Abstract
HLA (human leucocyte antigen)-A2 is an MHC Class I protein with primary functions in T-cell development and initi-ation of immune cell responses. MHC I proteins also play roles in intercellular adhesion, apoptosis, cell proliferation and neuronal plasticity. By utilizing a sequence comparison analysis, we recently identified HLA-A2 as a potential substrate for the Alzheimer's disease-associated PS1 (presenilin 1)/γ-secretase. α-Secretase-like membrane metalloproteinases are responsible for an initial shedding event, partially mediated by ADAM (a disinteg-rin and metalloproteinase)-10. Accordingly, activation or inhibition of α-secretase-like membrane metalloproteinases directly modulated levels of a 14 kDa HLA-A2 CTF (C-terminal frag-ment) in CHO (Chinese-hamster ovary) cells. To show that the HLA-A2 CTF is subsequently cleaved by PS1/γ-secretase, we re-duced its activity in cell lines stably expressing HLA-A2 and in Jurkat T-cells expressing endogenous MHC I. Treatment with specific PS1/γ-secretase inhibitors or expression of a dominant-negative construct led to a significant accumulation of HLA-A2 CTFs. We also identified the PS1/γ-secretase cleavage product of HLA-A2 CTF, termed HLA-A2 intracellular domain, in cell-free and cell-based experiments. In the absence of proteasome inhibitors, HLA-A2 intracellular domain underwent rapid degrad-ation. These data indicate that MHC I proteins undergo extra-cellular domain cleavage mediated by α-secretases and the cleavage product is subsequently cleaved by PS1/γ-secretase.
Keywords: α- and γ-secretases, a disintegrin and metallopro-teinase (ADAM), human leucocyte antigen, matrix metalloproteinase (MMP), MHC Class I, presenilin
Abbreviations: ADAM, a disintegrin and metalloproteinase; APP, amyloid precursor protein; CHO, Chinese-hamster ovary; CNS, central nervous system; CTF, C-terminal fragment; DAPT, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-(S)-phenylglycine t-butyl ester; HLA, human leucocyte antigen; ICD, intracellular domain; MHC I, MHC Class I; MMP, matrix metalloproteinase; PS1, presenilin 1; TNF, tumour necrosis factor; TAPI-1, TNFα processing inhibitor-1; TCR, T-cell receptor
INTRODUCTION
Along with at least three additional proteins, namely Aph-1, Nicastrin, and Pen-2, the presenilins [PS1 (presenilin 1) and PS2] form a proteolytic complex termed γ-secretase. γ-Secretase is responsible for cleavage of type I membrane proteins in a process termed regulated intramembrane proteolysis. Prior to regulated intramembrane proteolysis, shedding of ectodomain regions occurs by MMPs (matrix metalloproteinases). Once MMPs such as TNFα (tumour necrosis factor α)-converting enzyme or α-secretases ADAM (a disintegrin and metalloprotein-ase)-10 and -17 cleave their substrates, a catalytic aspartate resi-due in the presenilins allows γ-secretase complexes to cleave within the transmembrane domain. γ-Secretase cleavages liberate ICDs (intracellular domains) and several substrates [Notch-1, APP (amyloid precursor protein), CD44, E-cadherin and Erb-B4] have the ability to transduce nuclear signals and affect gene activation [1–7]. γ-Secretase cleavage within the transmembrane domain does not appear to require sequence specificity; however, one site in particular, the APP-ϵ/Notch S3 site near membrane–cytosol interface, has been used to identify novel substrates [8,9].
MHC I (MHC Class I) proteins are type I membrane proteins found on the cell surface of almost all nucleated cells. They largely form non-covalent heterodimeric complexes with β2-micro-globulin and present small cytosolic peptides of 9–11 amino acids to T-cells. During T-cell development, MHC I complexes are found on antigen-presenting cells where they play a central role in maturation of CD8+ cytotoxic T-lymphocytes. Additionally, when cells are invaded by foreign microbes such as viruses, MHC I complexes signal immune surveillance cells to initiate proper immune system responses. In both cases MHC I complexes are primary components of a highly dynamic immunological synapse where they ligate with TCRs (T-cell receptors) and CD8 molecules [10]. The immunological synapse involves additional proteins for proper stabilization between cells and communication: co-stimulatory molecules (CD28, CD80 and CD86), adhesion proteins [LFA-1 (lymphocyte function-associated antigen-1) and ICAM-1 (intercellular adhesion molecule 1)] and additional large glycoproteins to bridge contacts between cells (CD43, CD44 and CD45) [11]. It is interesting that both CD43 and CD44 have previously been identified as γ-secretase substrates [12,13].
In recent years it has been discovered that MHC I proteins are expressed in neurons throughout the CNS (central nervous system). MHC I expression was found to be regulated by neuronal activity during development, with peak expression occurring in the perinatal period [14,15]. Moreover, it has been shown that neuronal MHC I functions as a mediator of synaptic plasticity and activity-dependent refinement during development of the visual system [14,15]. Neuronal MHC I expression is regulated similarly to immune system MHC I, whereupon exposure to cytokines in-duces up-regulation of MHC I [16]. Additional studies suggest a role for neuronal MHC I during treatments such as axotomy where they may regulate the ability of neurons to maintain synapses [16,17].
The present study identifies a specific protein of the HLA (hu-man leucocyte antigen)-A locus, HLA-A2, as a substrate of both α- and γ-secretases. Cell-free and cell-based experiments show that HLA-A2 first undergoes an α-secretase-like shedding event, followed by a PS1/γ-secretase-mediated event that releases an ICD. Since MHC I proteins are important components of the immune system, the developing CNS and during activity-depend-ent synaptic remodelling, the present study provides new insights into the function of PS1/γ-secretase.
MATERIALS AND METHODS
Plasmids and transfections
An expression construct encoding full-length human HLA-A*0201 heavy chain (HLA-A2) was obtained from Dr Hidde Ploegh (Harvard Medical School). The construct contained a signal peptide from the mouse H-2Kb haplotype that enhances in vitro translation [18]. The primers used for PCR amplification were 5′-ACCATGGTACCGTGCACGCTGCTCCT-3′ and 5′-CACTTTACAAGCTGTGAGAGACACAT-3′. Subsequent cloning and transformation were performed using TOPO cloning vector (pcDNA3.1 containing a C-terminal V5/His tag) in One Shot TOP10 Chem competent Escherichia coli (Invitrogen). Sequenc-ing was later confirmed at Massachusetts General Hospital facil-ities. Effectene (Qiagen) was used for transfecting cell lines. We produced stably transfected CHO (Chinese-hamster ovary) cells as well as B104 rat neuroblastoma cells (Dr David Schubert, The Salk Institute, La Jolla, CA, U.S.A.). Jurkat cell line E6.1 was purchased from A.T.C.C.
Western-blot analysis, immunoprecipitation, antibodies and inhibitors
Cell extracts were prepared by directly lysing cells in a buffer containing 10 mM Tris/HCl (pH 6.8), 1 mM EDTA, 150 mM NaCl, 0.25% Nonidet P40, 1% Triton X-100 and a protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN, U.S.A.), followed by centrifugation at 16000 g (4 °C, 15 min). Samples were quantified using the BCA (bicinchoninic acid) protein assay kit (Pierce). Protein (20–100 μg) was resolved on 4–12% gradient Bis-Tris gels. Immunoprecipitations were done as described in [8]. Primary antibodies V5 (1:5000 dilution, Invitro-gen), anti-HLA (1:250), W6/32 were purchased from Biotrend Chemicals. HC10 antibodies were obtained from Dr Hidde Ploegh, and anti-HLA-A and -B (TA-17) were obtained from Dr Tanigaki Nobuyuki (Department of Immunology, Roswell Park Cancer Institute, Buffalo, NY, U.S.A.). The blots were developed using ECL® (enhanced chemiluminescence) with SuperSignal CL-HRP substrate (Pierce) according to the manufacturer's instructions. The γ-secretase inhibitors DAPT {N-[N-(3,5-difluorophenacetyl)-L-alanyl]-(S)-phenylglycine t-butyl ester} and L-685,458 were obtained from Calbiochem, and WPE31C was a gift from Dr Michael S. Wolfe (Department of Neurology, Brigham and Women's Hospital, Boston, MA, U.S.A.). The α-secretase inhibitor, TAPI-1 (TNFα processing inhibitor-1; IC-2), was from Biomol.
Cell-free generation of HLA-A2 ICD
Membrane preparation and cell-free generation of HLA-A2 proteolytic products were performed as described in [8]. The P2 and P3 fractions were resuspended in Buffer H (20 mM Hepes, 150 nM NaCl, 10% glycerol and 5 mM EDTA, pH 7.4) with pro-tease inhibitors. In vitro cleavage experiments were performed by incubating the membrane factions at 37 °C for 1 h in the presence or absence of indicated amounts of DAPT. After incubation, the soluble and membrane-associated fragments were separated by centrifugation of the reaction mixture at 120000 g for 45 min.
Immunohistochemistry
Cells were treated and fixed in 4% (w/v) paraformaldehyde for 10–20 min at room temperature (25 °C) Cells were permeabilized with Triton X-100, and primary and secondary antibodies were incubated for 1 h at room temperature. Anti-V5 (1:200) stained cells were visualized using confocal microscopy (Olympus).
RESULTS AND DISCUSSION
HLA-A2 forms functional MHC I complexes in CHO and B104 cells
HLA-A2 is one of the most commonly expressed MHC I proteins. To analyse proteolyic processing of HLA-A2, we stably transfected CHO and B104 cells with an HLA-A2 cDNA C-terminally tagged with V5/His. Two commonly used antibodies were chosen to characterize the overexpressed HLA-A2 protein. These anti-bodies distinguish between MHC I complexes containing β2-microglobulin (W6/32) and MHC I proteins lacking β2-microglobulin, termed β2-free MHC I (HC10) [19,20] (Figure 1a). Endogenous MHC I proteins are known to be found in complexes with β2-microglobulin. β2-free MHC I arise occasionally during biogenesis of MHC I complexes and after both β2-microglobulin and peptide dissociate from MHC I at the cell surface [21–23]. β2-free MHC I occur at low levels in naïve cells, but are known to accumulate during T-cell activation [21]. It was previously reported that expression of HLA-A2 in murine cells leads to easily detectable amounts of W6/32-reactive complexes [24].
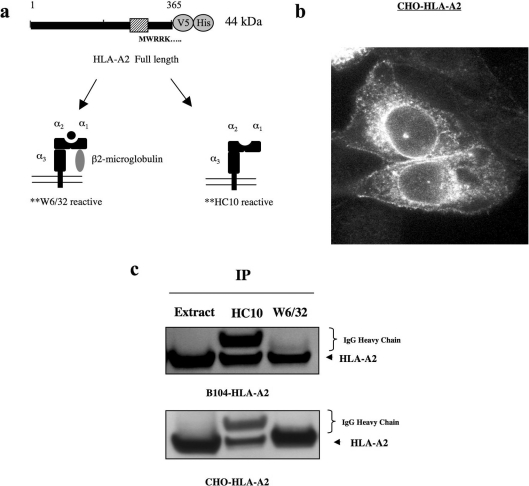
Figure 1. Stably expressed HLA-A2 in CHO and B104 cells.
(a) Expression construct of HLA-A2 containing a C-terminal V5/His tag. W6/32 and HC10 antibodies recognize dimerized and free forms of HLA-A2 respectively. (b) Anti-V5 immunostaining of CHO-HLA-A2 cells shows surface/early endosome localization of HLA-A2. (c) Immunoprecipitation (IP) of HLA-A2 from CHO and B104 cell lines stably expressing the protein indicates that HLA-A2 forms hybrid complexes with β2-microglobulin. Heavy chain of W6/32 antibody is not readily detected because of low concentrations used in the immunoprecipitations (0.2 μg for W6/32; 6.8 μg for HC10).
In stably transfected CHO cells we observed HLA-A2 protein at or near the cell surface by anti-V5 immunostaining (Figure 1b). Using the aforementioned antibodies, W6/32 and HC10, we tested whether HLA-A2 was complexed with β2-microglobulin in these cells. After immunoprecipitation, most HLA-A2 was found in W6/32-positive complexes in CHO-HLA-A2 cells (Figure 1c). This suggests that MHC I complexes containing β2-micro-globulin were formed. We also tested the B104 rat neuroblastoma cell line stably expressing HLA-A2. In these cells we found an equal distribution of MHC I complexes and β2-free MHC I after similar immunoprecipitations (Figure 1c). These results show that in hamster and rat cell lines, expression of HLA-A2 protein can form MHC I complexes containing β2-microglobulin.
HLA-A2 is processed by α-secretases
Prior to γ-secretase cleavage, all substrates undergo ectodomain shedding where they are cleaved by α-secretase-like MMPs at or near the cell surface. To establish a similar role for MMP activity in the generation of HLA-A2 CTFs (C-terminal fragments), we tested whether the α-secretase inhibitor TAPI-1 could affect HLA-A2 CTF generation in CHO-HLA-A2 stable cells. Stably transfected cells differed from transient HLA-A2 expression in that they showed constitutive HLA-A2 CTF generation at 14 kDa under untreated conditions. Constitutive HLA-A2 CTF produc-tion was significantly reduced by TAPI-1 treatment alone for 6 h (Figure 2a). Conversely, PS1/γ-secretase inhibition by DAPT treatment alone for 6 h increased HLA-A2 CTF levels. However, the increase due to γ-secretase inhibition was abolished during co-treatment of DAPT with TAPI-1 (Figure 2a). These data suggest that α-secretase activity is required for generation of HLA-A2 CTFs prior to γ-secretase cleavage.
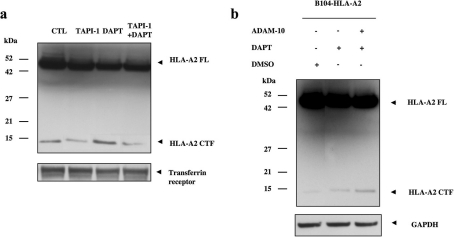
Figure 2. HLA-A2 is cleaved by α-secretase-like proteases.
(a) Treatment with TAPI-1 (50 μM for 3 h), an α-secretase inhibitor, attenuates HLA-A2 CTF production compared with untreated cells (CTL). Accumulation of HLA-A2 CTF with the PS1/γ-secretase inhibitor DAPT alone is significantly reduced upon co-treatment with TAPI-1 and DAPT. HLA-A2 is stained with an anti-V5 antibody. Transferrin receptor is shown as control. (b) HLA-A2 is cleaved by the α-secretase ADAM-10. Overexpression of ADAM-10 in B104 rat neuroblastoma cells stably expressing HLA-A2 shows that HLA-A2 CTF accumulation by PS1/γ-secretase inhibition is further enhanced in ADAM-10-expressing cells. HLA-A2 is stained with an anti-V5 antibody. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) is used as a loading control. FL, full-length.
To test whether a specific α-secretase, ADAM-10, contributes to HLA-A2 CTF generation, we used B104 neuroblastoma cells stably expressing HLA-A2. Transient expression of ADAM-10 in these cells significantly increased HLA-A2 CTF generation. DAPT treatment of cells expressing ADAM-10 resulted in in-creased HLA-A2 CTF accumulation when compared with cells with normal ADAM-10 levels (Figure 2b). Together, our results indicate that HLA-A2 CTFs are generated by MMP activity prior to PS1/γ-secretase cleavage and the α-secretase ADAM-10 con-tributes to the extracellular-domain shedding event.
HLA-A2 is cleaved by a PS1-dependent γ-secretase-like activity
Next, we characterized PS1/γ-secretase-mediated processing of HLA-A2. Our sequence comparison search using the APP-ϵ/Notch S3-like domain previously revealed γ-secretase substrates including nectin-1 and a voltage-gated sodium channel subunit (β2) [8,9]. Using the same method, we now found that HLA-A, -B and -C proteins also contain this site homology (Figure 3a). Western-blot analysis of CHO cells transiently transfected with HLA-A2 showed that the full-length HLA-A2 protein migrated at approx. 44 kDa (Figure 3c). In transiently transfected CHO cells treated for 3 h with PMA (‘TPA’), an activator for α-secretases such as ADAM-family metalloproteases, we did not see a consistent and significant accumulation of HLA-A2 CTFs (Figures 3c–3e). However, co-treatment of PMA with the γ-secretase inhibitor DAPT resulted in the accumulation of a C-terminally tagged HLA-A2 fragment at 14 kDa, suggesting that γ-secretase activity may be responsible for cleaving this fragment. In addition, a 6 h treatment of DAPT alone was sufficient to significantly increase HLA-A2 CTFs (Figure 3c). We confirmed these results using two additional γ-secretase inhibitors, L-685,458 and WPE31C. Each inhibitor independently increased HLA-A2 CTFs, which were augmented upon co-treatment with PMA in 3 h treatments (Figure 3d). We also stably expressed HLA-A2 in B104 rat neuroblastoma cells and found accumulation of HLA-A2 CTFs after PS1/γ-secretase inhibition. This effect was potentiated by co-treatment with PMA (Figure 3e). To further confirm that PS1/γ-secretase activity was responsible for cleaving HLA-A2, we tested whether a similar accumulation of the 14 kDa fragment occurs in cells stably expressing dominant-negative mutations in PS1 (D385A). Figure 3(f) shows that lack of γ-secretase activity in PS1 (D385A) cells resulted in accumulation of the HLA-A2 CTF. These results show that PS1/γ-secretase activity is responsible for cleavage of the HLA-A2 CTF detected at 14 kDa.
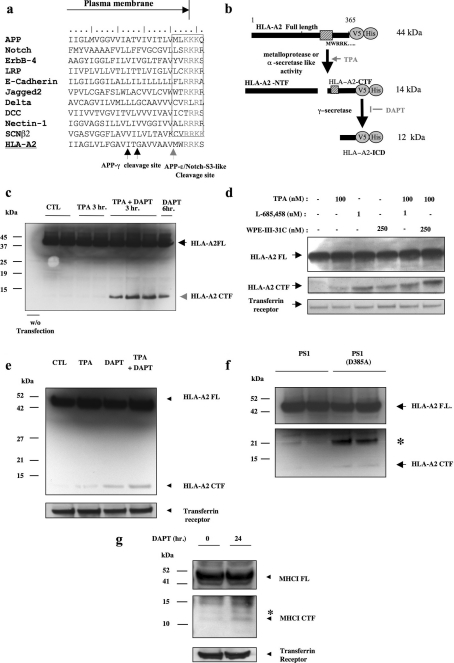
Figure 3. HLA-A2 is cleaved by PS1/γ-secretase.
(a) Sequence comparison of the APP-ϵ/Notch-S3 domains in ten known human PS1/γ-secretase-like substrates, and in HLA-A2. (b) Predicted fragment sizes before and after PS1/γ-secretase-mediated cleavage of HLA-A2 CTF. (c) CHO cells expressing HLA-A2 were resolved by SDS/12% PAGE and stained with anti-V5. Upon co-treatment with PMA (TPA) and DAPT, an HLA-A2 CTF accumulates around 14 kDa. (d) Anti-V5 immunostaining shows that HLA-A2 CTFs are increased by two additional γ-secretase inhibitor treatments, L-685,458 and WPE31. Transferrin receptor is shown as loading control. (e) Anti-V5 immunostaining of B104 rat neuroblastoma cells stably expressing HLA-A2 shows accumulation of HLA-A2 CTFs after treatment with PMA or DAPT, and further accumulation in co-treatments. Transferrin receptor is shown as loading control. (f) The dominant-negative form of PS1, D385A, elevates HLA-A2 CTF levels. Expression of PS1(D385A) results in the accumulation of the 14 kDa HLA-A2 CTF (arrowhead). The asterisk indicates an unidentified band that is not seen with PS1/γ-secretase inhibitor treatments. (g) Jurkat T-cells show accumulation of an endogenous MHC I CTF. A 24 h incubation of DAPT leads to accumulation of an approx. 12 kDa MHC I CTF. Immunostaining was with anti-HLA-A and -B (TA-17). Transferrin receptor is shown as a control.
Next we tested whether endogenous MHC I in human Jurkat T-cells exhibited a processing pattern similar to overexpressed HLA-A2. For these experiments we used a C-terminally directed antibody known to immunoprecipitate HLA-A and -B [25]. Endogenous MHC I CTF accumulated at approx. 12 kDa after a 24 h incubation with DAPT (Figure 3g). These data indicate that endogenous MHC I proteins are cleaved in cultured human T-cells.
γ-Secretase activity is required for generation of HLA-A2 ICD in vivo and in vitro
For additional confirmation that γ-secretase cleaves HLA-A2 CTFs, we performed a cell-free γ-secretase cleavage assay on CHO cells stably expressing high levels of HLA-A2. Cell-free assays have previously been successful for detection of ICDs, such as nectin-ICD [8]. CHO-HLA-A2 cell membranes were incubated with or without DAPT for 1 h and treated with various protease and proteasome inhibitors. Control samples, incubated at 0 °C, showed significant amounts of HLA-A2 CTF (Figure 4a). After incubation at 37 °C, a fragment of approx. 12 kDa was generated in cells with γ-secretase activity. This band was not observed in DAPT-treated cell extracts, indicating that the HLA-A2 ICD is generated by γ-secretase activity (Figure 4a).
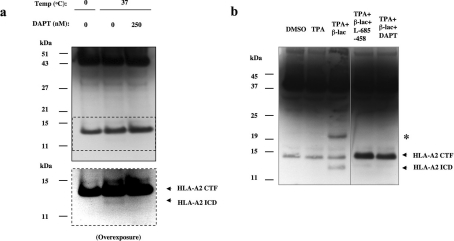
Figure 4. HLA-A2 ICD is produced under cell-free and in vivo conditions.
(a) Cell-free generation of a 12 kDa HLA-A2 ICD. Partially purified membrane fractions of CHO-HLA-A2 were subjected to a γ-secretase cleavage assay [8]. Membranes were incubated with protease inhibitors at 0 and 37 °C in the presence or absence of DAPT. Samples incubated at 37 °C generate a HLA-A2 ICD fragment. Blots were stained with an anti-V5 antibody. (b) In vivo generation of HLA-A2 ICD in CHO-HLA-A2 cells after incubation with a proteasomal inhibitor lactacystin. HLA-A2-ICD accumulation was blocked upon inhibition of PS1/γ-secretase activity with DAPT and L-685,458 respectively. The asterisk indicates an unidentified band that is not seen with PS1/γ-secretase inhibitor treatments.
To investigate whether HLA-A2 ICD can be detected in vivo, we utilized lactacystin β-lactone to inhibit potential proteasomal degradation of the ICD. CHO-HLA-A2 stable cells were first treated with PMA to activate α-secretases and increase both HLA-A2 CTF production and cleavage. Upon co-incubation with PMA and lactacystin β-lactone for 3 h, an HLA-A2 ICD was detected at approx. 12 kDa. The production of this fragment was blocked when similarly treated cells were also incubated with one of two γ-secretase inhibitors, DAPT and L-685,458 respectively (Figure 4b). Our results indicate that γ-secretase activity is required for generation of the HLA-A2 ICD fragment, which is highly unstable and quickly degraded by the proteasome system in vivo. We also observed an approx. 21 kDa band which was increased with PMA and lactacystin β-lactone treatment for 3 h, previously observed in PS1(D385A) cells transiently expressing HLA-A2. This band did not appear consistently in other experiments; per-haps it is a very unstable dimer of HLA-A2 CTFs or an intermediate degradation product. Additional studies would be required for further characterization.
Our study shows that MHC I proteins, such as HLA-A2, are substrates for both α- and γ-secretases in three different cell lines. Metalloprotease or α-secretase activities mediate ectodomain shedding, producing an HLA-A2 CTF, followed by sequential PS1/γ-secretase cleavage. We found that the PS1/γ-secretase cleavage product, HLA-A2 ICD, undergoes rapid proteasomal degradation upon generation by PS1/γ-secretase. The present study adds MHC I proteins to the growing list of PS1/γ-secretase substrates and provides further insight on PS1/γ-secretase func-tion in various tissues.
MHC I functions as a central protein involved in cell–cell inter-actions between antigen-presenting cells and cytotoxic T-lympho-cytes (CD8+) of the immune system. Interestingly, two additional PS1/γ-secretase substrate proteins, CD43 and CD44, are also involved in cell–cell interactions proximal to MHC I proteins [13,26]. Additionally, a novel function for MHC I in the CNS has been suggested in recent years. Several reports support a role for MHC-dependent signalling in development of the mammalian visual system, specifically activity-dependent remodelling and re-traction of inappropriate synapses [14,27,28]. Mice deficient in surface MHC I complexes show abnormal development of retinal projections in the visual system pathways [15]. In addition, these neurons also show perturbations in long-term potentiation and long-term depression in the adult hippocampus [15]. These studies suggest that PS1/γ-secretase cleavage of MHC I may occur not only in immunological cells but also in neuronal cells during synapse formation.
It is well established that the PS1/γ-secretase cleavage product of Notch, NICD (Notch intracellular domain), translocates to the nucleus to initiate transcription [1]. Recent work has shown that α- and PS1/γ-secretase-mediated cleavages of p75 neurotrophin receptors activate Rho and subsequent signals, resulting in axonal growth inhibition [29]. While MHC I-mediated signalling in the CNS is not known, it may play a role in T-cell activation. MHC I-mediated signalling in T-cells has been studied largely using antibody cross-linking, or ligation, of MHC I molecules. MHC I signalling pathways have been suggested to include induction of tyrosine kinase and PLCγ (phospholipase Cγ) activity as well as JAK (Janus kinase) Tyk2 and Stat-3 activation [33,34]. Further-more, MHC I-mediated signalling can activate ZAP-70 [ζ-chain (TCR)-associated protein kinase of 70 kDa] and Src kinase p56Lck, leading to induction of apoptosis in Jurkat T-cells [35]. Using a different cell type, it was suggested that MHC I-medi-ated signalling can prevent apoptosis and is mediated by intact p56Lck activity [36]. These data suggest that MHC I-mediated signalling is involved in both T-cell survival and death. However, previous work on MHC I proteins has revealed that all but four proximal amino acids from the ICD can be deleted with no effect on T-cell activation [30,31]. Similarly, membrane-associated MHC I cytoplasmic domains did not appear to affect cellular func-tions, including cytoskeletal association, aggregation and inter-nalization [32].
Our results suggest that α-secretase and PS1/γ-secretase activities are involved in degradation of MHC I proteins under physiological conditions. β2-free MHC I molecules have been reported as selectively cleaved and released from the plasma membrane by membrane-bound metalloproteinases, similar to α-secretase-mediated cleavages described in our study [37,38]. PS1/γ-secretase-like activity has also been described in virus-mediated down-regulation of MHC I proteins [39]. As previously suggested, PS1/γ-secretase may function as a proteosomal complex for a group of plasma membrane proteins [40]. Character-ization of novel substrate proteins such as MHC I contributes to a full understanding of PS1/γ-secretase function under physiological and pathological conditions.
Acknowledgments
We thank Dr S. Lichtenthaler (Ludwig-Maximilians-Universität) for the human ADAM10 construct, Dr David Schubert for the B104 rat neuroblastoma cells and Dr Tanigaki Nobuyuki for the HLA-A, -B (TA-17) antibody. This work is supported by grants from the NIH/NIA (National Institutes of Health/National Institute on Aging) and the John Douglas French Alzheimer's Foundation.
References
- 1.Schroeter E. H., Kisslinger J. A., Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 2.De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 3.Ni C. Y., Yuan H., Carpenter G. Role of the ErbB-4 carboxyl terminus in gamma-secretase cleavage. J. Biol. Chem. 2003;278:4561–4565. doi: 10.1074/jbc.M210504200. [DOI] [PubMed] [Google Scholar]
- 4.Marambaud P., Shioi J., Serban G., Georgakopoulos A., Sarner S., Nagy V., Baki L., Wen P., Efthimiopoulos S., Shao Z., et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammich S., Okochi M., Takeda M., Kaether C., Capell A., Zimmer A. K., Edbauer D., Walter J., Steiner H., Haass C. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an A β-like peptide. J. Biol. Chem. 2002;277:44754–44759. doi: 10.1074/jbc.M206872200. [DOI] [PubMed] [Google Scholar]
- 6.Murakami D., Okamoto I., Nagano O., Kawano Y., Tomita T., Iwatsubo T., De Strooper B., Yumoto E., Saya H. Presenilin-dependent gamma-secretase activity mediates the intramembranous cleavage of CD44. Oncogene. 2003;22:1511–1516. doi: 10.1038/sj.onc.1206298. [DOI] [PubMed] [Google Scholar]
- 7.Vidal G. A., Naresh A., Marrero L., Jones F. E. Presenilin-dependent gamma-secretase processing regulates multiple ERBB4/HER4 activities. J. Biol. Chem. 2005;280:19777–19783. doi: 10.1074/jbc.M412457200. [DOI] [PubMed] [Google Scholar]
- 8.Kim D. Y., Ingano L. A., Kovacs D. M. Nectin-1alpha, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/gamma-secretase-like cleavage. J. Biol. Chem. 2002;277:49976–49981. doi: 10.1074/jbc.M210179200. [DOI] [PubMed] [Google Scholar]
- 9.Kim D. Y., Ingano L. A., Carey B. W., Pettingell W. H., Kovacs D. M. Presenilin/gamma-secretase-mediated cleavage of the voltage-gated sodium channel beta2-subunit regulates cell adhesion and migration. J. Biol. Chem. 2005;280:23251–23261. doi: 10.1074/jbc.M412938200. [DOI] [PubMed] [Google Scholar]
- 10.Rosenstein Y., Ratnofsky S., Burakoff S. J., Herrmann S. H. Direct evidence for binding of CD8 to HLA class I antigens. J. Exp. Med. 1989;169:149–160. doi: 10.1084/jem.169.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huppa J. B., Davis M. M. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 12.Andersson C. X., Fernandez-Rodriguez J., Laos S., Baeckstrom D., Haass C., Hansson G. C. Shedding and gamma-secretase-mediated intramembrane proteolysis of the mucin-type molecule CD43. Biochem. J. 2005;387:377–384. doi: 10.1042/BJ20041387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson C. X., Fernandez-Rodriguez J., Laos S., Sikut R., Sikut A., Baeckstrom D., Hansson G. C. CD43 has a functional NLS, interacts with beta-catenin, and affects gene expression. Biochem. Biophys. Res. Commun. 2004;316:12–17. doi: 10.1016/j.bbrc.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Corriveau R. A., Huh G. S., Shatz C. J. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 15.Huh G. S., Boulanger L. M., Du H., Riquelme P. A., Brotz T. M., Shatz C. J. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linda H., Hammarberg H., Cullheim S., Levinovitz A., Khademi M., Olsson T. Expression of MHC class I and beta2-microglobulin in rat spinal motoneurons: regulatory influences by IFN-gamma and axotomy. Exp. Neurol. 1998;150:282–295. doi: 10.1006/exnr.1997.6768. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira A. L., Thams S., Lidman O., Piehl F., Hokfelt T., Karre K., Linda H., Cullheim S. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17843–17848. doi: 10.1073/pnas.0408154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Story C. M., Furman M. H., Ploegh H. L. The cytosolic tail of class I MHC heavy chain is required for its dislocation by the human cytomegalovirus US2 and US11 gene products. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8516–8521. doi: 10.1073/pnas.96.15.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stam N. J., Spits H., Ploegh H. L. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J. Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 20.Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J. Immunol. 1979;123:342–349. [PubMed] [Google Scholar]
- 21.Santos S. G., Powis S. J., Arosa F. A. Misfolding of major histocompatibility complex class I molecules in activated T cells allows cis-interactions with receptors and signaling molecules and is associated with tyrosine phosphorylation. J. Biol. Chem. 2004;279:53062–53070. doi: 10.1074/jbc.M408794200. [DOI] [PubMed] [Google Scholar]
- 22.Carreno B. M., Hansen T. H. Exogenous peptide ligand influences the expression and half-life of free HLA class I heavy chains ubiquitously detected at the cell surface. Eur. J. Immunol. 1994;24:1285–1292. doi: 10.1002/eji.1830240607. [DOI] [PubMed] [Google Scholar]
- 23.Demaria S., Schwab R., Bushkin Y. The origin and fate of beta 2m-free MHC class I molecules induced on activated T cells. Cell. Immunol. 1992;142:103–113. doi: 10.1016/0008-8749(92)90272-q. [DOI] [PubMed] [Google Scholar]
- 24.Rein R. S., Seemann G. H., Neefjes J. J., Hochstenbach F. M., Stam N. J., Ploegh H. L. Association with beta 2-microglobulin controls the expression of transfected human class I genes. J. Immunol. 1987;138:1178–1183. [PubMed] [Google Scholar]
- 25.Chersi A., Rosano L., Tanigaki N. Polystyrene beads coated with antibodies directed to HLA class I intracytoplasmic domain: the use in quantitative measurement of peptide–HLA class I binding by flow cytometry. Hum. Immunol. 2000;61:1298–1306. doi: 10.1016/s0198-8859(00)00187-7. [DOI] [PubMed] [Google Scholar]
- 26.Lammich S., Okochi M., Takeda M., Kaether C., Capell A., Zimmer A. K., Edbauer D., Walter J., Steiner H., Haass C. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Aβ-like peptide. J. Biol. Chem. 2002;277:44754–44759. doi: 10.1074/jbc.M206872200. [DOI] [PubMed] [Google Scholar]
- 27.Boulanger L. M., Huh G. S., Shatz C. J. Neuronal plasticity and cellular immunity: shared molecular mechanisms. Curr. Opin. Neurobiol. 2001;11:568–578. doi: 10.1016/s0959-4388(00)00251-8. [DOI] [PubMed] [Google Scholar]
- 28.Boulanger L. M., Shatz C. J. Immune signalling in neural development, synaptic plasticity and disease. Nat. Rev. Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- 29.Domeniconi M., Zampieri N., Spencer T., Hilaire M., Mellado W., Chao M. V., Filbin M. T. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron. 2005;46:849–855. doi: 10.1016/j.neuron.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Gur H., el-Zaatari F., Geppert T. D., Wacholtz M. C., Taurog J. D., Lipsky P. E. Analysis of T cell signaling by class I MHC molecules: the cytoplasmic domain is not required for signal transduction. J. Exp. Med. 1990;172:1267–1270. doi: 10.1084/jem.172.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gur H., Geppert T. D., Wacholtz M. C., Lipsky P. E. The cytoplasmic and the transmembrane domains are not sufficient for class I MHC signal transduction. Cell. Immunol. 1999;191:105–116. doi: 10.1006/cimm.1998.1417. [DOI] [PubMed] [Google Scholar]
- 32.Gur H., Geppert T. D., Lipsky P. E. Structural analysis of class I MHC molecules: the cytoplasmic domain is not required for cytoskeletal association, aggregation and internalization. Mol. Immunol. 1997;34:125–132. doi: 10.1016/s0161-5890(97)00007-2. [DOI] [PubMed] [Google Scholar]
- 33.Skov S., Odum N., Claesson M. H. MHC class I signaling in T cells leads to tyrosine kinase activity and PLC-γ1 phosphorylation. J. Immunol. 1995;154:1167–1176. [PubMed] [Google Scholar]
- 34.Skov S., Nielsen M., Bregenholt S., Odum N., Claesson M. H. Activation of Stat-3 is involved in the induction of apoptosis after ligation of major histocompatibility complex class I molecules on human Jurkat T cells. Blood. 1998;91:3566–3573. [PubMed] [Google Scholar]
- 35.Skov S., Bregenholt S., Claesson M. H. MHC class I ligation of human T cells activates the ZAP70 and p56lck tyrosine kinases, leads to an alternative phenotype of the TCR/CD3 zeta-chain, and induces apoptosis. J. Immunol. 1997;158:3189–3196. [PubMed] [Google Scholar]
- 36.Lamberth K., Claesson M. H. Ligation of major histocompatibility complex class I antigens (MHC-I) prevents apoptosis induced by Fas or SAPK/JNK activation in T-lymphoma cells. Tissue Antigens. 2001;58:171–180. doi: 10.1034/j.1399-0039.2001.580305.x. [DOI] [PubMed] [Google Scholar]
- 37.Demaria S., Schwab R., Gottesman S. R., Bushkin Y. Soluble beta 2-microglobulin-free class I heavy chains are released from the surface of activated and leukemia cells by a metalloprotease. J. Biol. Chem. 1994;269:6689–6694. [PubMed] [Google Scholar]
- 38.Demaria S., DeVito-Haynes L. D., Salter R. D., Burlingham W. J., Bushkin Y. Peptide-conformed beta2m-free class I heavy chains are intermediates in generation of soluble HLA by the membrane-bound metalloproteinase. Hum. Immunol. 1999;60:1216–1226. doi: 10.1016/s0198-8859(99)00113-5. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzo M. E., Jung J. U., Ploegh H. L. Kaposi's sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class major histocompatibility complexes to late endocytic compartments. J. Virol. 2002;76:5522–5531. doi: 10.1128/JVI.76.11.5522-5531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulz J. G., Annaert W., Vandekerckhove J., Zimmermann P., De Strooper B., David G. Syndecan 3 intramembrane proteolysis is presenilin/γ-secretase-dependent and modulates cytosolic signaling. J. Biol. Chem. 2003;278:48651–48657. doi: 10.1074/jbc.M308424200. [DOI] [PubMed] [Google Scholar]