Abstract
Diamond-Blackfan anemia (DBA) is a rare congenital red-cell aplasia characterized by anemia, bone-marrow erythroblastopenia, and congenital anomalies and is associated with heterozygous mutations in the ribosomal protein (RP) S19 gene (RPS19) in ∼25% of probands. We report identification of de novo nonsense and splice-site mutations in another RP, RPS24 (encoded by RPS24 [10q22-q23]) in ∼2% of RPS19 mutation–negative probands. This finding strongly suggests that DBA is a disorder of ribosome synthesis and that mutations in other RP or associated genes that lead to disrupted ribosomal biogenesis and/or function may also cause DBA.
Diamond-Blackfan anemia (DBA [MIM 105650]) is a congenital form of red-cell aplasia with marked clinical heterogeneity and an increased risk of malignancy.1–3 Affected individuals usually present in infancy or in early childhood with pallor due to severe macrocytic anemia. Although anemia is a prominent feature of DBA, the disease is also characterized by growth retardation and congenital anomalies, in particular of the head, neck, upper limbs, and urinary system, which are present in ∼40% of patients, reflecting the fact that DBA is a broad disorder of development.1,4–6 Laboratory findings such as increased mean corpuscular volume (MCV), elevated erythrocyte adenosine deaminase activity (eADA), and elevated hemoglobin F (HbF) are observed in a majority of but not all patients with DBA.7 In addition, the anemia may be mild or absent in some individuals within affected families, with only subtle indications of the erythroid abnormality, such as increased MCV and/or eADA. The first DBA gene, RPS19, was identified on chromosome 19q13.28 and was found to be mutated in ∼25% of probands with both sporadic and familial DBA.8–11 This highly conserved ribosomal protein (RP) gene encodes a 16-kDa protein, RPS19, that binds to the 40S ribosomal subunit as 1 of 33 associated proteins. Recently, the RPS19 protein was shown to play an important role in 18S rRNA ribosomal biogenesis,12 but its exact function(s) in translation and role(s) in erythropoiesis are unknown.
To identify other gene(s) involved in DBA, we performed a genomewide linkage screen and subsequently sequenced candidate genes. Here, we report that another RP gene, RPS24, is mutated in ∼2% of probands with DBA.
A total of 215 families participated in the study; 47 families were multiplex and 168 families comprised only one affected individual. Informed consent for genetic analyses was obtained from all subjects in the study. The diagnosis of DBA was based on the findings of normochromic anemia, increased eADA, reticulocytopenia, and a low number or lack of erythroid precursors in the bone marrow, often associated with congenital malformations. In a few individuals, the DBA diagnosis was made on the basis of a family history of the disease and elevated eADA and/or MCV. Blood samples were obtained from affected individuals and their family members, and genomic DNA (gDNA) was isolated according to standard procedures.
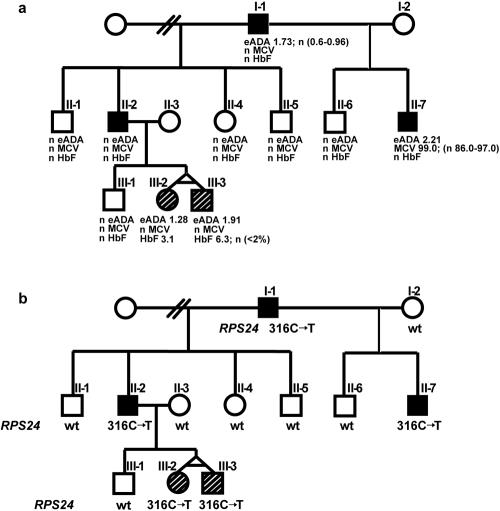
We performed a genomewide linkage screen, using GeneChip Human Mapping 10K Array Xba (Affymetrix) on an extensive family comprising 10 informative meioses (fig. 1a). The SNP mapping data were analyzed using a multipoint parametric model and the MERLIN pedigree-analysis package.13 The analysis was run assuming autosomal dominant inheritance, a gene frequency of 0.000001, and a penetrance of 1.0. We found evidence favoring linkage of the DBA phenotype to a 17.5-Mb region on chromosome 8q and to regions on chromosomes 10 and 6 (5.8 Mb and 3.8 Mb, respectively). We focused our attention on RP genes RPS20 and RPL7, present in the critical region on chromosome 8q, and on RPS24, located in the linked region on chromosome 10. We sequenced exons, intron-exon boundaries, and the promoter regions of these genes in the proband from this family. Sequence results for RPS20 and RPL7 were normal; in contrast, we found a heterozygous nonsense mutation (316C→T) in exon 4 of RPS24 (in sample D1) (table 1). As predicted by the linkage data, further sequencing of RPS24 in all family members revealed complete cosegregation of the 316C→T mutation with the DBA phenotype. Four other affected family members carry the same mutation, whereas five unaffected individuals are homozygous for the wild-type sequence (fig. 1b). There is no phenotype-genotype correlation among the affected family members, as shown in figure 1. The 316C→T mutation causes the change of glutamine to a stop codon and is predicted to result in formation of a truncated RPS24 protein.
Figure 1. .
Phenotype and genotype cosegregation in family MA-1. Squares represent males, circles represent females, blackened symbols represent clinically affected individuals who presented with severe anemia (I-1 and II-7 depend on small steroid doses, and II-2 has been in steroid-induced remission since childhood and presented with webbed neck), open symbols represent unaffected individuals, and line-shaded symbols represent clinically unaffected individuals with abnormal laboratory tests, such as elevated eADA and HbF. a, Phenotype of family MA-1. n = Normal range. b, Genotype of family MA-1. Nonsense mutation c.316C→T (Gln106STOP) was found in clinically affected individuals I-1, II-2, and II-7 and in two apparently healthy individuals, III-2 and III-3, with elevated eADA and HbF. wt = Wild type.
Table 1. .
Summary of RPS24 Mutations in Patients with DBA[Note]
| Patient | Inheritance | No. of Family Members with Mutation | DNA Mutation | Exon or Intron | RNA Change | Predicted Protein Change |
| D1 | Familial | 5 | c.316C→T | Exon 4 | r.316c→u | Gln106STOP |
| D2 | Sporadic | 1 | c.46C→T | Exon 2 | r.46c→u | Arg16STOP |
| D3 | Familial | 2 | c.4–14delGTTTATGTTTTCAG; c.4_6delACC/insTACGGATAG | Intron 1/Exon 2 | r.4_69del (skipped exon 2) | Del 22 aa (N1_M2del) |
Note.— Primer sequences and amplification conditions are available on request. Del = deletion.
Subsequently, we sequenced RPS24 (NCBI accession number NC_000010.9) in 215 unrelated probands with DBA, representing both familial and sporadic cases. Thirty had documented RPS19 mutations, whereas 185 had no known mutations. Among patients with no RPS19 mutations, we found another nonsense mutation in exon 2 in transfusion-dependent patient D2 and a combined deletion/insertion of the intron 1–exon 2 boundary resulting in skipped exon 2 in steroid-dependent patient D3 and in his father (table 1). The father does not currently have any sign of anemia; however, during childhood, he presented with multiple congenital heart anomalies, elevated eADA, and moderate anemia, which was resistant to iron treatment and, at the time, was attributed to his cardiac abnormalities. To control for sequence variation, we sequenced RPS24 in 210 control individuals from an ethnically matched population and did not find any of the above-mentioned sequence changes, consistent with our belief that these sequence variations are pathogenic mutations.
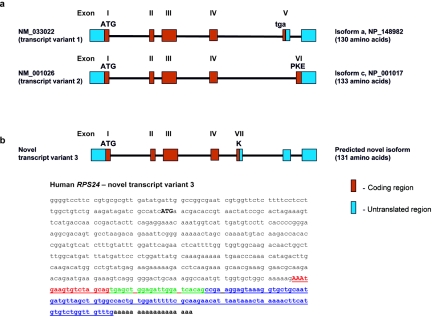
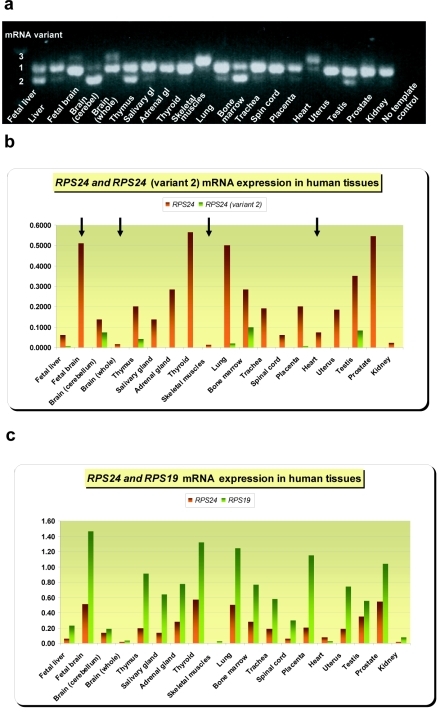
The human RPS24 gene includes six exons that encode an RP that is a component of the 40S ribosomal subunit.14 Xu and colleagues14 found that human RPS24 encodes RPS24 protein isoforms a and c, of length 130 and 133 aa, respectively, as a result of alternative 3′-end splicing into mRNA variants 1 and 2 (fig. 2a). These variants show tissue-specific differences in expression pattern.14 The murine Rps24 gene comprises seven exons with three alternatively spliced transcript variants 1, 2, and 3.14,15 To explore the normal role of RPS24 and to consider how its dysfunction might result in DBA, we performed RT-PCR on mRNA from 20 normal human tissues (Clontech), including whole bone marrow. Reverse transcription and PCR amplification were accomplished using One-step RT-PCR kit (Qiagen) with primers that span 240 bp at the 3′ end of the coding region (forward primer: gtggtggcaagacaactgg; reverse primer: agtggccacagctaacatca). Control reactions without reverse transcriptase were used to exclude contamination of the cDNA by gDNA. The size of the RT-PCR products was detected on 1.3% agarose gels, and RT-PCR products were purified and sequenced to determine exact splice junctions. The results revealed a third novel mRNA variant (variant 3) present in several human tissues—fetal and adult brain, skeletal muscle, and heart—and a lack of variant 2 in these tissues (fig. 3a). The mRNA variant 3 encodes a 131-aa isoform (fig. 2b) identical to murine Rps24 protein isoform 3 (NCBI accession number NP_207635).14 Quantitative real-time PCR (qrt-PCR), with primers and probes designed to exclusively amplify human variant 2 mRNA, confirmed lack of that mRNA in the four above-mentioned tissues (table 2 and fig. 3b). Interestingly, qrt-PCRs amplifying total human RPS24 and RPS19 mRNAs revealed a tissue-specific variation in expression level. Mature tissues, such as adult brain, skeletal muscle, heart, and kidney, expressed low levels of both transcripts, whereas tissues and organs with significant populations of proliferating cells, such as fetal brain, placenta, bone marrow, and various glandular organs, contained significantly higher levels, supporting the notion that absolute levels of RP synthesis correlate with cell proliferation.16 We found coordinate expression of both genes in the majority of tissues studied (fig. 3c and tables 3 and 4) as was previously found in yeast.17 RPS24 and RPS19 transcripts were quantified using Assays-by-Design or Assays-on-Demand gene-expression kits (Applied Biosystems) as described elsewhere.18
Figure 2. .
Structure of human RPS24 gene. a, Schema of the two variants of human RPS24, NCBI accession numbers NM_033022 (variant 1) and NM_001026 (variant 2), as a result of alternative 3′-end mRNA splicing.14 Variant 1 contains exons I–V and encodes RPS24 isoform a (130 aa) (NP_148982). Exon V encodes the stop codon (tga). Variant 2 contains exons I–IV and VI and encodes RPS24 isoform c (133 aa) (NP_001017). Exon VI encodes 3 aa, PKE. b, Schema of the novel human RPS24 variant 3, which contains exons I–IV and VII and encodes the 131-aa RPS24 isoform (isoform b). Below it is the sequence of the novel human RPS24 transcript variant 3. The ATG in bold uppercase letters represents the start codon. Alternatively spliced 3′ end of transcript variant 3 encodes Lys (AAA [red uppercase letters]) followed by stop codon tga. Red lowercase letters represent the untranslated region of exon VII, green letters represent untranslated exon V, and blue letters represent untranslated exon VI of the novel RPS24 transcript variant 3.
Figure 3. .
Expression of RPS24 mRNA in normal human tissues. a, RPS24 RT-PCR products from 20 human tissues visualized on a 1.3% agarose gel. Novel variant 3 is present in fetal brain, adult brain, heart, and skeletal muscle. gl = Gland. b, Tissue-specific expression of variant 2 and total RPS24 mRNA in human tissues, measured by qrt-PCR. Four arrows indicate four tissues where no variant 2 mRNA was detected. c, Tissue-specific expression of total RPS24 and RPS19 mRNA. (All results of qrt-PCR are normalized to reference gene GAPDH.)
Table 2. .
Expression of RPS24 Variant 2 mRNA in Human Tissues[Note]
| Human Tissue |
RPS24 Variant 2 Average CT |
GAPDH Average CT |
ΔCT RPS24 Variant 2 − GAPDH | RPS24 (Variant 2) Normalized to GAPDH (2-ΔCT) |
| Fetal liver | 28.32 ± .23 | 20.65 ± .09 | 7.67 ± .24 | .004927 (.004163–.005832) |
| Fetal brain | 40.00 ± .00 | 20.01 ± .11 | 19.99 ± .11 | .000001 (.000001–.000001) |
| Brain (cerebellum) | 22.30 ± .13 | 18.51 ± .32 | 3.79 ± .34 | .072293 (.056937–.091790) |
| Brain (whole) | 40.00 ± .00 | 19.29 ± .12 | 20.71 ± .12 | .000001 (.000001–.000001) |
| Thymus | 24.75 ± .27 | 20.16 ± .21 | 4.59 ± .34 | .041666 (.032954–.052680) |
| Salivary gland | 30.83 ± .51 | 20.49 ± .24 | 10.34 ± .57 | .000770 (.000520–.001142) |
| Adrenal gland | 34.68 ± 1.21 | 20.40 ± .21 | 14.28 ± 1.23 | .000050 (.000021–.000118) |
| Thyroid | 31.59 ± .97 | 20.81 ± .09 | 10.78 ± .98 | .000569 (.000289–.001120) |
| Skeletal muscles | 40.00 ± .00 | 17.31 ± .20 | 22.69 ± .20 | .000000 (.000000–.000000) |
| Lung | 27.07 ± .18 | 21.37 ± .14 | 5.70 ± .23 | .019303 (.016445–.022658) |
| Bone marrow | 24.17 ± .18 | 20.81 ± .14 | 3.36 ± .23 | .097396 (.083041–.114232) |
| Trachea | 32.33 ± .85 | 21.16 ± .17 | 11.17 ± .87 | .000434 (.000238–.000793) |
| Spinal cord | 32.95 ± 1.57 | 19.53 ± .38 | 13.41 ± 1.62 | .000092 (.000030–.000281) |
| Placenta | 28.27 ± .16 | 20.99 ± .27 | 7.28 ± .31 | .006434 (.005183–.007987) |
| Heart | 40.00 ± .00 | 18.30 ± .19 | 21.70 ± .19 | .000000 (.000000–.000000) |
| Uterus | 40.00 ± .00 | 20.98 ± .20 | 19.03 ± .20 | .000002 (.000002–.000002) |
| Testis | 24.03 ± .10 | 20.44 ± .42 | 3.59 ± .43 | .083043 (.061694–.111779) |
| Prostate | 37.84 ± 1.92 | 21.36 ± .42 | 16.48 ± 1.96 | .000011 (.000003–.000043) |
| Kidney | 32.81 ± .68 | 19.14 ± .17 | 13.67 ± .70 | .000077 (.000047–.000124) |
Note.— CT = number of quantitative PCR cycles.
Table 3. .
Expression of Total RPS24 mRNA in Human Tissues[Note]
| Human Tissue | RPS24 Average CT | GAPDH Average CT | ΔCT RPS24 − GAPDH | RPS24 Normalized to GAPDH (2-ΔCT) |
| Fetal liver | 24.64 ± .40 | 20.57 ± .39 | 4.07 ± .56 | .06 (.04–.09) |
| Fetal brain | 20.98 ± .15 | 20.01 ± .22 | .97 ± .26 | .51 (.42–.61) |
| Brain (cerebellum) | 21.16 ± .21 | 18.31 ± .23 | 2.86 ± .31 | .14 (.11–.17) |
| Brain (whole) | 25.22 ± .16 | 19.19 ± .20 | 6.02 ± .25 | .02 (.01–.02) |
| Thymus | 22.37 ± .17 | 20.05 ± .29 | 2.33 ± .34 | .20 (.16–.25) |
| Salivary gland | 23.42 ± .27 | 20.57 ± .30 | 2.86 ± .41 | .14 (.10–.18) |
| Adrenal gland | 22.12 ± .24 | 20.30 ± .15 | 1.82 ± .28 | .28 (.23–.34) |
| Thyroid | 21.39 ± .25 | 20.57 ± .32 | .82 ± .40 | .57 (.43–.75) |
| Skeletal muscles | 23.52 ± .11 | 17.13 ± .11 | 6.38 ± .16 | .01 (.01–.01) |
| Lung | 22.28 ± .26 | 21.29 ± .23 | .99 ± .35 | .50 (.39–.64) |
| Bone marrow | 22.27 ± .35 | 20.46 ± .38 | 1.82 ± .52 | .28 (.20–.41) |
| Trachea | 23.37 ± .20 | 20.98 ± .25 | 2.39 ± .32 | .19 (.15–.24) |
| Spinal cord | 23.12 ± .40 | 19.08 ± .46 | 4.04 ± .61 | .06 (.04–.09) |
| Placenta | 23.32 ± .26 | 21.01 ± .33 | 2.32 ± .42 | .20 (.15–.27) |
| Heart | 22.24 ± .07 | 18.51 ± .05 | 3.73 ± .09 | .08 (.07–.08) |
| Uterus | 23.34 ± .24 | 20.90 ± .21 | 2.44 ± .32 | .18 (.15–.23) |
| Testis | 21.62 ± .31 | 20.11 ± .16 | 1.51 ± .35 | .35 (.27–.45) |
| Prostate | 21.86 ± .28 | 20.99 ± .36 | .87 ± .46 | .55 (.40–.75) |
| Kidney | 24.57 ± .23 | 18.99 ± .36 | 5.59 ± .42 | .02 (.02–.03) |
Note.— CT = number of quantitative PCR cycles.
Table 4. .
Expression of RPS19 mRNA in Human Tissues[Note]
| Human Tissue | RPS19 Average CT | GAPDH Average CT | ΔCT RPS19 − GAPDH | RPS19 Normalized to GAPDH (2-ΔCT) |
| Fetal liver | 22.42 ± .17 | 20.30 ± .10 | 2.13 ± .20 | .23 (.20–.26) |
| Fetal brain | 19.41 ± .10 | 19.96 ± .04 | −.55 ± .10 | 1.46 (1.36–1.57) |
| Brain (cerebellum) | 20.80 ± .25 | 18.36 ± .26 | 2.44 ± .36 | .18 (.14–.24) |
| Brain (whole) | 23.99 ± .20 | 18.94 ± .26 | 5.05 ± .33 | .03 (.02–.04) |
| Thymus | 20.10 ± .19 | 19.97 ± .16 | .13 ± .24 | .91 (.77–1.08) |
| Salivary gland | 21.06 ± .14 | 20.42 ± .09 | .64 ± .17 | .64 (.57–.72) |
| Adrenal gland | 20.69 ± .28 | 20.33 ± .08 | .37 ± .29 | .78 (.63–.95) |
| Thyroid | 20.24 ± .13 | 20.64 ± .23 | −.40 ± .26 | 1.32 (1.10–1.58) |
| Skeletal muscles | 22.66 ± .48 | 17.42 ± .39 | 5.23 ± .62 | .03 (.02–.04) |
| Lung | 20.96 ± .10 | 21.28 ± .04 | −.31 ± .11 | 1.24 (1.15–1.34) |
| Bone marrow | 20.73 ± .22 | 20.34 ± .06 | .38 ± .23 | .77 (.65–.90) |
| Trachea | 21.60 ± .29 | 20.81 ± .27 | .79 ± .39 | .58 (.44–.76) |
| Spinal cord | 20.95 ± .31 | 19.22 ± .08 | 1.73 ± .32 | .30 (.24–.38) |
| Placenta | 20.51 ± .18 | 20.71 ± .22 | −.20 ± .28 | 1.15 (.94–1.40) |
| Heart | 23.39 ± .33 | 18.50 ± .17 | 4.89 ± .37 | .03 (.03–.04) |
| Uterus | 21.36 ± .22 | 20.93 ± .07 | .43 ± .23 | .74 (.63–.87) |
| Testis | 20.81 ± .19 | 19.96 ± .15 | .85 ± .24 | .56 (.47–.66) |
| Prostate | 20.72 ± .14 | 20.77 ± .07 | −.05 ± .16 | 1.04 (.93–1.15) |
| Kidney | 22.78 ± .16 | 19.00 ± .19 | 3.78 ± .24 | .07 (.06–.09) |
Note.— CT = number of quantitative PCR cycles.
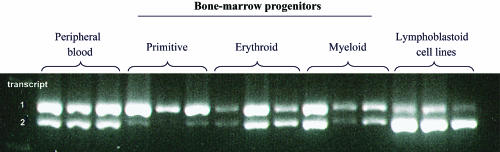
To determine splice-site pattern in specific subsets of bone marrow from three populations, the primitive (CD34+CD71−CD45RA−), erythroid (CD34+CD71+CD45RA−), and myeloid (CD34+CD71−CD45RA+) progenitors were separated as described elsewhere,18 and mRNA from these as well as peripheral blood and lymphoblastoid cell lines were tested by RT-PCR. We found that all tested hematopoietic cells express transcript variants 1 and 2, which encode isofoms a and c, in different proportions. There is an increasing expression of variant 2 with the maturation of the hematological cells (fig. 4).
Figure 4. .
Differently spliced mRNA variants of RPS24 in human hematological tissues. The three bone-marrow cell populations, primitive (CD34+CD71−CD45RA−), erythroid (CD34+CD71+CD45RA−), and myeloid (CD34+CD71−CD45RA+) progenitors, and peripheral blood and lymphoblastoid cell lines express variants 1 and 2, which encode isoforms a and c, in different proportions. There is increasing expression of variant 2 with maturation of hematological cells. RNA samples were obtained from three unrelated controls.
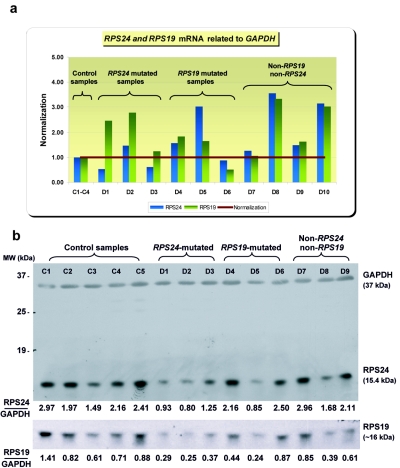
qrt-PCR showed a reduced level of total RPS24 mRNA from lymphoblastoid cell lines in both probands (D1 and D3) with nonsense mutations (fig. 5a and table 5) and premature stop codons, suggesting degradation of mutated transcript due to nonsense-mediated decay,19 whereas the RPS19 mRNA level in these patients was normal or elevated (fig. 5a and table 6). To correlate these findings with protein levels, western-blot experiments were performed as described elsewhere9; the RPS24 protein was detected with rabbit polyclonal antibodies raised to purified rat liver Rps24/2320 and with anti-rabbit IgG horseradish peroxidase-conjugated antibodies (Upstate Biotechnology). Subsequently, the membrane was stripped and was reprobed with the rabbit polyclonal RPS19 antibody9 and anti-rabbit IgG horseradish peroxidase-conjugated antibodies (Upstate Biotechnology) to detect the RPS19 protein. Compared with control samples, the results revealed a reduction of RPS24 protein in lymphoblastoid cell lines from all three mutated probands with nonsense and splice-site mutations (D1–D3), as well as in D5 with a splice-site mutation in RPS19 and a premature termination codon, which may result from nonsense-mediated decay. We found a similar pattern of RPS24 and RPS19 protein levels in these patients, indicating coordinate control of RP levels, as described elsewhere for several other species21,22 (fig. 5b). These data show that the steady-state levels of both proteins are decreased in RPS24-mutated samples, which may suggest that RPS24 is required for the assembly of RPS19 into ribosomal subunits. If not assembled properly, free RPS19 is most likely unstable. In contrast, in RPS19-mutated samples, RPS19 protein levels in some samples decrease while RPS24 levels remain high, suggesting that RPS24 may assemble into ribosomal subunits in the absence of RPS19, thereby achieving some level of stability.
Figure 5. .
Expression of RPS24 mRNA and RPS24 protein in control and diseased lymphoblastoid cell lines. (All results of qrt-PCR are normalized to reference gene GAPDH.) a, RPS24 and RPS19 mRNA expression in control and diseased lymphoblastoid cell lines. b, RPS24 and RPS19 protein expression in control and diseased lymphoblastoid cell lines. Quantification of the RPS24 and RPS19 protein levels normalized to GAPDH was performed with the Quantity One version 4.2.1 software (Bio-Rad Laboratories) on Image Station 440 (Kodak DS). C1–C4 are control samples; D1–D9 are diseased samples. MW = molecular weight.
Table 5. .
Expression of Total RPS24 mRNA Related to GAPDH in Lymphoblastoid Cell Lines in Diseased and Control Samples[Note]
| Sample Group and Sample ID(s) |
RPS24 Average CT | GAPDH Average CT | ΔCT RPS24 − GAPDH | ΔΔCT (ΔCT−ΔCT,C) | RPS24 Normalized to GAPDH (2-ΔΔCT) |
| Controls: | |||||
| C1–C4 | 20.13 ± .66 | 16.38 ± .36 | 3.94 ± .76 | .00 ± 1.07 | 1.0 (.48–2.10) |
| RPS24-mutated samples: | |||||
| D1 | 22.39 ± .28 | 17.56 ± .22 | 4.84 ± .36 | .90 ± .84 | .54 (.30–.96) |
| D2 | 20.83 ± .31 | 17.44 ± .17 | 3.39 ± .35 | −.55 ± .83 | 1.47 (.82–2.61) |
| D3 | 20.82 ± .33 | 16.25 ± .31 | 4.67 ± .45 | .73 ± .88 | .60 (.33–1.11) |
| RPS19-mutated samples: | |||||
| D4 | 19.68 ± .35 | 16.38 ± .24 | 3.30 ± .42 | −.64 ± .87 | 1.56 (.86–2.84) |
| D5 | 20.54 ± .38 | 18.21 ± .14 | 2.34 ± .41 | −1.60 ± .86 | 3.04 (1.67–5.51) |
| D6 | 20.50 ± .69 | 16.35 ± .27 | 4.14 ± .74 | .20 ± 1.06 | .87 (.42–1.81) |
| Non–RPS19/RPS24-mutated samples: | |||||
| D7 | 20.80 ± .82 | 17.20 ± .40 | 3.60 ± .91 | −.34 ± 1.18 | 1.27 (.56–2.87) |
| D8 | 20.80 ± .71 | 18.69 ± .36 | 2.11 ± .80 | −1.83 ± 1.10 | 3.56 (1.66–7.61) |
| D9 | 19.86 ± .33 | 16.49 ± .23 | 3.37 ± .40 | −.57 ± .85 | 1.49 (.82–2.69) |
| D10 | 19.45 ± .46 | 17.16 ± .35 | 2.29 ± .57 | −1.65 ± .95 | 3.15 (1.63–6.08) |
Note.— C1–C4 are control samples, and D1–D10 are diseased samples. CT = number of cycles; CT,C = number of control cycles.
Table 6. .
Expression of RPS19 mRNA Normalized to GAPDH in Lymphoblastoid Cell Lines in Diseased and Control Samples[Note]
| Sample Group and Sample ID(s) |
RPS19 Average CT | GAPDH Average CT | ΔCT RPS19 − GAPDH | ΔΔCT (ΔCT−ΔCT,C) |
RPS19 Normalized to GAPDH (2-ΔΔCT) |
| Controls: | |||||
| C1–C4 | 18.00 ± .56 | 16.46 ± .48 | 1.78 ± .74 | .00 ± 1.04 | 1.0 (.49–2.06) |
| RPS24-mutated samples: | |||||
| D1 | 18.30 ± .10 | 17.82 ± .13 | .48 ± .17 | −1.30 ± .76 | 2.46 (1.46–4.15) |
| D2 | 17.79 ± .16 | 17.49 ± .23 | .30 ± .28 | −1.48 ± .79 | 2.78 (1.61–4.81) |
| D3 | 17.81 ± .32 | 16.49 ± .30 | 1.47 ± .44 | −.31 ± .86 | 1.24 (.68–2.24) |
| RPS19-mutated samples: | |||||
| D4 | 17.60 ± .14 | 16.70 ± .05 | .90 ± .14 | −.87 ± .75 | 1.83 (1.09–3.09) |
| D5 | 19.25 ± .05 | 18.20 ± .05 | 1.06 ± .07 | −.72 ± .74 | 1.65 (.99–2.76) |
| D6 | 19.43 ± .21 | 16.64 ± .21 | 2.78 ± .30 | 1.01 ± .80 | .50 (.29–.87) |
| Non–RPS19/RPS24-mutated samples: | |||||
| D7 | 19.04 ± .31 | 17.33 ± .08 | 1.71 ± .32 | −.07 ± .80 | 1.05 (.60–1.83) |
| D8 | 19.15 ± .28 | 19.11 ± .11 | .04 ± .30 | −1.74 ± .80 | 3.33 (1.92–5.78) |
| D9 | 17.68 ± .47 | 16.61 ± .18 | 1.07 ± .51 | −.71 ± .89 | 1.63 (.88–3.04) |
| D10 | 17.46 ± .20 | 17.28 ± .24 | .18 ± .31 | −1.60 ± .80 | 3.03 (1.74–5.28) |
Note.— C1–C4 are control samples, and D1–D10 are diseased samples. CT = number of cycles; CT,C = number of control cycles.
To determine whether recruitment of mRNA to polysomes was impaired in patients with DBA, we separated lymphoblast cell-line lysates from nine diseased (D1–D9) and four control (C1–C4) individuals on sucrose gradients as described elsewhere.23 Polysome:free ribosome ratios were calculated using IgorPro software. We did not detect any significant difference in the RNA ratio of polysome-bound:free ribosomal subunits between diseased and control samples (P<.3; data not shown). It is likely that lymphoblasts, which are not defective in DBA, have a level of RPS24 or RPS19 encoded by one allele sufficient to form ribosomes and mask an abnormality in translation, even though the mRNA and protein levels are decreased in some patients.
In summary, our results indicate that the RPS24 gene is a second gene for DBA, mutated in ∼2% of probands. Since both RPS24 and RPS19 encode RPs, we consider other RP genes to be excellent candidate genes for DBA, despite the fact that a previous study found no mutations in three other RP genes, RPS3a, RPS13, and RPS16, in several DBA samples tested.24 It is likely that RP gene mutations are quite infrequent in patients with DBA, making it important to screen a large cohort of patients before concluding that any particular gene is not involved.
Our data reinforce the notion that DBA is a ribosomal disease with abnormal ribosomal biogenesis and, possibly, function. Considering the broad range of functions in which ribosomes are involved, our data support the notion that a better understanding of ribosomal biogenesis and function in DBA should lead to new insights into the pathogenesis and treatment of this disease.
Acknowledgments
This work was supported by the Diamond-Blackfan Anemia Foundation (to H.T.G. and C.A.S.), the Pediatric Cancer Foundation (to J.L.), National Institutes of Health (NIH) grant R01HL079571 (to J.M.L., A.V., and E.A.), Feinstein Institute for Medical Research General Clinical Research Center grant MO1 RR018535 (to J.M.L. and A.V.), the Max Reinhardt Charitable Trust (to S.E.B. and K.A.O.), DBA United Kingdom (to S.E.B. and K.A.O.), NIH grant 1R01HL07956501 (to L.D.C. and G.T.), NIH grant R01AR044345 (to A.H.B.), and the Maria Daniella Arturi Foundation (to C.A.S.). DNA sequencing was performed by Children’s Hospital Mental Retardation and Developmental Disabilities Research Center, supported by grant P30 HD18655. We thank Behzad Moghadaszadeh for inspirational discussion, Igor Splawski for control DNA samples, and Steven Boyden and Timothy Tran for excellent technical assistance. Special thanks to Marie Arturi; to Genethon, for French DNA samples; and to physicians and patients with DBA, for participating in the study.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- NCBI Entrez, http://www.ncbi.nlm.nih.gov/gquery/gquery.fcgi (for RPS24 genomic [accession number NC_000010.9], cDNA [accession numbers NM_001026 and NM_033022], human RPS24 protein [accession numbers NP_001017 and NP_148982], and murine Rps24 protein [accession number NP_207635] sequences)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for DBA) [PubMed]
References
- 1.Alter BP, Young NS (1998) The bone marrow failure syndromes. In: Nathan DG, Orkin HS (eds) Hematology of infancy and childhood. Vol 1. Saunders, Philadelphia, pp 237–335 [Google Scholar]
- 2.Janov AJ, Leong T, Nathan DG, Guinan EC (1996) Diamond-Blackfan anemia: natural history and sequelae of treatment. Medicine 75:77–78 10.1097/00005792-199603000-00004 [DOI] [PubMed] [Google Scholar]
- 3.Lipton JM, Federman N, Khabbaze Y, Schwartz CL, Hilliard LM, Clark JI, Vlachos A (2001) Osteogenic sarcoma associated with Diamond-Blackfan anemia: a report from the Diamond-Blackfan Anemia Registry. J Pediatr Hematol Oncol 23:39–44 10.1097/00043426-200101000-00009 [DOI] [PubMed] [Google Scholar]
- 4.Ball SE, McGuckin CP, Jenkins G, Gordon-Smith EC (1996) Diamond-Blackfan anaemia in the U.K.: analysis of 80 cases from a 20-year birth cohort. Br J Haematol 94:645–653 10.1046/j.1365-2141.1996.d01-1839.x [DOI] [PubMed] [Google Scholar]
- 5.Willig TN, Niemeyer CM, Leblanc T, Tiemann C, Robert A, Budde J, Lambiliotte A, Kohne E, Souillet G, Eber S, Stephan JL, Girot R, Bordigoni P, Cornu G, Blanche S, Guillard JM, Mohandas N, Tchernia G (1999) Identification of new prognosis factors from the clinical and epidemiologic analysis of a registry of 229 Diamond-Blackfan anemia patients: DBA group of Societe d’Hematologie et d’Immunologie Pediatrique (SHIP), Gesellshaft fur Padiatrische Onkologie und Hamatologie (GPOH), and the European Society for Pediatric Hematology and Immunology (ESPHI). Pediatr Res 46:553–561 [DOI] [PubMed] [Google Scholar]
- 6.Vlachos A, Klein GW, Lipton JM (2001) The Diamond Blackfan Anemia Registry: tool for investigating the epidemiology and biology of Diamond-Blackfan anemia. J Pediatr Hematol Oncol 23:377–382 10.1097/00043426-200108000-00015 [DOI] [PubMed] [Google Scholar]
- 7.Glader BE, Backer K, Diamond LK (1983) Elevated erythrocyte adenosine deaminase activity in congenital hypoplastic anemia. N Engl J Med 309:1486–1490 [DOI] [PubMed] [Google Scholar]
- 8.Draptchinskaia N, Gustavsson P, Andersson B, Pettersson M, Willig TN, Dianzani I, Ball S, Tchernia G, Klar J, Matsson H, Tentler D, Mohandas N, Carlsson B, Dahl N (1999) The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet 21:169–175 10.1038/5951 [DOI] [PubMed] [Google Scholar]
- 9.Gazda HT, Zhong R, Long L, Niewiadomska E, Lipton JM, Ploszynska A, Zaucha JM, Vlachos A, Atsidaftos E, Viskochil DH, Niemeyer CM, Meerpohl JJ, Rokicka-Milewska R, Pospisilova D, Wiktor-Jedrzejczak W, Nathan DG, Beggs AH, Sieff CA (2004) RNA and protein evidence for haplo-insufficiency in Diamond-Blackfan anaemia patients with RPS19 mutations. Br J Haematol 127:105–113 10.1111/j.1365-2141.2004.05152.x [DOI] [PubMed] [Google Scholar]
- 10.Orfali KA, Ohene-Abuakwa Y, Ball SE (2004) Diamond Blackfan anaemia in the UK: clinical and genetic heterogeneity. Br J Haematol 125:243–252 10.1111/j.1365-2141.2004.04890.x [DOI] [PubMed] [Google Scholar]
- 11.Willig TN, Draptchinskaia N, Dianzani I, Ball S, Niemeyer C, Ramenghi U, Orfali K, Gustavsson P, Garelli E, Brusco A, Tiemann C, Perignon JL, Bouchier C, Cicchiello L, Dahl N, Mohandas N, Tchernia G (1999) Mutations in ribosomal protein S19 gene and Diamond Blackfan anemia: wide variations in phenotypic expression. Blood 94:4294–4306 [PubMed] [Google Scholar]
- 12.Leger-Silvestre I, Caffrey JM, Dawaliby R, Alvarez-Arias DA, Gas N, Bertolone SJ, Gleizes PE, Ellis SR (2005) Specific role for yeast homologs of the Diamond Blackfan anemia associated Rps19 protein in ribosome synthesis. J Biol Chem 280:38177–38185 10.1074/jbc.M506916200 [DOI] [PubMed] [Google Scholar]
- 13.Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2002) Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30:97–101 10.1038/ng786 [DOI] [PubMed] [Google Scholar]
- 14.Xu WB, Roufa DJ (1996) The gene encoding human ribosomal protein S24 and tissue-specific expression of differentially spliced mRNAs. Gene 169:257–262 10.1016/0378-1119(96)88652-5 [DOI] [PubMed] [Google Scholar]
- 15.Xu L, He GP, Li A, Ro HS (1994) Molecular characterization of the mouse ribosomal protein S24 multigene family: a uniquely expressed intron-containing gene with cell-specific expression of three alternatively spliced mRNAs. Nucleic Acids Res 22:646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Da Costa L, Narla G, Willig TN, Peters LL, Parra M, Fixler J, Tchernia G, Mohandas N (2003) Ribosomal protein S19 expression during erythroid differentiation. Blood 101:318–324 10.1182/blood-2002-04-1131 [DOI] [PubMed] [Google Scholar]
- 17.Mager WH, Planta RJ (1991) Coordinate expression of ribosomal protein genes in yeast as a function of cellular growth rate. Mol Cell Biochem 104:181–187 10.1007/BF00229818 [DOI] [PubMed] [Google Scholar]
- 18.Gazda HT, Kho AT, Sanoudou D, Zaucha JM, Kohane IS, Sieff CA, Beggs AH (2006) Defective ribosomal protein gene expression alters transcription, translation, apoptosis, and oncogenic pathways in Diamond-Blackfan anemia. Stem Cells 24:2034–2044 10.1634/stemcells.2005-0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lykke-Andersen J, Shu MD, Steitz JA (2001) Communication of the position of exon-exon junctions to the mRNA surveillance machinery by the protein RNPS1. Science 293:1836–1839 10.1126/science.1062786 [DOI] [PubMed] [Google Scholar]
- 20.Lutsch G, Stahl J, Kargel HJ, Noll F, Bielka H (1990) Immunoelectron microscopic studies on the location of ribosomal proteins on the surface of the 40S ribosomal subunit from rat liver. Eur J Cell Biol 51:140–150 [PubMed] [Google Scholar]
- 21.Gorenstein C, Warner JR (1976) Coordinate regulation of the synthesis of eukaryotic ribosomal proteins. Proc Natl Acad Sci USA 73:1547–1551 10.1073/pnas.73.5.1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nomura M (1999) Regulation of ribosome biosynthesis in Escherichia coli and Saccharomyces cerevisiae: diversity and common principles. J Bacteriol 181:6857–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME (2004) BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci 24:7366–7377 10.1523/JNEUROSCI.1739-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cmejla R, Blafkova J, Stopka T, Jelinek J, Petrtylova K, Pospisilova D (2001) Ribosomal proteins S3a, S13, S16, and S24 are not mutated in patients with Diamond-Blackfan anemia. Blood 97:579–580 10.1182/blood.V97.2.579 [DOI] [PubMed] [Google Scholar]