Abstract
Deregulations in insulin and insulin-like growth factor (IGF) pathways may contribute to hepatocellular carcinoma. Although intracellular insulin receptor substrate-2 (IRS-2) is the main effector of insulin signaling in the liver, its role in hepatocarcinogenesis is unknown. Here, we show that IRS-2 was overexpressed in two murine models of hepatocarcinogenesis: administration of diethylnitrosamine and hepatic overexpression of SV40 large T antigen. In both models, IRS-2 overexpression was detected in preneoplastic lesions and at higher levels in tumoral nodules. IRS-2 overexpression associated with IGF-2 and IRS-1 overexpression and with GSK-3β inhibition. Increased expression of IRS-2 was also detected in human hepatocellular carcinoma specimens and hepatoma cell lines. In murine and human hepatoma cells, IRS-2 protein induction associated with increased IRS-2 mRNA levels. The functionality of IRS-2 was demonstrated in Hep3B cells, in which IRS-2 tyrosine phosphorylation and its association with phosphatidylinositol-3 kinase were induced by IGF-2. Moreover, down-regulation of IRS-2 expression increased apoptosis in these cells. In conclusion, we demonstrate that IRS-2 is overexpressed in human and murine hepatocellular carcinoma. The emergence of IRS-2 overexpression at preneoplastic stages during experimental hepatocarcinogenesis and its protective effect against apoptosis suggest that IRS-2 contributes to liver tumor progression.
Deregulations in insulin and insulin-like growth factor (IGF) pathways are implicated in the development and/or progression of hepatocellular carcinoma (HCC).1–3 Insulin, IGF-1, and IGF-2, acting through insulin and type 1 IGF (IGF-1R) receptors, promote a wide range of metabolic, growth-promoting, and survival functions in the liver. Early events in insulin and IGF actions involve tyrosine phosphorylation of intracellular insulin receptor substrate-1 and -2 (IRS-1 and IRS-2) by activated receptors. Phosphorylated IRS-1 and IRS-2 act as docking molecules and bind proteins containing Src homology 2 domains such as the p85 regulatory subunit of phosphatidylinositol (PI) 3-kinase and Grb2. These events lead to the activation of multiple signaling pathways including the Ras/MEK/ERK and PI 3-kinase/Akt/glycogen synthase kinase-3β (GSK-3β) pathways.4,5
Even though IRS-1 and IRS-2 bear structural and functional similarities, they can mediate distinct cellular responses.6–8 Evidence for a differential role of IRS-1 and IRS-2 has been obtained in the liver. In adult hepatocytes, IRS-2 is the main effector of the metabolic and proliferative signals triggered by insulin receptors, whereas IRS-1 mediates the mitogenic effects of IGF-1 receptors.9–12 In addition, IRS-2 functions as a major insulin-responsive molecule during fetal liver growth whereas IRS-1 is not involved in this process.13
Deregulations in insulin and IGF signaling pathways including re-expression of fetal IGF-2 mRNA,2,14 overexpression of IRS-1,1,15–17 IGF-1R,18 and of insulin receptor19,20 as well as inhibitory phosphorylation of GSK-3β21–23 have been reported in human and murine hepatocarcinogenesis. Along these lines, excessive storage of glycogen is a common finding in preneoplastic liver lesions.3
Because HCC is associated with the hyperactivation of insulin and IGF signaling pathways and because IRS-2 is a major effector of insulin signaling in the liver, the present study was designed to determine whether IRS-2 may undergo abnormal expression and contribute to tumor progression in hepatocellular carcinogenesis. To test this hypothesis, two mechanistically distinct murine models of hepatocarcinogenesis were analyzed as well as human HCC specimens and cell lines.
Materials and Methods
Animal Models
In the first model of liver carcinogenesis, male C57BL/6J-129Sv mice received a single intraperitoneal injection of diethylnitrosamine (DEN) (Sigma-Aldrich Co., St. Louis, MO) at a dose of 10 μg/g body weight at the age of 15 days as initially reported by Vesselinovitch and colleagues.24. Noninjected males were used as controls. The second model consisted in transgenic ASV mice (C57BL/6J-DBA2 hybrid background) that harbor the SV40 early region encoding the large T antigen under the control of the human antithrombin III regulatory sequences25 (generous gift from Dr. P. Briand, Institut Cochin, Paris, France). In these mice, the transgene is localized on the Y chromosome and all males develop HCC. Females do not develop HCC and were used as controls. Mice were maintained on normal diet and water ad libitum and they received humane care in compliance with the national ethical guidelines for the care and use of laboratory animals.
Cell Culture
Human hepatoma cell lines (HepG2, Hep3B, HuH7, Mahlavu) were maintained in minimal essential medium containing Earle’s salts, 1% nonessential amino acids, 1 mmol/L sodium pyruvate, and 10% fetal calf serum. Human hepatocytes in primary culture were prepared as reported elsewhere.26
Human HCC Specimens
Tumoral and nontumoral liver tissue specimens were collected from seven patients with HCC who underwent partial hepatectomy without previous anti-tumoral treatment. Patients (six men and one woman) were 51 to 77 years of age (mean, 61 years). Histological analyses of nontumoral liver tissues showed no fibrosis in three cases (patients 1 to 3), cirrhosis in three cases (patients 4 to 6), and periportal fibrosis without fibrous septa or bridging in one case (patient 7). Chronic liver disease was related to hepatitis C virus infection in three cases (patients 4, 5, and 7) and to hepatitis B virus infection in one case (patient 6). According to the World Health Organization classification of tumors,27 HCC was well-differentiated in patients 1, 3, 4, 5, and 6 and moderately differentiated in patients 2 and 7. All liver tissue samples were stored at −80°C until analysis.
Histological and Immunohistochemical Analyses
Human and murine liver tissue samples were fixed in 4% formaldehyde solution and embedded in paraffin. Four-μm tissue sections were stained with hematoxylin and phloxin before histological examination. Liver tumors in mice were classified according to the International Classification of Rodent Tumors.28
For immunohistochemistry, 4-μm-thick sections were incubated in 0.1% hydrogen peroxide in methanol for 30 minutes to inhibit endogenous peroxidase activity. Microwave antigen retrieval was performed using 750 W for 15 minutes followed by 150 W for 15 minutes in 10 mmol/L citrate buffer, pH 6. An avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA) was then used to prevent nonspecific binding. Immunolabeling was performed using the Supersensitive Link-Label immunohistochemistry detection system (Biogenex, San Ramon, CA) according to the manufacturer’s protocol. Briefly, after 1 hour of incubation at room temperature with an anti-IRS-2 primary antibody (1:1000) (Upstate Biotechnology, Lake Placid, NY), slides were rinsed and then incubated for 20 minutes in a prediluted biotinylated anti-immunoglobulin solution. Slides were rinsed again and incubated for 20 minutes in a prediluted horseradish peroxidase-labeled streptavidin solution. Peroxidase activity was revealed with a 3-amino-9-ethyl-carbazole solution that forms a reddish-brown end product. Sections were finally counterstained with hematoxylin and mounted with an aqueous mounting media (Glycergel; DAKO, Glostrup, Denmark). Negative controls included omission of the primary antibody.
Western Blotting and Immunoprecipitation
Whole cell lysates were obtained from human hepatocytes at day 2 of primary culture and from hepatoma cell lines as previously reported.29 Liver tissues were homogenized in RIPA buffer (150 mmol/L NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mmol/L/Tris-HCl, pH 7.5) and centrifuged at 10,000 × g at 4°C for 10 minutes. Samples were analyzed by Western blotting using the following antibodies: anti-phospho-GSK-3β (Ser9), anti-phospho-Akt (Ser473), anti-Akt, anti-phospho-PKCζ (Thr410) (Cell Signaling Technology Inc., Beverly, MA), anti-IRS-1, anti-p85, anti-insulin receptor, anti-IGF-1R (Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-IRS-2 (Upstate Biotechnology), anti-GSK-3β (Transduction Laboratories, BD Biosciences, San Diego, CA), anti-β-actin (Sigma-Aldrich Co.) and anti-phosphotyrosine (PY20; Stratagene, La Jolla, CA). Immune complexes were visualized by enhanced chemiluminescence (Amersham Biosciences, Saclay, France). For IRS-2 immunoprecipitation, cell extracts (500 μg) were incubated overnight at 4°C with the anti-IRS-2 antibody at a 1:200 dilution together with 30 μL of protein A/G PLUS-agarose (Santa Cruz Biotechnology Inc.). Immunoprecipitates were washed three times with lysis buffer, resuspended in gel loading buffer, and analyzed by Western blotting.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from liver tissue samples and from human hepatoma cell lines and hepatocytes with RNAqueous-4PCR (Ambion Inc., Austin, TX). RNA (2 μg) was then treated with DNase and reverse-transcribed by extension of random decamers using MMLV reverse transcriptase (RETROscript; Ambion Inc.). IGF-2, IRS-2, IGF-1R, and insulin receptor cDNAs were amplified by PCR using the following primers: IGF-2, 5′-CGTCGCAGCCGTGGCATCGTTGA-3′ and 5′-GCCCACGGGGTATCTGGGGAAGT-3′; murine IRS-2, 5′-TAGCCACAGGAGCAACACAC-3′ and 5′-CAGGCGTGGTTAGGGAGTAA-3′; human IRS-2, 5′-ACAATGGTGACTACACCGAG-3′ and 5′-CTGCTTTTCCTGAGAG-AGAC-3′; IGF-1R, 5′-CAATCTATTCACAAGCCTCC-3′ and 5′-GGAAAAAGAGAGGAGACGGA-3′ and insulin receptor, 5′-ACTCTCAGATCCTGAAGGAG-3′ and 5′-GACTCCTTGTTCACCAC-3′. PCR products were run on agarose gels containing ethidium bromide and revealed under ultraviolet illumination. Concurrent β-actin PCR products (β-actin primers, 5′-ATCATGTTTGAGACCTCCAA-3′ and 5′-TTGCGCTCAGGAGGAGCAAT-3′) were generated and analyzed in parallel to ensure that equivalent amounts of template were amplified.
RNA Interference
Hep3B cells were transfected with 200 nmol/L of a synthetic small interfering RNA (siRNA) against IRS-2 (sense 5′-GUACAUCAACAUCGACUUU-3′, anti-sense 5′-AAAGUCGAUGUUGAUGUAC-3′) or of an unrelated siRNA (Ambion Inc.) using LipofectAMINE 2000 (InVitrogen, Paisley, Scotland). Cells were analyzed 24 hours after transfection for IRS-2 expression by Western blotting and for apoptosis by staining with an annexin V-FITC conjugate and with propidium iodide (PI).
Flow Cytometric Assessment of Apoptosis by Annexin V Staining
Both adherent and floating cells were collected, washed, and stained with an annexin V-cyanine-5 conjugate (BD Biosciences Pharmingen) and with PI according to the manufacturer’s instructions. Hep3B cells were then analyzed by flow cytometry (LSR II; Becton Dickinson, Mountain View, CA). Annexin V binds to apoptotic cells that express phosphatidylserine on the outer layer of the cell membrane. Double-fluorescence analysis (annexin V and PI) allows the discrimination of living cells (unstained with either fluorochrome) from apoptotic cells (stained only with annexin V) and necrotic cells (stained with both annexin V and PI).
Results
IRS-2 Is Overexpressed in Murine Liver Tumors Induced by DEN or by SV40 Large T Antigen
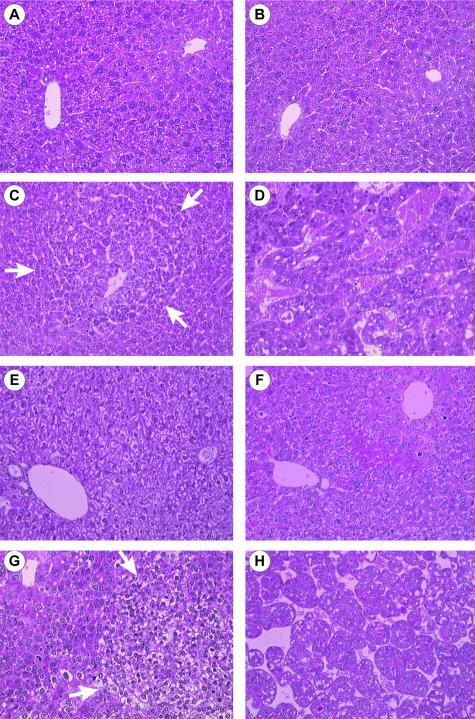
Two murine models were used in the following experiments to investigate IRS-2 expression during hepatocarcinogenesis. In the first model (Figure 1, A to D), hepatocarcinogenesis was chemically induced in males by a unique intraperitoneal injection of DEN at 15 days of age.24 In this model, no lesion was detected by histological analysis before 6 months. Figure 1 shows normal liver architecture in 3-month-old control (Figure 1A) and DEN-injected (Figure 1B) mice. Basophilic foci of altered hepatocytes (Figure 1C) with occasional eosinophilic cytoplasmic inclusions (data not shown) were identified at 6 months, hepatocellular adenomas at 8 months (data not shown), and multifocal HCC at 12 to 13 months (Figure 1D). In the second model (Figure 1, E to H), liver carcinogenesis resulted from hepatic expression of the SV40 large T antigen in males (ASV mice). Normal liver from a 3-month-old control female is shown in Figure 1E. In accordance with the initial model description,25 we observed hepatocellular atypias (anisocytosis, anisokaryosis), abnormal levels of mitosis, and apoptotic bodies within 2 months (Figure 1F). Foci of altered hepatocytes (clear cell type and mixed type) appeared at 3 months (Figure 1G) and diffuse HCC at 6 months (Figure 1H). At this stage, HCC invaded the whole liver parenchyma with no macroscopical evidence of nontumoral liver tissue. In the two murine models, HCC developed in a noncirrhotic liver.
Figure 1.
Histology of the liver from DEN-injected and ASV mice. Histological findings in liver sections stained with hematoxylin and phloxin: A, normal liver in a 3-month-old noninjected male; B, liver in a 3-month-old DEN-injected male with no evidence of either architectural or cytological modifications; C, liver in a 6-month-old DEN-injected male showing a basophilic foci of altered hepatocytes (arrows); D, HCC in a 12-month-old DEN-injected male; E, normal liver in a 3-month-old control female; F, liver in a 2-month-old ASV male with hepatocellular atypias and mitosis; G, liver in a 3-month-old ASV male with a clear foci of altered hepatocytes (arrows) and atypias around the foci; H, HCC in a 6-month-old ASV male. Original magnifications, ×200.
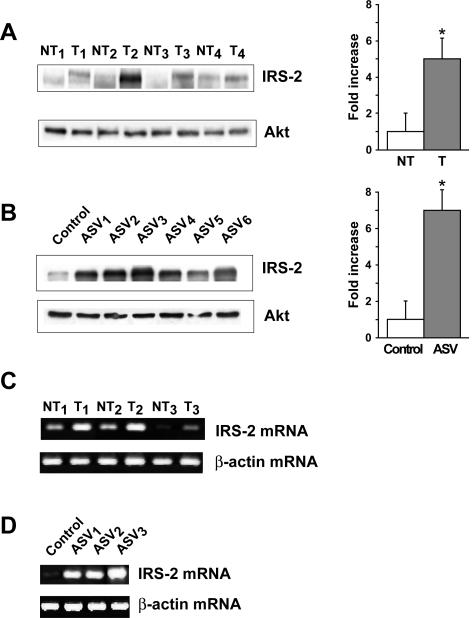
IRS-2 protein expression was examined by Western blotting in HCC nodules from 12- or 13-month-old DEN-injected mice (n = 20) and in HCC tissues from 6-month-old ASV mice (n = 13). We observed that HCC nodules from 17 of 20 DEN-injected mice (85%) exhibited increased expression of IRS-2 in comparison with nontumoral tissues. Four HCC nodules with IRS-2 overexpression are presented in Figure 2A. Quantification by densitometry scanning showed that IRS-2 protein levels were increased by fivefold in HCC as compared to nontumoral tissues. IRS-2 was also overexpressed in 11 of 13 HCCs (84%) from ASV mice in comparison with normal liver from an age-matched control female and was increased by sevenfold (Figure 2B). Semiquantitative RT-PCR experiments showed that increased expression of IRS-2 protein in HCC was associated with increased expression of IRS-2 mRNA, both in DEN-injected (Figure 2C) and ASV mice (Figure 2D).
Figure 2.
IRS-2 overexpression in HCC from DEN-injected and ASV mice. Protein extracts (40 μg) prepared from HCC (T) and nontumoral (NT) tissues from 12- and 13-month-old DEN-injected mice (A) and from 6-month-old ASV males (ASV 1 to 6) (B) were analyzed by Western blotting for IRS-2 protein expression. The liver was entirely tumoral in 6-month-old ASV males and the normal liver from an age-matched female was used as a control. Blots were reprobed with an anti-Akt antibody to ensure equivalent protein loading. Quantitative values are means ±SEM; *P < 0.05, compared with nontumoral tissue. Total RNA (2 μg) extracted from HCC (T) and nontumoral (NT) tissues from 12- and 13-month-old DEN-injected mice (C) and from 6-month-old ASV males (ASV 1 to 3) (D) and a control female were analyzed by semiquantitative RT-PCR using murine IRS-2 primers. β-Actin PCR products were run in parallel to ensure that equivalent amounts of cDNA were amplified.
IRS-2 Overexpression Is an Early Event of Murine Liver Tumorigenesis
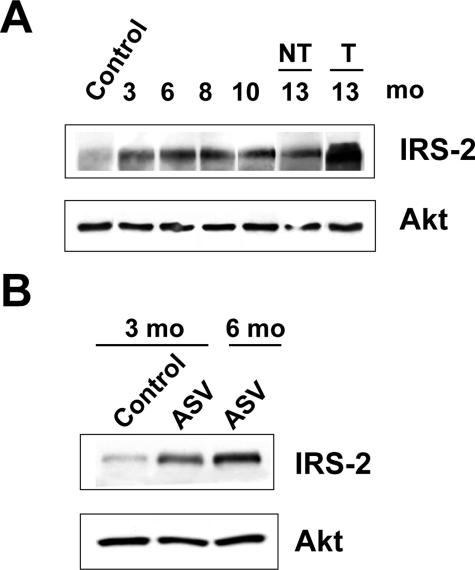
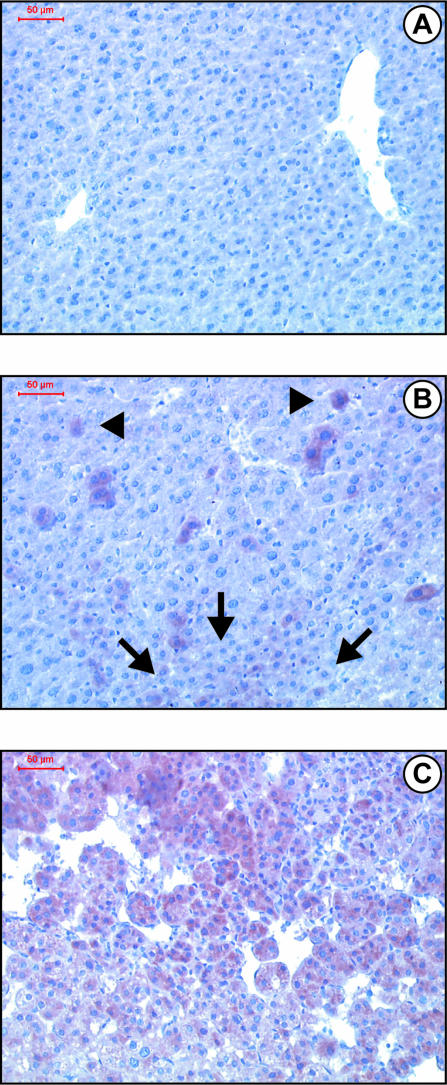
We next examined by Western blotting the time course of IRS-2 expression during murine liver tumorigenesis. As shown in Figure 3A, the expression of IRS-2 protein was increased in the liver from 3-month-old DEN-injected mice as compared with normal liver and was maintained at the same level between 3 and 10 months before the appearance of HCC. Thus, in the DEN model, IRS-2 overexpression occurred well before lesions were histologically detectable. In the ASV murine model, increased IRS-2 protein levels were detected in the liver from 3-month-old males at a stage when foci of transformed hepatocytes were detectable (Figure 3B). Importantly, in both experimental models, even if IRS-2 overexpression occurred at preneoplastic stages, higher levels of IRS-2 protein expression were detected in established HCC as compared with preneoplastic lesions (Figure 3, A and B). By using immunohistochemistry, we could show that as compared to normal liver (Figure 4A), liver tissue from 3-month-old DEN-injected mice displayed increased IRS-2 immunoreactivity in isolated hepatocytes or in small clusters (Figure 4B). In HCC nodules, most of the hepatocytes exhibited intense IRS-2 immunoreactivity (Figure 4C).
Figure 3.
Time course of IRS-2 expression in murine hepatocarcinogenesis. Protein extracts were prepared at different stages of DEN and ASV hepatocarcinogenesis and analyzed for IRS-2 protein expression by Western blotting. Blots were reprobed with an anti-Akt antibody to ensure equivalent protein loading. A: IRS-2 expression in the liver from 3- to 13-month-old males injected with DEN in comparison with normal liver from a 6-month-old noninjected male. NT, nontumoral tissue; T, tumoral. B: IRS-2 expression in the liver from 3- and 6-month-old ASV mice in comparison with normal liver from a 3-month-old control female. At 6 months of age, the liver sample was a HCC.
Figure 4.
Immunohistochemical detection of IRS-2 in the liver from control and DEN-injected mice. Liver tissue sections from control and DEN-injected mice were immunolabeled with an anti-IRS-2 antibody (reddish-brown labeling) and counterstained with hematoxylin. Histologically normal liver from a 3-month-old noninjected male (A) and from a 3-month-old DEN-injected male (B). IRS-2 immunoreactivity is detected in isolated hepatocytes (arrowheads) and in a small cluster (arrows). C: HCC nodules from a 12-month-old DEN-injected male. Original magnifications, ×200.
Characterization of the Insulin/IGF Signaling Axis in Murine Liver Tumors with IRS-2 Overexpression
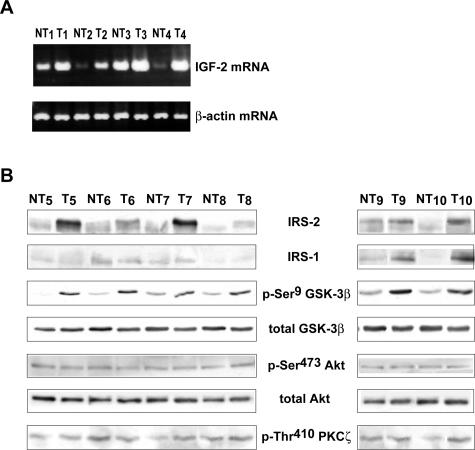
In murine DEN-induced HCC with IRS-2 overexpression, we analyzed the status of other components of the insulin and IGF signaling pathways that are deregulated in human and murine hepatocarcinogenesis, namely IGF-2,2,14 IRS-1,1,15–17 IGF-1R,18 insulin receptor,19,20 and GSK-3β.21–23 These results are summarized in Table 1. By semiquantitative RT-PCR analysis, we observed that 73% (8 of 11) of these tumors exhibited an increase in IGF-2 mRNA transcripts in comparison with nontumoral tissues (Figure 5A). Of note, IGF-2 mRNA transcripts were undetectable in age-matched control livers (data not shown). No change in the level of insulin receptor mRNA was observed in DEN-induced HCC as compared with nontumoral tissues (data not shown). By Western blotting, we observed that the insulin receptor was slightly increased in only 25% (4 of 16) of DEN-induced tumors (data not shown). Concerning the IGF-IR, we did not observe overexpression at the mRNA and protein levels (data not shown). IRS-1 was overexpressed in 13 of 17 tumors whereas 4 of 17 (24%) tumors with IRS-2 overexpression did not overexpress IRS-1 (Figure 5B). By using an anti-phospho-Ser9 GSK-3β antibody that allows detection of inactivated GSK-3β, we observed that 70% (12 of 17) of tumors with IRS-2 overexpression exhibited strong GSK-3β phosphorylation, ie, GSK-3β inactivation including the four tumors overexpressing IRS-2 but not IRS-1 (Figure 5B). Examination of the total GSK-3β content indicated that increased GSK-3β phosphorylation in HCC was not accompanied by increased GSK-3β expression. Different serine kinases including Akt and protein kinase C ζ (PKCζ) phosphorylate GSK-3β on the Ser9 residue.22 We next examined the activation levels of these kinases in HCC overexpressing IRS-2 and we observed that GSK-3β was hyperphosphorylated in these tumors in the absence of concomitant Akt and PKCζ activation as evaluated by using anti-activated phospho-Ser473 Akt and phospho-Thr410 PKCζ antibodies (Figure 5B). Similarly, in the ASV murine model, we identified a re-expression of IGF-2, an overexpression of IRS-1, and an inhibition of GSK-3β in IRS-2-overexpressing tumors (data not shown).
Table 1.
Expression of Different Components of the Insulin/IGF Signaling Axis in DEN-Induced Liver Tumors with IRS-2 Overexpression
| Molecules | Overexpression frequency |
|---|---|
| IGF-2 | 8 of 11 (73%) |
| Insulin receptor | 4 of 16 (25%) |
| IGF-1R | 0 of 16 |
| IRS-1 | 13 of 17 (76%) |
| p-Ser9GSK-3β | 12 of 17 (70%) |
Eighty-five percent of DEN-induced liver tumors exhibited IRS-2 overexpression and were examined for insulin receptor, IGF-1R, IRS-1, and phospho-Ser9 GSK-3β expression by Western blotting analysis and for IGF-2 mRNA expression by RT-PCR.
Figure 5.
Charaterization of the insulin/IGF signaling pathway in IRS-2-overexpressing HCC from DEN-injected mice. Total RNA and protein extracts were prepared from HCC (T) and nontumoral (NT) liver tissues obtained from 12- and 13-month-old mice injected with DEN. A: RNAs (2 μg) were analyzed by semiquantitative RT-PCR using IGF-2 primers. B: Protein extracts (40 μg) of six HCC-overexpressing IRS-2 were analyzed by Western blotting for IRS-1, phospho-Ser9, and total GSK-3β, phospho-Ser473 and total Akt, and phospho-Thr410 PKCζ protein expressions.
IRS-2 Is Overexpressed and Promotes Survival in Human HCC Cell Lines
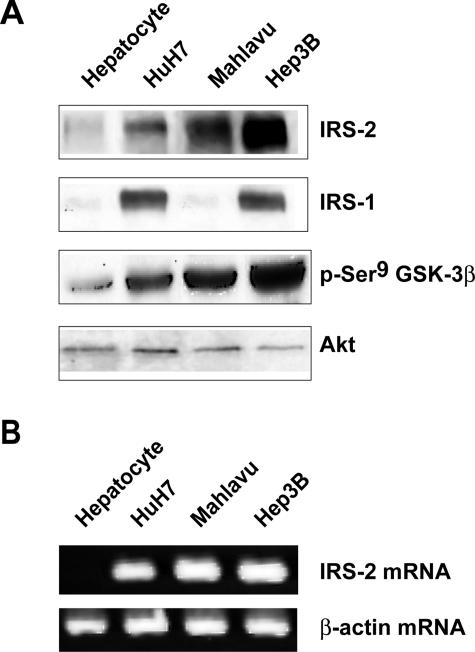
We then examined whether IRS-2 overexpression was relevant to human hepatocarcinogenesis. First, IRS-2 protein and mRNA expressions were examined in four human hepatoma cell lines (HepG2, HuH7, Mahlavu, Hep3B) in comparison with normal human hepatocytes in primary culture. HuH7, Mahlavu, and Hep3B cells overexpressed the IRS-2 protein, with the highest expression level observed in Hep3B cells (Figure 6A), whereas HepG2 cells displayed a low level of IRS-2 protein that was similar to that observed in normal hepatocytes (data not shown). RT-PCR experiments corroborated this finding by showing that HuH7, Mahlavu, and Hep3B cells (Figure 6B) but not HepG2 cells (data not shown) had increased IRS-2 mRNA levels as compared to normal hepatocytes. IRS-1 was overexpressed in HuH7 and Hep3B cells but not in the Mahlavu cell line. GSK-3β was hyperphosphorylated in the three cell lines including Mahlavu cells that overexpressed IRS-2 but not IRS-1 (Figure 6A).
Figure 6.
IRS-2 overexpression in human hepatoma cell lines. Protein extracts and total RNA were prepared from human hepatocytes maintained in primary culture and HCC cell lines (HuH7, Mahlavu, Hep3B) cultured for 17 hours in serum-free medium. A: Protein extracts (40 μg) were analyzed for IRS-2, IRS-1, and phospho-Ser9 GSK-3β protein expressions by Western blotting. Blots were reprobed with an anti-Akt antibody to ensure equivalent protein loading. B: RNA samples (2 μg) were analyzed by semiquantitative RT-PCR using human IRS-2 primers. β-Actin PCR products were run in parallel to ensure that equivalent amounts of cDNA were amplified.
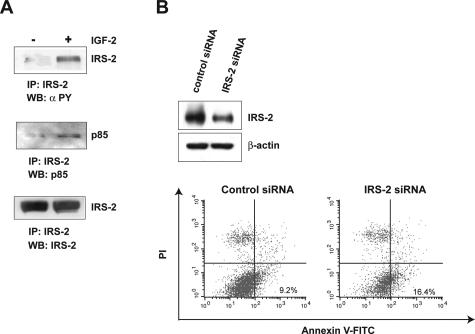
We next examined the functionality of IRS-2 in the Hep3B cell line. Cell treatment with IGF-2 induced a rapid increase in IRS-2 tyrosine phosphorylation content (Figure 7A, top) as well as IRS-2 association with the p85 regulatory subunit of PI 3-kinase (Figure 7A, middle) in anti-IRS-2 immunoprecipitates. IRS-2 mediates survival in different types of cell lines30–32 but no data have been reported in hepatoma cells. Then, we examined the potential role of IRS-2 overexpression in hepatoma cell survival by using a siRNA strategy. IRS-2 expression was decreased in Hep3B cells by the transfection of a siRNA duplex targeting IRS-2 as compared to cells transfected with an unrelated control siRNA (Figure 7B, top). The evaluation of annexin V staining (Annexin V+/PI−) revealed that the IRS-2 siRNA increased apoptosis by 78% compared with the control siRNA (Figure 7B, bottom). Similar results were obtained by the transient transfection of an IRS-2 anti-sense construct that increased by 45% the percentage of cells undergoing apoptosis as compared to an empty vector (data not shown).
Figure 7.
Functional analyses of IRS-2 in Hep3B cells. A: Hep3B cells were stimulated for 5 minutes with IGF-2 (10−8 mol/L) and lysed. Protein extracts (500 μg) were immunoprecipitated with an anti-IRS-2 antibody and analyzed by Western blotting with anti-phosphotyrosine (top) and anti-p85 (middle) antibodies. Blots were reprobed with an anti-IRS-2 antibody (bottom) to ensure that equivalent protein amounts were immunoprecipitated. B: Hep3B cells were transiently transfected with an IRS-2 or an unrelated control siRNA duplex. Twenty-four hours after transfection, cells were examined for IRS-2 protein expression by Western blotting (top) and for apoptosis by flow cytometry after staining with annexin V-FITC conjugate and PI (bottom). Data are representative of two independent experiments. Blots were reprobed with an anti-β-actin antibody to ensure equivalent protein loading.
IRS-2 Is Overexpressed in Human HCC Specimens
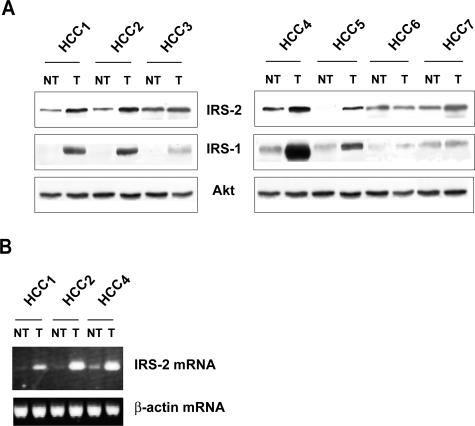
IRS-2 expression was investigated in seven human HCC specimens. Histological analyses indicated that HCC 1 to 3 developed in a noncirrhotic liver, HCC 4 to 6 arose in a cirrhotic liver, and HCC 7 in a fibrotic but noncirrhotic liver. Western blot analysis revealed that all samples except one (HCC 6) had increased IRS-2 protein expression in tumoral tissue as compared to nontumoral tissue (Figure 8A). Increased IRS-2 protein expression was associated with increased IRS-2 mRNA levels (Figure 8B). Among the six IRS-2-overexpressing tumors, five (HCC 1 to 5) displayed increased IRS-1 expression while one (HCC 7) did not overexpress IRS-1 showing that in human HCC as in experimental murine models, IRS-2 overexpression may occur in the absence of IRS-1 overexpression (Figure 8A).
Figure 8.
IRS-2 overexpression in human HCC specimens. Protein extracts and total RNA were prepared from human HCC (T) and nontumoral (NT) liver tissues from seven patients. A: IRS-2 and IRS-1 protein expressions were evaluated by Western blotting analysis. Blots were reprobed with an anti-Akt antibody to ensure equivalent protein loading. B: IRS-2 mRNA expression was evaluated by semiquantitative RT-PCR. β-Actin PCR products were run in parallel to ensure that equivalent amounts of cDNA were amplified.
Discussion
Until now, very limited data were available regarding the implication of IRS-2 in carcinogenesis. It has been previously shown that IRS-2 is overexpressed in metastatic variants of human breast cancer cell lines33 as well as in human pancreatic cancer.34,35 In the present study, we show that IRS-2 expression is deregulated during hepatocellular carcinogenesis. We found increased IRS-2 protein expression in HCC from two mechanistically distinct murine models of hepatocarcinogenesis and in both models, IRS-2 overexpression was detected at preneoplastic stages. In the DEN model, IRS-2 is even overexpressed long before preneoplastic lesions are histologically detectable, consistent with a role of IRS-2 in early steps of liver tumorigenesis. Moreover, in the two models, higher levels of IRS-2 expression were observed in HCC nodules than in preneoplastic lesions suggesting that IRS-2 is also implicated at later stages of liver carcinogenesis. In addition, we show that IRS-2 overexpression also occurs in human hepatocarcinogenesis because it was observed in human HCC cell lines as well as in human HCC specimens irrespective of whether the underlying liver was cirrhotic or not. Increased IRS-2 protein expression in human and murine HCC cells and tissues was accompanied by an increased IRS-2 mRNA level, suggesting that IRS-2 overexpression resulted from enhanced gene transcription during liver tumorigenesis.
In some murine HCC nodules overexpressing IRS-2, we observed that IRS-1 protein expression was also up-regulated. This finding corroborates previous studies reporting that IRS-1 overexpression is a marker of liver tumorigenesis in humans and rodents.1,15–17 However, 24% of HCCs with IRS-2 overexpression did not exhibit IRS-1 overexpression. This finding indicates that IRS-2 may be involved in hepatocarcinogenesis independently of IRS-1. Despite amino acid sequence homology and overlapping functions, IRS-1 and IRS-2 have the capacity to transduce distinct signals. The importance of IRS-2 vis-à-vis IRS-1 for insulin action in the liver has been shown in experiments using IRS-1 and/or IRS-2 knockout mice. In IRS-1 knockout mice that are glucose intolerant but do not develop diabetes, IRS-2 compensates IRS-1 deficiency in hepatocytes in mediating insulin’s effects on metabolism.9,36–39 In contrast, in IRS-2 knockout mice, the lack of IRS-2 in the liver is not compensated by enhanced IRS-1-dependent signaling and these mice are diabetic.9,10,40 By using hepatocytes isolated from IRS-2-deficient mice, it has been shown that IRS-2 is essential in mediating activation of proliferation and glycogen synthesis and repression of gluconeogenic enzymes expression in response to insulin,11,12 whereas IRS-1 seems to be the main effector of proliferative signals triggered by IGF-1 receptors.11,12 Taken together, these data suggest that IRS-1 and IRS-2 could have differential impacts on intracellular signaling during hepatocarcinogenesis. Consistent with this possibility, we observed that down-regulation of IRS-2 expression in Hep3B cells by the transient transfection of an IRS-2 siRNA duplex increased apoptosis despite a high expression level of IRS-1, indicating that IRS-1 and IRS-2 are not fully redundant in these cells. This suggests that IRS-2 overexpression could provide selective survival advantage to hepatocytes during transformation.
IGF-2 is a ligand for the insulin receptor and IGF-1R. In human and rodent HCC tissues, re-expression of fetal IGF-2 mRNA has been frequently reported2,14 while a few studies have mentioned increased expression of insulin receptor and IGF-1R.18–20 We observed that murine HCCs frequently exhibit overexpression of IGF-2 mRNA but not of insulin receptor and IGF-1R mRNA and protein. In this context, high levels of IRS-2 may amplify the potential of IGF-2 to signal through insulin receptor-dependent signaling pathways during hepatocarcinogenesis. In support of this hypothesis, we observed that, in Hep3B cells, IRS-2 undergoes tyrosine phosphorylation and associates with PI 3-kinase in response to IGF-2.
GSK-3β is a downstream target of insulin and IGF receptor-dependent pathways which result in GSK-3β phosphorylation on the serine 9 residue, GSK-3β inhibition, and subsequent stimulation of glycogen synthase activity.35 Recent findings from our laboratory and others indicate that hyperphosphorylation of GSK-3β on serine 9 is a hallmark of hepatocarcinogenesis21–23 and that, in human HCC cell lines, Akt and PKCζ phosphorylate and inactivate GSK-3β.22 In the present murine models, most HCC displayed high levels of phospho-Ser9 GSK-3β without concomitant Akt/PKCζ activation suggesting that, in these HCCs, Akt and PKCζ are not involved in GSK-3βSer9 phosphorylation. Alternatively, Akt- and/or PKCζ-mediated GSK-3βSer9 phosphorylation could be transient and an additional mechanism could contribute to maintaining GSK-3β under a phosphorylated state. Data obtained in vitro in different cell types including hepatocytes12,41 indicate that IRS-2 mediates GSK-3βSer9 phosphorylation in response to insulin. We found that in human HCC cell lines as well as in most murine HCCs with IRS-2 overexpression, GSK-3β is hyperphosphorylated even in the absence of IRS-1 overexpression thus reinforcing the assumption that IRS-2 and GSK-3β lie on the same intracellular signaling pathway.
In conclusion, this study is the first demonstration that IRS-2, which is the main mediator of insulin receptor in the normal liver, is overexpressed in human and murine HCCs. The emergence of IRS-2 overexpression at early preneoplastic stages during murine hepatocarcinogenesis and its protective effect against apoptosis suggest that IRS-2 contributes to liver tumor progression. Therefore, pharmacological or genetic interference with this pathway could have an important therapeutic potential in HCC.
Acknowledgments
We thank Dr. Pascale Briand (Institut Cochin, Paris, France) and Dr. Douglas Yee (University of Minnesota Cancer Center, Minneapolis, MN) for their generous gifts; Pierre Casanova, Marie-Cécile Samson, and Isabelle Renault for animal care; Roland Delelo (Centre de Recherches Chirurgicales, Hôpital Saint-Antoine, Paris, France) for making human hepatocytes available; Marie-José Blivet-Van Eggelpoël and Sylviane Moritz (INSERM U.680) for technical support; and Pr. J. Capeau (INSERM U.680) for critical reading of the manuscript.
Footnotes
Address reprint requests to Dr. Christèle Desbois-Mouthon, INSERM U.680, Faculté de Médecine Saint-Antoine, 27 rue Chaligny, 75571 Paris Cedex 12, France. E-mail: desbois@st-antoine.inserm.fr.
Supported by the Association pour la Recherche sur le Cancer (to M.B.) and the Ligue Nationale Contre le Cancer (to E.B.).
References
- Tanaka S, Sugimachi K, Maehara S, Harimoto N, Shirabe K, Wands JR. Oncogenic signal transduction and therapeutic strategy for hepatocellular carcinoma. Surgery. 2002;131:S142–S147. doi: 10.1067/msy.2002.119495. [DOI] [PubMed] [Google Scholar]
- Scharf JG, Braulke T. The role of the IGF axis in hepatocarcinogenesis. Horm Metab Res. 2003;35:685–693. doi: 10.1055/s-2004-814151. [DOI] [PubMed] [Google Scholar]
- Bannasch P, Klimek F, Mayer D. Early bioenergetic changes in hepatocarcinogenesis: preneoplastic phenotypes mimic responses to insulin and thyroid hormone. J Bioenerg Biomembr. 1997;29:303–313. doi: 10.1023/a:1022438528634. [DOI] [PubMed] [Google Scholar]
- Kaburagi Y, Yamauchi T, Yamamoto-Honda R, Ueki K, Tobe K, Akanuma Y, Yazaki Y, Kadowaki T. The mechanism of insulin-induced signal transduction mediated by the insulin receptor substrate family. Endocr J. 1999;46:S25–S34. doi: 10.1507/endocrj.46.suppl_s25. [DOI] [PubMed] [Google Scholar]
- White MF. IRS proteins and the common path to diabetes. Am J Physiol. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- Tseng YH, Ueki K, Kriauciunas KM, Kahn CR. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J Biol Chem. 2002;277:31601–31611. doi: 10.1074/jbc.M202932200. [DOI] [PubMed] [Google Scholar]
- Li L, Qi X, Williams M, Shi Y, Keegan AD. Overexpression of insulin receptor substrate-1, but not insulin receptor substrate-2, protects a T cell hybridoma from activation-induced cell death. J Immunol. 2002;168:6215–6223. doi: 10.4049/jimmunol.168.12.6215. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Winnay J, Cheatham B, Kahn CR. Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS-2 in IRS-1-deficient cells. Mol Cell Biol. 1997;17:1513–1521. doi: 10.1128/mcb.17.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest. 2000;105:199–205. doi: 10.1172/JCI7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- Rother KI, Imai Y, Caruso M, Beguinot F, Formisano P, Accili D. Evidence that IRS-2 phosphorylation is required for insulin action in hepatocytes. J Biol Chem. 1998;273:17491–17497. doi: 10.1074/jbc.273.28.17491. [DOI] [PubMed] [Google Scholar]
- Valverde AM, Burks DJ, Fabregat I, Fisher TL, Carretero J, White MF, Benito M. Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes. 2003;52:2239–2248. doi: 10.2337/diabetes.52.9.2239. [DOI] [PubMed] [Google Scholar]
- Khamzina L, Gruppuso PA, Wands JR. Insulin signaling through insulin receptor substrate 1 and 2 in normal liver development. Gastroenterology. 2003;125:572–585. doi: 10.1016/s0016-5085(03)00893-x. [DOI] [PubMed] [Google Scholar]
- Sedlaczek N, Hasilik A, Neuhaus P, Schuppan D, Herbst H. Focal overexpression of insulin-like growth factor 2 by hepatocytes and cholangiocytes in viral liver cirrhosis. Br J Cancer. 2003;88:733–739. doi: 10.1038/sj.bjc.6600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusaka A, Nishiyama M, Ohkawa K, Yamori T, Tsuruo T, Yonezawa K, Kasuga M, Hayashi S, Tanaka T. Expression of insulin receptor substrate-1 in hepatocytes: an investigation using monoclonal antibodies. Cancer Lett. 1994;84:85–92. doi: 10.1016/0304-3835(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Nehrbass D, Klimek F, Bannasch P, Mayer D. Insulin receptor substrate-1 is over-expressed in glycogenotic but not in amphophilic preneoplastic hepatic foci induced in rats by N-nitrosomorpholine and dehydroepiandrosterone. Cancer Lett. 1999;140:75–79. doi: 10.1016/s0304-3835(99)00095-6. [DOI] [PubMed] [Google Scholar]
- Nehrbass D, Klimek F, Bannasch P. Overexpression of insulin receptor substrate-1 emerges early in hepatocarcinogenesis and elicits preneoplastic hepatic glycogenosis. Am J Pathol. 1998;152:341–345. [PMC free article] [PubMed] [Google Scholar]
- Kim SO, Park JG, Lee YI. Increased expression of the insulin-like growth factor I (IGF-I) receptor gene in hepatocellular carcinoma cell lines: implications of the IGF-I receptor gene activation by hepatitis B virus X gene product. Cancer Res. 1996;56:3831–3836. [PubMed] [Google Scholar]
- Kurtaran A, Li SR, Raderer M, Leimer M, Muller C, Pidlich J, Neuhold N, Hubsch P, Angelberger P, Scheithauer W. Technetium-99m-galactosyl-neoglycoalbumin combined with iodine-123-Tyr-(A14)-insulin visualizes human hepatocellular carcinomas. J Nucl Med. 1995;36:1875–1881. [PubMed] [Google Scholar]
- Spector SA, Olson ET, Gumbs AA, Friess H, Buchler MW, Seymour NE. Human insulin receptor and insulin signaling proteins in hepatic disease. J Surg Res. 1999;83:32–35. doi: 10.1006/jsre.1998.5553. [DOI] [PubMed] [Google Scholar]
- Gotoh J, Obata M, Yoshie M, Kasai S, Ogawa K. Cyclin D1 over-expression correlates with beta-catenin activation, but not with H-ras mutations, and phosphorylation of Akt, GSK3 beta and ERK1/2 in mouse hepatic carcinogenesis. Carcinogenesis. 2003;24:435–442. doi: 10.1093/carcin/24.3.435. [DOI] [PubMed] [Google Scholar]
- Desbois-Mouthon C, Blivet-Van Eggelpoel MJ, Beurel E, Boissan M, Delelo R, Cadoret A, Capeau J. Dysregulation of glycogen synthase kinase-3beta signaling in hepatocellular carcinoma cells. Hepatology. 2002;36:1528–1536. doi: 10.1053/jhep.2002.37192. [DOI] [PubMed] [Google Scholar]
- Ban KC, Singh H, Krishnan R, Seow HF. GSK-3beta phosphorylation and alteration of beta-catenin in hepatocellular carcinoma. Cancer Lett. 2003;199:201–208. doi: 10.1016/s0304-3835(03)00421-x. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch SD, Koka M, Mihailovich N, Rao KV. Carcinogenicity of diethylnitrosamine in newborn, infant, and adult mice. J Cancer Res Clin Oncol. 1984;108:60–65. doi: 10.1007/BF00390974. [DOI] [PubMed] [Google Scholar]
- Dubois N, Bennoun M, Allemand I, Molina T, Grimber G, Daudet-Monsac M, Abelanet R, Briand P. Time-course development of differentiated hepatocarcinoma and lung metastasis in transgenic mice. J Hepatol. 1991;13:227–239. doi: 10.1016/0168-8278(91)90819-w. [DOI] [PubMed] [Google Scholar]
- Lakehal F, Dansette PM, Becquemont L, Lasnier E, Delelo R, Balladur P, Poupon R, Beaune PH, Housset C. Indirect cytotoxicity of flucloxacillin toward human biliary epithelium via metabolite formation in hepatocytes. Chem Res Toxicol. 2001;14:694–701. doi: 10.1021/tx0002435. [DOI] [PubMed] [Google Scholar]
- Hamilton S, Aaltonen L. Hamilton S, Aaltonen L, editors. Lyon: IARC Press,; Tumours of the Liver and Intrahepatic Bile Ducts. 2000:pp 157–202. [Google Scholar]
- Mohr U. Mohr U, editor. Berlin: Springer-Verlag; Liver, Gallbladder and Exocrine Pancreas. :pp 59–86. [Google Scholar]
- Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoel MJ, Bertrand F, Cherqui G, Perret C, Capeau J. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene. 2001;20:252–259. doi: 10.1038/sj.onc.1204064. [DOI] [PubMed] [Google Scholar]
- Ueno H, Kondo E, Yamamoto-Honda R, Tobe K, Nakamoto T, Sasaki K, Mitani K, Furusaka A, Tanaka T, Tsujimoto Y, Kadowaki T, Hirai H. Association of insulin receptor substrate proteins with Bcl-2 and their effects on its phosphorylation and antiapoptotic function. Mol Biol Cell. 2000;11:735–746. doi: 10.1091/mbc.11.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingohr MK, Dickson LM, Wrede CE, Briaud I, McCuaig JF, Myers MG, Jr, Rhodes CJ. Decreasing IRS-2 expression in pancreatic beta-cells (INS-1) promotes apoptosis, which can be compensated for by introduction of IRS-4 expression. Mol Cell Endocrinol. 2003;209:17–31. doi: 10.1016/j.mce.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Jackson JG, Zhang X, Yoneda T, Yee D. Regulation of breast cancer cell motility by insulin receptor substrate-2 (IRS-2) in metastatic variants of human breast cancer cell lines. Oncogene. 2001;20:7318–7325. doi: 10.1038/sj.onc.1204920. [DOI] [PubMed] [Google Scholar]
- Kornmann M, Maruyama H, Bergmann U, Tangvoranuntakul P, Beger HG, White MF, Korc M. Enhanced expression of the insulin receptor substrate-2 docking protein in human pancreatic cancer. Cancer Res. 1998;58:4250–4254. [PubMed] [Google Scholar]
- Schuppin GT, Pons S, Hugl S, Aiello LP, King GL, White M, Rhodes CJ. A specific increased expression of insulin receptor substrate 2 in pancreatic beta-cell lines is involved in mediating serum-stimulated beta-cell growth. Diabetes. 1998;47:1074–1085. doi: 10.2337/diabetes.47.7.1074. [DOI] [PubMed] [Google Scholar]
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, III, Johnson RS, Kahn CR. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, Sekihara H, Yoshioka S, Horikoshi H, Furuta Y, Ikawa Y, Kasuga M, Yazaki Y, Aizawa S. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- Patti ME, Sun XJ, Bruening JC, Araki E, Lipes MA, White MF, Kahn CR. 4PS/insulin receptor substrate (IRS)-2 is the alternative substrate of the insulin receptor in IRS-1-deficient mice. J Biol Chem. 1995;270:24670–24673. doi: 10.1074/jbc.270.42.24670. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Tobe K, Tamemoto H, Ueki K, Kaburagi Y, Yamamoto-Honda R, Takahashi Y, Yoshizawa F, Aizawa S, Akanuma Y, Sonenberg N, Yazaki Y, Kadowaki T. Insulin signalling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1-deficient mice. Mol Cell Biol. 1996;16:3074–3084. doi: 10.1128/mcb.16.6.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000;49:1880–1889. doi: 10.2337/diabetes.49.11.1880. [DOI] [PubMed] [Google Scholar]
- Oriente F, Formisano P, Miele C, Fiory F, Maitan MA, Vigliotta G, Trencia A, Santopietro S, Caruso M, Van Obberghen E, Beguinot F. Insulin receptor substrate-2 phosphorylation is necessary for protein kinase C zeta activation by insulin in L6hIR cells. J Biol Chem. 2001;276:37109–37119. doi: 10.1074/jbc.M104405200. [DOI] [PubMed] [Google Scholar]