Abstract
Prolactin (PRL) has been shown to be involved in the differen-tiation and proliferation of numerous tissues, including the prostate gland. Moreover, variations in [Ca2+]ER (calcium concentration within the endoplasmic reticulum) may play a role in cell growth. However, few studies have focused on the regulation of calcium homoeostasis by prolactin. The present study evaluates the regulation of calcium pools as well as the possible role of [Ca2+]ER variations as a signal for growth modulation by PRL. We show that PRL stimulates the proliferation of normal SV40 immortalized epithelial prostate (PNT1A) cells with a maximum effect at a dose of 100 ng/ml. We also show that 100 ng/ml PRL increases the [Ca2+]ER when measured either by indirect quantific-ation with Fura-2AM after application of 1 μM thapsigargin or by direct quantification with Mag-Fura-2AM within the endoplas-mic reticulum. Western blot analysis shows that the SERCA 2b (sarcoendoplasmic calcium ATPase 2b) is over-expressed in PNT1A cells treated with 100 ng/ml PRL for 24 h. A small inter-fering RNA SERCA 2a/b, used to down-regulate endogenous SERCA 2b expression, reduced both PNT1A cell proliferation and [Ca2+]ER. We thus identify [Ca2+]ER and SERCA 2b as protagonists in PRL-induced proliferation.
Keywords: calcium, prolactin, proliferation, prostate, sarcoendoplasmic calcium ATPase (SERCA)
Abbreviations: [Ca2+]cyt, cytosolic Ca2+ concentration; [Ca2+]ER, Ca2+ concentration within the endoplasmic reticulum; ER, endoplasmic reticulum; Fura-2 AM, fura-2 acetoxymethyl ester; HBSS, Hanks balanced salt solution; MTS, [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; PCNA, proliferating cell nuclear antigen; PRL, prolactin; SERCA 2b, sarcoendoplasmic calcium ATPase 2b; siRNA, small interfering RNA; TG, thapsigargin; TNT, Tris/NaCl/Tween 20 buffer; TRPC4, transient receptor potential channel 4
INTRODUCTION
Due to the increase in life expectancy, benign prostate hyperplasia and prostate cancer have become very common diseases. Prostate cancer is now the second highest cause of cancer-induced death among men. It has been clearly established that the growth, differentiation and programmed cell death of prostate cells are regulated by androgens [1]. For this reason, the main treatment for prostate tumours consists of cell growth inhibition by suppressing the action or production of endogenous androgens by chemical or surgical castration. This results only in a temporary regression of the disease, since almost all tumours continue to progress.
Therefore, other non-steroid factors or hormones, like prolactin (PRL), are considered to be important for the normal and patho-logical development of the prostate gland. Indeed, PRL serum levels increase with age, whereas androgen levels decrease, sug-gesting that the role of PRL in the development of prostate hyper-plasia and cancer becomes more important with age. PRL is a polypeptide hormone belonging to the cytokine super-family, produced mainly in the pituitary gland, but is also synthesized by peripheral tissues, such as the mammary or prostate glands. Studies by several groups, including our own, have shown that PRL is one of the non-steroidal factors involved both in prostate cell proliferation [2,3] and in the development of benign pros-tate hyperplasia and prostate cancer [4,5].
Previous research in our laboratory has demonstrated the crucial role played by Ca2+ pools in the prostate [6–8]. Indeed Ca2+-homoeostasis modulation is a mechanism common to a number of signal transduction pathways, regulating a wide range of cell phenomena. [Ca2+]cyt (cytosolic Ca2+ concentration) is known to control numerous cell functions, including contraction, gene expression and proliferation [9]. The maintenance of [Ca2+]ER [calcium concentration within the ER (endoplasmic reticulum)] is another critical factor for cell growth [10]. The ER is a dynamic environment designed perfectly for protein synthesis, post-trans-lational modifications and folding. Moreover, the maintenance of high intraluminal [Ca2+] is essential for these processes [11]. However the role of calcium signalling in PRL signal transduction has yet to be fully elucidated.
In the present study, we show that PRL stimulates the prolifer-ation of PNT1A prostate cells via a mechanism involving the increase in the [Ca2+]ER, due to the over-expression of SERCA 2b (sarcoendoplasmic calcium ATPase 2b). Using siRNA (small interfering RNA) to down-regulate the endogenous SERCA 2b expression in PNT1A cells, we show that a reduction in Ca2+-store content leads to a proportional decrease in cell growth. Thus, the present study provides the first direct evidence that SERCA 2b expression and [Ca2+]ER are major players in PRL signal transduction controlling prostate cell growth. Considering the recent evidence of PRL involvement in the aberrant proliferation of prostate tumours, the present results provide a new insight into the mechanisms of the age-related cancer development.
MATERIALS AND METHODS
Cell culture
The immortalized human prostate normal cell line PNT1A obtained from the European Collection and Cell Culture (E.C.A.C.C.) was maintained in culture in RPMI 1640 medium (Life Technologies, Inc.) supplemented with 10% (v/v) fetal calf serum (Seromed; Poly-Labo, Strasbourg, France) and 5 mM L-glutamine (Sigma) and 1% (v/v) kanamycin (Sigma). Cells were grown at 37 °C in a humidified 5% CO2/95% air atmosphere. The medium was replaced every 48 h.
Proliferation assays
The assay medium was the same as that used for culture, but supplemented with 1% (v/v) fetal calf serum. The 96-well plates (Nunc) had a final volume of 200 μl and 24-well plates (Nunc) had a final volume of 1 ml and were assayed 48 h after seeding. PNT1A cells were cultured for 4 days after treatment with or without reagents, as indicated. The medium was replaced every 48 h. Proliferation was determined with an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] reagent kit.
siRNA cell transfection
PNT1A cells were transfected overnight with 40 nM SERCA 2a/b siRNA, using JetSI™ENDO transfection reagent (Polyplus transfection SA, Illkrich, France). Ready-to-use SERCA 2a/b siRNA (processing option: A4) with a sense sequence of 5′-CAAAGUUCCUGCUGAUAUA(dTdT)-3′ was synthesized by Dharmacon Inc. Control siRNA experiments were performed either by applying the transfection reagent alone (vehicle) or by transfecting siRNA against TRPM8 (transient receptor potential melastatin 8) [12].
Ca2+ measurements using Fura-2 AM (fura-2 acetoxymethyl ester)
Cells were grown on glass coverslips and, before each imaging experiment, the culture medium was replaced with HBSS (Hanks balanced salt solution; 142 mM NaCl, 5.6 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 340 μM Na2PO4, 440 μM KH2PO4, 10 mM Hepes, 5.6 mM glucose and buffered to pH 7.4 with NaOH). Cells were loaded with Fura-2 by exposure to 2 μM Fura-2 AM (Calbiochem) in HBSS for 1 h at 37 °C. After incubation, cells were washed three times in the same dye-free solution. When a Ca2+-free medium was required, CaCl2 was omitted and replaced by equimolar MgCl2. Intracellular Ca2+ was measured by an imaging system (Quanticell 900; VisiTech, Sunderland, U.K.). The glass coverslip was mounted in a chamber on a Nikon fluorescence microscope. Fura-2 fluorescence was excited at 340 and 380 nm and emitted fluorescence was measured at 510 nm. The [Ca2+]cyt was derived from the ratio of the fluorescence intensities for each of the excitation wavelengths (λ340/λ380), and from the Grynkiewicz equation. TG (thapsigargin; Sigma), the most potent selective inhibitor of SERCA, is often used to estimate Ca2+ pool content. In a Ca2+-free medium, TG induces an increase in [Ca2+]cyt, due to Ca2+ release from intracellular stores. [Ca2+]ER could be estimated in Fura-2-loaded cells by measuring the difference between basal [Ca2+]cyt and maximum [Ca2+]cyt at the TG-induced peak.
Direct quantification of [Ca2+]ER
For imaging [Ca2+]ER, PNT1A cells were loaded with 2 μM Mag-Fura 2 AM for 45 min at 37 °C. After incubation with the dye, the cells were briefly rinsed in a high K+ intracellular buffer solution (125 mM KCl, 25 mM NaCl, 10 mM Hepes, pH 7.2, 0.1 MgCl2) and then exposed for 2 min to the intracellular buffer at 37 °C with 5 mg/ml digitonin. Digitonin-permeabilized cells were perfused continuously with digitonin-free intracellular buffer supplemented with 0.2 mM Mg2+−ATP and free [Ca2+] clamped to 170 nM using Ca2+/EGTA buffer. The Mag-Fura 2 fluorescence ratio was calibrated using exposure to 10 μM ionomycin and 15 mM Ca2+ or 10 mM EGTA, assuming a dissociation constant for Ca2+–Mag-Fura 2 at room temperature (21 °C) of 53 μM [13]. Ratio imaging measurements of Mag-Fura 2 fluorescence were made using a Quanticell 900 imaging system.
Western blot
Following the treatment with PRL or siRNA, PNT1A cells were lysed with RIPA buffer [1% (v/v) Triton X-100, 1% (w/v) Na deoxycholate, 150 mM NaCl and 20 mM sodium or potassium phosphate, pH 7.2] with 5 mM EDTA and anti-protease cocktail (P8340; Sigma) for 30 min on ice. Insoluble material was discarded by centrifugation at 10000 g for 10 min at 4 °C and the protein concentration was assessed by the bicinchoninic acid method (Pierce). Equal amounts of proteins were subjected to SDS/PAGE (10 or 16% gels). Finally, the proteins were transferred on to nitrocellulose membranes using a semi-dry electro-blotter (Bio-Rad). After the transfer, the membrane was stained with Ponceau Red and the membranes were cut into thin strips (2 mm wide) that were further processed for immuno-detection. The strips were blocked for 30 min at room temperature in TNT buffer (15 mM Tris/HCl, pH 8, 140 mM NaCl and 0.05% Tween 20) with 5% (w/v) dried skimmed milk. Following blocking, the membranes were incubated with mouse anti-actin antibody (pan Ab-5, Neomarkers, Fremont, CA, U.S.A.), rabbit anti-PCNA (proliferating cell nuclear antigen) antibody (sc-56S; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), rabbit anti-SERCA 2b antibody (a gift from Prof. Frank Wuytack, Department of Molecular Cell Biology, Katholieke Universiteit Leuven, Leuven, The Netherlands) or rabbit antibody anti-calreticulin (Stressgen Biotechnologies, San Diego, CA, U.S.A.) diluted in TNT with 5% (w/v) dried skimmed milk (1:500 for anti-actin; 1:1000 for anti-PCNA; 1:5000 for anti-SERCA 2b; or 1:10000 for anti-calreticulin) for 1 h at room temperature. After thorough washes in TNT buffer, the strips were treated with the corresponding horseradish peroxidase-linked secondary antibodies (anti-mouse AP192P or anti-rabbit AP182P; Chemicon, Temecula, CA, U.S.A.) diluted 1:20000 for 1 h at room temperature. After several washes in TNT buffer, the strips were processed for chemiluminescent detection using the SuperSignal West Pico chemiluminescent substrate (Pierce), according to the manufacturer's instructions. The blots were finally exposed to X-Omat AR films (Eastman Kodak Company, Rochester, NY, U.S.A.). The intensity of the signals was evaluated by densitometry and semi-quantified using the ratio between the intensity of protein of interest divided by the actin or JAK2 intensity for each experiment. Each experiment presented was repeated at least twice.
Indirect immunofluorescence
PNT1A cells were grown on glass coverslips. After permeabilization with cold (−20 °C) acetone for 10 min, the cells were blocked with PBS containing 1.2% gelatine for 30 min at 37 °C and then incubated with anti-PNCA antibody (diluted 1:100 in PBS/gelatine) for 1 h at 37 °C. After thorough washes in PBS/gelatine, the slides were treated with Alexa Fluor® 488-labelled secondary antibody (Molecular Probes) diluted in PBS/gelatine (1:4000) for 1 h at room temperature with Evans Blue (1:50000). After two washes in PBS/gelatine and in PBS, the slides were mounted with Mowiol® and examined using a confocal microscope (Zeiss LSM 510). The acquisition parameters were identical throughout the whole experiment to allow a comparison of the intensities of the fluorescence levels. The intensity of the signals was directly quantified on the confocal microscope with LSM examiner software (AIM 3.2; Zeiss, Le Pecq, France). Each experiment was repeated at least three times.
Reagents
Human recombinant PRL was provided by Vincent Goffin (Institut National de la Santé et de la Recherche Médicale; Université René Descartes-Paris V, Paris, France) [14].
Data analysis
The data were analysed using Origin 5.0 (Microcal Software Inc., Northampton, MA, U.S.A.). Results are expressed as means±S.E.M. Each experiment was repeated at least three times. Statistical analysis was performed using the Student's t test (P<0.05 considered as significant) and ANOVA tests followed by Tukey–Kramer post-tests.
RESULTS
PNT1A cell growth is stimulated by PRL
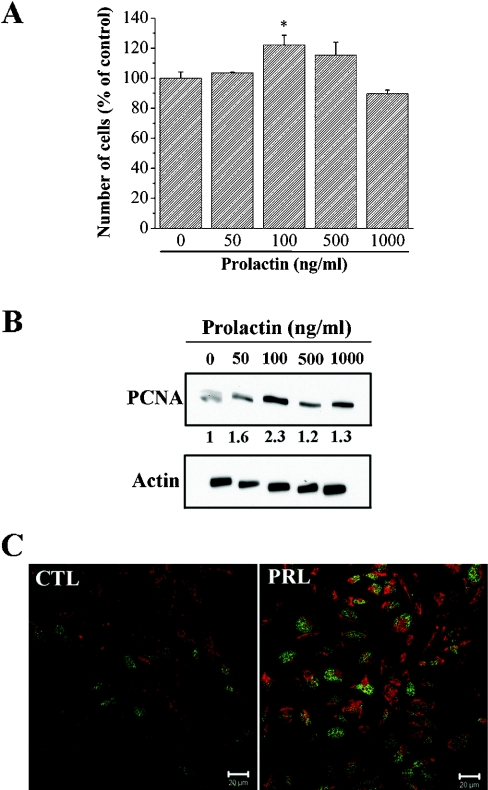
PRL stimulates PNT1A cell growth in a dose-dependent manner (Figure 1A). PRL (50–1000 ng/ml) increased cell growth after treatment for 4 days, as estimated by MTS assay. The maximum effect was observed at a dose of 100 ng/ml. At this concentration, PRL induced a proliferation increase of 122.2±6.5% compared with control conditions. We also checked the effect of PRL on the expression of PCNA, a marker of cell proliferation. Western blot analysis (Figure 1B) showed that PCNA was weakly expressed in PNT1A cells under control conditions. PCNA proteins were over-expressed 2.3-fold in PNT1A cells treated with 100 ng/ml PRL for 24 h (Figure 1B), corresponding to the maximum effect observed in the proliferation assay. The PRL-induced increase of PCNA expression was confirmed by immunofluoresence (Figure 1C). Indeed, in cells treated with 100 ng/ml PRL for 24 h, the number of cells positive for nuclear PCNA were greater in the PRL treated cells (45%) compared with control cells (22%). These results were confirmed using another proliferation marker, the nuclear antigen Ki67 (results not shown).
Figure 1. PRL stimulates cell growth.
(A) Cells were seeded in 96-well plates containing 200 μl culture medium with 1% (v/v) fetal calf serum. After 48 h, the cells were treated for 4 days with the indicated dose of PRL. The medium was replaced every 48 h. After 4 days, the number of cells in each well was determined by the MTS assay. (B) Western blot analysis of PCNA expression in cells treated with 100 ng/ml PRL for 24 h. (C) Regulation of PCNA protein expression was investigated by immunofluorescence. PCNA proteins were detected in basal conditions (CTL) and in cells treated with 100 ng/ml PRL for 24 h (PRL). PCNA, green; Evans Blue, red; scale bar, 20 μM. * Significantly different from the control value (P<0.05).
The [Ca2+]ER increases in PNT1A cells pretreated with PRL
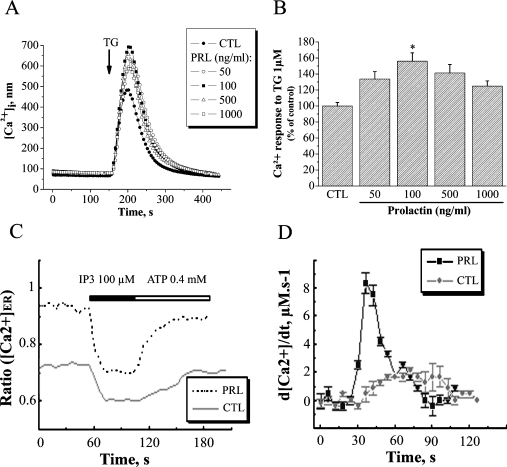
As the maintenance of luminal Ca2+ within the ER has been demonstrated to be essential for cell growth in different cell models [7,10,15], we investigated whether the Ca2+-pool content was modulated in PRL-stimulated PNT1A cells. The basal [Ca2+]cyt was not affected when PNT1A cell growth was stimulated by PRL (Figure 2A). Under control conditions, the [Ca2+]cyt of PNT1A cells was 49.7±1.2 nM. No significant increase in [Ca2+]cyt was observed in cells treated with 100 ng/ml PRL (50.2±1.2 nM). However, the Ca2+ release from the intracellular stores induced by 1 μM TG in cells treated for 24 h with 100 ng/ml PRL was greater than in control cells (Figure 2B). It is important to point out the remarkable similarity between the extent of cell proliferation (Figure 1A) and the amplitude of the TG-induced Ca2+ release from intracellular stores (Figure 2B). The maximum amplitude of TG-induced Ca2+ release (669.2±44.2 nM), corresponding to 156.1±10.3% of the TG response observed under control conditions (428.8±18.7 nM), was measured in cells with the highest growth rates (treated with 100 ng/ml PRL). This result suggests that there is a close connection between the Ca2+-pool content and the proliferation of PNT1A cells. Moreover, 50 μM AG490, a potent and specific inhibitor of JAK2 kinase activity (the major PRL-receptor-associated kinase) rendered PRL incapable of stimulating PNT1A cell growth, and the amplitude of the TG-induced Ca2+ release from intracellular stores in cells treated with AG490 and PRL was the same as observed for the control conditions (results not shown). The higher [Ca2+]ER in PRL treated cells, estimated using the TG-induced Ca2+ release method, was confirmed using the direct quantification of luminal [Ca2+]ER (Figure 2C). The luminal [Ca2+]ER was significantly higher in PRL-treated cells (0.95±0.02) compared with control cells (0.72±0.02). The functional activity of SERCA proteins has been confirmed by direct quantification of luminal [Ca2+]ER (Figure 2D) using the classical protocol to measure the Ca2+ re-uptake into the ER after inositol triphosphate-induced release [18]. As demonstrated by the d[Ca2+]/dtmax, the luminal [Ca2+]ER is more rapidly refilled in PRL-treated cells (8.5 μM·s−1) compared with control cells (2 μM·s−1) confirming that SERCA proteins are more active in PRL-treated cells than control cells. Thus, our results clearly demonstrate that PRL induces the increase in the [Ca2+]ER, suggesting that the cell growth stimulation by PRL could be linked to the high luminal [Ca2+]ER.
Figure 2. PRL increases [Ca2+]ER.
(A) Effect of 1 μM TG on the [Ca2+]cyt due to a Ca2+ release from intracellular stores ([Ca2+]i; ER). The effect of TG was recorded in a Ca2+-free HBSS. (●) Control cells and cells treated with (○) 50 ng/ml, (■) 100 ng/ml, (△) 500 ng/ml or (□) 1000 ng/ml PRL. (B) The histogram shows the amplitude of the TG-induced Ca2+ release, as an indication of the [Ca2+]ER, which was estimated by measuring the difference between basal [Ca2+]cyt and the peak induced by TG. * Significantly different from the control value (P<0.05). (C) Measurement of [Ca2+]ER in response to 100 μM IP3 (Ca2+ release), followed by 0.4 mM ATP (Ca2+ reload), as indicated in the Materials and methods section. Lower trace, control (CTL); upper trace, 100 ng/ml PRL. (D) Measurement of d[Ca2+]/dt reflecting the Ca2+ reload into the ER. (-●-), control (CTL); (-●-),100 ng/ml PRL.
SERCA 2b protein expression in PNT1A cells is regulated by PRL
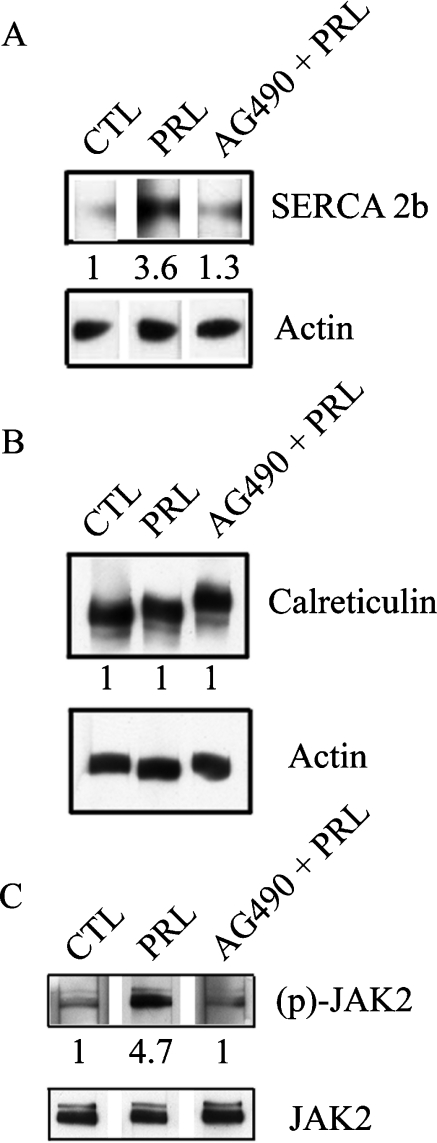
We investigated further the effect of PRL on the expression of SERCA proteins known to play an important role in the maintenance of luminal [Ca2+]ER [16]. Moreover, it has been suggested that SERCA proteins regulate cell growth by controlling the expression of growth and transformation of related genes [17]. We therefore investigated the effect of PRL on the expression of three SERCA isoforms: SERCA 2a (the cardiac/slow skeletal muscle isoform), SERCA 2b (the ubiquitously expressed isoform) and SERCA 3. In our experiments, neither SERCA 2a nor SERCA 3 protein expression were detected under control conditions or in PRL-treated cells. However, Western blot analysis showed that SERCA 2b was constitutively expressed in control cells and over-expressed (3.6-fold) in cells treated with 100 ng/ml PRL for 24 h (Figure 3A). This PRL-induced up-regulation of SERCA 2b expression in PNT1A cells was likely to be dependent on tyrosine kinase activity. After a brief (3 h) PRL treatment, Western blot analysis showed that JAK2 kinase was more strongly phosphorylated in PRL-treated cells than control cells (Figure 3C). When cells were treated with 100 ng/ml PRL in the presence of 50 μM AG490, the JAK2 kinase inhibitor, we did not observe an up-regulation of SERCA 2b expression (Figure 3A). Treating PNT1A cells with AG490 alone had no effect on SERCA 2b expression under control conditions (results not shown). We also studied expression of the luminal Ca2+ binding/storage chaperon calreticulin, to confirm that SERCA 2b over-expression was specific to PRL and was not due to the proliferation increase in protein synthesis. We therefore compared the expression of calreticulin in both control and PRL-treated cells, using semi-quantitative Western blot analysis. Over-expression of SERCA 2b induced by PRL treatment did not alter calreticulin expression (Figure 3B).
Figure 3. PRL induces SERCA 2b over-expression by JAK2 pathway.
(A) Western blot analysis of PRL (100 ng/ml) induced over-expression of SERCA 2b. AG490 (50 μM) prevents PRL-induced over-expression of SERCA 2b. (B) Western blot analysis of calreticulin expression. PRL (100 ng/ml) did not induce the over-expression of calreticulin. Treatment with 50 μM AG490 before the addition of 100 ng/ml PRL had no effect. (C) Western blot analysis of JAK2 and phospho-JAK2 expression. PRL (100 ng/ml) induced the over-phosphorylation of this JAK2. CTL, control; PRL, 100 ng/ml PRL; AG490+PRL, treatment with 50 μM AG490 before the addition of 100 ng/ml PRL.
SERCA 2b protein expression in PNT1A cells is closely linked to cell proliferation
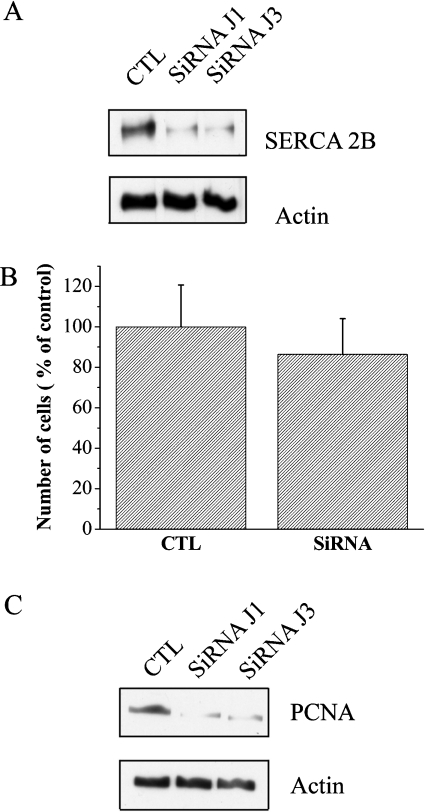
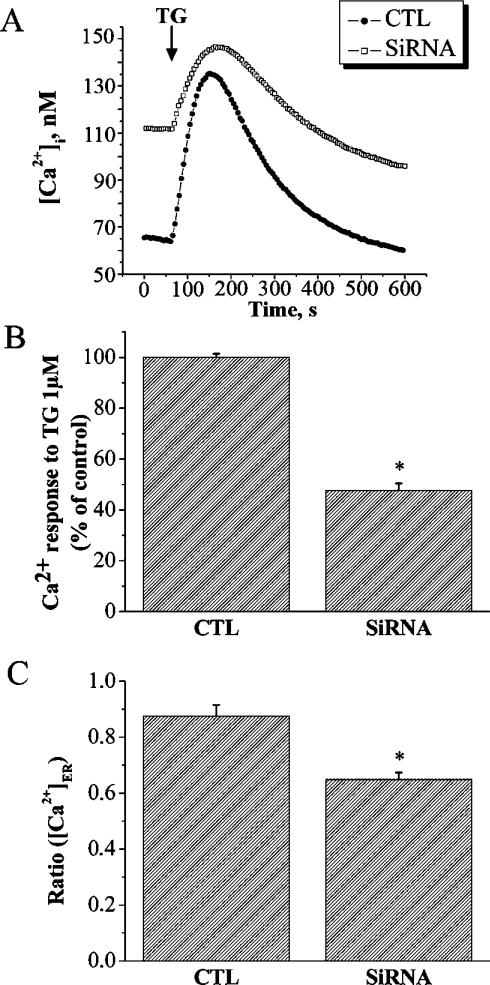
siRNA was used to down-regulate the endogenous SERCA 2b expression in PNT1A cells with a SERCA2a/b siRNA molecule. Western blotting with anti-SERCA 2b antibody on cells 1 and 3 days after transfection demonstrated that the level of SERCA 2b protein was 70% lower than the control cells (Figure 4A), indicating the effectiveness of the siRNA in down-regulating endogenous SERCA 2b expression. Then, we tested the SERCA2a/b siRNA effect on PNT1A proliferation. We observed a 13.6% reduction in the proliferation of the SiRNA SERCA 2a/b-treated cells compared with control cells (Figure 4B). The result was also confirmed by Western blot analysis, which shows the down-regulation of PCNA expression in SERCA2a/b siRNA-treated cells (Figure 4C).We next investigated whether the Ca2+-pool content was modulated in SERCA2a/b siRNA-treated PNT1A cells. The basal [Ca2+]cyt was affected and an increase in [Ca2+]cyt was observed in cells treated with SERCA2a/b siRNA (111.7±10.1 nM) compared with controls (64.9±2.8 nM; Figure 5A). The Ca2+ release induced by 1 μM TG in SERCA2a/b siRNA-treated cells was about 52% lower than that in control cells (Figure 5B). Because of the higher basal [Ca2+]cyt level, the resulting driving force may reduce the Ca2+ release in SERCA2a/b siRNA-treated cells. Therefore, these results were confirmed using the direct quantification of luminal [Ca2+]ER (Figure 5C). The luminal [Ca2+]ER was lower in SERCA2a/b siRNA-treated cells (0.64±0.02) compared with control cells (0.87±0.04). These results show that in normal human PNT1A prostate cells, SERCA 2b is a common target of physiological stimuli controlling cell growth by PRL.
Figure 4. SERCA2a/b siRNA reduces cell growth.
(A) Western blot analysis of SERCA 2b expression. SERCA 2a/b siRNA induced a down-regulation of SERCA 2b expression. CTL, control; J1, 1 day after transfection; and J3, 3 days after transfection. (B) Cells were seeded in 24-well plates and incubated for 48 h. PNT1A cells were transfected as indicated in the Materials and methods section and then cultured for a further 4 days. The medium was replaced every 48 h. After 4 days, the number of cells in each well was determined by the MTS assay. CTL, control; siRNA, SERCA 2a/b siRNA. (C) Western blot analysis of PCNA expression. SERCA 2a/b siRNA induced a down-regulation of PCNA expression. CTL, control; J1, 1 day after transfection; and J3, 3 days after transfection.
Figure 5. SERCA2a/b siRNA reduces [Ca2+]ER content.
(A) Effect of 1 μM TG on the [Ca2+]cyt due to Ca2+ release from intracellular stores ([Ca2+]i; ER). The effect of TG was recorded in a Ca2+-free HBSS. (●) control; (□) SERCA2a/b siRNA. (B) Histogram showing the amplitude of the TG-induced Ca2+ release as an indication of the [Ca2+]ER content, which was estimated by measuring the difference between basal [Ca2+]cyt and the Ca2+ peak induced by TG. CTL, control; siRNA, SERCA2a/b siRNA. * Significantly different from the control value (P<0.05). (C) Histogram showing the direct measurement of [Ca2+]ER in response to 100 μM IP3 as indicated in the Materials and methods section. CTL, control; siRNA, SERCA2a/b siRNA. * Significantly different from the control value (P<0.05).
DISCUSSION
It has been established that PRL [3,19] and its receptors [2,3,20–22] are expressed in the prostate. These findings have provided significant support for the existence of an autocrine/paracrine PRL-loop in prostate cells. Ahonen et al. [23] have shown that PRL was capable of inducing proliferation and survival of prostate epithelial cells in long-term organ culture of rat prostate tissue explants. In addition, Wennbo et al. [24] have reported a dramatic prostate enlargement in prolactin transgenic mice. In our previous studies [5], we have established that a chronic hyperprolactinaemia, caused by sulpiride, induced an enlargement and an inflammation of the lateral rat prostate. PRL stimulates the proliferation of LNCaP prostate cancer cells [2]. In the present study, we have shown that PRL stimulates the proliferation of the normal SV40 (simian virus 40) immortalized prostate PNT1A cells. Our experiments are consistent with those reported by Janssen et al. [25], who showed that PRL induced proliferation in LNCaP, DU145 and PC3 cancer cells. These authors also showed that PRL stimulates the proliferation of PC3 cells in a dose-dependent manner. However, the mechanisms by which PRL stimulates prostate cell proliferation are yet to be fully understood.
Ca2+ homoeostasis modulation is involved in a number of signal transduction pathways regulating a wide range of cellular phenomena. [Ca2+]cyt is known to control numerous cell functions, including contraction, gene expression and proliferation [9]. However, it has been documented that Ca2+-pools control numerous cell functions, including protein synthesis and cell proliferation [10,26], and the maintenance of Ca2+ levels within the lumen of the ER is critical for cell growth [10]. Indeed, a range of Ca2+-binding proteins within the ER lumen are involved in the folding and correct assembly of proteins. The increased effectiveness of these proteins may be the primary reason for growth progression. An alternative or additional possibility is that high [Ca2+]ER may promote important growth-factor-initiated, IP3-mediated Ca2+ signals that are generated through the release of Ca2+ from the ER and that are essential for cells to progress through the cell-cycle and to maintain growth. We therefore investigated the potential variation in PNT1A Ca2+-pool content linked to PRL-increased proliferation. The results of the present study show that PRL-induced proliferation of PNT1A cells is due, at least partially, to [Ca2+]ER increase and SERCA 2b over-expression by the JAK2 pathway. We did not observe any Ca2+ modifications in the cytoplasm or any modifications in the SERCA 2b activity following rapid PRL treatment (5–10 min). Thus, we suggest that PRL promotes, via the JAK2/Stat5 transduction signal, the transcription of SERCA 2b, which increases the [Ca2+]ER and subsequently cell growth. The intracellular calcium ATPase and the Ca2+-pool content have already been closely associated with cell growth and have been shown to be modulated by hormones or growth factors, such as platelet-derived growth factor [27]. We have shown previously that epidermal growth factor modulates prostate cancer LNCaP cell growth and is correlated with intracellular calcium pool content [7]. The rate of cell growth is correlated with an increase in the Ca2+-pool filling state, whereas growth-inhibited cells show a reduced Ca2+-pool load. In addition, we have demonstrated that insulin growth factor, which increases prostate cancer LNCaP cell growth, induces an increase in [Ca2+]ER and is associated with an over-expression of SERCA 2b [6]. To our knowledge, in the present study, we show for the first time that PRL stimulates cell proliferation via the over-expression of SERCA 2b and the subsequent up-regulation of [Ca2+]ER.
To study the consequence of a reduction in [Ca2+]ER on cell growth, and to confirm the involvement of SERCA 2b in PRL-induced proliferation, we used the siRNA to down-regulate SERCA 2b protein expression. The results show that a reduction in Ca2+-pool content leads to a proportional decrease in PNT1A cell growth. The correlation between the decrease in growth rate and the Ca2+-pool content decrease has already been demonstrated in other cell models. However, only artificial pharmacological agents have been used in these studies. Depletion of the [Ca2+]ER stores by the Ca2+-pump-inhibitor TG, or tBHQ [2,5-di-(tert-butyl)-hydroquinone] promotes growth arrest in DDT1MF-2 smooth muscle cells [15]. In a previous study, we have shown that a long-term treatment with ATP leads to a decrease in the intraluminal [Ca2+]ER and induces growth arrest in DU-145 androgen-independent human prostate cancer cells [28]. In the present study, we also found that [Ca2+]cyt in SERCA2a/b siRNA-treated cells was higher than in control cells. This could be due to the reduced Ca2+-pool load or to a compensation mechanism for Ca2+ signalling, via the up-regulation of membrane Ca2+ channels and exchangers [29]. The reduction of SERCA expression by gene silencing is associated with the up-regulation of TRPC4 (transient receptor potential channel 4) and TRPC5 and Na+/Ca2+ exchanger in cardiac myocytes. In the present study, store deficiency was compensated for by Ca2+ flux through the plasma membrane.
To summarize, the present study has identified and characterized, for the first time, the intracellular Ca2+ homoeostasis mechanisms involved in PRL regulation of PNT1A prostate cell growth. We have shown that PRL increases the intracellular Ca2+-pool in these proliferative cells through the over-expression of the SERCA 2b calcium pump. Using siRNA, we have specifically down-regulated SERCA 2b expression and shown that the intracellular Ca2+-pool was proportionally reduced. As a consequence, this reduces the PNT1A cell proliferation. We suggest that [Ca2+]ER exerts a strict control over cell growth and that it is a crucial element in PRL signal transduction.
Acknowledgments
Financial support to A.C. was from INSERM (Institut National de la Santé et de la Recherche Médicale) and Conseil Régional du Nord Pas-de-Calais.
References
- 1.Feldman B. J., Feldman D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 2.Van Coppenolle F., Skryma R., Ouadid-Ahidouch H., Slomianny C., Roudbaraki M., Delcourt P., Dewailly E., Humez S., Crépin A., Gourdou I., et al. Prolactin stimulates cell proliferation through a long form of prolactin receptor and K+ channel activation. Biochem. J. 2004;377:569–578. doi: 10.1042/BJ20030859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nevalainen M. T., Valve E. M., Ingleton P. M., Nurmi M., Martikainen P. M., Harkonen P. L. Prolactin and prolactin receptors are expressed and functioning in human prostate. J. Clin. Invest. 1997;99:618–627. doi: 10.1172/JCI119204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Coppenolle F., Le Bourhis X., Carpentier F., Delaby G., Cousse H., Raynaud J. P., Dupouy J. P., Prevarskaya N. Pharmacological effects of the lipidosterolic extract of Serenoa repens (Permixon®) on rat prostate hyperplasia induced by hyperprolactinemia: comparison with finasteride. Prostate. 2000;43:49–58. doi: 10.1002/(sici)1097-0045(20000401)43:1<49::aid-pros7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Van Coppenolle F., Slomianny C., Carpentier F., Le Bourhis X., Ahidouch A., Croix D., Legrand G., Dewailly E., Fournier S., Cousse H., et al. Effects of hyperprolactinemia on rat prostate growth: evidence of androgen-dependence. Am. J. Physiol. Endocrinol. Metab. 2001;280:E120–E129. doi: 10.1152/ajpendo.2001.280.1.E120. [DOI] [PubMed] [Google Scholar]
- 6.Humez S., Legrand G., Vanden-Abeele F., Monet M., Marchetti P., Lepage G., Crépin A., Dewailly E., Wuytack F., Prevarskaya N. Role of endoplasmic reticulum calcium content in prostate cancer cell growth regulation by IGF and TNFα. J. Cell. Physiol. 2004;201:201–213. doi: 10.1002/jcp.20049. [DOI] [PubMed] [Google Scholar]
- 7.Legrand G., Humez S., Slomianny C., Dewailly E., Vanden-Abeele F., Mariot P., Wuytack F., Prevarskaya N. Ca2+ pools and cell growth: evidence for sarcoendoplasmic Ca2+-ATPases 2B involvement in human prostate cancer cell growth control. J. Biol. Chem. 2001;276:47608–47614. doi: 10.1074/jbc.M107011200. [DOI] [PubMed] [Google Scholar]
- 8.Humez S., Monet M., Legrand G., Lepage G., Delcourt P., Prevarskaya N. Epidermal growth factor-induced neuroendocrine differentiation and apoptotic resistance of androgen-independent human prostate cancer cells. Endocr.-Relat. Cancer. 2006;13:181–195. doi: 10.1677/erc.1.01079. [DOI] [PubMed] [Google Scholar]
- 9.Berridge M. J. Calcium signalling and cell proliferation. Bioessays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- 10.Waldron R. T., Short A. D., Meadows J. J., Ghosh T. K., Gill D. L. Endoplasmic reticulum calcium pump expression and control of cell growth. J. Biol. Chem. 1994;269:11927–11933. [PubMed] [Google Scholar]
- 11.Michalak M., Mariani P., Opas M. Calreticulin, a multifunctional Ca2+ binding chaperone of the endoplasmic reticulum. Biochem. Cell Biol. 1998;76:779–785. doi: 10.1139/bcb-76-5-779. [DOI] [PubMed] [Google Scholar]
- 12.Thebault S., Lemonnier L., Bidaux G., Flourakis M., Bavencoffe A., Gordienko D., Roudbaraki M., Delcourt P., Panchin Y., Shuba Y., et al. Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. J. Biol. Chem. 2005;280:39423–39435. doi: 10.1074/jbc.M503544200. [DOI] [PubMed] [Google Scholar]
- 13.Hofer A. M., Schulz I. Quantification of intraluminal free [Ca] in the agonist-sensitive internal calcium store using compartmentalized fluorescent indicators: some considerations. Cell Calcium. 1996;20:235–242. doi: 10.1016/s0143-4160(96)90029-9. [DOI] [PubMed] [Google Scholar]
- 14.Paris N., Rentier-Delrue F., Defontaine A., Goffin V., Lebrun J. J., Mercier L., Martial J. A. Bacterial production and purification of recombinant human prolactin. Biotechnol. Appl. Biochem. 1990;12:436–449. [PubMed] [Google Scholar]
- 15.Short A. D., Bian J., Ghosh T. K., Waldron R. T., Rybak S. L., Gill D. L. Intracellular Ca2+ pool content is linked to control of cell growth. Proc. Natl. Acad. Sci. U.S.A. 1993;90:4986–4990. doi: 10.1073/pnas.90.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanden-Abeele F., Skryma R., Shuba Y., Van Coppenolle F., Slomianny C., Roudbaraki M., Mauroy B., Wuytack F., Prevarskaya N. Bcl-2-dependent modulation of Ca2+ homoeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 2002;1:169–179. doi: 10.1016/s1535-6108(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 17.Stokes D. L., Wagenknecht T. Calcium transport across the sarcoplasmic reticulum: structure and function of Ca2+-ATPase and the ryanodine receptor. Eur. J. Biochem. 2000;267:5274–5279. doi: 10.1046/j.1432-1327.2000.01569.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheng G., Liu B. F., Yu Y., Diglio C., Kuo T. H. The exit from G(0) into the cell cycle requires and is controlled by sarco(endo)plasmic reticulum Ca2+ pump. Arch. Biochem. Biophys. 1996;329:65–72. doi: 10.1006/abbi.1996.0192. [DOI] [PubMed] [Google Scholar]
- 19.Nevalainen M. T., Valve E. M., Ahonen T., Yagi A., Paranko J., Harkonen P. L. Androgen-dependent expression of prolactin in rat prostate epithelium in vivo and in organ culture. FASEB J. 1997;11:1297–1307. doi: 10.1096/fasebj.11.14.9409549. [DOI] [PubMed] [Google Scholar]
- 20.Leav I., Merk F. B., Lee K. F., Loda M., Mandoki M., McNeal J. E., Ho S. M. Prolactin receptor expression in the developing human prostate and in hyperplastic, dysplastic, and neoplastic lesions. Am. J. Pathol. 1999;154:863–870. doi: 10.1016/S0002-9440(10)65333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nevalainen M. T., Valve E. M., Ingleton P. M., Harkonen P. L. Expression and hormone regulation of prolactin receptors in rat dorsal and lateral prostate. Endocrinology. 1996;137:3078–3088. doi: 10.1210/endo.137.7.8770934. [DOI] [PubMed] [Google Scholar]
- 22.Peirce S. K., Chen W. Y. Quantification of prolactin receptor mRNA in multiple human tissues and cancer cell lines by real time RT-PCR. J. Endocrinol. 2001;171:R1–R4. doi: 10.1677/joe.0.171r001. [DOI] [PubMed] [Google Scholar]
- 23.Ahonen T. J., Harkonen P. L., Laine J., Rui H., Martikainen P. M., Nevalainen M. T. Prolactin is a survival factor for androgen-deprived rat dorsal and lateral prostate epithelium in organ culture. Endocrinology. 1999;140:5412–5421. doi: 10.1210/endo.140.11.7090. [DOI] [PubMed] [Google Scholar]
- 24.Wennbo H., Kindblom J., Isaksson O. G., Tornell J. Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology. 1997;138:4410–4415. doi: 10.1210/endo.138.10.5461. [DOI] [PubMed] [Google Scholar]
- 25.Janssen T., Darro F., Petein M., Raviv G., Pasteels J. L., Kiss R., Schulman C. C. In vitro characterization of prolactin-induced effects on proliferation in the neoplastic LNCaP, DU145, and PC3 models of the human prostate. Cancer. 1996;77:144–149. doi: 10.1002/(SICI)1097-0142(19960101)77:1<144::AID-CNCR24>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Gill D. L., Waldron R. T., Rys-Sikora K. E., Ufret-Vincenty C. A., Graber M. N., Favre C. J., Alfonso A. Calcium pools, calcium entry, and cell growth. Bioscience Rep. 1996;16:139–157. doi: 10.1007/BF01206203. [DOI] [PubMed] [Google Scholar]
- 27.Magnier-Gaubil C., Herbert J. M., Quarck R., Papp B., Corvazier E., Wuytack F., Levy-Toledano S., Enouf J. Smooth muscle cell cycle and proliferation: relationship between calcium influx and sarco-endoplasmic reticulum Ca2+-ATPase regulation. J. Biol. Chem. 1996;271:27788–27794. doi: 10.1074/jbc.271.44.27788. [DOI] [PubMed] [Google Scholar]
- 28.Vanoverberghe K., Mariot P., Vanden Abeele F., Delcourt P., Parys J. B., Prevarskaya N. Mechanisms of ATP-induced calcium signalling and growth arrest in human prostate cancer cells. Cell Calcium. 2003;34:75–85. doi: 10.1016/s0143-4160(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 29.Seth M., Sumbilla C., Mullen S. P., Lewis D., Klein M. G., Hussain A., Soboloff J., Gill D. L., Inesi G. Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) gene silencing and remodeling of the Ca2+ signaling mechanism in cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16683–16688. doi: 10.1073/pnas.0407537101. [DOI] [PMC free article] [PubMed] [Google Scholar]