Abstract
The Wilson protein (ATP7B) is a copper-translocating P-type ATPase that mediates the excretion of excess copper from hep-atocytes into bile. Excess copper causes the protein to traffic from the TGN (trans-Golgi network) to subapical vesicles. Using site-directed mutagenesis, mutations known or predicted to abrogate catalytic activity (copper translocation) were introduced into ATP7B and the effect of these mutations on the intracellular traf-ficking of the protein was investigated. Mutation of the critical aspartic acid residue in the phosphorylation domain (DKTGTIT) blocked copper-induced redistribution of ATP7B from the TGN, whereas mutation of the phosphatase domain [TGE (Thr-Gly-Glu)] trapped ATP7B at cytosolic vesicular compartments. Our findings demonstrate that ATP7B trafficking is regulated with its copper-translocation cycle, with cytosolic vesicular localization associated with the acyl-phosphate intermediate. In addition, mut-ation of the six N-terminal metal-binding sites and/or the trans-membrane CPC (Cys-Pro-Cys) motif did not suppress the consti-tutive vesicular localization of the ATP7B phosphatase domain mutant. These results suggested that copper co-ordination by these sites is not essential for trafficking. Importantly, copper-chelation studies with these mutants clearly demonstrated a requirement for copper in ATP7B trafficking, suggesting the presence of an additional copper-binding site(s) within the protein. The results presented in this report significantly advance our understanding of the regulatory mechanism that links copper-translocation activity with copper-induced intracellular trafficking of ATP7B, which is central to hepatic and hence systemic copper homoeostasis.
Keywords: ATP7B, copper translocation, metal-binding site, P-type ATPase, Wilson disease, WND
Abbreviations: BCS, bathocuproinedisulfonic acid; CHO-K1, Chinese-hamster ovary K1; CPC motif, Cys-Pro-Cys motif; MBS, metal-binding site; TGE, Thr-Gly-Glu; TGN, trans-Golgi network; wt, wild-type
INTRODUCTION
The P-type ATPase ATP7B plays a pivotal role in mammalian copper homoeostasis and defects in this protein cause the copper-toxicity disorder Wilson disease [1]. P-type ATPases are an exten-sive family of transmembrane proteins that mediate the transloc-ation of various cations, including Mg2+, Ca2+, Na+, K+, Zn2+, Ag+ and Cu+, across cellular membranes and often against concentration gradients [2]. Mammals have two copper-translocating P-type ATPases: ATP7B and its paralogue ATP7A. Both proteins contain the characteristic features of P-type ATPases which in-clude an ATP-binding site (GDGIND), a phosphorylation domain [DKTGT(I,L)T], a phosphatase domain [TGE (Thr-Gly-Glu)] and eight transmembrane regions (Figure 1) [1–3]. In addition, heavy-metal-transporting P-type ATPases, such as ATP7A and ATP7B, form a subfamily termed CPX-type ATPases [4]. Members of the subfamily are characterized by a variable number of heavy MBSs (metal-binding sites) (GMXCXXC) in the N-terminal region and a conserved Cys-Pro-Xaa motif (Xaa=cysteine, histidine or serine) in the proposed cation transduction channel (Figure 1) [4]. Both ATP7A and ATP7B have six N-terminal GMXCXXC motifs, which have been shown to preferentially bind copper with a stoi-chiometry of one copper atom per binding motif [5]. The CPC (Cys-Pro-Cys) motif situated in the sixth transmembrane domain has also been shown to bind copper, which may facilitate trans-location of the cation through the membrane [6,7].
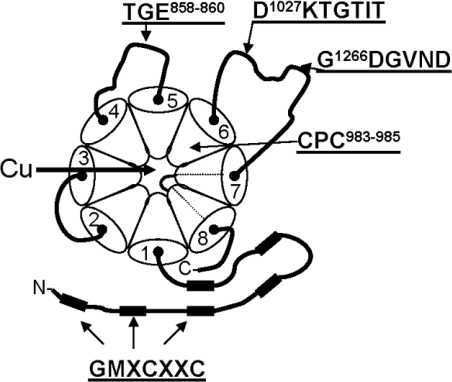
Figure 1. Schematic representation of ATP7B.
Transmembrane domains are numbered 1 to 8. Filled rectangles represent the six N-terminal MBSs (GMXCXXC). The amino acids mutated in the present study and their positions in ATP7B are shown. The conserved domains are as follows: phosphorylation domain, D1027KTGTIT; phosphatase domain, TGE858–860; ATP-binding domain, G1266DGVND; CPC motif, CPC983–985. Protein orientation is from cytosolic side facing to luminal side, the direction in which ATP7B translocates copper across the membrane.
The enzymatic properties of both copper-ATPases conform to the classical P-type ATPase model [2,8]. Phosphorylation of the aspartic acid residue in the phosphorylation domain is required for copper translocation and is dependent on the presence of cation (copper) and ATP [9,10]. The transiently phosphorylated intermediate (acyl-phosphate) is sensitive to basic pH, ADP and treatment with hydroxylamine and the enzymes are inhibited by the structural homologue of Pi, orthovanadate [9,10]. Acyl-phosphorylation drives protein conformational changes leading to the translocation of the bound cations across the lipid bilayer [2]. The reaction cycle is completed with hydrolysis of the acyl-phosphate (dephosphorylation) by an intrinsic phosphatase activity (via TGE motif) and the pump is returned back to its original conformation to allow further cation binding and transloc-ation [2,8,11].
ATP7A and ATP7B are likely to have evolved from a single ancestral copper-ATPase to perform specific functions in different cell types. ATP7B is expressed predominantly in liver hepatocytes [12] but also in regions of the brain [13], breast [14] and placenta [15], while ATP7A is expressed in most extra-hepatic tissues [16]. Studies on the subcellular localization of the copper-ATPases have shown that both proteins reside at the final compartment of the Golgi apparatus, the TGN (trans-Golgi network) [17–19]. At this location, copper is incorporated into various cuproenzymes such as lysyl oxidase in fibroblasts (mediated by ATP7A) [20] or ceruloplasmin in hepatocytes (mediated by ATP7B) [21]. In hep-atocytes, an increase in copper levels stimulates trafficking of ATP7B to pericanalicular vesicles and ATP7B recycles back to the TGN when copper concentrations are decreased [22,23]. The target vesicles for ATP7B have not been characterized and are possibly novel compartments that sequester excess copper and undergo a mechanism similar to lysosomal exocytosis to expel their luminal contents into bile [23–25]. ATP7A displays similar copper-induced trafficking to ATP7B, but relocates from the TGN to the basolateral membrane in polarized cells [22], where it can efflux copper directly from the cell [26]. In non-polarized cell types, ATP7A traffics to the plasma membrane [19], while ATP7B traffics to a dispersed population of cytosolic vesicles [17,18]. The predominant subcellular localization of each copper-ATPase depends on copper levels, although recycling between the compartments is likely to be constitutive in the presence of either high or low concentrations of copper [26].
We have previously shown that the copper-induced trafficking of ATP7A is regulated with its catalytic cycle [11]. Formation of the acyl-phosphorylated intermediate (catalytic activation) is required for ATP7A redistribution to the plasma membrane, while hydrolysis of the acyl-phosphate (dephosphorylation) is essential for the retrieval of trafficked ATP7A molecules back to the TGN [11]. In the present study, we show that the trafficking of ATP7B is also coupled with its catalytic activity, with the vesicular localization associated with its acyl-phosphate intermediate. Surprisingly, copper co-ordination by either the six N-terminal MBSs or the CPC motif is not essential for ATP7B trafficking even though the trafficking process is dependent on copper. This observation suggests the presence of an additional copper-binding site(s) in the molecule. The results presented in this report represent significant progress towards understanding the mechanism by which copper regulates the subcellular localization of its own transporter.
EXPERIMENTAL
Cells, reagents and antibodies
CHO-K1 (Chinese-hamster ovary K1) cells were cultured as monolayers in Eagle's Basal Medium (Trace BioSciences, Nobel Park, VIC, Australia) supplemented with 0.2 mM proline, 10% (v/v) foetal calf serum, 2 mM L-glutamine, 1.2 mM NaHCO3 and 20 mM Hepes (Commonwealth Serum Laboratories, Broadmedows, VIC, Australia). Transfection of CHO-K1 cells with plasmid DNA was performed using Lipofectamine™ (Life Technologies, Mount Waverley, VIC, Australia) and the manufac-turer's recommendations. Transfected cells were selected using 500 μg/ml G418 (Life Technologies) for 14 days. The Saccharo-myces cerevisiae mutant strain, ccc2Δ (MATα, his3-200, trp-1-101, ura3-52, leu2, ade5, ccc2Δ::LEU2) [27], was cultured on YPD solid medium containing 2% (w/v) trypticase peptone, 1% (w/v) yeast extract, 2% (w/v) glucose and 2% (w/v) agar. This strain was transformed with plasmid DNA using a lithium acetate method as previously described [28] and transformants were selected on DOB-Ura medium composed of 0.66% (w/v) yeast nitrogen base without amino acids, 2% glucose and 0.08% (w/v) CSM (complete supplement mixture minus uracil). The sheep polyclonal anti-ATP7B antibody (NC36) was raised against amino acids 1–199 (N-terminal), 1309–1315 and 1376–1465 (C-terminal) of human ATP7B as previously described [29]. All re-agents used in the present study were purchased from Sigma (Castle Hill, NSW, Australia) unless otherwise stated.
Generation of ATP7B key domain mutants
The Asp1027 residue in the phosphorylation domain was changed to glutamate (D-E) by PCR-based mutagenesis of the ATP7B cDNA as previously described [25]. PCR-based mutagenesis was also used to change the Thr858, Gly859 and Glu860 residues in the phos-phatase domain to alanine residues (TGE-AAA). In this case, the oligonucleotides 5′-CAATACCATGGCTGATGAGTCCCTCAT-CGCGGCCGCTGCCA-3′ (hWND#20) and 5′-TCTGTTCC-AAGTTCCTCTT-3′ (hWND#19) were used to amplify nt 2544–3259 of ATP7B. The oligonucleotide hWND no. 20 intro-duced the desired codon changes, with the codons encoding the alanine residues chosen to form a NotI restriction endonuclease site. The PCR product was digested with HinfI and PinAI to produce a 193 bp fragment (nt 2561–2753), which together with an StuI/HinfI fragment isolated from pCMB278 (nt 2516–2753 of ATP7B) was then ligated into PinAI/StuI-digested pCMB278. The resultant plasmid was designated pCMB515. The ATP7B mutant containing both the phosphorylation- and phosphatase-domain changes (D-E+TGE-AAA) was generated by isolating the TGE-AAA mutation from the pCMB515 plasmid using StuI/PinAI and using this fragment to replace the corresponding wt (wild-type) region in the D-E cDNA in pCMB514. The resultant plasmid was designated pCMB516.
The ATP7B construct with the cysteine residues of the six N-terminal MBSs mutated to serine residues (MBS1-6c/s) was created as previously described [29]. To generate ATP7B with the cysteine residues in the CPC983–985 motif mutated to serine residues (CPC-SPS), the oligonucleotide 5′-CGGTTTGCTTTC-CAGACGTCCATCACGGTGCTGTGCATTGCCTCACCCTC-GTCCCTGGGGCTG-3′ (hWND#22) introduced the codon changes when used in combination with 5′-GCTGACATCGCTA-GAAAT-3′ (hWND#14) to amplify nt 2905–3499 of ATP7B. The PCR product was digested with AatII and AocI to produce a 234 bp fragment (nt 2924–3159), which together with an AocI/SalI fragment isolated from pCMB411 (nt 3160–4395 of ATP7B) was then ligated into AatII/SalI-digested pCMB411. The resulting plasmid was designated pCMB517. To create the final expression construct, the entire CPC-SPS cDNA was isolated from pCMB517 using XbaI/SalI and cloned into the Nhel/SalI sites of pCMB77, generating pCMB518.
The following strategy was employed to create two ATP7B double mutants, one containing the six N-terminal MBS mutations and the phosphatase domain mutation (MBS1-6c/s+TGE-AAA) and another in which the CPC motif and the phosphatase domain are mutated (CPC-SPS+TGE-AAA). The TGE-AAA mutation (nt 2754–4395) was isolated from pCMB515 using PinAI/SalI. This fragment was used to replace the corresponding wt region in pCMB398 (resulting plasmid designated pCMB520) and in pCMB515 (resulting plasmid designated pCMB519) res-pectively. The ATP7B construct with the six N-terminal MBSs, the CPC motif and the phosphatase domain mutated (MBS1-6c/s+CPC-SPS+TGE-AAA) was engineered by isolating the CPC-SPS and TGE-AAA mutations together from pCMB519 (nt 2153–4395) using StuI and SalI. This fragment was then cloned into the StuI/SalI sites of the MBS1-6c/s cDNA in pCMB398, replacing the corresponding wt region (resulting plasmid design-ated pCMB522). The ATP7B construct with the six N-terminal MBSs and the CPC motif mutated (MBS1-6c/s+CPC-SPS) was generated by using StuI/PinAI to drop out the region in pCMB522 containing the TGE-AAA mutation and by replacing this fragment with an StuI/PinAI fragment isolated from wtATP7B (from pCMB278). The resultant plasmid was designated pCMB521. The PCR-derived inserts and ligation sites in all constructs generated were sequenced to confirm integrity. The plasmid constructs generated and used in the present study are outlined in Table 1.
Table 1. Plasmids generated and used in the present study.
| Plasmid | Characteristics | Reference |
|---|---|---|
| pWSK29 | Bacterial propagation vector | [45] |
| pCMB77 | Mammalian expression vector | [19] |
| pVT-103U | Yeast expression vector | [30] |
| pCMB278 | wtATP7B in pCMB77 | [29] |
| pCMB514 | D-E in pCMB77 | [25] |
| pCMB515 | TGE-AAA in pCMB77 | Present study |
| pCMB516 | D-E+TGE-AAA in pCMB77 | Present study |
| pCMB517 | CPC-SPS in pCMB411 | Present study |
| pCMB518 | CPC-SPS in pCMB77 | Present study |
| pCMB519 | CPC-SPS+TGE-AAA in pCMB77 | Present study |
| pCMB398 | MBS1-6c/s in pCMB77 | [29] |
| pCMB520 | MBS1-6c/s+TGE-AAA in pCMB77 | Present study |
| pCMB521 | MBS1-6c/s+CPC-SPS in pCMB77 | Present study |
| pCMB522 | MBS1-6c/s+CPC-SPS+TGE-AAA in pCMB77 | Present study |
| pCMB554 | wtATP7B in pVT-103U | [29] |
| pCMB555 | MBS1-2c/s in pVT-103U | [29] |
| pCMB594 | D-E in pVT-103U | Present study |
| pCMB595 | TGE-AAA in pVT-103U | Present study |
| pCMB596 | D-E+TGE-AAA in pVT-103U | Present study |
| pCMB557 | MBS1-6c/s in pVT-103U | [29] |
| pCMB597 | MBS1-6c/s+TGE-AAA in pVT-103U | Present study |
| pCMB598 | CPC-SPS in pVT-103U | Present study |
| pCMB599 | CPC-SPS+TGE-AAA in pVT-103U | Present study |
| pCMB600 | MBS1-6c/s+CPC-SPS in pVT-103U | Present study |
| pCMB601 | MBS1-6c/s+CPC-SPS+TGE–AAA in pVT-103U | Present study |
The ATP7B mutant constructs described above were incorpor-ated into the yeast expression vector pVT-103U. This vector con-tains the ADH1 (alcohol dehydrogenase 1) gene promoter, the 2 μ origin of replication for propagation in yeast, the URA3 marker for plasmid selection in yeast, and the ori and intergenic region of the phage f1 (f1 ori). In addition, pVT-103U also contains an origin of replication and the β-lactamase gene for propagation and selection (ampicillin resistance) in Escherichia coli [30]. ATP7B cDNA constructs can be unstable in high copy number vectors. Although pVT-103U is only an intermediate copy number vector [30], ATP7B constructs generated using this vector were still pro-pagated in the Able®-K E. coli strain (Stratagene, La Jolla, CA, U.S.A.) to reduce the copy number 10-fold to ensure stable propagation of ATP7B.
The ATP7B constructs were cloned into pVT-103U using the following strategy. Nucleotides 494–4395 were removed from wtATP7B in pVT-103U (pCMB554) or from MBS 1+2c/s in pVT-103U (pCMB555) (previously described in [29]) using BseAI and XhoI. These fragments were replaced with the corresponding region from each ATP7B mutant cDNA, which was isolated from their respective expression plasmids (shown in Table 1) using BseAI and SalI. Constructs with MBS 1–2 mutated were inserted into pCMB555. Digestion profiles, exploiting endo-nuclease sites introduced during the generation of each mutant, were used to verify that the mutant cDNAs replaced the wtATP7B segment of pCMB554. Final plasmid constructs were designated as shown in Table 1.
Protein preparation and Western-blot analysis
Yeast protein extracts were prepared using a trichloroacetic acid method as described in Clontech's Yeast Protocols Handbook (PT3024-1). Whole cell protein extracts (30 μg) were fraction-ated by SDS/PAGE (7.5% polyacrylamide). Following electrophoretic separation, proteins were transferred to nitrocellulose (Amersham, Rydalmere, NSW, Australia) using a Trans-Blot SD semi-dry electrophoretic transfer apparatus (Bio-Rad, Regents Park, NSW, Australia) and immunoblot analysis was carried out using the Lumi-Light chemiluminescence blotting kit (Roche, Castle Hill, NSW, Australia) according to the manufacturer's protocol. ATP7B was detected using the ammonium sulfate-precip-itated NC36 antibody (diluted 1:1000) [29], followed by horse-radish peroxidase-conjugated donkey anti-sheep IgG (Chemicon, Boronia, VIC, Australia) (1:4000). Exposed Hyperfilm™ (Amersham) was fixed and developed using a Fuji medical film processor (Fuji Photo Film).
Indirect immunofluorescence microscopy
Transfected CHO-K1 cells were seeded on to 13 mm glass cover-slips in a 24-well tray and cultured for 16–72 h to approx. 50–70% confluence. The growth medium was replaced with basal medium (0.5–1 μM Cu), 200 μM CuCl2-supplemented medium or a medium containing the copper chelators BCS (bathocuproine-disulfonic acid; 200 μM) and D-penicillamine (200 μM) as indi-cated in the results. Following treatment, cells were fixed using 4% (w/v) paraformaldehyde in PBS for 10 min and then permeabilized with 0.1% (v/v) Triton X-100 in PBS for 10 min. Cells were blocked in 1% (w/v) BSA and 1% (w/v) gelatin in PBS at 4 °C overnight. For the detection of ATP7B, coverslips were in-cubated with ammonium sulfate-precipitated NC36, diluted 1:10000 in blocking solution for 1 h. Following four PBS washes (15 min each) coverslips were incubated (room temperature for 1 h) with the secondary antibody, donkey anti-sheep IgG Alexa Fluor® 488 (1:4000; Chemicon) and again washed with PBS as above. Coverslips were mounted on glass slides using 2.6% (w/v) Dabco (Sigma) in 90% (v/v) glycerol. Immunolabelled cells were analysed using a ×60 oil objective with an Olympus Provis AX70 microscope.
Complementation of S. cerevisiae ccc2Δ
The S. cerevisiae ccc2Δ yeast strains that expressed wt and mutant ATP7B proteins were each inoculated into 5 ml of DOB-Ura and grown at 30 °C overnight to saturation. Serial 10-fold dilutions in sterile distilled water were made in a 96-well plate starting from 10−1. For each dilution, 5 μl was applied in triplicate on to iron-limited DOB-Ura agar plates containing 50 mM Mes and 250 μM ferrozine and on to normal DOB-Ura plates to verify cell viability and then incubated at 30 °C for 3 days. Images were taken using a Canon digital IXUS V camera.
RESULTS
Effect of mutations in the phosphorylation and phosphatase domains on the subcellular localization and trafficking of ATP7B
Under basal copper conditions, the localization of ATP7B to the TGN has been well established [17,18,25]. Despite previous attempts [18,31], the cytosolic vesicular compartments to which ATP7B traffics in elevated copper concentrations remain undefined. In the present study, the effect of various mutations within ATP7B on the ability of the protein to traffic from the perinuclear region to cytosolic vesicles was assessed.
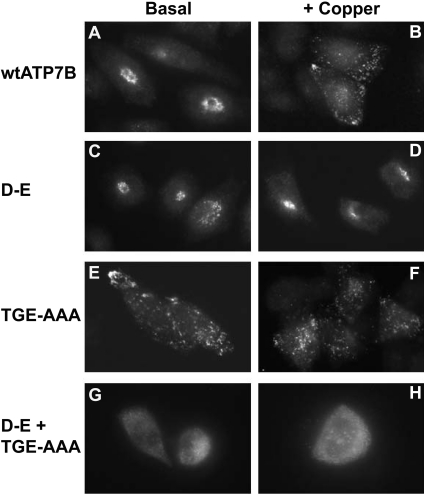
The invariant aspartate (Asp1027) in the phosphorylation domain of ATP7B (DKTGTIT) has been shown to be essential for catalytic activity [29]. To determine if this aspartate is also required for the copper-induced trafficking of the protein, it was mutated to glutamate (D-E) and the ability of the mutant protein to traffic was studied in CHO-K1 cells (Figure 2). In cells cultured under basal copper conditions (0.5–1 μM Cu), the D-E protein was detected in the perinuclear region (Figure 2C), similar to wtATP7B under the same conditions (Figure 2A). However, in cells exposed to 200 μM copper for 2 h, the D-E protein remained perinuclear (Figure 2D), while most of the wtATP7B molecules redistributed to cytosolic vesicles (Figure 2B). Increasing copper concentration to 400 μM and extending the exposure time to 4 h had no observable effect on the localization of the D-E protein (results not shown). These results demonstrate that Asp1027 is required for the trafficking of ATP7B and support the hypothesis that the copper-induced trafficking process is dependent on acyl-phosphate formation [11].
Figure 2. Effect of mutating the phosphorylation and phosphatase domains on the trafficking of ATP7B.
CHO-K1 cells transfected with wt or mutant ATP7B constructs [amino acid substitution(s) indicated on the left] were cultured in basal medium (0.5–1 μM Cu) or a medium supplemented with 200 μM CuCl2 (+Copper). Detection of ATP7B was performed by immunofluorescence analysis using ammonium sulfate-precipitated NC36 (diluted 1:10000) and donkey anti-sheep IgG Alexa Fluor® 488 (diluted 1:4000) (Chemicon). Photographs were taken using a ×60 oil objective with an Olympus Provis AX70 microscope.
Mutation of the phosphatase domain (TGE) in several P-type ATPases, including both H+- and Ca2+-ATPase, has been shown to prevent cation translocation and resulted in the accumulation of the acyl-phosphorylated intermediate [32,33]. Mutation of the TGE858–860 sequence in ATP7B to alanine residues (TGE-AAA) caused constitutive vesicular localization of the protein (Fig-ure 2E), resembling the distribution of wtATP7B in elevated levels of copper (Figure 2B). There was no distinguishable difference in the vesicular staining of the TGE-AAA protein when cells were cultured in either basal medium or in a medium supplemented with 200 μM CuCl2 (Figures 2E and 2F). This observation sug-gested that even in basal copper concentrations acyl-phosphate formation occurs, and in the absence of the intrinsic phosphatase activity, most of the ATP7B molecules remain phosphorylated and consequently are trapped at vesicular compartments.
To investigate whether the trafficking and vesicular localization of the TGE-AAA protein was dependent on the presence of the invariant aspartate (Asp1027), an ATP7B double mutant containing the D-E and TGE-AAA mutations was generated. The D-E+TGE-AAA protein localized abnormally in a hazy punctate pattern throughout the cytosol when expressed in CHO-K1 cells (Fig-ure 2G). This phenotype was observed in all transfected cells in the absence and in the presence of 200 μM CuCl2 (Figure 2H). In addition, there were clearly fewer cells in which the D-E+TGE-AAA protein was detected in comparison with all other transfected cell lines and the cells that expressed the mutant protein displayed weak immunofluorescence staining (results not shown).
The D-E+TGE-AAA mutant protein is temperature-sensitive
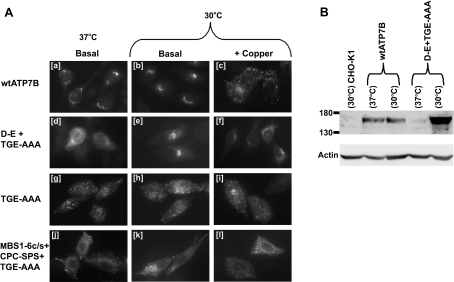
Temperature-sensitive proteins that contain tsf (temperature-sen-sitive folding) mutations, which destabilize an intermediate in the folding pathway, often can fold correctly and maintain normal function when biosynthesized at a lower temperature [34]. To determine if the aberrant localization of the D-E+TGE-AAA protein was due to temperature-sensitive misfolding, CHO-K1 cells that express this mutant protein were incubated at 30 °C for 3 days before being evaluated by immunofluorescence micro-scopy (Figure 3A) and Western-blot analysis (Figure 3B). The decrease in temperature resulted in the perinuclear staining of the D-E+TGE-AAA protein [Figure 3A (e)], which was clearly dis-tinct from the hazy punctate pattern observed at 37 °C [Figure 3A (d)]. Elevated copper (200 μM) had no effect on the perinuclear localization of D-E+TGE-AAA [Figure 3A (f)], whereas under the same conditions most of the wtATP7B molecules trafficked to cytosolic vesicles [Figure 3A (c)]. The reduction in temperature also resulted in an increase in the number of cells in which the D-E+TGE-AAA protein was detected (results not shown), consistent with an increase in the stability of the D-E+TGE-AAA protein at 30 °C. Western-blot analysis confirmed that culturing the cells for 3 days at 30 °C increased the amount of intact, stable D-E+TGE-AAA protein, while having no obvious effect on the amount of wtATP7B (Figure 3B). Allowing the D-E+TGE-AAA protein to assemble at a permissive temperature (30 °C) not only corrected the subcellular localization of the protein [Figure 3A (e)], but also revealed that the D-E substitution suppressed the effect of the TGE-AAA mutation as the double mutant was incapable of trafficking from the perinuclear region [Figure 3A (f)]. To demonstrate that the vesicular localization of the TGE-AAA protein did not arise from a similar temperature (sensitive) effect, the effect of protein biosynthesis and assembly at 30 °C on the subcellular localization of TGE-AAA was assessed [Figure 3A (h and i)]. In cells cultured at 30 °C in either basal medium or a medium supplemented with 200 μM copper, the TGE-AAA pro-tein retained its cytosolic vesicular localization. Therefore the vesicular localization of TGE-AAA is unlikely to have arisen from protein misfolding.
Figure 3. Effect of temperature reduction on the subcellular localization and stability of ATP7B mutants.
(A) Immunofluorescence analysis of CHO-K1 cells expressing wt or mutant ATP7B constructs [amino acid substitution(s) indicated on the left] cultured at 30 °C. Cells were cultured at 30 °C for 3 days and then were incubated for 8 h (at 30 °C) with basal medium or a medium supplemented with 200 μM CuCl2 (+Copper) before being analysed by immunofluorescence microscopy. Detection of ATP7B was performed as described in Figure 2. (B) Western-blot analysis comparing the amount of wtATP7B and D-E+TGE-AAA expression in CHO-K1 cells. Cells were cultured for 3 days at either 37 or 30 °C before total cellular protein (∼50 μg) was subjected to SDS/PAGE (7.5% polyacrylamide) and transferred to nitrocellulose. The membrane was incubated with ammonium sulfate-precipitated NC36 (diluted 1:1000), followed by horseradish peroxidase-conjugated donkey anti-sheep IgG (diluted 1:4000) and analysed by chemiluminescence. The positions of the molecular mass markers (Bio-Rad) are indicated on the left in kDa. The nitrocellulose was then stripped using Re-Blot Plus-Mild (Chemicon) and reblotted with mouse anti-β-actin monoclonal antibody (diluted 1:5000) and horseradish peroxidase-conjugated sheep anti-mouse IgG (diluted 1:4000).
Copper binding to the GMXCXXC and CPC motifs is not essential for ATP7B trafficking
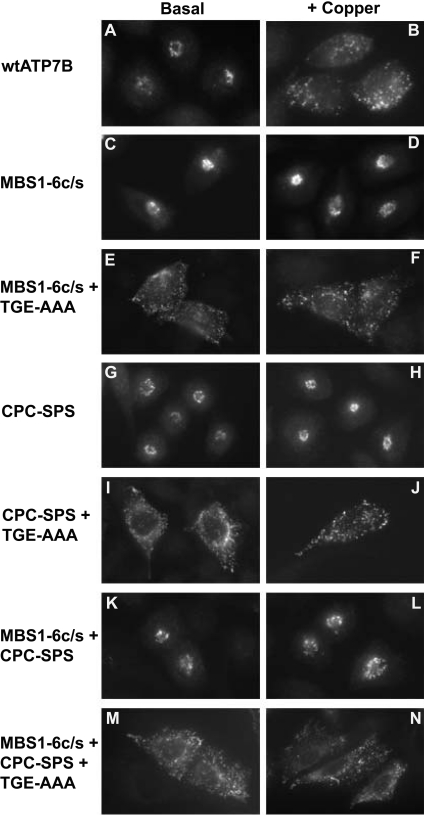
Mutation of the cysteine residues in all six N-terminal copper-binding sites (GMXCXXC) to serine residues inhibited the trafficking of ATP7B [29]. This observation was consistent with copper binding to these sites being required for catalytic activation (formation of the acyl-phosphate) leading to trafficking (Fig-ures 4C and 4D). However, the failure of this and other ATP7B variants to traffic [17,31] could be due to either (i) the mutation preventing formation of the acyl-phosphorylated intermediate or (ii) slowing of the rate of catalysis to such an extent that the rate of anterograde trafficking is less than the rate of retrieval back to the TGN. In the latter case, ATP7B would not accumulate at the cyto-solic vesicles. To distinguish between these two possibilities, the six N-terminal copper-binding site mutations were combined with the phosphatase domain mutation (MBS1-6c/s+TGE-AAA). The mutant protein localized to vesicular compartments in both basal medium (Figure 4E) and medium supplemented with 200 μM copper (Figure 4F), with a pattern of labelling indistingu-ishable from that of the TGE-AAA protein (Figures 2E and 2F). These results suggested that mutation of the six N-terminal copper-binding sites alone perturbs, but does not completely abrogate the trafficking of ATP7B.
Figure 4. Effect of mutating copper-binding sites on the trafficking of ATP7B.
CHO-K1 cells transfected with wt or mutant ATP7B constructs [amino acid substitution(s) indicated on the left] were cultured in basal medium (0.5–1 μM Cu) or a medium supplemented with 200 μM CuCl2 (+Copper). Detection of ATP7B was performed as described in Figure 2.
If copper binding to ATP7B is responsible for initiating catalytic activation and trafficking, then mutation of the copper-binding site(s) involved would be expected to abolish these activities. The CPC motif in the copper-ATPases (ATP7A and ATP7B) is 43 amino acids upstream of the phosphorylation domain [DKTGT(I,L)T] (Figure 1) and is critical for the function of both copper-ATPases [6,17,35,36]. It has been proposed that the CPC motif may facilitate the translocation of copper through the transmembrane region [7]. It is conceivable that binding of copper to the CPC motif leads directly to acyl-phosphate formation. Mut-ation of both cysteine residues in the CPC motif to serine residues (CPC-SPS) prevented trafficking of ATP7B in CHO-K1 cells (Figures 4G and 4H). The CPC-SPS protein localized to the perinu-clear region under both basal and elevated copper conditions (Figures 4G and 4H), consistent with previous observations [6]. Surprisingly, when the TGE-AAA mutation was introduced into the CPC-SPS protein, the double mutant localized to vesicles in the absence and presence of added CuCl2 (Figures 4I and 4J). This observation suggested that mutation of the CPC motif retards rather than completely blocks the trafficking process, as observed with the six N-terminal MBS mutations.
Possibly copper binding to either the N-terminal MBSs or the CPC would be sufficient for acyl-phosphate formation and hence trafficking. To investigate this hypothesis, a mutant protein in which the six N-terminal MBSs, the CPC motif and the phosphat-ase domain were mutated (MBS1-6c/s+CPC-SPS+TGE-AAA) was expressed in CHO-K1 cells. This mutant protein localized to vesicular compartments in both basal and 200 μM CuCl2-supple-mented medium (Figures 4M and 4N). When the six N-terminal MBSs and the CPC motif were mutated but the phosphatase domain was left unchanged (MBS1-6c/s+CPC-SPS), the protein localized to the perinuclear region (Figures 4K and 4L). This result indicated that the combination of the MBS1-6c/s and the CPC-SPS mutations was not responsible for the constitutive vesicular localization of the MBS1-6c/s+CPC-SPS+TGE-AAA protein. To demonstrate that the vesicular localization of this protein was not due to folding irregularities caused by the multiple mutations, CHO-K1 cells that expressed this mutant protein were incubated at 30 °C for 3 days before being evaluated by immunofluorescence microscopy [Figure 3A (k and l)]. Reducing the temperature had no effect on the subcellular localization of the MBS1-6c/s+CPC-SPS+TGE-AAA protein [Figure 3A (k and l)]. These results suggested that while the six N-terminal MBSs and the CPC motif are required for the efficient trafficking of ATP7B, surprisingly they are not essential for trafficking to occur and, when mutated together with the phosphatase domain (TGE-AAA), lead to accu-mulation of ATP7B at vesicular compartments.
Copper chelation restores TGN localization of TGE-AAA mutants
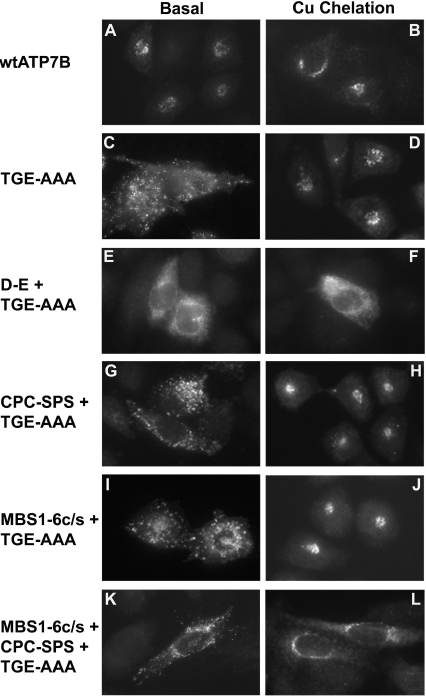
If ATP7B is required to bind copper to initiate catalytic activation and trafficking, then depleting intracellular copper should prevent, or slow, the redistribution of the TGE-AAA mutants. To test this hypothesis, cell lines that expressed the TGE-AAA mutants were cultured in a medium containing the copper chelators BCS (200 μM) and D-penicillamine (200 μM). With the exception of the D-E+TGE-AAA protein (Figure 5F), all TGE-AAA mutants localized to the perinuclear region when cells were incubated for 3 days with the copper chelators (Figure 5). Three days of incub-ation were necessary to shift the steady-state localization of the TGE-AAA mutants from cytosolic vesicles to the perinuclear region (Figure 5); after only one or two days the proteins were still observed in vesicles (results not shown). The D-E+TGE-AAA protein retained the hazy punctate pattern under copper-depleted conditions (Figure 5F). The overall number and appearance of the cells was unchanged by the copper chelation treatment, suggest-ing that the cells were still viable. Taken together, these results demonstrate that copper availability was necessary for the consti-tutive vesicular localization of the TGE-AAA mutants. Since the two known copper-binding regions were mutated in the MBS1-6c/s+CPC-SPS+TGE-AAA protein, these results raise the possibility of an additional unrecognized copper-binding site(s) in ATP7B.
Figure 5. Effect of copper chelation on the subcellular localization of the TGE-AAA mutants.
CHO-K1 cells expressing the indicated wt or TGE-AAA mutant ATP7B proteins were cultured in basal medium or a medium containing the copper chelators BCS (200 μM) and D-penicillamine (200 μM). Detection of ATP7B was performed as described in Figure 2.
The role of the key domains in the copper-translocation activity of ATP7B
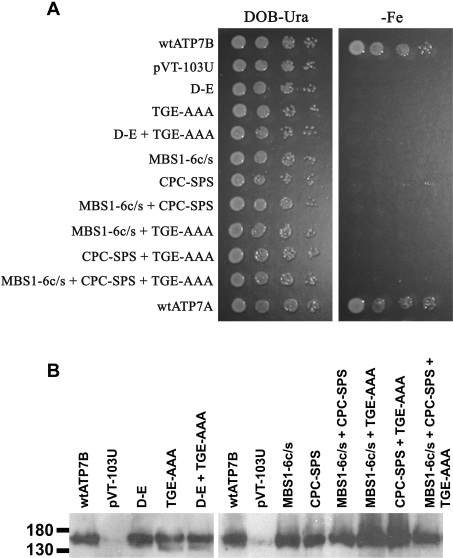
Any mutation that blocks a step in the catalytic cycle of ATP7B will inhibit the translocation of copper by the protein. Thus mut-ation of the phosphorylation (D-E) or the phosphatase domain (TGE-AAA), both of which are required for ATP7B to complete the copper-translocation cycle, should result in a non-functional protein. The aspartate residue in the phosphorylation domain of ATP7B is essential for catalytic activation [37]; however, the importance of the phosphatase domain has not been evaluated. The ATP7B key domain mutants were expressed in S. cerevisiae ccc2Δ and evaluated for their copper-transport ability by assessing the growth of the transformed yeast on iron-limited medium (Figure 6A, –Fe). The ccc2Δ strain that expressed wtATP7B (or wtATP7A) grew uniformly on iron-limited agar plates, while the ccc2Δ strains that expressed the mutant proteins did not grow on the same medium (Figure 6A, –Fe). The viability of the yeast strains was confirmed on iron-sufficient medium (Figure 6A, DOB-Ura). The failure of the ATP7B mutant proteins to comple-ment the copper-transport defect of ccc2Δ was not due to low expression levels, which were equivalent to or greater than wtATP7B (Figure 6B).
Figure 6. The ability of the ATP7B key domain mutants to complement S. cerevisiae ccc2Δ.
(A) S. cerevisiae ccc2Δ transformed with wt or mutant ATP7B constructs [amino acid substitution(s) indicated on the left] were analysed for their ability to grow on iron-depleted medium (–Fe). Each yeast strain was inoculated in 5 ml of DOB-Ura and grown at 30 °C overnight to reach saturation. Serial 10-fold dilutions in sterile distilled water were made starting from 1:10 and 5 μl of each dilution was applied on to the appropriate medium. Yeast strain viability was demonstrated by growth on iron-sufficient plates (DOB-Ura). (B) The ccc2Δ strains used in the copper-transport assay described above were analysed for ATP7B expression by Western-blot analysis. Total cellular protein (30 μg) was fractionated by SDS/PAGE (7.5% polyacrylamide) and transferred to nitrocellulose. Protein was immunolabelled with ammonium sulfate-precipitated NC36 (diluted 1:1000), followed by horseradish peroxidase-conjugated donkey anti-sheep IgG (diluted 1:4000) and analysed by chemiluminescence.
DISCUSSION
In cells exposed to high concentrations of copper, both ATP7A and ATP7B shift their steady-state localization from the TGN to either the plasma membrane (ATP7A) or to cytosolic vesicles (ATP7B) [17,19,23–25]. The copper-induced trafficking of ATP7A pre-viously was shown to be regulated with the catalytic cycle of the ATPase [11]. Consistent with this report, we show here that acyl-phosphorylation was required for the trafficking of ATP7B and dephosphorylation via the phosphatase domain was essential for TGN retrieval of trafficked protein.
The combination of the D-E and TGE-AAA mutations led to instability of the protein at 37 °C. Temperature-sensitive mutations have been identified in ATP7B and include the patient muta-tions H1069Q and M769V and several C- and N-terminal trunc-ations [6,29,38,39]. Analysis at reduced temperatures previously was used to determine the functional effect of the H1069Q and M769V mutations [6,39]. In the present study, the subcellular localization of D-E+TGE-AAA was corrected at the permissive temperature (30 °C) and we demonstrated that the constitutive vesicular accumulation of the TGE-AAA protein required the aspartate residue (Asp1027), consistent with the requirement for acyl-phosphorylation for trafficking of ATP7B to occur.
Copper binding by the copper-ATPases is believed to be res-ponsible for initiating catalytic activation and regulating their subcellular distribution [29,37,40]. The six N-terminal MBSs (GMXCXXC) and the CPC motif are currently the only recog-nized copper-binding motifs in both copper-ATPases. The TGE-AAA (phosphatase) mutation provided a valuable tool to determine whether the N-terminal GMXCXXC sites or the CPC are essential for trafficking, because it results in trafficked molecules accumulating at cytosolic vesicles. Although the MBS1-6c/s and CPC-SPS mutant proteins predominantly had perinuclear local-ization in basal and elevated copper, when each was combined with the TGE-AAA mutation to produce double mutants or together to produce a triple mutant, the resultant proteins were found at cytosolic vesicular compartments. These results show that mutation of the N-terminal MBSs (MBS1-6c/s) and/or the CPC motif (CPC-SPS) did not suppress the effect of the TGE-AAA mutation, suggesting that copper co-ordination by these sites is not essential for the formation of the acyl-phosphate interme-diate required for trafficking. Nevertheless, copper was required for the vesicular localization of the TGE-AAA mutants since copper chelation confined the proteins to the perinuclear region (Figure 5). These observations suggest that copper may bind to another region of ATP7B leading to acyl-phosphate formation and trafficking. Alternatively, the trafficking of these mutants may be stimulated by another copper-binding protein(s) (such as a copper-dependent kinase) that might interact with ATP7B to provide a signal for acyl-phosphorylation to occur. Although the presence of copper was previously shown to be critical for acyl-phosphate formation [9], our results demonstrate that acyl-phosphorylation and trafficking can occur in the absence of N-terminal or CPC copper binding, albeit this process seems less efficient. This result was unexpected, as most models of ATP7B (and ATP7A) function envisage that copper is delivered to the N-terminal MBSs and is then transferred to the CPC residues. This latter step is thought to be critical for initiating copper-dependent acyl-phosphorylation [4,6,7]. However, the results obtained in the present study clearly suggest an additional mechanism, namely that there is another copper-binding site(s) to induce acyl-phosphate formation in addition to the N-terminal MBS and the CPC motif. Several methionine and cysteine residues located within transmembrane regions are candidates for additional copper-binding sites, in particular the highly conserved Met1393 in transmembrane domain 7, since mutation of this residue is responsible for the Wilson disease phenotype in the toxic milk mouse [41] and is important for copper translocation and acyl-phosphate formation in ATP7A [10]. However, there is currently no direct evidence that any of these residues co-ordinate copper.
Further studies are required to distinguish if the anterograde movement of the copper-ATPases is directly linked to formation of the acyl-phosphorylated intermediate or whether further down-stream protein modifications are required. Possibly, a specific conformation associated with the acyl-phosphate intermediate exposes a trafficking signal or occludes a TGN retention sequence (not mutually exclusive). Vanderwerf et al. [42] have reported a correlation between kinase-dependent phosphorylation (distinct from acyl-phosphorylation) of ATP7B and its trafficking to cytosolic vesicles. These authors demonstrated that there are at least two different sites in ATP7B that are phosphorylated by unknown kinases and that elevated copper concentrations result in hyper-phosphorylation of ATP7B coinciding with vesicular redistribution [42]. Previously, we discovered that a putative targeting motif exists within the sixth metal-binding domain of ATP7B, which directs the protein to vesicular compartments [43]. Possibly the exposure or modification (e.g. kinase-dependent phosphorylation) of this region subsequent to acyl-phosphoryl-ation directs both copper-ATPases into the trafficking pathway and the difference in this region between both proteins is responsible for their respective post-Golgi destinations.
Previously, we used complementation of S. cerevisiae ccc2Δ to show that mutation of the six N-terminal MBSs inhibits the copper-translocation ability of both ATP7A and ATP7B [29,43]. Using a different assay based on copper translocation into isolated vesicles, Voskoboinik et al. [44] demonstrated that mutation of all MBSs in ATP7A reduced, but did not abolish, copper-transloc-ation activity. This observation is consistent with the constitutive vesicular localization of the ATP7B MBS1-6c/s+TGE-AAA protein, which suggested that the MBS1-6c/s protein possessed reduced activity and this activity was detected by the introduction of the TGE-AAA mutation that trapped the acyl-phosphate inter-mediate at cytosolic vesicles. Thus we propose that the predomin-ant perinuclear localization of the MBS1-6c/s and CPC-SPS mutant proteins resulted from a reduction in the rate of cata-lytic activation rather than complete abolition of activity. By slowing down acyl-phosphate formation, the rate of anterograde traf-ficking becomes less than the rate of perinuclear retrieval of trafficked protein and therefore the mutant proteins display a steady-state perinuclear localization.
Intracellular trafficking represents a switch in function for the copper-ATPases from a role in copper delivery to cuproenzymes at the TGN, to a protective role involving the transport of excess copper into vesicles (ATP7B) and across the plasma membrane (ATP7A) for excretion from the cell. The shift in the steady-state localization of the copper-ATPases from the TGN can be explained by an increase in the number of molecules that are catalytically activated (acyl-phosphorylated). By coupling trafficking with catalytic activity, this provides both autoregulation and an ap-propriate distribution of the copper-ATPase between the TGN and post-Golgi compartments following copper uptake [11]. When intracellular copper is raised to a certain level, the number of cata-lytically activated molecules is sufficient to maintain a steady-state post-Golgi subcellular localization. Trafficking allows the removal of excess copper, which results in the shift in the distri-bution of the copper-ATPases back to the TGN.
The findings presented in this report substantiate the fact that the intracellular trafficking of ATP7B requires acyl-phosphate formation. Importantly, formation of the acyl-phosphate inter-mediate was not entirely dependent on copper co-ordination by the six N-terminal MBSs or the CPC motif, although these sites clearly enhance the efficiency of the trafficking process. We have demonstrated that copper was indeed still essential for the trafficking of ATP7B and thus our data allude to the presence of an as yet unrecognized copper-binding site(s) that contributes to the efficiency of ATP7B trafficking.
Acknowledgments
This work was supported in part by the NHMRC (National Health and Medical Research Council) of Australia, the Australian Research Council and the International Copper Association. S.L.F. was supported by an NHMRC R. Douglas Wright Fellowship.
References
- 1.Petrukhin K., Lutsenko S., Chernov I., Ross B. M., Kaplan J. H., Gilliam T. C. Characterization of the Wilson disease gene encoding a copper transporting ATPase: genomic organization, alternative splicing, and structure/function predictions. Hum. Mol. Genet. 1994;3:1647–1656. doi: 10.1093/hmg/3.9.1647. [DOI] [PubMed] [Google Scholar]
- 2.Moller J. V., Juul B., le Maire M. Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim. Biophys. Acta. 1996;1286:1–51. doi: 10.1016/0304-4157(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 3.Vulpe C., Levinson B., Whitney S., Packman S., Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat. Genet. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- 4.Solioz M., Vulpe C. CPX-type ATPases: a class of P-type ATPases that pump heavy metals. Trends Biochem. Sci. 1996;21:237–241. [PubMed] [Google Scholar]
- 5.Lutsenko S., Petrukhin K., Cooper M. J., Gilliam C. T., Kaplan J. H. N-terminal domains of human copper-transporting adenosine triphosphatases (the Wilson's and Menkes disease proteins) bind copper selectively in vivo and in vitro with stoichiometry of one copper per metal-binding repeat. J. Biol. Chem. 1997;272:18939–18944. doi: 10.1074/jbc.272.30.18939. [DOI] [PubMed] [Google Scholar]
- 6.Forbes J. R., Cox D. W. Functional characterization of missense mutations in ATP7B: Wilson disease mutation or normal variant? Am. J. Hum. Genet. 1998;63:1663–1674. doi: 10.1086/302163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myari A., Hadjiliadis N., Fatemi N., Bibudhendra S. Copper(I) interaction with model peptides of WD6 and TM6 domains of Wilson ATPase: regulatory and mechanistic implications. J. Inorg. Biochem. 2004;98:1483–1494. doi: 10.1016/j.jinorgbio.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Scarborough G. A. Structure and function of the P-type ATPases. Curr. Opin. Cell Biol. 1999;11:517–522. doi: 10.1016/S0955-0674(99)80075-1. [DOI] [PubMed] [Google Scholar]
- 9.Lutsenko S., Tsivkovskii R., Walker J. M. Functional properties of the human copper-transporting ATPase ATP7B (the Wilson's disease protein) and regulation by metallochaperone Atox1. Ann. N.Y. Acad. Sci. 2003;986:204–211. doi: 10.1111/j.1749-6632.2003.tb07161.x. [DOI] [PubMed] [Google Scholar]
- 10.Voskoboinik I., Mar J., Strausak D., Camakaris J. The regulation of catalytic activity of the Menkes copper-translocating P-type ATPase. J. Biol. Chem. 2001;276:28620–28627. doi: 10.1074/jbc.M103532200. [DOI] [PubMed] [Google Scholar]
- 11.Petris M. J., Voskoboinik I., Cater M., Smith K., Kim B.-E., Llanos R. M., Strausak D., Camakaris J., Mercer J. F. B. Copper-regulated trafficking of the Menkes disease copper ATPase is associated with formation of a phosphorylated catalytic intermediate. J. Biol. Chem. 2002;277:46736–46742. doi: 10.1074/jbc.M208864200. [DOI] [PubMed] [Google Scholar]
- 12.Bull P. C., Thomas G. R., Rommens J. M., Forbes J. R., Cox D. C. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993;5:327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- 13.Tanzi R. E., Petrukhin K., Chernov I., Pellequer J. L., Wasco W., Ross B., Romano D. M., Parano E., Pavone L., Brzustowicz L. M., et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 14.Ackland M. L., Anikijenko P., Michalczyk A., Mercer J. F. B. Expression of Menkes copper-transporting ATPase, MNK, in the lactating human breast: possible role in copper transport into milk. J. Histochem. Cytochem. 1999;47:1553–1561. doi: 10.1177/002215549904701207. [DOI] [PubMed] [Google Scholar]
- 15.Hardman B., Manuelpillai U., Wallace M. E., Vaan de Waasenberg S., Cater M., Mercer F. B. J., Ackland M. L. Expression and localization of the Menkes and Wilson copper transporting ATPases in human placenta. Placenta. 2004;24:512–517. doi: 10.1016/j.placenta.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Paynter J. A., Grimes A., Lockhart P., Mercer J. F. B. Expression of the Menkes gene homologue in mouse tissues lack of effect of copper on the mRNA levels. FEBS Lett. 1994;351:186–190. doi: 10.1016/0014-5793(94)00868-x. [DOI] [PubMed] [Google Scholar]
- 17.Forbes J. R., Cox D. W. Copper-dependent trafficking of Wilson disease mutant ATP7B proteins. Hum. Mol. Genet. 2000;9:1927–1935. doi: 10.1093/hmg/9.13.1927. [DOI] [PubMed] [Google Scholar]
- 18.Hung I. H., Suzuki M., Yamaguchi Y., Yuan D. S., Klausner R. D., Gitlin J. D. Biochemical characterization of the Wilson disease protein and functional expression in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1997;272:21461–21466. doi: 10.1074/jbc.272.34.21461. [DOI] [PubMed] [Google Scholar]
- 19.Petris M. J., Mercer J. F. B., Culvenor J. G., Lockhart P., Gleeson P. A., Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- 20.Royce P. M., Steinmann B. Markedly reduced activity of lysyl oxidase in skin and aorta from a patient with Menkes' disease showing unusually severe connective tissue manifestations. Pediatr. Res. 1990;28:137–141. doi: 10.1203/00006450-199008000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Terada K., Nakako T., Yang X.-L., Iida M., Aiba N., Minamiya Y., Nakai M., Sakaki T., Miura N., Sugiyama T. Restoration of holoceruloplasmin synthesis in LEC rat after infusion of recombinant adenovirus bearing WND cDNA. J. Biol. Chem. 1998;273:1815–1820. doi: 10.1074/jbc.273.3.1815. [DOI] [PubMed] [Google Scholar]
- 22.Greenough M., Pase L., Voskobionik I., Petris M., Wilson Obrien A., Camakaris J. Signals regulating trafficking of the Menkes (MNK; ATP7A) copper translocating P-type ATPase in polarized MDCK cells. Am. J. Physiol. Cell Physiol. 2004;287:C1463–C1471. doi: 10.1152/ajpcell.00179.2004. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer M., Hopkins R. G., Failla M. A., Gitlin J. D. Hepatocyte-specific localization and copper-dependent trafficking of the Wilson's disease protein in the liver. Am. J. Physiol. 1999;276:G639–G646. doi: 10.1152/ajpgi.1999.276.3.G639. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer M., Roelofsen H., Wolters H., Hofmann W. J., Müller M., Kuipers F., Stremmel W., Vonk R. J. Localization of the Wilson's disease protein in human liver. Gastroenterology. 1999;117:1380–1385. doi: 10.1016/s0016-5085(99)70288-x. [DOI] [PubMed] [Google Scholar]
- 25.Cater M., La Fontaine S., Shield K., Deal Y., Mercer F. B. J. ATP7B mediates vesicular sequestration of copper; insight into biliary copper excretion. Gastroenterology. 2006;130:493–506.. doi: 10.1053/j.gastro.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 26.Petris M., Mercer J. F. B. The Menkes protein (ATP7A, MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C-terminal di-leucine endocytic signal. Hum. Mol. Genet. 1999;8:2107–2115. doi: 10.1093/hmg/8.11.2107. [DOI] [PubMed] [Google Scholar]
- 27.Fu D., Beeler T., Dunn T. Sequence, mapping and disruption of CCC2, a gene that cross-complements the Ca2+ sensitive phenotype of csg1 mutants and encodes a P-type ATPase belonging to the Cu2+ ATPase subfamily. Yeast. 1995;11:283–292. doi: 10.1002/yea.320110310. [DOI] [PubMed] [Google Scholar]
- 28.Elble R. A simple and efficient procedure for transformation of yeasts. BioTechniques. 1992;13:18–20. [PubMed] [Google Scholar]
- 29.Cater M., Forbes J. R., La Fontaine S., Cox D. C., Mercer J. F. B. Intracellular trafficking of the human Wilson protein: the role of the six N-terminal metal binding sites. Biochem. J. 2004;380:805–813. doi: 10.1042/BJ20031804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vernet T., Dignard D., Thomas D. Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52:225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- 31.La Fontaine S., Theophilos M. B., Firth S. D., Gould R., Parton R. G., Mercer J. F. B. Effect of the toxic milk mutation (tx) on the function and intracellular localization of Wnd, the murine homologue of the Wilson copper ATPase. Hum. Mol. Genet. 2001;10:361–370. doi: 10.1093/hmg/10.4.361. [DOI] [PubMed] [Google Scholar]
- 32.Clarke D. M., Loo T. W., MacLennan D. H. Functional consequences of alterations to amino acids located in the nucleotide binding domain of the Ca(2+)-ATPase of sarcoplasmic reticulum. J. Biol. Chem. 1990;25:22223–22227. [PubMed] [Google Scholar]
- 33.Portillo F., Serrano R. Dissection of functional domains of the yeast proton-pumping ATPase by direct mutagenesis. EMBO J. 1989;6:1793–1798. doi: 10.1002/j.1460-2075.1988.tb03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matraki A., King J. Amino acid substitutions influencing intracellular protein folding pathways. FEBS Lett. 1992;307:20–25. doi: 10.1016/0014-5793(92)80894-m. [DOI] [PubMed] [Google Scholar]
- 35.Hass R., Gutierrez-Rivero B., Knoche J., Boker K., Manns M. P., Schmidt H. H. Mutation analysis in patients with Wilson disease: identification of 4 novel mutations. Hum. Mutat. 1999;14:88. doi: 10.1002/(SICI)1098-1004(1999)14:1<88::AID-HUMU15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 36.Tumer Z., Moller L. B., Horn N. Mutation spectrum of ATP7A, the gene defective in Menkes disease. Adv. Exp. Med. Biol. 1999;448:83–95. doi: 10.1007/978-1-4615-4859-1_7. [DOI] [PubMed] [Google Scholar]
- 37.Huster D., Lutsenko S. The distinct roles of the N-terminal copper-binding sites in regulation of catalytic activity of the Wilson's disease protein. J. Biol. Chem. 2003;278:32212–32218. doi: 10.1074/jbc.M305408200. [DOI] [PubMed] [Google Scholar]
- 38.Hsi G., Lara M., Cullen D., Moira G., Cox D. C. Functional assessment of the carboxy-terminus of the Wilson disease copper-transporting ATPase, ATP7B. Genomics. 2004;83:473–481. doi: 10.1016/j.ygeno.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Payne A. S., Kelly E. J., Gitlin J. D. Functional expression of the Wilson disease protein reveals mislocalization and impaired copper-dependent trafficking of the common H1069Q mutation. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10854–10859. doi: 10.1073/pnas.95.18.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strausak D., Fontaine S. L., Hill J., Firth S. D., Lockhart P. J., Mercer J. F. B. The role of GMXCXXC metal binding sites in the copper-induced redistribution of the Menkes protein. J. Biol. Chem. 1999;274:11170–11177. doi: 10.1074/jbc.274.16.11170. [DOI] [PubMed] [Google Scholar]
- 41.Theophilos M. B., Cox D. W., Mercer J. F. B. The toxic milk mouse is a murine model of Wilson disease. Hum. Mol. Genet. 1996;5:1619–1624. doi: 10.1093/hmg/5.10.1619. [DOI] [PubMed] [Google Scholar]
- 42.Vanderwerf S. M., Cooper M. J., Stetsenko I. V., Lutsenko S. Copper specifically regulates intracellular phosphorylation of the Wilson's disease protein, a human copper-transporting ATPase. J. Biol. Chem. 2001;276:36289–36294. doi: 10.1074/jbc.M102055200. [DOI] [PubMed] [Google Scholar]
- 43.Mercer J. F. B., Barnes N., Steverson J., Strausak D., Llanos R. Copper-induced trafficking of the Cu-ATPases: a key mechanism for copper homeostasis. Biometals. 2003;16:175–184. doi: 10.1023/a:1020719016675. [DOI] [PubMed] [Google Scholar]
- 44.Voskoboinik I., Strausak D., Greenough M., Brooks H., Petris M., Smith S., Mercer J. F., Camakaris J. Functional analysis of the N-terminal CXXC metal-binding motifs in the human Menkes copper-transporting P-type ATPase expressed in cultured mammalian cells. J. Biol. Chem. 1999;274:22008–22012. doi: 10.1074/jbc.274.31.22008. [DOI] [PubMed] [Google Scholar]
- 45.Wang R. F., Kushner S. R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]