Abstract
Proteins of the SOCS (suppressors of cytokine signalling) family are characterized by a conserved modular structure with pre-SH2 (Src homology 2), SH2 and SOCS-box domains. Several members, including CIS (cytokine-inducible SH2 protein), SOCS1 and SOCS3, are induced rapidly upon cytokine receptor activation and function in a negative-feedback loop, attenuating signalling at the receptor level. We used a recently developed mammalian two-hybrid system [MAPPIT (mammalian protein–protein interaction trap)] to analyse SOCS protein-interaction patterns in intact cells, allowing direct comparison with biological function. We find that, besides the SH2 domain, the C-terminal part of the CIS SOCS-box is required for functional interaction with the cytokine receptor motifs examined, but not with the N-terminal death domain of the TLR (Toll-like receptor) adaptor MyD88. Mutagenesis revealed that one single tyrosine residue at position 253 is a critical binding determinant. In contrast, substrate binding by the highly related SOCS2 protein, and also by SOCS1 and SOCS3, does not require their SOCS-box.
Keywords: cytokine-inducible Src homology 2 protein (CIS), cytokine receptor, mammalian protein–protein interaction trap (MAPPIT), myeloid differentiation marker 88 (MyD88), signal transduction, suppressor of cytokine signalling box (SOCS-box)
Abbreviations: CIS, cytokine-inducible Src homology 2 protein; CSF, colony-stimulating factor; DD, death domain; EMSA, electrophoretic mobility-shift assay; Epo, erythropoietin; EpoR, Epo receptor; GH, growth hormone; GHR, GH receptor; gp130, glycoprotein 130; HEK-293, human embryonic kidney; HIF, hypoxia inducible factor; IL, interleukin; JAK, Janus kinase; KIR, kinase inhibitory region; LPS, lipopolysaccharide; LR, leptin receptor; MAPPIT, mammalian protein–protein interaction trap; MyD88, myeloid differentiation marker 88; NF-κB, nuclear factor κB; ODDD, oxygen-dependent degradation domain; rPAPI, rat pancreatitis associated protein I; SH2, Src homology 2; SOCS, suppressor of cytokine signalling; STAT, signal transducer and activator of transcription; TAP2, tandem affinity purification 2; TIR, Toll/IL-1 receptor; TLR, Toll-like receptor; VHL, von Hippel–Lindau
INTRODUCTION
A wide spectrum of α-helical bundle cytokines contributes to growth, differentiation and survival of haemopoietic cells. Examples include the CSFs (colony-stimulating factors), Epo (erythropoietin) and several ILs (interleukins) such as IL-5. More recently, leptin, a hormone-like member of this family, was also shown to promote proliferation of haemopoietic progenitors [1–3]. All of these cytokines activate the highly conserved JAK (Janus kinase)/STAT (signal transducer and activator of transcription) signalling pathway upon receptor binding. Signalling via these receptors is under tight control, including negative feedback by rapidly induced SOCS (suppressor of cytokine signalling) proteins. CIS [cytokine-inducible SH2 (Src homology 2) protein] was the founding member of the SOCS protein family that consists of eight members: SOCS1–SOCS7 and CIS [4,5]. All SOCS proteins comprise an SH2-domain responsible for association with phosphotyrosine motifs, an N-terminal pre-SH2 domain and a C-terminal SOCS-box [5]. They can modulate receptor activation and signalling via at least three distinct mechanisms.
CIS can inhibit Epo and GH (growth hormone) signalling by competition for STAT5-docking sites at the receptor level [6–8]. Consistent with this, CIS suppresses Epo-induced cell proliferation and promotes apoptosis of erythroid progenitor cells [9,10]. Phenotypes of CIS-transgenic mice and of STAT5a- and/or STAT5b-knockout mice show clear similarities, lending further support for CIS as a specific negative-feedback regulator of STAT5-mediated cytokine signalling [4,6,7,11]. Direct interference with STAT5 recruitment has also been suggested for SOCS2-mediated inhibition of GH action [12,13]. Interestingly, SOCS2 shows a dual effect on GH signalling. Mice lacking SOCS2 and SOCS2-transgenic mice both exhibit increased growth [12,14,15]. This may be explained through direct binding and functional interference between SOCS proteins [16,17].
CIS-dependent receptor degradation was reported for the EpoR (Epo receptor) and GHR (GH receptor) [6,18]. The SOCS-box of SOCS proteins can interact with the Elongins B and C [19], which form a complex with proteins of the Cullin and Rbx families. This assembly is an E3 ubiquitin ligase complex that is responsible for specific targeting of associated proteins for polyubiquitination [20]. This way, several SOCS proteins can inhibit signalling by marking associated signalling components for proteasomal degradation.
SOCS1 and SOCS3 carry a KIR (kinase inhibitory region) domain in their N-terminal region that acts as a pseudo-substrate for direct inhibition of JAK activity. SOCS1 interacts directly with the phosphorylated activation loop of JAK2 via its SH2 domain [21], whereas SOCS3 shows only weak affinity for JAK2 and is thought to bind to the receptor in close proximity to the kinase [22]. This is exemplified for SOCS3, which was identified recently as a potent inhibitor of LR (leptin receptor) signalling involved in regulation of energy balance: SOCS3 haplo-insufficient mice or neural-cell-specific SOCS3-knockout mice show augmented leptin sensitivity in the hypothalamus associated with a remarkable attenuation of diet-induced obesity, suggesting a key role for SOCS3 in leptin resistance [23,24].
SOCS proteins are also involved in regulating JAK/STAT-independent pathways such as insulin and TNF-α (tumour necrosis factor α) signalling [25–27]. Also, triggering of TLRs (Toll-like receptors), which are key players in innate immunity, leads to the induction of CIS, SOCS1 and SOCS3 [28–30]. Evidence linking SOCS proteins to TLR signalling arose from the analysis of SOCS1-deficient mice that show enhanced sensitivity to LPS (lipopolysaccharide)-induced sepsis [31,32] and from SOCS1-deficient mice that lack endotoxin tolerance. Moreover, macrophages lacking SOCS1 produce increased levels of nitric oxide and pro-inflammatory cytokines in response to LPS. Conversely, SOCS1 overexpression in macrophages suppresses LPS-induced NF-κB (nuclear factor κB) activation. On the basis of these data, SOCS1 was considered to be a negative regulator of TLR signalling, although no direct target of SOCS1 could be identified. Recently, two groups have reported that SOCS1 has an indirect inhibitory effect on TLR signalling, targeting the secondary type I interferon signalling pathway and not the main NF-κB pathway [30,33].
In the present study, we examined the binding modalities of SOCS proteins in more detail. We demonstrate that the SOCS-box of CIS, and more particularly its C-terminal tyrosine residue, is essential for interaction with recruitment motifs in the EpoR and LR, and for its inhibitory role on STAT5 activation. In contrast, the SOCS-box is not required for SOCS2 receptor interaction or for signalling inhibition by SOCS1 and SOCS3. Furthermore, we identified the universal TLR adaptor MyD88 (myeloid differentiation marker 88) as a target for CIS. This interaction is SOCS-box-independent, indicating a different binding modus compared with that of the cytokine receptors.
EXPERIMENTAL
Constructs
Generation of the mutant murine LRs by mutagenesis and cloning in the pMET7 expression vector has been published elsewhere [34]. EpoR-bait constructs containing two extra leucine residues in the transmembrane region were described previously [35]. The pXP2d2-rPAPI-luciferase reporter, originating from the rPAPI (rat pancreatitis associated protein I) promoter was used as described previously [36]. The pGL3-β-casein-luciferase reporter consisting of five repeats of the STAT5-responsive motif of the β-casein promoter was a gift from Dr Ivo Touw. Generation of the prey constructs pMG2-CIS and pMG2-SOCS2 was described in [35].
CISd5, CISdbox (amino acids 1–221), CISY249F and CISY253F prey constructs were generated by site-directed mutagenesis on pMG1-CIS using the following primer pairs. CISd5: 5′-GACTACCTCCGACAGTGATATCTCCAACTCTGATCTAG-3′ and 5′-CTAGATCAGAGTTGGAGATATCACTGTCGGAGGTAGTC-3′. CISdbox: 5′-GTGCGCAGGAGCAGTTGATATCGCTTACAACATCTGTG-3′ and 5′-CACAGATGTTGTAAGCGATATCAACTGCTCCTGCGCAC-3′. CISY249F: 5′-GGCGTATGGCCGACTTCTTAAGACAGTACCCCTTCC-3′ and 5′-GGAAGGGGTACTGTCTTAAGAAGTCGGCCATACGCC-3′. CISY253F: 5′-GACTACCTCCGACAGTTCCCCTTCCAGCTGTGATCTAGAGAAAAAACCTCC-3′ and 5′-GGAGGTTTTTTCTCTAGATCACAGCTGGAAGGGGAACTGTCGGAGGTAGTC-3′.
The CIS mutants were then transferred to the pMG2 vector and the pMET7-FLAG expression vector by EcoRI/XbaI cloning. SOCS2Y194F was generated using the previously described pMG1-SOCS2 as template [36] and the primer set 5′-GCAGAATTCACCCTGCGGTGCCTGGAGCC-3′ and 5′-CGCTGCGGCCGCTTATACCTGGAATTTGAATTCTTCCAAGTAATC-3′, and was first cloned in the pMG1 vector as an EcoRI/NotI fragment, and then transferred to the pMG2 vector using EcoRI/XbaI. SOCS2Y190F and SOCS2dbox (amino acids 1–159) were amplified from the pEF-FLAG-I/mSOCS2 construct (a gift from Dr Robyn Starr) using the 5′-GCAGAATTCACCCTGCGGTGCCTGGAGCC-3′ and the 5′-GGTCGTCTAGAGCGGCCGCTTATACCTGGAATTTATATTCTTCCAAGAAATCTTTTAGTCTTGTTG-3′ and 5′-GCTGGGCGGCCGCTTATGATGTATACAGAGGTTTGG-3′ primers respectively, and were also cloned into the pMG2 vector. SOCS2 was transferred from the pMG2 vector to the pMET7-FLAG expression vector as an EcoRI/XbaI or EcoRI/NotI fragment.
The pMET7-FLAG-SOCS3 expression vector was described previously [37]. SOCS3dbox was amplified from the pMET7-FLAG-SOCS3 template using primers 5′-GCGAGATCTCAGAATTCGTCACCCACAGCAAGTTTCC-3′ and 5′-CGCTTCTAGATTAGTTGGAGGAGAGAGGTCGG-3′ allowing EcoRI/XbaI-based cloning in the pMet7-FLAG vector.
The pMet7-FLAG/mSOCS1 and pMet7-FLAG/mSOCS1dbox constructs were generated by amplifying SOCS1 and SOCS1dbox from the pEF-FLAG-I/mSOCS1 construct (gift from Dr Robyn Starr) with the 5′-CCAGCGAATTCATGGCGCGCCAGGACTACAAGGAC-3′ and 5′-GGTCGTCTAGATCAGATCTGGAAGGGGAAGGAAC-3′ or 5′-GGTCGTCTAGATCAGCGGCGCTGGCGCAGCGGGGCCCCCAAC-3′ primer sets respectively and EcoRI/XbaI cloning in the pMet7-FLAG vector.
EpoR cDNA was amplified from TF1-derived using primers 5′-CGGGGTACCATGGACCACCTCGGGGCGTCC-3′ and 5′-CGCTCTAGACTAAGAGCAAGCCACATAGC-3′ and was cloned in the pSVsport vector by KpnI/XbaI cloning. The pECE-STAT5B expression vector was a gift from Dr Walter Becker.
Mouse full-length MyD88 was amplified using the 5′-GCGCGAGCTCAATGTCTGCGGGAGACCCCCGCG-3′ and 5′-GCGTGCGGCCGCTCAGGGCAGGGACAAAGCC-3′ primer pair on a pCAGGSE-mMyD88 expression vector (a gift from Dr Rudy Beyaert). After SacI/NotI digestion, the fragment was cloned in the pCEL(2L) vector, which was described previously [35]. This resulted in the mMyD88 bait construct. The mMyD88(N) and the mMyD88TIR bait vectors were made in an analogous manner using primer pairs 5′-GCGCGAGCTCAATGTCTGCGGGAGACCCCCG-3′/5′-GCTCGCGGCCGCTTACGTTTGTCCTAGGGGGTC-3′ and 5′-GCGCGAGCTCAATGCCGGAACTTTTCGATGCC-3′/5′-GCTCGCGGCCGCTCAGGGCAGGGACAAAGCC-3′ respectively.
The pCAGGSE-mMyD88(N) and pCAGGSE-mMyD88 DD (death domain) expression vectors were generated by amplification using oligonucleotide pairs 5′-GGCAAAGAATTGAATTCCACCATGGGTGCGC-3′ and 5′-GCGCCTCGAGTCAAAGTTCCGGCGTTTGTCCTAGGGGGTC-3′, and 5′-GGCAAAGAATTGAATTCCACCATGGGTGCGC-3′ and 5′-GCGCCTCGAGTCAGACAGACGCGCCAGAGCGCCCCTGCC-3′ respectively on pCAGGSE-mMyD88, followed by a EcoRI/XhoI digestion and ligation into the pCAGGSE vector.
Generation of the pMET7TAP2 construct was described in [17]. CIS, CISdbox, CISY249F and CISY253F were introduced in a TAP2 (tandem affinity purification 2) construct via EcoRI/KpnI cloning from the respective pMG2 constructs.
An overview of the bait constructs used in this study is given in Table 1.
Table 1. Overview of the bait constructs used in the present study.
(a) Extracellular EpoR–intracellular LRF3 (Y985F/Y1077F/Y1138F) bait constructs
| Protein | Construction |
|---|---|
| Mock | No bait |
| EpoR Tyr402 | Tyr402 motif of the EpoR |
| EpoR Tyr430/Tyr432 | Tyr430/Tyr402 motif of the EpoR |
| mMyD88 | MyD88 |
| mMyD88(N) | N-terminal part of MyD88 (DD and intermediate domain) |
| mMyD88(TIR) | C-terminal TIR domain of MyD88 |
| (b) Extracellular LR–intracellular LR bait constructs | |
| Protein | Construction |
| LR(F3) | LR (Y985F/Y1077F/Y1138F) |
| LR(YYF) | LR (Y1138F) |
| LR(YFF) | LR (Y1077F/Y1138F) |
| LR(FYF) | LR (Y985F/Y1138F) |
| (c) Non-MAPPIT receptor constructs | |
| Protein | Construction |
| EpoR | WT EpoR |
| LR(FFY) | LR (Y985F/Y1077F) |
| LR(YFY) | LR (Y1077F) |
Cell culture, transfection and reporter assays
Cell culture conditions, transfection procedures and luciferase assays for HEK-293T (human embryonic kidney) cells were described previously [37]. For a typical luciferase experiment, 4×105 cells were seeded in six-well plates 24 h before overnight transfection with the desired constructs together with the luciferase reporter gene. Cells were left untreated (negative control) or were stimulated overnight with 100 ng/ml leptin or 3.3 ng/ml Epo, and luciferase activity of the transfected cells was measured by chemiluminescence.
Co-immunoprecipitation
Approx. 2×106 HEK-293T cells were transfected with different combinations of mMyD88-E, mMyD88(N), mMyD88 DD, mCIS–FLAG, mCISY253F and mCISdbox. Cleared lysates [modified RIPA lysis buffer: 200 mM NaCl, 50 mM Tris/HCl, pH 8, 0.05% SDS, 2 mM EDTA, 1% Nonidet P40, 0.5% sodium deoxycholate and Complete™ Protease Inhibitor Cocktail (Roche)] were incubated with 4 μg/ml anti-FLAG mouse monoclonal antibody (Sigma) and Protein G–Sepharose (Amersham Biosciences). After immunoprecipitation, SDS/PAGE and Western blotting, interactions were detected using anti-E-Tag antibody (Amersham Biosciences) and horseradish-peroxidase-conjugated anti-(mouse IgG) antibody (Amersham Biosciences).
Phosphopeptide affinity chromatography
The phosphopeptide affinity chromatography procedure was as described previously [37].
EMSA (electrophoretic mobility-shift assay)
HEK-293T cells transiently transfected with the desired constructs were starved for 4 h in serum-free medium and were subsequently stimulated with 5 ng/ml Epo for 15 min or were left untreated. Protein concentrations of the nuclear extracts were measured with the Bio-Rad protein assay. Double-stranded oligonucleotides based on the β-casein promoter (sense: 5′-CAGATTTCTAGGAATTC-3′; antisense: 5′-GGATTTGAATTCCTAGAAATC-3′) were labelled by filling in 5′ protruding ends with Klenow enzyme using [α-32P]dATP (3000 Ci/mmol; 10 mCi/ml). This probe binds STAT5 homodimers. Nuclear extracts (5 μg of protein) were incubated with approx. 10 fmol (20000 c.p.m.) of probe in gel-shift incubation buffer [10 mM Hepes, pH 7.8, 1 mM EDTA, 5 mM MgCl2, 5% glycerol, 5 mM dithiothreitol, 2 mM Pefabloc® SC, 1 mg/ml BSA and 0.1 mg/ml poly(dI-dC)·(dI-dC)] for 10 min at room temperature (25 °C). The supershifting anti-STAT5 antibodies were incubated with the nuclear extracts for 10 min at room temperature before addition of the radiolabelled β-casein probe. The protein–DNA complexes were separated on a 4.5% (w/v) polyacrylamide gel containing 7.5% glycerol in 0.5-fold TBE (Tris/borate/EDTA) at 20 V/cm for 90 min. Gels were fixed in water/methanol/ethanoic (acetic) acid (80:10:10, by vol.) for 30 min, dried and autoradiographed.
TAP2 purification and MS
HEK-293T cells were transfected with the appropriate TAP2 constructs. The TAP2 purification procedure was followed as described previously [17]. Proteins were visualized on a polyacrylamide gel by silver staining, or for MS analysis with Sypro Ruby protein gel stain (Molecular Probes) according to the manufacturer's instructions. Proteins of interest were excised, prepared for MS and applied for nano-LC-MS/MS analysis on an UltiMate™ system (Dionex) connected inline to an Esquire HCT (high-capacity trap) ion trap (Bruker Daltonics).
Modelling method
Molecular models were built for the CIS–Elongin B–Elongin C complex, using the crystal structure of the SOCS2–Elongin B–Elongin C complex as a template [38]. The sequences of CIS and SOCS2 were aligned automatically using the sequence-alignment editor of MOE (Molecular Operating Environment; Chemical Computing Group). Using this alignment, 150 models were built for the CIS–Elongin B–Elongin C complex in MODELLER version 8.1 [39], and ten models with the best DOPE (discrete optimized protein energy) and molpdf (molecular probability density function) scores were selected and evaluated.
RESULTS
Design of MAPPIT (mammalian protein–protein interaction trap) experiments
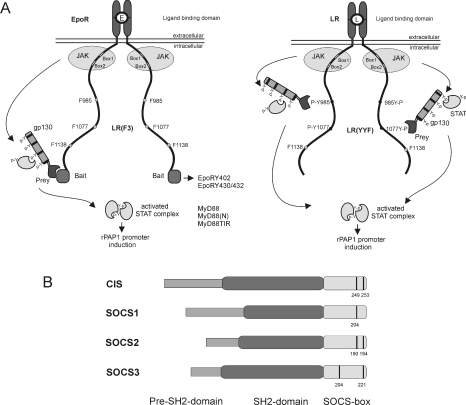
We previously reported a new two-hybrid method to study protein–protein interactions in intact mammalian cells, termed MAPPIT [36]. Briefly, a bait protein is C-terminally linked to a chimaeric EpoR/LR that is deficient in STAT3-recruitment sites, while a prey protein is attached to the string of four functional STAT3-recruitment sites of gp130 (glycoprotein 130). Association of bait and prey leads to STAT3 activation and subsequent activation of the STAT3-responsive rPAPI promoter–luciferase reporter. To examine interactions with the EpoR, we used its intracellular receptor tyrosine motifs as baits. We also analysed interactions with the LR itself by mutating the STAT3-recruiting Tyr1138 to phenylalanine. One or both of the two membrane-proximal tyrosine residues at positions 985 and 1077 were mutated to phenylalanine to examine tyrosine-specific interactions. These LR mutants were termed LR(YFF) and LR(FYF). MAPPIT configurations used in this manuscript are shown in Figure 1(A).
Figure 1. MAPPIT.
(A) MAPPIT, a cytokine receptor-based two-hybrid method, is displayed in the left-hand panel, with the various receptor motifs used in the present study. The right-hand panel shows a variant of the MAPPIT technique using the STAT3 signalling-deficient LR as bait. Both MAPPIT methods are described in more detail in the Results section. (B) Schematic structure of SOCS proteins. Conserved tyrosine residues in the SOCS-box are indicated with a black line.
The C-terminus of CIS but not that of SOCS2 is required for receptor binding
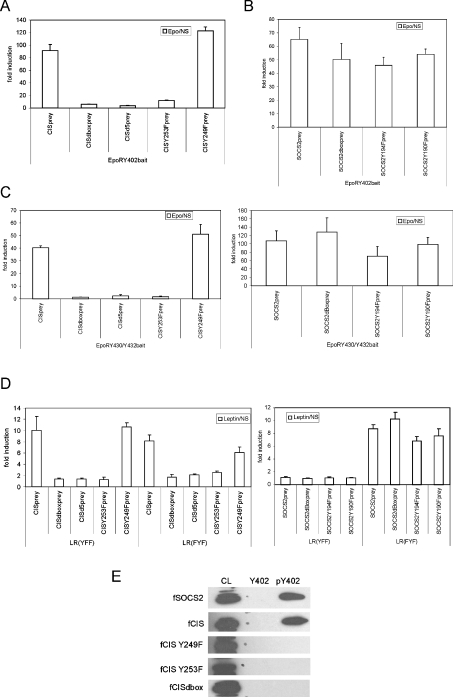
We recently showed interaction of CIS and SOCS2 with the EpoR and the LR [17,35]. CIS and SOCS2 both interact with Tyr402, and to a lesser extent also with Tyr344 and the double Tyr430/Tyr432 motifs of the EpoR, and with Tyr1077 in the LR. Although both are highly related, only CIS associated with Tyr985 of the LR and only SOCS2 with the pTyr480 motif of the EpoR [17,35]. In the present study, we examined the binding modus of CIS and SOCS2 in more detail. It is well established that interaction of SOCS proteins with their receptor targets depends on their SH2 domains [40,41]. Figure 1(B) shows a diagrammatic representation of the structure of SOCS proteins. For MAPPIT use, CIS and SOCS2 preys were generated by N-terminal fusion with part of gp130. Figure 2(A) shows the critical requirement of the CIS SOCS-box for CISprey binding to the EpoR Tyr402 motif in a MAPPIT experiment. C-terminal deletion of the entire SOCS-box resulted in complete loss of MAPPIT signalling. Detailed mapping showed that deletion of the five C-terminal amino acids and even a single Y253F mutation leads to impaired MAPPIT signalling (Figure 2A). In contrast, no effect was observed upon replacing the second conserved tyrosine residue at position 294 with phenylalanine. We next performed a similar analysis for SOCS2. Here, deletion of the entire SOCS-box, or tyrosine-to-phenylalanine mutation of both conserved tyrosine residues did not significantly affect signalling (Figure 2B). Very similar observations were obtained in MAPPIT experiments for the EpoR Tyr430/Tyr432 motif (Figure 2C). In Figure 2(D), we expand this dataset further to the LR Tyr985 and Tyr1077 positions, demonstrating that these findings are not limited to the EpoR system.
Figure 2. The CIS C-terminus is critical for interaction with EpoR and LR.
(A) Interaction of CISprey mutants with EpoR Tyr402. HEK-293T cells were transiently co-transfected with plasmids encoding the EpoR Tyr402 bait, various mutants of the pMG2-CIS prey construct and with the pXP2d2-rPAPI-luciferase reporter. After transfection, cells were left untreated (NS) or were stimulated with Epo for 24 h. Luciferase activities were measured in triplicate. All preys were also tested for interaction with a ‘mock bait’ lacking an EpoR tyrosine motif, and consistently showed absence of any signalling (results not shown). Data are expressed as the stimulated/NS ratio+S.D. for triplicate measurements. (B) Interaction of SOCS2 prey mutants with EpoR Tyr402. As in (A), except HEK-293T cells were transiently co-transfected with plasmids encoding the EpoR Tyr402 bait, various mutants of the pMG2-SOCS2 prey construct and with the pXP2d2-rPAPI-luciferase reporter. (C) Interaction of CISprey or SOCS2prey mutants with EpoR Tyr430/Tyr432. As in (A), except HEK-293T cells were transiently co-transfected with plasmids encoding the EpoR Tyr430/Tyr432 bait, various mutants of the pMG2-CIS and pMG2-SOCS2 prey constructs and with the pXP2d2-rPAPI-luciferase reporter. (D) Interaction of CIS/SOCS2 prey mutants with LR(YFF) and LR(FYF). HEK-293T cells were transiently co-transfected with plasmids encoding different LR tyrosine mutants, various mutants of the pMG2-CIS or pMG2-SOCS2 prey constructs and with the pXP2d2-rPAPI-luciferase reporter. The transfected cells were either stimulated for 24 h with leptin or left untreated (NS). Luciferase measurements were performed in triplicate. All preys were also tested for interaction with the LR lacking intracellular tyrosine residues and consistently showed absence of any signaling (results not shown). Data are expressed as the stimulated/NS ratio+S.D. (E) Peptide-affinity chromatography. HEK-293T cells were transfected with various mutants of CIS or SOCS2. The lysates were incubated with the (phospho-)tyrosine peptides corresponding to the Tyr402 motif of the EpoR. Specific protein binding was revealed by SDS/PAGE and immunoblotting using the anti-FLAG antibody.
CIS binding to the EpoR pTyr402 motif was also evaluated by phosphopeptide affinity chromatography. Figure 2(E) clearly shows loss of CIS binding by deletion of the SOCS-box or by introduction of the single Y253F mutation. In contrast to the MAPPIT dataset, complete loss of binding is also observed with the Y249F mutant. This may be explained by a lowered binding affinity so that the interaction with the EpoR pTyr402 motif is still detected with MAPPIT, but not with peptide affinity chromatography. In line with such an assumption, MAPPIT detects interactions without the need for any purification step.
No role for the SOCS1 and SOCS3 SOCS-box for receptor binding
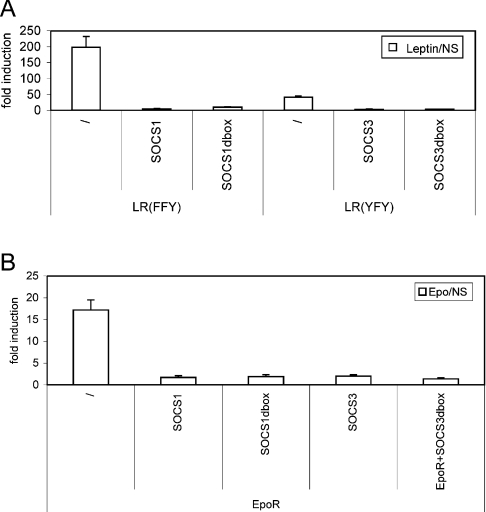
We also examined the role of the SOCS-box for the inhibitory function of SOCS1 and SOCS3. In this assay system, with clear inhibition of EpoR and LR signal transduction by co-expression of SOCS1 and SOCS3, deletion of the complete SOCS-box of SOCS1 or SOCS3 did not significantly alter the inhibitory effect (Figure 3). We conclude that the SOCS-box of SOCS1 and SOCS3, in analogy with SOCS2, does not contain critical determinants involved in substrate binding. This highlights the unique new property of the CIS SOCS-box, which we next evaluated in more detail.
Figure 3. The C-terminus of SOCS1 and SOCS3 is not essential for signalling inhibition.
Inhibition of EpoR (A) and LR (B) signalling by SOCS1/SOCS3 mutants. HEK-293T cells were transiently co-transfected with plasmids encoding different LR tyrosine mutants or EpoR, various mutants of SOCS1 and SOCS3 and with the pXP2d2-rPAPI-luciferase reporter. The LR tyrosine mutants were used to minimize interference of other inhibitors. The transfected cells were either stimulated for 24 h with ligand or left untreated (NS). Luciferase measurements were performed in triplicate. Data are expressed as the stimulated/NS ratio+S.D.
Critical role of Tyr253 in CIS function
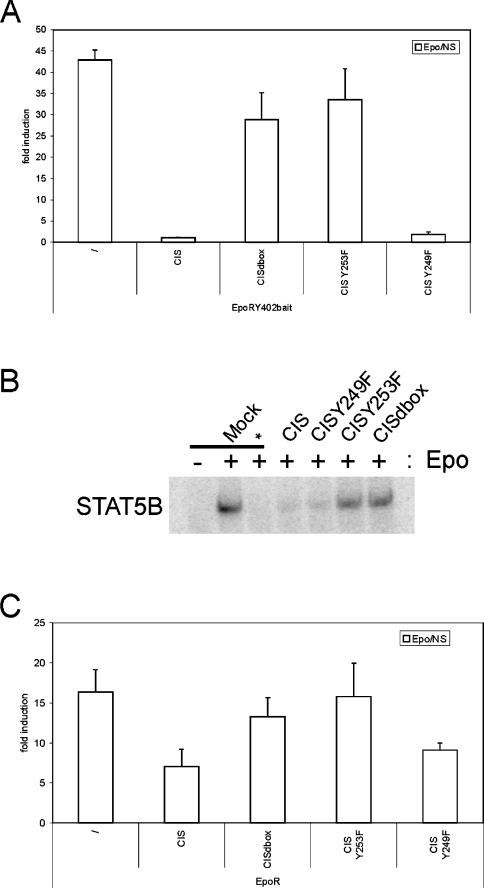
We first investigated the functional implications of the CIS SOCS-box mutations. CIS operates in a classical negative-feedback loop on EpoR signalling: it is rapidly and strongly induced by activated STAT5 upon EpoR activation, subsequently binds to the Tyr402 site in the EpoR, and there inhibits STAT5 activation. We first looked at the effect of CIS mutations on STAT5-dependent β-casein promoter-reporter activity using the EpoR Tyr402 bait. Wild-type CIS clearly abrogated reporter induction as expected. In contrast, co-expression of mutant proteins with a C-terminal deletion of the SOCS-box, or with the Y253F mutation was unable to impair reporter induction. The CISY249F mutant inhibited signalling to a similar extent as did wild-type CIS protein (Figure 4A). Expression of all CIS variants was verified via Western blot (results not shown).
Figure 4. Critical role of CIS C-terminus in blocking STAT5 activation.
(A) STAT5 reporter assay using the EpoR Tyr402 motif. HEK-293T cells were transiently co-transfected with plasmids encoding the EpoR Tyr402 bait, various mutants of FLAG-tagged CIS and with the pGL3-β-casein-luciferase reporter. After transfection, cells were left untreated (NS) or were stimulated with Epo for 24 h. Luciferase activities were measured in triplicate. Data are expressed as the stimulated/NS ratio+S.D. (B) EMSA using the EpoR Tyr402 motif. HEK-293 Flp-In cells were transiently co-transfected with plasmids encoding the EpoR Tyr402 bait, various FLAG-tagged CIS mutants and STAT5B. Nuclear lysates were incubated with 32P-labelled probe corresponding to a β-casein STAT5-binding site to reveal active STAT5 complexes. The EpoRy402F bait (*) was used as a negative control. (C) STAT5 reporter assay using the wildtype EpoR. HEK-293T cells were transiently co-transfected with plasmids encoding the EpoR, various mutants of FLAG-tagged CIS and with the pGL3-β-casein-luciferase reporter. After transfection, cells were left untreated (NS) or were stimulated with Epo for 24 h. Luciferase activities were measured in triplicate. Data are expressed as the stimulated/NS ratio+S.D.
Confirmation was obtained using EMSAs. A 32P-labelled probe corresponding to a β-casein STAT5-binding site was used to visualize bound STAT5 complexes. Whereas wild-type CIS clearly suppressed the formation of nuclear STAT5–DNA complexes, deletion of the complete SOCS-box, as well as the Y253F mutant, resulted in loss of inhibition (Figure 4B). Again, the Y249F mutant behaved as wild-type CIS. Supershift with anti-FLAG antibody confirmed the presence of STAT5B in the complexes (results not shown).
Reporter assays were also performed on the wild-type EpoR. Although the effects were less pronounced, the tendencies clearly corresponded to what we observed for the EpoR bait construct (Figure 4C). This weaker effect is most likely explained by the incomplete overlap of STAT5- and CIS-binding sites [6,35].
CIS interaction with MyD88 does not depend on its SOCS-box
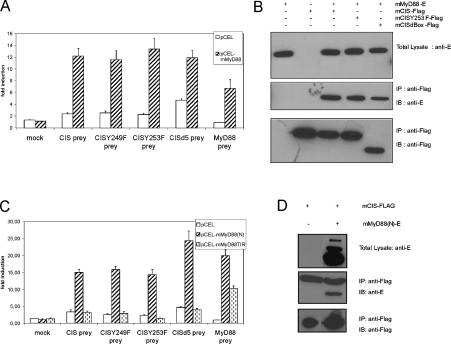
SOCS proteins are also rapidly induced after TLR stimulation. However, no interaction partner of the SOCS proteins in TLR signalling has been described so far. A possible target of SOCS proteins in TLR signalling is the adaptor protein MyD88, which is used by most TLRs. To investigate this possibility, we performed MAPPIT analysis using MyD88 as bait and the CISprey. As shown in Figure 5(A), we observed clear induction of luciferase activity implying an interaction between CIS and MyD88. To investigate the role of the SOCS-box of CIS in this interaction, we next analysed the effect of the above mentioned CIS mutants. Much in contrast with association of CIS with the EpoR and LR, interaction between MyD88 and CIS was not affected by any CIS mutation, including deletion of its entire SOCS-box. These data were confirmed by co-immunoprecipitation (Figure 5B). Here, we transiently co-expressed E-tagged MyD88 (MyD88-E) and FLAG-tagged CIS (CIS–FLAG), CISY253F (CISY253F–FLAG) or CIS lacking its SOCS-box (CISdbox–FLAG). In every case, MyD88-E was co-immunoprecipitated using an anti-FLAG antibody.
Figure 5. The SOCS-box of CIS is not critical for interaction with MyD88.
(A) Interaction of MyD88 and CIS prey constructs. HEK-293T cells were transiently co-transfected with the MAPPIT mock bait or the mMyD88 bait plasmid (0.1 μg), various CIS or CIS-mutant prey (0.5 μg) constructs and with the pXP2d2-rPAPI-luciferase reporter (0.2 μg). MyD88 prey was used as a positive control. The transfected cells were either stimulated for 24 h with Epo or left untreated (NS). Luciferase measurements were performed in triplicate. Data are expressed as the stimulated/NS ratio+S.D. (B) Co-immunoprecipitation analysis. HEK-293T cells were transiently co-transfected with combinations of mMyD88-E, mCIS–FLAG, mCISY253F and mCISdbox. Cell lysates were immunoprecipitated (IP) with anti-FLAG and subsequently immunoblotted (IB) with anti-E. (C) Role for the N-terminal domain of MyD88 in CIS binding. HEK-293T cells were transiently co-transfected with the MAPPIT mock bait, the mMyD88(N) bait or the mMyD88TIR bait vector (0.1 μg), various CIS or CIS-mutant prey (0.5 μg) constructs and with the pXP2d2-rPAPI-luciferase reporter (0.2 μg). The MyD88 prey construct was used as a positive control. The transfected cells were either stimulated for 24 h with Epo or left untreated (NS). Luciferase measurements were performed in triplicate. Data are expressed as the stimulated/NS ratio+S.D. (D) Co-immunoprecipitation analysis. HEK-293T cells were transiently co-transfected with combinations of mMyD88(N)-E and mCIS–FLAG. Cell lysates were immunoprecipitated (IP) with anti-FLAG and subsequently immunoblotted (IB) with anti-E.
MyD88 consists of two interaction domains: a C-terminal ‘TIR’ (Toll/IL-1 receptor) domain and a N-terminal DD, linked by a short intermediate domain. To examine the role of either domain in CIS binding, we created MAPPIT baits containing the N-terminal part of MyD88 encompassing the DD and the intermediate domain, or the C-terminal TIR domain. MAPPIT analysis showed clearly that only the N-terminal part of MyD88 interacted with CIS (Figure 5C). Again, co-immunoprecipitation studies confirmed these findings (Figure 5D). Taken together, we clearly document a role for the MyD88 DD in CIS recruitment, and show that this interaction depends solely on the CIS SH2 domain.
C-terminal mutations in CIS do not affect Elongin B/C and Cullin 5 recruitment
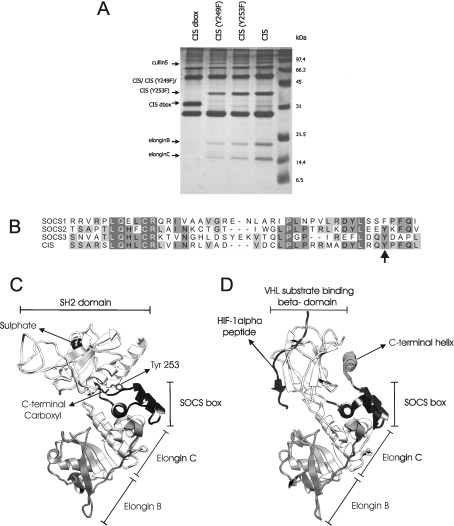
We used a variant, called TAP2, of the tandem affinity purification method developed by Puig et al. [42] (S. Eyckerman, unpublished work) to analyse protein complexes of CIS and its mutants. Cullin 5 and Elongins B and C were identified as interacting partners of CIS. While deletion of the complete SOCS-box of CIS abrogates their association, mutation of the tyrosine residues at position 249 or 253 to phenylalanine did not significantly influence Cullin 5 or Elongin B and C binding (Figure 6A).
Figure 6. Role of the CIS C-terminus in Elongin B/C association.
(A) TAP2 purification using CIS mutants. HEK-293T cells were transiently transfected with various mutants of the pMet7TAP2-CIS construct. Cell lysates were purified by the TAP2 method and were then loaded on a 14% polyacrylamide gel. After silver-staining, the protein bands indicated with an arrow were analysed by MS and identified as the annotated protein. (B) Sequence alignment of SOCS-boxes. SOCS-box sequences of murine SOCS1, SOCS2, SOCS3 and CIS were aligned using the t_coffee algorithm. An arrow indicates Tyr253. Increasing grey shading corresponds with increasing percentage identity. (C) Homology model of mouse CIS, in complex with Elongin C and B. The CIS SOCS-box is shown in black. The position of the phosphopeptide substrate in the model is indicated by a sulfate ion, copied from the SOCS2 template structure, that mimics the phosphate group of the phosphopeptide substrate. Tyr253 hydrogen bonds with the C-terminal carboxy group. (D) Crystal structure of VHL protein (PDB code 1LQB), bound to its hydroxylated HIF-1α substrate (black) and to Elongins C and B. The pVHL SOCS-box and the hydroxyproline-binding domain are in black. The extra C-terminal helix (dark grey) is indicated.
Modelling of the SOCS-box of CIS
We used the crystal structure of the SOCS2–Elongin C–Elongin B complex [38] to build a molecular model for CIS bound to Elongin B and C (Figure 6C). This model, together with sequence alignment with other SOCS proteins (Figure 6B), showed that residues involved in Elongin C binding are highly, conserved. As for SOCS2, the C-terminal residues of CIS mutated in this work are not part of its actual Elongin C-binding site and mutation of the C-terminus of CIS is thus not predicted to affect Elongin C binding directly. As in SOCS2, the C-terminus of CIS is buried in the interface between the SH2 domain and he SOCS-box domain. The hydroxy group of the completely buried Tyr253 hydrogen bonds to the buried C-terminal carboxy group. The C-terminus of CIS or SOCS2 is not able to make direct contact with a phosphopeptide substrate bound to the SH2 domain (Figure 6C).
DISCUSSION
SOCS proteins typically consist of a phosphotyrosine-binding SH2 domain, a C-terminal SOCS-box involved in proteasome recruitment and a pre-SH2 domain that only in the case of SOCS1 and SOCS3 contains a JAK-blocking KIR domain. Association of SOCS proteins with their target substrates is believed to occur solely via their SH2 domain. In the present study, we took a closer look at the binding modus of SOCS proteins using the MAPPIT approach, combined with biochemical and functional analyses.
A key finding is that the SOCS-box of CIS is essential for association with recruitment motifs in cytokine receptors, including the EpoR and LR. Deletion of the entire SOCS-box abrogated binding completely, and more detailed mutagenesis analysis revealed the critical role of the single C-terminal Tyr253. These findings were confirmed by peptide-affinity chromatography using the phosphorylated or non-phosphorylated EpoR Tyr402 motif. Furthermore, reporter assays and EMSAs extended these findings to functional activation of STAT5. Indirect effects of the mutations on the structural integrity of CIS could be ruled out, since clear SOCS-box-independent interaction was observed with the unrelated MyD88 protein as bait. Association of the CISY249F mutant with the EpoR pTyr402 motif could not be demonstrated by peptide-affinity chromatography, suggesting that Tyr249 might serve a similar role as Tyr253. However, MAPPIT experiments showed indisputable association of this CIS mutant with the same EpoR Tyr402 motif. Only this latter interaction was functionally confirmed by the clear inhibitory effect seen in EpoR Tyr402-dependent STAT5 recruitment and activation. The Y249F mutant thus only modestly reduced binding affinity compared with wild-type CIS. This reduced binding affinity of CISY249F completely abolished binding with the EpoR pTyr402 motif in a peptide-affinity chromatography experiment, much in contrast with MAPPIT. The MAPPIT technique therefore reveals itself as a sensitive tool for the identification of weaker, but functionally highly relevant, protein interactions.
In line with our findings that the SOCS-box of CIS is essential for EpoR association, Ketteler et al. [10] reported previously that the SOCS-box of CIS is essential for the apoptotic effect of CIS on erythroid progenitor cells. Seemingly contradictory to our observations, they also found that the SOCS-box of CIS was not required for inhibition of EpoR-induced proliferative responses [10]. However, this anti-proliferative effect may be due to CIS interference with intermediate signalling molecules coupling to the cell cycle. CIS can indeed associate with downstream effector molecules in a SOCS-box-independent modus as we showed for MyD88.
This critical role of the CIS SOCS-box in substrate binding may be a unique feature of CIS, and was not seen for the highly related SOCS2 protein, or for SOCS1 and SOCS3. The corresponding mutation of the conserved tyrosine residue in the SOCS-box of SOCS2 or even deletion of its entire SOCS-box did not show any significant effect on receptor association. Likewise, the inhibitory effect of SOCS1 and SOCS3 on cytokine receptor signalling was hardly affected by removal of the SOCS-box. Previously, the SOCS-box of SOCS1 was also reported to be dispensable for LIF, IL-6 and GHR signalling inhibition but not for G-CSF (granulocyte CSF) signal transduction [40,41,43,44]. In vivo deletion of the entire SOCS-box of SOCS1, however, leads to partial loss of SOCS1 function [45], most likely reflecting its role in Elongin B/C binding, thus establishing an E3 ubiquitin ligation complex leading to proteasomal degradation of associated receptor complexes.
Using MAPPIT, we could also demonstrate the association of CIS with the universal TLR adaptor MyD88. This interaction was confirmed by co-immunoprecipitation. In contrast with the data described above, mutation of the conserved C-terminal Tyr253 to phenylalanine or deletion of the complete SOCS-box of CIS had no effect at all on MyD88 binding. Further analysis of this association revealed a critical role for the DD of MyD88 in CIS binding. More studies are required to elucidate the functional consequences of this interaction. We also observed interaction of other members of the SOCS protein family with MyD88 and its splicing variant lacking the intermediate domain (P. Ulrichts, unpublished work), and structural and functional analyses of these interactions are ongoing. Interestingly, our results imply differential modulation by CIS of signalling via cytokine receptors and TLRs.
A crystal structure for SOCS2 in a complex with Elongin C and B was determined recently [38]. A molecular model was built for the CIS–Elongin C–Elongin B complex in order to get structural insight into the role of the CIS C-terminus on substrate recognition. In the SOCS2 structure and the CIS model, the C-terminus is buried in the interface between the SH2 domain and the SOCS-box, excluding the possibility that this C-terminus could make direct contact with a phosphopeptide substrate bound to the SH2 domain (Figure 6C). In both CIS and SOCS2, the hydroxy group of the last tyrosine residue hydrogen bonds to the buried C-terminal carboxy group. The Y253F mutation in CIS can thus be expected to influence the protein structure or folding: removing the tyrosine hydroxy group may render burial of the C-terminus energetically unfavourable. Tyr253 of CIS may therefore play a structural role. One can speculate that this affects stability of the packing between SOCS-box and SH2 domain, but how this might affect binding to a phosphopeptide substrate remains unclear. Allosteric effects on the substrate-binding pocket cannot be excluded. However, the direct environment of the C-termini in the CIS model and SOCS structure are very similar, hinting that the same phenomenon would be expected for the Y194F mutation in SOCS2, whereas this mutation has no effect on interaction with its phosphopeptide substrates.
Tyrosine phosphorylation of SOCS proteins has been reported previously: Cacalano and co-workers [46,47] showed Epo-induced phosphorylation of the two conserved tyrosine residues in the SOCS-box of SOCS3, including Tyr221 that corresponds to Tyr253 in CIS. Interestingly, the C-terminal pTyr221 allowed binding and functional coupling to the Ras signalling pathway on the one hand, while both phosphorylated tyrosine residues, situated centrally and C-terminally in the SOCS-box, were involved in abrogation of Elongin C interaction [46,47]. Intriguingly, as in CIS and SOCS2, Tyr221 in SOCS3 is also predicted (results not shown) to be buried in the interface between the SH2 domain and the SOCS-box, and its hydroxy group hydrogen bonds to the buried C-terminal carboxy group. It is therefore likely that phosphorylation of Tyr221 in SOCS3, and possibly Tyr253 in CIS, requires changes in the conformation of the C-terminus. One possibility is that burial of the C-terminus as seen in the SOCS2 crystal structure depends on binding of the Elongin complex. In the absence of Elongin binding, the C-terminal tyrosine motifs may be accessible for phosphorylation. In this structural modus, phosphorylation-dependent interactions may occur with signalling molecules or with accessory proteins that facilitate interactions with (a subset of) substrates. Mutating Tyr253 in CIS may then prevent the phosphorylation-driven structural change required for downstream interactions. Deletion of the four C-terminal amino acids in the CISprey, eliminating the putative phosphorylation context, leads to impaired MAPPIT signalling, adding evidence to this phosphorylation hypothesis (results not shown). Of note, it seems unlikely that disturbed Elongin binding causes the substrate-binding defects in the CIS mutants: TAP2 purification of wild-type CIS and of its C-terminal tyrosine mutants showed clear association with Elongins B and C and with Cullin 5, which was lost completely when the entire SOCS-box of CIS was deleted. Thus Elongin C binding does not involve the C-terminus of CIS and has no role in the effect of mutating Tyr253.
The role of the C-terminus of CIS in substrate binding is remarkably similar to the role of the C-terminal helix of the VHL (von Hippel–Lindau) tumour-suppressor protein. The VHL protein is part of a VHL–E3 ligase complex involved in ubiquitination of transcription factors such as HIF (hypoxia inducible factor)-1α, targeting them for degradation. The VHL–E3 ligase complex binds and ubiquitinates two oxygen-dependent degradation domains (HIF-ODDD). The crystal structure of VHL protein, bound to a hydroxylated HIF-1α peptide, and to the Elongins C and B has been determined (Figure 6D) [48]. This revealed that the SOCS-box of VHL is followed by a C-terminal helix, which is not directly involved in binding to the hydroxylated HIF-1α peptide. Like the C-terminus of CIS, this helix interacts tightly with the SH2 domain. Lewis et al. [49] reported that this C-terminal helix is critical for ubiquitination of HIF-1α. Deletion of this C-terminal peptide impaired VHL binding and ubiquitination of the C-terminal HIF-ODDD, while ubiquitination and degradation of the N-terminal HIF-ODDD is hardly affected [49]. The role of the C-terminal peptide in VHL thus shows some striking parallels with the CIS C-terminus. As for CIS, deletion of the VHL C-terminus specifically affects certain functions/interactions, leaving other functions unaltered. It was suggested that deletion of the C-terminus might affect VHL substrate binding by secondary folding effects.
In brief, we have shown that the SOCS-box of CIS is essential for interaction with target cytokine receptors but not with the universal TLR adaptor MyD88. It appears that the biological role of the SOCS-box is more complex than simple recruitment of a ubiquitin–ligation complex, and is also involved in (regulated) substrate binding. Depending on the type of SOCS protein, this may include receptor recruitment motifs, alternative signalling pathways and other SOCS proteins [16,17]. The precise underlying controls that are involved in these diverse functions of the SOCS-box remain to be clarified.
Acknowledgments
We greatly acknowledge the following colleagues for sharing research tools: Robyn Starr (CIS, SOCS1 and SOCS2 constructs), Ivo Touw (pGL3-β-casein-luciferase reporter), Walter Becker (pECE-STAT5B expression vector) and Rudy Beyaert (pCAGGSE-mMyD88 expression vector). We also thank Marc Goethals for peptide synthesis, and An Staes and Evy Timmerman for MS analysis. This work was supported by grants from the Flanders Institute of Science and Technology (grant GBOU 010090, and a personal grant to P.U.), from the Fund for Scientific Research – Flanders (FWO-V grant number 1.5.446.98, and personal grants to D.L. and S.E.) and from Ghent University (GOA 12051401).
References
- 1.Bennett B. D., Solar G. P., Yuan J. Q., Mathias J., Thomas G. R., Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 1996;6:1170–1180. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- 2.Umemoto Y., Tsuji K., Yang F. C., Ebihara Y., Kaneko A., Furukawa S., Nakahata T. Leptin stimulates the proliferation of murine myelocytic and primitive hematopoietic progenitor cells. Blood. 1997;90:3438–3443. [PubMed] [Google Scholar]
- 3.Fantuzzi G., Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J. Leukocyte Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- 4.Yoshimura A., Ohkubo T., Kiguchi T., Jenkins N. A., Gilbert D. J., Copeland N. G., Hara T., Miyajima A. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995;14:2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starr R., Willson T. A., Viney E. M., Murray L. J., Rayner J. R., Jenkins B. J., Gonda T. J., Alexander W. S., Metcalf D., Nicola N. A., Hilton D. J. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 6.Verdier F., Chretien S., Muller O., Varlet P., Yoshimura A., Gisselbrecht S., Lacombe C., Mayeux P. Proteasomes regulate erythropoietin receptor and signal transducer and activator of transcription 5 (STAT5) activation: possible involvement of the ubiquitinated Cis protein. J. Biol. Chem. 1998;273:28185–28190. doi: 10.1074/jbc.273.43.28185. [DOI] [PubMed] [Google Scholar]
- 7.Ram P. A., Waxman D. J. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J. Biol. Chem. 1999;274:35553–35561. doi: 10.1074/jbc.274.50.35553. [DOI] [PubMed] [Google Scholar]
- 8.Hansen L. H., Wang X., Kopchick J. J., Bouchelouche P., Nielsen J. H., Galsgaard E. D., Billestrup N. Identification of tyrosine residues in the intracellular domain of the growth hormone receptor required for transcriptional signaling and Stat5 activation. J. Biol. Chem. 1996;271:12669–12673. doi: 10.1074/jbc.271.21.12669. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto A., Masuhara M., Mitsui K., Yokouchi M., Ohtsubo M., Misawa H., Miyajima A., Yoshimura A. CIS, a cytokine inducible SH2 protein, is a target of the JAK–STAT5 pathway and modulates STAT5 activation. Blood. 1997;89:3148–3154. [PubMed] [Google Scholar]
- 10.Ketteler R., Moghraby C. S., Hsiao J. G., Sandra O., Lodish H. F., Klingmuller U. The cytokine-inducible Scr homology domain-containing protein negatively regulates signaling by promoting apoptosis in erythroid progenitor cells. J. Biol. Chem. 2003;278:2654–2660. doi: 10.1074/jbc.M211236200. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto A., Seki Y., Kubo M., Ohtsuka S., Suzuki A., Hayashi I., Tsuji K., Nakahata T., Okabe M., Yamada S., Yoshimura A. Suppression of STAT5 functions in liver, mammary glands, and T cells in cytokine-inducible SH2-containing protein 1 transgenic mice. Mol. Cell. Biol. 1999;19:6396–6407. doi: 10.1128/mcb.19.9.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenhalgh C. J., Bertolino P., Asa S. L., Metcalf D., Corbin J. E., Adams T. E., Davey H. W., Nicola N. A., Hilton D. J., Alexander W. S. Growth enhancement in suppressor of cytokine signaling 2 (SOCS-2)-deficient mice is dependent on signal transducer and activator of transcription 5b (STAT5b) Mol. Endocrinol. 2002;16:1394–1406. doi: 10.1210/mend.16.6.0845. [DOI] [PubMed] [Google Scholar]
- 13.Greenhalgh C. J., Rico-Bautista E., Lorentzon M., Thaus A. L., Morgan P. O., Willson T. A., Zervoudakis P., Metcalf D., Street I., Nicola N. A., et al. SOCS2 negatively regulates growth hormone action in vitro and in vivo. J. Clin. Invest. 2005;115:397–406. doi: 10.1172/JCI22710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metcalf D., Greenhalgh C. J., Viney E., Willson T. A., Starr R., Nicola N. A., Hilton D. J., Alexander W. S. Gigantism in mice lacking suppressor of cytokine signalling-2. Nature. 2000;405:1069–1073. doi: 10.1038/35016611. [DOI] [PubMed] [Google Scholar]
- 15.Greenhalgh C. J., Metcalf D., Thaus A. L., Corbin J. E., Uren R., Morgan P. O., Fabri L. J., Zhang J. G., Martin H. M., Willson T. A., et al. Biological evidence that SOCS-2 can act either as an enhancer or suppressor of growth hormone signaling. J. Biol. Chem. 2002;277:40181–40184. doi: 10.1074/jbc.C200450200. [DOI] [PubMed] [Google Scholar]
- 16.Tannahill G. M., Elliott J., Barry A. C., Hibbert L., Cacalano N. A., Johnston J. A. SOCS2 can enhance interleukin-2 (IL-2) and IL-3 signaling by accelerating SOCS3 degradation. Mol. Cell. Biol. 2005;25:9115–9126. doi: 10.1128/MCB.25.20.9115-9126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavens D., Montoye T., Piessevaux J., Zabeau L., Vandekerckhove J., Gevaert K., Becker W., Eyckerman S., Tavernier J. A complex interaction pattern of CIS and SOCS2 with the leptin receptor. J. Cell Sci. 2006;119:2214–2224. doi: 10.1242/jcs.02947. [DOI] [PubMed] [Google Scholar]
- 18.Landsman T., Waxman D. J. Role of the Cytokine-induced SH2 domain-containing protein CIS in growth hormone receptor internalization. J. Biol. Chem. 2005;280:37471–37480. doi: 10.1074/jbc.M504125200. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J. G., Farley A., Nicholson S. E., Willson T. A., Zugaro L. M., Simpson R. J., Moritz R. L., Cary D., Richardson R., Hausmann G., et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamura T., Burian D., Yan Q., Schmidt S. L., Lane W. S., Querido E., Branton P. E., Shilatifard A., Conaway R. C., Conaway J. W. Muf1, a novel Elongin BC-interacting leucine-rich repeat protein that can assemble with Cul5 and Rbx1 to reconstitute a ubiquitin ligase. J. Biol. Chem. 2001;276:29748–29753. doi: 10.1074/jbc.M103093200. [DOI] [PubMed] [Google Scholar]
- 21.Giordanetto F., Kroemer R. T. A three-dimensional model of suppressor of cytokine signalling 1 (SOCS-1) Protein Eng. 2003;16:115–124. doi: 10.1093/proeng/gzg015. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki R., Sakamoto H., Yasukawa H., Masuhara M., Wakioka T., Sasaki A., Yuge K., Komiya S., Inoue A., Yoshimura A. CIS3 and JAB have different regulatory roles in interleukin-6 mediated differentiation and STAT3 activation in M1 leukemia cells. Oncogene. 1998;17:2271–2278. doi: 10.1038/sj.onc.1202143. [DOI] [PubMed] [Google Scholar]
- 23.Howard J. K., Cave B. J., Oksanen L. J., Tzameli I., Bjorbaek C., Flier J. S. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat. Med. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 24.Mori H., Hanada R., Hanada T., Aki D., Mashima R., Nishinakamura H., Torisu T., Chien K. R., Yasukawa H., Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 25.Morita Y., Naka T., Kawazoe Y., Fujimoto M., Narazaki M., Nakagawa R., Fukuyama H., Nagata S., Kishimoto T. Signals transducers and activators of transcription (STAT)-induced STAT inhibitor-1 (SSI-1)/suppressor of cytokine signaling-1 (SOCS-1) suppresses tumor necrosis factor α-induced cell death in fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5405–5410. doi: 10.1073/pnas.090084797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Sepulveda P., Okkenhaug K., La Rose J., Hawley R., Dubreuil P., Rottapel R. Socs1 binds to multiple signalling proteins and suppresses Steel factor-dependent proliferation. EMBO J. 1999;18:904–915. doi: 10.1093/emboj/18.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawazoe Y., Naka T., Fujimoto M., Kohzaki H., Morita Y., Narazaki M., Okumura K., Saitoh H., Nakagawa R., Uchiyama Y., et al. Signal transducer and activator of transcription (STAT)-induced STAT inhibitor 1 (SSI-1)/suppressor of cytokine signaling 1 (SOCS1) inhibits insulin signal transduction pathway through modulating insulin receptor substrate 1 (IRS-1) phosphorylation. J. Exp. Med. 2001;193:263–270. doi: 10.1084/jem.193.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoiber D., Kovarik P., Cohney S., Johnston J. A., Steinlein P., Decker T. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-γ. J. Immunol. 1999;163:2640–2647. [PubMed] [Google Scholar]
- 29.Dalpke A. H., Opper S., Zimmermann S., Heeg K. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. J. Immunol. 2001;166:7082–7089. doi: 10.4049/jimmunol.166.12.7082. [DOI] [PubMed] [Google Scholar]
- 30.Baetz A., Frey M., Heeg K., Dalpke A. H. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate Toll-like receptor signaling in innate immune cells. J. Biol. Chem. 2004;279:54708–54715. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- 31.Kinjyo I., Hanada T., Inagaki-Ohara K., Mori H., Aki D., Ohishi M., Yoshida H., Kubo M., Yoshimura A. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 2002;17:583–591. doi: 10.1016/s1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa R., Naka T., Tsutsui H., Fujimoto M., Kimura A., Abe T., Seki E., Sato S., Takeuchi O., Takeda K. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 33.Gingras S., Parganas E., de Pauw A., Ihle J. N., Murray P. J. Re-examination of the role of suppressor of cytokine signaling 1 (SOCS1) in the regulation of Toll-like receptor signaling. J. Biol. Chem. 2004;279:54702–54707. doi: 10.1074/jbc.M411043200. [DOI] [PubMed] [Google Scholar]
- 34.Eyckerman S., Waelput W., Verhee A., Broekaert D., Vandekerckhove J., Tavernier J. Analysis of Tyr to Phe and fa/fa leptin receptor mutations in the PC12 cell line. Eur. Cytokine Network. 1999;10:549–556. [PubMed] [Google Scholar]
- 35.Montoye T., Lemmens I., Catteeuw D., Eyckerman S., Tavernier J. A systematic scan of interactions with tyrosine motifs in the erythropoietin receptor using a mammalian 2-hybrid approach. Blood. 2005;105:4264–4271. doi: 10.1182/blood-2004-07-2733. [DOI] [PubMed] [Google Scholar]
- 36.Eyckerman S., Verhee A., der Heyden J. V., Lemmens I., Ostade X. V., Vandekerckhove J., Tavernier J. Design and application of a cytokine-receptor-based interaction trap. Nat. Cell Biol. 2001;3:1114–1119. doi: 10.1038/ncb1201-1114. [DOI] [PubMed] [Google Scholar]
- 37.Eyckerman S., Broekaert D., Verhee A., Vandekerckhove J., Tavernier J. Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. FEBS Lett. 2000;486:33–37. doi: 10.1016/s0014-5793(00)02205-5. [DOI] [PubMed] [Google Scholar]
- 38.Bullock A. N., Debreczeni J. E., Edwards A. M., Sundstrom M., Knapp S. Crystal structure of the SOCS2–elongin C–elongin B complex defines a prototypical SOCS box ubiquitin ligase. Proc. Natl. Acad. Sci. U.S.A. 2006;103:7637–7642. doi: 10.1073/pnas.0601638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sali A., Blundell T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 40.Narazaki M., Fujimoto M., Matsumoto T., Morita Y., Saito H., Kajita T., Yoshizaki K., Naka T., Kishimoto T. Three distinct domains of SSI-1/SOCS-1/JAB protein are required for its suppression of interleukin 6 signaling. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13130–13134. doi: 10.1073/pnas.95.22.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholson S. E., Willson T. A., Farley A., Starr R., Zhang J. G., Baca M., Alexander W. S., Metcalf D., Hilton D. J., Nicola N. A. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 1999;18:375–385. doi: 10.1093/emboj/18.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puig O., Caspary F., Rigaut G., Rutz B., Bouveret E., Bragado-Nilsson E., Wilm M., Seraphin B. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 2001;24:218–229. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- 43.Hansen J. A., Lindberg K., Hilton D. J., Nielsen J. H., Billestrup N. Mechanism of inhibition of growth hormone receptor signaling by suppressor of cytokine signaling proteins. Mol. Endocrinol. 1999;13:1832–1843. doi: 10.1210/mend.13.11.0368. [DOI] [PubMed] [Google Scholar]
- 44.van de Geijn G. J., Gits J., Touw I. P. Distinct activities of suppressor of cytokine signaling (SOCS) proteins and involvement of the SOCS box in controlling G-CSF signaling. J. Leukocyte Biol. 2004;76:237–244. doi: 10.1189/jlb.0104041. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J. G., Metcalf D., Rakar S., Asimakis M., Greenhalgh C. J., Willson T. A., Starr R., Nicholson S. E., Carter W., Alexander W. S., et al. The SOCS box of suppressor of cytokine signaling-1 is important for inhibition of cytokine action in vivo. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13261–13265. doi: 10.1073/pnas.231486498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cacalano N. A., Sanden D., Johnston J. A. Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat. Cell Biol. 2001;3:460–465. doi: 10.1038/35074525. [DOI] [PubMed] [Google Scholar]
- 47.Haan S., Ferguson P., Sommer U., Hiremath M., McVicar D. W., Heinrich P. C., Johnston J. A., Cacalano N. A. Tyrosine phosphorylation disrupts elongin interaction and accelerates SOCS3 degradation. J. Biol. Chem. 2003;278:31972–31979. doi: 10.1074/jbc.M303170200. [DOI] [PubMed] [Google Scholar]
- 48.Hon W. C., Wilson M. I., Harlos K., Claridge T. D., Schofield C. J., Pugh C. W., Maxwell P. H., Ratcliffe P. J., Stuart D. I., Jones E. Y. Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature. 2002;417:975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 49.Lewis M. D., Roberts B. J. Role of the C-terminal α-helical domain of the von Hippel–Lindau protein in its E3 ubiquitin ligase activity. Oncogene. 2004;23:2315–2323. doi: 10.1038/sj.onc.1207384. [DOI] [PubMed] [Google Scholar]