Abstract
Transcription of the milk protein β-casein gene is induced by the lactogenic hormones Prl (prolactin) and glucocorticoids. Multiple transcription factors involved in this induction have been identified, including the STAT5 (signal transducer and activator of transcription 5) and the GR (glucocorticoid receptor). Our previous studies have identified a binding site for the ubiquitous Oct-1 (octamer-binding transcription factor 1) protein in the lactogenic hormonal regulatory region of the mouse β-casein promoter. In the present study, we report that Oct-1 is indeed expressed and binds to the β-casein promoter in mammary epithelial cells. Oct-1 activates hormonally induced β-casein promoter activity in a dose-dependent manner. Hormonal induction of promoter activity was decreased not only by mutating the Oct-1-binding site from ATTAGCAT to GCTAGCAT, which abolishes Oct-1 binding (50% decrease, P<0.01), but also by changing the site to the consensus Oct-1-binding motif ATTTGCAT (40% decrease, P<0.01). Reversing the Oct-1-binding site reduced hormonal induction by 70% (P<0.01), showing that orientation of Oct-1 binding is also critical in hormonal action. In transient transfection experiments, Oct-1 collaboratively transactivated the β-casein gene promoter with STAT5 and/or GR in the presence of Prl receptor in cells treated with the lactogenic hormones. The C-terminus of Oct-1 was not essential to its function. The results of the present study provide biochemical evidence that the ubiquitous Oct-1 transcription factor may be involved in hormonally regulated, tissue-specific β-casein gene expression.
Keywords: β-casein promoter, gene expression, hormonal regulation, octamer-binding transcription factor 1 (Oct-1), prolactin, transcriptional regulation
Abbreviations: ChIP, chromatin immunoprecipitation; Dex, dexamethasone; GnRH, gonadotropin-releasing hormone; GR, glucocorticoid receptor; GRE, glucocorticoid response element; LHRR, lactogenic hormonal regulatory region; MMTV, murine-mammary-tumour virus; Oct-1, octamer-binding transcription factor 1; Prl, prolactin; Prl-R, Prl receptor; STAT5, signal transducer and activator of transcription 5; TFII, transcription factor II
INTRODUCTION
Expression of the major milk protein β-casein gene in the mammary gland is primarily regulated at the transcriptional level under the control of the synergistic action of the lactogenic hormones insulin, glucocorticoid and Prl (prolactin) [1,2]. Thus it provides an attractive model for elucidating the molecular mechanisms of hormonally regulated, tissue-specific gene expression and the interaction of the signalling pathways regulated by two classes of hormones. Transfection studies using reporter plasmids containing nested deletions of the 5′-flanking region of the β-casein gene demonstrated that in the rodent approx. 300 nt of the proximal region are sufficient for induction of β-casein gene transcription by insulin, glucocorticoid and Prl [1,3,4]. This LHRR (lactogenic hormonal regulatory region) contains composite positive and negative response elements with multiple binding sites for several transcription factors [1,2], including the STAT5 (signal transducer and activator of transcription 5) and the GR (glucocorticoid receptor). Surprisingly, none of these transcription factors are mammary-specific or even lactation stage-specific.
Sequence alignment of the LHRR of mouse, rat, bovine and rabbit casein genes reveals three highly conserved sequences, designated as blocks A, B and C [5]. Blocks A and B contain STAT5-binding sites that are essential for Prl-induced β-casein gene expression [6]. Block C is also critical for hormonal induction of β-casein gene expression. Mutation at block C caused an 84% reduction in the lactogenic hormone response in primary mammary epithelial cells [7]. Our previous study identified an Oct-1 (octamer-binding transcription factor 1)-binding site that could be responsible for the role of block C in hormonal activation [8]. In addition to blocks A, B and C, the rodent LHRR also contains multiple half-palindromic GREs (glucocorticoid response elements) [9]. Although it does not contain a full GRE, the half-GRE does function in the presence of activated STAT5 to mediate the action of GR on transcription. GR is able to synergize with STAT5 by directly binding to STAT5 and enhancing its DNA binding ability by prolonging the dephosphorylation process [10–13].
The Oct proteins are a group of transcription factors that bind specifically to the octamer motif (ATGCAAAT) and related sequences. These cis-acting transcriptional regulatory elements are found in promoters and enhancers of a wide variety of both ubiquitously expressed and cell-type-specific genes [14]. Oct transcription factors belong to a family of structurally related POU domain factors found throughout the eukaryotes. All POU factors share a highly conserved bipartite DNA-binding domain, consisting of an approx. 75-amino-acid N-terminal-specific subdomain (POUS) and a 60-amino-acid C-terminal homeo-subdomain (POUH), tethered by a variable 14–26 amino acid linker [15,16]. Oct-1 is the most studied member of the POU factors. It is expressed in all eukaryotic cells and regulates, either positively or negatively, the expression of a variety of genes. These include the RNA polymerase II-transcribed, ubiquitously expressed histone H2B gene [17] and tissue-specific immunoglobulin genes [18,19], as well as RNA polymerase II- or III-transcribed snRNA (small nuclear RNA) genes [20,21]. In regulating basal promoter activity, Oct-1 has been shown to directly interact with several basal transcription factors, including TBP (TATA-box-binding protein) [22], a component of TFIID (transcription factor IID), TFIIB [23] and MAT1 (cyclin-dependent kinase-activating kinase assembly factor) [24], a subunit of TFIIH. It has also been shown to interact with many other general or tissue-specific transcription factors and cofactors in mediating specific gene expression. Examples include the interaction with various steroid receptors to mediate hormonal activation of the MMTV (murine-mammary-tumour virus) and GnRH (gonadotropin-releasing hormone) promoters [25,26] and the interaction with STAT5 on the cyclin D1 promoter in mediating Prl signalling [27,28]. The crystal structure of Oct-1 shows that the larger POUS preferably binds to ATGC, whereas the smaller POUH contacts the AAAT site on the octamer motif. The central (A/T) pair (ATGCAAAT) is contacted by both POUS and POUH domains [29].
In the present study, we examined Oct-1 expression and binding to the β-casein promoter in mammary epithelial cells and systematically investigated the role of Oct-1 in hormonal induction of β-casein promoter activity. We demonstrate that the ubiquitous Oct-1 transcription factor may play a critical role in lactogenic hormone-regulated β-casein gene expression.
EXPERIMENTAL
Immunofluorescence microscopy
Mammary gland tissues from a mid-lactating mouse were fixed in 4% (w/v) paraformaldehyde at 4 °C for 4 h followed by immersion in 0.5 M sucrose in PBS overnight. Following cryoprotection, fixed tissues were embedded in OCT compound (Sakura Finetek, Torrance, CA, U.S.A.), frozen in liquid nitrogen-chilled 2-methylbutane and stored at −80 °C. For immunohistochemical staining, tissue sections (10 μm) were cut, thaw-mounted on the surface of gelatin-coated slips and incubated in 10% (v/v) normal goat serum for 30 min at room temperature (22 °C) to block non-specific antibody binding. The sections were then incubated with either diluted (2 μg/ml in PBS with 1% BSA) anti-Oct-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) or normal rabbit IgG for 1 h at 37 °C. For an additional control, some sections were incubated with anti-Oct-1 antibody that had been pre-incubated with a 5-fold excess control polypeptide (Santa Cruz Biotechnology) at 4 °C overnight. After washing twice in PBS with 1% BSA for 5 min, the sections were incubated in the dark with an Alexa Fluor® 647-conjugated secondary antibody (Molecular Probes, Eugene, OR, U.S.A.) at 1:400 dilution for 1 h at room temperature. Following washing three times in PBS with 1% BSA for 5 min, some sections were counterstained with SYTOX (Molecular Probes) with 1:10000 dilution in PBS for 5 min at room temperature. Finally, the sections were washed twice in PBS with 1% BSA for 15 min, once in PBS and once in distilled water before being mounted on glass microscope slips and examined under a confocal microscope (Bio-Rad, Hercules, CA, U.S.A.).
Mouse mammary epithelial cell line HC11 cells were grown in RPMI 1640 supplemented with 10% (v/v) heat-inactivated fetal calf serum (Gibco, Carlsbad, CA, U.S.A.), 10 ng/ml murine epidermal growth factor (Sigma, St. Louis, MO, U.S.A.), 5 μg/ml bovine insulin (Sigma) and 50 μg/ml gentamicin (Gibco). Cells were grown on glass coverslips to confluency and fixed with 4% paraformaldehyde in PBS at room temperature for 20 min. The cells were then washed twice in PBS and permeabilized in 0.1% Triton X-100 (in PBS with 0.5% BSA) for 15 min at room temperature, followed by washing twice in PBS with 1% BSA for 10 min. The immunofluorescence staining of the cells was carried out as described above, except using an Alexa Fluor® 568-conjugated secondary antibody (1:1000 dilution in PBS with 1% BSA).
ChIP (chromatin immunoprecipitation) assay
The ChIP assay was carried out according to the instructions of the ChIP assay kit (Upstate Biotechnology, Lake Placid, NY, U.S.A.) with minor modifications. HC11 cells were grown as described above to confluency in 150 mm plates. One group of cells was treated with the lactogenic hormones for 48 h by the addition of 0.1 μM Dex (dexamethasone; Sigma) and 5 μg/ml ovine Prl (Sigma) in the presence of bovine insulin but in the absence of epidermal growth factor. Cells were then incubated with 1% formaldehyde for 10 min at 37 °C to cross-link proteins to the DNA and washed with cold PBS buffer twice before lysis with SDS lysis buffer containing protease inhibitor cocktail (1 mM PMSF, 1 μg/ml aprotinin and 1 μg/ml pepstatin A). Cell lysates were sonicated on ice to shear chromatin to an average DNA length of 200–1000 bp as verified by agarose gel electrophoresis. The sheared chromatin was evenly divided and diluted in ChIP dilution buffer [0.01% (w/v) SDS, 1.1% (v/v) Triton X-100, 1.2 mM EDTA, 16.7 mM Tris/HCl (pH 8.1) and 167 mM NaCl]. A small portion of each diluted chromatin was collected, protein–DNA cross-links were reversed, and samples were used as the input DNA control. The rest of chromatin suspension was precleared with Protein A–agarose slurry containing salmon sperm DNA and then incubated with either anti-Oct-1 antibody or normal rabbit IgG (2 μg) overnight at 4 °C with continual rotation. The Protein A–agarose slurry containing salmon sperm DNA was added to each chromatin solution and incubated for another 2 h at 4 °C with constant rotation. The agarose beads were collected by centrifugation, washed and the antibody-bound chromatin was released from the agarose beads according to the manufacturer's instructions. The eluted chromatin was digested with RNase A, cross-links were reversed, samples were digested with protease K and finally purified by phenol/chloroform extraction and ethanol precipitation. The β-casein promoter was detected by PCR with the forward primer 5′-TAGAATTTCTTGGGAAAGAC-3′ and the reverse primer 5′-CTTTAGTGGAGGACAAGAGA-3′. The amplicon is 193 bp. For a negative control, the β-actin sequence was also amplified from the eluted chromatin using primers mβActin-F and mβActin-R [30]. The PCR conditions for ChIP assay were 5 cycles of 94 °C for 30s, 60 °C for 30s, 72 °C for 1 min and 30 cycles (23 cycles for β-actin) of 94 °C for 30 s, 50 °C for 30 s and 72 °C for 1 min.
Plasmid constructs
Mouse Oct-1B (mOct-1B/pcDNA3.1) and Oct-1Z expression plasmids (mOct-1Z/pcDNA3.1) and the wild-type mouse β-casein promoter (−258/+7) construct (LHRRWT/pGL3) have been described previously [30]. Mouse STAT5A (mSTAT5a/pECE) expression plasmid and its corresponding vector plasmid pECE were provided by Dr Wolfgang Doppler (Institute of Medical Chemistry and Biochemistry, University of Innsbruck, Innsbruck, Austria) [31]. Mouse long-form Prl-R (Prl receptor) expression plasmid (mPRLR/pEF6C) was provided by Dr Russell Hovey (Department of Animal Science, University of Vermont). Mouse GR expression plasmid (mGR/pSV2Wrec) was provided by Dr John Cidlowski (Molecular Endocrinology Group, Laboratory of Signal Transduction, National Institute of Environmental Health Science, Research Triangle Park, NC, U.S.A.) [32]. The cDNA sequences of mouse GR was amplified from the mGR/pSV2Wrec by PCR using Pfu DNA polymerase (Stratagene, La Jolla, CA, U.S.A.) and primers mGR-F (5′-ATAAGAATGCGGCCGCTAATATTTGCCAATGGAC-3′) and mGR-R (5′-GGGGTACCCAAACAAAAAGAACGACAAAACATC-3′), which contain NotI and KpnI restriction enzyme sites respectively. The PCR product was gel-purified and cloned into the TA cloning vector pCR2.1 (Invitrogen). The cDNA sequence of mGR was then excised from the pCR2.1 vector and subcloned into a mammalian expression vector pcDNA3.1(–) (Invitrogen) to form mGR/pcDNA3.1. The mutant mouse β-casein LHRR luciferase constructs were generated by a PCR-based mutagenesis method [33] and the PCR products were cloned into the pGL3 basic vector (Promega, Madison, WI, U.S.A.). A C-terminal truncation of Oct-1, which contains the first 13 exons of mouse Oct-1 [30], was amplified from mOct-1B/pcDNA3.1 using primers (5′-GCTCTAGAGCGAGTCAAGATGAGAGTTC-3′ and 5′-TTGGTACCTCATGTAAGAGAGAGATTAGTC-3′), and then subcloned into pcDNA3.1(–) to form Oct-1N13/pcDNA3.1. All sequences generated by PCR were confirmed by sequencing.
Transient transfection and luciferase assay
COS-7 cells were grown in Dulbecco's modified Eagle's medium (Gibco) adjusted to contain 4 mM L-glutamine, 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 1.0 mM sodium pyruvate, 10% fetal calf serum, 100000 units/l penicillin, 100000 μg/l streptomycin and 250 μg/l amphotericin B (Gibco). Before transfection, COS-7 cells were seeded 1.5×105 cells/well in 12-well plates or 3×105 cells/well in 6-well plates and grown in a humidified incubator at 37 °C and 5% CO2 overnight to 70–80% confluence. In basal promoter activity studies, COS-7 cells were co-transfected with varying amounts of Oct-1 expression plasmid and wild-type or mutant β-casein promoter construct using Lipofectamine™ (Invitrogen) following the manufacturer's instructions. After 5 h, transfection medium was replaced with normal growth medium, and cells were harvested after 36 h. In the hormonal induction experiments, wild-type or mutant β-casein promoter constructs and expression plasmids of STAT5A, Prl-R and GR were co-transfected into COS-7 cells with or without Oct-1 plasmid for 5 h. Transfection medium was then replaced by starvation medium (serum-free normal medium). After 13 h, the starvation medium was replaced by the hormone treatment medium [serum-free normal medium plus 5 μg/ml Prl (Sigma) and 0.1 μM Dex (Sigma)]. Cells were harvested after 24 h of hormone treatment. In all experiments, TK-Renilla luciferase control plasmid (pRL-TK, Promega) was used as an internal control to normalize transfection efficiency. The total amount of transfected DNA in each experiment was balanced by corresponding empty vectors. For the luciferase assay, cells were lysed using Promega Passive Lysis Buffer. The activities of firefly luciferase and Renilla luciferase in cell lysates were quantified by the Promega Dual-Luciferase Reporter Assay system using a Promega Turner Designs Luminometer Model TD-20/20 Genetic Reporter system.
Western-blot analysis
Whole cell protein lysates were prepared from COS-7 cells by the addition of RIPA lysis buffer consisting of 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% (v/v) Triton X-100, 1% (w/v) sodium deoxycholate and 0.1% (w/v) SDS with freshly added protease inhibitor cocktail (Sigma). The protein concentration of cell lysates was determined using the Bio-Rad Protein Assay kit. Equal amounts of protein from each treatment were subjected to Western-blot analysis as described previously [8] using specific antibodies against Oct-1, STAT5 or GR (Santa Cruz Biotechnology).
Statistical analysis
The statistical analyses of normalized luciferase activities between treatments were carried out using Minitab 14 statistical software. Where comparisons were among more than two groups, Tukey's one-way ANOVA was performed. Comparisons between two groups were carried out using t test.
RESULTS
Oct-1 is expressed and binds to the β-casein promoter in the mammary epithelial cells
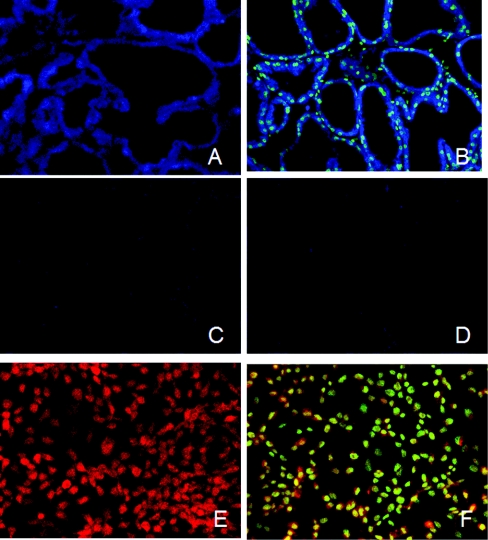
Previous in vivo and in vitro studies suggested a role for Oct-1 in regulating β-casein gene expression by lactogenic hormones [8]. To study this role, we first used immunohistochemical staining to examine Oct-1 expression and localization in mammary epithelial cells in a tissue section and in a mouse mammary epithelial cell line, HC11. As shown in Figure 1, Oct-1 is expressed in the alveolus epithelial cells of the mammary gland with subcellular localization to the nucleus and cytoplasm (Figures 1A and 1B) and in HC11 cells with subcellular localization to the nucleus and perinucleus regions (Figures 1E and 1F). Control staining using normal rabbit IgG or anti-Oct-1 pre-incubated with the control peptide reduced the signal to autofluorescence levels (Figures 1C and 1D; autofluorescence data and HC11 control data not shown).
Figure 1. Expression and localization of Oct-1 by immunocytochemistry in mouse mammary gland and HC11 cells.
The sections of mammary tissue from a mid-lactating mouse (A–D) and HC11 cells (E, F) were incubated with either normal rabbit IgG (D) or anti-Oct-1 (A–C, E, F). In (C), the anti-Oct-1 antibody was pre-incubated with corresponding control peptide before applying to the tissue section. The immunostaining was detected by fluorochrome-coupled secondary antibodies and analysed with a confocal microscope. In (B, F), the tissue section or cells were counterstained with SYTOX nuclear stain.
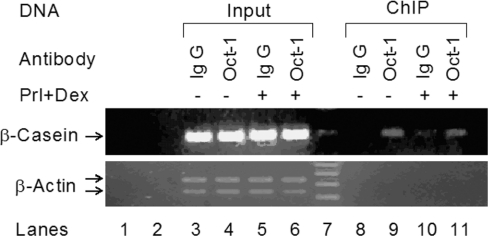
In order to examine the occupancy of Oct-1 on the endogenous β-casein promoter in mammary epithelial cells, we performed ChIP assays in HC11 cells in the presence or absence of the lactogenic hormones Prl and Dex. Based on the location of the Oct-1 motif, primers were prepared to amplify the LHRR region of the β-casein promoter (Figure 2). Specific binding of endogenous Oct-1 was demonstrated using a specific anti-Oct-1 antibody in both hormone-treated and non-treated cells (Figure 2, lanes 9 and 11). As negative controls, normal rabbit IgG was either unable to immunoprecipitate the same DNA–protein complexes or did so at a very low level (Figure 2, lanes 8 and 10) and β-actin primers were not able to amplify any product from the anti-Oct-1 immunoprecipitated DNA–protein complexes (Figure 2, lower panel). These results demonstrated that Oct-1 interacts with the LHRR region of the β-casein promoter in native chromatin regardless of Prl and Dex treatment.
Figure 2. Binding of Oct-1 to the LHRR of the β-casein gene promoter by ChIP in the mammary epithelial cells.
Cell lysates from HC11 cells treated either with or without the lactogenic hormones, Prl and glucocorticoid (Dex), were analysed by ChIP assay. Lysates were immunoprecipitated with either Oct-1 antibody or normal rabbit IgG. The LHRR of the β-casein gene promoter (upper panel) and the β-actin sequence (lower panel, negative control) were amplified by PCR from both input and immunoprecipitated DNAs. Lanes 1, 2 and 7 are the negative PCR control, empty lane and DNA ladder respectively.
Oct-1 functionally interacts with STAT5 and GR in the hormonal induction of β-casein promoter activity, and this interaction is Prl signalling-dependent
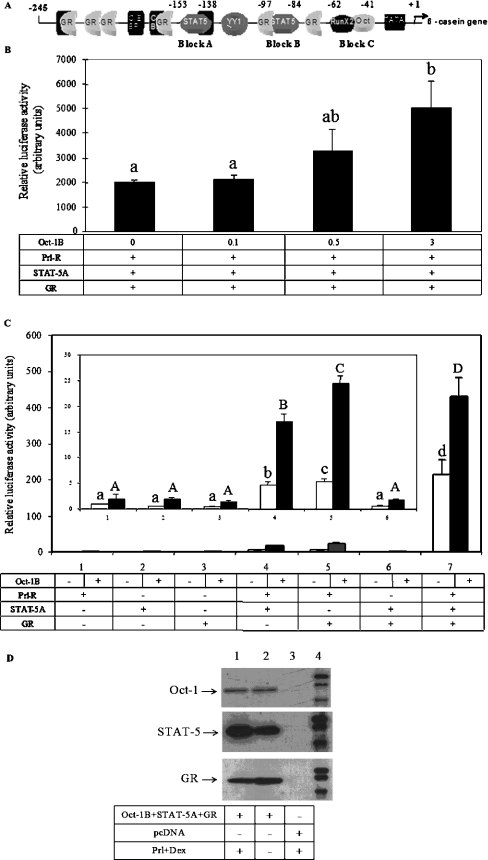
Transcription of the β-casein gene in the mammary gland is under the control of the synergistic action of the lactogenic hormones glucocorticoid and Prl, mediated through the interaction between STAT5 and GR on the LHRR (Figure 3A) [34]. In order to study the role of Oct-1 in this process, co-transfection experiments were performed. We adapted a widely used reconstituted COS-7 cell system in which COS-7 cells were reconstituted to be lactogenic hormone-responsive by the transfection of STAT5, Prl-R and GR expression plasmids [34]. The reconstituted COS-7 cells were co-transfected with the β-casein promoter/luciferase construct and treated with lactogenic hormones (Prl and Dex). The reporter luciferase activity could be induced over 200-fold by hormonal treatment in our experiments (results not shown). When increasing amounts of Oct-1 expression plasmid were transfected into the reconstituted COS-7 cells, the reporter luciferase activity under hormonal treatment was further increased by up to 150% (Figure 3B). This indicated that Oct-1 also activates hormonal induction of β-casein promoter activity and that this activation is dose-dependent. These results also suggested that Oct-1 may functionally interact with one of the hormone-responsive factors, Prl-R, STAT5 and GR, or with the combined action of these factors in hormonal induction. To explore these interactions, individual expression plasmids of Prl-R, STAT5 and GR, or various combinations of these plasmids, were transfected into COS-7 cells along with or without the Oct-1 plasmid, and the cells were then treated with lactogenic hormones. Consistent with previous studies [34,35], the hormonal induction of the promoter activity was only marginal when either Prl-R, STAT5 or GR was missing, regardless of whether Oct-1 was co-transfected (Figure 3C). When Prl-R, STAT5 and GR (Groups 1, 2 and 3) individually or both STAT5 and GR (Group 6) were transfected with or without Oct-1, the hormonal induction of the promoter activity remained at the basal activity levels. However, Oct-1 showed a strong synergy in the groups where Prl-R was transfected with either STAT5 or GR (Groups 4 and 5) (P<0.05) in addition to the group with all of the three components transfected (Group 7) (P<0.01). Thus Oct-1 can functionally interact with both STAT5 and GR individually. However, these interactions depend on the presence of the Prl signalling pathway, and the most dramatic activation requires the interactions of Oct-1 with both STAT5 and GR together. Figure 3(D) demonstrates the expression of the transiently expressed Oct-1, STAT5 and GR in COS-7 cells with or without hormonal treatment.
Figure 3. Synergism between Oct-1 and Prl-R, STAT5 and GR in hormonal induction of β-casein promoter activity.
(A) Diagrammatic representation of the defined transcription factor-binding sites in the LHRR of the rodent β-casein gene. GR, half-palindromic GRE; C/EBP, CCAAT/enhancer-binding protein; TATA, TATA box; YY1, Ying and Yang 1 (a transcription factor). (B) COS-7 cells were transfected with various amounts of mOct-1B/pcDNA3.1 (0–3.0 μg) and expression plasmids of Prl-R, STAT5A and GR together with the LHRRWT/pGL3, and reporter luciferase activities were determined in extracts from cells stimulated by Prl and Dex for 24 h. (C) In 12-well plates, COS-7 cells were transfected with 0.2 μg of LHRRWT/pGL3 and 0.2 μg of individual expression plasmids of Prl-R, STAT5A or GR or various combinations of these constructs. The cells were co-transfected with or without mOct-1B/pcDNA3.1, followed by Prl (5 μg/ml) and Dex (0.1 μM) treatment. In all groups, the total amount of DNA was balanced by corresponding vector DNA. Reporter luciferase activities were expressed as the means±S.E.M. for one experiment performed in triplicate. Three independent experiments were carried out. Data from one representative experiment are shown. Bars with different letters are significantly different (P<0.05). The inset in (C) shows the data from Groups 1–6 expanded. (D) Western-blot analysis of whole cell lysates of COS-7 cells transfected with expression plasmids of Oct-1B, STAT5, GR and Prl-R or pcDNA3.1 vector alone and treated with or without Prl and Dex.
Both the integrity and orientation of the Oct-1-binding site in the β-casein promoter are critical to basal promoter activity and hormonal induction
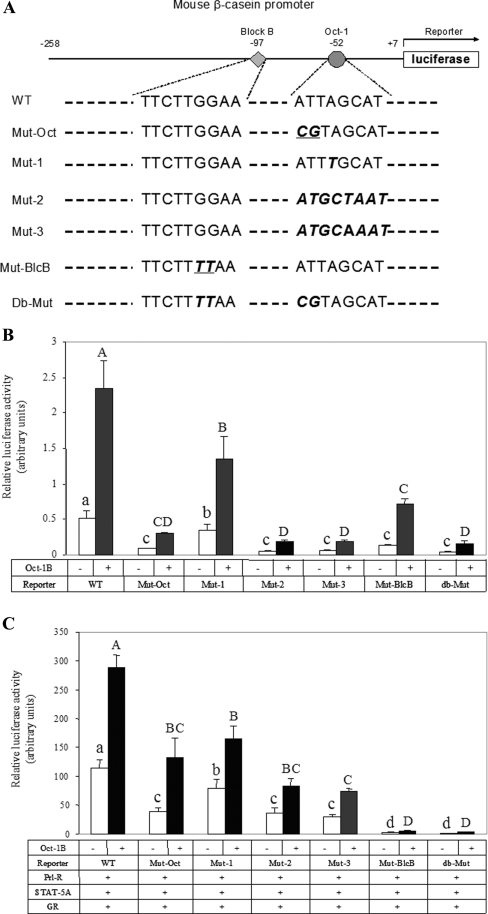
The core Oct-1-binding site in the mouse β-casein promoter (ATTAGCAT) varies by one base-pair from the complement of the classic octamer motif (ATGCAAAT). DNA-binding studies have shown that the binding of the Oct-1 POU domain has sequence specificity with the binding consensus of a(a/t)TATGC(A/T)AAT(t/a)t [16]. To study the role of Oct-1 binding in the hormonal induction of β-casein promoter activity, a promoter construct (Mut-Oct, Figure 4A) was made with mutations at the Oct-1-binding site (CGTAGCAT) which abolished Oct-1 binding [8]. The activity of this promoter construct was compared with the wild-type promoter construct (WT, Figure 4A), a construct with mutations in block B of the WT which abolished STAT5 binding (Mut-BlcB, Figure 4A), and a construct carrying both the Mut-Oct and Mut-BlcB mutations (db-Mut, Figure 4A). To study the significance of the discrepancy in sequence and orientation of the mouse β-casein promoter Oct-1-binding site relative to the classic octamer motif, three further mutations, Mut-1, Mut-2 and Mut-3, were generated (Figure 4A). In Mut-1, the exact complement of the classic octamer motif was made by changing A to T at the fourth position. Based on an in vitro binding study, Oct-1 has a slight binding preference for T versus A at this position [14]. In Mut-2, the octamer site orientation was reversed to ATGCTAAT. In Mut-3, the octamer site was reversed in its orientation and mutated at the fourth position to exactly match the classic octamer motif.
Figure 4. Effect of the integrity and orientation of the Oct-1 binding site on the β-casein promoter activity.
(A) Diagram of the β-casein promoter constructs. The core binding sequences of STAT5 and Oct-1 in blocks B and C are shown. The mutated sites of these sequences in different constructs are printed in bold italic face. (B) Effect of the integrity and orientation of the Oct-1-binding site on basal activity of the β-casein promoter. COS-7 cells were plated on 12-well plates and transfected with 0.2 μg of different β-casein promoter constructs shown in (A) with or without 0.3 μg of mOct-1B/pcDNA3.1. (C) Effect of the integrity and orientation of the Oct-1-binding site on the lactogenic hormonal induction of β-casein promoter activity; 0.2 μg of different promoter constructs shown in (A) and 0.2 μg of each expression plasmid of Prl-R, STAT5A and GR were transiently transfected into COS-7 cells on 12-well plates with or without mOct-1B/pcDNA3.1. The cells were then treated with Prl (5 μg/ml) and Dex (0.1 μM) for 24 h. Reporter luciferase activities were expressed as the means±S.E.M. for one experiment performed in triplicate. Three independent experiments were carried out. Results from one representative experiment are shown. Bars not sharing a common letter are significantly different (P<0.05).
First, the basal activities of different promoter constructs with or without exogenous Oct-1 were compared (Figure 4B). In COS-7 cells without transfection of Oct-1 plasmid (open bars), the basal promoter activities of Mut-Oct, Mut-1, Mut-2, Mut-3, Mut-BlcB and db-Mut were reduced by 83, 33, 89, 86, 75 and 93% respectively compared with the wild-type promoter (P<0.01). In COS-7 cells transfected with exogenous Oct-1 (closed bars), the basal promoter activity increased 1–4-fold in all constructs, including a weaker activation in the constructs with mutations to block Oct-1 binding (Mut-Oct and db-Mut) (P<0.05). However, the relative activity of the different mutations remained the same: the basal activities of these constructs decreased by 87, 42, 92, 92, 69 and 93% respectively.
Secondly, the response of these β-casein promoter mutation constructs to hormonal induction was investigated (Figure 4C). The reconstituted COS-7 cells were co-transfected with the individual promoter constructs, followed by Prl and Dex treatment for 24 h. The hormonal induction of Mut-Oct, Mut-1, Mut-2, Mut-3, Mut-BlcB and db-Mut was reduced by 66, 31, 69, 74, 97 and 98% respectively compared with the wild-type construct (open bars). Similarly, in the presence of exogenous Oct-1 expression, the hormonal induction was reduced by 54, 43, 71, 74, 98 and 99% respectively (closed bars).
These results indicate that Oct-1 binding and the integrity of the Oct-1-binding site in block C are critical for both the basal activity and the hormonal induction of the β-casein promoter. Prevention of Oct-1 binding to block C reduced the promoter activity and the hormonal induction by 54–87% (Mut-Oct) (P<0.05). However, at least part of the active role of Oct-1 in these processes was not dependent on its binding to block C since exogenous Oct-1 still enhanced the activity of Mut-Oct. Both the basal activity and hormonal induction decreased when the Oct-1-binding site was mutated to the exact classic octamer motif (Mut-1 and Mut-3) (P<0.05), although in vitro binding assays showed that the POU domain has a higher binding affinity to the octamer motif [14]. Our results also clearly demonstrate that the orientation of the Oct-1-binding site in the β-casein promoter is critical for promoter activity as reversal of the octamer site to the same orientation of the classical octamer motif severely reduced both the basal activity and its hormonal induction (Mut-2 and Mut-3) (P<0.01). The essential role of the STAT5-binding site in hormonal induction (Mut-BlcB and db-Mut) has been demonstrated in several studies [11,12]. Our results also show that the STAT5-binding site also plays an essential role in basal promoter activity.
The C-terminal sequence of Oct-1 has different effects on the basal activity of the β-casein promoter and its hormonal induction
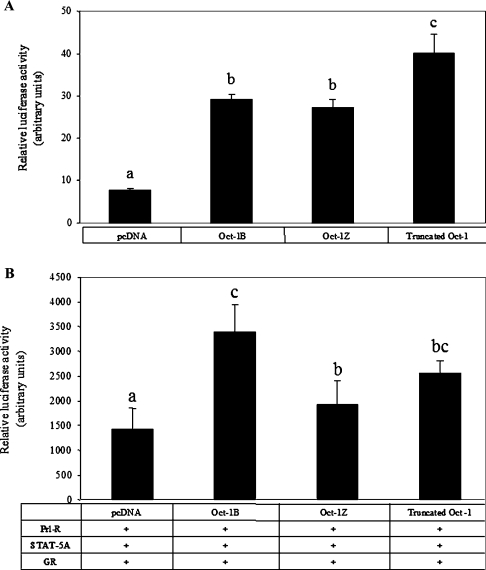
Our previous study reported that multiple splicing isoforms of the Oct-1 gene are expressed in mammalian cells [30]. The major difference among these isoforms occurs at the C-terminus, which contains activation domains for various gene promoters [30]. In the mammary gland, at least two isoforms, Oct-1B and Oct-1Z, are expressed [8,30]. Oct-1Z has a much shorter and different C-terminal tail after the POU homeodomain compared with the major isoform, Oct-1B. To study the role of the C-terminal sequence of Oct-1 in activation of the β-casein promoter, we compared the activities of Oct-1B, Oct-1Z and a truncated Oct-1, which contains a C-terminal deletion after the POU domain, by transfection assays (Figure 5). Consistent with our previous observations, Oct-1B and Oct-1Z showed no difference in activating basal promoter activity [30], whereas the truncated Oct-1 increased activation by 37% over Oct-1B (Figure 5A) (P<0.05). This implies that the activation domain of Oct-1 involved in the regulation of β-casein promoter basal activity is located in the N-terminal region and/or in the POU domain. The C-terminal sequences of Oct-1B and Oct-1Z slightly repress the activation. In contrast, different effects of these isoforms were seen in the hormonal induction of the β-casein promoter (Figure 5B). Exogenous Oct-1Z increased the hormonal induction only by 30%, 100% less compared with Oct-1B (P<0.01), whereas truncated Oct-1 did not show a significant difference. These results indicate that the C-terminal domain of Oct-1 has a more specific role in hormonal regulation than in basal activation. Although the C-terminal sequence of Oct-1 is not essential for the activation of hormonal induction by Oct-1, the shorter C-terminal sequence in Oct-1Z showed significant decreases in this activation.
Figure 5. Functional properties of the Oct-1 C-terminus on basal β-casein promoter activity (A) and its hormonal induction (B).
(A) In 12-well plates, COS-7 cells were transfected with 0.2 μg of the wild-type β-casein promoter construct (LHRRWT/pGL3) and an equal molar amount of mOct-1B/pcDNA3.1, mOct-1Z/pcDNA3.1 or the C-terminal truncated Oct-1 (Oct-1N13/pcDNA3.1). (B) COS-7 cells transfected with LHRRWT/pGL3 and expression plasmids of Prl-R, STAT5A and GR were co-transfected with or without Oct-1B/pcDNA3.1, Oct-1Z/pcDNA3.1 or Oct-1N13/pcDNA3.1, followed by Prl (5 μg/ml) and Dex (0.1 μM) treatment for 24 h. In both (A) and (B), reporter firefly luciferase activities were expressed as the means±S.E.M. for one experiment performed in triplicate. Three independent experiments were carried out. Data from one representative experiment are shown. Bars not sharing a common letter are significantly different (P<0.05).
DISCUSSION
Previous in vitro and in vivo studies suggest that Oct-1 may play a role in the hormonal induction of β-casein expression [7,8]. In the present study, we first examined Oct-1 expression and localization in mammary epithelial cells where the milk protein β-casein is synthesized and verified the binding of endogenous Oct-1 to β-casein chromatin in mammary epithelial cells. A COS-7 cell co-transfection assay was then adapted to study the role of Oct-1 in the hormonal regulation of β-casein transcription. COS-7 cells do not express endogenous Prl-R and STAT5 and only express trace amounts of GR [34], but can be reconstituted to become lactogenic hormone-responsive by transfection with these factors. Thus COS-7 cells have been widely used in co-transfection assays for studying the synergistic interactions between these factors in Prl and glucocorticoid signalling [10,12,31,34]. In the present study, the COS-7 cell co-transfection assays clearly demonstrated an active role for Oct-1 in the regulation of β-casein transcription by the lactogenic hormones Prl and GR.
The active role of Oct-1 in the regulation of β-casein transcription by lactogenic hormones in addition to its active role in basal promoter activity is supported by the following evidence: (i) the induction of β-casein promoter activity by Prl and Dex is enhanced by Oct-1 in a dose-dependent manner; (ii) all mutations of the Oct-1-binding site that either block Oct-1 binding, match the classical octamer motif, or reverse the orientation reduce the hormonal response by up to 87%. A similar reduction of the hormone response was observed in primary mammary cells [7]; (iii) the hormonal response of the promoter is differentially affected by alternative Oct-1 isoforms; and finally (iv) lactogenic hormones induce Oct-1 binding to the β-casein promoter in mammary gland tissue [8] and primary mammary epithelial cells [7]. Although our ChIP assay did not detect increased Oct-1 binding to the endogenous β-casein promoter after hormonal treatment for 48 h, any increasing Oct-1 binding may not have been sustained over this time period. Kabotyanski et al. [36] have recently reported that the binding of STAT5 and GR to the β-casein promoter rapidly increases in the first half hour following hormonal induction, then decreases dramatically and shows either no increase or a small increase after 24 h.
Although our results show clearly that binding of Oct-1 to block C is critical for hormonal induction of the β-casein gene promoter, the binding itself is not sufficient for induction of transcription. Transfection of the Oct-1 expression plasmid into COS-7 cells results in a large increase in Oct-1 binding activity to block C, while the promoter activity only increases by a few fold [8]. However, the promoter activity increases by several hundred fold in the presence of STAT5, Prl-R, GR and hormone treatment. Therefore it is the functional partnership of Oct-1 with Prl-R, STAT5 and GR that efficiently activates the promoter, and this partnership requires lactogenic hormone signalling. This is also in agreement with many reports that Oct-1 alone is a weak transcriptional activator and its activation is potentiated by interaction with other factors [37–42].
The role of STAT5 and GR in Prl and glucocorticoid signalling in LHRR induction has been well studied. The synergistic co-operation of Prl and glucocorticoid is mainly mediated by physical interaction of STAT5 and GR [34,43]. This interaction helps GR to bind to the half-GREs, enhance STAT5 DNA binding, protect STAT5 from inactivation by dephosphorylation, and recruit other co-activators. Our study indicates that Oct-1 is also integrated in Prl and glucocorticoid signalling and may interact with both STAT5 and GR. Previous studies have already shown that Oct-1 can directly interact with GR either positively or negatively in a promoter-specific manner. For example, on MMTV and mouse GnRH promoters, the interaction of Oct-1 and GR is required for transactivation [25,26], while interaction of Oct-1 with GR is involved in the repression of the histone H2b promoter [44]. Recently, Oct-1 was shown to form a stable complex with STAT5 on the cyclin D1 promoter, and Oct-1 is essential for recruitment of STAT5 to cyclin D1 GAS2 (γ-interferon activation sequence 2) elements [27,28]. Thus it is possible that Oct-1 physically interacts with both STAT5 and GR on the β-casein promoter.
Our observations indicate that the effect of exogenous Oct-1 depends on the original strength of the promoter activity before introducing Oct-1 into the cell, and exogenous Oct-1 functions as an amplifier of the original hormonal response. Oct-1 even amplifies the activity of the promoter with a mutation to block Oct-1 binding although the overall hormonal induction is significantly lower in this promoter. This may indicate that the role of Oct-1 is much stronger when Oct-1 binds to the promoter, but that binding may not be essential. In addition, our results showed that the orientation of the Oct-1-binding site in the promoter is critical in hormonal regulation. The Oct-1-binding site in the β-casein promoter is in reverse orientation to the classic octamer motif. Switching its orientation to match with the classic octamer motif severely reduced the promoter's response to hormones. Structural and biochemical studies of Oct-1 POU domain–DNA interactions have shown that the binding of Oct-1 to the octamer motif has a specific orientation: the larger Oct-1 POUS preferably binds to ATGC, whereas the smaller POUH contacts the AAAT site [29]. Thus changing the orientation of the Oct-1 site in the β-casein promoter would change the orientation of Oct-1 when binding to the promoter, which may disrupt the interactions of Oct-1 with STAT5, GR and the transcription machinery, resulting in a reduced hormonal response.
In summary, the results of the present study provided biochemical evidence that Oct-1 functionally interacts with lactogenic hormone-responsive factors STAT5 and GR and play an important role in lactogenic hormone activation of β-casein gene expression.
Acknowledgments
We thank Dr John Cidlowski, Dr Wolfgang Doppler, Dr Russell Hovey and Dr Jeff Rosen (Department of Cell Biology, Baylor College of Medicine, Houston, TX, U.S.A.) for providing us with expression plasmids of Prl-R, STAT5 and GR, Dr Brooke Mossman for useful discussion and Peter Miller and Dr Aileen Keating for editorial assistance before submission.
References
- 1.Doppler W., Groner B., Ball R. K. Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat β-casein gene promoter constructs in a mammary epithelial cell line. Proc. Natl. Acad. Sci. U.S.A. 1989;86:104–108. doi: 10.1073/pnas.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen J. M., Wyszomierski S. L., Hadsell D. Regulation of milk protein gene expression. Annu. Rev. Nutr. 1999;19:407–436. doi: 10.1146/annurev.nutr.19.1.407. [DOI] [PubMed] [Google Scholar]
- 3.Kanai A., Nonomura N., Yoshimura M., Oka T. DNA-binding proteins and their cis-acting sites controlling hormonal induction of a mouse β-casein: CAT fusion protein in mammary epithelial cells. Gene. 1993;126:195–201. [PubMed] [Google Scholar]
- 4.Liu X., Robinson G. W., Gouilleux F., Groner B., Hennighausen L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8831–8835. doi: 10.1073/pnas.92.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshimura M., Oka T. Transfection of β-casein chimeric gene and hormonal induction of its expression in primary murine mammary epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1990;87:3670–3674. doi: 10.1073/pnas.87.10.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groner B., Altiok S., Meier V. Hormonal regulation of transcription factor activity in mammary epithelial cells. Mol. Cell. Endocrinol. 1994;100:109–114. doi: 10.1016/0303-7207(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 7.Saito H., Oka T. Hormonally regulated double- and single-stranded DNA-binding complexes involved in mouse β-casein gene transcription. J. Biol. Chem. 1996;271:8911–8918. doi: 10.1074/jbc.271.15.8911. [DOI] [PubMed] [Google Scholar]
- 8.Zhao F.-Q., Adachi K., Oka T. Involvement of Oct-1 in transcriptional regulation of β-casein gene expression in mouse mammary gland. Biochim. Biophys. Acta. 2002;1577:27–37. doi: 10.1016/s0167-4781(02)00402-5. [DOI] [PubMed] [Google Scholar]
- 9.Welte T., Philipp S., Cairns C., Gustafsson J. A., Doppler W. Glucocorticoid receptor binding sites in the promoter region of milk protein genes. J. Steroid Biochem. Mol. Biol. 1993;47:75–81. doi: 10.1016/0960-0760(93)90059-6. [DOI] [PubMed] [Google Scholar]
- 10.Doppler W., Windegger M., Soratroi C., Tomasi J., Lechner J., Rusconi S., Cato A. C., Almlof T., Liden J., Okret S., et al. Expression level-dependent contribution of glucocorticoid receptor domains for functional interaction with STAT5. Mol. Cell. Biol. 2001;21:3266–3279. doi: 10.1128/MCB.21.9.3266-3279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lechner J., Welte T., Doppler W. Mechanism of interaction between the glucocorticoid receptor and Stat5: role of DNA-binding. Immunobiology. 1997;198:112–123. doi: 10.1016/S0171-2985(97)80032-0. [DOI] [PubMed] [Google Scholar]
- 12.Wyszomierski S. L., Rosen J. M. Cooperative effects of STAT5 (signal transducer and activator of transcription 5) and C/Upbeat (CCAAT/enhancer-binding protein-β) on β-casein gene transcription are mediated by the glucocorticoid receptor. Mol. Endocrinol. 2001;15:228–240. doi: 10.1210/mend.15.2.0597. [DOI] [PubMed] [Google Scholar]
- 13.Wyszomierski S. L., Yeh J., Rosen J. M. Glucocorticoid receptor/signal transducer and activator of transcription 5 (STAT5) interactions enhance STAT5 activation by prolonging STAT5 DNA binding and tyrosine phosphorylation. Mol. Endocrinol. 1999;13:330–343. doi: 10.1210/mend.13.2.0232. [DOI] [PubMed] [Google Scholar]
- 14.Verrijzer C. P., Alkema M. J., van Weperen W. W., Van Leeuwen H. C., Strating M. J., van der Vliet P. C. The DNA binding specificity of the bipartite POU domain and its subdomains. EMBO J. 1992;11:4993–5003. doi: 10.1002/j.1460-2075.1992.tb05606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholer H. R. Octamania: the POU factors in murine development. Trends Genet. 1991;7:323–329. doi: 10.1016/0168-9525(91)90422-m. [DOI] [PubMed] [Google Scholar]
- 16.Veenstra G. J., van der Vliet P. C., Destree O. H. POU domain transcription factors in embryonic development. Mol. Biol. Rep. 1997;24:139–155. doi: 10.1023/a:1006855632268. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher C., Heintz N., Roeder R. G. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell. 1987;51:773–781. doi: 10.1016/0092-8674(87)90100-0. [DOI] [PubMed] [Google Scholar]
- 18.Dreyfus M., Doyen N., Rougeon F. The conserved decanucleotide from the immunoglobulin heavy chain promoter induces a very high transcriptional activity in B-cells when introduced into an heterologous promoter. EMBO J. 1987;6:1685–1690. doi: 10.1002/j.1460-2075.1987.tb02418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirth T., Staudt L., Baltimore D. An octamer oligonucleotide upstream of a TATA motif is sufficient for lymphoid-specific promoter activity. Nature. 1987;329:174–178. doi: 10.1038/329174a0. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M., Lai J. S., Herr W. Promoter-selective activation domains in Oct-1 and Oct-2 direct differential activation of an snRNA and mRNA promoter. Cell. 1992;68:755–767. doi: 10.1016/0092-8674(92)90150-b. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M., Grossniklaus U., Herr W., Hernandez N. Activation of the U2 snRNA promoter by the octamer motif defines a new class of RNA polymerase II enhancer elements. Genes Dev. 1988;2:1764–1778. doi: 10.1101/gad.2.12b.1764. [DOI] [PubMed] [Google Scholar]
- 22.Zwilling S., Annweiler A., Wirth T. The POU domains of the Oct1 and Oct2 transcription factors mediate specific interaction with TBP. Nucleic Acids Res. 1994;22:1655–1662. doi: 10.1093/nar/22.9.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakshatri H., Nakshatri P., Currie R. A. Interaction of Oct-1 with TFIIB: implications for a novel response elicited through the proximal octamer site of the lipoprotein lipase promoter. J. Biol. Chem. 1995;270:19613–19623. doi: 10.1074/jbc.270.33.19613. [DOI] [PubMed] [Google Scholar]
- 24.Inamoto S., Segil N., Pan Z. Q., Kimura M., Roeder R. G. The cyclin-dependent kinase-activating kinase (CAK) assembly factor, MAT1, targets and enhances CAK activity on the POU domains of octamer transcription factors. J. Biol. Chem. 1997;272:29852–29858. doi: 10.1074/jbc.272.47.29852. [DOI] [PubMed] [Google Scholar]
- 25.Chandran U. R., Warren B. S., Baumann C. T., Hager G. L., DeFranco D. B. The glucocorticoid receptor is tethered to DNA-bound Oct-1 at the mouse gonadotropin-releasing hormone distal negative glucocorticoid response element. J. Biol. Chem. 1999;274:2372–2378. doi: 10.1074/jbc.274.4.2372. [DOI] [PubMed] [Google Scholar]
- 26.Prefontaine G. G., Walther R., Giffin W., Lemieux M. E., Pope L., Hache R. J. Selective binding of steroid hormone receptors to octamer transcription factors determines transcriptional synergism at the mouse mammary tumor virus promoter. J. Biol. Chem. 1999;274:26713–26719. doi: 10.1074/jbc.274.38.26713. [DOI] [PubMed] [Google Scholar]
- 27.Brockman J. L., Schuler L. A. Prolactin signals via Stat5 and Oct-1 to the proximal cyclin D1 promoter. Mol. Cell. Endocrinol. 2005;239:45–53. doi: 10.1016/j.mce.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magne S., Caron S., Charon M., Rouyez M. C., Dusanter-Fourt I. STAT5 and Oct-1 form a stable complex that modulates cyclin D1 expression. Mol. Cell. Biol. 2003;23:8934–8945. doi: 10.1128/MCB.23.24.8934-8945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klemm J. D., Pabo C. O. Oct-1 POU domain–DNA interactions: cooperative binding of isolated subdomains and effects of covalent linkage. Genes Dev. 1996;10:27–36. doi: 10.1101/gad.10.1.27. [DOI] [PubMed] [Google Scholar]
- 30.Zhao F.-Q., Zheng Y., Dong B., Oka T. Cloning, genomic organization, expression, and effect on β-casein promoter activity of a novel isoform of the mouse Oct-1 transcription factor. Gene. 2004;326:175–187.. doi: 10.1016/j.gene.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 31.Lechner J., Welte T., Tomasi J. K., Bruno P., Cairns C., Gustafsson J., Doppler W. Promoter-dependent synergy between glucocorticoid receptor and Stat5 in the activation of β-casein gene transcription. J. Biol. Chem. 1997;272:20954–20960. doi: 10.1074/jbc.272.33.20954. [DOI] [PubMed] [Google Scholar]
- 32.Webster J. C., Jewell C. M., Bodwell J. E., Munck A., Sar M., Cidlowski J. A. Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. J. Biol. Chem. 1997;272:9287–9293. doi: 10.1074/jbc.272.14.9287. [DOI] [PubMed] [Google Scholar]
- 33.Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stocklin E., Wissler M., Gouilleux F., Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726–728. doi: 10.1038/383726a0. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita H., Nevalainen M. T., Xu J., LeBaron M. J., Wagner K. U., Erwin R. A., Harmon J. M., Hennighausen L., Kirken R. A., Rui H. Role of serine phosphorylation of Stat5a in prolactin-stimulated β-casein gene expression. Mol. Cell. Endocrinol. 2001;183:151–163. doi: 10.1016/s0303-7207(01)00546-9. [DOI] [PubMed] [Google Scholar]
- 36.Kabotyanski E. B., Huetter M., Xian W., Rijnkels M., Rosen J. M. Integration of prolactin and glucocorticoid signaling at the β-casein promoter and enhancer by ordered recruitment of specific transcription factors and chromatin modifiers. Mol. Endocrinol. 2006;20:2355–2368. doi: 10.1210/me.2006-0160. [DOI] [PubMed] [Google Scholar]
- 37.Botting C. H., Hay R. T. Characterisation of the adenovirus preterminal protein and its interaction with the POU homeodomain of NFIII (Oct-1) Nucleic Acids Res. 1999;27:2799–2805. doi: 10.1093/nar/27.13.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coenjaerts F. E., van Oosterhout J. A., van der Vliet P. C. The Oct-1 POU domain stimulates adenovirus DNA replication by a direct interaction between the viral precursor terminal protein–DNA polymerase complex and the POU homeodomain. EMBO J. 1994;13:5401–5409. doi: 10.1002/j.1460-2075.1994.tb06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakshatri H., Nakshatri P., Currie R. A. Interaction of Oct-1 with TFIIB. Implications for a novel response elicited through the proximal octamer site of the lipoprotein lipase promoter. J. Biol. Chem. 1995;270:19613–19623. doi: 10.1074/jbc.270.33.19613. [DOI] [PubMed] [Google Scholar]
- 40.O'Connor M., Bernard H. U. Oct-1 activates the epithelial-specific enhancer of human papillomavirus type 16 via a synergistic interaction with NFI at a conserved composite regulatory element. Virology. 1995;207:77–88. doi: 10.1006/viro.1995.1053. [DOI] [PubMed] [Google Scholar]
- 41.van Leeuwen H. C., Rensen M., van der Vliet P. C. The Oct-1 POU homeodomain stabilizes the adenovirus preinitiation complex via a direct interaction with the priming protein and is displaced when the replication fork passes. J. Biol. Chem. 1997;272:3398–3405. doi: 10.1074/jbc.272.6.3398. [DOI] [PubMed] [Google Scholar]
- 42.Vigano M. A., Staudt L. M. Transcriptional activation by Oct-3: evidence for a specific role of the POU-specific domain in mediating functional interaction with Oct-1. Nucleic Acids Res. 1996;24:2112–2118. doi: 10.1093/nar/24.11.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cella N., Groner B., Hynes N. E. Characterization of Stat5a and Stat5b homodimers and heterodimers and their association with the glucocortiocoid receptor in mammary cells. Mol. Cell. Biol. 1998;18:1783–1792. doi: 10.1128/mcb.18.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kutoh E., Stromstedt P. E., Poellinger L. Functional interference between the ubiquitous and constitutive octamer transcription factor 1 (OTF-1) and the glucocorticoid receptor by direct protein-protein interaction involving the homeo subdomain of OTF-1. Mol. Cell. Biol. 1992;12:4960–4969. doi: 10.1128/mcb.12.11.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]