Abstract
Metallo-β-lactamases are native zinc enzymes that catalyse the hydrolysis of β-lactam antibiotics, but are also able to function with cobalt(II) and require one or two metal-ions for catalytic activity. The hydrolysis of cefoxitin, cephaloridine and benzylpenicillin catalysed by CoBcII (cobalt-substituted β-lactamase from Bacillus cereus) has been studied at different pHs and metal-ion concentrations. An enzyme group of pKa 6.52±0.1 is found to be required in its deprotonated form for metal-ion binding and catalysis. The species that results from the loss of one cobalt ion from the enzyme has no significant catalytic activity and is thought to be the mononuclear CoBcII. It appears that dinuclear CoBcII is the active form of the enzyme necessary for turnover, while the mononuclear CoBcII is only involved in substrate binding. The cobalt-substituted enzyme is a more efficient catalyst than the native enzyme for the hydrolysis of some β-lactam antibiotics suggesting that the role of the metal-ion is predominantly to provide the nucleophilic hydroxide, rather than to act as a Lewis acid to polarize the carbonyl group and stabilize the oxyanion tetrahedral intermediate.
Keywords: Bacillus cereus, cobalt substitution, β-lactan hydrolysis, metallo-β-lactamase, pH-rate profile
Abbreviations: MBL, metallo-β-lactamase; BcII, β-lactamase from Bacillus cereus
INTRODUCTION
The major bacterial defence mechanism against the normally lethal action of β-lactam antibiotics consists of bacteria producing a class of enzymes, the β-lactamases, which catalyse the hydrolysis of the β-lactam ring, rendering the drugs inactive (Scheme 1).
Scheme 1.
From the mechanistic point of view, β-lactamases are divided into two classes: serine-β-lactamases (classes A, C and D β-lactamases), which use an active site serine residue for hydrolysis, and MBLs (metallo-β-lactamases; class B β-lactamases), which require one or two zinc ions for catalytic activity [1]. MBLs have been further divided into three subclasses, B1, B2 and B3, based on their amino acid sequences, substrate profiles and metal-ion requirement [2]. Subclass B1 of the MBLs is the largest and contains four well-studied β-lactamases: BcII from Bacillus cereus [3–5], CcrA from Bacteroides fragilis [6–9], IMP-1 from Pseudomonas aeruginosa [10–12] and BlaB from Chryseobacterium meningosepticum [13]. They efficiently hydrolyse a wide range of substrates, including penicillins, cephalosporins and carbapenems [14]. The most common representatives of subclass B2 are CphA from Aeromonas hydrophila [15] and ImiS from Aeromonas veronii [16], which preferentially hydrolyse carbapenems, e.g. imipenem and meropenem [17], and have poor activity against penicillins and cephalosporins [18,19]. Finally, subclass B3 contains the only known tetrameric zinc-β-lactamase, the L1 enzyme from Stenotrophomonas maltophilia [20], and the monomeric FEZ-1 from Legionella gormanii [21]. Both enzymes hydrolyse a wide spectrum of β-lactam antibiotics [18,19].
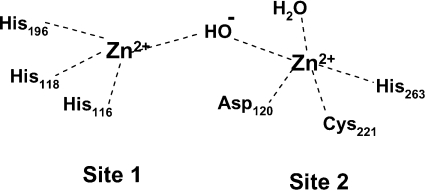
There are two zinc-binding sites [2–4,6,12,13] but their occupancy has different effects in different MBLs. Furthermore, the zinc ligands in the two sites are not the same and are not fully conserved between the different MBLs. In the B1 enzymes, such as the B. cereus MBL, BcII (Figure 1), the zinc in site 1 (the histidine site or His3 site) is four-co-ordinated through the nitrogens of the imidazoles of three histidine residues (116, 118 and 196) and a water molecule, Wat1. The zinc in the second site (site 2, the DCH or the Cys site) is co-ordinated to five ligands: His263, Asp120, Cys221 and one water molecule, carbonate [22] or another water, often referred to as the apical water, or Wat2 [3,6,23,24]. The two metal-ions are relatively close to each other, but the distance between them varies from 3.4 to 4.4 Å (1 Å=0.1 nm) in different structures of the BcII and CcrA enzymes [3,6,22–24]. The CcrA enzyme shows a bridging water ligand between the two metals that is thought to exist as a hydroxide ion [6,24] as does BcII at pH 7.5 [25], although at lower pH this solvent molecule is strongly associated to the zinc in site 1 [3,26].
Figure 1. Schematic representation of the two zinc-binding sites in the MBL from B. cereus, BcII.
MBLs have different metal-ion affinities for the two binding sites and apparently different metal-ion requirement for catalysis. The class B1 enzyme BcII, from B. cereus, has very different dissociation constants for the two metal-binding sites, based on fluorescence spectroscopy using a chromophoric chelator. For the loss of metal-ion from the mononuclear enzyme, the dissociation constant, Kmono, is 6.2×10−10 M and that for the loss of one metal-ion from the dinuclear MBL, Kdi, is 1.5×10−6 M [27]. Kmono decreases significantly, from nanomolar to picomolar values in the presence of substrate, whereas Kdi decreases by only 2-fold [28]. This suggests that the mono-zinc enzyme is responsible for the catalytic activity under physiological conditions, where the concentration of free zinc ions is in the picomolar range [28]. Conversely, and despite the very close similarity with BcII, the class B1 enzyme CcrA (from Bact. fragilis) binds both zinc ions very tightly [29]. Kinetic studies of CcrA with nitrocefin have shown that only the di-nuclear species is active and that a previously observed mono-zinc CcrA enzyme [7] was a mixture of the di-zinc and the apo (metal-free) enzyme [30].
Based on the pH-dependence of kcat/Km for the ZnBcII catalysed hydrolysis of benzylpenicillin and cephaloridine, and of the Ki (competitive inhibition constant) of BcII with a thiol inhibitor, it has been proposed that there are three catalytically important groups in the enzyme: two of pKa 5.6 which are assigned to Asp120 and the zinc-bound water, and are required in their deprotonated form for activity, and one of pKa 9.5 which is required in its protonated form for maximum activity [31]. Increasing the metal-ion concentration (from 10−6 to 10−3 M) had no effect on the rate constant at neutral pH but restored the enzyme activity at lower pHs (pH≤5.5), with a corresponding decrease of the apparent pKa values (from 5.6 to 4.5). Decreasing the pH liberates metal-ion from the enzyme due to protonation of one of the metal ligands. In terms of the equilibrium between mono- and di-nuclear enzymes, binding of the second zinc ion lowers the pKa of the catalytically important groups on the acidic limb [31]. The proposed mechanism involves the metal-bound hydroxide attacking the carbonyl to generate a mono-anionic tetrahedral intermediate, which is followed by proton abstraction by the Asp120 to give a di-anionic species (Scheme 2).
Scheme 2.
It was suggested that the same aspartic acid residue then functions as a proton donor, to facilitate C–N bond fission and opening of the β-lactam ring [31].
Changing the metal-ion in zinc enzymes gives the opportunity to explore the structural and mechanistic role of the metal [32–37]. The zinc of MBLs can be replaced with cadmium, cobalt and manganese to give catalytically active enzymes [25,38,39]. The metal environment of cobalt-substituted MBLs has been studied by UV-visible spectroscopy, NMR and EPR [4,29,40–43]. The kinetic effect of cobalt substitution in BcII is to make the progress curves for the hydrolysis of benzylpenicillin biphasic, with an initial burst followed by a transient to a steady-state rate [44,45]. The size of the burst was greater than the concentration of enzyme, which led the authors to propose a branched kinetic hydrolysis pathway involving two enzyme–substrate intermediates, ES1 and ES2, which we have recently reinterpreted as mono- and di-nuclear forms of the enzyme [46]. The pH-dependencies of the Michaelis–Menten constants kcat/Km and kcat for the CoBcII-catalysed hydrolysis of benzylpenicillin were performed at saturating concentrations of cobalt ion [38]. However, given more recent studies [31], which have shown that the pKa value of the ionization on the acidic limb of the ZnBcII pH-rate profile is metal-ion-concentration-dependent, we have studied the pH-rate profiles for CoBcII at different cobalt ion concentrations. Cobalt has approx. 100-fold less affinity than zinc for the two binding sites of BcII, i.e. the dissociation constants are higher, which makes it easier, from an experimental point of view, for studying the differences between the catalytic properties of the mono- and di-nuclear forms of the enzyme.
EXPERIMENTAL
Materials
The reagents used in all kinetic experiments were of analytical or an equivalent grade. Cephaloridine was supplied by GlaxoSmithKline and used without further purification. Buffers, benzylpenicillin, cefoxitin, Chelex 100 and CoCl2 (99.9999%) were purchased from Sigma. Deionized ultrapure water (18 MΩ·cm) was used for the preparation of buffers and other aqueous solutions. The buffers used were: acetate (pKa 4.75), Mes (pKa 6.15), Mops (pKa 7.20), Taps [N-tris(hydroxymethyl)methyl-3-aminopropane] (pKa 8.40) and Ches [2-(N-cyclohexylamino)ethanesulfonic acid] (pKa 9.2). Buffer solutions were prepared just prior to the experiment, and their ionic strength was kept constant by means of potassium chloride.
The apo B. cereus 569/H/9 enzyme (metal-free BcII) was prepared using the following procedure: ZnBcII (prepared and purified as described in [47]) was dialysed, while stirring, against two changes of 0.015 M Mes (pH 6.5), containing 0.1 M NaCl and 0.02 M EDTA over a 12 h period. EDTA was removed from the resulting apo-enzyme solution using four dialysis steps against the same buffer containing 1 M NaCl and Chelex 100 and finally two dialysis steps against 0.015 M Mes (pH 6.5) containing 0.1 M NaCl and Chelex 100. The resulting apo-enzyme contained less than 3% zinc ions, as determined by atomic absorption spectroscopy, and less than 10% free EDTA, as shown by 1H-NMR.
Equipment
pH measurements were made using a φ40 pH meter (Beckman) with a calomel glass electrode (Beckman). A two point calibration of the pH meter was taken at 30 °C prior to use, with a pH 7 phosphate ‘green’ buffer (Beckman) and a pH 4 or pH 10 calibration buffer (BDH). UV spectrometry was performed on a Cary 1E UV-visible spectrometer equipped with a twelve compartment cell block thermostatically controlled using a Peltier system (Varian). Rate constants were estimated using the Cary Win UV kinetics application version 02.00(26).
The concentration of free EDTA in the apo-enzyme solution was determined by 1H-NMR spectra on a 400 MHz instrument (Brucker), using the standard addition method.
The residual zinc content of apoBcII was determined by atomic absorption spectroscopy on a PerkinElmer AAnalyst 100 atomic absorption spectrometer. The hollow cathode lamp wavelength was set at 213.9 nm, with a current of 7 mA, and a slit wave of 0.7 nm. Zinc sulphate solutions of different concentrations were used as standards.
General kinetic procedure
In a typical experiment, the apoBcII (1×10−7–2×10−6 M) was incubated for 5 min at 30 °C in the buffer (2 ml) containing the corresponding cobalt ion concentration (10−4–10−3 M), in a quartz cuvette (200–2500 nm; Hellma). Unless otherwise specified, the reaction was initiated by adding the substrate and was followed by the decrease in absorbance at 235 nm for benzylpenicillin (Δϵ=820 M−1·cm−1) and 260 nm for cefoxitin (Δϵ=4000 M−1·cm−1) and cephaloridine (Δϵ=8000 M−1·cm−1).
Steady-state kinetics
The Michaelis–Menten kinetic parameters kcat, Km and kcat/Km were determined as follows. Below saturation, where [S]≪Km, the curves were fitted to a simple first-order rate law to obtain the pseudo-first-order rate constants, kobs, which were shown to be first-order in enzyme concentration. The second-order rate constant, kcat/Km, was obtained by dividing kobs by the concentration of enzyme used. For the determination of kcat and Km, the substrate concentrations used were in the range of the Km values. The initial rates, measured for at least seven substrate concentrations, were fitted directly to the Michaelis–Menten equation using SCIENTIST software (MicroMath Scientific Software), to obtain the apparent kcat and Km values for each pH and each metal-ion concentration.
RESULTS AND DISCUSSION
pH-rate profiles for CoBcII catalysed hydrolysis of cefoxitin, cephaloridine and benzylpenicillin
The kinetic studies of metallo-enzymes that involve the replacement of the native enzyme should ensure that the contribution from any remaining wild-type enzyme to the measured activity is as small as possible. In the present paper, a background hydrolysis rate of all substrates [cefoxitin (1), cephaloridine (2) and benzylpenicillin (3)] in the presence of the apo-enzyme was always determined and was insignificant, unless otherwise stated. In the following experiments, two cobalt ion concentrations were used, 10−3 M and 10−4 M, which should ensure that the mono-cobalt species was always present and that a variable di-cobalt species could be present as the dissociation constants for loss of the metal-ion from the mono- (Kmono) and di- (Kdi) cobalt species were 9.3×10−8 M and 6.0×10−5 M respectively [27]. Under our experimental conditions at 30 °C only clean first- or zero-order kinetics were observed over the entire progress curves for the hydrolysis of the substrates. The biphasic behaviour described in the Introduction is only observed at low temperatures or low metal-ion concentrations [44–46]. For the three substrates studied here, cefoxitin (1), cephaloridine (2) and benzylpenicillin (3), the Michaelis constant, Km, is much lower for the cobalt-substituted enzyme than for the native zinc enzyme. This makes it difficult to obtain kinetic data below saturation conditions in order to determine Km and kcat/Km, especially at lower pHs, as Km decreases with decreasing pH.
Cefoxitin (1)
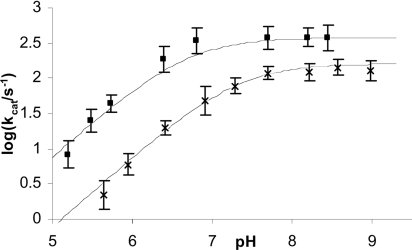
The catalytic constant, kcat, for CoBcII-catalysed hydrolysis of cefoxitin varies with pH and cobalt ion concentration as shown in Figure 2, indicative of activity being dependent on an ionizable group. Between pH 8 and 9 the reaction rate was pH-independent, but decreased at lower pHs. In the pH-rate-dependent region, kcat decreased with a first-order dependence on hydronium ion concentration, i.e. the slope of log kcat against pH was 1. At low pH a 10-fold decrease in cobalt ion concentration decreased kcat approx. 10-fold, whereas in the pH-rate independent region kcat changed less than 3-fold. The kinetically important ionization in the kcat-pH profile had apparent pKa values of 7 and 6.5 for CoBcII-catalysed hydrolysis of cefoxitin at 10−4 and 10−3 M CoCl2 respectively (Figure 2).
Figure 2. pH-rate profile for CoBcII-catalysed hydrolysis of cefoxitin.
Plot of log kcat against pH for CoBcII-catalysed hydrolysis of cefoxitin in 0.025 M buffer, I=0.25 M, in the presence of 10−4 M cobalt ions (×) and 10−3 M cobalt ions (■); the solid lines are the calculated values using eqn (1) and the parameters in Table 1 (Scheme 4).
Although the Michaelis constant, Km, could not be determined very accurately due to its very low value, especially at low pH, to a first approximation, Km was also pH-independent above pH 8 and there was an evident trend of Km decreasing with decreasing pH (Figure 3). The apparent sigmoidal dependence of pKm on pH generates apparent pKa values of 7.2 and 6.5, at 10−4 M and 10−3 M CoCl2 respectively. The values of Km may also be dependent on the concentration of metal-ion but the data are not definitive. At pH 8–9 and 5–6 there is little or no dependence on cobalt ion concentration, but, between pH 6 and 7, Km does vary approx. 6–7 fold when the concentration of CoCl2 varies from 10−4 M to 10−3 M (Figure 3).
Figure 3. pH-Km profile for CoBcII-catalysed hydrolysis of cefoxitin.
Plot of pKm against pH for CoBcII-catalysed hydrolysis of cefoxitin in 0.025 M buffer, I=0.25 M, in the presence of 10−4 M cobalt ions (×) and 10−3 M cobalt ions (■); the solid lines are the calculated values using eqn (2) and the parameters in Table 1 (Scheme 4).
Similarly, the values of the second-order rate constant, kcat/Km, for the CoBcII-catalysed hydrolysis of cefoxitin, are subject to considerable error (due to the errors involved in determining the Km) but show an apparent bell-shape pH-rate profile, with a decrease in rate at high and low pH and an intermediate pH-independent region. The rate constant shows a first-order dependence on both metal-ion and acid concentration at low pH, whereas in the pH-independent region, kcat/Km increases less than 2-fold for a 10-fold increase in metal-ion concentration. The apparent pKa values for the acidic ionization in the pH-kcat/Km profile are 6.3 and 5.8 for 10−4 and 10−3 M CoCl2 respectively.
Cephaloridine (2)
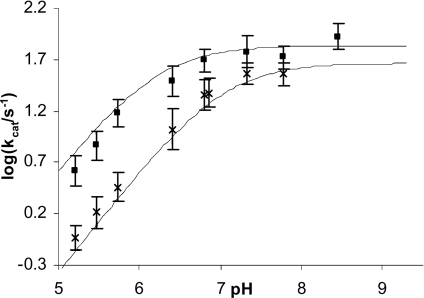
Using cephaloridine as a substrate, a similar trend for the variation of kcat (Figure 4), Km and kcat/Km (results not shown) with pH at different CoCl2 concentrations was observed. At low pH, the log kcat versus pH profile showed a first-order decrease of kcat with increasing hydronium ion concentration, which was again metal-ion-concentration-dependent, with apparent pKa values of 6.8 and 6.2 for 10−4 and 10−3 M CoCl2 respectively (Figure 4). The rate increased approx. 6-fold at low pH and less than 2-fold in the pH-independent region with increasing CoCl2 concentration from 10−4 to 10−3 M.
Figure 4. pH-rate profile for CoBcII-catalysed hydrolysis of cephaloridine.
Plot of log kcat against pH for CoBcII-catalysed hydrolysis of cephaloridine in 0.025 M buffer, I=0.25 M, in presence of 10−4 M cobalt ions (×) and 10−3 M cobalt ions (■); the solid lines are the calculated values using eqn (1) and the parameters in Table 1 (Scheme 4).
The decrease of Km with decreasing pH also appeared to be metal-ion-concentration-dependent, and Km varied from approx. 3×10−5 and 5×10−5 M at high pHs to approx. 3×10−6 and 8×10−6 M at low pHs, at 10−4 and 10−3 M CoCl2 respectively. Although the quality of the data is poor, (due to the difficulties of attaining below saturation conditions), it is possible to see that the second-order rate constant, kcat/Km, decreased at pHs less than 6 and is metal-ion-concentration-independent in the pH-independent region. Unlike cefoxitin, for cephaloridine there is no obvious evidence of a decrease in kcat/Km at higher pH.
Benzylpenicillin (3)
For CoBcII-catalysed hydrolysis of benzylpenicillin only the kcat versus pH profile was determined at different metal-ion concentrations ([cobalt ions]=10−3 M and 10−4 M; Figure 5), due to the very low Km values (<10−4 M). At lower pH values, kcat decreased with decreasing metal-ion concentration, with apparent pKa values of 6.8 and 6.2 for 10−4 M and 10−3 M CoCl2 respectively. As with the other substrates, at low pH, kcat showed a first-order dependence on acid and on metal-ion concentration, while in the pH-independent region, kcat increased less than 2-fold on increasing the CoCl2 concentration from 10−4 and 10−3 M (Figure 5).
Figure 5. pH-rate profile for CoBcII-catalysed hydrolysis of benzylpenicillin.
Plot of log kcat against pH for CoBcII-catalysed hydrolysis of benzylpenicillin in 0.025 M buffer, I=0.25 M, in the presence of 10−4 M cobalt ions (×) and 10−3 M cobalt ions (■); the solid lines are the calculated values using eqn (1) and the parameters in Table 1 (Scheme 4).
Mechanistic models
The variation of kcat and kcat/Km with pH and cobalt ion concentration was similar for the three substrates used, which indicates that the phenomena are an intrinsic property of the enzyme and independent of the nature of the substrate. The decrease in the apparent pKa values with increasing cobalt ion concentration suggests that the inverse first-order effect of the acid concentration on the rate of hydrolysis at lower pHs is due to the protonation of a metal ligand, which is responsible for the loss of one cobalt ion from the enzyme active site, rather than to the protonation of a catalytically important group or to a change in the rate-limiting step. The decrease in the values of kcat and Km with decreasing pH and metal-ion concentration suggests that there are at least two enzyme species, one of which has greater values of kcat and Km, and is dominant at high pH and metal-ion concentration. The other species results from the loss of a cobalt ion and has a lower Km and kcat, and is probably, effectively, inactive. Several possible explanations for the metal-ion concentration and pH-dependencies have been considered (Scheme 3):
Scheme 3.
In model (i), the apo-enzyme is the dominant species present at low pH and low metal-ion concentration and is catalytically inactive, while the mono-cobalt enzyme is responsible for the catalytic activity. Even though this model explains the variation of kcat and kcat/Km with pH and metal-ion concentration, it does not explain the decrease in Km with decreasing pH (tighter substrate binding) as it has been shown that the apo-enzyme does not bind the substrate, at least in the case of benzylpenicillin and some cephalosporins [48]. It follows that the enzyme species involved in the tight substrate binding (low Km), at low pH and metal-ion concentration, is probably the mono-cobalt BcII. The observed rate dependence on cobalt ion concentration can be explained if the catalytically active species is formed by the uptake of one cobalt ion i.e. is a di-cobalt enzyme species [models (ii), (iii) or (iv)]. The increase in rate with increasing pH can be explained by the ionization of a group involved in the binding of the second cobalt ion: this can be either the metal-bound water [model (ii)] or a protein residue, in which case the bridging water can be either deprotonated [model (iii)] or protonated [model (iv)]. The interconversion between mono- and di-cobalt enzyme species is also supported by the biphasic kinetics observed at lower temperatures [46].
A more detailed pathway involves the mono-cobalt deprotonated (ECo) enzyme, which forms at higher pH values, in substrate binding and possibly catalysis (Scheme 4):
Scheme 4.
The experimental data for the three substrates were fitted to eqns (1) and (2), which describe the variation of kcat and Km with pH and metal-ion concentration, according to Scheme 4.
 |
(1) |
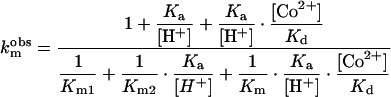 |
(2) |
The data for the pKm–pH and log kcat–pH dependencies were fitted to eqn (1) and eqn (2) using the literature value of Kdi for Kd, 6.0×10−5 M [27], in order to obtain the substrate-dependent parameters, Km, Km1 and Km2, kcat, kcat1 and kcat2 and the substrate-independent parameter, Ka. It was found that the kcat values for the mono-cobalt enzyme, in both the protonated and deprotonated forms, (kcat1 and kcat2 respectively), were close to zero (<0.1 s−1). The only catalytically active species is the di-cobalt enzyme. For benzylpenicillin, for which only the values of kcat were available, the data were fitted to eqn (1), fixing the value for Km as 1.6×10−4 M. The derived constants for the three substrates are summarized in Table 1. The pKa value for the kinetically important ionization was found to be 6.52±0.1. This pKa may correspond either to the cobalt-bound water (hydroxide ion), whose protonation results in dissociation of a cobalt ion from the enzyme active site, or to a metal-binding enzyme residue, such as Cys221. The pKa of Cys221 (or the Cys221–His263 pair) in the apoBcII was found to be 7.85 [46], but may decrease in the mononuclear species.
Table 1. Calculated values of the rate and dissociation constants from Scheme 4.
Rate and dissociation constants for CoBcII-catalysed hydrolysis of cefoxitin (1), cephaloridine (2) and benzylpenicillin (3) in 0.025 M buffer, I=0.25 M, at 30 °C, (Kd=6.0×10−5 M). The corresponding values, determined in similar conditions for the native ZnBcII, are given for comparison.
| kcat/Km (106 M−1·s−1) | kcat (s−1) | Km (10−6 M) | pKa | Km1 (10−6 M) | Km2 (10−6 M) | ||
|---|---|---|---|---|---|---|---|
| 1 | Co2+ | 0.052±0.009 | 16±2.0 | 310±10 | 6.52±0.1 | 12±1.0 | 100±20 |
| Zn2+ | 0.00027±0.00005 | 0.57±0.1 | 2100±200 | ||||
| 2 | Co2+ | 1.7±0.3 | 67±10 | 40±10 | 6.52±0.1 | 3.0±0.3 | 25±5.0 |
| Zn2+ | 0.15±0.02 | 160±20 | 1060±150 | 5.5±0.05a | |||
| 3 | Co2+ | 2.4±0.4 | 390±20 | 160±20 | 6.52±0.1 | 12±2.0 | 500±100 |
| Zn2+ | 0.87±0.1 | 950±120 | 1100±200 | 5.8±0.05a |
a Values taken from [31]
It is commonly suggested that the role of the metal-ion in metalloproteases is to act as a Lewis acid by co-ordination to the peptide carbonyl oxygen giving a more electron deficient carbonyl carbon which then facilitates nucleophilic attack and stabilizes the negative charge developed on the carbonyl oxygen of the tetrahedral intermediate anion. Another role of the metal-ion is to lower the pKa of the co-ordinated water so that the concentration of metal-bound ‘hydroxide ion’ is increased relative to bulk solvent hydroxide ion at neutral pH. This metal-bound conjugate base is also a better nucleophile than water, but obviously not better than ‘free’ hydroxide ion. The dissociation of an acid with a low pKa generates a weaker base which usually corresponds to a weaker nucleophile. Hence the order of basic strength and so nucleophilicity is: aqueous hydroxide ion, metal-bound hydroxide ion and then water. The stronger the Lewis acid (i.e. a more electron deficient metal-ion centre) is in stabilizing the negative charge developed on the tetrahedral intermediate from the β-lactam carbonyl oxygen, the greater will also be the effect on the acidity of bound water i.e. the lower the pKa. However, a lower pKa of zinc-bound water gives rise to a more weakly nucleophilic hydroxide ion even though it may become the dominant species even at lower pH. Conversely, a weaker Lewis acid would be less effective at reducing the pKa of the zinc-bound water but, at pHs above the pKa, would produce a more nucleophilic hydroxide ion. Similarly, a high pKa water implies a weak Lewis acid and so the zinc ion will be less efficient at stabilizing the tetrahedral intermediate. The main factors controlling the charge density on metal-ions with the same formal positive charge are ionic radius and the number and type of its ligands. Replacement of the native zinc in the MBLs by other metal-ions enables an exploration of different Lewis acidities on catalytic activity.
A comparison of the pH-kcat/Km profiles for cobalt-substituted BcII species with that of the native zinc enzyme [31], for the hydrolysis of cefoxitin and cephaloridine, shows the cobalt enzyme to have a higher maximum activity at their pH optimum and to be more active than ZnBcII at pH 7. The cobalt enzyme is remarkably approx. 200- and 10-fold more active than the native ZnBcII with cefoxitin and cephaloridine as substrate respectively (Table 1). Assuming that the pKa of the cobalt-bound water is 6.5 and that of the zinc-bound water is 5.6 in BcII [31], then cobalt has a smaller effective positive charge, so it is a weaker Lewis acid than zinc, but it gives a more nucleophilic metal-bound hydroxide ion. At pH 7, 96% of ZnBcII and 76% of CoBcII will be in their active deprotonated forms. The fact that the cobalt-substituted BcII is more active than the native ZnBcII (Table 1) suggests that the role of the metal-ion is predominantly to provide the nucleophilic hydroxide, rather than to act as a Lewis acid to polarize the carbonyl group and stabilize the oxyanion tetrahedral intermediate.
Acknowledgments
This research was supported by the European Union research network on MBLs within the TMR (Training and Mobility of Researchers) Programme, contract number HPRN-CT-2002-00264 and the University of Huddersfield.
References
- 1.Frère J. M. β-Lactamases and bacterial resistance to antibiotics. Mol. Microbiol. 1995;16:385–395. doi: 10.1111/j.1365-2958.1995.tb02404.x. [DOI] [PubMed] [Google Scholar]
- 2.Galleni M., Lamotte-Brasseur J., Rossolini G. M., Spencer J., Dideberg O., Frère J. M. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 2001;45:660–663. doi: 10.1128/AAC.45.3.660-663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabiane S. M., Sohi M. K., Wan T., Payne D. J., Bateson J. H., Mitchell T., Sutton B. J. Crystal structure of the zinc-dependent β-lactamase from Bacillus cereus at 1.9 Å resolution: binuclear active site with features of a mononuclear enzyme. Biochemistry. 1998;37:12404–12411. doi: 10.1021/bi980506i. [DOI] [PubMed] [Google Scholar]
- 4.Orellano E. G., Girardini J. E., Cricco J. A., Ceccarelli E. A., Vila A. J. Spectroscopic characterization of a binuclear metal site in Bacillus cereus β-lactamase II. Biochemistry. 1998;37:10173–10180. doi: 10.1021/bi980309j. [DOI] [PubMed] [Google Scholar]
- 5.Paul-Soto R., Bauer R., Frère J. M., Galleni M., Meyer-Klaucke W., Nolting H., Rossolini G. M., de Seny D., Hernandez-Valladares M., Zeppezauer M., Adolph H. W. Mono- and binuclear Zn2+-β-lactamase. Role of the conserved cysteine in the catalytic mechanism. J. Biol. Chem. 1999;274:13242–13249. doi: 10.1074/jbc.274.19.13242. [DOI] [PubMed] [Google Scholar]
- 6.Concha N. O., Rasmussen B. A., Bush K., Herzberg O. Crystal structure of the wide-spectrum binuclear zinc β-lactamase from Bacteroides fragilis. Structure. 1996;4:823–836. doi: 10.1016/s0969-2126(96)00089-5. [DOI] [PubMed] [Google Scholar]
- 7.Paul-Soto R., Hernadez-Valladares M., Galleni M., Bauer R., Zeppezauer M., Frère J. M., Adolph H. W. Mono- and binuclear Zn2+-β-lactamase from Bacteroides fragilis: catalytic and structural roles of the zinc ions. FEBS Lett. 1998;438:137–140. doi: 10.1016/s0014-5793(98)01289-7. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y., Keeney D., Tang X., Canfield N., Rasmussen B. A. Kinetic properties and metal content of the metallo-β-lactamase CcrA harboring selective amino acid substitutions. J. Biol. Chem. 1999;274:15706–15711. doi: 10.1074/jbc.274.22.15706. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z., Fast W., Benkovic S. J. On the mechanism of the Bacteroides fragilis metallo-β-lactamase. Biochemistry. 1999;38:10013–10023. doi: 10.1021/bi990356r. [DOI] [PubMed] [Google Scholar]
- 10.Laraki N., Franceschini N., Rossolini G. M., Santucci P., Meunier C., de Pauw E., Amicosante G., Frère J. M., Galleni M. Biochemical characterisation of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 1999;43:902–906. doi: 10.1128/aac.43.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haruta S., Yamaguchi H., Yamamoto E. T., Eriguchi Y., Nukaga M., O'Hara K., Sawai T. Functional analysis of the active site of a metallo-β-lactamase proliferating in Japan. Antimicrob. Agents Chemother. 2000;44:2304–2309. doi: 10.1128/aac.44.9.2304-2309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Concha N. O., Janson C. A., Rowling P., Pearson S., Cheever C. A., Clarke B. P., Lewis C., Galleni M., Frère J. M., Payne D. J., et al. Crystal structure of the IMP-1 metallo-β-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent, broad-spectrum inhibitor. Biochemistry. 2000;39:4288–4298. doi: 10.1021/bi992569m. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Saez I., Hopkins J., Papamicael C., Franceschini N., Amicosante G., Rossolini G. M., Galleni M., Frère J. M., Dideberg O. The 1.5 Å structure of Chryseobacterium meningosepticum zinc β-lactamase in complex with the inhibitor, D-captopril. J. Biol. Chem. 2003;278:23868–23873. doi: 10.1074/jbc.M301062200. [DOI] [PubMed] [Google Scholar]
- 14.Crowder M. W., Walsh T. R. Structure and function of metallo-β-lactamases. Recent Res. Dev. Antimicrob. Agents Chemother. 1999;3:105–132. [Google Scholar]
- 15.Hernandez Valladares M., Felici A., Weber G., Adolph H. W., Zeppezauer M., Rossolini G. M., Amicosante G., Frère J. M., Galleni M. Zn(II) dependence of the Aeromonas hydrophila AE036 metallo-β-lactamase activity and stability. Biochemistry. 1997;36:11534–11541. doi: 10.1021/bi971056h. [DOI] [PubMed] [Google Scholar]
- 16.Crawford P. A., Yang K. W., Sharma N., Bennett B., Crowder M. W. Spectroscopic studies on cobalt(II)-substituted metallo-β-lactamase ImiS from Aeromonas veronii bv. sobria. Biochemistry. 2005;44:5168–5176. doi: 10.1021/bi047463s. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen B. A., Bush K. Carbapenem hydrolysing β-lactamases. Antimicrob. Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felici A., Amicosante G., Oratore A., Strom R., Ledent P., Joris B., Fanuel L., Frère J. M. An overview of the kinetic parameters of class B β-lactamases. Biochem. J. 1993;291:151–155. doi: 10.1042/bj2910151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felici A., Amicosante G. Kinetic analysis of extension of substrate specificity with Xanthomonas maltophilia, Aeromonas hydrophila, and Bacillus cereus metallo-β-lactamases. Antimicrob. Agents Chemother. 1995;39:192–199. doi: 10.1128/aac.39.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowder M. W., Walsh T. R., Banovic L., Pettit M., Spencer J. Overexpression, purification, and characterization of the cloned metallo-β-lactamase (L1) from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 1998;42:921–926. doi: 10.1128/aac.42.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercuri P. S., Bouillenne F., Boschi L., Lammote-Brasseur J., Amicosante G., Devreese B., Van Beeumen J., Frère J. M., Rossolini G. M., Galleni M. Biochemical characterization of the FEZ-1 metallo-β-lactamase of Legionella gormanii ATCC 33297T produced in Escherichia coli. Antimicrob. Agents Chemother. 2001;45:1254–1262. doi: 10.1128/AAC.45.4.1254-1262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carfi A., Duée E., Galleni M., Frère J. M., Dideberg O. 1.85 Å resolution structure of the zinc (II) β-lactamase from Bacillus cereus. Acta Crystallogr. Sect. D Biol. Crystallogr. 1998;54:313–323. doi: 10.1107/s0907444997010627. [DOI] [PubMed] [Google Scholar]
- 23.Carfi A., Duee E., Paul-Soto R., Galleni M., Frère J. M., Dideberg O. X-ray structure of the Zn(II) β-lactamase from Bacteroides fragilis in an orthorhombic crystal form. Acta Crystallogr. Sect. D Biol. Crystallogr. 1998;54:45–57. doi: 10.1107/s090744499700927x. [DOI] [PubMed] [Google Scholar]
- 24.Concha N. O., Rasmussen B. A., Bush K., Herzberg O. Crystal structure of the cadmium- and mercury-substituted metallo-β-lactamase from Bacteroides fragilis. Protein Sci. 1997;6:2671–2676. doi: 10.1002/pro.5560061225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul-Soto R., Zeppezauer M., Adolph H. W., Galleni M., Frère J. M., Carfi A., Dideberg O., Wouter J., Hemmingsen L., Bauer R. Preference of Cd(II) and Zn(II) for the two metal sites in Bacillus cereus β-lactamase II: a perturbed angular correlation of γ-rays (PAC) spectroscopy study. Biochemistry. 1999;38:16500–16506. doi: 10.1021/bi9911381. [DOI] [PubMed] [Google Scholar]
- 26.Carfi A., Pares S., Duee E., Galleni M., Duez C., Frère J. M., Dideberg O. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Seny D., Heinz U., Wommer S., Kiefer M., Meyer-Klaucke W., Galleni M., Frère J. M., Bauer R., Adolph H. W. Metal ion binding and coordination geometry for wild type and mutants of metallo-β-lactamase from Bacillus cereus 569/H/9 (BcII); a combined thermodynamic, kinetic and spectroscopic approach. J. Biol. Chem. 2001;276:45065–45078. doi: 10.1074/jbc.M106447200. [DOI] [PubMed] [Google Scholar]
- 28.Wommer S., Rival S., Heinz U., Galleni M., Frère J. M., Franceschini N., Amicosante G., Rasmussen B., Bauer R., Adolph H. W. Substrate activated zinc binding of metallo-β-lactamases; physiological importance of the mononuclear enzymes. J. Biol. Chem. 2002;277:24142–24147. doi: 10.1074/jbc.M202467200. [DOI] [PubMed] [Google Scholar]
- 29.Crowder M. W., Wang Z., Franklin S. L., Zovinka E. P., Benkovic S. J. Characterization of the metal-binding sites of the β-lactamase from Bacteroides fragilis. Biochemistry. 1996;35:12126–12132. doi: 10.1021/bi960976h. [DOI] [PubMed] [Google Scholar]
- 30.Fast W., Wang Z., Benkovic S. J. Familial mutations and zinc stoichiometry determine the rate-limiting step of nitrocefin hydrolysis by metallo-β-lactamase from Bacteroides fragilis. Biochemistry. 2001;40:1640–1650. doi: 10.1021/bi001860v. [DOI] [PubMed] [Google Scholar]
- 31.Bounaga S., Laws A. P., Galleni M., Page M. I. The mechanism of catalysis and the inhibition of the Bacillus cereus zinc-dependent β-lactamase. Biochem. J. 1998;331:703–711. doi: 10.1042/bj3310703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auld D. S. Removal and replacement of metal ions in metallopepatidases. Methods Enzymol. 1995;248:228–242. doi: 10.1016/0076-6879(95)48016-1. [DOI] [PubMed] [Google Scholar]
- 32a.Maret W., Vallee B. L. Cobalt as probe and label of proteins. Methods Enzymol. 1993;226:52–71. doi: 10.1016/0076-6879(93)26005-t. [DOI] [PubMed] [Google Scholar]
- 33.Vila A. J., Fernandez C. O. Alkaline transition of Rhus vernicifera stellacyanin, an unusual Blue copper protein. Biochemistry. 1997;36:10566–10570. doi: 10.1021/bi970504i. [DOI] [PubMed] [Google Scholar]
- 33a.Guo J. Q., Wang S. K., Dong J., Qiu H. W., Scott R. A., Giedroc D. P. X-ray and visible absorption spectroscopy of wild-type and mutant T4 gene 32 proteins: His61, not His81 is the non-thiolate zinc ligand. J. Am. Chem. Soc. 1995;117:9437–9440. [Google Scholar]
- 34.Bertini I., Johnsson B. H., Luchinat C., Pierattelli R., Vila A. J. Strategies of signal assignments in paramagnetic metalloproteins. An NMR investigation of the thiocyanate adduct of the cobalt (II) substituted human carbonic anhydrase II. J. Magn. Reson. Ser. B. 1994;104:230–239. doi: 10.1006/jmrb.1994.1080. [DOI] [PubMed] [Google Scholar]
- 35.Oz G., Pountney D. L., Armitage I. M. NMR spectroscopic studies of I=1/2 metal ions in biological systems. Biochem. Cell Biol. 1998;76:223–234. doi: 10.1139/bcb-76-2-3-223. [DOI] [PubMed] [Google Scholar]
- 36.Bennet B., Holz R. C. EPR studies on the mono- and dicobalt(II)-substituted forms of the aminopeptidase from Aeromonas proteolytica. Insight into the catalytic mechanism of dinuclear hydrolases. J. Am. Chem. Soc. 1997;119:1923–1933. [Google Scholar]
- 37.Bauer R., Adolph H. W., Andersson I., Danielsen E., Formicka G., Zeppezauer M. Coordination geometry for cadmium in the catalytic zinc site of horse liver alcohol dehydrogenase: studies by PAC spectroscopy. Eur. Biophys. J. 1991;20:215–221. doi: 10.1007/BF00183458. [DOI] [PubMed] [Google Scholar]
- 38.Bicknell R., Knott-Hunziker Y., Waley S. G. The pH-dependence of class B and class C β-lactamases. Biochem. J. 1983;213:61–66. doi: 10.1042/bj2130061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldwin G. S., Edwards G. F., Kiener P. A., Tully M. J., Waley S. G., Abraham E. P. Production of a variant of β-lactamase II with selectively decreased cephalosporinase activity by a mutant of Bacillus cereus 569/H/9. Biochem. J. 1980;191:111–116. doi: 10.1042/bj1910111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z., Benkovic S. J. Purification, characterization, and kinetic studies of a soluble Bacteroides fragilis metallo-β-lactamase that provides multiple antibiotic resistance, J. Biol. Chem. 1998;273:22402–22408. doi: 10.1074/jbc.273.35.22402. [DOI] [PubMed] [Google Scholar]
- 41.Myers J. L., Shaw R. W. Production, purification and spectral properties of metal-dependent β-lactamase from Bacillus cereus. Biochim. Biophys. Acta. 1989;995:264–272. doi: 10.1016/0167-4838(89)90045-9. [DOI] [PubMed] [Google Scholar]
- 42.Garrity J. D., Bennet B., Crowder M. W. Direct evidence that the reaction intermediate of metallo-β-lactamase L1 is metal bound. Biochemistry. 2005;44:1078–1087. doi: 10.1021/bi048385b. [DOI] [PubMed] [Google Scholar]
- 43.Crawford P. A., Sharma N., Chandrasekar S., Sigdel T., Walsh T. R., Spencer J., Crowder M. W. Over-expression, purification, and characterization of metallo-β-lactamase ImiS from Aeromonas veronii bv. sobria. Protein Expression Purif. 2004;36:272–279. doi: 10.1016/j.pep.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Bicknell R., Waley S. G. Cryoenzymology of Bacillus cereus β-lactamase II. Biochemistry. 1985;24:6876–6887. doi: 10.1021/bi00345a021. [DOI] [PubMed] [Google Scholar]
- 45.Bicknell R., Schaffer A., Waley S. G., Auld D. S. Changes in the coordination geometry of the active-site metal during catalysis of benzylpenicillin hydrolysis by Bacillus cereus β-lactamase II. Biochemistry. 1986;25:7208–7215. doi: 10.1021/bi00370a066. [DOI] [PubMed] [Google Scholar]
- 46.Badarau A. Ph.D. Thesis. Huddersfield, U.K.: University of Huddersfield; 2006. Reactivity and inhibition of metallo-β-lactamases. [Google Scholar]
- 47.Damblon C., Jensen M., Ababou A., Barsukov I., Papamicael C., Schofield C. J., Olsen L., Bauer R., Roberts G. C. The inhibitor thiomandelic acid binds to both metal ions in metallo-β-lactamase and induces positive cooperativity in metal binding. J. Biol. Chem. 2003;31:29240–29251. doi: 10.1074/jbc.M301562200. [DOI] [PubMed] [Google Scholar]
- 48.Rasia R. M., Vila A. J. Structural determinants of substrate binding to Bacillus cereus metallo-β-lactamase. J. Biol. Chem. 2004;279:26046–26051. doi: 10.1074/jbc.M311373200. [DOI] [PubMed] [Google Scholar]