Abstract
Hereditary angioedema (HAE) is characterized clinically by recurrent acute skin swelling, abdominal pain, and potentially life-threatening laryngeal edema. Three forms of HAE have been described. The classic forms, HAE types I and II, occur as a consequence of mutations in the C1-inhibitor gene. In contrast to HAE types I and II, HAE type III has been observed exclusively in women, where it appears to be correlated with conditions of high estrogen levels—for example, pregnancy or the use of oral contraceptives. A recent report proposed two missense mutations (c.1032C→A and c.1032C→G) in F12, the gene encoding human coagulation factor XII (FXII, or Hageman factor) as a possible cause of HAE type III. Here, we report the occurrence of the c.1032C→A (p.Thr328Lys) mutation in an HAE type III–affected family of French origin. Investigation of the F12 gene in a large German family did not reveal a coding mutation. Haplotype analysis with use of microsatellite markers is compatible with locus heterogeneity in HAE type III. To shed more light on the pathogenic relevance of the HAE type III–associated p.Thr328Lys mutation, we compared FXII activity and plasma levels in patients carrying the mutation with that of healthy control individuals. Our data strongly suggest that p.Thr328Lys is a gain-of-function mutation that markedly increases FXII amidolytic activity but that does not alter FXII plasma levels. We conclude that enhanced FXII enzymatic plasma activity in female mutation carriers leads to enhanced kinin production, which results in angioedema. Transcription of F12 is positively regulated by estrogens, which may explain why only women are affected with HAE type III. The results of our study represent an important step toward an understanding of the molecular processes involved in HAE type III and provide diagnostic and possibly new therapeutic opportunities.
Hereditary angioedema (HAE [MIM #106100]) is an autosomal dominant condition characterized clinically by recurrent attacks of facial, genital, or peripheral skin edema without urticaria. The angioedema episodes are potentially life threatening because of occasional attacks of laryngeal and/or intra-abdominal swelling.1,2 The majority of HAE cases are attributable to inadequate C1-inhibitor protein function as a consequence of mutations in SERPING1, the gene encoding C1 inhibitor.3,4 At the molecular level, two clinically indistinguishable categories are recognized: HAE type I, characterized by dramatically reduced levels of the C1 inhibitor (e.g., as a consequence of SERPING1 deletions), and HAE type II, characterized by normal C1-inhibitor levels but impaired protein function as a consequence of SERPING1 missense mutations. HAE swelling is independent of histamine release but is related to excessive generation of vasoactive kinin peptide hormones, in particular bradykinin, in affected tissues.2,5
We and others have recently described pedigrees of individuals exhibiting HAE who have normal C1-inhibitor concentration and function.1,6,7 Patients from these pedigrees are exclusively women, and their episodes of angioedema are precipitated or worsened by high estrogen levels (e.g. during pregnancy or treatment with oral contraceptives). This new type of HAE has been termed “HAE type III” (MIM %300268) or, more comprehensively, “estrogen-related HAE” or “estrogen-sensitive HAE.”6 Binkley and Davis6 have excluded the possibility that mutations at the C1-inhibitor locus are the genetic cause of HAE type III and have suggested that a different locus must be responsible for this phenotype.
We have previously performed a genomewide linkage study of four German families with HAE type III reported by Bork et al.1 and have found evidence of the presence of a disease-causing gene in chromosomal region 5q35.2-q35.3 (authors' unpublished data). For three of these families, Dewald and Bork8 recently reported a missense mutation (c.1032C→A, which results in a p.Thr328Lys substitution) in F12, the gene for blood-coagulation factor XII (FXII, or Hageman factor), which is located in this particular chromosomal region. The F12 gene can be considered a strong candidate for HAE type III for two reasons: FXII proteolytic activity is involved in generation of kinins, which increase vascular leakage and trigger edema formation, and FXII expression and plasma levels are known to be regulated by estrogens.9,10 These results suggest that mutated F12 could be the sought-after disease-causing gene for HAE type III.
In the present study, we report additional genetic and functional data that shed more light on the molecular basis of HAE type III. We investigated the F12 locus in two new families with HAE type III, one of French and the other of German origin. Whereas the French family segregates a c.1032C→A (p.Thr328Lys) mutation previously identified in German families, thus supporting its potential pathogenic relevance, no F12-coding mutation could be detected in the German family. We performed haplotype analyses compatible with the presence of locus heterogeneity. The occurrence of the same mutation (c.1032C→A) in three German families and one French family prompted us to investigate whether this mutation had arisen independently or whether it originates from a common founder. Haplotype analyses with use of SNPs at the F12 locus provided evidence that the German and French families share a common founder. Finally, we present comprehensive functional data that demonstrate the pathogenic relevance of the p.Thr328Lys missense mutation in FXII. Our data strongly suggest that p.Thr328Lys in FXII is an activating mutation that most probably causes angioedema through a bradykinin-driven pathomechanism.
In a first step, we investigated the F12 locus in two extended pedigrees with HAE type III, one from Germany, which two of us had previously reported independently,1,7 and the other from France (fig. 1). Before sequencing the F12 gene, we genotyped microsatellite markers around the F12 locus, at 5q35.2-q35.3, in all available family members and performed haplotype analysis to confirm linkage of the disease to this locus.
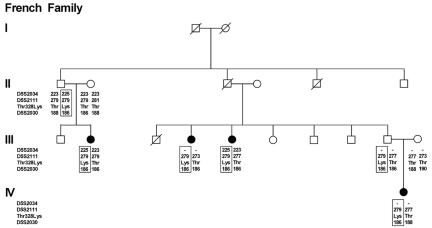
Figure 1. .
Haplotype analysis with use of genetic markers at chromosomal region 5q35.2-q35.5 in a French family with HAE type III. The presence of a C or an A in the second position of codon 328 of F12 (located between D5S2111 and D5S2030) is indicated by the resulting amino acid (Thr or Lys). To avoid disclosure of carrier status of unaffected individuals, only affected individuals, obligate carriers, and founder individuals are depicted. The putative disease-associated haplotype(s) is boxed. A missing genotype is indicated by a hyphen (-). The presence of 328Lys is associated with a history of angioedema attacks.
In the French family, analysis of markers D5S2034, D5S2111, and D5S2030 identified a putative disease-associated haplotype that was present in all affected females and obligate carriers (fig. 1). We subsequently undertook a systematic sequence analysis of the F12 coding region. All 14 exons and exon-flanking intronic regions were amplified by standard PCR in two affected women and in one healthy spouse in both the French and German families. PCR products were sequenced directly on an automated ABI 3130 XL capillary sequencer (Applied Biosystems). The obtained nucleotide sequences were compared between affected and unaffected individuals and with the human F12 cDNA sequence (gi:9961354). We identified only one sequence change affecting the primary structure of the protein: a C→A change in position 1032 (c.1032C→A) that resulted in a p.Thr328Lys substitution in the proline-rich domain (fig. 2). All affected women were carriers of the p.Thr328Lys mutation. Six unaffected women from the last generation also carried the mutation, as predicted from the segregation of disease-associated haplotypes. The mutation was not present in 1,000 control chromosomes.
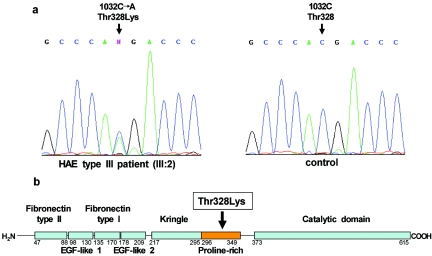
Figure 2. .
Thr328Lys mutation in factor XII in patients with HAE type III. a, Sequencing profiles showing part of exon 9 (nucleotide positions 1027–1037, according to cDNA sequence gi:9961354) in an affected female (III:2) from the French family (left panel) and in a control individual (right panel). b, Schematic picture of the primary structure of the factor XII protein, with known functionally important domains indicated as boxes. Amino acid ranges covering functional domains are given below the primary structure. The location of the Thr328Lys mutation in the proline-rich domain is indicated by an arrow.
Haplotype analysis in the German family (F10) was performed using markers D5S2034, D5S2111, and D5S2030 as well as two additional markers (D5S498 and D5S408) that had been genotyped in the course of a genome scan. We could not identify a disease haplotype that was present in all affected women (data not shown). Assignment of a putative disease haplotype was possible only under the assumption that one affected female represented a phenocopy and that one unaffected female (an obligate carrier) was a nonpenetrant case. For another affected female, limited marker information meant that it was not possible to decide whether she had inherited the putative disease haplotype.
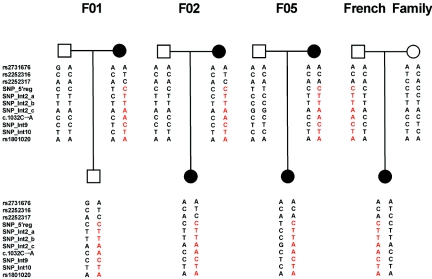
The occurrence of the same mutation (c.1032C→A) in a French family and in three German families described elsewhere (F01, F02, and F05 in this study; designated “family 005,” “family 004,” and “family 003” in the study by Dewald and Bork8) is striking and raises the question of whether independent mutational events have taken place in these families or whether they share a common founder. We addressed this question by performing haplotype analysis with use of 10 SNPs and the c.1032C→A mutation in F12 (fig. 3). Our results are compatible with the hypothesis that the p.Thr328Lys mutation in the one French and the three German families can be traced to a common founder. In a next step, we estimated the age of the most recent common ancestor (MRCA) of the c.1032C→A mutation-bearing chromosomes. The presence of significant linkage disequilibrium (LD) (δ>0.1, calculated according to the method of Bengtsson and Thompson11) at four markers (D5S498, D5S2034, D5S2111, and D5S408) as well as the availability of defined map distances for each of them allowed the estimation of the coalescence time. Age was first estimated by two moment methods,12,13 both of which are based on the “genetic clock” equation, lnQ=-θg, which relates the time (in generations [g]) since the MRCA of mutant chromosomes, the frequency of recombination between the disease locus and the marker (θ), and the probability that a marker’s allele on a disease chromosome is the ancestral one (Q).14 The sex-averaged θ between each microsatellite and F12 gene was estimated on the basis of the physical distance (in Mb) (National Center for Biotechnology Information build 36.1; UCSC Genome Browser March 2006 assembly). The genetic distance:physical distance ratios (2.36:2.98 cM/Mb) were taken from the deCODE high-resolution recombination map.15 An unbiased estimate of Q is the proportion of observed haplotypes that are ancestral. The genetic clock was set following the approach of Luria and Delbrück,16 which takes into account the population growth rate (d). Accordingly, the estimated age was corrected by adding g0=-(1/d)ln(θ/d) to the g value.17 Under the second moment method,13 it was possible to correct the decay of LD over generations for the mutation rate (μ=0.00056 for dinucleotide repeats18) at marker loci. LD data were also used to infer the MRCA age, with use of the Bayesian Markov chain–Monte Carlo method of Rannala and Reeve,19 implemented in the DMLE+ program, version 2.14 (DMLE+ Disease Mapping Using Linkage Disequilibrium Web site). The posterior probability distribution (P) of the c.1032C→A mutation age was inferred assuming a proportion of mutation-bearing chromosomes in our sample that conservatively represents the expected allele frequency and allows us to obtain good convergence of P. The population growth parameter (d=0.08) rests on the demographic history of Western Europe.
Figure 3. .
Haplotype analysis in representative parents-offspring triads from three German families (F01, F02, and F05) and one French family, showing the p.Thr328Lys mutation, with use of 10 SNPs covering the complete genomic region of the F12 gene and the disease-causing mutation (c.1032C→A). SNPs without an “rs” number were identified as informative markers by sequencing analysis of F12 in the families (flanking sequences and assay conditions for the SNPs are available on request). Our results show that the disease-causing mutation is located on the same haplotype (red) in all four families segregating the Thr328Lys mutation, which is compatible with the hypothesis that the mutation goes back to a common founder.
The mean (± SD) overall age estimate for the c.1032C→A mutation is 31.8±18.9 g (95% CI 10.4–52.3 g) with use of the first algorithm12 and is 29.1±18.3 g (95% CI 8.3–49.8 g) with use of the iterative method of Reich and Goldstein.13
Because simple parametric age estimators obtained analytically from the genetic-clock equation suffer from the uncertainty about the intra-allelic genealogy, LD data were reanalyzed in a Bayesian perspective with use of a Markov chain–Monte Carlo method.19 Under the assumption that d=0.08, the Bayesian inference provided an estimation of 34.2 g (95% CI 15.1–92.7 g), an age similar to the one obtained by the moment methods. However, the upper limit of the CI is higher than the corresponding confidence limits resulting from parametric analysis of LD decay over generations.
Under the assumption that g=25–30 years, our results date the MRCA bearing the c.1032C→A mutation to the 11th century (minimum 95% CI 720–1510 a.d.; maximum 95% CI 820 b.c.–1750 a.d.).
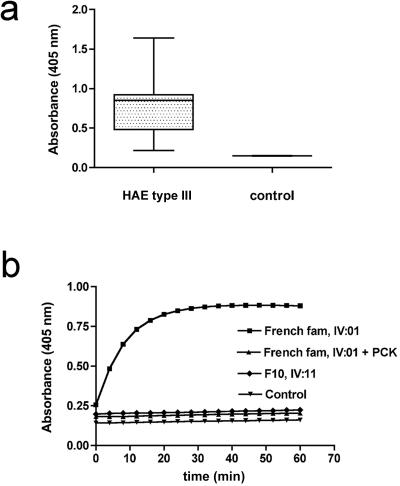
In the absence of studies demonstrating a functional effect, discussions regarding the pathogenic effect of missense mutations are always hypothetical. We therefore aimed to investigate the functional consequences of the p.Thr328Lys mutation in FXII. We determined FXII plasma levels in patients, using an Dade Behring automated Behring Coagulation System and Dade Behring reagents, according to the manufacturer’s instructions. FXII plasma concentrations in patients with HAE type III were similar to those in healthy controls (89%±15% vs. 97%±12%; two-tailed Mann-Whitney test: P is not significant; n=10) and within the normal range (70%–150% [100%=30 μg/ml]). Using the chromogenic substrate S-2302 (D-Pro-Phe-Arg-p-nitroanilide, 6 mM, 20-min reaction time), which is specifically hydrolyzed by active FXII (FXIIa), we compared amidolytic activity in plasma from patients with HAE type III with that from healthy individuals. Mutation p.Thr328Lys largely increases FXII amidolytic activity (more than fourfold), as compared with controls (0.73±0.34 vs. 0.15±0.01; P<.005; n=10) (fig. 4a). Increased enzymatic activity in patient plasma was completely blocked by FXIIa inhibitor H-D-Pro-Phe-Arg-chloromethylketone (2 mM), which specifically inhibits FXII activation in human plasma (fig. 4b).20 Our data strongly suggest that Thr328Lys is a gain-of-function mutation that results in increased FXII amidolytic activity but that does not alter FXII plasma levels.
Figure 4. .
Analysis of FXII activity in plasma from patients with HAE type III. a, Relative FXII amidolytic activity in plasma from HAE type III and healthy individuals (control), determined using the FXIIa-specific chromogenic substrate S-2303. Substrate turnover was measured photometrically via absorbance at a 405-nm wavelength. Data are presented in box-and-whisker plots showing the median (dark line in the box), 25th–75th percentiles (box), and 5th–95th percentiles (whiskers). b, Time course of S-2303 turnover in plasma of a representative patient with HAE type III (“French fam, IV:01”) from the French family, in the absence or presence of the FXIIa inhibitor PCK (2 mM) and in a patient with HAE type III (“F10, IV:11”) from German family F10. For comparison, FXII substrate cleavage in a plasma sample from a healthy control is plotted. Whereas FXII amidolytic activity is markedly increased because of the p.Thr328Lys mutation in the French patient, FXII activity is normal in the German patient.
In patient IV:11 of family F10, who shows neither the Thr328Lys mutation nor any other coding F12 gene mutation, both FXII amidolytic activity and plasma level were indistinguishable from those of healthy control patients (fig. 4b).
HAE types I and II are associated with mutations of the C1-inhibitor gene (SERPING1), located in chromosomal region 11q12-q13.1. It has recently been suggested that a c.1032C→A substitution in the F12 gene on 5q35.3, leading to a threonine→lysine amino acid change in the proline-rich domain of factor XII, caused HAE type III.8 The identification of the same mutation in a French family further supports this notion. In fact, there are several arguments in favor of the hypothesis that factor XII bearing Lys328 is a molecular cause of HAE type III: on the basis of the striking clinical similarity between classic HAE (types I and II) and HAE type III, it can be hypothesized that the three HAE types are caused by similar pathophysiological mechanisms. In this respect, F12 is a strong candidate gene, since it acts on the same biochemical pathway as does the C1 inhibitor—namely, the production of bradykinin, which is a strong inducer of vasodilation and increased vasopermeability. An overproduction of kinins, especially bradykinin, is thought to be responsible for the localized swelling reactions observed in HAE.2,3,5 Factor XII is a serine protease that circulates in the blood as a zymogen. FXII zymogen is activated by contact with negatively charged surfaces, which gives rise to the name of the FXII-driven cascade as a “contact activation system.” In vitro, such negatively charged surfaces may be phospholipids released from activated platelets and damaged cells. In vivo, it has been demonstrated that FXII activation is linked to platelet activation.21 FXIIa cleaves plasma prekallikrein to form plasma kallikrein, which, in turn, generates bradykinin from high-molecular-weight kininogen.22 Under physiological conditions, the C1 inhibitor controls this cascade by inactivating FXIIa and plasma kallikrein. In HAE types I and II, C1-inhibitor deficiency results in an uncontrolled proteolytic activity of FXII and plasma kallikrein as well as consecutive excessive bradykinin formation. Our data strongly suggest that the HAE type III–associated p.Thr328Lys mutation in FXII might also cause angioedema through a bradykinin-driven pathomechanism. However, in contrast to HAE types I and II, in which excessive protease activity is due to deficiency of the endogenous FXII inhibitor, Thr328Lys is a gain-of-function mutation that increases enzymatic activity of FXII. Pathological FXII activity may trigger excessive bradykinin generation, which results in an increase of endothelial permeability and vascular leakage.9 Although the in-depth structural consequences of p.Thr328Lys remain to be identified, the location of the mutation in the proline-rich domain (aa positions 296–349 [fig. 2])—one of three domains involved in the binding of factor XII to negatively charged surfaces23—strongly suggests that the substitution of a neutral threonine residue by a positively charged lysine in this domain may enhance activation of FXII as a consequence of more-effective binding to negatively charged surfaces.24
Further support for an involvement of FXII in HAE type III is derived from the fact that the expression of FXII is increased by estrogens via estrogen-responsive elements in the promoter region.10 This may explain why only women are affected in our HAE type III–affected families and why there is a correlation between episodes of angioedema and periods of elevated estrogen-levels—for example, during pregnancy or treatment with oral contraceptives or estrogen-replacement therapy. The functional properties and estrogen-dependent expression of FXII prompted Binkley and Davis6 to search for possible gain-of-function mutations in estrogen-responsive elements in the promoter region of F12 in a large HAE type III kindred from southern Italy. They did not identify mutations and speculated that other putative estrogen-responsive sequences outside the immediate 5′-flanking region might contain a mutation. It would certainly be worthwhile to examine this family for the presence of coding gain-of-function mutations, such as p.Thr328Lys. It is also noteworthy that we did not observe altered FXII plasma levels in patients with HAE type III who have the p.Thr328Lys mutation, in comparison with control individuals. This observation shows that there is no undetected mutation in the regulatory region of F12 that is in complete LD with p.Thr328Lys, which could be the underlying mutation for disease development through an influence on expression levels of FXII.
Interestingly, it has recently been demonstrated that FXII is essential for pathological thrombus formation in mouse models of ischemic stroke.20 Given the fact that FXII activity will trigger thrombosis in humans via similar pathways, it will be interesting to correlate FXII-driven edema formation and risk of venous and/or arterial thrombosis in patients suffering from HAE type III. Furthermore, since PCK efficiently interferes with pathological FXII activation in postischemic vessels,20 this substance might be a promising candidate for treatment of the acute swelling attacks suffered by patients with HAE type III.
Surprisingly, we did not find a mutation in the coding region of F12 in German family F10. Haplotype analysis with microsatellite markers including the F12 gene were compatible with the presence of a disease-causing mutation only at this locus, under the assumption that one of the affected women represents a phenocopy and that another unaffected woman is a nonpenetrant carrier. Functional studies in one patient showed normal FXII amidolytic activity and normal FXII plasma levels. These findings suggest that a so-far-unknown gene locus may be responsible for the HAE type III phenotype in this family. Promising candidate genes include those that encode proteins involved in FXII activation, FXII-driven bradykinin signaling, or downstream effectors. Future studies will aim to identify the disease-causing mutation in this and other known families with HAE type III.
Interestingly, individual families with HAE and normal C1-inhibitor concentration and function have been reported in which males are also affected with the disease.25–27 Whether the disease affecting these families can be classified as HAE type III—and, consequently, whether it would be preferable to describe the observed sex effect as occurring “predominantly in females” rather than “exclusively in females”—has been discussed.27 Alternatively, there may be at least two different forms of HAE with a normal C1 inhibitor, one limited to females (HAE type III) and the other occurring in both sexes.27 The investigation of F12 in families with affected males will allow this question to be addressed at the molecular level.
Striking clinical variability is observed among carriers of the p.Thr328Lys mutation. Environmental factors, such as exposure to triggering factors, as well as additional genetic factors may account for this variability. Clearly, genetic variants that influence factor XII levels will be promising candidates in the search for the factors underlying clinical expression of the p.Thr328Lys mutation.
In conclusion, the results of our study provide important insights into the molecular causes of HAE type III and have important implications for the diagnosis of this potentially life-threatening condition and possibly also for the development of new therapies. Families carrying the p.Thr328Lys mutation should receive immediate benefit from our results. Predictive testing in presymptomatic females will help prepare carriers for the recognition and treatment of potential episodes of angioedema and may influence individual decisions to avoid conditions of high estrogen levels—for example, the use of oral contraceptives.
Acknowledgments
We thank Sonia DeZutter, Ramona Eisenreich, and Birgitta Schinke, for their excellent technical assistance, and Dr. Christine Schmael, for critically reading the manuscript. M.M.N. was supported by the Alfried Krupp von Bohlen und Halbach-Stiftung. The work was supported by Deutsche Forschungsgemeinschaft SFB 688 (to T.R.). Above all, we are grateful to the invaluable help of the families who cooperated in this study.
Web Resources
The URLs for data presented herein are as follows:
- DMLE+ Disease Mapping Using Linkage Disequilibrium, http://dmle.org/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for HAE and HAE type III)
References
- 1.Bork K, Barnstedt S-E, Koch P, Traupe H (2000) Hereditary angioedema with normal C1-inhibitor activity in women. Lancet 356:213–217 10.1016/S0140-6736(00)02483-1 [DOI] [PubMed] [Google Scholar]
- 2.Agostoni A, Aygoren-Pursun E, Binkley KE, Blanch A, Bork K, Bouillet L, Bucher C, et al (2004) Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol 114:S51–S131 10.1016/j.jaci.2004.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tosi M (1998) Molecular genetics of C1 inhibitor. Immunobiology 199:358–365 [DOI] [PubMed] [Google Scholar]
- 4.Bowen B, Hawk JJ, Sibunka S, Hovick S, Weiler JM (2001) A review of the reported defects in the human C1 esterase inhibitor gene producing hereditary angioedema including four new mutations. Clin Immunol 98:157–163 10.1006/clim.2000.4947 [DOI] [PubMed] [Google Scholar]
- 5.Nussberger J, Cugno M, Cicardi M (2002) Bradykinin-mediated angioedema. N Engl J Med 347:621–622 10.1056/NEJM200208223470820 [DOI] [PubMed] [Google Scholar]
- 6.Binkley KE, Davis A (2000) Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol 106: 546–550 10.1067/mai.2000.108106 [DOI] [PubMed] [Google Scholar]
- 7.Martin L, Degenne D, Toutain A, Ponard D, Watier H (2001) Hereditary angioedema type 3: an additional French pedigree with autosomal dominant transmission. J Allergy Clin Immunol 107:747 10.1067/mai.2001.114242 [DOI] [PubMed] [Google Scholar]
- 8.Dewald G, Bork K (2006) Missense mutations in the coagulation factor XII (Hageman factor) gene in hereditary angioedema with normal C1 inhibitor. Biochem Biophys Res Commun 343:1286–1289 10.1016/j.bbrc.2006.03.092 [DOI] [PubMed] [Google Scholar]
- 9.Renné T, Schuh K, Muller-Esterl W (2005) Local bradykinin formation is controlled by glycosaminoglycans. J Immunol 175:3377–3385 [DOI] [PubMed] [Google Scholar]
- 10.Farsetti A, Misiti S, Citarella F, Felici A, Andreoli M, Fantoni A, Sacchi A, Pontecorvi A (1995) Molecular basis of estrogen regulation of the Hageman factor XII gene expression. Endocrinology 136:5076–5083 10.1210/en.136.11.5076 [DOI] [PubMed] [Google Scholar]
- 11.Bengtsson BO, Thomson G (1981) Measuring the strength of associations between HLA antigens and diseases. Tissue Antigens 18:356–363 [DOI] [PubMed] [Google Scholar]
- 12.Risch N, de Leon D, Ozelius L, Kramer P, Almasy L, Singer B, Fahn S, Breakefield X, Bressman S (1995) Genetic analysis of idiopathic torsion dystonia in Ashkenazi Jews and their recent descent from a small founder population. Nat Genet 9:152–159 10.1038/ng0295-152 [DOI] [PubMed] [Google Scholar]
- 13.Reich DE, Goldstein DB (1999) Estimating the age of mutations using variation at linked markers. In: Goldstein GB, Schlötterer C (eds) Microsatellites: evolution and applications. Oxford University Press, Oxford, United Kingdom, pp 129–138 [Google Scholar]
- 14.Labuda D, Zietkiewicz E, Labuda M (1997) The genetic clock and the age of the founder effect in growing populations: a lesson from French Canadians and Ashkenazim. Am J Hum Genet 61:768–771 [PMC free article] [PubMed] [Google Scholar]
- 15.Kong A, Gudbjartsson DF, Sainz J, Jonsdottir GM, Gudjonsson SA, Richardsson B, Sigurdardottir S, Barnard J, Hallbeck B, Masson G, Shlien A, Palsson ST, Frigge ML, Thorgeirsson TE, Gulcher JR, Stefansson K (2002) A high-resolution recombination map of the human genome. Nat Genet 31:241–247 [DOI] [PubMed] [Google Scholar]
- 16.Luria SE, Delbrück M (1943) Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28:491–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labuda M, Labuda D, Korab-Laskowska M, Cole DE, Zietkiewicz E, Weissenbach J, Popowska E, Pronicka E, Root AW, Glorieux FH (1996) Linkage disequilibrium analysis in young populations: pseudo-vitamin D–deficiency rickets and the founder effect in French Canadians. Am J Hum Genet 59:633–643 [PMC free article] [PubMed] [Google Scholar]
- 18.Weber JL, Wong C (1993) Mutation of human short tandem repeats. Hum Mol Genet 2:1123–1128 [DOI] [PubMed] [Google Scholar]
- 19.Rannala B, Reeve JP (2001) High-resolution multipoint linkage-disequilibrium mapping in the context of a human genome sequence. Am J Hum Genet 69:159–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer H-U, Burfeind P, Renné C, Gailani D, Nieswandt B, Renné T (2006) Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med 203:513–518 10.1084/jem.20052458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renné T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B (2005) Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med 202:271–281 10.1084/jem.20050664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colman RW (1984) Surface-mediated defense reactions: the plasma contact activation system. J Clin Invest 73:1249–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Citarella F, Ravon DM, Pascucci B, Felici A, Fantoni A, Hack CE (1996) Structure/function analysis of human factor XII using recombinant deletion mutants. Evidence for an additional region involved in the binding to negatively charged surfaces. Eur J Biochem 238:240–249 10.1111/j.1432-1033.1996.0240q.x [DOI] [PubMed] [Google Scholar]
- 24.Hojima Y, Cochrane CG, Wiggins RC, Austen KF, Stevens RL (1984) In vitro activation of the contact (Hageman factor) system of plasma by heparin and chondroitin sulfate E. Blood 63:1453–1459 [PubMed] [Google Scholar]
- 25.Cicardi M, Bergamaschini L, Zingale LC, Gioffré D, Agostini A (1999) Idiopathic nonhistaminergic angioedema. Am J Med 106:650–654 10.1016/S0002-9343(99)00123-0 [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Yu F, Klaustermeyer WB (2004) New-variant hereditary angioedema in three brothers with normal C1 esterase inhibitor level and function. Allergy 59:557–558 10.1111/j.1398-9995.2004.00428.x [DOI] [PubMed] [Google Scholar]
- 27.Bork K, Gül D, Dewald G (2006) Hereditary angio-edema with normal C-inhibitor in a family with affected women and men. Br J Dermatol 154:542–545 10.1111/j.1365-2133.2005.07048.x [DOI] [PubMed] [Google Scholar]