Abstract
Two common disorders of the elderly are heart failure and Alzheimer disease (AD). Heart failure usually results from dilated cardiomyopathy (DCM). DCM of unknown cause in families has recently been shown to result from genetic disease, highlighting newly discovered disease mechanisms. AD is the most frequent neurodegenerative disease of older Americans. Familial AD is caused most commonly by presenilin 1 (PSEN1) or presenilin 2 (PSEN2) mutations, a discovery that has greatly advanced the field. The presenilins are also expressed in the heart and are critical to cardiac development. We hypothesized that mutations in presenilins may also be associated with DCM and that their discovery could provide new insight into the pathogenesis of DCM and heart failure. A total of 315 index patients with DCM were evaluated for sequence variation in PSEN1 and PSEN2. Families positive for mutations underwent additional clinical, genetic, and functional studies. A novel PSEN1 missense mutation (Asp333Gly) was identified in one family, and a single PSEN2 missense mutation (Ser130Leu) was found in two other families. Both mutations segregated with DCM and heart failure. The PSEN1 mutation was associated with complete penetrance and progressive disease that resulted in the necessity of cardiac transplantation or in death. The PSEN2 mutation showed partial penetrance, milder disease, and a more favorable prognosis. Calcium signaling was altered in cultured skin fibroblasts from PSEN1 and PSEN2 mutation carriers. These data indicate that PSEN1 and PSEN2 mutations are associated with DCM and heart failure and implicate novel mechanisms of myocardial disease.
A great deal of effort has been expended to understand mechanisms of degenerative disease. Two common diseases—heart failure1 and Alzheimer disease2 (AD [MIM 104300])—increase dramatically in likelihood with age and cause considerable morbidity and mortality. Identification of the genes that cause early-onset familial AD has greatly facilitated the understanding of potential disease mechanisms for patients, of all ages, with AD. Since the discovery of the presenilin 1 gene (PSEN1 [MIM 104311]) in 1995,3 >100 mutations have been reported in cases of early-onset familial AD.4 Presenilin 2 (PSEN2 [MIM 600759]),5 highly homologous to PSEN1, is a less common cause of familial AD.6 It has been suggested that amyloid fibril accumulation is in part responsible for the neurodegeneration observed in AD.7–10 Other postulated disease mechanisms include apoptosis, aberrant calcium signaling, or other disordered intracellular signaling, such as the β-catenin/Wnt pathway.7–10 Recent studies have focused on genetic variation that increases the risk of AD.11 Initially thought to be a rare, noninherited cause of dementia, AD is now recognized as a common disease with important underlying genetic factors.12
Dilated cardiomyopathy (DCM [MIM 115200]) resulting from any cause, including ischemic disease, is the most common antecedent to heart failure.1 DCM of unknown etiology after exclusion of ischemic cardiomyopathy and all other known causes is termed “idiopathic dilated cardiomyopathy” (IDC). IDC has been shown to occur as familial DCM (FDC) in 20%–50% of cases,13 implicating genetic causation. Since 1998, mutations in >15 genes have been shown to cause autosomal dominant FDC13,14; they account for approximately one-quarter of all cases13 and encode proteins essential either to the contractile apparatus or cytoskeleton,13,14 to calcium handling,15 or to potassium16 or sodium17 channels. A notable exception is the pleiotropic lamin A/C, a component of the inner nuclear membrane, mutations of which cause DCM, conduction system disease, and several other disorders.13,18 To date, there is no unifying hypothesis of DCM that integrates these disparate genetic pathways.
Regardless of the initiating insult, DCM resembles a progressive, degenerative disease of the myocardium. Notably, the AD genes PSEN1 and PSEN2 are expressed in the heart,19,20 and gene targeting in murine models has shown the genes' importance in cardiac development.21,22 Given the possibility that mutations in these genes cause myocardial disease, we screened the DNA specimens from our cohort of 132 FDC-affected families and 183 subjects with IDC or possible FDC23 for sequence variations in the coding exons of the PSEN1 and PSEN2 genes. We identified a novel PSEN1 mutation in one family and a single PSEN2 missense mutation in two other families. In all three cases, the mutations were present in all clinically affected subjects and segregated with DCM and heart failure.
Material and Methods
Clinical Evaluation
Written, informed consent was obtained from all subjects, and the Oregon Health & Science University (OHSU) Institutional Review Board approved the study. Clinical data for the 315 probands were obtained through our own evaluations, as described elsewhere,23,24 or through medical-record or death-certificate review.23,24 IDC was defined by left ventricular (LV) enlargement—with systolic dysfunction (ejection fraction ⩽0.50)—and the exclusion of other causes of cardiomyopathy. FDC was defined by a documented diagnosis of IDC in two or more family members. The genotyped members of the three families presented here were evaluated by us (history, exam, electrocardiogram [ECG], and echocardiogram).
Genetic Studies
DNA was prepared from fresh whole blood. The human PSEN1 spans >83 kb and comprises 11 protein-coding exons. The human PSEN2 is >24 kb and also consists of 11 coding exons. PCR was used to amplify each coding exon of both genes, as well as of the following known DCM-causative genes encoding lamin A/C (LMNA [MIM 150330]), β-myosin heavy chain (MYH7 [MIM 160760]), troponin T (TNNT2 [MIM 191045]), the cardiac sodium channel (SCN5A [MIM 600163]), the muscle LIM domain protein (CSRP3 [MIM 600824]), and phospholamban (PLN [MIM 172405]). PCR products were sequenced in the forward and reverse directions (primer sequences and PCR conditions are available at the OHSU Familial Dilated Cardiomyopathy Research Project Web site). DNA samples, used as unaffected controls with no cardiovascular or neurological disease, totaled 413 and included 207 African American individuals. Haplotypes were constructed for families B and C from genotypes of nine markers (D1S237–D1S2785) flanking the PSEN2 locus.
Tissue Studies
All tissues were fixed in formalin, were embedded in paraffin, and were stained by routine histological procedures. Immunoperoxidase studies were performed using the Vector ABC horseradish peroxidase kit with use of primary antibodies against the following: amyloid-β peptide (4G8 [Signet], 1:1,000), hyperphosphorylated tau (Tau-2 [Sigma], 1:1,000), α-synuclein (Ab [Novocastra], 1:500), and oligomerized protein (A11 [Biosource], 1:500). Sections from patients with AD, Lewy body disease, or amyloidogenic cardiomyopathy were obtained from the Oregon Brain Bank and were used as positive controls.
Fibroblast Calcium Studies
Measurement of [Ca2+]i was performed using fluorescence microscopy and the Ca2+-sensitive indicator fura-2, as described elsewhere.25 In brief, fibroblasts from presenilin mutation–positive patients and unaffected controls (70%–90% confluent) were grown on uncoated glass-bottom dishes (MatTek) and were incubated for 30–45 min with fura-2/AM 2.5–5.0 μM with Pluronic 0.01%–0.05% (Molecular Probes). After washing twice and allowing 30–60 min to complete ester hydrolysis, fibroblasts were perfused (1.6 ml/min) with 300 mOsm buffer (pH 7.4, at room temperature) containing 138 mM NaCl, 5 mM KCl, 10 mM HEPES, 1.2 mM MgCl2, 2.4 mM CaCl2, 1.2 mM NaH2PO4, and 10 mM glucose. The tip of the perfusion device was positioned at the edge of a 10-mm well that maintained a volume of 50–75 μl by suction. Fibroblasts were illuminated using an inverted microscope (Nikon TE2000E) with 340 nm and 380 nm light (Chroma) alternated by a Lamda 10−3 filterwheel (Sutter Instruments), reflected by a 400-nm dichroic longpass filter, and were monitored at 510±40 nm. Images were visualized using an ORCA ER CCD camera (Hamamatsu Photonics) and were binned and stored for subsequent analysis (MetaFluor [Molecular Devices]). Estimated Ca2+ concentration (EST [Ca2+]i) was determined as described elsewhere,25 with background correction, in vitro calibration, and the dissociation constant of 224 nm.
Protein Alignments
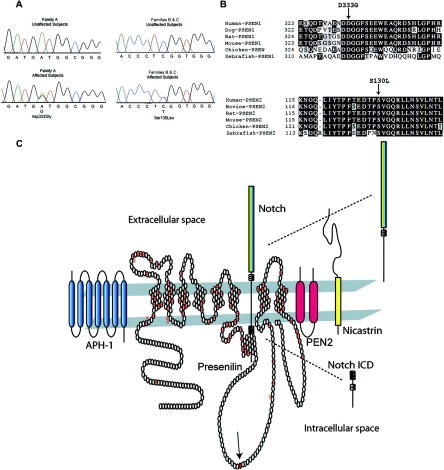
Multiple sequence alignments were performed with the CLUSTALW algorithm and BOXSHADE program. In figure 1, identical residues are indicated by the blackened background, and the conserved residues are shown on the gray background. Protein sequences were obtained from GenBank. The accession numbers for PSEN1 are as follows: NP_000012 (human), AAR97724 (dog), NP_062036.2 (rat), NP_032969.1 (mouse), NP_989494.1 (chicken), and CAB40386 (zebrafish). The accession numbers for PSEN2 are as follows: BC006365 (human), NM_174440 (bovine), NM_031087 (rat), BC010403 (mouse), NM_204302 (chicken), and BC065382 (zebrafish).
Figure 1. .
Presenilin sequencing electropherograms, sequence alignments, and protein structure, with known mutations and components of the γ-secretase complex. A, DNA-sequencing electropherograms for PSEN1 and PSEN2. The sequencing electropherogram for family A demonstrated heterozygosity in PSEN1 at nucleotide 1539, which changed the wild-type aspartic acid to glycine. For families B and C, heterozygosity was demonstrated in PSEN2 at nucleotide 756, which changed the wild-type serine to a leucine. B, PSEN1 and PSEN2 sequence alignments. The amino acid sequences of PSEN1 and PSEN2 from various species are shown and indicate that the mutations occurred in highly conserved residues. C, Presenilin 1 protein structure and mutation locations and the γ-secretase complex. The putative transmembrane structure of the 467-aa presenilin 1 protein is shown, including known AD mutations4,7,8 denoted by the crossed symbols. Most AD mutations reside in the transmembrane domains,4 whereas the DCM mutation (solid symbol with arrow) identified in family A resides in the large, intracytoplasmic loop. The proposed topology of the 448-aa presenilin 2 protein (not shown) closely resembles that of presenilin 1. The PSEN2 mutation identified in families B and C resides at position 130 in its first extracellular domain, near a previously reported AD mutation at aa 122,26 which is also in the first extracellular domain. PSEN1 is shown accompanied by APH-1, nicastrin, and PEN2, proteins that constitute the γ-secretase complex and are required for γ-secretase activity.9,20 Notch is shown as a representative member of the type I membrane protein family. Notch, following extracellular binding of its ligands Delta or Jagged, undergoes presenilin-mediated γ-secretase cleavage, which generates its active, intracellular signaling domain that translocates to the nucleus and acts as a transcriptional regulator in diverse systems, including cardiovascular development and disease. APP27 and several other transmembrane protein substrates, including Erb-B4 and E- and N-cadherins, are similarly processed.10
Results
Genetic Studies
The PSEN1 and PSEN2 genes were evaluated for sequence variations in the probands of 132 independent families with FDC and 183 patients with IDC or possible FDC, whose identification has been described elsewhere.23 A single-nucleotide variant (1539A→G) (GenBank accession number L76517) in exon 10 of PSEN1 was detected in the index patient of family A (fig. 1A). The variant was predicted to change a highly conserved aspartic acid at residue 333 to glycine (D333G) (fig. 1B and 1C).26,27 This change from a negatively charged aspartic acid to an uncharged glycine could be expected to significantly impact the structure and function of PSEN1.
A C→T transition at nucleotide 756 (GenBank accession number NM_000447) was found in exon 5 of PSEN2 in the index patients from families B and C (fig. 1A). The variant was predicted to substitute a highly conserved serine at residue 130 with a leucine (S130L) (fig. 1B). Serine is a polar hydrophilic amino acid, whereas leucine is a nonpolar hydrophobic residue. A new StyI restriction site was created by the variant and was used to independently confirm the presence of the alteration.
The PSEN1 and PSEN2 sequence variants were not detected in 413 unaffected unrelated individuals, of whom 206 were white and 207 were African American. Haplotype analysis of families B and C indicated that this sequence variant did not originate from a common ancestor (data not shown).
The coding sequences and intron/exon junctions of six known FDC genes—LMNA, MYH7, TNNT2, SCN5A, CSRP3, and PLN—were sequenced from DNA from the probands of families A, B, and C. No mutations were identified.
Clinical Studies
Family A
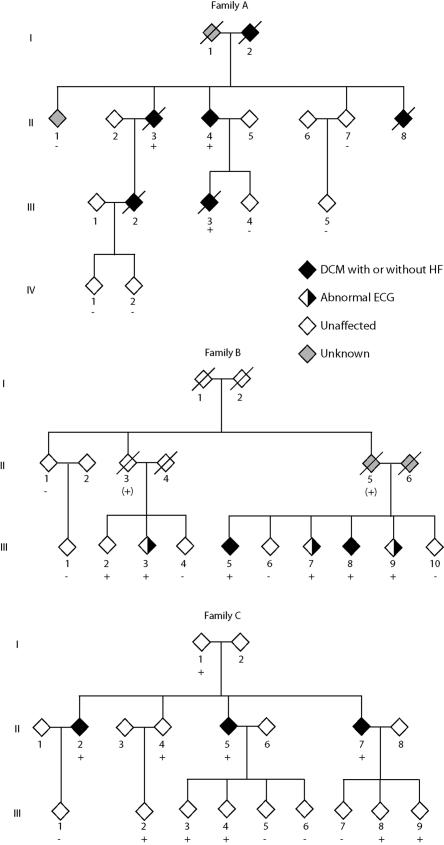
Family A is an African American family in which the onset of DCM and heart failure ranged from age 24 to 69 years (table 1 and fig. 2). Mortality from progressive heart failure usually followed within a few years of diagnosis. The index patient (II.4) presented with symptomatic heart failure due to IDC at age 52 years. Family member II.3 presented with IDC and heart failure at age 51 years, which progressed and required cardiac transplantation at age 62 years. On the basis of these two patients, a diagnosis of FDC was established. Subsequent family evaluation revealed that three additional family members (I.2, II.8, and III.2) had died of DCM and heart failure before the family came to our attention. Inheritance was consistent with an autosomal dominant pattern. Extensive hospital records showed that family member I.2 had received the diagnosis of heart failure and nonischemic DCM at age 69 years and with dementia at age 71 years. Both the DCM and dementia progressed and resulted in repeated hospitalizations until the patient’s death at age 78 years, in advanced heart failure. Hospital records contained a medical diagnosis of “Alzheimer’s dementia.” No autopsy was performed. Family member II.8 died at age 39 years of advanced heart failure despite medical therapy; an ejection fraction was 0.10, and coronary angiography was normal. The endomyocardial biopsy sample was negative for iron or amyloid deposition (data not shown). Family member III.2 presented at age 24 years in advanced heart failure with an ejection fraction of 0.20 and an LV dimension, in diastole, of 75 mm. Coronary angiography was normal. Death resulted at age 30 years from advanced refractory heart failure despite full medical therapy. Family member III.3 died of progressive heart failure at age 44 years. Coronary heart disease was excluded by nuclear imaging, and the ejection fraction was 0.10, with an LV dimension, in diastole, of 77 mm. All clinically affected members of family A for whom DNA was available were positive for the PSEN1 mutation.
Table 1. .
Clinical Characteristics of Family Members with Presenilin Mutations
| Family and Subject | Agea (years) |
ECG Abnormalities | DCM | Comments |
| A: | ||||
| II.3 | 51 | Left bundle branch block | Yes | Ejection fraction 23%; LV dimension, in diastole, of 66 mm by echocardiography; negative coronary angiography; heart transplant at age 62 years; death related to transplant complications |
| II.4 | 52 | First-degree atrioventricular block; LV hypertrophy | Yes | Ejection fraction 15%; LV dimension, in diastole, of 66 mm by echocardiography; negative coronary angiography; implantable cardiac defibrillator for syncope at age 55 years; stable on medical therapy at age 61 years |
| III.3 | 39 | LV hypertrophy | Yes | Ejection fraction 10%; LV dimension, in diastole, of 77 mm; progressive heart failure until death at age 44 years |
| B: | ||||
| II.3 | 80 | No records | No records | Dementia onset at age 75 years; death from progressive dementia at age 80 years |
| II.5 | 62 | No records | No records | Died in 7th decade after history of multiple episodes of syncope; no medical records available |
| III.2 | 54 | No | No | Normal cardiovascular screening |
| III.3 | 50 | Anteroseptal infarct pattern | No | Negative stress echocardiography for ischemia |
| III.5 | 55 | First-degree atrioventricular block; atrial fibrillation; LV hypertrophy | Yes | Presented in heart failure; ejection fraction 10%; LV dimension, in diastole, of 60 mm; negative coronary angiography |
| III.7 | 55 | Anterior infarct pattern; nonspecific ST-T changes | No | LV dimension, in diastole, of 56 mm (>99th percentile), with normal systolic function; ejection fraction estimated at 60%; negative coronary angiography; no heart failure |
| III.8 | 48 | Anterior infarct pattern; nonspecific ST-T changes | Yes | Initial ejection fraction 38%; negative coronary angiography; heart failure 3 years later; ejection fraction 24%; LV dimension, in diastole, of 80 mm |
| III.9 | 50 | Anterior infarct pattern; nonspecific ST-T changes | No | Negative stress echocardiography for ischemia |
| C: | ||||
| I.1 | 72 | No | No | Poor echocardiographic window, but probably within normal limits; no heart failure |
| II.2 | 45 | No | Yes | LV dimension, in diastole, of 53 mm (97.5 percentile); ejection fraction estimated at 40%, with diffuse global hypokinesis; no heart failure |
| II.4 | 44 | No | No | Data from outside medical records; no cardiovascular abnormalities observed; no heart failure |
| II.5 | 36 | Anterior infarct pattern; nonspecific ST-T changes | Yes | Presented in heart failure; ejection fraction 23%; LV dimension, in diastole, of 73 mm; negative coronary angiography; progressive heart failure stabilized with full medical therapy at age 45 years |
| II.7 | 40 | Nonspecific ST-T changes | Yes | Ejection fraction 35%; LV dimension, in diastole, of 59 mm (>99th percentile); negative coronary angiography; no heart failure; LV function improved with medical therapy |
| III.2, 3, 4, 8, and 9 | <35 | No | No |
Age at diagnosis of cardiovascular disease or age at cardiovascular screening.
Figure 2. .
Pedigrees of families A, B, and C with presenilin mutations. Diamonds represent either males or females. Symbols with a diagonal line represent deceased family members. Black symbols indicate family members affected with DCM with or without heart failure, half–filled symbols represent those with abnormal ECGs, gray symbols represent family members who were of unknown status, and white symbols represent family members who were unaffected. The presence (+) or absence (−) of PSEN1 (family A) or PSEN2 (families B and C) mutations are indicated for those from whom DNA was available. For family B, a plus sign within parentheses (+) indicates obligate carriers of the PSEN2 mutation.
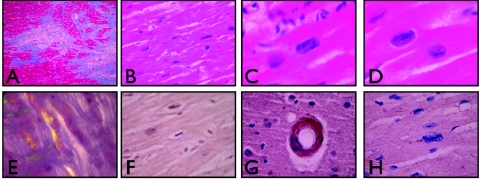
Histological examination of cardiac tissue from the explanted heart of an affected family member (II.3) demonstrated multiple abnormalities consistent with DCM (fig. 3). Regions of replacement of normal myocardium with variably dense fibrous tissue were identified. No inflammation was present in these areas; only rare macrophages were identified. The myocardial cells contained variably enlarged, hyperchromatic, and pleomorphic nuclei with frequent rectangular forms (known as “boxcar nuclei”). No intracellular or extracellular deposits suggestive of amyloid were identified with staining for Congo Red or with immunoperoxidase stains for amyloid-β peptide, hyperphosphorylated tau, α-synuclein, or oligomerized peptide.
Figure 3. .
Histological examination of LV myocardium from an affected member of family A and controls. Histological examination was performed on LV myocardial tissue from the explanted heart taken at the time of cardiac transplantation of an affected member (II.3) of family A, who carried the PSEN1 mutation. A, Regions of myocardial dropout and irregular fibrosis, consistent with remote ischemic damage (Gomori’s modified trichrome; 2×). B–D, Pleomorphic hyperchromatic myocardial nuclei. Panel B demonstrates the diffuse nature of this change (20×). Panels C and D demonstrate rectangular boxcar nuclei (hematoxylin and eosin staining; 60×). E, Control ventricular myocardium, from a patient with amyloid cardiomyopathy, that demonstrates birefringent amyloid deposits (Congo Red under polarized light; 40×). F, Myocardium from subject II.3, in which no amyloid was observed (Congo Red under polarized light; 40×). G, Immunoperoxidase staining of control tissue from a patient with AD and congophilic amyloid angiopathy, which demonstrates strong reactivity with the antioligomeric antibody (AHB0052) (40×). H, Ventricular myocardium from subject II.3, which demonstrated no evidence of reactivity with AHB0052 (40×).
Family B
Family B was a white family with onset of cardiovascular disease at age 48–55 years (table 1 and fig. 2). The index patient (III.5) presented with IDC and decompensated heart failure requiring hospitalization, which stabilized and improved with medical therapy. Shortly thereafter, family member III.8 received the diagnosis of IDC, which established the diagnosis of FDC. Both carried the PSEN2 mutation. On research screening, four additional surviving mutation carriers were identified (fig. 2 and table 1). One family member (III.7) had an anterior infarct pattern on ECG, negative coronary angiography, and LV enlargement without systolic dysfunction; two (III.3 and III.9) had anterior infarct patterns on ECG but no prior history of ischemic heart disease and negative results of screening tests for myocardial ischemia. ECG abnormalities13,24,28 and LV enlargement28 have been suggested as early signs of FDC. One mutation-positive family member (III.2) had normal results on cardiovascular screening, suggesting reduced penetrance, as has been observed elsewhere in FDC.13 One obligate carrier from the second generation (II.3) had the onset of dementia at age 75 years without evidence of cardiovascular disease.
Family C
Family C was of white origin. Three siblings (II.2, II.5, and II.7) received the diagnosis of DCM on the basis of ventricular dilatation and systolic dysfunction and had the onset of cardiovascular disease between ages 36 and 45 years (table 1 and fig. 2); they were found to have the PSEN2 mutation. Two of the five mutation carriers aged >35 years (I.1 and II.4) did not show evidence of cardiovascular disease, suggesting reduced penetrance and/or variable age at onset, as has been observed elsewhere in FDC.13,28 None of the mutation carriers from the third generation (all aged <35 years) showed evidence of DCM or heart failure.
Fibroblast Calcium Studies
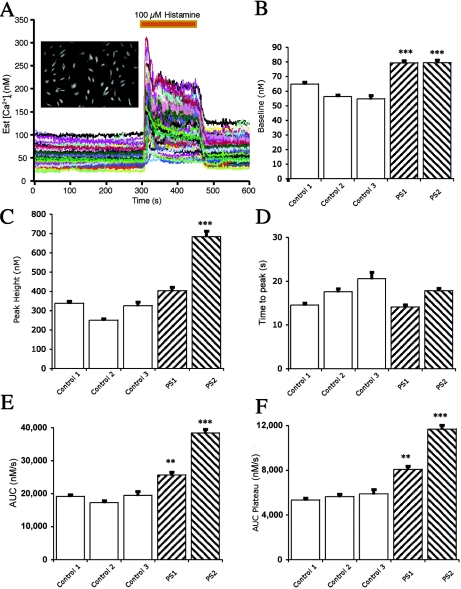
Previous studies have established that intracellular calcium signaling is altered with AD-related presenilin mutations.29,30 Histamine-induced calcium signaling occurs in both myofibroblasts31 and skin fibroblasts.32 We therefore evaluated intracellular calcium signaling in cultured skin fibroblasts from PSEN1 and PSEN2 mutation carriers in comparison with fibroblasts from unaffected subjects (fig. 4). Resting intracellular calcium concentrations were elevated in cultured fibroblasts from patients with PSEN1 or PSEN2 mutations, as were histamine-stimulated maximal calcium concentrations and the area under the curve, suggesting that the identified presenilin mutations may have functionally disrupted intracellular calcium signaling in these cells.
Figure 4. .
Histamine-induced alteration of intracellular calcium concentration ([Ca2+]i) in cultured skin fibroblasts derived from control subjects and those with a PSEN1 or PSEN2 mutation. A, Representative experiment from cells loaded with fura-2/AM. A photomicrograph from one dish of control cells is shown (inset). The baseline was recorded for 300 s before exposure to 100 μM histamine for 150 s. Each tracing represents the measured [Ca2+]i from a single fibroblast. B, Baseline [Ca2+]i measured before the application of histamine. Baseline [Ca2+]i was higher in fibroblasts cultured from patients with the PSEN1 or PSEN2 mutations than in fibroblasts cultured from control subjects. The data shown include the mean (± SEM) responses from control patient 1 (six dishes; 453 cells), control patient 2 (four dishes; 241 cells), control patient 3 (three dishes; 98 cells), a PSEN1 mutation carrier (eight dishes; 440 cells), and a PSEN2 mutation carrier (eight dishes; 561 cells). There was an average of 62 cells per dish, with a range of 21–92 cells. C, Maximal [Ca2+]i response to histamine. The histamine-induced [Ca2+]i response was increased in fibroblasts with the PSEN2 mutation compared with those of three controls. The response from fibroblasts with the PSEN1 mutation was different from one of three controls (P<.0001). The results shown are the peak responses in [Ca2+]i to 100 μM histamine. D, Elapsed time to the maximal [Ca2+]i responses following histamine application. No difference was observed between the fibroblasts derived from control patients and those with a PSEN1 or PSEN2 mutation. The elapsed time was measured in seconds from the histamine application to the maximal response of [Ca2+]i. The perfusion flow rate was high compared with the dish volume; therefore, cells were exposed to 100 μM histamine in <3 s. E, Area under the curve (AUC) of the histamine-induced increase in [Ca2+]i. The total AUC of the histamine-induced increase in [Ca2+]i was higher in fibroblasts from patients with the PSEN1 and PSEN2 mutation than in fibroblasts from control patients. F, AUC during the plateau phase of the histamine-induced increase in [Ca2+]i. The mean plateau phase [Ca2+]i response in fibroblasts cultured from patients with the PSEN1 or PSEN2 mutations was greater than the responses from fibroblasts cultured from control patients. The AUC during the plateau phase of the histamine application was measured from 390 to 450 s. A triple asterisk (***) indicates P<.0001 in three of three controls (Bonferroni/Dunn t test); a double asterisk (**) indicates P<.0001 in two of three controls.
Discussion
We identified a novel PSEN1 mutation in one family and a single PSEN2 missense mutation in two families from a large cohort of probands with FDC or IDC. Both mutations altered highly conserved amino acids, and neither was observed in DNA samples from 413 unrelated unaffected controls. Altered calcium signaling was observed in cultured skin fibroblasts derived from mutation carriers. The mutations were present in all clinically affected subjects and segregated with DCM and heart failure. The cardiovascular disease phenotype attributed to the PSEN1 mutation in family A appeared to be fully penetrant and was manifest as aggressive DCM and advanced heart failure that usually resulted in the necessity of cardiac transplantation or in death. A milder cardiovascular disease phenotype was associated with the PSEN2 mutation in families B and C, which did not predispose to lethality or the need for cardiac transplantation, was less commonly associated with heart failure, and was associated with a more favorable response to medical therapy. FDC has been shown elsewhere to demonstrate widely variable clinical presentations, penetrance, and age at onset associated with locus and allelic heterogeneity.13 We conclude that these data strongly suggest but do not prove that presenilin mutations are causative of DCM and that additional studies will be required to clarify the degree of cardiovascular risk they incur.
Two cases of dementia were identified within the three families. One subject (I.2 from family A) experienced DCM and heart failure at age 69 years, followed by dementia at age 71 years. No DNA was available from that subject, precluding genetic testing to determine whether that family member carried the PSEN1 mutation. The second subject (II.3 from family B), an obligate carrier of the PSEN2 mutation, had the onset of dementia at age 75 years. This PSEN2 mutation has been briefly reported in association with AD in a small kindred33 and is near a different PSEN2 mutation associated with early-onset familial AD.34 Thus, it is possible that the identified presenilin mutations may have contributed to the dementia observed in these subjects.
It remains to be determined whether the disease mechanisms suggested elsewhere to cause AD are relevant for the DCM observed in our study families. The presenilins, in concert with other proteins, form the γ-secretase complex (fig. 1C) that acts on numerous protein substrates. A notable type I protein substrate of the γ-secretase complex is the amyloid precursor protein (APP). Although rare, APP mutations causing early-onset familial AD were the first identified genetic causes of AD.7 Not surprisingly, PSEN1 and PSEN2 mutations have been associated with disordered proteolysis of APP.7 The result is an increased ratio of the fibrillar amyloid peptides Aβ-42:Aβ-40 and increased amyloid deposition in the CNS.7–10 Interestingly, amyloid deposition has been observed in tissues beyond the CNS in patients with familial AD.35 Since the genes for the presenilins19,20 and APP36 are expressed in the heart, as is γ-secretase activity,20 an APP-based pathogenesis of DCM cannot be excluded. However, no evidence of amyloid-induced DCM was found by direct examination of ventricular myocardium from the explanted heart of an affected PSEN1 mutation carrier.
Considerable evidence suggests that disordered γ-secretase–dependent signaling pathways other than APP may have caused DCM in these families. The presenilins interact with >20 proteins10 that couple to diverse signaling pathways, several of which may be relevant for myocardial disease. For example, a key transcriptional regulator of cardiac growth and development is the Notch protein family.37 Phenotypes such as syndromic cardiovascular disease, congenital heart disease, valvular abnormalities, and myocyte dysfunction have been associated with disordered Notch,38 Notch activating ligand,39 and downstream signaling such as Hey2.40 It was therefore of interest that two PSEN2 mutation carriers had congenital cardiovascular disease (ventricular septal defect and bicuspid aortic valve) (data not shown).
It is also possible that the observed presenilin-associated DCM is not related to γ-secretase activity. For example, β-catenin signaling is required for normal cardiac growth and development,41 and PSEN1 mutations have been shown to cause defective β-catenin signaling.42 The PSEN1 mutation identified in family A was positioned in a large cytoplasmic loop between transmembrane domains six and seven7,8 (fig. 1C). This domain is essential for the association of PSEN1 and β-catenin.43 It is possible that the mutation in PSEN1 disrupted its interaction with β-catenin, contributing to a cardiovascular disease phenotype. Another possibility also unrelated to γ-secretase activity is disordered calcium handling, which has been associated with genetic cardiomyopathy.15 Our cellular experiments demonstrated that histamine-induced calcium signaling was significantly altered in skin fibroblasts expressing presenilin mutations. A similar pattern of calcium transients was observed in evoked heart papillary muscle from PSEN2-knockout mice.44 Furthermore, alterations of calcium signaling induced by presenilin mutations may be reversed by calsenilin, a protein that associates with the C terminus of both PSEN1 and PSEN2.30 The abnormal calcium signaling that we identified in skin fibroblasts from presenilin mutation carriers supports a hypothesis of disordered calcium handling as a possible disease mechanism in these families.
In summary, we have identified two distinct presenilin mutations among DCM-affected families and have linked genetic defects of presenilins with myocardial disease. Additional studies exploring presenilin-related disease pathways may lead to novel understandings of cardiovascular disease.
Acknowledgments
We are indebted to the many families who participated in the OHSU Familial Dilated Cardiomyopathy Research Program; without them, these studies would not have been possible. We thank David Clark, for assistance with the immunohistochemistry studies, and Michael Peoples, for assistance with the calcium-imaging studies. We acknowledge Patti Kramer, Ph.D., with the African American Dementia and Aging Project, and we thank the Project for providing unaffected African American DNA samples; the Project is supported by the Layton Aging and Alzheimer’s Disease Research Center, National Institute on Aging, National Institutes of Health (NIH) (grant P30 AG08017) and by Public Health Service grant M01 RR000334. We also acknowledge Sue Richards, Ph.D., OHSU, and John Belmont, M.D., Ph.D., Baylor College of Medicine, for providing unaffected African American DNA samples. This work was supported by NIH awards RO1-HL58626 and 5 M01 RR000334.
Web Resources
The URLs for data presented herein are as follows:
- OHSU Familial Dilated Cardiomyopathy Research Project, http://www.fdc.to/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for AD, PSEN1, PSEN2, DCM, LMNA, MYH7, TNNT2, SCN5A, CSRP3, and PLN)
References
- 1.Hunt SA (2005) ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 46:e1–e82 10.1016/j.jacc.2005.08.022 [DOI] [PubMed] [Google Scholar]
- 2.Nussbaum RL, Ellis CE (2003) Alzheimer’s disease and Parkinson’s disease. N Engl J Med 348:1356–1364 10.1056/NEJM2003ra020003 [DOI] [PubMed] [Google Scholar]
- 3.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, et al (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375:754–760 10.1038/375754a0 [DOI] [PubMed] [Google Scholar]
- 4.Hardy J, Crook R (2001) Presenilin mutations line up along transmembrane α-helices. Neurosci Lett 306:203–205 10.1016/S0304-3940(01)01910-3 [DOI] [PubMed] [Google Scholar]
- 5.Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, Mar L, Sorbi S, Nacmias B, Piacentini S, Amaducci L, Chumakov I, Cohen D, Lannfelt L, Fraser PE, Rommens JM, St George-Hyslop PH (1995) Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 376:775–778 10.1038/376775a0 [DOI] [PubMed] [Google Scholar]
- 6.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu YH, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE (1995) Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 269:973–977 10.1126/science.7638622 [DOI] [PubMed] [Google Scholar]
- 7.Czech C, Tremp G, Pradier L (2000) Presenilins and Alzheimer’s disease: biological functions and pathogenic mechanisms. Prog Neurobiol 60:363–384 10.1016/S0301-0082(99)00033-7 [DOI] [PubMed] [Google Scholar]
- 8.Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- 9.De Strooper B (2003) Aph-1, Pen-2, and nicastrin with presenilin generate an active gamma-secretase complex. Neuron 38:9–12 10.1016/S0896-6273(03)00205-8 [DOI] [PubMed] [Google Scholar]
- 10.Thinakaran G, Parent AT (2004) Identification of the role of presenilins beyond Alzheimer’s disease. Pharmacol Res 50:411–418 10.1016/j.phrs.2003.12.026 [DOI] [PubMed] [Google Scholar]
- 11.Bertram L, Hiltunen M, Parkinson M, Ingelsson M, Lange C, Ramasamy K, Mullin K, Menon R, Sampson AJ, Hsiao MY, Elliott KJ, Velicelebi G, Moscarillo T, Hyman BT, Wagner SL, Becker KD, Blacker D, Tanzi RE (2005) Family-based association between Alzheimer’s disease and variants in UBQLN1. N Engl J Med 352:884–894 10.1056/NEJMoa042765 [DOI] [PubMed] [Google Scholar]
- 12.Bird TD (2005) Genetic factors in Alzheimer’s disease. N Engl J Med 352:862–864 10.1056/NEJMp058027 [DOI] [PubMed] [Google Scholar]
- 13.Burkett EL, Hershberger RE (2005) Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol 45:969–981 10.1016/j.jacc.2004.11.066 [DOI] [PubMed] [Google Scholar]
- 14.Morita H, Seidman J, Seidman CE (2005) Genetic causes of human heart failure. J Clin Invest 115:518–526 10.1172/JCI200524351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE (2003) Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299:1410–1413 10.1126/science.1081578 [DOI] [PubMed] [Google Scholar]
- 16.Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O’Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A (2004) ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet 36:382–387 10.1038/ng1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson TM, Michels VV, Ballew JD, Reyna SP, Karst ML, Herron KJ, Horton SC, Rodeheffer RJ, Anderson JL (2005) Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA 293:447–454 10.1001/jama.293.4.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatkin D, MacRae C, Sasaki T, Wolff M, Porcu M, Frenneaux M, Atherton J, Vidaillet H, Spudich S, Girolami U, Seidman J, Seidman C (1999) Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med 341:1715–1724 10.1056/NEJM199912023412302 [DOI] [PubMed] [Google Scholar]
- 19.Levy-Lahad E, Poorkaj P, Wang K, Fu YH, Oshima J, Mulligan J, Schellenberg GD (1996) Genomic structure and expression of STM2, the chromosome 1 familial Alzheimer disease gene. Genomics 34:198–204 10.1006/geno.1996.0266 [DOI] [PubMed] [Google Scholar]
- 20.Hebert SS, Serneels L, Dejaegere T, Horre K, Dabrowski M, Baert V, Annaert W, Hartmann D, De Strooper B (2004) Coordinated and widespread expression of gamma-secretase in vivo: evidence for size and molecular heterogeneity. Neurobiol Dis 17:260–272 10.1016/j.nbd.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 21.Nakajima M, Moriizumi E, Koseki H, Shirasawa T (2004) Presenilin 1 is essential for cardiac morphogenesis. Dev Dyn 230:795–799 10.1002/dvdy.20098 [DOI] [PubMed] [Google Scholar]
- 22.Donoviel DB, Hadjantonakis AK, Ikeda M, Zheng H, Hyslop PS, Bernstein A (1999) Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev 13:2801–2810 10.1101/gad.13.21.2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kushner JD, Nauman D, Burgess D, Ludwigsen S, Parks S, Pantely G, Burkett EL, Hershberger R (2006) Clinical characteristics of 304 kindreds evaluated for familial dilated cardiomyopathy. J Cardiac Fail 12:422–429 10.1016/j.cardfail.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 24.Crispell K, Wray A, Ni H, Nauman D, Hershberger R (1999) Clinical profiles of four large pedigrees with familial dilated cardiomyopathy: preliminary recommendations for clinical practice. J Am Coll Cardiol 34:837–847 10.1016/S0735-1097(99)00276-4 [DOI] [PubMed] [Google Scholar]
- 25.Irwin RP, Maragakis NJ, Rogawski MA, Purdy RH, Farb DH, Paul SM (1992) Pregnenolone sulfate augments NMDA receptor mediated increases in intracellular Ca2+ in cultured rat hippocampal neurons. Neurosci Lett 141:30–34 10.1016/0304-3940(92)90327-4 [DOI] [PubMed] [Google Scholar]
- 26.Finckh U, Alberici A, Antoniazzi M, Benussi L, Fedi V, Giannini C, Gal A, Nitsch RM, Binetti G (2000) Variable expression of familial Alzheimer disease associated with presenilin 2 mutation M239I. Neurology 54:2006–2008 [DOI] [PubMed] [Google Scholar]
- 27.Annaert W, De Strooper B (2002) A cell biological perspective on Alzheimer’s disease. Annu Rev Cell Dev Biol 18:25–51 10.1146/annurev.cellbio.18.020402.142302 [DOI] [PubMed] [Google Scholar]
- 28.Baig MK, Goldman JH, Caforio AP, Coonar AS, Keeling PJ, McKenna WJ (1998) Familial dilated cardiomyopathy: cardiac abnormalities are common in asymptomatic relatives and may represent early disease. J Am Coll Cardiol 31:195–201 10.1016/S0735-1097(97)00433-6 [DOI] [PubMed] [Google Scholar]
- 29.Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL (1994) Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci USA 91:534–538 10.1073/pnas.91.2.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leissring MA, Yamasaki TR, Wasco W, Buxbaum JD, Parker I, LaFerla FM (2000) Calsenilin reverses presenilin-mediated enhancement of calcium signaling. Proc Natl Acad Sci USA 97:8590–8593 10.1073/pnas.97.15.8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang W, McDonald P, McManus B, van Breemen C, Wang X (2003) Histamine-induced Ca(2+) signaling in human valvular myofibroblasts. J Mol Cell Cardiol 35:379–388 10.1016/S0022-2828(03)00010-5 [DOI] [PubMed] [Google Scholar]
- 32.Johnson CL, Johnson CG, Bazan E, Garver D, Gruenstein E, Ahluwalia M (1990) Histamine receptors in human fibroblasts: inositol phosphates, Ca2+, and cell growth. Am J Physiol 258:C533–C543 [DOI] [PubMed] [Google Scholar]
- 33.Tedde A, Nacmias B, Ciantelli M, Forleo P, Cellini E, Bagnoli S, Piccini C, Caffarra P, Ghidoni E, Paganini M, Bracco L, Sorbi S (2003) Identification of new presenilin gene mutations in early-onset familial Alzheimer disease. Arch Neurol 60:1541–1544 10.1001/archneur.60.11.1541 [DOI] [PubMed] [Google Scholar]
- 34.Finckh U, Müller-Thomsen T, Mann U, Eggers C, Marksteiner J, Meins W, Binetti G, Alberici A, Hock C, Nitsch RM, Gal A (2000) High prevalence of pathogenic mutations in patients with early-onset dementia detected by sequence analyses of four different genes. Am J Hum Genet 66:110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joachim CL, Mori H, Selkoe DJ (1989) Amyloid β-protein deposition in tissues other than brain in Alzheimer’s disease. Nature 341:226–230 10.1038/341226a0 [DOI] [PubMed] [Google Scholar]
- 36.Sandbrink R, Masters CL, Beyreuther K (1994) Beta A4-amyloid protein precursor mRNA isoforms without exon 15 are ubiquitously expressed in rat tissues including brain, but not in neurons. J Biol Chem 269:1510–1517 [PubMed] [Google Scholar]
- 37.Harper JA, Yuan JS, Tan JB, Visan I, Guidos CJ (2003) Notch signaling in development and disease. Clin Genet 64:461–472 10.1046/j.1399-0004.2003.00194.x [DOI] [PubMed] [Google Scholar]
- 38.Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, Shahidi R, Dorovini-Zis K, Li L, Beckstead B, Durand RE, Hoodless PA, Karsan A (2004) Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res 94:910–917 10.1161/01.RES.0000124300.76171.C9 [DOI] [PubMed] [Google Scholar]
- 39.Przemeck GK, Heinzmann U, Beckers J, Hrabe de Angelis M (2003) Node and midline defects are associated with left-right development in Delta1 mutant embryos. Development 130:3–13 10.1242/dev.00176 [DOI] [PubMed] [Google Scholar]
- 40.Donovan J, Kordylewska A, Jan YN, Utset MF (2002) Tetralogy of fallot and other congenital heart defects in Hey2 mutant mice. Curr Biol 12:1605–1610 10.1016/S0960-9822(02)01149-1 [DOI] [PubMed] [Google Scholar]
- 41.Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E (2004) Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol 166:359–367 10.1083/jcb.200403050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimura M, Yu G, Levesque G, Zhang DM, Ruel L, Chen F, Milman P, et al (1999) Presenilin mutations associated with Alzheimer disease cause defective intracellular trafficking of beta-catenin, a component of the presenilin protein complex. Nat Med 5:164–169 10.1038/5526 [DOI] [PubMed] [Google Scholar]
- 43.Murayama M, Tanaka S, Palacino J, Murayama O, Honda T, Sun X, Yasutake K, Nihonmatsu N, Wolozin B, Takashima A (1998) Direct association of presenilin-1 with beta-catenin. FEBS Lett 433:73–77 10.1016/S0014-5793(98)00886-2 [DOI] [PubMed] [Google Scholar]
- 44.Takeda T, Asahi M, Yamaguchi O, Hikoso S, Nakayama H, Kusakari Y, Kawai M, Hongo K, Higuchi Y, Kashiwase K, Watanabe T, Taniike M, Nakai A, Nishida K, Kurihara S, Donoviel DB, Bernstein A, Tomita T, Iwatsubo T, Hori M, Otsu K (2005) Presenilin 2 regulates the systolic function of heart by modulating Ca2+ signaling. FASEB J 19:2069–2071 [DOI] [PubMed] [Google Scholar]