194.185.165/dgpi/
- 36.Eisenhaber B., Bork P., Eisenhaber F. Prediction of potential GPI-modification sites in proprotein sequences. J. Mol. Biol. 1999;292:741–758. doi: 10.1006/jmbi.1999.3069. [DOI] [PubMed] [Google Scholar]
- 37.Gore-Langton R. E., Tung P. S., Fritz I. B. The absence of specific interactions of Sertoli-cell-secreted proteins with antibodies directed against H-Y antigen. Cell. 1983;32:289–301. doi: 10.1016/0092-8674(83)90519-6. [DOI] [PubMed] [Google Scholar]
- 38.Müllegger J., Lepperdinger G. Degradation of hyaluronan by a Hyal2-type hyaluronidase affects pattern formation of vitelline vessels during embryogenesis of Xenopus laevis. Mech. Dev. 2002;111:25–35. doi: 10.1016/s0925-4773(01)00593-7. [DOI] [PubMed] [Google Scholar]
- 39.Rai S. K., Duh F. M., Vigdorovich V., Danilkovitch-Miagkova A., Lerman M. I., Miller A. D. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4443–4448. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seaton G. J., Hall L., Jones R. Rat sperm 2B1 glycoprotein (PH20) contains a C-terminal sequence motif for attachment of a glycosyl phosphatidylinositol anchor. Effects of endoproteolytic cleavage on hyaluronidase activity. Biol. Reprod. 2000;62:1667–1676. doi: 10.1095/biolreprod62.6.1667. [DOI] [PubMed] [Google Scholar]
- 41.Chain E., Duthie E. S. Identity of hyaluronidase and spreading factor. Brit. J. Expl. Pathol. 1940;21:324. [Google Scholar]
- 42.Lin Y., Mahan K., Lathrop W. F., Myles D. G., Primakoff P. A hyaluronidase activity of the sperm plasma membrane protein PH-20 enables sperm to penetrate the cumulus cell layer surrounding the egg. J. Cell Biol. 1994;125:1157–1163. doi: 10.1083/jcb.125.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin Y., Kimmel L. H., Myles D. G., Primakoff P. Molecular cloning of the human and monkey sperm surface protein PH-20. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10071–10075. doi: 10.1073/pnas.90.21.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Markovic-Housley Z., Miglierini G., Soldatova L., Rizkallah P. J., Muller U., Schirmer T. Crystal structure of hyaluronidase, a major allergen of bee venom. Structure. 2000;8:1025–1035. doi: 10.1016/s0969-2126(00)00511-6. [DOI] [PubMed] [Google Scholar]
- 45.Arming S., Strobl B., Wechselberger C., Kreil G. In vitro mutagenesis of PH-20 hyaluronidase from human sperm. Eur. J. Biochem. 1997;247:810–814. doi: 10.1111/j.1432-1033.1997.t01-1-00810.x. [DOI] [PubMed] [Google Scholar]
- 46.Markovic-Housley Z., Schirmer T. Structural Evidence for Substrate Assisted Catalytic Mechanism of Bee Venom Hyaluronidase, a Major Allergen of Bee Venom. Cambridge, U.K.: The Royal Society; 2002. [Google Scholar]