Abstract
Intracellular and secreted cAMPs play crucial roles in controlling cell movement and gene regulation throughout development of the social amoeba Dictyostelium discoideum. cAMP is produced by three structurally distinct ACs (adenylate cyclases), ACA, ACG and ACB, which have distinctive but overlapping patterns of expression and, as concluded from gene disruption studies, seemingly overlapping functions. In addition to gene disruption, acute pharmacological abrogation of protein activity can be a powerful tool to identify the protein's role in the biology of the organism. We analysed the effects of a range of compounds on the activity of ACA, ACB and ACG to identify enzyme-specific modulators. Caffeine, which was previously used to specifically block ACA function, also inhibited cAMP accumulation by ACB and ACG. IPA (2′,3′-O-isopropylidene adenosine) specifically inhibits ACA when measured in intact cells, without affecting ACB or ACG. All three enzymes are inhibited by the P-site inhibitor DDA (2′,5′-dideoxyadenosine) when assayed in cell lysates, but not in intact cells. Tyrphostin A25 [α-cyano-(3,4,5-trihydroxy)cinnamonitrile] and SQ22536 [9-(tetrahydro-2′-furyl)adenine] proved to be effective and specific inhibitors for ACG and ACA respectively. Both compounds acted directly on enzyme activity assayed in cell lysates, but only SQ22536 was also a specific inhibitor when added to intact cells.
Keywords: adenylate cyclase, caffeine, cAMP, enzyme-specific inhibitor, P-site inhibition, Dictyostelium discoideum
Abbreviations: AC, adenylate cyclase; cAR1, cAMP receptor 1; DA, 2′-deoxyadenosine; DcAMP, 2′-deoxyadenosine 3′,5′-monophosphate; DDA, 2′,5′-dideoxyadenosine; DTT, dithiothreitol; GTP[S], guanosine 5′-[γ-thio]triphosphate; IBMX, isobutylmethylxanthine; IPA, 2′,3′-O-isopropylidene adenosine; PDE, phosphodiesterase; PdeE, phosphodiesterase E; PdsA, phosphodiesterase A; PKA-R, protein kinase A regulatory subunit; RdeA, phospho-relay intermediate A; RegA, phosphodiesterase 2
INTRODUCTION
The evolution of social amoebas or Dictyostelids was accompanied by extensive elaboration of cAMP signalling pathways [1]. In Dictyostelium discoideum, cAMP acts as a classical second messenger for external stimuli. In this role, cAMP controls the initiation of multicellular development, the maturation of stalk and spore cells and the germination of spores. cAMP is also secreted in a highly regulated manner. As an extracellular signal, it co-ordinates the aggregation of starving cells and the directional movement of cells in multicellular structures. In addition, extracellular cAMP acts as a trigger for gene regulation at different stages of development [2,3].
D. discoideum has three structurally distinct ACs (adenylate cyclases), ACA, ACB and ACG, for synthesis of cAMP. ACA produces cAMP for cell aggregation. It is structurally similar to the mammalian ACs with two different catalytic domains that are interspersed by two sets of six transmembrane helices [4]. Similar to mammalian ACs, ACA is activated by a serpentine receptor, in this case the cAMP receptor, cAR1 (cAMP receptor 1), that interacts with a heterotrimeric G-protein, G2. However, in contrast with mammalian ACs [5], the G-protein does not interact directly with ACA. Instead the G2 βγ-subunit activates a phospholipid inositol kinase that generates plasma membrane-binding sites for the CRAC (cytosolic regulator of AC), which activates ACA upon recruitment to the plasma membrane [6].
ACG has an extracellular sensor domain, one or two transmembrane helices, and a single intracellular catalytic domain. ACG is an osmosensor that controls the germination of spores [7,8], and has an overlapping role with ACA and ACB in triggering prespore differentiation (E. Alvarez-Curto and P. Schaap, unpublished work). The catalytic domain of ACB [9], encoded by the AcrA gene [10], is homologous with that of the bicarbonate-regulated bacterial ACs [11]. Similar to the CyaC ACs from the cyanobacteria Anaebena spirulensis and Spirulina platensis, ACB also harbours a response regulator domain and a histidine kinase domain. ACB is required for the maturation of spores [10].
Information on the role of each of the enzymes in particular aspects of the developmental programme has been derived from studies with null mutants in their respective genes [4,7,10]. However, this approach precludes the demonstration of late developmental roles for those enzymes that are essential for an early stage of development. Moreover, recent studies indicate that the Dictyostelium ACs negatively regulate each other's expression. As a consequence, gene disruption in each of the genes will lead to overexpression of the others and partial or full restoration of the function of the abrogated gene (E. Alvarez-Curto and P. Schaap, unpublished work). The use of enzyme-specific inhibitors with acute effects circumvents such problems.
Similar to the mammalian ACs, the D. discoideum enzyme ACG is active as a dimer, potentially creating two binding sites for ATP binding and catalysis [8]. For ACA, random mutagenesis studies have identified amino acids that are either essential for catalysis or for regulation by upstream components in the signalling pathway [12,13]. However, apart from these data, no structural information on enzyme regulation is available. In addition to studies of the protein crystal structure, the elucidation of the catalytic mechanism of the mammalian ACs has benefited greatly from pharmacological interference with enzyme activity. Notably the use of some ribose-modified adenosine analogues, known as P-site inhibitors, and of the AC activator forskolin, have contributed considerably to the understanding of how ATP interacts with the catalytic site and how the catalytically active dimer is formed [14,15]. In Dictyostelium, ACA is not activated by forskolin, but its activity in intact cells is inhibited by caffeine and by ribose-modified adenosine analogues [16–18]. However, it is not clear whether the target for the analogues is ACA itself or the cAMP receptor that activates ACA [19,20]. Neither these compounds nor any of the drugs known to directly modulate the activity of the mammalian ACs have yet been tested on ACG or ACB.
In the present study, we perform a systematic investigation of the effects of caffeine, ribose-modified adenosine analogues and other known regulators of mammalian ACs on the activities of ACA, ACB and ACG. Our study identifies two enzyme-specific inhibitors for the Dictyostelium ACs and indicate that the effects of caffeine on AC inhibition is mediated by two different targets.
EXPERIMENTAL
Materials, cell lines and cell culture
GTP[S] (guanosine 5′-[γ-thio]triphosphate), DcAMP (2′-deoxyadenosine 3′,5′-monophosphate), IPA (2′,3′-O-isopropylidene adenosine), IBMX (isobutylmethylxanthine), DTT (dithiothreitol), sodium PPi and G418 were from Sigma (St. Louis, MO, U.S.A.). DDA (2′,5′-dideoxyadenosine), tyrphostin A25 [α-cyano-(3,4,5-trihydroxy)cinnamonitrile], SQ22536 [9-(tetrahydro-2′-furyl)adenine], MDL-12,330A [cis-N-(2-phenylcyclopentyl)azacyclotridec-1-en-2-amine, HCl] and NKY80 [2-amino-7-(furanyl)-7,8-dihydro-5(6H)-quinazolinone] were from Calbiochem (San Diego, CA, U.S.A.). [2,8-3H]cAMP was from Amersham Biosciences (Little Chalfont, Bucks., U.K.). Naja messambica snake venom was from SA venom suppliers (Louis Trichardt, South Africa).
Wild-type NC4 cells, aca–/A15::ACG [4] and aca–/rdeA– [9] mutants were grown in standard axenic medium [20a], which was supplemented with 20 μg/ml G418 for aca–/A15::ACG cells. A pdeE–/regA– double null mutant was created by transforming pdeE– cells [21] with the pRegAKO construct [22]. Null mutants were selected from blasticidin-resistant transformed clones by two PCR reactions and Southern-blot analysis of genomic digests. While pdeE– cells develop normally [21], the pdeE–/regA– cells displayed the rapidly developing phenotype of regA– mutants [22].
aca–/A15::ACG, aca–/rdeA– and pdeE–/regA– cells were harvested during exponential growth, washed once with PB (10 mM sodium/potassium phosphate buffer, pH 6.5) and resuspended in either PB or lysis buffer (2 mM MgCl2 and 250 mM sucrose in 10 mM Tris, pH 8.0) to 108 cells/ml. Wild-type cells were plated on PB agar [1.5% (w/v) agar in PB] at 2.5×106 cells/cm2, starved for 6–8 h at 22 °C until aggregation territories were formed, and subsequently collected and resuspended in PB or lysis buffer to 108 cells/ml.
AC assays in intact cells
Cells were resuspended in PB and exposed to either 5 mM DTT (ACB, ACG) or stimulated with 5 μM DcAMP in 5 mM DTT (ACA) in a total volume of 30 μl in microtitre plate wells at 22 °C under gentle agitation. After various time periods, the reaction was terminated by addition of 30 μl of 3.5% (v/v) HClO4. Lysates were neutralized by addition of 15 μl of 50% saturated KHCO3 and 75 μl of cAMP assay buffer (4 mM EDTA in 150 mM sodium phosphate, pH 7.5). Microtitre plates were centrifuged for 5 min at 3000 g to precipitate protein and KClO4. cAMP was assayed in 30 μl of the supernatant fraction by isotope dilution assay, using purified PKA-R (protein kinase A regulatory subunit) from beef muscle as cAMP-binding protein [23] and [2,8-3H]cAMP as competitor. Since several of the compounds used in the present study to alter AC activity have some structural similarity to cAMP, and could potentially compete with [2,8-3H]cAMP for binding to PKA-R, we compared and show t=0 time points for each assay without and with the highest concentration of the compound. No significant interference of any of the compounds with the cAMP assay could be detected.
AC assays in cell lysates
Cells were resuspended in ice-cold lysis buffer and lysed through nuclepore filters (pore size, 3 μM), in the presence or absence of 30 μM GTP[S] for NC4 cells. Aliquots of 10 μl cell lysate were added to 5 μl of variables at 4× the desired final concentration and 5 μl of assay mix (2 mM ATP, 0.8 mM IBMX and 40 mM DTT in lysis buffer), which was supplemented with 8 mM MnCl2 for ACG and 38 mM MgCl2 for ACB assays. After 5 min of incubation on ice, reactions were started by transferring the samples to a 22 °C water bath. Reactions were terminated by adding 10 μl of 0.4 M EDTA (pH 8.0) followed by boiling for 1 min [23]. cAMP was assayed directly in the boiled lysate. For all assays the cAMP levels were standardized on the protein content of the lysate or cell suspension.
cAMP PDE (phosphodiesterase) assay
Cells were resuspended to 108 cells/ml in 10 mM DTT in PB, and incubated for 30 min at 22 °C with 10−7 M [2,8-3H]cAMP, caffeine and IBMX as indicated in a total volume of 20 μl. Reactions were stopped by boiling, and the reaction product was converted into [2,8-3H]adenosine by incubation for 30 min with 10 μg of N. messambica snake venom (which contains 5′ nucleotidase). [2,8-3H]Adenosine was separated from [2,8-3H]cAMP by adsorption of the latter to Dowex anion-exchange resin and mea-sured by scintillation counting.
RESULTS
Effects of caffeine on ACA, ACB and ACG
The modified purine caffeine acts as an antagonist for adenosine A1 and A2A receptors in human brain [24], and also inhibits some mammalian cAMP PDEs [25]. In D. discoideum, caffeine is commonly used to inhibit ligand-induced ACA activation [17]. Its mode of action is not clear. It was suggested that caffeine could act by increasing cytosolic Ca2+ levels [17], but later studies showed that ACA activity was not inhibited by Ca2+ [26]. To study whether caffeine is a specific inhibitor of ACA, we compared its effect on the activities of ACA, ACB and ACG in intact cells.
To measure each enzyme separately, we chose the following conditions: ACA was measured in wild-type NC4 cells that were starved for 6 h to induce maximal expression of ACA. Cells were stimulated with the cAMP receptor agonist DcAMP in the presence of DTT that acts here as an inhibitor of the extracellular PDE PdsA (phosphodiesterase A) [27]. In Dictyostelium, cAMP is rapidly secreted after synthesis, and its extracellular accumulation can therefore be readily measured when PdsA is inhibited.
ACB is closely associated with the intracellular cAMP PDE RegA (phosphodiesterase 2). It appears to be constitutively active, but its activity can only be measured when RegA or the RegA activator RdeA (phospho-relay intermediate A) is absent [9]. ACB shows significant activity in vegetative aca–/rdeA– cells, which is not obscured by the presence of ACA. ACG is maximally expressed in spores, which are virtually inaccessible for measurement of AC activity. This enzyme was therefore measured in vegetative aca– cells that express ACG from the constitutive A15 promoter (aca–/A15::ACG) [4]. Although ACB is also present in vegetative cells, its activity [0.83 pmol·min−1·(mg of protein)−1] is negligible compared with that of ACG [38 pmol·min−1·(mg of protein)−1]. cAMP production by both ACB or ACG can be measured during exposure of intact cells to DTT.
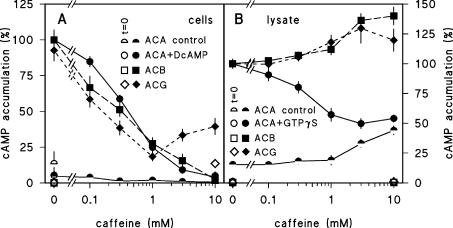
Figure 1(A) shows that caffeine inhibits ACB, ACG and DcAMP-stimulated ACA activity equally effectively with an IC50 (effective concentration that produces half-maximal inhibition) of 0.2–0.4 mM. Inhibition is complete at 10 mM, except for ACG where the higher caffeine concentrations become less effective. We next measured whether caffeine inhibited the three enzymes directly by testing its effect on the conversion of ATP into cAMP in cell lysates. Figure 1(B) shows that under these conditions caffeine does not inhibit ACB or ACG activity, nor the basal activity of ACA, and even slightly stimulates the three enzymes at 3–10 mM. However, caffeine does inhibit GTP[S]-induced activation of ACA. These results indicate that none among ACA, ACB and ACG is a direct target for the inhibitory effects of caffeine. They furthermore suggest that there are at least two different caffeine targets that mediate either its effect on GTP[S] stimulation of ACA in lysates, or on cAMP production by at least two of the three ACs in intact cells.
Figure 1. Effects of caffeine on ACA, ACB and ACG activity in intact cells and lysates.
(A) Intact cells. For assay of ACA-mediated cAMP accumulation, 6 h-starved NC4 cells were incubated for 0 and 3 min with 5 mM DTT in the presence and absence of 5 μM DcAMP. For assay of ACG, vegetative aca–/A15::ACG cells were incubated for 0 and 5 min with 5 mM DTT and, for assay of ACB, vegetative aca–/rdeA– cells were incubated for 0 and 30 min with 5 mM DTT. Caffeine was present during incubation at the indicated concentrations. Reactions were terminated by addition of HClO4 to 1.75% and cAMP was assayed in the neutralized cell lysates. Data are expressed as percentage of cAMP accumulation measured at the maximal incubation time for each enzyme in the absence of caffeine, and for ACA in the presence of DcAMP. This was 43.3±15.5, 24.6±5.1 and 190±53 pmol of cAMP/mg of protein for ACA+DcAMP, ACB and ACG respectively. (B) Cell lysates. Six hour-starved NC4 cells were filter-lysed in the presence and absence of 30 μM GTP[S] and incubated for 0 and 5 min with 0.5 mM ATP, 2 mM MgCl2 and 10 mM DTT for assay of ACA. Vegetative aca–/A15::ACG cells were lysed and incubated for 0 and 5 min with 0.5 mM ATP, 2 mM MnCl2 and 10 mM DTT for assay of ACG. Vegetative aca–/rdeA– cell lysates were assayed for 0 and 30 min with 0.5 mM ATP, 10 mM MgCl2 and 10 mM DTT for assay of ACB. Caffeine was present during incubation at the indicated concentrations. Reactions were terminated by addition of EDTA to 0.13 M and boiling, and cAMP was assayed in the boiled lysate. Data are expressed as percentage of cAMP accumulation measured at the maximal incubation time for each enzyme in the absence of caffeine, and for ACA in the presence of GTP[S]. This was 34.0±6.0, 27.1±4.5 and 193±51 pmol of cAMP/mg of protein for ACA+GTP[S], ACB and ACG respectively. Means±S.E.M. for at least three experiments performed in triplicate are presented.
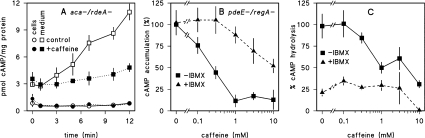
A global effect of caffeine on cAMP accumulation could occur if caffeine inhibited cAMP secretion, in which case cAMP would be degraded by intracellular cAMP PDEs, or if caffeine strongly stimulated a cAMP PDE. Figure 2(A) shows that when ACB activity is measured in cells and the medium separately, caffeine does not increase the amount of cAMP associated with the cell fraction. The small amount of cAMP that is produced in the presence of caffeine is fully secreted. Therefore caffeine is unlikely to inhibit cAMP secretion. D. discoideum has two intracellular cAMP PDEs, RegA [22] and PdeE (phosphodiesterase E) [21], and two cell-surface-associated enzymes, PdsA and PDE4 [28]. PdsA is in our assays inhibited by DTT, while RegA requires RdeA for activity [9] and should not be active in the aca–/rdeA– mutants that are used to assay ACB. PDE4 can be inhibited by the common PDE inhibitor IBMX [28]. To test whether caffeine inhibition of cAMP accumulation is due to activation of any of the four cAMP PDEs, we measured its effect on cAMP accumulation by ACB in PdeE–/RegA– double null mutants with DTT to inhibit PdsA and IBMX to inhibit PDE4. Figure 2(B) shows that in the absence of IBMX, cAMP accumulation is effectively inhibited by caffeine, which rules out PdeE, RegA and PdsA as caffeine targets. However, in the presence of IBMX, caffeine inhibition is reduced. This suggests that PDE4 could be activated by caffeine. To test this directly, we measured the effect of caffeine on [3H]cAMP hydrolysis by intact cells, with DTT added to inhibit PdsA. Figure 2(C) shows that the IBMX-sensitive PDE activity, which is most likely PDE4, is inhibited instead of stimulated by caffeine. This indicates that caffeine does not inhibit cAMP accumulation by activating PDE4 either. Inhibition by IBMX of the effects of caffeine on cAMP accumulation as observed in Figure 2(B) is perhaps due to true antagonism. IBMX and caffeine (1,3,7-trimethylxanthine) are similar in structure and may both bind to the target of caffeine, which, for the time being, remains obscure.
Figure 2. Effects of caffeine on cAMP secretion and cAMP PDE activity.
(A) cAMP secretion. Vegetative aca–/rdeA– cells were incubated at 108 cells/ml with 5 mM DTT in PB in the presence and absence of 2 mM caffeine. At the indicated time periods 50 μl aliquots of cell suspension were centrifuged for 5 s at 10000 g. Supernatant (medium) and pellet (cells) fractions were rapidly separated and boiled for 30 s. cAMP was assayed in both fractions. (B) cAMP production in PDE-null mutants. Vegetative pdeE–/regA– cells were incubated for 30 min with 10 mM DTT in PB in the presence and absence of 3 mM IBMX and the indicated caffeine concentrations and assayed for cAMP. Data are expressed as percentage of cAMP accumulation in the absence of IBMX and caffeine. (C) PDE activity. Vegetative wild-type cells were incubated with 10 mM DTT and 10−7 M [3H]cAMP in the presence and absence of IBMX and caffeine as indicated. After 30 min the samples were assayed for [3H]5′-AMP production. Data are presented as percentage of [3H]cAMP hydrolysis obtained in the absence of IBMX and caffeine. Means±S.E.M. for two experiments performed in duplicate for (A) and triplicate for (B, C) are presented.
Effects of P-site inhibitors on AC activity in intact cells and cell lysates
In Dictyostelium, most if not all cAMP-induced responses that are mediated by the cAMP receptor cAR1, including the activation of ACA, are inhibited by adenosine [18,20,29,30]. Adenosine analogues with modifications in the purine moiety are generally less effective than adenosine. Ribose-modified adenosine analogues are more effective and this is particularly the case for IPA [18,20,30–32]. These effects were attributed to inhibition by adenosine of cAMP binding to cAR1, which shows a similar adenosine analogue specificity [20,29].
Many mammalian ACs are directly inhibited by adenosine. Also, here, modification of the purine moiety reduces efficacy, while some ribose-modified analogues, such as DA (2′-deoxyadenosine) and particularly DDA and 2′,5′-dideoxy-3′-AMP are more active than adenosine [33]. Owing to its dependence on an intact purine moiety, this type of AC inhibition is known as P-site inhibition [34]. Co-crystallization of the C1 and C2 catalytic domains of mammalian AC with P-site inhibitors and PPi showed that the complex occupied the ATP-binding pocket of the enzyme, mimicking the enzyme–product complex in the transition state. Apart from this direct interaction, adenosine also has both stimulatory and inhibitory indirect effects on mammalian ACs that are mediated by a large class of G-protein-coupled adenosine receptors [35]. These receptors require an intact ribose-moiety and were traditionally called R-sites [34]. However, this class of adenosine receptor has never been detected in Dictyostelium.
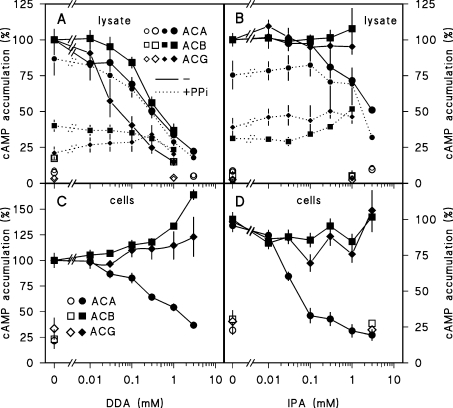
In Dictyostelium, ACA inhibition by DA and DDA was previously reported [19], but effects of P-site inhibitors on ACB or ACG were never investigated. It is also not known whether IPA inhibits Dictyostelium ACs directly. We therefore tested the effect of both DDA and IPA on the three Dictyostelium ACs. PPi was added at 1 mM. Figure 3(A) shows that DDA inhibits ACG activity in cell lysates most effectively (IC50≈75 μM), and inhibits ACA and ACB at 3–5-fold higher concentrations. In crude lysates the inhibitory effects of DDA are not dependent on added PPi, although the compound itself markedly inhibits both ACG and ACB activities. IPA has no effect on ACG and ACB and only slightly inhibits ACA activation in lysates (Figure 3B).
Figure 3. Effects of IPA and DDA on AC activities.
(A, B) Cell lysates. For assay of ACB, ACG or ACA activity, aca–/rdeA–, aca–/A15::ACG or NC4 cells were lysed without or with 30 μM GTP[S] (NC4). Lysates were incubated in the presence (small symbols, dotted lines) and absence (large symbols, solid lines) of 1 mM PPi and the indicated concentrations of IPA or DDA. Accumulated cAMP levels were determined after 0 min (open symbols), 5 min (closed symbols, ACA, ACG) or 30 min (closed symbols, ACB). (C, D) Intact cells. aca–/rdeA–, aca–/A15::ACG and NC4 cells were incubated with DTT or DcAMP/DTT (NC4) in the presence of the indicated concentrations of DDA and IPA. Accumulated cAMP levels were determined after 0 min (open symbols), 3 min (●, ACA), 5 min (◆, ACG) or 30 min (■, ACB). Data are standardized on cAMP levels obtained in the absence of DDA, IPA or PPi. Means±S.E.M. for two or three experiments performed in triplicate are presented.
When the three enzymes are measured in intact cells, the effects of DDA and IPA are quite different. Neither of the two analogues inhibits ACG or ACB activity (Figures 3C and 3D). IPA strongly inhibits DcAMP-induced ACA activation (IC50≈30 μM), while at least 15-fold higher concentrations of DDA are required to inhibit this response. These results indicate that the effects of IPA on ACA activation in intact cells are due to inhibition of cAMP binding to cAR1 as previously proposed [20]. ACG, ACB and ACA are inhibited by the ‘classical’ P-site inhibitor DDA when assayed in lysates, but DDA is apparently not sufficiently membrane-permeable to inhibit the enzymes when added to intact cells.
Searching for compounds that specifically inhibit ACA, ACB or ACG
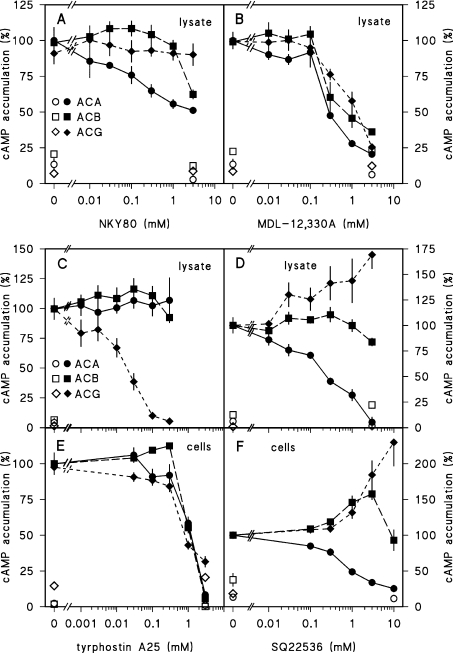
To identify inhibitors that act specifically on ACA, ACB or ACG, we tested a range of compounds that inhibit ACs in other organisms, such as NKY80 [36], MDL-12,330A [37] and SQ22536 [38]. In addition, we tested tyrphostin A25, a compound that was initially identified as a tyrosine kinase inhibitor, but also proved to inhibit a variety of mammalian guanylate cyclases and ACs [39]. NKY80 and tyrphostin A25 both act directly at the AC catalytic core [36,39]. We first tested the effects of the four inhibitors on ACA, ACB and ACG activity measured in cell lysates. Figure 4(A) shows that NKY80 does not inhibit ACG and has only a partial inhibitory effect on ACA and ACB. On the other hand, MDL-12,330A inhibits all three enzymes with IC50 values that vary between 0.3 and 0.9 mM (Figure 4B and Table 1). Tyrphostin A25 did not alter ACA or ACB activity (Figure 4C), but was an effective ACG inhibitor with an IC50 of approx. 16 μM in cell lysates. However, when added to intact cells, ACG inhibition required a 40-fold higher concentration. At these concentrations ACA and ACB also started to be inhibited, suggesting that this inhibition is due to a pleiotropic effect (Figure 4E). SQ22536 effectively inhibited both GTP[S]-stimulated ACA activity in cell lysates and DcAMP-stimulated ACA activity in intact cells (Figures 4D and 4F). Remarkably, SQ22536 stimulated ACG activity strongly and ACB activity weakly, both when assayed in lysates and intact cells. To conclude, both tyrphostin A25 and SQ22536 are specific inhibitors for ACG and ACA respectively when assayed in lysates. However, only SQ22536 can be used to inhibit ACA specifically in intact cells.
Figure 4. Effects of NKY80, MDL-12,330A, tyrphostin A25 and SQ22536 on ACA, ACB and ACG activities.
(A–D) Cell lysates. For assay of ACB, ACG or ACA activity, aca–/rdeA–, aca–/A15::ACG or NC4 cells were lysed without or with 30 μM GTP[S] (NC4) and incubated with the indicated concentrations of NKY80 (A), MDL-12,330A (B), tyrphostin A25 (C) or SQ22536 (D). Accumulated cAMP levels were determined after 0 min (open symbols), 5 min (closed symbols, ACA, ACG) or 30 min (closed symbols, ACB). Data were standardized on control cAMP levels. (E, F) Intact cells. aca–/rdeA–, aca–/A15::ACG or NC4 cells were incubated with DTT and/or DcAMP/DTT in the presence of the indicated concentrations of tyrphostin A25 or SQ22536. Accumulated cAMP levels were determined after 0 min (open symbols), 3 min (ACA), 5 min (ACG) or 30 min (ACB). Data were standardized on levels obtained in the absence of inhibitor, and in case of ACA in the presence of DcAMP. Means±S.E.M. for two or three experiments performed in triplicate are presented.
Table 1. Pharmacological profiles of the three Dictyostelium ACs.
ND, not determined; NA, not applicable; 0, no effect; −, inhibition; −−, strong inhibition; +, stimulation; ++, strong stimulation; Cm, concentration that yields maximal effect. IC50 values were determined by non-linear curve fitting of the data presented in Figures 1–4 to y=100–[I]×100/(IC50+[I]), where y denotes percentage cAMP accumulation, with t=0 cAMP levels subtracted, and [I] the inhibitor concentration. IC50 values are presented as means ±95% confidence interval. The goodness-of-fit (r2) is indicated in parentheses.
| ACA (6 h starvation) | ACB (vegetative) | ACG (vegetative) | ||||
|---|---|---|---|---|---|---|
| Cells | Lysates | Cells | Lysates | Cells | Lysates | |
| GTP[S] | NA | ++ [43] | NA | 0 [9] | NA | 0 [4] |
| Mg2+ | NA | + [23] | NA | ++ Cm=10 mM [9] | NA | + [23] |
| Mn2+ | NA | ++ Cm=20 mM [23] | NA | + [9] | NA | ++ Cm=1–2 mM [23] |
| Caffeine | −− | −* | −− | + | −− | + |
| IC50=361±113 μM | IC50=825±114 μM | IC50=300±107 μM | EC50=1–2 mM | IC50=207±312 μM | EC50=1–2 mM | |
| (0.99) [17] | (0.99) | (0.99) | (0.79) | |||
| IPA | −− | 0/− | 0 | 0 | 0 | 0 |
| IC50=31±13 μM | IC50=2.18±0.65 mM | |||||
| (0.98) | (0.96) | |||||
| DDA | − | − | + | − | 0/+ | −− |
| IC50=465±217 μM | IC50=274±153 μM | EC50>1 mM | IC50=336±82 μM | IC50=76±25 μM | ||
| (0.97) | (0.98) | (0.99) | (0.99) | |||
| PPi | NA | 0/− | NA | − | NA | − |
| NKY80 | ND | 0/− | ND | 0/− | ND | 0 |
| IC50=1.31±1.36 mM | IC50=5.78±4.97 mM | |||||
| (0.78) | (0.89) | |||||
| MDL- | ND | − | ND | − | ND | −− |
| 12,330A | IC50=329±174 μM | IC50=497±383 μM | IC50=885±258 μM | |||
| (0.95) | (0.93) | (0.99) | ||||
| Tyrphostin | 0/− | 0 | 0/− | 0 | 0/− | −− |
| A25 | 1119±879 μM | 1336±715 μM | IC50=602±336 μM | IC50=16.6±7.4 μM | ||
| (0.94) | (0.89) | (0.95) | (0.97) | |||
| SQ22536 | − | −* | + | 0 | ++ | + |
| IC50=812±280 μM | IC50=247±134 μM | |||||
| (0.99) | (0.96) | |||||
* GTP[S]-stimulated activity.
DISCUSSION
The present study was initiated to identify specific inhibitors for the three Dictyostelium ACs in order to identify and study specific roles of each of the enzymes during the life cycle of the organism. We first studied the effects of caffeine, which has long been used to study specific roles of ACA in cell aggregation and development [17,40–42]. Our experiments showed that in intact cells caffeine inhibited ACB and ACG as efficiently as ACA (Figure 1). The effects of caffeine on the three enzymes must be indirect, since it does not directly inhibit their basal activities when measured in cell lysates. In the case of ACA, it does prevent activation of the enzyme by GTP[S], which activates the G-protein G2, that is part of the signal transduction cascade that normally activates ACA [2,3]. However, neither ACB nor ACG is activated by G-proteins [4,9]. It therefore appears that there is one target for caffeine that is at or downstream from the G-protein G2, and another that acts globally to prevent cAMP accumulation by either ACB or ACG and possibly also ACA.
We explored the possibility that caffeine could inhibit cAMP accumulation by either activating a cAMP PDE activity or by inhibiting cAMP secretion, causing cAMP to be degraded intracellularly. However, no effects of caffeine on cAMP secretion were observed (Figure 2A), and caffeine still inhibited cAMP accumulation when three out of the four cAMP PDEs were inactivated (Figure 2B). The fourth cAMP PDE was inhibited rather than activated by caffeine (Figure 2C), in agreement with the known effect of caffeine on mammalian cAMP PDEs [25]. The observation that caffeine still inhibits cAMP accumulation in the pdeE–/regA– mutant that lacks intracellular cAMP PDEs, confirms that it does not act on cAMP secretion, since in this mutant cAMP should then accumulate intracellularly.
We next analysed the potential of ribose-modified adenosine analogues as specific AC inhibitors. DDA is a characteristic P-site inhibitor for mammalian ACs that acts directly at the catalytic core of the enzymes [15]. It also inhibited the three Dictyostelium ACs, and particularly ACG, when measured in cell lysates, indicating that also here it may interact directly with the catalytic region of the enzymes. However, DDA did not inhibit ACB or ACG activity when added to intact cells, which implies that there is insufficient uptake of the compound. There was an inhibitory effect of DDA on ligand-induced ACA activation, but this, as discussed below, could be due to interference with ligand binding. IPA is another ribose-modified adenosine analogue that is very effective in inhibiting cAR1-mediated responses in Dictyostelium [30–32]. However, it has no reported merits as P-site inhibitor for mammalian ACs and had no effects on ACB and ACG, and only modest effects on ACA activity measured in cell lysates (Figure 3C). IPA was however a very effective inhibitor of cAR1-mediated ACA activation in intact cells (Figure 3D). Other ribose modified cAMP analogues, such as 2′-chloroadenosine and 2′-O-methyladenosine, also inhibit cAMP-induced ACA activation in intact cells [18]. These compounds as well as IPA and DA are also effective inhibitors of cAMP binding to cAR1 [20]. This strongly suggests that all inhibitory effects of ribose-modified analogues on cAMP-induced responses in intact cells are due to inhibition of cAMP binding to cAR1.
Lastly, we tested four known inhibitors of ACs in other organisms for effects on ACA, ACB and ACG. One compound, NKY80, was not effective, while another, MDL-12,330A, was effective, but not specific for either of the three enzymes. The third compound tyrphostin A25 proved to be a specific and effective inhibitor of ACG in lysates. However, in intact cells it required much higher concentrations to have an effect, which was then no longer specific to ACG. The fourth inhibitor, SQ22536, was a specific ACA inhibitor, acting both in lysates and in intact cells. SQ22536 is highly lipophilic and may prove to be a useful agent for investigation of specific roles of ACA in Dictyostelium chemotaxis and development.
The present study added a large number of traits that distinguish the ACs from each other. The novel traits are summarized with previously reported distinguishing features in Table 1. They will prove useful for enzyme identification in specific cell types or during specific stages in development of D. discoideum or to recognize similar activities in other organisms.
Acknowledgments
We thank Robert R. Kay (MRC Laboratory of Molecular Biology, Cambridge, U.K.), Julian Gross (Department of Biochemistry, University of Oxford, Oxford, U.K.) and Peter N. Devreotes (Department of Cell Biology, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A.) for their gifts of the pRegAKO vector, aca–/rdeA– cells and aca–/A15::ACG cells respectively. This research was supported by an NWO (Netherlands Organization for Scientific Research) grant 805.17.047, Wellcome Trust University Award Grant 057137 and Wellcome Trust Project Grant 076618.
References
- 1.Alvarez-Curto E., Rozen D. E., Ritchie A. V., Fouquet C., Baldauf S. L., Schaap P. Evolutionary origin of cAMP-based chemoattraction in the social amoebae. Proc. Natl. Acad. Sci. U.S.A. 2005;102:6385–6390. doi: 10.1073/pnas.0502238102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubry L., Firtel R. Integration of signaling networks that regulate Dictyostelium differentiation. Annu. Rev. Cell Dev. Biol. 1999;15:469–517. doi: 10.1146/annurev.cellbio.15.1.469. [DOI] [PubMed] [Google Scholar]
- 3.Saran S., Meima M. E., Alvarez-Curto E., Weening K. E., Rozen D. E., Schaap P. cAMP signaling in Dictyostelium – complexity of cAMP synthesis, degradation and detection. J. Muscle Res. Cell Motil. 2002;23:793–802. doi: 10.1023/a:1024483829878. [DOI] [PubMed] [Google Scholar]
- 4.Pitt G. S., Milona N., Borleis J., Lin K. C., Reed R. R., Devreotes P. N. Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell. 1992;69:305–315. doi: 10.1016/0092-8674(92)90411-5. [DOI] [PubMed] [Google Scholar]
- 5.Tesmer J. J., Sunahara R. K., Gilman A. G., Sprang S. R. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 6.Comer F. I., Lippincott C. K., Masbad J. J., Parent C. A. The PI3K-mediated activation of CRAC independently regulates adenylyl cyclase activation and chemotaxis. Curr. Biol. 2005;15:134–139. doi: 10.1016/j.cub.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Van Es S., Virdy K. J., Pitt G. S., Meima M., Sands T. W., Devreotes P. N., Cotter D. A., Schaap P. Adenylyl cyclase G, an osmosensor controlling germination of Dictyostelium spores. J. Biol. Chem. 1996;271:23623–23625. doi: 10.1074/jbc.271.39.23623. [DOI] [PubMed] [Google Scholar]
- 8.Saran S., Schaap P. Adenylyl cyclase G is activated by an intramolecular osmosensor. Mol. Biol. Cell. 2004;15:1479–1486. doi: 10.1091/mbc.E03-08-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H. J., Chang W. T., Meima M., Gross J. D., Schaap P. A novel adenylyl cyclase detected in rapidly developing mutants of Dictyostelium. J. Biol. Chem. 1998;273:30859–30862. doi: 10.1074/jbc.273.47.30859. [DOI] [PubMed] [Google Scholar]
- 10.Soderbom F., Anjard C., Iranfar N., Fuller D., Loomis W. F. An adenylyl cyclase that functions during late development of Dictyostelium. Development. 1999;126:5463–5471. doi: 10.1242/dev.126.23.5463. [DOI] [PubMed] [Google Scholar]
- 11.Cann M. J., Hammer A., Zhou J., Kanacher T. A defined subset of adenylyl cyclases is regulated by bicarbonate ion. J. Biol. Chem. 2003;278:35033–35038. doi: 10.1074/jbc.M303025200. [DOI] [PubMed] [Google Scholar]
- 12.Parent C. A., Devreotes P. N. Isolation of inactive and G protein-resistant adenylyl cyclase mutants using random mutagenesis. J. Biol. Chem. 1995;270:22693–22696. doi: 10.1074/jbc.270.39.22693. [DOI] [PubMed] [Google Scholar]
- 13.Parent C. A., Devreotes P. N. Constitutitively active adenylyl cyclase mutant requires neither G proteins nor cytosolic regulators. J. Biol. Chem. 1996;271:18333–18336. doi: 10.1074/jbc.271.31.18333. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G., Liu Y., Ruoho A. E., Hurley J. H. Structure of the adenylyl cyclase catalytic core. Nature. 1997;386:247–253. doi: 10.1038/386247a0. [DOI] [PubMed] [Google Scholar]
- 15.Dessauer C. W., Tesmer J. J. G., Sprang S. R., Gilman A. G. The interactions of adenylate cyclases with P-site inhibitors. Trends Pharmacol. Sci. 1999;20:205–210. doi: 10.1016/s0165-6147(99)01310-3. [DOI] [PubMed] [Google Scholar]
- 16.Brenner M., Padh H. Forskolin does not activate cyclic AMP synthesis in Dictyostelium discoideum in vivo or in vitro. J. Cycl. Nucl. Protein Phosphor. Res. 1983;9:297–303. [PubMed] [Google Scholar]
- 17.Brenner M., Thoms S. D. Caffeine blocks activation of cyclic AMP synthesis in Dictyostelium discoideum. Dev. Biol. 1984;101:136–146. doi: 10.1016/0012-1606(84)90124-6. [DOI] [PubMed] [Google Scholar]
- 18.Theibert A., Devreotes P. Adenosine and its derivatives inhibit the cAMP signaling response in Dictyostelium discoideum. Dev. Biol. 1984;106:166–173. doi: 10.1016/0012-1606(84)90072-1. [DOI] [PubMed] [Google Scholar]
- 19.Khachatrian L., Klein C., Howlett A. Regulation of Dictyostelium discoideum adenylate cyclase by manganese and adenosine analogs. Biochim. Biophys. Acta. 1987;927:235–246. doi: 10.1016/0167-4889(87)90140-6. [DOI] [PubMed] [Google Scholar]
- 20.Van Lookeren Campagne M. M., Schaap P., Van Haastert P. J. M. Specificity of adenosine inhibition of cAMP-induced responses in Dictyostelium resembles that of the P-site of higher organisms. Dev. Biol. 1986;117:245–251. [Google Scholar]
- 20a.Sussman R., Sussman M. Cultivation of Dictyostelium discoideum in axenic medium. Biochem. Biophys. Res. Commun. 1967;29:53–55. doi: 10.1016/0006-291x(67)90539-6. [DOI] [PubMed] [Google Scholar]
- 21.Meima M. E., Weening K. E., Schaap P. Characterization of a cAMP-stimulated cAMP phosphodiesterase in Dictyostelium discoideum. J. Biol. Chem. 2003;278:14356–14362. doi: 10.1074/jbc.M209648200. [DOI] [PubMed] [Google Scholar]
- 22.Thomason P. A., Traynor D., Cavet G., Chang W.-T., Harwood A. J., Kay R. R. An intersection of the cAMP/PKA and two-component signal transduction systems in Dictyostelium. EMBO J. 1998;17:2838–2845. doi: 10.1093/emboj/17.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meima M. E., Schaap P. Fingerprinting of adenylyl cyclase activities during Dictyostelium development indicates a dominant role for adenylyl cyclase B in terminal differentiation. Dev. Biol. 1999;212:182–190. doi: 10.1006/dbio.1999.9352. [DOI] [PubMed] [Google Scholar]
- 24.Cauli O., Morelli M. Caffeine and the dopaminergic system. Behav. Pharmacol. 2005;16:63–77. doi: 10.1097/00008877-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Chasin M., Harris D. N. Inhibitors and activators of cyclic nucleotide phosphodiesterase. Adv. Cycl. Nucl. Res. 1976;7:225–264. [PubMed] [Google Scholar]
- 26.Schaap P., Brandt R., Van Es S. Regulation of Dictyostelium adenylyl cyclases by morphogen-induced modulation of cytosolic pH or Ca2+ levels. Dev. Biol. 1995;168:179–188. doi: 10.1006/dbio.1995.1070. [DOI] [PubMed] [Google Scholar]
- 27.Pannbacker R. G., Bravard L. J. Phosphodiesterase in Dictyostelium discoideum and the chemotactic response to cyclic adenosine monophosphate. Science. 1972;175:1014–1015. doi: 10.1126/science.175.4025.1014. [DOI] [PubMed] [Google Scholar]
- 28.Bader S., Kortholt A., Snippe H., Van Haastert P. J. DdPDE4, a novel cAMP-specific phosphodiesterase at the surface of Dictyostelium cells. J. Biol. Chem. 2006;281:20018–20026. doi: 10.1074/jbc.M600040200. [DOI] [PubMed] [Google Scholar]
- 29.Newell P. C., Ross F. M. Inhibition by adenosine of aggregation centre initiation and cyclic AMP binding in Dictyostelium. J. Gen. Microbiol. 1982;128:2715–2724. [Google Scholar]
- 30.Soede R. D. M., Hopper N. A., Williams J. G., Schaap P. Extracellular cAMP depletion triggers stalk gene expression in Dictyostelium: disparities in developmental timing and dose dependency indicate that prespore induction and stalk repression by cAMP are mediated by separate signaling pathways. Dev. Biol. 1996;177:152–159. doi: 10.1006/dbio.1996.0152. [DOI] [PubMed] [Google Scholar]
- 31.Verkerke-VanWijk I., Kim J. Y., Brandt R., Devreotes P. N., Schaap P. Functional promiscuity of gene regulation by serpentine receptors in Dictyostelium discoideum. Mol. Cell. Biol. 1998;18:5744–5749. doi: 10.1128/mcb.18.10.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dormann D., Abe T., Weijer C. J., Williams J. Inducible nuclear translocation of a STAT protein in Dictyostelium prespore cells: implications for morphogenesis and cell-type regulation. Development. 2001;128:1081–1088. doi: 10.1242/dev.128.7.1081. [DOI] [PubMed] [Google Scholar]
- 33.Johnson R. A., Yeung S. M., Stubner D., Bushfield M., Shoshani I. Cation and structural requirements for P site-mediated inhibition of adenylate cyclase. Mol. Pharmacol. 1989;35:681–688. [PubMed] [Google Scholar]
- 34.Londos C., Wolff J. Two distinct adenosine-sensitive sites on adenylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 1977;74:5482–5486. doi: 10.1073/pnas.74.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fredholm B. B., IJzerman A. P., Jacobson K. A., Klotz K.-N., Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 36.Onda T., Hashimoto Y., Nagai M., Kuramochi H., Saito S., Yamazaki H., Toya Y., Sakai I., Homcy C. J., Nishikawa K., Ishikawa Y. Type-specific regulation of adenylyl cyclase. Selective pharmacological stimulation and inhibition of adenylyl cyclase isoforms. J. Biol. Chem. 2001;276:47785–47793. doi: 10.1074/jbc.M107233200. [DOI] [PubMed] [Google Scholar]
- 37.Guellaen G., Mahu J. L., Mavier P., Berthelot P., Hanoune J. RMI 12330 A, an inhibitor of adenylate cyclase in rat liver. Biochim. Biophys. Acta. 1977;484:465–475. doi: 10.1016/0005-2744(77)90102-4. [DOI] [PubMed] [Google Scholar]
- 38.Harris D. N., Asaad M. M., Phillips M. B., Goldenberg H. J., Antonaccio M. J. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J. Cycl. Nucl. Res. 1979;5:125–134. [PubMed] [Google Scholar]
- 39.Jaleel M., Shenoy A. R., Visweswariah S. S. Tyrphostins are inhibitors of guanylyl and adenylyl cyclases. Biochemistry. 2004;43:8247–8255. doi: 10.1021/bi036234n. [DOI] [PubMed] [Google Scholar]
- 40.Siegert F., Weijer C. Digital image processing of optical density wave propagation in Dictyostelium discoideum and analysis of the effects of caffeine and ammonia. J. Cell Sci. 1989;93:325–335. [Google Scholar]
- 41.Yoshida H., Yamada Y., Okamoto K. DC6, a novel type of Dictyostelium discoideum gene regulated by secreted factors but not by cAMP. Differentiation. 1991;46:161–166. doi: 10.1111/j.1432-0436.1991.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 42.So J. S., Weeks G. The effect of extracellular cyclic AMP on differentiation inducing factor (DIF)-dependent prestalk cell gene expression in monolayers of Dictyostelium is complex. Differentiation. 1994;56:131–135. doi: 10.1046/j.1432-0436.1994.5630131.x. [DOI] [PubMed] [Google Scholar]
- 43.Theibert A., Devreotes P. Surface receptor-mediated activation of adenylate cyclase in Dictyostelium. J. Biol. Chem. 1986;261:15121–15125. [PubMed] [Google Scholar]