Abstract
Synthesis of VLCFAs (very long chain fatty acids) and biosynthesis of DHS (dihydrosphingosine) both are of vital importance for Saccharomyces cerevisiae. The bulk of VLCFAs and DHS are used for ceramide synthesis by the Lag1p (longevity-assurance gene 1)/Lac1p (longevity-assurance gene cognate 1)/Lip1p (Lag1p/Lac1p interacting protein) ceramide synthase. LAG1 and LAC1 are redundant but LIP1 is essential. Here we show that 4Δ (lag1Δlac1Δypc1Δydc1Δ) cells devoid of all known endogenous ceramide synthesis pathways are unviable but can be rescued by the expression of Lass5, a mouse LAG1 homologue. Ceramide synthase activity of 4Δ.Lass5 cells only utilizes C16 and C18 fatty acids and does not require the help of Lip1p, an essential cofactor of Lag1p/Lac1p. HPLC-electrospray ionization-MS/MS analysis demonstrated that in IPCs (inositolphosphorylceramides) of 4Δ.Lass5, the very long chain fatty acids (C26 and C24) account for <1% instead of the normal >97%. Notwithstanding, IPCs incorporated into glycosylphosphatidylinositol anchors of 4Δ.Lass5 show normal mobility on TLC and the ceramide- and raft-dependent traffic of Gas1p (glycophospholipid-anchored surface protein) from endoplasmic reticulum to Golgi remains almost normal. Moreover, the biosynthesis of C24:0 fatty acids remains essential. Thus, C24:0 and dihydrosphingosine are both necessary for survival of yeast cells even if they utilize C16 and C18 fatty acids for sphingolipid biosynthesis.
Keywords: ceramide, ceramide synthase, fatty acid elongation, longevity-assurance gene 1 (LAG1), Lass5, sphingolipid
Abbreviations: 4Δ, lag1Δlac1Δypc1Δydc1Δ; DHS, dihydrosphingosine; ER, endoplasmic reticulum; ESI, electrospray ionization; Gas1p, glycophospholipid-anchored surface protein; GPI, glycosylphosphatidylinositol; IPC, inositolphosphorylceramide; LAC1, longevity-assurance gene cognate 1; LAG1, longevity-assurance gene 1; LCB, long chain base; LCFA, long chain fatty acid; LM, Lester medium; Lip1p, Lag1p/Lac1p interacting protein 1; MIPC, mannosylated IPC; M(IP)2C, inositolphosphoryl-MIPC; PE, phosphatidylethanolamine; PHS, phytosphingosine; PI, phosphatidylinositol; SLC1, sphingolipid compensation 1; ts, temperature sensitive; TSC13, temperature-sensitive Csg2 suppressor; URA3, URAcil requiring; VLCFA, very long chain fatty acid; WT, wild-type; Ydc1p, yeast dihydroceramidase; Ypc1p, yeast phytoceramidase
INTRODUCTION
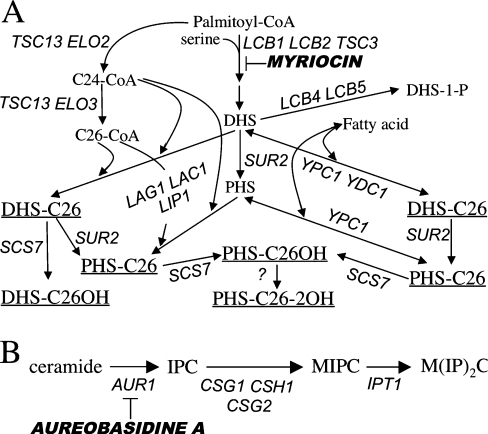
Sphingolipids of the yeast Saccharomyces cerevisiae, the IPCs (inositolphosphorylceramides), MIPCs (mannosyl-IPCs) and M(IP)2Cs (inositolphosphoryl-MIPCs) (Figure 1) are major components of the plasma membrane and the biosynthesis of IPCs is vital [1]. Contrary to humans, yeast only makes sphingolipids containing VLCFAs (very long chain fatty acids), namely C26:0 and minor amounts of C24:0, C22:0, C22:1, C20:0, C20:1, but no LCFAs (long chain fatty acids) such as C18 and C16 [2,3]. Yeast sphingolipids contain PHS (phytosphingosine) and small amounts of DHS (dihydrosphingosine), but no sphingosine, the desaturated LCB (long chain base) typically present in mammals. Yeast sphingolipids act not only as structural components of membranes and rafts but DHS and PHS as well as their 1-phosphorylated derivatives are also proposed to act as signal transduction molecules governing heat stress responses, endocytosis, ubiquitin-dependent degradation of membrane channels and progression through G1-phase [1,4]. While signal pathways controlled by PHS are quite well understood, much less is known about signalling functions of ceramide. On the other hand, the ER (endoplasmic reticulum) to Golgi transport of GPI (glycosylphosphatidylinositol) proteins has been shown to be dependent on ongoing sphingolipid synthesis, and the critical role is played by ceramides and/or inositolphospholipids [5–7]. Gas1p (glycophospholipid-anchored surface protein) has been shown to associate with lipid rafts in the ER and the formation of these rafts is dependent on sphingolipid biosynthesis [8]. Thus, the role of sphingolipids in GPI protein transport may be related to raft formation [7,8]. In further support of this, the transport of Gas1p to the Golgi is specifically and severely retarded if the elongation of C24 to C26 fatty acids by Elo3p is abolished [9], and it is also slowed when ceramide synthesis is blocked in lag1 (longevity-assurance gene 1)Δ lac1 (longevity-assurance gene cognate 1) Δ cells [10].
Figure 1. Sphingolipid biosynthesis in Saccharomyces cerevisiae.
(A) Biosynthesis of ceramides (underlined). (B) Generation of mature sphingolipids from ceramide. Gene names and inhibitors are in italics.
The biosynthesis of ceramide in yeast is dependent on LAG1 (Figure 1), which is a member of a large eukaryotic gene family [11–14]. The simultaneous deletion or depletion of LAG1 and its close homologue LAC1 in yeast eliminates all detectable acyl-CoA-dependent ceramide biosynthesis in microsomes. The lag1Δ lac1Δ cells show a dramatic reduction of IPCs and a marked accumulation of free DHS and PHS [11]. The concomitant deletion of LAG1 and LAC1 causes a significant growth defect in the genetic background of W303 cells and the same double deletion does not yield viable cells in the YPK9 background, which was used for the present study [10–12]. Lag1p and Lac1p interact with each other and with Lip1p (Lag1p/Lac1p interacting protein 1), a single span membrane protein that is equally required for acyl-CoA-dependent ceramide biosynthesis [15]. Lip1p is essential for S. cerevisiae but no mammalian homologue of LIP1 has been identified (Figure 1). In the absence of the acyl-CoA-dependent ceramide synthase, two highly homologous ceramidases, Ypc1p (yeast phytoceramidase) and Ydc1p (yeast dihydroceramidase), can synthesize ceramides from free fatty acids and LCBs by a reverse ceramidase reaction (Figure 1) [13,16,17].
In certain conditions, yeast cells can survive without sphingolipids. Indeed, deletion of LCB1 (Figure 1A) can be suppressed by a gain of function mutation in SLC1 (sphingolipid compensation 1) allowing for the incorporation of C26:0 into the sn-2 position of PI (phosphatidylinositol) [18]. The resulting VLCFA–PI is mannosylated, indicating that later acting enzymes of the sphingolipid biosynthetic pathway mistake VLCFA–PI for IPC [19]. These sphingolipid-like, PI-based phosphoinositides are believed to rescue lcb1Δ cells by restoring essential properties to membranes.
The elaboration of C26- and C24-CoA depends on ELO3 and ELO2 respectively, as well as TSC13 (temperature-sensitive Csg2 suppressor), which encodes an enoyl reductase acting in each cycle of fatty acid elongation (Figure 1A) [3,20]. Simultaneous deletion of ELO2 and ELO3 is lethal, deletion of ELO2 strongly diminishes the amounts of VLCFAs made, while deletion of ELO3 abolishes the synthesis of C26-CoA, but this is compensated by an increase of C24- and C22-CoA. Deletion of TSC13 is also lethal. ELO3 mutants have decreased amounts of complex sphingolipids, and exhibit a large panel of abnormal phenotypes and genetic interactions [3,9,20–24].
Lac1p and Lag1p dependent ceramide synthesis strongly prefers C26- and C24-CoA over shorter fatty acid CoA derivatives [25]. Human LAG1 homologues LASS1, LASS2 and LASS4 can rescue lag1Δ lac1Δ and have, when expressed in yeast, a preference for C26:0- or C24:0-CoA (Table 1). Overexpression of the murine Lass5 in human cells, however, showed a clear preference of this homologue for C16 and C18 fatty acids [26,27]. Here we show that the same is true, when Lass5 is expressed in budding yeast and that yeast cells survive without the normal set of VLCFA-containing sphingolipids.
Table 1. Reported fatty acyl-CoA preferences of mammalian LASS family members.
LASS genes having the same number are very closely related. Shown are human and mouse homologues, the human ones in capitals.
| Current name | Old name | Accession number | Mammalian cells in vivo | Mammalion cells in vitro | Yeast in vitro |
|---|---|---|---|---|---|
| LASS1 | UOG1 | NM_021267 | – | – | C26:0 [25] |
| Lass1 | uog1 | NM_138647 | C18:0 [55] | C18:0 [27,55] | – |
| LASS2 | clone 1 | BC010032 | – | – | C24:0 [25] |
| Lass2 | trh3 | BC006847 | VLCFA> | C22:0>C24:0>C26:0 ≈ | – |
| LCFA [27] | C18:0 [27] | ||||
| Lass3 | XM_907730 | C24>C22:0≈C18:0 | C18:0≈C22:0≈C24> | – | |
| C26:0≈C16:0>C20:0 [54] | |||||
| LASS4 | clone 4 | AK022151 | – | – | C24:0>C20:0>C18:0 [25] |
| Lass4 | trh1 | BC003946 | C18:0> | C22:0>C24:0≈C18:0> | – |
| C20:0> | C26:0>C16:0>C14:0 [27] | ||||
| C22:0 [26] | and C18:0>C20:0>C22:0 [26] | ||||
| Lass5 | trh4 | BC046797 | C16:0 only [26] | C14:0≈C16:0≥C18:0≈ | C12:0≈14:0≈ |
| C18:1>C12:0 [27] | C16:0≈C18:0≈C18:1 this study | ||||
| Lass6 | trh1 like | BC057629 | LCFA>VLCFA [27] | C14:0≈C16:0≥C18:0≈C12:0 [27] | – |
MATERIALS AND METHODS
Strains, growth conditions and materials
S. cerevisiae strains used are listed in Table 2. Mutants bearing single deletions of non-essential genes were obtained from EUROSCARF (EUROpean S. Cerevisiae ARchive for Functional analysis, Frankfurt, Germany). Cells were grown at 30 °C on rich medium {YPD [1% (w/v) yeast extract/2% (w/v) peptone/2% (w/v) glucose] or YPG [1% (w/v) yeast extract/2% (w/v) peptone/2% (w/v) galactose]} or minimal media (SD or SG) [28] containing 2% (w/v) glucose or galactose as a carbon source and uracil, adenine and amino acids as required. Alternatively, LM (Lester medium), a medium specially designed for growing cells unable to make sphingolipids, was used [29]. Methionine was added at 20 mg/l, which allowed the reduction of transcription from the MET17 promoter by 40% [30]. Plates containing haloperidol (H1512; Sigma) were supplemented with 0.1% Tween 20 and 0.9% ethanol to facilitate drug solubilization. Haloperidol was prepared as a 50 mM stock solution in ethanol. To assess chitin production, cells were streaked on YPD plates containing 0, 25 or 50 μg/ml of Calcofluor White (Fluorescent Brightener 28, F-3543; Sigma). To stain cells with Calcofluor White, cells were kept in continuous exponential growth for >6 generations, 1 A600 unit of cells was washed with water, stained in Calcofluor White (1 mg/ml) and observed under an Axioplan 2 (Zeiss) microscope.
Table 2. Yeast saccharomyces cerevisiae strains.
| Strains | Genotype | Reference |
|---|---|---|
| W303-1A | MATa can1-100 ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 | [11] |
| YPK9 | MATa ade2-101ochre his3-Δ200 leu2-Δ1 lys2-801ambertrp1-Δ63 ura3-52 | [11] |
| YPK11 | MATα ade2-101ochre his3-Δ200 leu2-Δ1 lys2-801ambertrp1-Δ63 ura3-52 | Michal Jazwinski |
| 2Δ.LAG1 | Same as YPK9 but lag1::TRP1 lac1::LEU2 containing pBM150:LAG1* | [11] |
| 4Δ.LAG1 | Same as YPK9 but lag1::TRP1 lac1::LEU2 | This study |
| ydc1::natMX ypc1::kanMX4 containing pBM150:LAG1* | ||
| 4Δ.Lass5 | Same as 4Δ.LAG1 but containing p413MET:Lass5 instead of pBM150:LAG1* | This study |
| 4Δ.Lass5-ADE2 | Same as YPK9 but lag1::TRP1 lac1::LEU2 | This study |
| ydc1::natMX ypc1::kanMX4::ADE2 containing p413MET:Lass5 | ||
| 4Δ.Lass5 elo3Δ | Same as 4Δ.Lass5-ADE2 containing elo3::kanMX | This study |
| 2Δ.Lass5 | Same as YPK9, but MATα lac1Δ::LEU2 | This study |
| lag1Δ::TRP1 and containing p413MET:Lass5 | ||
| 4Δ.Lass5-URA3 | Same as YPK9 but lag1::TRP1 lac1::LEU2 | This study |
| ydc1::natMX ypc1::kanMX4::URA3 containing p413MET:Lass5 | ||
| 4Δ.Lass5 lip1Δ | Same as 4Δ.Lass5-URA3 but lip1::kanMX4 | This study |
| lcb1-100 | MATa end8-1 leu2 ura3 his4 bar1 | R. Schneiter |
| 4R3 | lcb1::URA3 SLC1-1 ura3-52 leu2-3,112 ade1 MEL1 | R. Dickson |
| SJ21R | ura3-52 leu2-3,112 ade1 | K. Athenstaedt |
| BY4742 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | EUROSCARF |
| vma4Δ | YOR332w::kanMX4 in BY4742 | EUROSCARF |
| elo3Δ-1 | YLR372w::kanMX4 in BY4742 | EUROSCARF |
| elo3Δ-2 | YLR372w::his5+ in YPK11 | This study |
| ypc1Δ ydc1Δ | Same as YPK9 but ypc1::kanMX4 ydc1::kanMX4::natMX | This study |
| sec18 | MATα sec18ts | Howard Riezman |
*pBM150 is a CEN-ARS, URA3 vector possessing the yeast GAL1,10 promoter.
Construction of strains and plasmids
The Lass5 (product IRAVp968F0719D) cloned into the pCMV-SPORT6 plasmid was from the RZPD (German Resource Center for Genome Research; http://www.rzpd.de) and linearized with ScaI. The Lass5 open reading frame of IRAVp968F0719D was amplified by PCR using primers Lass5_BamHI.F1 (5′-CACGGATCCTATATAATGGCGACTGCAGCAGCG-3′) and Lass5_EcoRI.R3 (5′-GTTCGTAATAGAATTCGCTAGTCACAGGAGTGTAGAT-3′). The PCR product and the p413MET25 plasmid [30] were digested with EcoRI and BamHI, and ligated to generate p413MET-Lass5. This plasmid was transfected into Escherichia coli HB101 cells. The inserted Lass5 sequence was verified by sequencing and exactly matched the 414 amino acid protein sequence predicted by the NCBI entry number AY029533. To obtain the 4Δ.Lass5 strain we used 2Δ.LAG1 [11], a lac1Δ lag1Δ strain viable on galactose because it contains pBM150-LAG1, a CEN-ARS-URA3 (URAcil requiring) vector possessing LAG1 behind the yeast GAL1,10 promoter. YDC1 was deleted in 2Δ.LAG1 using a ydc1::KanMX4 deletion cassette, KanMX4 was then replaced by NatMX4 from pAG25 [31], and finally, YPC1 was deleted using a ypc1::KanMX4 cassette to generate 4Δ.LAG1. The 2Δ.LAG1 and 4Δ.LAG1 strains were transfected with p413MET-Lass5 and growing His+ clones were streaked out on 5-fluoro-orotic acid to shuffle out pBM150-LAG1. Whereas, non-transfected 2Δ.LAG1 and 4Δ.LAG1 were unable to grow on 5-fluoro-orotic acid, 12 out of 12 His+ clones immediately grew in the presence of the drug (results not shown). Replacement of ELO3, TSC13, and LIP1 open reading frames by KanMX4 respectively, were done using standard procedures in 4Δ.Lass5 derivatives, in which KanMX4 was replaced by either ADE2 (ADEnine requiring) or URA3 (Table 2) [31]. The ELO3 gene was also deleted in YPK11, a strain isogenic with YPK9, using an elo3::his5+ cassette obtained by PCR using the pFA6a-His3MX6 plasmid [32] as a template. All deletions were verified by PCR using DNA preparations of the various strains as templates.
Analysis of [3H]myo-inositol labelled lipids
Unless stated otherwise, metabolic labelling, cell disruption, lipid extraction and analysis were carried out as described previously [33]. Where indicated, lipids were subjected to mild alkaline treatment using 0.1 M NaOH in methanol for 1 h at 37 °C. Solvent system 1 [CHCl3/CH3OH/NH4OH, (40:10:1 by vol.)] or system 2 [CHCl3/CH3OH/0.25% KCl, (55:45:5 by vol.)] were used in ascending TLC on silica gel plates (Silica Gel 60, Merck). Radioactivity was detected and quantified by one-dimensional radioscanning (Berthold) and fluorography. Preparation and analysis of [3H]myo-inositol labelled GPI anchor lipids was done as described previously [34].
MS analysis of lipid
For supplementary Figure S1 (see http://www.BiochemJ.org/bj/401/bj4010205add.htm for details) and a series of similar experiments, exponentially growing cells were resuspended in CHCl3/CH3OH (1:1, v/v), and thoroughly disrupted by freeze/thawing and shaking with glass beads. Lipids were in glass vials throughout and were dried with nitrogen. Lipids were left untreated or were deacylated with NaOH, and were then partitioned between butanol and 0.1 mM EDTA and 5 mM Tris/HCl, pH 7. Lipids were resuspended in CHCl3/CH3OH (1:1, v/v) and analysed with a Bruker 4.7 T BioAPEX II FT/ICR mass spectrometer in the negative ion mode. Lipids were injected at various concentrations at a flow rate of 2 μl/min with a tension of −250 V at the capillary exit. For each spectrum, 20–30 scans were averaged. All tandem MS experiments were performed with argon as collision gas at a pressure of 8 mbar.
In another series of experiments, a different lipid extraction method allowing to also extract MIPCs and M(IP)2Cs and HPLC-ESI (electrospray ionization)-MS/MS technology were used. Exponentially growing cells (D600 of 1–2) were extracted as described [35], using procedure IIIA. Aliquots in CHCl3/CH3OH corresponding to 1 A600 unit of cells were heated to 65 °C for 15 min to solubilize M(IP)2C and immediately injected into a PVA SIL HPLC column (1 mm×150 mm; YMC Europe GmbH, D46514 Scherbeck, Germany), and eluted at 45 μl/min. Solvents A, hexane/propan-2-ol (98:2, v/v); B, CHCl3/propan-2-ol (65:35, v/v); and, C, CH3OH were changed linearly over time to give ratios A:B:C as follows: Start, 100:0:0; 22 min, 12:88:0; 30 min, 10:74:16; 45 min, 8:61:31; 50 through 65 min, 0:0:100; 70 through 72.5 min, 0:100:0; 75 through 82.5 min, 100:0:0. Lipids from WT (wild-type) cells grown in [13C]glucose and [12C]inositol were used as internal standards. Ions in the effluent were ionized by ESI with an electrode potential of 3500 V and the masses of negative ions were detected by a Bruker Esquire-LC ion trap mass spectrometer.
Maturation of secretory proteins
For pulse–chase analysis, cells were metabolically labelled with 35S-containing amino acids as described previously [36], with one modification: after the addition of SDS to 1% (w/v), the lysate was incubated at 95 °C for 5 min.
Membrane association assay
Cells were grown at 30 °C in YPD to exponential phase, and 10–20 A600 units were collected, washed once in water and worked up for the membrane association assay as described before [7]. Proteins were precipitated with trichloroacetic acid and analysed by reducing SDS/PAGE (7.5 % gels). Western blots were probed with rabbit anti-Pma1p (1:10000), rabbit anti-Gas1p (1:20000) or mouse anti-CPY (1:1000) antisera and AlexaFluor®680 anti-rabbit (1:5000) or AlexaFluor®680 anti-mouse (1:5000) secondary antibodies for detection with the fluorescence Odyssey Imaging system (LI-COR).
RESULTS
YPK9 4Δ (lag1Δ lac1Δ ypc1Δ ydc1Δ) cells are rescued by Lass5 expression
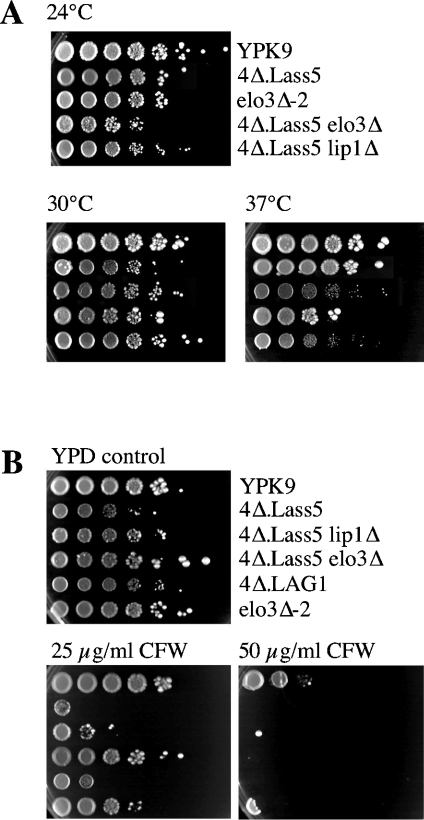
The simultaneous deletion of acyl-CoA-dependent ceramide synthases (LAG1 and LAC1) in the YPK9 background is lethal [11]. YPK9 4Δ cells harbouring LAG1 on an URA3 vector (4Δ.LAG1 cells) in which the acyl-CoA-independent ceramide biosynthesis enzymes are also deleted, could not lose their covering plasmid either (results not shown). Transfection of a single copy vector harbouring Lass5 under the control of the MET17 promoter allowed 2Δ.LAG1 and 4Δ.LAG1 cells to readily form colonies on 5-fluoro-orotic acid-containing media and hence to lose LAG1. The resulting 2Δ.Lass5 and 4Δ.Lass5 cells grew at all temperatures between 24 and 37 °C (Figure 2A). Cells grew best in YPD medium, grew reasonably in LM, but only very slowly in minimal medium and were strictly inositol auxotrophic. When the MET17 promoter was induced (about 1.75-fold) by removal of methionine from the standard growth medium, there was no effect on the growth rate. As shown in Figure 2(B), the 4Δ.Lass5 cells were hypersensitive to Calcofluor White, indicating increased synthesis of chitin. Strains containing additional deletions (4Δ.Lass5.elo3Δ, 4Δ.Lass5.lip1Δ) will be discussed below.
Figure 2. Temperature and Calcofluor White sensitivity of 4Δ.Lass5.
Serial 10-fold dilutions of cells were plated on YPD + uracil + adenine agar plates. (A) Plates were incubated at 24, 30 or 37 °C. (B) Plates contained 0, 25 or 50 μg/ml of Calcofluor White (CFW) and were incubated at 30 °C. All plates were photographed after 3 days.
Biosynthesis of sphingolipids in 4Δ.Lass5 cells
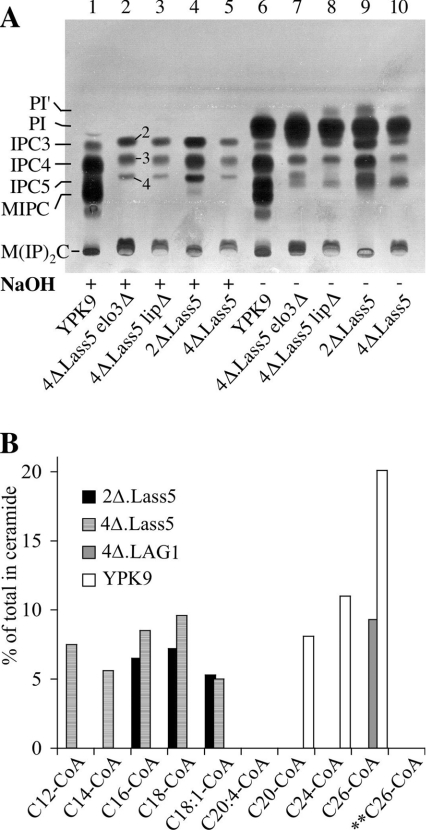
To understand whether 4Δ.Lass5 cells had the ability to make sphingolipids, the cells were labelled with [3H]inositol and lipid extracts were analysed by TLC. As shown in Figure 3(A), 4Δ.Lass5 cells made several base resistant lipids that migrated in a similar manner to the IPCs and MIPC of WT cells, but with slightly different Rf values. The ensuing HPLC–ESI-MS analysis, however, suggested that the 4Δ.Lass5 sphingolipids co-migrating with WT IPCs, having a total of three, four or five hydroxyl groups in their ceramide moiety (IPC3, IPC4 and IPC5), are in fact IPC2, IPC3 and IPC4 forms respectively, which migrate less than the similarly hydroxylated WT species because they contain shorter fatty acids than WT IPCs (see below). Figure 3(A) and Table 3 also show that 4Δ.Lass5 cells make reduced amounts of sphingolipids in metabolic labelling experiments, which is also true for 4Δ.Lass5.elo3Δ and 4Δ.Lass5.lip1Δ (discussed further below). The specificity of the Lass5-dependent ceramide synthesis was explored further in microsomal ceramide synthase assays. As shown in Figure 3(B), ceramides were synthesized in the presence of C12:0–, C14:0–, C16:0–, C18:0– or C18:1–CoA, but not with C20–, C24:0– or C26:0–CoA. The last two are the preferred substrates of yeast LAG1- and LAC1-, and human LASS1-, LASS2- and LASS4-dependent ceramide synthases, when assayed in yeast microsomes under the same conditions [25] (Table 1). Thus, it appears that the fatty acid specificity of Lass5 overexpressed in human and yeast cells is very similar (Table 1).
Figure 3. 4Δ.Lass5 make abnormal IPCs and use C16– and C18–CoA for ceramide synthesis.
(A) The indicated strains were labelled with [3H]inositol, washed, and lipids (plus intracellular free [3H]inositol) were extracted. Aliquots of extract corresponding to 106 c.p.m. (counts per min) were treated with NaOH (lanes 1–5) or control incubated (lanes 6–10) and desalted lipids were separated by TLC in solvent system 2. IPC2, IPC3, IPC4 and IPC5 and numbers 2, 3 and 4 next to lane 2 designate types of IPC, which contain a total number of 2 to 5 hydroxy groups in their ceramide moiety. The numbers next to lane 2 are rendered likely by HPLC–ESI-MS/MS results (Figure 4 and supplementary Figure S1). (B) Microsomes of 4Δ.Lass5 were radiolabelled for 2 h with [3H]DHS in the presence of the indicated acyl-CoAs (0.1 mM) or free fatty acids (0.1 mM) for control. The radiolabelled lipids were extracted, analysed by TLC using solvent system 1 and quantitated by radioscanning. Counts present in ceramide were given as the percentage of total counts present in the lane. No ceramides were made when microsomes were incubated with free fatty acids (results not shown). ** Tubes containing boiled microsomes were used as a further control.
Table 3. Incorporation of [3H]inositol into sphingolipids.
The experiment shown in Figure 3(A) was performed two to seven times and the radioactivity of sphingolipids (as percentage of total labelled lipids) and of individual IPC types (as percentage of total IPCs) were calculated on the basis of radioscans of the TLC plates.
| Strain | No. of experiments | Sphingolipids (% of lipids) | IPC2 (% of IPCs) | IPC3 (% of IPCs) | IPC4 (% of IPCs) |
|---|---|---|---|---|---|
| 2ΔLass5 | 2 | 22.6±7.2 | 46.9±3.5 | 36.8±1.4 | 16.3±2.2 |
| 4ΔLass5 | 5 | 11±4.1 | 46.3±2.6 | 42.2±2.8 | 11.5±1.8 |
| 4ΔLass5 elo3Δ | 5 | 13.8±4.7 | 46.9±5.2 | 38.9±5.2 | 14.2±2.2 |
| 4ΔLass5 lip1Δ | 5 | 10.3±2.3 | 50.9±2.2 | 38.5±2.0 | 10.5±1.8 |
| YPK9 | 7 | 64.4±11.9 | - | - | - |
Sphingolipid profile of 4Δ.Lass5 cells in HPLC-ESI-MS
To analyse further what sphingolipids the yeast cells could make, when they depended on a LCFA-specific ceramide synthase such as Lass5 and on endogenous acyl-CoA biosynthesis, the lipids of exponentially growing cells were extracted and analysed by HPLC–ESI-MS (supplementary Figure S1). In all experiments of this type, IPCs of WT cells contained base resistant m/z peaks corresponding to IPCs with 42 or 44 C atoms in their ceramide moiety. Since most yeast sphingolipids contain LCBs with 18 C atoms, this suggests that the IPCs of YPK9 contained the usual C24 and C26 fatty acids. In contrast, the main IPCs of 2Δ.Lass5 and 4Δ.Lass5 contained 34 or 36 C atoms suggesting that they contained C16 and C18 fatty acids (supplementary Figures S1A and S1B). On the other hand, the profile of glycerophospholipids was similar in WT and 4Δ.Lass5 (supplementary Figure S1A). The most abundant IPC of 4Δ.Lass5 and 2Δ.Lass5 cells (IPC16-2 in supplementary Figure S1) contains DHS, although previous analysis of lipids in WT cells shows that IPCs contain PHS, not DHS [2,37]. However, DHS is the natural substrate for mammalian ceramide synthases [38]. The relative predominance of IPC2 over the more polar IPC3 and IPC4 species (supplementary Figure S1) is also reflected in Figure 3A and Table 3, where the most hydrophobic (farthest migrating) species are quantitatively major, thus allowing the tentative assignments shown in Figure 3A, lane 2. Furthermore, the predominance of C16 and C18 fatty acids in IPCs of 4Δ.Lass5 cells was in close agreement with the specificity of the activity measured in microsomes (Figure 3B).
Sphingolipid profile of 4Δ.Lass5 cells in HPLC-ESI-MS/MS
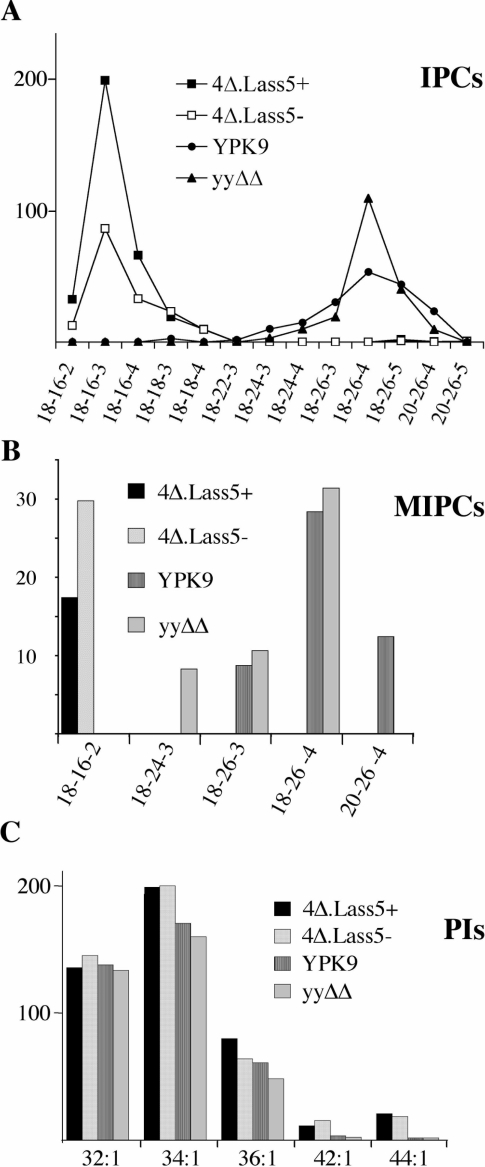
The ESI-MS results were confirmed by a further series of experiments using HPLC-ESI-MS/MS to reduce the risk of ion suppression. Thereby we also used a different lipid extraction protocol designed for quantitative extraction of all classes of yeast sphingolipids and at the same time compared 4Δ.Lass5 with ypc1Δydc1Δ, a strain that is more closely related to 4Δ.Lass5 than the YPK9 WT strain. Figure 4 presents selected data from several experiments yielding very similar results, and again shows that most IPCs and MIPCs of 4Δ.Lass5 contained between 34 and 36 carbon atoms and two, three or four hydroxy groups in their ceramide moiety (labelled 18-16-2, 18-16-3, 18-16-4, 18-18-3 and 18-18-4 to indicate the probable number of carbon atoms in the LCB, C atoms of the fatty acid and the sum of hydroxyls in the ceramide moiety respectively). Greater than 97% of IPCs and MIPCs of YPK9 and ypc1Δydc1Δ control cells contained fatty acids with 22 to 26 carbon atoms. In Figure 4(A), lipid extracts from WT cells grown in [13C]glucose were used as internal standards and HPLC-ESI-MS/MS analysis detected the corresponding [13C]IPC peaks of similar magnitude in all runs. This shows that the striking absence in 4Δ.Lass5 cells of IPC18–24, IPC18–26 and IPC20–26 cannot be due to some specific ion suppression in this strain. While ceramide moieties of most IPCs of 4Δ.Lass5 contained C16 and C18 fatty acids, small amounts of IPCs with VLCFAs were still present (barely noticeable in Figure 4A). To quantitate them we used high concentrations of mild alkaline treated lipids: IPC18-26-5 and 20-26-5 amounted to 0.6% and 0.22% of total detectable IPCs respectively.
Figure 4. Sphingolipids from 4Δ.Lass5 cells contain mainly C16:0 and C18:0 fatty acids by HPLC–ESI-MS/MS.
All sphingolipid classes were extracted quantitatively [35] from cells growing exponentially in LM at 30 °C and analysed by HPLC–ESI-MS/MS. YPK9, ypc1Δ ydc1Δ (yyΔΔ) and 4Δ.Lass5 cells were all grown with methionine (4Δ.Lass5+), 4Δ.Lass5 also without methionine (4Δ.Lass5−). Parent ion and fragment ion masses (m/z) were recorded throughout the HPLC run and were screened for all theoretically possible sphingolipids containing different saturated, monounsaturated, mono- or di-hydroxylated fatty acids combined with DHS or PHS as well as for conventional phospholipids [40]. The vertical axis in all panels gives the ion counts in thousands. All species shown in (A) were identified as phosphoinositides by characteristic fragment ions at m/z 259 and 241, corresponding to inositolphosphate and inositolphosphate–H2O, except for those that were less than 1% of the most predominant IPC. However, the LCB could not be identified by fragmentation and sphingolipids were annotated by three consecutive numbers to indicate the probable number of carbon atoms in the LCB, the probable number of carbon atoms in the fatty acid, and the sum of hydroxy groups in the ceramide moiety respectively. (A) The lipid extracts were mixed with lipid extracts from WT cells grown in [13C]glucose as the only carbon source and the results were normalized using this internal standard. The normalized number of ions derived from IPCs present in extracts of 4Δ.Lass5+, 4Δ.Lass5− and ypc1Δ ydc1Δ amounted to 183%, 92% and 106% of the WT (YPK9) respectively. (C) The PIs are specified by two consecutive numbers indicating the total number of carbon atoms and double bonds present in the two fatty acids respectively. The Figure shows one representative of several experiments yielding very similar results.
The only MIPCs we found in 2Δ.Lass5 and 4Δ.Lass5 cells contained DHS–C16:0, whereas in WT or ypc1Δydc1Δ only MIPCs with three or four hydroxy groups were detectable (Figure 4B). Thus, the mannosyltransferases Csg1p and Csh1p are able to act on C16- and DHS-containing IPCs (IPC1816-2) while their physiological substrates probably are IPC18-26-3 and IPC18-26-4 (Figure 4B) [39,40]. The predominance of MIPC18-16-2 over other MIPCs in 4Δ.Lass5 cells may reflect a relative abundance of IPC18-16-2, but it cannot be ruled out that in 4Δ.Lass5 cells Csg1p and Csh1p prefer IPC18-16-2 to other abnormal substrates or that the specificity of these enzymes is changed as a consequence of the abnormal sphingolipid content of surrounding membranes.
Yeast harbours Ole1p (OLEic acid requiring), a desaturase producing C16:1–CoA and C18:1–CoA and a majority of the fatty acids in glycerophospholipids are unsaturated. Since C18:1 was a good substrate for Lass5 in vitro (Figure 2B), we expected to see subtantial amounts of unsaturated fatty acids in the IPCs of 4Δ.Lass5 cells. Yet, while mono-unsaturated fatty acids were predominant in PI and PE (phosphatidylethanolamine), as expected (supplementary Figure S1), the most prominent unsaturated IPC of 4Δ.Lass5 cells (m/z=810 for IPC18-16-4 with one double bond) only amounted to 3.3% of the corresponding saturated species (m/z=812). Also, we could not detect any ceramides with unsaturated fatty acids in 4Δ.Lass5 cells and the profile of ceramides in these cells closely resembled the ceramides that were found in IPCs (results not shown). Thus, Lass5 does not appear to use unsaturated fatty acids in vivo.
C24 fatty acids remain essential in 4Δ.Lass5 cells
Overall, the HPLC-ESI-MS/MS results showed that 4Δ.Lass5 cells made large amounts of sphingolipids, but that most of them did not contain VLCFAs. In this context, we wondered if the VLCFAs were still required for cell survival. We therefore tried to delete the ELO3 and TSC13 genes (Figure 1). Clones with the correct replacement of ELO3 by KanMX4 were obtained easily. In liquid YPD medium, the 4Δ.Lass5 elo3Δ cells grew much more slowly than 4Δ.Lass5, but they grew better in LM. On agar plates, however, 4Δ.Lass5 elo3Δ cells grew as well as 4Δ.Lass5 cells (Figure 2). Metabolic labelling indicates that 4Δ.Lass5 elo3Δ cells incorporate [3H]inositol into sphingolipids as fast as 4Δ.Lass5 cells (Figure 3A, lane 2 compared with lane 5; Table 3). In contrast, an attempt to replace TSC13 with KanMX4 in 4Δ.Lass5 failed, in that 12 out of 12 clones growing on the selective medium still contained TSC13. This suggests that, although not present in sphingolipids, the VLCFAs continue to fulfil some vital function in 4Δ.Lass5. This function possibly is provided by PI′, a PI form having C26:0. PI(44:1) and PI(42:1) containing C26 in sn-1 have been described as a minor fraction, making up less than 1% of total PI in WT cells [41]. PI′ is increased in extracts of [3H]inositol labelled 4Δ.Lass5 cells (Figure 3A, lanes 6–10) and in the HPLC-ESI-MS/MS lipid profile, 4Δ.Lass5 cells contain 10-fold more PI(44:1) (m/z=975.5) and 3-fold more PI(42:1) (m/z=947.7) than WT cells (Figure 4C), while no corresponding species were found for PE and PS (phosphatidylserine; results not shown). Fragmentation showed that the peak at 975.5 contained C26:0 and C18:1, and that the peak at 947.7 contained C26:0. During fragmentation the m/z peak 975 lost C18:1 much more readily than C26:0, indicating that C18:1 is in position sn-2 and C26:0 in sn-1.
4Δ.Lass5 cells transport Gas1p from ER to Golgi at an almost normal rate
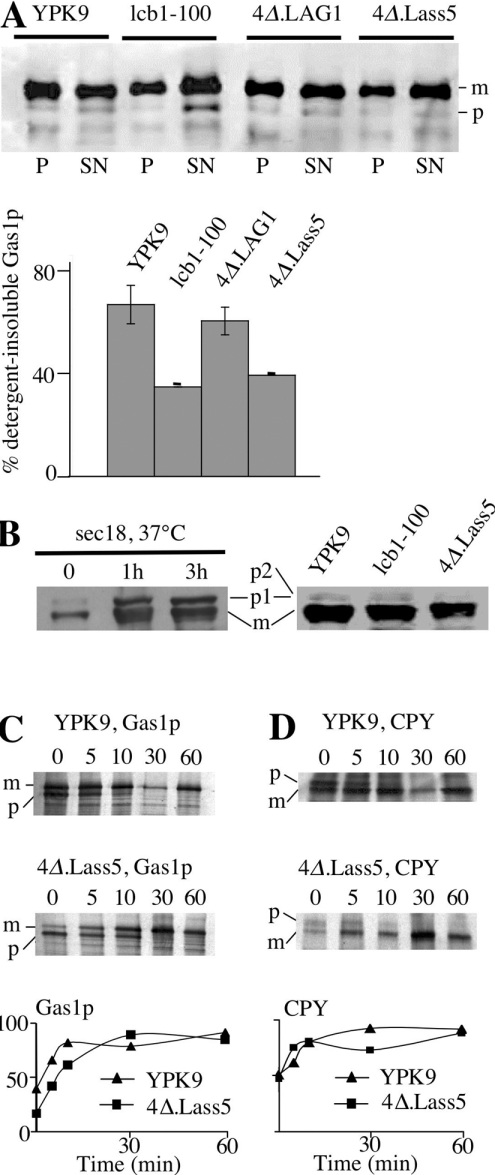
The transport of GPI proteins from ER to Golgi depends on ongoing ceramide synthesis and the most popular hypothesis proposes that sphingolipids are required for the formation of microdomains or rafts, into which GPI proteins have to get integrated prior to their incorporation into transport vesicles ([5,7,8] and discussed in [42]). Sphingolipids are necessary for raft formation and can be assumed to have an influence on the membrane thickness of lipid rafts, as yeast sphingolipids normally contain C26 and C24 fatty acids. We therefore investigated if the C16- and C18-containing sphingolipids of 4Δ.Lass5 could still form functional rafts and assist GPI protein export from the ER in a normal way. Raft association of Gas1p is reflected by its resistance to Triton X-100 extraction at 4 °C [7,8]. Of the mature form of Gas1p, 62% was Triton X-100 resistant in WT cells, whereas only 36% mature Gas1p was detergent insoluble in 4Δ.Lass5 (Figure 5A). For comparison, the corresponding percentage was only 32% in the temperature-sensitive lcb1-100 strain grown at 24 °C on rich medium, from which it could take up some LCBs (Figure 5A). In addition, the blots showed an abnormal accumulation of immature Gas1p in lcb1-100, but not in 4Δ.Lass5 cells. Blotting with anti-CPY indicated that there is no accumulation of the ER and Golgi proforms p1 and p2 in 4Δ.Lass5, whereas its ER form p1 readily accumulated in sec18 cells at restrictive temperature (37 °C) [43] (Figure 5B). Pulse–chase experiments detect a delay in Gas1p transport in a more sensitive manner than blotting experiments. In a pulse–chase experiment, Gas1p was transported at a slightly reduced rate from the ER to the Golgi in 4Δ.Lass5, whereas CPY was transported normally (Figures 5C and 5D). The retardation of Gas1p transport was, however, only very small in comparison with the dramatic delay revealed by pulse–chase experiments in elo3Δ or myriocin-treated cells [5,9]. Also, no accumulation of the immature 105 kDa form of Gas1p was observed by Western blotting in extracts of elo3Δ-2 or 4Δ.Lass5 elo3Δ cells, in three experiments (results not shown). These results indicate that the ceramides and IPCs of 4Δ.Lass5 allow for a relatively efficient transport of Gas1p from the ER to the Golgi, although the incorporation or retention of Gas1p in rafts is somewhat compromised. As the thickness of membranes might influence sorting in the secretory pathway [44,45], we tested for secretion of the vacuolar hydrolase CPY, the sorting of which is dependent on the correct sorting of the integral membrane protein Vps10p (carboxypeptidase Y-deficient 10). 4Δ.Lass5 cells did not secrete CPY as determined by Western blotting, and also did not secrete Kar2p (karyogamy 2), an ER chaperone that is secreted when cells react to stress by an unfolded protein response [46] (results not shown). The plasma membrane H+-ATPase Pma1p, a classical raft marker, was found to be completely resistant to extraction with Triton X-100 in 4Δ.Lass5 cells grown at 30 °C, indicating raft association, but there was approx. 3-fold less Pma1p compared with WT, suggesting that the rafts of 4Δ.Lass5 cells are only partially achieving Pma1p stabilization at 30 °C (results not shown).
Figure 5. Raft association and transport of Gas1p in 4Δ.Lass5.
(A) 4Δ.Lass5 was grown in LM (His−, Met−), 4Δ.LAG1 in LM (−ura) containing galactose instead of glucose, YPK9 and lcb1-100 in YPD. All cells were grown at 30 °C except for lcb1-100, which was kept at 24 °C. Exponentially growing cells were lysed with glass beads and aliquots of lysate were solubilized in Triton X-100 for a membrane association assay (see the Materials and methods section). Triton X-100 lysates were subjected to ultracentrifugation, the soluble (SN) and the pellet (P) fractions were processed for Western blotting using anti-Gas1p (A) or anti-CPY antibodies (B), allowing the detection of mature (m) and precursor (p) forms. (A) Quantitation of results of top panel and of a second identical experiment is also shown. In (B) sec18Δ cells were incubated at 37 °C for 0, 1 and 3 h before lysis. For kinetic analysis of Gas1p (C) and CPY (D) maturation, yeast cells were metabolically labelled with [35S]methionine and [35S]cysteine, the label was chased for 0–60 min and Gas1p and CPY were immunoprecipitated from cell extracts, resolved by SDS/PAGE, and detected by phosphoimaging and by fluorography. Relative amounts of mature proteins are indicated in bottom panels.
4Δ.Lass5 cells remodel the lipid moieties of GPI proteins normally
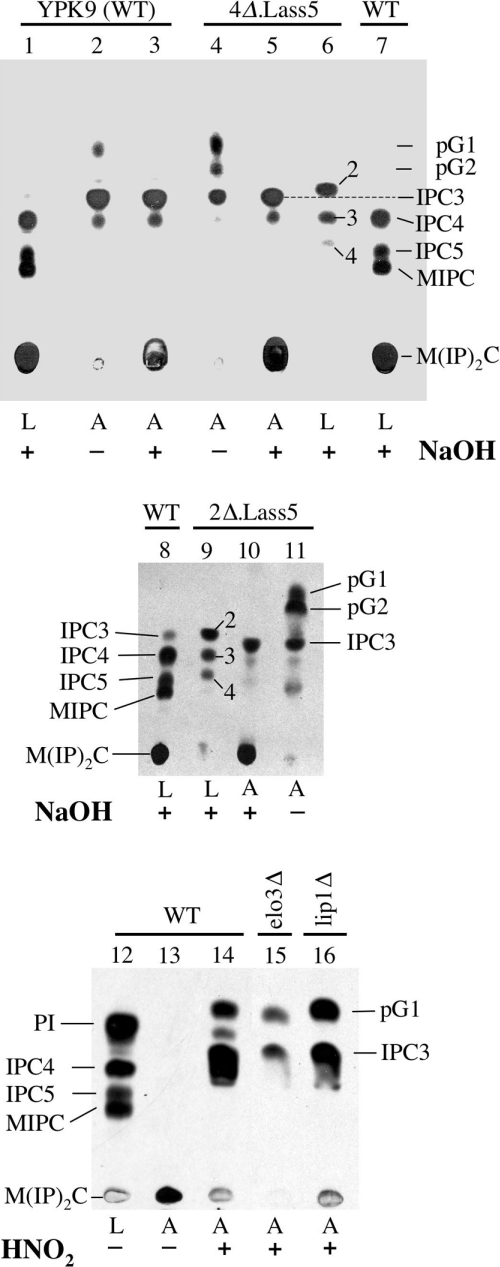
The lipid moieties of mature GPI-anchors do not usually contain the diacylglycerol, which is present on the GPI lipid at the stage when it is transferred by the transamidase to GPI proteins, and which probably contains the C16 and C18 fatty acids typical of yeast PI. Most mature GPI proteins of yeast contain a ceramide moiety, whereas a minor fraction contains a modified diacylglycerol containing C26:0 in sn-2 [47,48]. Yet, certain yeast mutants do not incorporate any ceramides in their GPI anchors [49]. We thus wondered if GPI anchors of 4Δ.Lass5 cells contained any ceramides. For this, cells were labelled with [3H]inositol and the labelled lipid moiety of GPI anchors was analysed by TLC. As shown in Figure 6 (lane 2), the anchors of WT cells contained the usual pG1, a form of PI having C26:0 in sn-2, IPC3 (containing PHS–C26:0) and minor amounts of IPC4 (containing PHS–C26:0-OH) [33,48]. pG1 was sensitive to mild alkaline conditions, IPCs were not, as expected (Figure 6, lanes 2 and 3). Very surprisingly, the profile of anchor lipids of 4Δ.Lass5 was normal. The presence of some not yet remodelled pG2 is also occasionally seen in WT cells (Figure 6, lanes 4 and 5). The anchor-derived IPCs of 4Δ.Lass5 cells migrated differently from the free IPCs isolated from the very same cells (Figure 6, compare lane 5 with lane 6). The same difference between anchor-derived and free IPCs was also found in 2Δ.Lass5 cells (Figure 6, lanes 9 and 10). Thus, in spite of making large amounts of abnormal IPCs, 4Δ.Lass5 cells seem to incorporate normal ceramides into their GPI anchors. It may be that the GPI ceramide remodelase exclusively utilizes the small amounts of C26-containing ceramides, which also are present in the small amounts of IPC18-26-5 and IPC20-26-5 of 4Δ.Lass5 cells (see above).
Figure 6. GPI anchor lipids of WT and mutant 4Δ.Lass5 cells are similar.
Labelling of 25 A600 units of YPK9 (WT), 4Δ.Lass5, 2Δ.Lass5, 4Δ.Lass5 elo3Δ (lane 15) and 4Δ.Lass5 lip1Δ (lane 16) cells for 2 h with 100 μCi of [3H]inositol. Free lipids were extracted, GPI proteins were completely delipidated and their lipid moieties were liberated using HNO2, with the exception of lane 13. Free lipids (L, lanes 1, 6–9 and 12) and liberated anchor lipids (A, lanes 2–5, 10, 11 and 13–16) were treated with NaOH (+) or mock incubated (−) for deacylation, separated by TLC and exposed for fluorography. IPCs were annotated as in Figure 3(A).
Lip1p is not required for Lass5p-dependent ceramide synthase activity in yeast
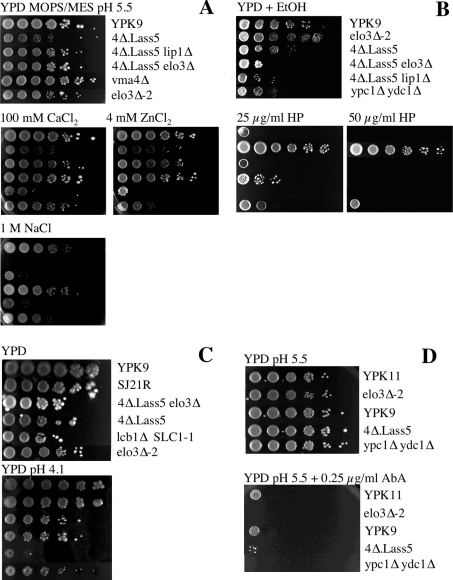
Lag1p and Lac1p interact with each other and with a third membrane protein, Lip1p, the latter being equally required for acyl-CoA-dependent ceramide biosynthesis [15]. To see if Lip1p is required for acyl-CoA-dependent ceramide synthase activity of the LAG1 homologue Lass5, we tried to delete the essential LIP1 gene in 4Δ.Lass5 cells. Transformation of the KanMX4-containing deletion cassette yielded numerous geneticin resistant clones and subsequent PCR reactions confirmed that in some of them KanMX4 had successfully replaced LIP1 (results not shown). Thus, replacement of LAG1 and LAC1 by Lass5 renders LIP1 non-essential. As shown in Figure 3(A), the sphingolipid profile of 4Δ.Lass5 lip1Δ was similar to the one of 4Δ.Lass5 (Figure 3A, lanes 3 and 5), and quantitation by radioscanning of TLC plates in several experiments of the type shown in Figure 3(A) showed that the incorporation of [3H]inositol into sphingolipids in 4Δ.Lass5 lip1Δ cells was not slower than in 4Δ.Lass5 cells (Table 3). Moreover, the relative amounts of the three mild alkaline resistant lipid species observed in [3H]inositol-labelled lipid extracts of 4Δ.Lass5 and 4Δ.Lass5 lip1Δ were similar (Table 3). Lip1p was also not required for the incorporation of sphingolipids into GPI anchors (Figure 6, lane 16). Although 4Δ.Lass5 lip1Δ cells were slightly thermosensitive at 37 °C (Figure 2A), they were otherwise as robust as 4Δ.Lass5 cells (Figure 7A), in keeping with the idea that the main function of Lip1p is to stabilize yeast Lag1p and Lac1p.
Figure 7. Phenotypic analysis of 4Δ.Lass5 cells under various stress conditions.
Serial 10-fold dilutions of cells were plated on buffered (A and D) or unbuffered YPD + uracil + adenine plates containing the indicated ingredients. Plates were then incubated at 30 °C and photographed after 3 days except for panel (D), where they were grown at 24 °C for 3 days. 4Δ.LAG1 cells were grown in YPG before being plated on YPD, where they strongly reduce sphingolipid biosynthesis but, nevertheless, continue to grow for several days. The vma4Δ cells served as a positive control and exhibited the same Vma− phenotype as described for vma2Δ and vma13Δ [23].
Several phenotypes of elo3Δ and lcb1 mutants are not present in 4Δ.Lass5 cells
The fact that TSC13 could not be deleted in 4Δ.Lass5 cells suggested that VLCFAs continued to play an essential role in yeast cells, even though most of them were no longer associated with sphingolipids. In an attempt to identify functions, which LCBs and VLCFAs may only be able to exert, when combined with each other, we monitored 4Δ.Lass5 cells for the presence of several of the well-documented abnormal phenotypes of elo3Δ or lcb1Δ cells. The V1 subunit of the vacuolar H+-ATPase was reported to be defective in elo3Δ cells and this resulted in a set of phenotypes referred to as Vma−, which comprises a reduced uptake of quinacrine into vacuoles, calcium and zinc hypersensitivity and inability of cells to grow at high pH or on glycerol [23]. Furthermore, lcb1Δ SLC1-1 cells have difficulty to grow in high salt, low pH or at 37 °C [50]. We thus tested the growth of 4Δ.Lass5 cells under these conditions. As shown in Figure 7(A), elo3Δ-2, carrying deletion of ELO3 in the YPK9 background, were hypersensitive to Zn2+ but not to Ca2+; the 4Δ.Lass5 cells were not hypersensitive to these two cations and deletion of ELO3 in this background did not cause any cation hypersensitivity (Figure 7A). Thus, for elo3Δ cells to exhibit Zn2+ hypersensitivity, the VLCFA and LCB may have to be linked, and it may be the predominance of C22- and C24-containing sphingolipids, rather than the absence of C26-containing sphingolipids that affects the function of the vacuolar H+-ATPase of elo3Δ-2 cells [23]. Also, in the YPK9 background, ELO3 was not required for the accumulation of quinacrine in vacuoles. The 4Δ.Lass5 and 4Δ.Lass5 elo3Δ cells also accumulated quinacrine quite normally, although there was more heterogeneity in vacuolar morphology and quinacrine uptake in those mutants than in the YPK9 WT (results not shown). Similarly, neither elo3Δ-2 nor 4Δ.Lass5 cells were found to be sensitive to pH 6.7, and at higher pH (7.3) not only 4Δ.Lass5, but also the parental YPK9 cells showed reduced growth (results not shown). Thus, apart from Zn2+ hypersensitivity, we were unable to detect Vma− phenotypes in elo3Δ mutants generated in either the YPK9 or BY4742 background and no Vma− phenotypes were present in 4Δ.Lass5 cells or 4Δ.Lass5 elo3Δ cells.
As for lcb1 phenotypes, 4Δ.Lass5 cells grew well at low pH (4.1; Figure 7C) and at 37 °C (Figure 2A), but they grew badly on 1M NaCl (Figure 7A). Moreover, they were Calcofluor White hypersensitive (Figure 2B), but they were not more sensitive to SDS than WT cells (results not shown), indicating that they were not osmotically fragile [51]. Thus, 4Δ.Lass5 cells expressed some but not all of the phenotypes described in lcb1Δ SLC1-1 cells. Remarkably, the salt and Calcofluor White sensitivity of 4Δ.Lass5 was rescued by deletion of ELO3 (Figures 2B and 7A), suggesting that under stress conditions C26:0 fatty acids, which cannot be integrated into sphingolipids, exert toxic effects that are not seen with C22:0 and C24:0 fatty acids.
Deletion of ELO3 provides resistance to haloperidol in WT as well as 4Δ.Lass5 cells
Deletion of ELO3 has also been reported to confer resistance to SR31747 and fenpropimorph, two inhibitors of the ergosterol biosynthesis enzymes Erg2p and Erg24p, and to abolish the growth defect of erg2Δ mutants [21,22]. As SR31747 was not available to us, we used haloperidol, another specific inhibitor of Erg2p [52]. As shown in Figure 7(B), elo3Δ-2 cells indeed were resistant to haloperidol, while 4Δ.Lass5 cells were as sensitive as the WT strain. The ypc1Δ ydc1Δ strain was also more resistant than WT cells, but far less than elo3Δ-2 cells. The elimination of ELO3 from 4Δ.Lass5 cells significantly increased their haloperidol resistance. The result suggests that C26:0 fatty acids are inhibitory to the growth of erg2Δ, even if they are not present in sphingolipids, while C22 and C24:0 fatty acids are better tolerated by erg2Δ.
The 4Δ.Lass5 cells were sensitive to aureobasidine A, an inhibitor of IPC synthase Aur1p, as were WT and ypc1Δ ydc1Δ cells (Figure 7D). This suggests that the proposed toxicity of ceramides [13] is exerted not only by the natural VLCFA-ceramides but also by C16- and C18-ceramides. The proposed toxicity of ceramides can also explain the aureobasidine A hypersensitivity of ypc1Δ ydc1Δ cells (Figure 7D).
Bud-site selection has been reported to be deficient in both diploid and haploid elo3Δ cells [24]. While the presence of this defect was confirmed for the strain used by Ni et al. [24], we could not find a similar effect of ELO3 deletion in the background of YPK11 (see supplementary Figure S2 at http://www.BiochemJ.org/bj/401/bj4010205add.htm; see elo3Δ-2), even when growing cells at 37 °C (results not shown). Also, neither 4Δ.Lass5 nor 4Δ.Lass5 elo3Δ cells showed the defect.
Globally, it looks as if several of the described phenotypes of elo3Δ cells do not manifest themselves in the YPK9 background, and that of those that are present, Zn2+ hypersensitivity and haloperidol resistance, only the latter is also observed when ELO3 is deleted in the 4Δ.Lass5 background.
DISCUSSION
Our study was initiated to confirm that the fatty acyl-CoA specificity of the acyl-CoA-dependent ceramide synthase activities is determined by the Lag and Lass proteins, and not by some other proteins. This idea was supported by the finding that overexpression of different Lass proteins in mammalian cells resulted in ceramide synthase activities with preferences for acyl-CoAs of different chain length and the analogous findings obtained through the expression of different human LASS proteins in yeast (Table 1). The idea received further support through two recent studies showing that the detergent-purified yeast Lag1p–Lac1p–Lip1p complex, as well as purified murine Lass5 protein exhibit the same preferences for fatty acid chain lengths that were observed in microsomal assays [15,53]. It, nevertheless, still seems possible that the membrane environment could also influence the specificity of ceramide synthases. Indeed, the Lag1 complex could only be obtained in an active form when solubilized by digitionin [15], a detergent that is not apt to remove all lipids from membrane proteins. Similarly, the activity of purified Lass5 was strongly enhanced by the addition of glycerophospholipids, whereby it is not known if the fatty acid composition of added lipids has any influence on the acyl-CoA specificity of the enzyme [53]. In this context, it is interesting to note that Lass5 expressed in yeast cells exhibits a very similar specificity in vitro and in vivo as was measured for the same enzyme expressed in mammalian HEK-293 T cells (Figure 3B; Table 1). (In vivo we did not find C12- and C14-sphingolipids in 4Δ.Lass5 cells, probably because C12– and C14–CoA are present in very low concentrations in living cells). Thus, LAG1 and Lass5 expressed in the very same background, induce ceramide synthases with very different fatty acids specificities. However, even this finding cannot definitively eliminate a role of the membrane environment in fatty acid specificity of ceramide synthases, since the very absence of Lag1p in 4Δ.Lass5 cells may alter the membrane environment in the ER where these enzymes act. Analysis of the acyl-CoA specificity of purified enzyme reconstituted into different lipid environments may shed further light on this question. The abnormally high proportion of DHS-based sphingolipids in 4Δ.Lass5 cells suggests that Lass5 also somehow dictates the LCB specificity of the ceramide synthase.
It should be noted, that of the five murine LAG1 homologues, Lass5 is the only one able to utilize oleic acid (Δ9-C18:1) [27,54]. Oleyl–CoA was efficiently utilized by Lass5 present in yeast microsomes (Figure 3B), but C18:1-CoA was apparently not utilized very efficiently in living cells, since no PHS-C18:1 and DHS-C18:1 ceramides were detected (results not shown). It still is possible that PHS-C18:1 and DHS-C18:1 were degraded rapidly. Such degradation could however only be operated by ceramidases unrelated to Ypc1p and Ydc1p, as these enzymes were deleted in 4Δ.Lass5 cells. Indeed, the present study did not reveal any role of these ER based alkaline ceramidases in determining the fatty acid profile of sphingolipids, since the same spectrum of IPCs was present in YPK9 WT and ypc1Δ ydc1Δ cells (Figure 4) and similarly, no major differences were observed between 2Δ.Lass5 and 4Δ.Lass5 cells (see supplementary Figures S1A and S1B). However, hypersensitivity of ypc1Δ ydc1Δ cells to aureobasidine A (Figure 7D), suggests that Ypc1p and Ydc1p allow degrading excess ceramides in the ER.
While Lip1p is an essential component of the Lag1p–Lac1p complex in yeast, there is no homologous or analogous protein presently known for mammals. An enzymatically active, affinity-purified Lass5p preparation consisted of Lass5p and only trace amounts of other proteins, as judged by SDS/PAGE and Silver Nitrate staining [53]. The present study has led to the conclusion that Lass5 does not require additional subunits for its activity, a conclusion which is further supported by our finding that LIP1 can be deleted in 4Δ.Lass5 cells without any apparent consequences for sphingolipid biosynthesis. Indeed, the 4Δ.Lass5 lip1Δ cells incorporated as much [3H]inositol into sphingolipids as 4Δ.Lass5 cells (Figure 3A, lane 3 compared with lane 5; Table 3). This finding also suggests that Lip1p is not required for protecting the ceramides from degradation, for channelling of ceramides into secretory vesicles or for other ceramide-related functions that could only be required specifically in yeast. Thus, the function of Lip1p seems to be intimately related to the Lag1p–Lac1p enzyme itself, but other unrelated functions cannot be excluded at this stage. A similar conclusion was reached in a recent report showing that murine Lass3 can rescue lip1Δ cells [54].
Our study clearly indicates that C22:0 or longer fatty acids play a crucial role for cell survival, even if these fatty acids can be integrated into sphingolipids only very inefficiently. Several results also suggest that VLCFAs, when outside the normal sphingolipid context, can have adverse effects, and this is much more pronounced for C26:0 than for C22:0 and C24:0 fatty acids. Thus, C26:0 seems to become toxic when 4Δ.Lass5 cells are stressed by NaCl (Figure 7A), when they are exposed to Calcofluor White (Figure 2B) and also when the ergosterol C8 isomerase Erg2p is inhibited (Figure 7B).
We believe that 4Δ.Lass5 cells will be useful for dissecting the sphingolipid-dependent signal transduction pathways in yeast further, as well as for a better understanding of the role of sphingolipids and VLCFAs in forming the membrane structure. 4Δ.Lass5 cells may become useful, not only for the analysis of other ceramide synthases, but also for the study of other mammalian enzymes involved in the biosynthesis of complex sphingolipids, since the ceramide profile of 4Δ.Lass5 cells is similar to the one found in various human tissues.
Online data
Acknowledgments
We would like to thank Fredy Nydegger (Department of Chemistry, University of Fribourg, Fribourg, Switzerland) for excellent and expert HPLC–ESI-MS/MS work. This project was supported by a grant from the Swiss National Science Foundation (31-67188.01) and a donation from the Novartis Stiftung für Medizinisch-Biologische Forschung, Basel, Switzerland.
References
- 1.Dickson R. C., Sumanasekera C., Lester R. L. Functions and metabolism of sphingolipids in Saccharomyces cerevisiae. Prog. Lipid Res. 2006;45:447–465. doi: 10.1016/j.plipres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Smith S. W., Lester R. L. Inositol phosphorylceramide, a novel substance and the chief member of a major group of yeast sphingolipids containing a single inositol phosphate. J. Biol. Chem. 1974;249:3395–3405. [PubMed] [Google Scholar]
- 3.Oh C. S., Toke D. A., Mandala S., Martin C. E. ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J. Biol. Chem. 1997;272:17376–17384. doi: 10.1074/jbc.272.28.17376. [DOI] [PubMed] [Google Scholar]
- 4.Sims K. J., Spassieva S. D., Voit E. O., Obeid L. M. Yeast sphingolipid metabolism: clues and connections. Biochem. Cell Biol. 2004;82:45–61. doi: 10.1139/o03-086. [DOI] [PubMed] [Google Scholar]
- 5.Horvath A., Sutterlin C., Manning-Krieg U., Movva N. R., Riezman H. Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J. 1994;13:3687–3695. doi: 10.1002/j.1460-2075.1994.tb06678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skrzypek M., Lester R. L., Dickson R. C. Suppressor gene analysis reveals an essential role for sphingolipids in transport of glycosylphosphatidylinositol-anchored proteins in Saccharomyces cerevisiae. J. Bacteriol. 1997;179:1513–1520. doi: 10.1128/jb.179.5.1513-1520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe R., Funato K., Venkataraman K., Futerman A. H., Riezman H. Sphingolipids are required for the stable membrane association of glycosylphosphatidylinositol-anchored proteins in yeast. J. Biol. Chem. 2002;277:49538–49544. doi: 10.1074/jbc.M206209200. [DOI] [PubMed] [Google Scholar]
- 8.Bagnat M., Keranen S., Shevchenko A., Shevchenko A., Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenkolb M., Zenzmaier C., Leitner E., Schneiter R. A specific structural requirement for ergosterol in long-chain fatty acid synthesis mutants important for maintaining raft domains in yeast. Mol. Biol. Cell. 2002;13:4414–4428. doi: 10.1091/mbc.E02-02-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barz W. P., Walter P. Two endoplasmic reticulum (ER) membrane proteins that facilitate ER-to-Golgi transport of glycosylphosphatidylinositol-anchored proteins. Mol. Biol. Cell. 1999;10:1043–1059. doi: 10.1091/mbc.10.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillas I., Kirchman P. A., Chuard R., Pfefferli M., Jiang J. C., Jazwinski S. M., Conzelmann A. C26-CoA-dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p. EMBO J. 2001;20:2655–2665. doi: 10.1093/emboj/20.11.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang J. C., Kirchman P. A., Zagulski M., Hunt J., Jazwinski S. M. Homologs of the yeast longevity gene LAG1 in Caenorhabditis elegans and human. Genome Res. 1998;8:1259–1272. doi: 10.1101/gr.8.12.1259. [DOI] [PubMed] [Google Scholar]
- 13.Schorling S., Vallee B., Barz W. P., Riezman H., Oesterhelt D. Lag1p and Lac1p are essential for the acyl-CoA-dependent ceramide synthase reaction in Saccharomyces cerevisae. Mol. Biol. Cell. 2001;12:3417–3427. doi: 10.1091/mbc.12.11.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter E., Ponting C. P. TRAM, LAG1 and CLN8: members of a novel family of lipid-sensing domains? Trends Biochem. Sci. 2002;27:381–383. doi: 10.1016/s0968-0004(02)02154-0. [DOI] [PubMed] [Google Scholar]
- 15.Vallee B., Riezman H. Lip1p: a novel subunit of acyl-CoA ceramide synthase. EMBO J. 2005;24:730–741. doi: 10.1038/sj.emboj.7600562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao C., Xu R., Bielawska A., Obeid L. M. Cloning of an alkaline ceramidase from Saccharomyces cerevisiae: an enzyme with reverse (CoA-independent) ceramide synthase activity. J. Biol. Chem. 2000;275:6876–6884. doi: 10.1074/jbc.275.10.6876. [DOI] [PubMed] [Google Scholar]
- 17.Mao C., Xu R., Bielawska A., Szulc Z. M., Obeid L. M. Cloning and characterization of a Saccharomyces cerevisiae alkaline ceramidase with specificity for dihydroceramide. J. Biol. Chem. 2000;275:31369–31378. doi: 10.1074/jbc.M003683200. [DOI] [PubMed] [Google Scholar]
- 18.Nagiec M. M., Wells G. B., Lester R. L., Dickson R. C. A suppressor gene that enables Saccharomyces cerevisiae to grow without making sphingolipids encodes a protein that resembles an Escherichia coli fatty acyltransferase. J. Biol. Chem. 1993;268:22156–22163. [PubMed] [Google Scholar]
- 19.Lester R. L., Wells G. B., Oxford G., Dickson R. C. Mutant strains of Saccharomyces cerevisiae lacking sphingolipids synthesize novel inositol glycerophospholipids that mimic sphingolipid structures. J. Biol. Chem. 1993;268:845–856. [PubMed] [Google Scholar]
- 20.Kohlwein S. D., Eder S., Oh C. S., Martin C. E., Gable K., Bacikova D., Dunn T. Tsc13p is required for fatty acid elongation and localizes to a novel structure at the nuclear-vacuolar interface in Saccharomyces cerevisiae. Mol. Cell Biol. 2001;21:109–125. doi: 10.1128/MCB.21.1.109-125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revardel E., Bonneau M., Durrens P., Aigle M. Characterization of a new gene family developing pleiotropic phenotypes upon mutation in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1995;1263:261–265. doi: 10.1016/0167-4781(95)00124-y. [DOI] [PubMed] [Google Scholar]
- 22.Silve S., Leplatois P., Josse A., Dupuy P. H., Lanau C., Kaghad M., Dhers C., Picard C., Rahier A., Taton M., et al. The immunosuppressant SR 31747 blocks cell proliferation by inhibiting a steroid isomerase in Saccharomyces cerevisiae. Mol. Cell Biol. 1996;16:2719–2727. doi: 10.1128/mcb.16.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung J. H., Lester R. L., Dickson R. C. Sphingolipid requirement for generation of a functional v1 component of the vacuolar ATPase. J. Biol. Chem. 2003;278:28872–28881. doi: 10.1074/jbc.M300943200. [DOI] [PubMed] [Google Scholar]
- 24.Ni L., Snyder M. A genomic study of the bipolar bud site selection pattern in Saccharomyces cerevisiae. Mol. Biol. Cell. 2001;12:2147–2170. doi: 10.1091/mbc.12.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillas I., Jiang J. C., Vionnet C., Roubaty C., Uldry D., Chuard R., Wang J., Jazwinski S. M., Conzelmann A. Human homologues of LAG1 reconstitute Acyl-CoA-dependent ceramide synthesis in yeast. J. Biol. Chem. 2003;278:37083–37091. doi: 10.1074/jbc.M307554200. [DOI] [PubMed] [Google Scholar]
- 26.Riebeling C., Allegood J. C., Wang E., Merrill A. H. J., Futerman A. H. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J. Biol. Chem. 2003;278:43452–43459. doi: 10.1074/jbc.M307104200. [DOI] [PubMed] [Google Scholar]
- 27.Mizutani Y., Kihara A., Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 2005;390:263–271. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- 29.Pinto W. J., Srinivasan B., Shepherd S., Schmidt A., Dickson R. C., Lester R. L. Sphingolipid long-chain-base auxotrophs of Saccharomyces cerevisiae: genetics, physiology, and a method for their selection. J. Bacteriol. 1992;174:2565–2574. doi: 10.1128/jb.174.8.2565-2574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mumberg D., Muller R., Funk M. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 1994;22:5767–5768. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voth W. P., Jiang Y. W., Stillman D. J. New ‘marker swap’ plasmids for converting selectable markers on budding yeast gene disruptions and plasmids. Yeast. 2003;20:985–993. doi: 10.1002/yea.1018. [DOI] [PubMed] [Google Scholar]
- 32.Wach A., Brachat A., Alberti-Segui C., Rebischung C., Philippsen P. Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast. 1997;13:1065–1075. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1065::AID-YEA159>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 33.Reggiori F., Canivenc-Gansel E., Conzelmann A. Lipid remodeling leads to the introduction and exchange of defined ceramides on GPI proteins in the ER and Golgi of Saccharomyces cerevisiae. EMBO J. 1997;16:3506–3518. doi: 10.1093/emboj/16.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillas I., Pfefferli M., Conzelmann A. Analysis of ceramides present in glycosylphosphatidylinositol anchored proteins of Saccharomyces cerevisiae. Methods Enzymol. 2000;312:506–515. doi: 10.1016/s0076-6879(00)12935-0. [DOI] [PubMed] [Google Scholar]
- 35.Hanson B. A., Lester R. L. The extraction of inositol-containing phospholipids and phosphatidylcholine from Saccharomyces cerevisiae and Neurospora crassa. J. Lipid Res. 1980;21:309–315. [PubMed] [Google Scholar]
- 36.Gaigg B., Timischl B., Corbino L., Schneiter R. Synthesis of sphingolipids with very long chain fatty acids but not ergosterol is required for routing of newly synthesized plasma membrane ATPase to the cell surface of yeast. J. Biol. Chem. 2005;280:22515–22522. doi: 10.1074/jbc.M413472200. [DOI] [PubMed] [Google Scholar]
- 37.Haak D., Gable K., Beeler T., Dunn T. Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J. Biol. Chem. 1997;272:29704–29710. doi: 10.1074/jbc.272.47.29704. [DOI] [PubMed] [Google Scholar]
- 38.Rother J., van Echten G., Schwarzmann G., Sandhoff K. Biosynthesis of sphingolipids: dihydroceramide and not sphinganine is desaturated by cultured cells. Biochem. Biophys. Res. Commun. 1992;189:14–20. doi: 10.1016/0006-291x(92)91518-u. [DOI] [PubMed] [Google Scholar]
- 39.Lisman Q., Pomorski T., Vogelzangs C., Urli-Stam D., de Cocq van Delwijnen W., Holthuis J. C. Protein sorting in the late Golgi of Saccharomyces cerevisiae does not require mannosylated sphingolipids. J. Biol. Chem. 2004;279:1020–1029. doi: 10.1074/jbc.M306119200. [DOI] [PubMed] [Google Scholar]
- 40.Ejsing C. S., Moehring T., Bahr U., Duchoslav E., Karas M., Simons K., Shevchenko A. Collision-induced dissociation pathways of yeast sphingolipids and their molecular profiling in total lipid extracts: a study by quadrupole TOF and linear ion trap-orbitrap mass spectrometry. J. Mass Spectrom. 2006;41:372–389. doi: 10.1002/jms.997. [DOI] [PubMed] [Google Scholar]
- 41.Schneiter R., Brugger B., Amann C. M., Prestwich G. D., Epand R. F., Zellnig G., Wieland F. T., Epand R. M. Identification and biophysical characterization of a very-long-chain-fatty-acid-substituted phosphatidylinositol in yeast subcellular membranes. Biochem. J. 2004;381:941–949. doi: 10.1042/BJ20040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conzelmann A. In: Biosynthesis, remodeling and targeting of GPI proteins in the yeast Saccharomyces cerevisiae. Daum G., editor. Trivandrum, Kerala: Research Signpost; 2005. pp. 133–159. [Google Scholar]
- 43.Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981;25:461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- 44.Rayner J. C., Pelham H. R. Transmembrane domain-dependent sorting of proteins to the ER and plasma membrane in yeast. EMBO J. 1997;16:1832–1841. doi: 10.1093/emboj/16.8.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine T. P., Wiggins C. A., Munro S. Inositol phosphorylceramide synthase is located in the Golgi apparatus of Saccharomyces cerevisiae. Mol. Biol. Cell. 2000;11:2267–2281. doi: 10.1091/mbc.11.7.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belden W. J., Barlowe C. Deletion of yeast p24 genes activates the unfolded protein response. Mol. Biol. Cell. 2001;12:957–969. doi: 10.1091/mbc.12.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fankhauser C., Homans S. W., Thomas-Oates J. E., McConville M. J., Desponds C., Conzelmann A., Ferguson M. A. Structures of glycosylphosphatidylinositol membrane anchors from Saccharomyces cerevisiae. J. Biol. Chem. 1993;268:26365–26374. [PubMed] [Google Scholar]
- 48.Sipos G., Reggiori F., Vionnet C., Conzelmann A. Alternative lipid remodelling pathways for glycosylphosphatidylinositol membrane anchors in Saccharomyces cerevisiae. EMBO J. 1997;16:3494–3505. doi: 10.1093/emboj/16.12.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y., Vionnet C., Conzelmann A. Ethanolaminephosphate side chain added to GPI anchor by Mcd4p is required for ceramide remodeling and forward transport of GPI proteins from ER to Golgi. J. Biol. Chem. 2006;281:19830–19839. doi: 10.1074/jbc.M601425200. [DOI] [PubMed] [Google Scholar]
- 50.Patton J. L., Srinivasan B., Dickson R. C., Lester R. L. Phenotypes of sphingolipid-dependent strains of Saccharomyces cerevisiae. J. Bacteriol. 1992;174:7180–7184. doi: 10.1128/jb.174.22.7180-7184.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Packeiser A. N., Urakov V. N., Polyakova Y. A., Shimanova N. I., Shcherbukhin V. D., Smirnov V. N., Ter-Avanesyan M. D. A novel vacuolar protein encoded by SSU21/MCD4 is involved in cell wall integrity in yeast. Yeast. 1999;15:1485–1501. doi: 10.1002/(SICI)1097-0061(199910)15:14<1485::AID-YEA477>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 52.Moebius F. F., Bermoser K., Reiter R. J., Hanner M., Glossmann H. Yeast sterol C8-C7 isomerase: identification and characterization of a high-affinity binding site for enzyme inhibitors. Biochemistry. 1996;35:16871–16878. doi: 10.1021/bi961996m. [DOI] [PubMed] [Google Scholar]
- 53.Lahiri S., Futerman A. H. LASS5 is a bona fide dihydroceramide synthase that selectively utilizes palmitoyl-CoA as acyl donor. J. Biol. Chem. 2005;280:33735–33738. doi: 10.1074/jbc.M506485200. [DOI] [PubMed] [Google Scholar]
- 54.Mizutani Y., Kihara A., Igarashi Y. LASS3 (longevity assurance homologue 3) is a mainly testis-specific (dihydro)ceramide synthase with relatively broad substrate specificity. Biochem. J. 2006;398:531–538. doi: 10.1042/BJ20060379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venkataraman K., Riebeling C., Bodennec J., Riezman H., Allegood J. C., Sullards M. C., Merrill A. H. J., Futerman A. H. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J. Biol. Chem. 2002;277:35642–35649. doi: 10.1074/jbc.M205211200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.