Abstract
Activation of both PAR-1 (proteinase-activated receptor-1) and PAR-2 resulted in release of the chemokine GRO (growth-regulated oncogene)/CINC-1 (cytokine-induced neutrophil chemoattractant-1), a functional counterpart of human interleukin-8, from rat astrocytes. Here, we investigate whether the two PAR receptor subtypes can signal separately. PAR-2-induced GRO/CINC-1 release was independent of protein kinase C, phosphoinositide 3-kinase and MEK (mitogen-activated protein kinase kinase)-1/2 activation, whereas these three kinases were involved in PAR-1-induced GRO/CINC-1 release. Despite such clear differences between PAR-1 and PAR-2 signalling pathways, JNK (c-Jun N-terminal kinase) was identified in both signalling pathways to play a pivotal role. By isoform-specific loss-of-function studies using small interfering RNA against JNK1–3, we demonstrate that different JNK isoforms mediated GRO/CINC-1 secretion, when it was induced by either PAR-1 or PAR-2 activation. JNK2 and JNK3 isoforms were both activated by PAR-1 and essential for chemokine GRO/CINC-1 secretion, whereas PAR-1-mediated JNK1 activation was mainly responsible for c-Jun phosphorylation, which was not involved in GRO/CINC-1 release. In contrast, PAR-2-induced JNK1 activation, which failed to phosphorylate c-Jun, uniquely contributed to GRO/CINC-1 release. Therefore our results show for the first time that JNK-mediated chemokine GRO/CINC-1 release occurred in a JNK isoform-dependent fashion and invoked PAR subtype-specific mechanisms. Furthermore, here we demonstrate that activation of PAR-2, as well as PAR-1, rescued astrocytes from ceramide-induced apoptosis via regulating chemokine GRO/CINC-1 release. Taken together, our results suggest that PAR-1 and PAR-2 have overlapping functions, but can activate separate pathways under certain pathological conditions to rescue neural cells from cell death. This provides new functional insights into PAR/JNK signalling and the protective actions of PARs in brain.
Keywords: apoptosis, brain, c-Jun N-terminal kinase (JNK), growth-regulated oncogene (GRO)/cytokine-induced neutrophil chemoattractant-1 (CINC-1), proteinase-activated receptor (PAR), small interfering RNA (siRNA)
Abbreviations: AP, activating peptide; CINC-1, cytokine-induced neutrophil chemoattractant-1; CXCR2, CXC chemokine receptor 2; DMEM, Dulbecco's modified Eagle's medium; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GRO, growth-regulated oncogene; GST, glutathione S-transferase; IL8, interleukin-8; JNK, c-Jun N-terminal kinase; LDH, lactate dehydrogenase; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; PAR, proteinase-activated receptor; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; PTX, pertussis toxin; RT, reverse transcriptase; SAPK, stress-activated protein kinase; siRNA, small interfering RNA; TRag, thrombin receptor agonist peptide; WT, wild-type
INTRODUCTION
PARs (proteinase-activated receptors), a family of G-protein-coupled receptors, are activated by proteolytic cleavage of their N-termini by serine proteinases, such as thrombin, trypsin or tryptase. Thus a new N-terminus is unmasked acting as a tethered ligand which can interact with the second extracellular loop of the receptor and thereby initiate multiple signal transductions [1,2]. Four members (PAR-1, -2, -3 and -4) of the PAR family have been identified. PAR-1, PAR-3 and PAR-4 are thrombin receptors. Thrombin, which has been reported to be significantly increased in the brain as a result of the infusion of the bloodstream through the disrupted blood–brain barrier and, under certain pathophysiological conditions, is produced locally in the central nervous system [3–5], protects neurons and astrocytes from cell death mainly via activating PAR-1 [6,7]. Although trypsin is a main physiological agonist of PAR-2, it is not present in brain. On the other hand, mast-cell tryptase and p22 both have been identified as two potential candidates for neural PAR-2 activation [8,9]. So far, PAR-2 activation has received great attention for its functional significance in the context of inflammation. Our recent results [10] have demonstrated that PAR-1 activation regulated the release of the chemokine GRO (growth-regulated oncogene)/CINC-1 (cytokine-induced neutrophil chemoattractant-1), which is a rat chemokine with structural and functional homology to human IL8 (interleukin-8). Further, previous data from our laboratory have shown that all four types of PARs are functionally expressed in astrocytes [11]. However, the role of the three other PARs on chemokine GRO/CINC-1 secretion remains unknown.
JNK (c-Jun N-terminal kinase), an important subfamily of the MAPK (mitogen-activated protein kinase) superfamily, plays a pivotal role in the process of PAR-1-induced release of the chemokine GRO/CINC-1 [10]. Three JNK isoforms encoded by different genes have been identified. JNK1 and JNK2 are ubiquitously expressed in most tissues, whereas JNK3 is expressed selectively in the nervous system and displays low levels of expression in heart and testis [12,13]. These JNKs have two or four sub-isoforms resulting from alternative splicing [13]. It has been shown that JNKs are involved in numerous physiological or pathological processes, including proliferation [14,15], differentiation [16–18] and cell death [19–21].
Increasing evidence shows that distinct JNK isoforms might have identical or completely different functions, depending on the physiological or pathological context. JNK2 has been identified as the main contributor for c-Jun activation induced by lipopolysaccharide in microglia with pro-inflammatory actions [22]. Moreover, JNK2 can mediate tumour necrosis factor-induced cell death in mouse embryonic fibroblasts [23]. However, there is also evidence that JNK1, but not JNK2, which is activated by tumour necrosis factor α and UV light, is required for c-Jun activation and apoptosis in mouse fibroblasts [24]. JNK3 appears to have an important role for ischaemic apoptosis [25]. Transgenic knockouts reveal that single knockouts of jnk1, jnk2 or jnk3 and double mutants of jnk1/jnk3 or jnk2/jnk3 do not show clear structural abnormalities. However, the double knockout of jnk1/jnk2 leads to embryonic lethality [26]. Therefore until now the precise roles of each JNK isoform still remain largely unknown. Our recent data have identified the involvement of JNK in PAR-1-induced GRO/CINC-1 secretion, but it is not known which JNK isoforms play critical roles in this process.
In the present study, we show that activation of both PAR-1 and PAR-2 increased chemokine GRO/CINC-1 release from astrocytes. However, neither PAR-3 nor PAR-4 activation could mediate GRO/CINC-1 secretion. We demonstrate that the three JNK isoforms clearly differentially mediate PAR-1- and PAR-2-induced GRO/CINC-1 release. Moreover, we show here that activation of both PAR-1 and PAR-2 rescued cells from C2-ceramide-induced apoptosis through the regulation of GRO/CINC-1 secretion. These findings provide important implications for understanding the biological functions of individual JNK isoforms and the protective roles of PARs in brain.
EXPERIMENTAL
Materials
The synthetic TRag (thrombin receptor agonist peptide; Ala-pFluoro-Phe-Arg-Cha-HomoArg-Tyr-NH2), rat PAR-2AP (PAR-2 activating peptide; Ser-Leu-Ile-Gly-Arg-Leu) and mouse PAR-4AP (Gly-Tyr-Pro-Gly-Lys-Phe) were purchased from NeoMPS SA (Strasbourg, France). Human PAR-3AP (Thr-Phe-Arg-Gly-Ala-Pro) was from Bachem (Heidelberg, Germany). PTX (pertussis toxin), SP600125, wortmannin, GF109203X, U0126, SB203580 and C2-ceramide were purchased from Calbiochem (La Jolla, CA, U.S.A.). Rat GRO/CINC-1 ELISA kit was purchased from GE Healthcare (Freiburg, Germany). Non-radioactive SAPK (stress-activated protein kinase)/JNK assay kit, anti-[phospho-JNK (Thr183/Tyr185)], anti-JNK, anti-[phospho-c-Jun (Ser63)] and anti-c-Jun antibodies were from Cell Signaling Technology (Beverly, MA, U.S.A.). Anti-JNK1 (F-3) and anti-JNK2 (D-2) antibodies were from Santa Cruz Biotechnology (Heidelberg, Germany). Anti-JNK3/SAPK1b (clone C05T) antibody was obtained from Upstate (Charlottesville, VA, U.S.A.). Monoclonal anti-β-tubulin I (clone SAP.4G5) antibody was from Sigma (Deisenhofen, Germany). Cytochrome c Ab-2 (clone 7H8.2C12), horseradish peroxidase-conjugated goat anti-rabbit IgG and goat anti-mouse antibodies were from Dianova (Hamburg, Germany). DMEM (Dulbecco's modified Eagle's medium), foetal calf serum, penicillin and streptomycin were from Biochrom (Berlin, Germany). SB-332235 was generously provided by Dr H. Sarau (GlaxoSmithKline, King of Prussia, PA, U.S.A.).
Cell culture
Primary astrocyte-enriched cell cultures were obtained from new-born rats as reported previously [10]. The cells were cultured in DMEM containing 10% (v/v) heat-inactivated foetal calf serum, 100 units/ml penicillin and 100 μg/ml streptomycin in a humidified incubator with 10% CO2 at 37 °C. The medium was changed every 2–3 days, depending on the cell density. For experiments cells were used between day 10 and 13 in culture.
Semi-quantitative RT (reverse transcriptase)–PCR
Total RNA was extracted and reverse-transcribed. The resulting cDNA was amplified by PCR with different reaction cycles using HotStarTaq™ Master Mix kit (Qiagen) for 15 min at 95 °C, followed by repeated cycles, 30 s at 94 °C, 90 s at 53–60 °C and 60 s at 72 °C, and a final 10 min extension at 72 °C. Specific primers, annealing temperatures, reaction cycles and length of different PCR products for GRO/CINC-1, JNK1, JNK2, JNK3 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) are shown in Supplementary Table 1 (http://www.BiochemJ.org/bj/401/bj4010065add.htm) The reaction products were analysed by electrophoresis with 1% agarose gel containing ethidium bromide, and visualized using the Bio-Rad gel document system. The intensity of PCR products was semi-quantified by Quantity One quantification software (Bio-Rad).
Rat GRO/CINC-1 protein determination
Extracellular GRO/CINC-1 protein was measured using rat GRO/CINC-1 ELISA kit, according to the manufacturer's protocol. Briefly, serum-starved astrocytes, including normal primary cultured astrocytes and astrocytes which had been transfected with appropriate siRNA (small interfering RNA) for 48 h, were stimulated with TRag, trypsin or PAR-2AP for indicated time intervals (2, 3 or 6 h), and then the supernatant was collected for analysis of GRO/CINC-1 levels. For inhibitor studies, astrocytes were pretreated with the inhibitors, which are indicated in the Results section, for 30 min prior to trypsin or PAR-2AP stimulation. The levels of GRO/CINC-1 were assayed at 450 nm. In addition, cells were lysed for measuring the amount of total cellular protein.
Preparation of whole cell lysate and cell cytosol fraction
Serum-starved astrocytes were treated with agonists or inhibitors at 37 °C as indicated in Results section. After stimulation, cells were washed with ice-cold PBS, then lysed in modified RIPA buffer [50 mM Tris/HCl, pH 7.4, 1% Igepal, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF and a proteinase inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany; one tablet per 50 ml)]. After a brief sonication, the cell lysate was centrifuged at 15000 g for 15 min at 4 °C. The whole cell lysate was collected from the resulting supernatant. For cytochrome c detection, the cytosol fraction was prepared. Astrocytes were incubated with 20 μM C2-ceramide in the absence or presence of PAR-2AP at the indicated concentrations for 7 h, and lysed by 20 strokes of a Dounce homogenizer in hypo-osmotic buffer containing 10 mM Hepes (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol and proteinase inhibitor cocktail. The cell lysate was centrifuged at 50000 g for 30 min at 4 °C, to remove nuclei, membranes and mitochondria. The cytosol fraction was collected from the supernatant. The protein content of both whole cell lysate and cytosol fraction was determined by the Bradford method [48] using BSA as the standard.
Kinase assays
Serum-starved astrocytes were treated with 10 μM TRag or 500 μM PAR-2AP for the indicated times and then whole cell lysate was harvested as described above. The JNK activity in cell lysates was determined by using non-radioactive kinase assay. Briefly, equal amounts of cell lysates were incubated with GST (glutathione S-transferase)–c-Jun1–89 fusion protein immobilized on glutathione–agarose beads at 4 °C overnight. After washing, the bound proteins were incubated with the kinase buffer containing ATP (200 μM) for 30 min at 30 °C. Afterwards, the reaction was terminated with sample buffer and the proteins were separated by SDS/10% PAGE. The JNK activity was determined by Western blotting with the [phospho-c-Jun (Ser63)] antibody (provided in the kit).
Western blot
Samples containing equal amounts of protein were separated by SDS/PAGE (10 or 15% gels), followed by electro-transfer to nitrocellulose membrane. The membrane was blocked and probed with the antibodies against phospho-JNK (1:2000), phospho-c-Jun (Ser63) II (1:2000), JNK1 (1:1000), JNK2 (1:2500), JNK3 (1:5000) or cytochrome c (1:1000) overnight at 4 °C. After washing, the membrane was further incubated with peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG (1:10000) and visualized by the SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL, U.S.A.).
After stripping, the membranes were reprobed with anti-JNK (1:2000), c-Jun (1:2000) or β-tubulin (1:40000) antibody. Films were analysed densitometrically by the GS-800 calibrated densitometer (Bio-Rad) by referring the optical density values to a range of preset grey values. The intensity of the Western blot bands was quantified by Quantity One quantification software (Bio-Rad).
siRNA
siRNA against JNK1, JNK2 and JNK3 (siRNAs 1–3) and non-silencing siRNA labelled with fluorescein as scrambled control were from Qiagen (Heidelberg, Germany). siRNA sequences are provided in Supplementary Table 2 (http://www.BiochemJ.org/bj/401/bj4010065add.htm). In the present study, we mainly used JNK1 siRNA 1, JNK2 siRNA 1 and JNK3 siRNA 1, unless indicated otherwise. Rat astrocytes seeded on the 6-well or 24-well plate were transfected at 80% density with siRNA using MATra-A (magnet-assisted transfection for adherent cells) reagent (IBA, Göttingen, Germany) according to the manufacturer's protocol. JNK knockdown was determined by both RT–PCR and Western blotting 48 h after transfection.
LDH (lactate dehydrogenase) release
Serum-starved astrocytes in Phenol Red-free DMEM were treated with C2-ceramide in the absence or presence of TRag or PAR-2AP at the indicated concentrations for 7 h. The cell-culture medium of each well was removed for the LDH release assay using a cytotoxicity detection kit, following the manufacturer's protocol. The relative absorbance of all samples was measured at 490 nm. Cells treated with C2-ceramide alone were taken as control (100%). Cytotoxicity was calculated as a relative value normalized with the control group.
Statistical analysis
Statistical evaluation was carried out by Student's t test between two groups and by one-way ANOVA with GraphPrism software within multiple groups. Results are presented as means±S.E.M. and P<0.05 was considered significant.
RESULTS
PAR-1 and PAR-2 have similar capacity to up-regulate the chemokine GRO/CINC-1 secretion in astrocytes
Our recent studies have already demonstrated that activation of PAR-1 induced release of the chemokine GRO/CINC-1, a functional counterpart of human IL8, from rat astrocytes [10]. To determine whether activation of the three other PARs affected GRO/CINC-1 at the mRNA level, we treated serum-starved astrocytes with trypsin, PAR-2AP (SLIGRL), PAR-3AP (TFRGAP) and PAR-4AP (GYPGKF) for 3 h and then extracted the total RNA. As shown by RT–PCR, PAR-2 activation induced by trypsin and PAR-2AP, with increasing concentration, increased the GRO/CINC-1 mRNA level (Figure 1A). However, PAR-3 and PAR-4 activation both failed to up-regulate GRO/CINC-1 mRNA level (results not shown).
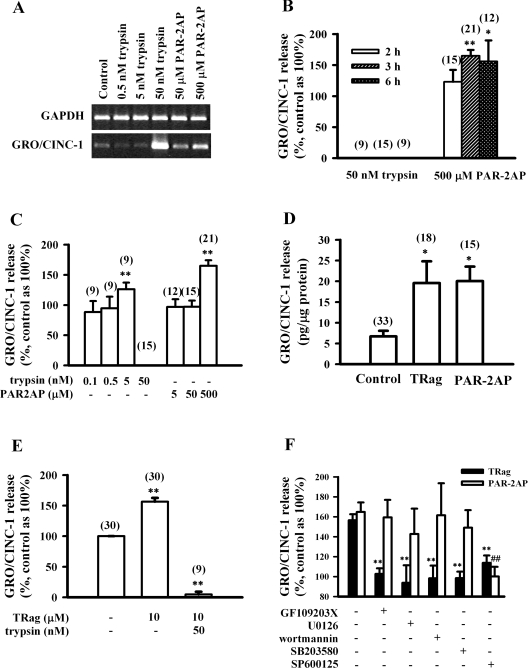
Figure 1. PAR-2 activation results in GRO/CINC-1 up-regulation at both mRNA and protein levels in rat astrocytes.
(A) Representative gel analysis of GRO/CINC-1 mRNA level induced by trypsin (0.5–50 nM) or PAR-2AP (50–500 μM) for 3 h. (B) Time dependence of GRO/CINC-1 release induced by 50 nM trypsin or 500 μM PAR-2AP for 2, 3 or 6 h. (C) Concentration dependence of GRO/CINC-1 release induced by 3 h incubation with trypsin (0.1–50 nM) or PAR-2AP (5–500 μM). (D) The amount of GRO/CINC-1 release induced by 3 h incubation of 10 μM TRag or 500 μM PAR-2AP. (E) Degradation of GRO/CINC-1 by trypsin. Cells were firstly treated with 10 μM TRag for 2 h, then treated with 50 nM trypsin together with TRag for another 1 h. Cells stimulated by 10 μM TRag alone for 3 h served as the positive control. Cells without treatment were taken as basal control (100%). (F) JNK activation contributes to both PAR-1- and PAR-2-induced GRO/CINC-1 release. Serum-starved cells were pre-incubated with PKC inhibitor GF109203X (5 μM), MEK-1/2 inhibitor U0126 (25 μM), PI3K inhibitor wortmannin (5 μM), p38 MAPK inhibitor SB203580 (10 μM) or JNK inhibitor SP600125 (30 μM) for 30 min prior to 3 h stimulation with 10 μM TRag or 500 μM PAR-2AP. Astrocytes without any treatment were taken as baseline (100%). Cells treated only with TRag or PAR-2AP served as positive control for GRO/CINC-1 release. (B–E) Numbers given in parentheses above the respective columns represent the number of samples. Results in (B–F) show the means±S.E.M. for at least three independent experiments. **P<0.01, *P<0.05 as compared with control in (B–E). **P<0.01 as compared with the cells exposed to TRag alone in (F). ##P<0.01 as compared with the cells exposed to PAR-2AP alone in (F).
On the other hand, the effect of PAR-2 activation on the release of GRO/CINC-1 protein was also investigated. The ELISA results demonstrate that PAR-2AP (500 μM) time-dependently up regulated GRO/CINC-1 release (Figure 1B). Moreover, release of GRO/CINC-1 was regulated by both trypsin and PAR-2AP in a concentration-dependent manner (Figure 1C). The magnitude of GRO/CINC-1 secretion induced by both PAR-1 and PAR-2 activation were compared here. As Figure 1(D) shows, PAR-2AP treatment increased GRO/CINC-1 release from astrocytes with levels of approx. 17–23 pg per μg of cellular protein, which was much higher than that found with untreated control cells. Similarly, PAR-1 activation, caused by TRag treatment, also induced comparable amounts of GRO/CINC-1 secretion from astrocytes by approx. 15–25 pg/μg of cellular protein. These results demonstrate that activation of PAR-1 and PAR-2 have similar capacities to increase the release of the chemokine GRO/CINC-1 from astrocytes.
To our surprise, no GRO/CINC-1 was detected after treatment with 50 nM trypsin for 2, 3 or 6 h, although 5 nM trypsin significantly induced GRO/CINC-1 secretion (Figures 1B and 1C). It has been established that trypsin cleaves peptides C-terminally to arginine or lysine residues, and prefers glycine or proline residues in the P2 position [27]. Sequence analysis of rat GRO/CINC-1 (accession number: NM_030845) revealed one potential cleavage site for trypsin: Gly71-Arg72. Thus it is possible that trypsin degrades GRO/CINC-1 by cleaving at position Gly71-Arg72. To test this, we stimulated cells with 10 μM TRag for 2 h, then further treated the cells with 50 nM trypsin together with TRag for another 1 h. Cells treated with TRag (10 μM) alone for 3 h served as positive control, and cells without any treatment were taken as basal value. As Figure 1(E) shows, the application of trypsin completely abolished TRag-induced GRO/CINC-1 release, which confirms that trypsin has the capability to degrade GRO/CINC-1. Nevertheless, our results clearly indicate that PAR-2 activation like PAR-1 activation is able to up-regulate GRO/CINC-1 at both mRNA and protein levels.
JNK activation is involved in both PAR-1- and PAR-2-induced GRO/CINC-1 release
Because PAR-1 and PAR-2 activations both resulted in chemokine GRO/CINC-1 release from astrocytes, it is important to study whether the same or different mechanisms are involved in PAR-1- and PAR-2-induced GRO/CINC-1 secretion. PKC (protein kinase C), PI3K (phosphoinositide 3-kinase), MEK (MAPK kinase)-1/2, p38 MAPK and JNK activations have been reported to be involved in multiple processes [10,28,29]. Therefore here we applied GF109203X (PKC-specific inhibitor), U0126 (MEK-1/2 inhibitor), wortmannin (PI3K inhibitor), SB203580 (p38 MAPK inhibitor) or SP600125 (JNK inhibitor) to astrocytes for 30 min prior to a 3 h PAR-2AP stimulation. For comparison, the same treatments with inhibitors were also performed in parallel prior to PAR-1 stimulation by TRag. As Figure 1(F) shows, inhibitors for PKC, PI3K, MEK-1/2, p38 MAPK and JNK significantly reduced PAR-1-induced GRO/CINC-1 release. However, PAR-2-induced GRO/CINC-1 release was only abolished by the specific JNK inhibitor SP600125. Inhibitors for PKC, PI3K, MEK-1/2 and p38 MAPK did not significantly affect PAR-2-induced GRO/CINC-1 release. These results indicate that PAR-1- and PAR-2-induced GRO/CINC-1 releases are apparently mediated by different mechanisms, although JNK seems to be involved in both signalling pathways.
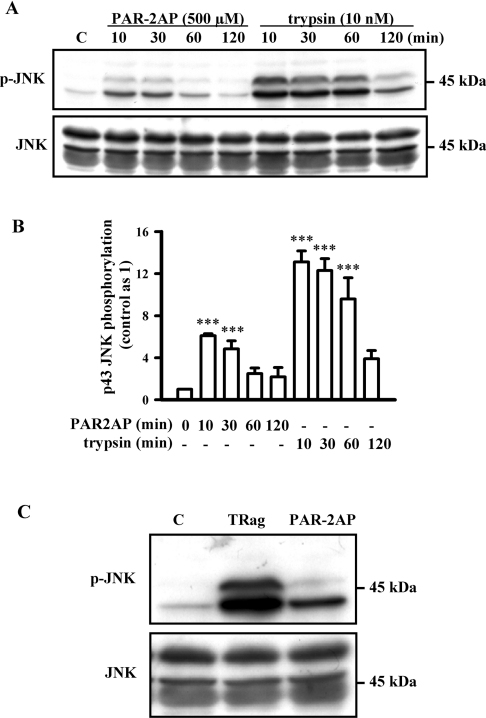
Next, we tested JNK activation with PAR-2 agonist stimulation in astrocytes in order to further confirm that JNK is involved in the PAR-2 signalling pathway. As Figures 2(A) and 2(B) show, both 43 and 46 kDa JNK isoforms were time-dependently phosphorylated by treatment with trypsin (10 nM). It is well known that JNKs splicing at the C-terminus mainly yield proteins of 46 and 54 kDa [30–32]. However, here we found that the JNK isoform with 54 kDa was not phosphorylated by trypsin treatment. These results are in line with our recent data [10] that showed PAR-1 activation induced by thrombin resulted in phosphorylation of the 43 and 46 kDa JNK isoforms, but not the 54 kDa JNK isoform. Trypsin, one main agonist of PAR-2, has also been reported to activate PAR-1 [1]. To rule out any unspecific proteolytic activity of trypsin and its possible side effect on PAR-1 activation, the specific PAR-2AP was also applied here. PAR-2AP (500 μM) stimulation significantly phosphorylated JNK isoform of 43 kDa, but not 46 kDa, which occurred in a time-dependent manner. The pronounced activation of JNK isoforms induced by PAR-2AP and trypsin were both observed at 10 min. These results suggest that the 43 kDa JNK isoform, but not the 46 kDa isoform, was activated upon PAR-2 activation. Unlike PAR-2AP-induced JNK phosphorylation, the PAR-1-specific peptide TRag activated both 43 and 46 kDa JNK isoforms (Figure 2C), indicating a difference between PAR-1- and PAR-2-induced JNK activations.
Figure 2. PAR-1- and PAR-2-induced JNK phosphorylation in rat astrocytes.
(A) Serum-starved cells were exposed to 500 μM PAR-2AP or 10 nM trypsin for 10, 30, 60 and 120 min respectively. Representative blots for JNK phosphorylation (upper panel) and total JNK (lower panel) from one experiment are shown. (B) The phosphorylation of 43 kDa JNK isoform induced by PAR-2 activation was quantified and normalized relative to that of control. Results represent the means±S.E.M. for at least three independent experiments. ***P<0.001 as compared with control. (C) Serum-starved cells were stimulated with 10 μM TRag or 500 μM PAR-2AP for 10 min. Representative blots of JNK phosphorylation (upper panel) and total JNK (lower panel) from one experiment are shown.
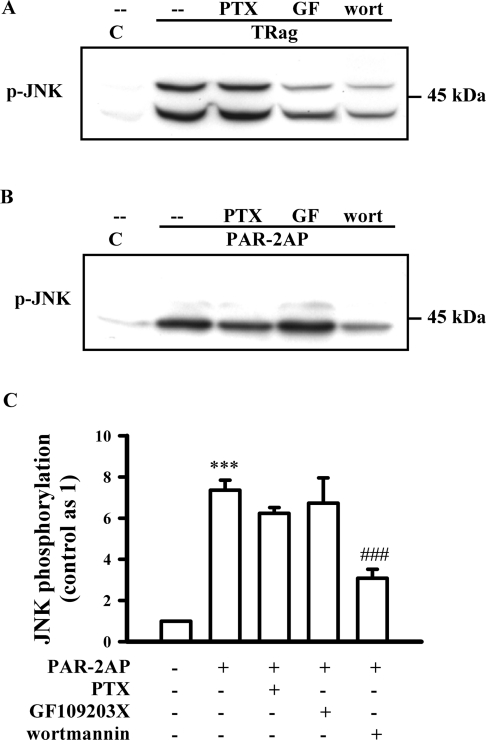
Further experiments demonstrate that both PKC and PI3K inhibition, but not PTX, significantly reduced PAR-1-induced JNK phosphorylation, suggesting that PKC and PI3K are two main upstream activators of PAR-1-induced JNK phosphorylation (Figure 3A). However, PAR-2-induced JNK activation was apparently not affected by treatment with the PKC inhibitor GF109203X (Figures 3B and 3C). Application of the PI3K inhibitor wortmannin only partially reduced PAR-2-induced JNK activation (Figures 3B and 3C), and PTX treatment also slightly reduced PAR-2-induced JNK phosphorylation (Figures 3B and 3C). These results suggest that PAR-2-induced JNK activation was partially mediated by PTX-sensitive G-protein and PI3K activation, but apparently independently of PKC activation, indicating that the upstream components of JNK activation induced by PAR-1 and PAR-2 are remarkably different.
Figure 3. Effects of PTX, GF109203X and wortmannin on PAR-1- and PAR-2-induced JNK activation in rat astrocytes.
Serum-starved cells were pre-incubated with PTX (200 ng/ml) for 24 h and PKC inhibitor GF109203X (GF, 5 μM) or PI3K inhibitor wortmannin (wort, 5 μM) for 30 min prior to 10 min incubation with 10 μM TRag or 500 μM PAR-2AP. (A) Representative blot of PAR-1-induced JNK phosphorylation is shown. (B) Representative blot of PAR-2-induced JNK phosphorylation is shown. (C) The phosphorylation of 43 kDa JNK isoform induced by PAR-2 activation was quantified and normalized relative to that of control. Results represent the means±S.E.M. for at least three independent experiments. ***P<0.001 as compared with control; ###P<0.001 as compared with the cells exposed to PAR-2AP alone.
PAR-1- and PAR-2-induced JNK activities causing the phosphorylation of the downstream transcription factor c-Jun are different
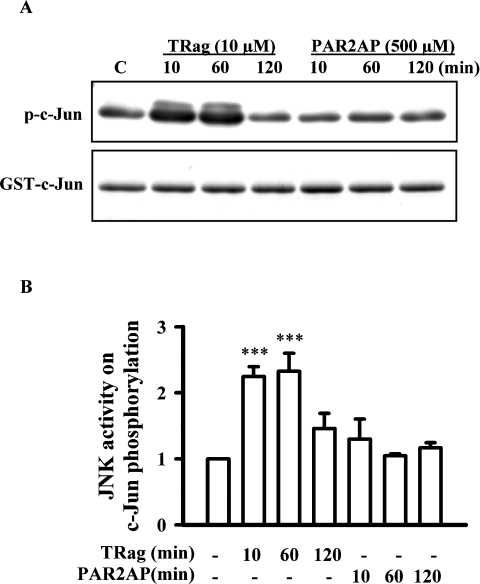
PAR-1-induced JNK activation has been shown to further phosphorylate c-Jun [10]. However, here we found that JNK activation induced by PAR-2AP failed to activate c-Jun (results not shown). To investigate further whether different JNK isoforms, which have different properties, were activated in the PAR-1 and PAR-2 signalling pathways, we examined and compared the JNK activity by the JNK kinase assay. We stimulated astrocytes for different times varying from 10 min to 2 h. The cell extracts were incubated with c-Jun–GST fusion protein immobilized on glutathione–agarose beads, which were used to pull down the JNK enzyme from cell extracts. Upon addition of kinase buffer and ATP, activated JNK could phosphorylate the substrate c-Jun. The JNK activity was determined by checking c-Jun phosphorylation by immunoblotting.
Figure 4 shows TRag-induced JNK activity. PAR-1 activation caused time-dependent phosphorylation of c-Jun. However, PAR-2AP-induced JNK activation was unable to phosphorylate c-Jun. These results indicate that different JNK isoforms with distinct activities are possibly involved in either PAR-1 or PAR-2 signalling pathways. Therefore we next investigated the role of the different JNK isoforms in both PAR-1 and PAR-2 signalling pathways.
Figure 4. Time course of JNK activity in response to TRag or PAR-2AP treatment in rat astrocytes.
(A) JNK activity in response to 10 μM TRag or 500 μM PAR-2AP treatment for the indicated times was evaluated by non-radioactive kinase assay. The upper panel shows the phosphorylation of c-Jun induced by TRag and PAR-2AP time-dependently; the lower panel demonstrates the equal loading of each sample by Coomassie Blue staining. (B) The amount of c-Jun phosphorylation was quantified and normalized relative to that of control. Results are presented as means±S.E.M. for at least three independent experiments. ***P<0.001 as compared with the control.
Expression and silencing of three JNK isoforms in astrocytes
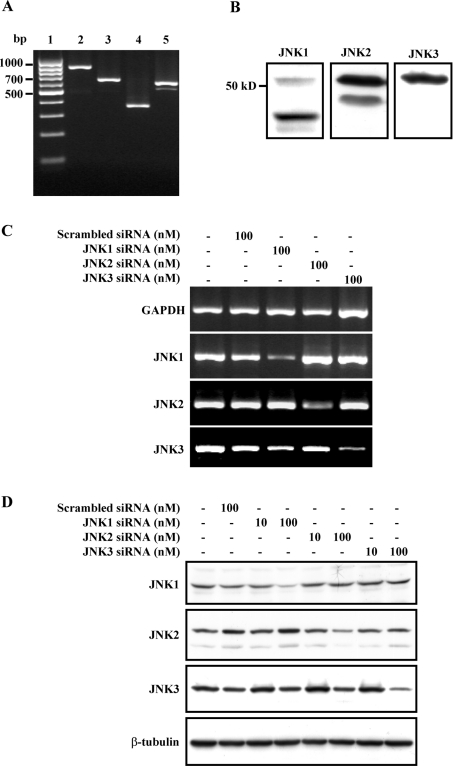
It is well known that there are three JNK genes (jnk1, jnk2 and jnk3) expressed in different tissues [30–32]. However, it is still not known whether all three JNK isoforms are expressed in astrocytes. Therefore, before investigating which JNK isoforms were specifically activated in the PAR-1 and PAR-2 signalling pathways, here we first studied the expression of three JNK isoforms in astrocytes. RT–PCR was performed using specific primers designed according to the respective conserved motif for jnk1, jnk2 and jnk3, which could recognize different sub-isoforms of the individual gene but could not cross-recognize isoforms of the two other genes (Supplementary Table 1). As shown in Figure 5(A), all three JNK isoforms were expressed in astrocytes. The PCR products of JNKs were confirmed by DNA sequencing. In addition, all three JNK isoforms were also detected at the protein level in astrocytes (Figure 5B).
Figure 5. Expression and silencing of JNK1, JNK2 and JNK3 in rat astrocytes.
(A) RT–PCR determination of JNK1, JNK2 and JNK3 expression. Lanes 1–5 represent marker, GAPDH, JNK1, JNK2 and JNK3 respectively. (B) Expression of JNK1, JNK2 and JNK3 in rat astrocytes by Western blot. (C) The mRNA levels of JNK isoforms detected by RT–PCR after transfection with scrambled siRNA (100 nM, lane 2), JNK1 siRNA 1 (100 nM, lane 3), JNK2 siRNA 1 (100 nM, lane 4) or JNK3 siRNA 1 (100 nM, lane 5) for 48 h. Cells without any treatment served as the control (lane 1). Representative gels for GAPDH, JNK1, JNK2 and JNK3 are shown. (D) The protein levels of JNK isoforms detected by Western blot after transfection with scrambled siRNA (100 nM, lane 2), JNK1 siRNA 1 (10–100 nM, lanes 3 and 4), JNK2 siRNA 1 (10–100 nM, lanes 5 and 6) or JNK3 siRNA 1 (10–100 nM, lanes 7 and 8) for 48 h. Cells without any treatment served as control (lane 1). Representative blots for JNK1, JNK2, JNK3 and β-tubulin are shown.
To investigate the distinct physiological roles of the JNK isoforms, we applied siRNA to knock down the respective JNK isoforms. The specific siRNAs against the JNK isoforms were designed from the respective conserved motif for jnk1, jnk2 and jnk3, which could recognize different sub-isoforms of the respective gene but could not cross-recognize isoforms of the two other genes (Supplementary Table 2), and primary cultured astrocytes were transfected using magnet-assisted transfection. As Figure 5(C) shows, JNK1 siRNA 1 significantly knocked down the expression of JNK1 without interfering with JNK2 or JNK3 mRNA expression. Similarly, JNK2 siRNA 1 and JNK3 siRNA 1 also only specifically knocked down their own mRNA expression. The transfection of non-silencing siRNA, which was given as scrambled siRNA control, did not disturb the mRNA expression of any of the three JNK isoforms or the housekeeping gene GAPDH. The effects of JNK siRNA were also studied at the protein level by Western blot. As shown in Figure 5(D), JNK1 siRNA 1, JNK2 siRNA 1 and JNK3 siRNA 1 concentration-dependently reduced the expression of the respective JNK isoform, without interfering with the expression of the two other JNK isoforms. Scrambled siRNA control also did not affect the expression of the three JNK isoforms, confirming the specificity of the three JNK siRNAs. Similar to JNK1–3 siRNA 1, other JNK siRNAs (including JNK1 siRNA 2, JNK2 siRNA 2–3 and JNK3 siRNA 2–3) targeting the three different JNK isoforms also specifically reduced the respective JNK isoforms at both mRNA and protein levels (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/401/bj4010065add.htm). This further confirmed the specificity of the three JNK siRNAs. Taken together, these results indicate that siRNA for the three JNK isoforms at the indicated concentrations could specifically knock down the respective JNK isoforms at both mRNA and protein levels.
PAR-1 and PAR-2 activate different JNK isoforms in astrocytes
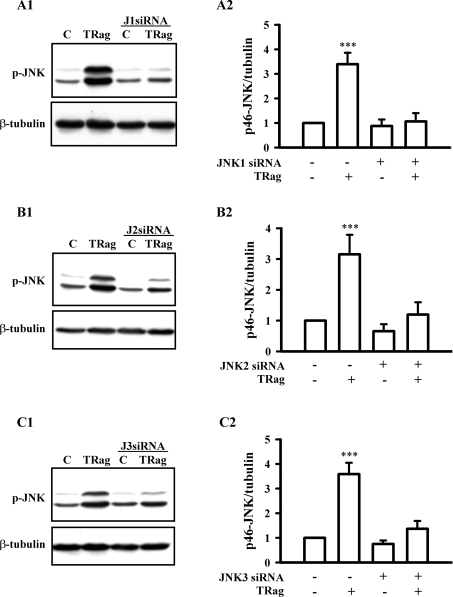
To investigate which JNK isoforms are involved in the PAR-1 signalling pathway, we examined JNK activation after knocking down certain JNK isoforms by siRNA. Figures 6(A1 and A2) show the PAR-1-specific peptide TRag induced JNK phosphorylation of both the 43 and 46 kDa isoforms. These were apparently impaired in JNK1-deficient cells. Similarly, TRag-induced JNK phosphorylation, especially with the 46 kDa isoform, remarkably was eliminated in both JNK2-deficient and JNK3-deficient cells (Figures 6B1, 6B2, 6C1 and 6C2). These results suggest that all three JNK isoforms (JNK1, JNK2 and JNK3) were activated by PAR-1 agonist stimulation.
Figure 6. Three different JNK isoforms are activated by PAR-1 in rat astrocytes.
Cells, transfected with (A1 and A2) JNK1 siRNA 1 (J1 siRNA, 100 nM), (B1 and B2) JNK2 siRNA 1 (J2 siRNA, 100 nM) or (C1 and C2)JNK3 siRNA 1 (J3 siRNA, 100 nM) for 48 h, were treated with or without 10 μM TRag in the serum-free medium for 10 min. Cells without transfection served as the control (C), and non-transfected cells stimulated with 10 μM TRag for 10 min were regarded as the positive control. (A1, B1 and C1) Show representative blots for JNK phosphorylation (p-JNK, upper panel) and β-tubulin (lower panel). (A2, B2 and C2) show the relative phosphorylation of 46 kDa JNK normalized to β-tubulin. Results are presented as means±S.E.M. for at least three independent experiments. ***P<0.001 as compared with the control cells.
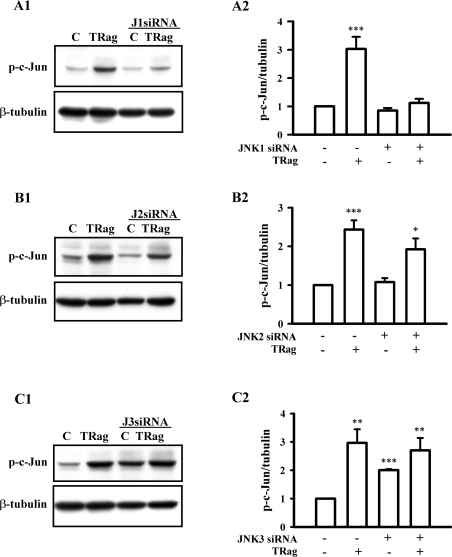
To further elucidate which JNK isoform was responsible for PAR-1-dependent c-Jun phosphorylation, we compared the TRag-induced c-Jun phosphorylation between WT (wild-type) cells and the JNK isoform-deficient cells. As Figures 7(A1 and A2) show, TRag stimulation (1 h) significantly increased c-Jun phosphorylation in WT cells, which notably was eliminated in JNK1-deficient cells. In JNK2-deficient cells, TRag stimulation was still able to significantly activate c-Jun (Figures 7B1 and 7B2). However, compared with that in WT cells, TRag-induced c-Jun phosphorylation in JNK2-deficient cells was slightly decreased. Interestingly, c-Jun activation was significantly enhanced even in non-stimulated JNK3-deficient cells compared with that in WT cells (Figures 7C1 and 7C2). These results suggest that PAR-1-induced JNK1 activation mainly mediated c-Jun phosphorylation, whereas JNK3 was able to negatively regulate c-Jun activation.
Figure 7. Different effects of JNK isoforms on PAR-1-dependent c-Jun activation in rat astrocytes.
Cells, transfected with (A1 and A2) JNK1 siRNA 1 (J1 siRNA, 100 nM), (B1 and B2) JNK2 siRNA 1 (J2 siRNA, 100 nM) or (C1 and C2) JNK3 siRNA 1 (J3 siRNA, 100 nM) for 48 h, were treated with 10 μM TRag or without in serum-free medium for 1 h. Untransfected cells served as control (C), non-transfected cells stimulated with 10 μM TRag for one hour were regarded as positive control. (A1, B1 and C1) show representative blots for c-Jun phosphorylation (p-c-Jun, upper panel) and β-tubulin (lower panel). (A2, B2 and C2) show the relative values of c-Jun phosphorylation normalized to β-tubulin. Results are presented as means±S.E.M. for at least three independent experiments. ***P<0.001, **P<0.01, *P<0.05 as compared with the control cells.
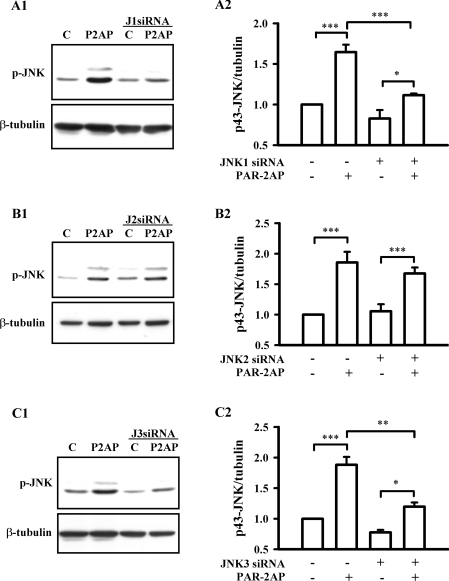
Similarly, we investigated which JNK isoforms were activated in the PAR-2 signalling pathway by knocking down JNK expression with siRNA. As Figures 8(A1 and A2) show, the 43 kDa JNK isoform was significantly phosphorylated after PAR-2AP treatment for 10 min in WT cells. However, PAR-2AP-induced JNK activation in JNK1-deficient cells was strikingly reduced compared with that in WT cells, although JNKs still can be significantly phosphorylated in these cells. Unlike the role of JNK1 in the PAR-2 signalling pathway, JNK2 knockdown with siRNA did not significantly affect PAR-2AP-induced JNK phosphorylation (Figures 8B1 and 8B2). However, JNK3 knockdown partially eliminated PAR-2AP-induced JNK activation (Figures 8C1 and 8C2). The reduction was comparable with that in JNK1-deficient cells. Taken together, these results indicate that JNK1 and JNK3, but not JNK2, were involved in the PAR-2 signalling pathway.
Figure 8. JNK1 and JNK3 isoforms are activated by PAR-2 in rat astrocytes.
Cells, transfected with (A1 and A2) JNK1 siRNA 1 (J1 siRNA, 100 nM), (B1 and B2) JNK2 siRNA 1 (J2 siRNA, 100 nM) or (C1 and C2) JNK3 siRNA 1 (J3 siRNA, 100 nM) for 48 h, were treated with or without 500 μM PAR-2AP (P2AP) in the serum-free medium for 10 min. Cells without transfection served as control (C), and non-transfected cells stimulated with 500 μM PAR-2AP for 10 min were regarded as positive control. (A1, B1 and C1), show representative blots for JNK phosphorylation (p-JNK, upper panel) and β-tubulin (lower panel). (A2, B2 and C2) show the relative value of 43 kDa JNK phosphorylation normalized to β-tubulin. Results are presented as means±S.E.M. for at least three independent experiments. ***P<0.001, **P<0.01, *P<0.05.
Distinct JNK isoforms are essential for PAR-1- and PAR-2-induced physiological responses
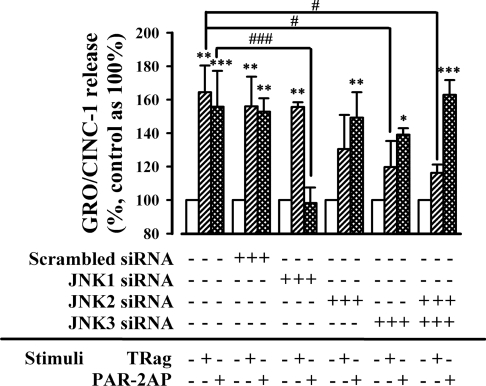
Considering that diverse JNK isoforms were activated in both PAR-1 and PAR-2 signalling pathways, as our results above show, here we investigated further the effects of individual JNK isoforms on the chemokine GRO/CINC-1 secretion by knocking down different JNK isoforms. The activation of PAR-1 and PAR-2 both significantly up-regulated the release of the chemokine GRO/CINC-1 in WT cells as well as in scrambled siRNA-transfected cells (Figure 9).
Figure 9. Distinct JNK isoforms are essential for PAR-1- and PAR-2-induced release of GRO/CINC-1.
Astrocytes were transfected with scrambled siRNA (100 nM), JNK1 siRNA 1 (100 nM), JNK2 siRNA 1 (100 nM) or JNK3 siRNA 1(100 nM) respectively for 48 h. Then, transfected and non-transfected cells were stimulated with or without 10 μM TRag or 500 μM PAR-2AP in the serum-free medium for 6 h. The supernatant medium was analysed for GRO/CINC-1 level by ELISA. The amount of GRO/CINC-1 release from non-stimulated cells (including transfected and non-transfected cells) was regarded as baseline (100%). Results represent the means±S.E.M. for at least three independent experiments. ***P<0.001, **P<0.01, *P<0.05 as compared with the respective control cells. ###P<0.001, #P<0.05 as compared with non-transfected cells exposed to TRag or PAR-2AP alone respectively.
Importantly, knockdown with JNK1 siRNA 1 did not affect PAR-1-induced GRO/CINC-1 release (Figure 9). However, PAR-2-induced GRO/CINC-1 release was greatly reduced in JNK1-deficient cells compared with WT cells. Unlike the stimulation in JNK1-deficient cells, PAR-1-induced GRO/CINC-1 release was apparently eliminated by both JNK2 siRNA 1 and JNK3 siRNA 1. This effect is clearer in JNK2/3-double-deficient cells. However, PAR-2-induced GRO/CINC-1 release was not affected by JNK2 or JNK3 silencing or JNK2/3 double silencing. Similar biological effects were also observed when using other JNK siRNAs (see Supplementary Figure 2 at http://www.BiochemJ.org/bj/401/bj4010065add.htm). These results demonstrate that PAR-1-induced GRO/CINC-1 release was apparently mediated by JNK2 and JNK3 activation, whereas PAR-1-induced JNK1/c-Jun activation was not essential for the secretion of GRO/CINC-1. In contrast, in the PAR-2 signalling pathway, JNK1 activation mainly contributed to GRO/CINC-1 release.
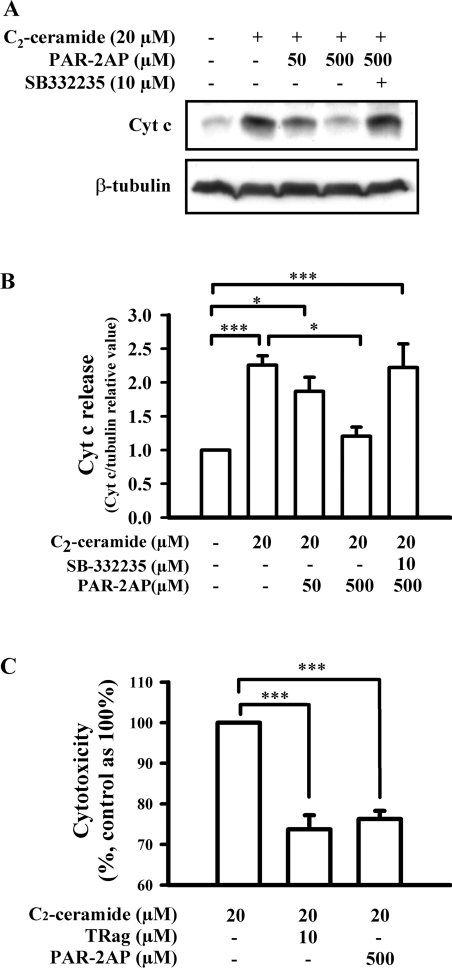
The chemokine GRO/CINC-1 release could prevent C2-ceramide-induced cytochrome c release from mitochondria, and thus rescue astrocytes from cell death, as we have found recently [10]. Importantly, our results further demonstrated that PAR-1 activation by TRag was also able to suppress the release of cytochrome c from mitochondria in a concentration-dependent manner via regulating chemokine GRO/CINC-1 secretion. Therefore, here we investigated whether PAR-2AP could exert an effect similar to that of TRag. PAR-2 activation by PAR-2AP concentration-dependently prevented C2-ceramide-induced cytochrome c release from mitochondria (Figures 10A and 10B). Furthermore, the protective effect of PAR-2 on suppressing C2-ceramide-induced cytochrome c release from mitochondria was remarkably abolished by treatment with the antagonist of the unique GRO/CINC-1 receptor CXCR2 (CXC chemokine receptor 2), SB-332235 (Figures 10A and 10B). These results demonstrate that PAR-2 activation by PAR-2AP likewise prevented cytochrome c release from mitochondria via regulating the secretion of the chemokine GRO/CINC-1.
Figure 10. Activation of PAR-1 and PAR-2 prevents C2-ceramide-induced cytochrome c release from mitochondria and rescues astrocytes from cell death by regulating GRO/CINC-1 release.
Serum-starved cells were treated with 20 μM C2-ceramide in the absence or presence of TRag (10 μM) or PAR-2AP (50 or 500 μM) together with CXCR2 antagonist SB-332235 (10 μM) for 7 h. Then, the cell cytosol fraction was isolated for measuring cytochrome c release, and the cell-culture medium was collected for LDH measurement. (A) Representative blots for cytochrome c (Cyt c, upper panel) and tubulin (lower panel) from the same experiment are shown. (B) The intensities of cytochrome c were quantified and normalized relative to β-tubulin. (C) Cytotoxicity assessment by measuring LDH release. Results shown in (B and C) are presented as means±S.E.M. for at least three independent experiments. ***P<0.001, *P<0.05.
The release of LDH, a stable cytoplasmic enzyme present in all cells, is a well-known cell death marker [33]. Upon damage of the plasma membrane, LDH is rapidly released into the cell-culture supernatant. Here, to investigate and compare the protective effects of PAR-1 and PAR-2 on astrocytes, we stimulated cells with the pro-apoptotic lipid C2-ceramide in the presence or absence of TRag or PAR-2AP, and then tested the LDH activity in the culture medium. As Figure 10(C) shows, C2-ceramide treatment significantly increased the LDH release. The increase in LDH release caused by C2-ceramide was apparently reduced by both PAR-1 activation by TRag and PAR-2 activation by PAR-2AP (Figure 10C). The protective capacity of PAR-2 is comparable with that of PAR-1 (Figure 10 C). These results demonstrate that PAR-2 activation, like PAR-1 activation, is able to protect neural cells from toxic insults by regulating chemokine GRO/CINC-1 release.
DISCUSSION
JNK is a central mediator of PAR-induced chemokine GRO/CINC-1 secretion
In the present study, our results clearly indicate that both PAR-1 and PAR-2 activation, but not PAR-3 or PAR-4, induced the release of the chemokine GRO/CINC-1, a functional counterpart of human IL8, from astrocytes. JNKs play a pivotal role in both PAR-1- and PAR-2-induced GRO/CINC-1 secretion, as seen from our ELISA data (Figure 1F) and Western-blot data (Figure 2). Pharmacological inhibition of PKC or PI3K not only significantly reduced PAR-1-induced JNK activation (Figure 3A), but also remarkably attenuated PAR-1-induced GRO/CINC-1 secretion (Figure 1F). These results suggest that PKC- or PI3K-mediated JNK activation mainly contributed to PAR-1-induced GRO/CINC-1 release. However, PAR-2-mediated JNK activation was not suppressed by the PKC inhibitor, and only partially reduced by the PI3K inhibitor (Figures 3B and 3C). This indicates that different upstream activators mediated JNK activation in the PAR-1 and PAR-2 signalling pathways. Importantly, PAR-2-induced GRO/CINC-1 release, unlike PAR-1-induced GRO/CINC-1 secretion, was independent of both PKC and PI3K activation (Figure 1F). Therefore PI3K-mediated JNK activation should not be essential for PAR-2-induced GRO/CINC-1 release. Furthermore, we show that MEK-1/2 activation, which contributed to PAR-1-induced GRO/CINC-1 release, was also not involved in PAR-2-induced GRO/CINC-1 secretion. Taken together, PKC, PI3K and MEK-1/2 were only involved in PAR-1-induced GRO/CINC-1 release but not PAR-2-induced GRO/CINC-1 secretion (Figure 1F). This indicates a clear difference between PAR-1 and PAR-2 signalling pathways.
Three different JNK genes (jnk1, jnk2 and jnk3) have been identified. Splicing at the C-terminus of the JNK isoforms yields 46- and 54-kDa polypeptides of JNK1, JNK2 and JNK3 [13]. The treatment with the PAR-1-specific agonist TRag could strongly phosphorylate JNK with both 46- and 43-kDa JNK isoforms. However, PAR-2AP could only induce the phosphorylation of the 43-kDa JNK isoform (Figure 2C). This indicates another clear difference between the PAR-1 and PAR-2 signalling pathways. Moreover, the JNK activity on c-Jun phosphorylation induced by PAR-1 and PAR-2 activation was completely different (Figure 4). Therefore our analysis, by comparing the JNK upstream activators, JNK phosphorylation and JNK activity on its downstream transcription factor c-Jun, evidently demonstrates that different JNK isoforms with distinct JNK properties were involved in the PAR-1 and PAR-2 signalling pathways. Next, we analysed whether they might contribute to the chemokine GRO/CINC-1 secretion.
Role of three JNK isoforms in PAR-induced chemokine GRO/CINC-1 release and c-Jun activation
Here, we established that all three JNK isoforms (JNK1, JNK2 and JNK3) were expressed in astrocytes (Figures 5A and 5B). It is well known that all three JNK isoforms are able to phosphorylate the transcription factor c-Jun and to play distinct biological functions in different systems [15,19–21,24,34–36]. We found that PAR-1 and PAR-2 activations both resulted in JNK1 phosphorylation. In contrast, PAR-2-induced JNK1 activation failed to activate c-Jun (Figure 4) and PAR-1-mediated JNK1 activation was mainly responsible for c-Jun phosphorylation (Figures 7A1 and 7A2). Our previous results [10] have shown that c-Jun phosphorylation was not suppressed after blocking the activation of JNK upstream factors, PI3K and PKC, suggesting that PAR-1-mediated JNK1/c-Jun phosphorylation was independent of PI3K and PKC activation. On the other hand, the loss-of-function studies here clearly demonstrate that PAR-1-dependent JNK1 activation was not involved in chemokine GRO/CINC-1 release (Figure 9). However, PAR-2-mediated JNK1 activation, which failed to phosphorylate c-Jun, was essential for GRO/CINC-1 release (Figure 9). Therefore our results suggest that JNK1 exerted completely different functions in the PAR-1 and PAR-2 signalling pathways.
JNK3 was likewise activated by both PAR-1 and PAR-2, but exerted different functions in these two signalling pathways. Here, we show that PAR-1-induced c-Jun phosphorylation was pronouncedly increased in JNK3-deficient cells without TRag stimulation (Figures 7C1 and 7C2). This indicates that JNK3 might be a negative regulator for JNK1-mediated c-Jun phosphorylation. We have already seen that PAR-1-induced c-Jun phosphorylation was significantly up-regulated after the pretreatment with the PI3K inhibitor which is a JNK upstream factor [10]. From these data we hypothesize that PI3K is likely to activate JNK3, which further negatively regulates JNK1-induced c-Jun phosphorylation by competing for the common substrate c-Jun.
It has been reported that the inactive JNKs caused degradation of transcription factors such as c-Jun, ATF2 (activating transcription factor 2) and p53 [37], while phosphorylated JNK stabilized c-Jun in vivo by suppressing multi-ubiquitination process [38]. Therefore our data suggest that JNK3 is likely to have higher affinity to bind to c-Jun and to cause c-Jun degradation at the resting state in our system, so as to keep the levels of c-Jun phosphorylation low. However, in JNK3-deficient cells, JNK1 has higher chances to bind to c-Jun. JNK1 is primarily responsible for the basal JNK activity in brain [25]. Thus, without competition with JNK3, active JNK1 significantly increases the phosphorylation of c-Jun. On the other hand, after stimulation, the active JNKs are expected to have a higher binding affinity for c-Jun than inactive JNKs. Although PI3K-mediated JNK3 activation is unable to phosphorylate c-Jun, it could compete with JNK1 to bind to c-Jun and negatively regulate JNK1-mediated c-Jun activation. Future studies are needed to test this model.
We show here that PAR-1-induced PI3K/JNK3 activation not only further negatively regulated c-Jun phosphorylation, but also prominently contributed to GRO/CINC-1 release. This finding could contribute to the explanation of the physiological role of the different JNK isoforms (Figure 9). However, PAR-2-induced GRO/CINC-1 release was independent of PI3K-mediated JNK3 activation (Figure 9), which is consistent with our data that PI3K was not involved in PAR-2-induced GRO/CINC-1 release (Figure 1F).
PKC and PI3K were identified as two important upstream factors of JNK activation that are required for the PAR-1-induced release of GRO/CINC-1 [10,39]. As the discussion above shows, PAR-1-mediated JNK1 activation was independent of PKC and PI3K. Therefore PKC is likely to be the activator of JNK2. Here, the JNK2 isoform has been shown to be activated by only PAR-1 and not by PAR-2 (Figures 6 and 8). These results are consistent with our data showing that the JNK2 upstream activator PKC was not involved in the PAR-2 signalling pathway (Figures 3B and 3C). Furthermore, loss-of-function studies clearly confirm that PKC/JNK2 activation contributed to PAR-1-induced GRO/CINC-1 release (Figure 9). In contrast, JNK2 knockdown apparently did not interfere with the secretion of GRO/CINC-1 induced by PAR-2 (Figure 9), which further supports our data that PKC-mediated JNK2 activation was not involved in the PAR-2 signalling pathway (Figure 8).
Taken together the results presented here so far show that activation of PAR-1 and PAR-2 both result in increase of secretion of the chemokine GRO/CINC-1, and JNK is a central mediator for PAR-induced GRO/CINC-1 secretion. The three JNK isoforms function differently and even the same JNK isoform, depending on PAR-1 or PAR-2 activation, can exert different functions. PAR-1-induced GRO/CINC-1 release is mainly mediated by PKC/JNK2 and PI3K/JNK3. However, PAR-2-induced GRO/CINC-1 release is mainly mediated by JNK1 activation.
Therefore our results indicate for the first time that JNK mediates chemokine GRO/CINC-1 release in a JNK isoform-dependent fashion in astrocytes and invokes PAR subtype-dependent mechanisms. The results presented here provide novel functional insights into the physiological role of different JNK isoforms in astrocytes.
Physiological significance of PAR-1 and PAR-2 signalling in brain
In addition to unravelling the biochemical mechanism of PAR/JNK subtype-specific activation and the signalling pathways to chemokine release, we further aimed at shedding light on the pathophysiological significance of these results. Brain injury, such as trauma, Alzheimer's disease, Parkinson's disease and ischaemia/stroke, has been reported to induce accumulation of the apoptotic mediator ceramide in neural astrocytes, which causes neural cell death [40–43]. On the other hand, brain injury might also increase the serine proteinases thrombin, tryptase or p22 levels in the brain tissue [3,5,9,44,45]. Subsequently, increased levels of proteinases cause activation of PAR-1, PAR-2, or both receptors simultaneously. Thrombin at low concentrations has been reported to prevent neural cell death in ischaemic and traumatic brain injury by activating its receptor PAR-1 [5]. The protective effect of PAR-2 in brain has not received much attention for a long time. Although recently a few reports appeared showing that PAR-2 might prevent neural cell death during HIV infection and acute focal ischaemic brain injury [46,47], but the protective action of PAR-2 is still poorly understood. In this context, our results here are of high significance showing that activation of PAR-2, as well as PAR-1, rescued neural cells from ceramide-induced cell death by regulating chemokine GRO/CINC-1 release (Figure 10). Possibly, these protective actions only occur at the early state after mild brain injury, since we found that the protection by PAR-1 and PAR-2 after C2-ceramide treatment was decreased after 24 h compared with that at 5–10 h (results not shown). Therefore, here we provide a new mechanism that is likely to explain the protective action of low concentrations of thrombin after brain injury. Moreover, our results suggest that, besides the PAR-1 signal, the PAR-2 signalling pathway could be an additional protective pathway in brain. These results provide important implications for our understanding of the protective role of PARs.
Online data
Acknowledgments
We thank Dr T. Hanck and Dr R. Stricker for helpful suggestions and discussions. We thank Dr H. Sarau for kindly providing us with the CXCR2 antagonist SB-332235. This work was supported by grants from the Deutsche Forschungsgemeinschaft 253/5 (Graduiertenkolleg für ‘Biologische Grundlagen von Erkrankungen des Nervensystems’), Land Sachsen-Anhalt (grant 2923A/0028H) and Bundesministerium für Bildung und Forschung (01-ZZ 9505).
References
- 1.Ossovskaya V. S., Bunnett N. W. Protease-activated receptors: contribution to physiology and disease. Physiol. Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 2.Wang H., Reiser G. Thrombin signaling in the brain: the role of protease-activated receptors. Biol. Chem. 2003;384:193–202. doi: 10.1515/BC.2003.021. [DOI] [PubMed] [Google Scholar]
- 3.Arai T., Miklossy J., Klegeris A., Guo J. P., McGeer P. L. Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. J. Neuropathol. Exp. Neurol. 2006;65:19–25. doi: 10.1097/01.jnen.0000196133.74087.cb. [DOI] [PubMed] [Google Scholar]
- 4.Dihanich M., Kaser M., Reinhard E., Cunningham D., Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron. 1991;6:575–581. doi: 10.1016/0896-6273(91)90060-d. [DOI] [PubMed] [Google Scholar]
- 5.Xi G., Reiser G., Keep R. F. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J. Neurochem. 2003;84:3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 6.Donovan F. M., Cunningham D. D. Signalling pathways involved in thrombin-induced cell protection. J. Biol. Chem. 1998;273:12746–12752. doi: 10.1074/jbc.273.21.12746. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan P. J., Pike C. J., Cotman C. W., Cunningham D. D. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J. Neurosci. 1995;15:5389–5401. doi: 10.1523/JNEUROSCI.15-07-05389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noorbakhsh F., Vergnolle N., Hollenberg M. D., Power C. Proteinase-activated receptors in the nervous system. Nat. Rev. Neurosci. 2003;4:981–990.. doi: 10.1038/nrn1255. [DOI] [PubMed] [Google Scholar]
- 9.Sawada K., Nishibori M., Nakaya N., Wang Z., Saeki K. Purification and characterization of a trypsin-like serine proteinase from rat brain slices that degrades laminin and type IV collagen and stimulates protease-activated receptor-2. J. Neurochem. 2000;74:1731–1738. doi: 10.1046/j.1471-4159.2000.0741731.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Luo W., Stricker R., Reiser G. Protease-activated receptor-1 protects rat astrocytes from apoptotic cell death via JNK-mediated the release of the chemokine GRO/CINC-1. J. Neurochem. 2006;98:1046–1060. doi: 10.1111/j.1471-4159.2006.03950.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Ubl J. J., Reiser G. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia. 2002;37:53–63. doi: 10.1002/glia.10012. [DOI] [PubMed] [Google Scholar]
- 12.Kyriakis J. M., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 13.Waetzig V., Herdegen T. Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage. Trends Pharmacol. Sci. 2005;26:455–461. doi: 10.1016/j.tips.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Sabapathy K., Hochedlinger K., Nam S. Y., Bauer A., Karin M., Wagner E. F. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol. Cell. 2004;15:713–725. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Sabapathy K., Wagner E. F. JNK2: a negative regulator of cellular proliferation. Cell Cycle. 2004;3:1520–1523. doi: 10.4161/cc.3.12.1315. [DOI] [PubMed] [Google Scholar]
- 16.Leppa S., Saffrich R., Ansorge W., Bohmann D. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J. 1998;17:4404–4413. doi: 10.1093/emboj/17.15.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park K. S., Lee R. D., Kang S. K., Han S. Y., Park K. L., Yang K. H., Song Y. S., Park H. J., Lee Y. M., Yun Y. P., et al. Neuronal differentiation of embryonic midbrain cells by upregulation of peroxisome proliferator-activated receptor-gamma via the JNK-dependent pathway. Exp. Cell Res. 2004;297:424–433. doi: 10.1016/j.yexcr.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 18.Tawadros T., Martin D., Abderrahmani A., Leisinger H. J., Waeber G., Haefliger J. A. IB1/JIP-1 controls JNK activation and increased during prostatic LNCaP cells neuroendocrine differentiation. Cell. Signalling. 2005;17:929–939. doi: 10.1016/j.cellsig.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Keramaris E., Vanderluit J. L., Bahadori M., Mousavi K., Davis R. J., Flavell R., Slack R. S., Park D. S. c-Jun N-terminal kinase 3 deficiency protects neurons from axotomy-induced death in vivo through mechanisms independent of c-Jun phosphorylation. J. Biol. Chem. 2005;280:1132–1141. doi: 10.1074/jbc.M410127200. [DOI] [PubMed] [Google Scholar]
- 20.Morishima Y., Gotoh Y., Zieg J., Barrett T., Takano H., Flavell R., Davis R. J., Shirasaki Y., Greenberg M. E. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. 2001;21:7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang D. D., Kuan C. Y., Whitmarsh A. J., Rincon M., Zheng T. S., Davis R. J., Rakic P., Flavell R. A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 22.Waetzig V., Czeloth K., Hidding U., Mielke K., Kanzow M., Brecht S., Goetz M., Lucius R., Herdegen T., Hanisch U. K. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia. 2005;50:235–246. doi: 10.1002/glia.20173. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich N., Thastrup J., Holmberg C., Gyrd-Hansen M., Fehrenbacher N., Lademann U., Lerdrup M., Herdegen T., Jaattela M., Kallunki T. JNK2 mediates TNF-induced cell death in mouse embryonic fibroblasts via regulation of both caspase and cathepsin protease pathways. Cell Death Differ. 2004;11:301–313. doi: 10.1038/sj.cdd.4401353. [DOI] [PubMed] [Google Scholar]
- 24.Liu J., Minemoto Y., Lin A. c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor alpha-induced c-Jun kinase activation and apoptosis. Mol. Cell. Biol. 2004;24:10844–10856. doi: 10.1128/MCB.24.24.10844-10856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuan C. Y., Whitmarsh A. J., Yang D. D., Liao G., Schloemer A. J., Dong C., Bao J., Banasiak K. J., Haddad G. G., Flavell R. A., et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuan C. Y., Yang D. D., Samanta Roy D. R., Davis R. J., Rakic P., Flavell R. A. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Richter-Landsberg C., Reiser G. Expression of protease-activated receptors (PARs) in OLN-93 oligodendroglial cells and mechanism of PAR-1-induced calcium signaling. Neuroscience. 2004;126:69–82.. doi: 10.1016/j.neuroscience.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Wang H., Ubl J. J., Stricker R., Reiser G. Thrombin (PAR-1)-induced proliferation in astrocytes via MAPK involves multiple signaling pathways. Am. J. Physiol. Cell Physiol. 2002;283:C1351–C1364. doi: 10.1152/ajpcell.00001.2002. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J., Krishnegowda G., Gowda D. C. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: the requirement of extracellular signal-regulated kinase, p38, c-Jun N-terminal kinase and NF-kappaB pathways for the expression of proinflammatory cytokines and nitric oxide. J. Biol. Chem. 2005;280:8617–8627. doi: 10.1074/jbc.M413539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barr R. K., Bogoyevitch M. A. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs) Int. J. Biochem. Cell Biol. 2001;33:1047–1063. doi: 10.1016/s1357-2725(01)00093-0. [DOI] [PubMed] [Google Scholar]
- 31.Enomoto A., Suzuki N., Morita A., Ito M., Liu C. Q., Matsumoto Y., Yoshioka K., Shiba T., Hosoi Y. Caspase-mediated cleavage of JNK during stress-induced apoptosis. Biochem. Biophys. Res. Commun. 2003;306:837–842. doi: 10.1016/s0006-291x(03)01050-7. [DOI] [PubMed] [Google Scholar]
- 32.Sugden P. H., Clerk A. ‘Stress-responsive’ mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ. Res. 1998;83:345–352. doi: 10.1161/01.res.83.4.345. [DOI] [PubMed] [Google Scholar]
- 33.Koh J. Y., Choi D. W. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J. Neurosci. Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs S. Y., Fried V. A., Ronai Z. Stress-activated kinases regulate protein stability. Oncogene. 1998;17:1483–1490. doi: 10.1038/sj.onc.1202184. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S., Barrett T., Whitmarsh A. J., Cavanagh J., Sluss H. K., Derijard B., Davis R. J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 36.Kallunki T., Su B., Tsigelny I., Sluss H. K., Derijard B., Moore G., Davis R., Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs S. Y., Xie B., Adler V., Fried V. A., Davis R. J., Ronai Z. c-Jun NH2-terminal kinases target the ubiquitination of their associated transcription factors. J. Biol. Chem. 1997;272:32163–32168. doi: 10.1074/jbc.272.51.32163. [DOI] [PubMed] [Google Scholar]
- 38.Musti A. M., Treier M., Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science. 1997;275:400–402. doi: 10.1126/science.275.5298.400. [DOI] [PubMed] [Google Scholar]
- 39.Slepko N., Patrizio M., Levi G. Expression and translocation of protein kinase C isoforms in rat microglial and astroglial cultures. J. Neurosci. Res. 1999;57:33–38. doi: 10.1002/(SICI)1097-4547(19990701)57:1<33::AID-JNR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 40.Ariga T., Jarvis W. D., Yu R. K. Role of sphingolipid-mediated cell death in neurodegenerative diseases. J. Lipid Res. 1998;39:1–16. [PubMed] [Google Scholar]
- 41.Barrier L., Ingrand S., Piriou A., Touzalin A., Fauconneau B. Lactic acidosis stimulates ganglioside and ceramide generation without sphingomyelin hydrolysis in rat cortical astrocytes. Neurosci. Lett. 2005;385:224–229. doi: 10.1016/j.neulet.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 42.Blazquez C., Galve-Roperh I., Guzman M. De novo-synthesized ceramide signals apoptosis in astrocytes via extracellular signal-regulated kinase. FASEB J. 2000;14:2315–2322. doi: 10.1096/fj.00-0122com. [DOI] [PubMed] [Google Scholar]
- 43.Blazquez C., Geelen M. J., Velasco G., Guzman M. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001;489:149–153. doi: 10.1016/s0014-5793(01)02089-0. [DOI] [PubMed] [Google Scholar]
- 44.Lozada A., Maegele M., Stark H., Neugebauer E. M., Panula P. Traumatic brain injury results in mast cell increase and changes in regulation of central histamine receptors. Neuropathol. Appl. Neurobiol. 2005;31:150–162. doi: 10.1111/j.1365-2990.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- 45.Rozniecki J. J., Hauser S. L., Stein M., Lincoln R., Theoharides T. C. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann. Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- 46.Jin G., Hayashi T., Kawagoe J., Takizawa T., Nagata T., Nagano I., Syoji M., Abe K. Deficiency of PAR-2 gene increases acute focal ischemic brain injury. J. Cereb. Blood Flow Metab. 2005;25:302–313. doi: 10.1038/sj.jcbfm.9600021. [DOI] [PubMed] [Google Scholar]
- 47.Noorbakhsh F., Vergnolle N., McArthur J. C., Silva C., Vodjgani M., Andrade-Gordon P., Hollenberg M. D., Power C. Proteinase-activated receptor-2 induction by neuroinflammation prevents neuronal death during HIV infection. J. Immunol. 2005;174:7320–7329. doi: 10.4049/jimmunol.174.11.7320. [DOI] [PubMed] [Google Scholar]
- 48.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.