Abstract
T cells infiltrating the inflamed liver express high levels of CXCR3 and show enhanced migration to CXCR3 ligands in chemotactic assays. Moreover, CXCR3 ligands are up-regulated on hepatic endothelium at sites of T-cell infiltration in chronic hepatitis, and their presence correlates with outcome of inflammatory liver disease. We used a flow-based adhesion assay with human hepatic endothelium to investigate the function of CXCR3 on lymphocyte adhesion to and transmigration through hepatic endothelium under physiological conditions of blood flow. To more accurately model the function of in vivo activated CXCR3high lymphocytes, we isolated T cells from human liver tissue and studied their behavior in flow-based adhesion assays. We demonstrate that CXCR3 not only promoted the adhesion of effector T cells to endothelium from flow but also drove transendothelial migration. Moreover, these responses could be stimulated either by endogenous CXCR3 ligands secreted by the endothelium or by exogenous CXCR3 ligands derived from other cell types and presented by the endothelium. This study thus demonstrates that activation of CXCR3 promotes lymphocyte adhesion and transendothelial migration under flow and that human hepatic endothelium can present functionally active chemokines secreted by other cell types within the liver.
Chronic inflammation occurs as a consequence of the recruitment and retention of lymphocytes in tissue. The recruitment of effector lymphocytes from the circulation is dependent on interactions between lymphocytes and specific cell surface molecules expressed on endothelial cells. Once captured the retention and positioning of leukocytes within tissue requires signals to localize and retain them at sites of target cell damage. These receptors and chemokine signals can be organized into the accepted multistep paradigm of leukocyte adhesion to vascular endothelium, which is relevant to most organ systems, although the specific signals involved differ between tissues.1,2 Initial transient interactions between flowing lymphocytes and endothelium tether the lymphocyte and induce it to roll on the vessel wall where it comes into contact with chemokines in the endothelial glycocalyx. In the presence of an appropriate chemokine, specific G-protein-coupled receptors on the lymphocyte are activated triggering high-affinity β1 and β2 integrins on the leukocyte surface to bind endothelial ligands resulting in arrest and stable adhesion.3 This is followed by shape change of the lymphocyte associated with migration on and through the endothelium and into underlying tissue where cells accumulate at the focus of inflammation, a process driven by chemotactic signals.4,5
Chemokines are critical components of this adhesion cascade and are believed to play two crucial roles: triggering integrin-mediated stable adhesion and directing migration. Chemokines can bind to endothelial glycosaminoglycans allowing them to be presented to flowing leukocytes and also providing a mechanism for the paracrine presentation of chemokines secreted by other cells within the microenvironment, a process termed “posting”.6–11 Similar mechanisms are believed to be involved in both normal immune surveillance and in inflammatory disease although the chemokines involved differ with constitutive chemokines playing the dominant role in physiological trafficking and inducible inflammatory cytokines involved in inflammation.1
Chemokines can be classified into four groups according to their amino acid sequence.12 The two largest groups are the CC chemokines, where conserved cysteine residues lie adjacent to each other, and CXC chemokines where an amino acid separates the first two cysteine residues. This group can be further classified by the presence of a glutamic acid-leucine-arginine (ELR) sequence near the N terminus. The ELR-containing CXC chemokines are potent chemoattractants for neutrophils and include interleukin (IL)-8.13 CXC chemokines lacking the ELR motif are CXCL10 or interferon (IFN)-inducible protein (IP-10), CXCL9 or monokine induced by IFN-γ (MIG), and CXCL11 or IFN-inducible T-cell α-chemoattractant (ITAC), all of which display potent lymphocyte chemotactic activity.14–17 These three chemokines bind a common receptor, CXCR3, the expression of which is increased on tissue-infiltrating T cells and Th1-polarized T cells. The observation that CXCR3 ligands require IFN-γ for their expression and are localized to sites of inflammation led to the assumption that CXCR3 is important for effector lymphocyte recruitment into inflamed tissue. Studies using CXCR3-deficient or IP-10-deficient mice show reduced tissue infiltration of effector cells in several inflammatory and transplantation models18,19 supporting the hypothesis but the role played by CXCR3 remains unclear. CXCR3 ligands have been shown to promote adhesion of lymphoblasts to human umbilical vein endothelial cells in vitro20 but have not previously been shown to be involved in transendothelial migration under conditions of physiological flow.
We have previously reported that T cells infiltrating the inflamed liver express high levels of CXCR3 and show enhanced migration to CXCR3 ligands in chemotactic assays.21–23 Moreover CXCR3 ligands are up-regulated on hepatic endothelium at sites of T-cell infiltration in chronic hepatitis and their presence has been correlated with outcome of inflammatory liver disease.24–27 We have developed a flow-based adhesion assay using human hepatic endothelium which allows us to model leukocyte interactions with hepatic endothelium under physiological conditions of blood flow.28 In the present study we used this assay to investigate the function of CXCR3 ligands in promoting lymphocyte adhesion and transendothelial migration. To more accurately model the function of in vivo-activated CXCR3high lymphocytes, we isolated T cells from human liver tissue and studied their behavior in flow-based adhesion assays. We demonstrate that CXCR3 not only promotes the adhesion of effector T cells to endothelium from flow but also drives transendothelial migration. Moreover, these responses can be stimulated either by endogenous CXCR3 ligands secreted by the endothelium or by exogenous CXCR3 ligands derived from other cell types and presented by the endothelium. This study thus demonstrates the ability of either endogenously or exogenously secreted CXCR3 ligands on human hepatic endothelium to promote lymphocyte adhesion and transendothelial migration under flow.
Materials and Methods
Liver tissue and peripheral blood samples were obtained during liver transplantation at the Liver Unit at the Queen Elizabeth Hospital, Edgbaston, Birmingham, UK. All samples were collected with appropriate patient consent and local ethical committee approval.
Immunohistochemistry and Dual Immunofluorescence
Six-μm cryostat sections of 1-cm3 liver blocks were cut for immunohistochemistry and immunofluorescence. These sections were air-dried on slides pretreated with Fro-tissue pen (The Binding Site, Birmingham, UK), then fixed for 10 minutes in acetone before staining.
Immunohistochemistry
Tissue sections were initially incubated with avidin/biotin blocking kit (DAKO, Ely, UK) and washed in Tris-buffered saline (TBS) buffer. Subsequently, the sections were incubated with 20% normal goat serum in TBS buffer for 30 minutes before the addition of a mouse anti-human monoclonal primary antibody (CXCL9: 49106, 5 μg/ml; CXCL10: 6D4, 2 μg/ml; CXCL11: 87328, 5 μg/ml; all from R&D Systems Europe, Abingdon, UK) in TBS for 60 minutes at 37°C in a humidified container. Control sections were incubated without the primary antibody or relevant isotype-matched control. Primary antibody binding was assessed using the StreptABComplex Duet peroxidase kit (DAKO) and developed with diaminobenzidine chromogen (Sigma FastDAB with 1 mg/L of sodium azide; Sigma, Poole, UK) for 5 minutes. The sections were washed in distilled water, counterstained with hematoxylin, and mounted. Positive staining was identified by the presence of a dark brown reaction product. All washes were performed with Tris-buffered saline, pH 7.6.
Immunofluorescence
Tissue sections were initially incubated with 20% normal goat serum in TBS buffer for 30 minutes before the addition of both mouse anti-human monoclonal CXCL10 IgG2a (6D4, 2 μg/ml) and CD31 IgG1 (JC70A, 5 μg/ml; DAKO) antibodies in TBS for 60 minutes at 37°C in a humidified container. The slides were then washed in TBS buffer and incubated with goat anti-mouse fluorescein isothiocyanate (IgG2a) and TXR (IgG1) (both Southern Biotechnology, Birmingham, AL) in TBS for 60 minutes at 37°C and protected from light. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (Sigma) and the sections mounted with fluorescence mounting medium (DAKO). Dual immunofluorescence was assessed using AxioVision software (Zeiss, Germany).
Isolation of Liver-Infiltrating Lymphocytes
Lymphocytes were isolated from a variety of diseased livers (hepatitis C virus, primary biliary cirrhosis, alcoholic liver disease, primary sclerosing cholangitis) using nonenzymatic, mechanical methods to preserve chemokine receptor expression. Resected tissue was reduced to 5-mm3 cubes and placed for 5 minutes at 230rpm in a Stomacher 400 circulator (Seward, UK). The resultant homogenized tissue was filtered through a fine gauze mesh and lymphocytes were separated by layering over a 33%/77% Percoll (Amersham Biosciences, UK) density gradient and centrifugation at 650 × g for 30 minutes. Isolated lymphocytes were resuspended in RPMI 1640 (Invitrogen, Paisley, UK) with 10% fetal calf serum at 4°C.
Isolation of Peripheral Blood Lymphocytes
Peripheral blood lymphocytes were isolated as previously described29 by density gradient centrifugation over Lymphoprep (Nycomed Pharma, Norway) for 30 minutes at 650 × g. Harvested lymphocytes were washed and resuspended in RPMI 1640/10% fetal calf serum at 4°C before use.
Flow Cytometric Analysis of Lymphocytes
Lymphocytes were resuspended in cold fluorescence-activated cell sorting media consisting of 0.5 mmol/L MgCl2, 1.0 mmol/L CaCl2, phosphate-buffered saline (Sigma), pH 7.4, with 10% fetal calf serum and 1 mg/ml sodium azide. Cells were subsequently blocked with 3 mg/ml of mouse immunoglobulins (Sigma) and labeled with the addition of three directly conjugated primary monoclonal antibodies in relevant combinations [CXCR3-RPE: 49801; 2.5 μg/ml (R&D Systems); CD3-RPE-Cy5: UCHT1; 1:10, CD8-fluorescein isothiocyanate: DK25; 1:10 (both DAKO); CD4-ECD: SFCI12T4D11; 1:10, CD3-ECD: HIT3a; 1:10 (both Beckman Coulter UK Ltd.); CD45RA-RPE-Cy5: MEM56; 1:20, CD45R0-fluorescein isothiocyanate: UCHL1; 10 μg/ml (both Serotec, Oxford, UK)]. Irrelevant isotype-matched antibodies were used to label control samples. Cells were fixed in 1% paraformaldehyde before three-color analysis on a Coulter Epics XL flow cytometer (Coulter Electronics Ltd., UK). Results were analyzed on Summit software (Dako Cytomation, UK).
Isolation and Culture of Hepatic Sinusoidal Endothelial Cells (HSECs) and Biliary Epithelial Cells (BECs)
HSECs and BECs were isolated according to our previously described methods.28,30 Briefly, ∼30 g of liver tissue was removed from explanted livers of patients undergoing transplantation. The tissue was finely chopped and subjected to enzymatic digestion (1 mg/ml, collagenase type IV; Sigma) and metrizamide density gradient centrifugation (25%, w/v; Sigma). The nonparenchymal cell band was then removed and cells were further purified by immunomagnetic selection. Cells positive for mAb HEA-125 (Progen, Germany) were classified BECs, whereas those cells negative for HEA-125 but positive for CD31 (DAKO) were classified HSECs. After isolation HSECs and BECs were plated in collagen-coated 25-cm2 tissue culture flasks (Corning). HSECs were cultured in human endothelial basal media (PAA Laboratories, Somerset, UK) containing 10% human serum (H&D Supplies, Bucks, UK), penicillin, and streptomycin (100 U/ml), hepatocyte growth factor (5 ng/ml, PromoCell) and vascular endothelial growth factor (10 ng/ml, R&D Systems). BECs were cultured in Dulbecco’s modified Eagle’s medium, Ham’s F12 (Invitrogen), containing 10% human serum, penicillin, and streptomycin (100 IU/ml), glutamine (2 mmol/L), epidermal growth factor (10 ng/ml, R&D Systems), hydrocortisone (2 mg/ml), cholera toxin (10 ng/ml), triiodothyronine (2 nmol/L), all Sigma, insulin (0.124 U/ml, Novo Nordisk, W. Sussex, UK), hepatocyte growth factor (5 ng/ml). Cells were cultured to confluence and in all experiments used between passages 2 and 6.
Measurement of Cell Surface Adhesion Molecules
Cells were plated at an initial count of 1 × 105/ml and grown to confluence in collagen-coated 96-well flat-bottom plates (Falcon). Cells were then left under basal conditions or stimulated with cytokines for 24 hours (10 ng/ml recombinant tumor necrosis factor (TNF)-α, TNF-β, IL-1β, or oncostatin M all from PeproTech, London, UK) or lipopolysaccharide (Sigma). For all stimulations the cells were incubated in the presence or absence of IFN-γ (10 ng/ml, PeproTech). After stimulation supernatant from relevant wells was pooled and stored at −70°C until analyzed and the cell monolayers fixed in methanol. Nonspecific binding of mAb was inhibited by preincubation of cells for 1 hour at 37°C with 4% goat serum or rabbit serum as required (Sigma) before the addition of mouse anti-human mAb [ICAM-1: M7063, 1.85 μg/ml; VCAM-1: M7106, 1.75 μg/ml; E-selectin: M7105, 1.6 μg/ml; CD31: M0823, 2.25 μg/ml; cytokeratin 19: M0888, 0.5 μg/ml (all from DAKO) and ICAM-2: sc-1512, 1 μg/ml (Autogen Bioclear UK Ltd., Wiltshire, UK)] for 1 hour at 37°C. The cells were then washed thoroughly before incubation with peroxidase-conjugated goat anti-mouse or rabbit anti-goat secondary Ab as required (P0447 and P0449, respectively, 1/5000; DAKO). The enzyme-linked immunosorbent assay (ELISA) was developed using O-phenylenediamine substrate (OPD: S2045; DAKO) according to the manufacturer’s instructions and the enzymatic reaction was stopped using 0.5 mol/L H2SO4 (Fisher Scientific, Lei-cestershire, UK). Colorimetric analysis was performed by measuring absorbance values at 490 nm using a Dynatech Laboratories MRX plate reader. All treatments were performed in triplicate for each experiment.
Measurement of Cell-Specific Chemokine Production
The concentration of CXCL9, -10, and -11 in supernatant samples was determined by sandwich ELISA31 using commercially available kits (R&D Systems). Briefly, relevant capture antibodies were prepared in 0.1 mol/L carbonate/bicarbonate buffer, pH 9.6, added to 96-well immunosorp plates (Nunc) and allowed to coat overnight at 4°C. Protein standards of known concentration or test samples were then added to relevant wells and incubated at room temperature for 1 hour before washing and a further 1-hour incubation with relevant biotinylated detection antibodies. The plates were then washed thoroughly before incubation with peroxidase-conjugated streptavidin (1/5000; Zymed, Cambs, UK). The ELISA was developed using TMB substrate [100 μg/ml 3,5,3,5-tetramethylbenzidine (TMB; Sigma) in 0.11 mol/L sodium acetate, pH 5.5, and 0.0003% H2O2 (both BDH)] and the enzymatic reaction stopped using 2.5 mol/L H2SO4. Colorimetric analysis was performed by measuring absorbance values at 450 nm using a Dynatech Laboratories MRX plate reader. All measurements were performed in duplicate for each experiment.
Adhesion Assays
Static Adhesion Assays
Lymphocytes isolated from chronically inflamed liver samples or matched peripheral blood samples were resuspended to a count of 8 × 104/ml. Eighteen-well Teflon-coated slides (Erie Scientific, Portsmouth, NH) were incubated with rhICAM-1 (5 μg/ml), rhVCAM-1 (5 μg/ml), or bovine serum albumin (1 μg/ml) and then incubated with CXCL9, -10, or -11 at 400 ng/ml for 90 minutes at 37°C. Some lymphocytes were preincubated with pertussis toxin (PTX), 100 ng/ml, to inhibit G-protein-coupled signaling. In control wells adhesion was triggered by the addition of the integrin-activator MnCl2 (100 nmol/L/ml). After nonadherent cells were removed slides were fixed and mounted before counting adherent lymphocytes in three representative high-power fields per well.
Flow-Based Adhesion Assays
To study the pattern of lymphocyte adhesion under the influence of physiological blood flow, HSECs were cultured to confluence in glass capillary tubes, stimulated for 24 hours with TNF-α and IFN-γ (both 10 ng/ml; R&D Systems) and connected to the flow-based system as described previously.29 LILs were perfused through microslides at wall shear stresses relevant to those found in the liver sinusoids (0.05 Pa). Adherent cells were visualized microscopically under phase contrast and a video link was used to record assays for off-line analysis at a later date. The number of adherent cells was converted to adherent cells per millimeter squared and corrected for the number of cells perfused. The pattern of adhesion was analyzed to determine the number of cells rolling, statically adherent, or transmigrated. Cells appearing phase bright were above the endothelial monolayer whereas those appearing phase dark had migrated through the monolayer. For studies requiring the addition of exogenous chemokine the initial HSEC culture time was extended to allow development of cell surface glycosaminoglycans. HSECs were stimulated with TNF-α alone (10 ng/ml) 24 hours before assay and exogenous chemokine added for 15 minutes immediately before assay. For functional studies HSEC monolayers were incubated with blocking antibodies raised against ICAM-1 (11C81: 10 μg/ml) and VCAM-1 (4B2: 10 μg/ml) (both R&D Systems) or lymphocytes were incubated with a blocking antibody raised against CXCR3 (1C6: 10 μg/ml; kind gift of M. Briskin, Millennium Pharmaceuticals Inc., USA) or pertussis toxin (100 ng/ml; Sigma Aldrich Ltd., Aldrich). Adhesion of CXCR3-expressing lymphocytes to VCAM-1 in the presence of CXCL9, -10, and -11 was performed by first immobilizing recombinant human VCAM-1 (5 μg/ml; R&D Systems) in microslides followed by recombinant human chemokine at a variety of concentrations. Incubations were for 90 minutes at 37°C.
Western Blotting
Fresh tissue was homogenized in loading sample buffer, normalized for total protein using Coomassie blue, and loaded on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. After electrophoresis and transfer onto Hybond membranes (Amersham-Pharmacia) blocked with 10% skimmed milk, CXCR3 ligands were detected using mouse anti-human mAbs [CXCL9: 49106, 5 μg/ml; CXCL11: 87328, 5 μg/ml (both R & D Systems), CXCL10: 6D4. 5 μg/ml (Abcam, Cambs, UK)]. Rabbit anti-mouse horseradish peroxidase-conjugated antibody was used as a secondary step and demonstrated using the ECL detection system (Amersham Pharmacia).
Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR)
CXCL9, -10, and -11 chemokine gene expressions were analyzed by RT-PCR amplification of RNA from HSECs, comparing stimulated and unstimulated cells. Total RNA was extracted from cells using RNAzol extraction (Biogenesis, Bournemouth, UK), and single-stranded cDNA synthesis was conducted as previously described.32 The PCR reaction was conducted using 1 μl of cDNA per reaction. Reaction conditions were 1× (95°C, 5 minutes), 1× (55°C, 5 minutes), 35× (94°C, 30 seconds; 55°C, 30 seconds; 72°C, 2 minutes), and 1× (72°C, 5 minutes). Primers were designed from GenBank sequences for β-actin, CXCL9, CXCL10 and CXCL11: β-actin forward, 5′-CAT CAC CAT TGG CAA TGA GC-3′; β-actin reverse, 5′-CGA TCC ACA CGG AGT ACT TG-3′; CXCL9 forward, 5′-GGC AAC CAG ACC ATT GTC TC-3′; CXCL9 reverse, 5′-TTC TGG CCA CAG ACA ACC TC-3′; CXCL10 forward, 5′-CAG AAT CGA CCA TCA AG-3′; CXCL10 reverse, 5′-GGC AGT GGA AGT CCA TGA AG-3′; CXCL11 forward, 5′-AAC AGC ACC AGC AGC AAC AG-3′; CXCL11 reverse 5′-GTG CAC TGT TGC CAG TAT CC. Product sizes for each set of primers were: β-actin, 284 bp; CXCL9, 643 bp; CXCL10, 506 bp; CXCL11, 1041 bp. Positive and negative controls were included in each assay. Amplified products were analyzed on 2% agarose gel containing ethidium bromide.
Statistical Analysis
t-Tests (paired or independent) were used to assess data normally distributed whereas nonnormally distributed data were compared using Wilcoxon signed ranks test (for related samples) or Mann-Whitney U-tests (for unrelated samples). P values ≤0.1 were indicated by (*), ≤0.01; (**), and ≤0.001 (***). Statistics were performed using the software package SPSS version 11.0 (SPSS, Chicago, IL).
Results
HSECs and BECs Increase Expression of CAMs and Secrete CXCR3 Chemokines in Response to Cytokine Stimulation in Vitro
Cultured HSECs and BECs displayed typical cellular morphology in culture and were positive for characteristic phenotypic markers. Thus BECs stained positively for the epithelial markers HEA-125 and cytokeratin 19, but were negative for CD31. HSECs stained positively for antibodies raised against CD31 and LYVE-133,34 and took up acetylated low-density lipoprotein with a cytoplasmic staining pattern35(data not shown).
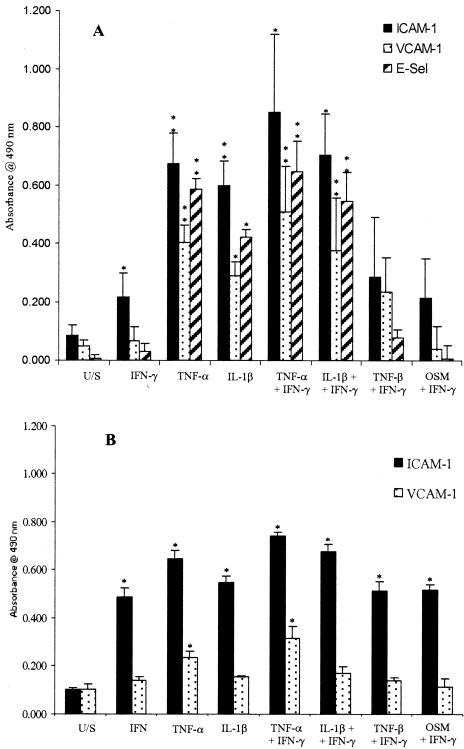
Because IFN-γ is required to induce secretion of CXCR3 ligands, all our cells were treated with 10 ng/ml of IFN-γ and the effect of additional cytokine combinations was tested. Figure 1 shows that IFN-γ alone induced increased expression of ICAM-1 on HSECs and ICAM-1 and VCAM-1 on BECs. The addition of TNF-α or IL-1β further increased expression of ICAM-1, E-selectin, and VCAM-1 on HSECs but the addition of TNF-β or oncostatin M had no additional effect. The addition of TNF-α to IFN-γ increased expression of ICAM-1 and VCAM-1 slightly on BECs whereas the addition of TNF-β or oncostatin M had no additional effect (Figure 1).
Figure 1.
Expression of adhesion molecules determined by ELISA on HSECs (A) and BECs (B) in response to stimulation with a variety of cytokines. Data represent the mean ± SEM of four replicated experiments using different cell isolates. Values represent the mean absorbance of three replicate wells minus the absorbance of an isotype-matched control antibody. All cytokine treatments were at 10 ng/ml for 24 hours before assay. U/S, unstimulated; TNF-α/β, tumor necrosis factor-α/β; IL-1β, interleukin-1β; OSM, oncostatin M.
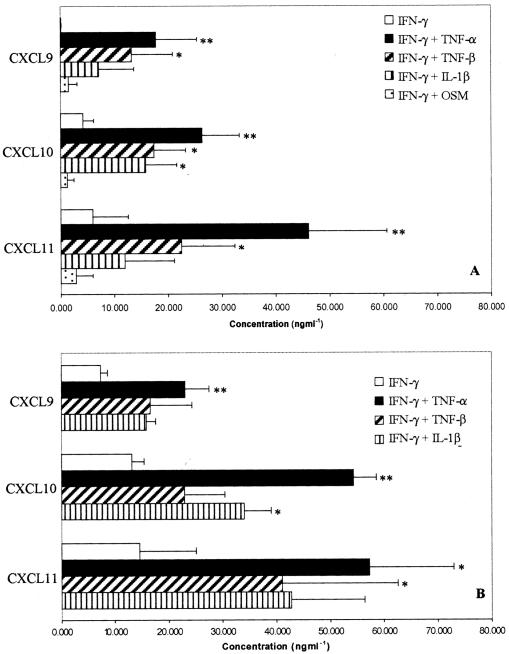
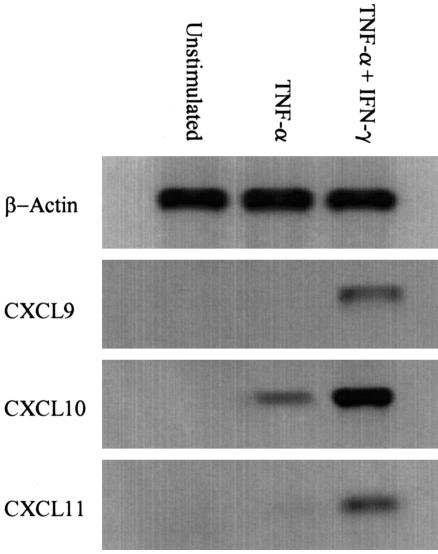
Cultured HSECs and BECs secreted CXCL9, -10, and -11 in response to IFN-γ, although the levels of CXCL9 secreted by HSECs were very low. In common with previous studies, secretion increased further if TNF-β, TNF-α, or IL-1β were added36,37 with the maximal secretion of all three chemokines seen with IFN-γ and TNF-α. The addition of oncostatin M suppresse the secretion of CXCL10 and CXCL11 in response to IFN-γ in HSECs (Figure 2). We confirmed that the combination of TNF-α and IFN-γ resulted in chemokine release into the supernatant as a result of de novo synthesis by the demonstration of mRNA by RT-PCR in stimulated cells in vitro. Whereas TNF-α alone induced only weak mRNA for CXCL10 the combination of TNF and IFN-γ induced a marked increase in transcripts for all three chemokines (Figure 3).
Figure 2.
Secretion of CXCR3 ligands by HSECs (A) and BECs (B) in response to stimulation with a variety of cytokines. Data represent the mean ± SEM of four replicate capture ELISA experiments using different cell isolates. Values represent the mean absorbance of three replicate wells minus the absorbance of a blank well. All cytokine treatments were at 10 ng/ml for 24 hours before assay. No detectable chemokine was secreted by cytokine-stimulated cells in the absence of IFN-γ treatment.
Figure 3.
De novo synthesis of CXCR3 ligands by human sinusoidal endothelial cells was confirmed by RT-PCR. In the absence of any stimulus no product was detected. Stimulation with TNF-α had little or no effect on CXCL9/11 production but did up-regulate CXCL10. Stimulation with TNF-α concomitant with IFN-γ consistently up-regulated all three CXCR3L. Representative data from one of three replicate experiments are shown.
CXCL9, CXCL10, and CXCL11 Are Expressed in Human Liver Tissue
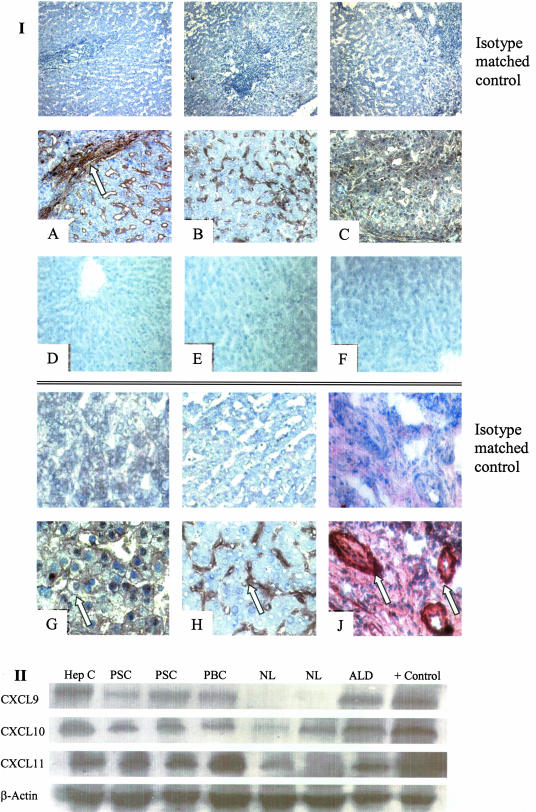
Immunohistochemical analysis of normal and diseased human liver demonstrated expression of all three CXCR3 ligands in inflammatory disease tissue. Staining was most marked on sinusoidal endothelium, particularly at areas of active inflammation and fibrosis and was also seen on hepatocyte membranes and on inflamed bile ducts and ductules (Figure 4I). There was little detectable CXCR3L on normal liver tissue and staining with isotype-matched controls was negative. Expression of CXCL9, -10, and -11 in liver tissue samples was confirmed by Western blot analysis, which demonstrated low-level expression in normal liver with a clear up-regulation in inflammatory liver disease (Figure 4II).
Figure 4.
Expression of CXCR3 ligands in human liver samples. I: Top: Low-power photomicrographs demonstrating expression (brown pigment) of CXCL9 (A), CXCL10 (B), and CXCL11 (C) in chronically inflamed human liver (primary biliary cirrhosis) tissue by immunohistochemistry. All three ligands were detected on sinusoids with particularly strong staining at areas of inflammation and active fibrosis (A, arrow). Little or no expression was detected in normal liver tissue (D–F). I: Bottom: High-power photomicrographs demonstrating CXCL11 expression on hepatocyte membranes (G) and CXCL10 expression on liver sinusoids (H). J: CXCL11 expression was also detected on bile ducts and proliferating bile ductules. Staining with an isotype-matched control antibody was negative for all samples. II: Western blot analysis of whole liver tissue lysates (B) was used to confirm expression. Marked levels of CXCL9, -10, and -11 were recorded in viral as well as chronically inflamed liver samples whereas little chemokine was detected in normal liver samples. PSC, primary sclerosing cholangitis; PBC, primary biliary cirrhosis; Hep C, chronic hepatitis C infection; ALD, alcoholic liver disease; NL, normal liver. Original magnifications: ×200 (I); ×400 (II).
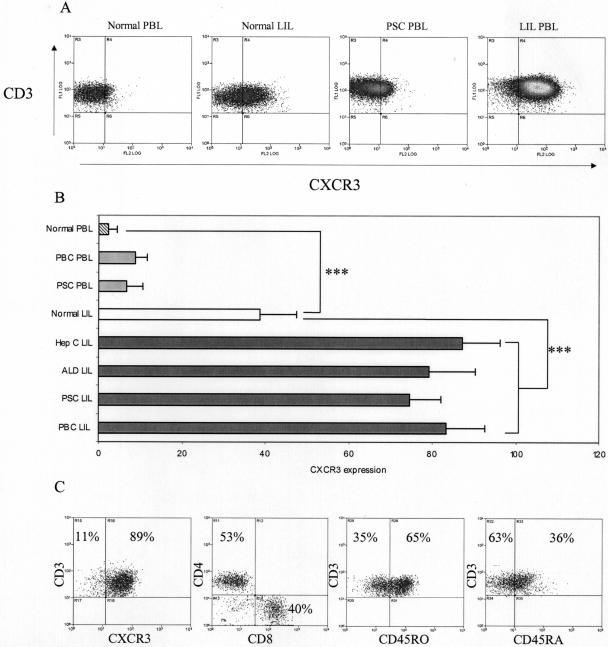
Liver-Infiltrating Lymphocytes (LILs) Show Increased Expression of CXCR3
Because all three CXCR3 ligands were expressed in inflamed human liver sections we investigated the expression of CXCR3 on lymphocytes isolated from both peripheral blood and liver tissue (Figure 5). CXCR3 was expressed at low levels on peripheral blood lymphocytes (PBLs) isolated from normal patients (2.45 ± 2% of cells stained positively) and was detected on up to 10% of PBLs in patients with inflammatory liver disease (7.74 ± 3.27%, P = 0.021, compared to normal PBL). An increased number of lymphocytes isolated from normal liver (LILs) expressed CXCR3 when compared with normal PBL (38.6 ± 8.76%, P < 0.001) but the highest numbers of CXCR3+ lymphocytes were seen in LILs isolated from inflamed liver (74.54 to 87.24 ± 9.21%, P < 0.001). Phenotypic analysis of liver-infiltrating T-lymphocytes (CD3+) revealed the ratio of CD4- to CD8-positive lymphocytes on CXCR3+ T cells was 55:45, with 65% displaying classical memory phenotype (CD45RO+). The 35% CD45RA+ cells probably represent terminally differentiated effector cells that have re-expressed CD45RA rather than true naïve cells.
Figure 5.
Little CXCR3 was detected on peripheral blood lymphocytes (PBLs) from normal patients with a small but significant increase on peripheral blood from patients with chronic inflammatory liver diseases. The number of cells expressing high levels of CXCR3 was significantly increased on LILs (P < 0.001) and further increased on LILs isolated from diseased livers (P < 0.001). A: Representative dot plots of CXCR3- and CD3-labeled LILs from normal and diseased livers. B: The percentage of cells that expressed CXCR3 on lymphocytes isolated from normal liver and chronically inflamed liver is derived from at least three experiments for each group shown as mean ± SEM. C: Phenotypic analysis of liver-infiltrating T lymphocytes (CD3+) revealed that on average 89% were CXCR3+, compared to just 4% in peripheral blood. The ratio of CD4- to CD8-positive lymphocytes on CXCR3+ T cells was 55:45, with 65% displaying classical memory phenotype (CD45RO+). The 35% CD45RA+ cells probably represent terminally differentiated effector cells that have re-expressed CD45RA rather than true naïve cells. Representative data from one of three replicate experiments. PSC, primary sclerosing cholangitis; PBC, primary biliary cirrhosis; Hep C, chronic hepatitis C infection; ALD, alcoholic liver disease.
Immobilized CXCR3 Ligands Trigger Adhesion of CXCR3high LILs to ICAM-1 or VCAM-1
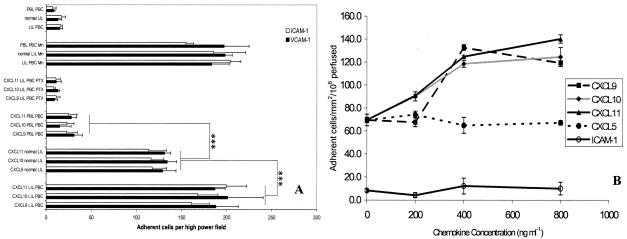
In static adhesion assays the adhesion of LILs to immobilized ICAM-1 or VCAM-1 was triggered by the addition of CXCL9, -10, and -11 (Figure 6A). Preincubation of lymphocytes with pertussis toxin, a Gi protein receptor inhibitor abrogated any effect seen after the addition of chemokine. Addition of manganese, a potent activator of integrins that acts in a receptor-independent manner, caused increased adhesion comparable to that seen after chemokine treatment.
Figure 6.
CXCR3 ligands trigger integrin-mediated adhesion of LILs to ICAM-1 and VCAM-1 under static conditions (A) and to VCAM-1 under flow (B). A: Lymphocytes isolated from chronically inflamed liver samples or matched peripheral blood samples were resuspended to a count of 8 × 104 ml and applied to individual wells of an 18-well slide, precoated with ICAM-1 (5 μg/ml). Co-incubation with CXCL9, -10, or -11 significantly increased the binding of LILs, although this effect was abrogated by the addition of pertussis toxin (PTX). Co-incubation of peripheral blood lymphocytes (PBLs) with CXCR3 ligands did not trigger adhesion. Adhesion of LILs and peripheral blood lymphocytes was increased in the presence of the integrin activator MnCl2. Data represent mean ± SEM for five replicate experiments. Statistical significance was calculated using paired t-tests, comparing lymphocytes isolated from diseased or normal livers (grouped) with those from the peripheral circulation. B: Adhesion of LILs to immobilized VCAM-1 and ICAM-1 under flow. VCAM-1 or ICAM-1 was immobilized (5 μg/ml) onto glass microslides and adhesion triggered by co-immobilization of the chemokines CXCL9, -10, and -11. CXCL5 was used to control for any nonspecific effects of chemokine co-immobilization because very few lymphocytes express its receptor CXCR2. No adhesion from flow was detected with ICAM-1, even in the presence of CXCR3 ligands. VCAM-1 was able to capture lymphocytes efficiently from flow and co-immobilization of CXCR3 ligands enhanced this effect significantly adhesion being maximal at a chemokine concentration of 400 ng/ml (P < 0.001). Data represent mean ± SEM for three replicate experiments performed at 0.05 Pa.
Immobilized CXCR3 Ligands Promote Stable Adhesion of LILs to VCAM-1 under Conditions of Flow
We investigated the role of CXCR3 under conditions of shear stress using a flow-based adhesion assay (Figure 6B). Experiments were done at 0.05 Pa to model physiological blood flow in the hepatic sinusoids. Immobilized ICAM-1 was unable to capture lymphocytes from flow whereas VCAM-1 was efficient in promoting adhesion of flowing lymphocytes. Co-immobilized purified CXCR3 ligands increased adhesion from flow to VCAM-1 in a dose-dependent manner with optimal adhesion seen at a chemokine concentration of 400 ng/ml.
CXCR3 Activation Promotes Stable Adhesion of Liver-Infiltrating Lymphocytes to Cytokine-Activated Endothelial Cells under Conditions of Flow
We have previously shown that cytokine-stimulated human sinusoidal endothelial cells support adhesion of lymphocytes under conditions of flow.28 This, together with the demonstration that human sinusoidal endothelial cells express ICAM-1, VCAM-1, and produce CXCR3L allowed us to investigate the role of CXCR3 in a more physiological setting using primary human endothelial cells. Because stimulation of HSECs with combinations of TNF-α and IFN-γ resulted in maximal secretion of CXCR3L as well as induction of ICAM-1 and VCAM-1 we used this cytokine combination to investigate the role of CXCR3 and its ligands in lymphocyte adhesion to hepatic endothelium under conditions of shear stress.
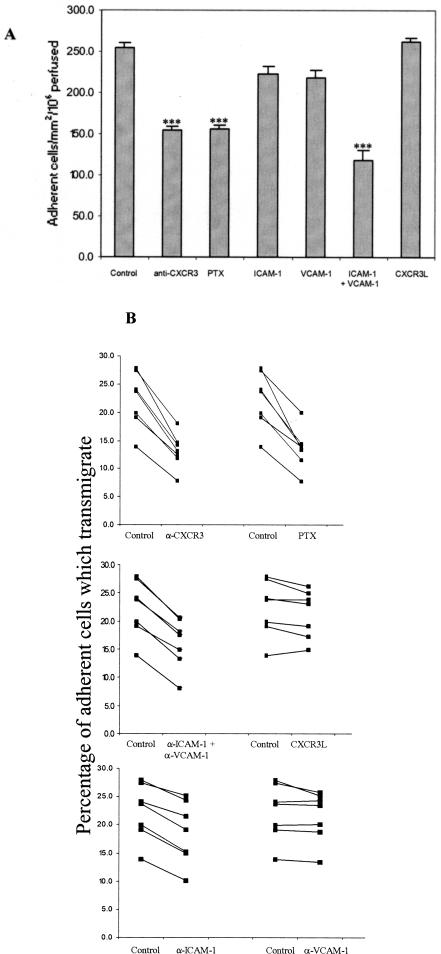
Adhesion of LILs to TNF-α- and IFN-γ-stimulated HSECs was consistently reduced by ∼40% by pretreating lymphocytes with anti-CXCR3 mAb (P < 0.001) (Figure 7A). A comparable reduction in adhesion was demonstrated when LILs were pretreated with pertussis toxin. Moreover, adhesion was further reduced by the addition of blocking antibodies to ICAM-1/VCAM-1. These data suggest that most if not all of the chemokine-mediated effect was due to CXCR3 but that some integrin-binding was chemokine-independent consistent with the activated state of the LILs. To make sure that our endothelial cells were synthesizing and presenting optimal concentrations of CXCR3 ligands we added recombinant CXCR3L to the endothelial cells before performing the adhesion assay (Figure 7A). This did not increase total adhesion further suggesting that we had achieved optimal presentation of endogenous chemokines to induce adhesion.
Figure 7.
CXCR3 ligands promote lymphocyte adhesion from flow and subsequent transmigration on TNF-α/IFN-γ-stimulated human HSECs. A: Inhibition of CXCR3 with a function blocking antibody or a Gi protein block (PTX) significantly reduces total adhesion of LILs from flow to TNF-α/IFN-γ-stimulated HSECs. Adhesion was further reduced by the addition of blocking antibodies against ICAM-1/VCAM-1. Data represent number of adherent cells per mm2 per million perfused and are the mean ± SEM of seven experiments. B: Transmigration of adherent lymphocytes through TNF-α/IFN-γ-stimulated HSECs was significantly reduced after CXCR3 and ICAM-1 blockade whereas anti-VCAM-1 mAb had no affect either when used alone or when used in combination. Migrating cells were calculated using frame-by-frame analysis of experimental videos to count the percentage of phase dark (transmigrated) lymphocytes. Data represent paired samples from individual experiments. All experiments were performed at 0.05 Pa.
CXCR3 Mediates Transmigration of Liver-Infiltrating Lymphocytes under Conditions of Flow
We then studied the proportion of adherent cell that subsequently underwent transendothelial migration under conditions of flow in our adhesion experiments. On average 22% (range, 13.9 to 27.9) of adherent LILs transmigrated through HSEC monolayers within 5 minutes of initial capture under flow. This was significantly reduced after the addition of an anti-CXCR3 mAb or incubation of the lymphocytes with pertussis toxin (P < 0.001 for both). As previously reported the addition of anti-ICAM-1 but not anti-VCAM-1 mAb also reduced transmigration (P < 0.001) although the effect was less than that seen with CXCR3 blockade. Addition of an isotype-matched control antibody did not have any effect on transmigration and the number of adherent liver LILs transmigrating could not be increased by the addition of exogenous recombinant CXCR3L (Figure 7B).
Chemokine Secreted by BECs Induces Transmigration of LILs across TNF-α-Stimulated HSECs
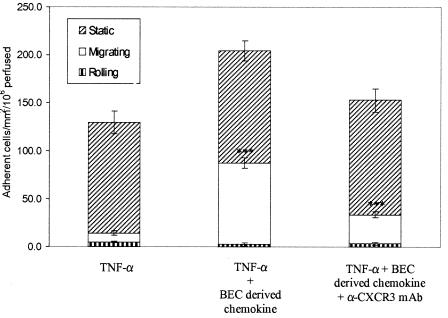
Because CXCR3 ligands are secreted by other nonendothelial cell types within the liver including cholangiocytes we next tested whether exogenous chemokines secreted by other liver cell types could be presented on liver endothelium and trigger adhesion and transmigration. Because on its own TNF-α stimulation of HSECs induces good levels of ICAM-1 and VCAM-1 but does not induce secretion of CXCR3 ligands this provided us with a model in which we could test the effects of exogenous CXCR3 ligands. The incubation of HSECs for 10 minutes with supernatant taken from BECs previously stimulated with IFN-γ and TNF-α to stimulate optimal CXCR3 ligand secretion significantly increased the total levels of lymphocyte adhesion from 129.5 to 204.1 cells/mm2/106 perfused. An average of 7.3% of adherent LILs transmigrated across TNF-α-stimulated HSECs within 5 minutes under flow and after incubation with BEC culture supernatants this increased significantly to 41.4% (P = 0.001). We demonstrated that this increased migration was due to activation of CXCR3 by studies in which preincubation of LILs with CXCR3-blocking mAb significantly reduced the level of transmigration (P = 0.002) seen after treatment of HSECs with BEC supernatant (Figure 8).
Figure 8.
Incubation of TNF-α-stimulated HSECs with BEC-derived CXCR3 ligands increased total adhesion and transendothelial migration of liver-derived lymphocytes under conditions of flow. Pretreatment of HSEC monolayers with BEC supernatant containing CXCL9, -10, and -11 increased the number of adherent lymphocytes. No variation in the number of static nonmigrating lymphocytes was recorded but the number of lymphocytes transmigrating was significantly increased (P = 0.001). Pretreatment of lymphocytes with a CXCR3 blocking antibody significantly reduced the number of lymphocytes migrating across the HSECs. Data represent the mean of three replicate experiments ± SEM. All experiments were performed at 0.05 Pa.
Discussion
Tissue-infiltrating lymphocytes express high levels of CXCR3, which is believed to enable them to respond to CXCR3 ligands expressed at sites of inflammation including interface hepatitis in chronic liver disease. However, the ability of CXCR3 ligands to promote the transendothelial migration of effector lymphocytes under flow has not been reported. Here, we report that CXCR3 activation by endogenously presented CXCR3 ligands promotes adhesion and transmigration of in vivo-activated T cells across human liver endothelium under flow. These studies suggest that CXCR3 is the dominant chemokine receptor mediating the recruitment of tissue-infiltrating lymphocytes in chronic inflammatory liver disease. We also show that liver endothelium can present functional CXCR3 ligands secreted by other liver cell types. Thus, paracrine secretion and posting of CXCR3 ligands on hepatic endothelium contributes to the complement of functional chemokines presented at the endothelial surface at sites of inflammation.
We previously demonstrated increased expression of CXCL9, -10, and -11 associated with tissue infiltration by CXCR3high T cells in chronic hepatitis C infection and allograft rejection.22,23 Furthermore, others have demonstrated that increased expression of CXCL10 is associated with accelerated progression of inflammatory liver disease suggesting that CXCR3 is an important receptor in regulating chronic liver inflammation.38,39 Studies in diverse tissues including the brain, liver, and lung suggest that CXCR3 does not provide a tissue-specific signal for lymphocyte recruitment but is associated with infiltration of inflamed tissues regardless of the anatomical site.36,40,41 Although these studies have shown expression of CXCR3 ligands associated with CXCR3+ lymphocytes in inflammatory disease, functional studies have been limited to demonstrating that lymphocytes isolated from diseased tissues undergo chemotaxis to CXCR3 ligands.21
In the present study we found increased expression of all three CXCR3 ligands in several different chronic inflammatory liver diseases whereas the levels in normal liver were minimal. CXCR3 ligands were detected on inflamed hepatic endothelium at sites of lymphocyte infiltration and also on inflamed bile ducts and proliferating bile ductules. These observations are consistent with studies in chronic hepatitis C showing increased expression of CXCR3 ligands and CXCL10 mRNA on hepatocytes at sites of interface hepatitis26,39,42,43 but suggest that CXCR3 ligands are induced at sites of lymphocyte infiltration in chronic hepatitis regardless of the underlying cause. We compared levels of the CXCR3 receptor on LILs isolated from normal liver and a variety of chronic inflammatory liver diseases. CXCR3 was detected on 20 to 35% of T cells infiltrating normal liver compared with >80% of T cells isolated from chronic inflamed livers. These observations suggest a role for CXCR3 and its ligands in liver inflammation but do not, on their own, provide evidence that this receptor can promote lymphocyte recruitment.
We investigated the function of CXCR3 on tissue-infiltrating lymphocytes using a flow-based adhesion assay in which lymphocyte responses to CXCR3 ligands were studied under physiological levels of wall shear stress on either immobilized purified ligands or primary human hepatic endothelium.28 To develop a relevant cell culture model we first demonstrated that cultured human intrahepatic endothelial cells express ICAM-1 and VCAM-1 and secrete CXCL9, -10, and -11 in response to IFN-γ and TNF-α thereby resembling the phenotype of inflamed hepatic endothelium in patients with chronic hepatitis. The fact that stimulation with TNF-α alone increased expression of ICAM-1 and VCAM-1 but did not stimulate CXCR3 ligand expression meant that we could compare responses to activated endothelium in the presence and absence of endogenously secreted CXCR3 ligands.
CXCR3 activation by its ligands has been shown to induce shear-resistant adhesion of in vitro-generated lymphoblasts to umbilical vein endothelium.15,44 However, in vitro-generated lymphoblasts differ from lymphocytes that infiltrate tissues in vivo. In particular the high levels of integrin activation on in vitro-activated lymphoblasts mean that they rapidly arrest on endothelium under flow but then remain firmly adherent with relatively few cells undergoing subsequent transendothelial migration (S.M. Curbishley, P. Lalor, and D.H. Adams, unpublished). These observations convinced us of the importance of studying cells that have been activated to infiltrate tissues in vivo. We initially tested the response of LILs to CXCR3 ligands co-immobilized with the endothelial adhesion molecule VCAM-1. Because VCAM-1 has been reported to bind and present chemokines we did not use chemokines as soluble factors but rather as co-immobilized ligands with VCAM-1.45 All three CXCR3 ligands activated concentration-dependent adhesion of lymphocytes to VCAM-1 from flow providing further evidence that these chemokines can act as adhesion triggers when immobilized. Previous studies have suggested that CXCL11 is a more potent activator of CXCR3 than the other CXCR3 ligands when used as soluble factors15 but our studies suggest that when immobilized they have similar potencies. These experiments demonstrated that LILs respond to CXCR3 activation under flow and we then went on to show that lymphocytes isolated from inflamed human tissue show CXCR3-dependent adhesion and transmigration on activated endothelium under flow.
Under low-flow conditions, comparable with those present in sinusoids in vivo, lymphocytes isolated from human livers interacted with cytokine-activated HSECs. Although lymphocytes isolated from normal human livers did bind, the level of adhesion was significantly higher when lymphocytes isolated from chronically inflamed human liver were used, consistent with the high levels of CXCR3 detected on these cells. The lymphocytes exhibited only brief attenuated rolling on the endothelial monolayer and the majority arrested rapidly from flow. This may reflect the lower levels of classical rolling receptors such as selectins present on sinusoidal endothelium and the reduced requirement for initial capture and rolling interactions within the low-shear environment of the sinusoids. In addition we used in vivo-activated lymphocytes, which are l-selectin-negative and LFA-1high when compared with blood lymphocytes.46 Inhibition of CXCR3 reduced adhesion to TNF-α/IFN-γ-stimulated HSECs under flow in all conditions by ∼50%. The level of inhibition was similar to that seen using the global Gpi inhibitor pertussis toxin suggesting that most of the chemokine-mediated effect in this system was through CXCR3. This is despite the fact that cultured sinusoidal endothelial cells can secrete other chemokines for which liver lymphocytes express receptors, including CCL5 and CCR5.22
Inhibition of CXCR3 also significantly reduced the number of adherent lymphocytes that underwent trans-endothelial migration. Blockade of ICAM-1/VCAM-1 had a similar effect but an isotype-matched control antibody had no effect. For these analyses we measured the number of transmigrating lymphocytes as a percentage of the total number of lymphocytes adherent to the endothelial monolayer allowing us to assess transmigration in isolation, independent of the initial adhesion steps. The molecular basis of lymphocyte trans-endothelial migration is still unclear. Some studies, including our own using liver endothelial cells, suggest that β2 integrins and their ligands are important28,47,48 whereas others suggest that these receptor ligand pairings are primarily involved in the firm adhesion step and do not play a critical role in transmigration.49 The posting of chemokines on the apical surface of vessels enables rapid activation of adherent lymphocytes50 in the presence of shear stress in vitro.51,52 We now demonstrate that CXCR3 and its ligands work together with ICAM-1 to effect the transmigration of adherent lymphocytes across activated endothelium independently of their effects on initial adhesion.
The chemokines presented by inflamed endothelium may be secreted by the endothelium itself or by other cells in the local microenvironment and then posted on the endothelial cell surface where they play a role in the recruitment of lymphocytes.11,53 This may be particularly true of CXCL9 because mRNA for this chemokine has been detected in hepatocytes at areas of interface hepatitis.24,27 To demonstrate the ability of liver endothelium to present chemokines from a paracrine source we stimulated HSECs with TNF-α alone, resulting in an activated endothelial phenotype in the absence of CXCR3 ligands and then incubated these HSECs with supernatants from human BECs (cholangiocytes) that had been stimulated with cytokines to secrete all three CXCR3 ligands. The supernatants were incubated with the HSECs for 10 minutes before being used in the flow assay. This treatment resulted in an increase in lymphocyte adhesion and transmigration that could be inhibited by the addition of a CXCR3 antibody confirming that human liver endothelial cells can present CXCR3 ligands secreted by other cell types within the liver. We were unable to completely inhibit the effect of the exogenous chemokines with anti-CXCR3 despite using supersaturating concentrations of the antibody suggesting that other chemokines in the cell culture supernatant may also contribute to the effect. Cholangiocytes secrete a broad range of chemokines in response to cytokine stimulation54 including CCL5 and CCL2 and it is possible that these or other chemokines may also contribute to the effect. CXCR3 ligands are secreted by several cells types in the liver in addition to HSECs and cholangiocytes including hepatocytes and stellate cells.26,55 Thus such posting of chemokines allows the endothelium to present chemokines that reflect the inflammatory status throughout the local liver microenvironment as has been demonstrated for lymph node chemokine presentation.7,8 This may be an important amplification step for increasing cell recruitment at inflammatory sites.
In summary, we have shown that CXCR3 expression is increased on lymphocytes isolated from chronically inflamed human liver and that activation of this receptor by chemokines on liver endothelial cells can promote lymphocyte adhesion and transendothelial migration across human hepatic endothelium under flow. We propose that CXCR3 is a critical factor in the recruitment of effector lymphocytes to sites of inflammation including the hepatic lobule.
Footnotes
Address reprint requests to Dr. Stuart M. Curbishley or Prof. D. H. Adams, Liver Research Group, Institute of Biomedical Research, The University of Birmingham Medical School, Edgbaston, Birmingham, UK B15 2TT. E-mail: s.m.curbishley@bham.ac.uk or d.h.adams@bham.ac.uk.
Supported by an educational grant from Pfizer Inc.
References
- Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Pachynski RK, Wu SW, Gunn MD, Erle DJ. Secondary lymphoid-tissue chemokine (SLC) stimulates integrin alpha 4 beta 7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J Immunol. 1998;161:952–956. [PubMed] [Google Scholar]
- Foxman EF, Kunkel EJ, Butcher EC. Integrating conflicting chemotactic signals. The role of memory in leukocyte navigation. J Cell Biol. 1999;147:577–588. doi: 10.1083/jcb.147.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Adams DH, Shaw S. Proteoglycan on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol Today. 1993;14:111–114. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- Gretz JE, Kaldjian EP, Anderson AO, Shaw S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J Immunol. 1996;157:495–499. [PubMed] [Google Scholar]
- Ebnet K, Kaldjian EP, Anderson AO, Shaw S. Orchestrated information-transfer underlying leukocyte-endothelial interactions. Annu Rev Immunol. 1996;14:155–177. doi: 10.1146/annurev.immunol.14.1.155. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1β. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Meyer M, Hensbergen PJ, van der Raaij-Helmer EM, Brandacher G, Margreiter R, Heufler C, Koch F, Narumi S, Werner ER, Colvin R, Luster AD, Tensen CP, Werner-Felmayer G. Cross reactivity of three T cell attracting murine chemokines stimulating the CXC chemokine receptor CXCR3 and their induction in cultured cells and during allograft rejection. Eur J Immunol. 2001;31:2521–2527. doi: 10.1002/1521-4141(200108)31:8<2521::aid-immu2521>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Longo DL, Murphy WJ. Human interferon-inducible protein-10 induces mononuclear cell infiltration in mice and promotes the migration of human T-lymphocytes into the peripheral-tissues of human peripheral-blood lymphocytes-SCID mice. Blood. 1996;87:1423–1431. [PubMed] [Google Scholar]
- Farber JM. HuMig: a new human member of the chemokine family of cytokines. Biochem Biophys Res Commun. 1993;192:223–230. doi: 10.1006/bbrc.1993.1403. [DOI] [PubMed] [Google Scholar]
- Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine re-ceptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan K, Ding Z, Hanly J, Issekutz TB. IFN-gamma-inducible T cell alpha chemoattractant is a potent stimulator of normal human blood T lymphocyte transendothelial migration: differential regulation by IFN-gamma and TNF-alpha. J Immunol. 2002;168:6420–6428. doi: 10.4049/jimmunol.168.12.6420. [DOI] [PubMed] [Google Scholar]
- Yoong KF, Afford SC, Jones R, Aujla P, Qin S, Price K, Hubscher SG, Adams DH. Expression and function of CXC and CC chemokines in human malignant liver tumors: a role for human monokine induced by gamma-interferon in lymphocyte recruitment to hepatocellular carcinoma. Hepatology. 1999;30:100–111. doi: 10.1002/hep.510300147. [DOI] [PubMed] [Google Scholar]
- Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–6243. [PubMed] [Google Scholar]
- Goddard S, Williams A, Morland C, Qin S, Gladue R, Hubscher SG, Adams DH. Differential expression of chemokines and chemokine receptors shapes the inflammatory response in rejecting human liver transplants. Transplantation. 2001;72:1957–1967. doi: 10.1097/00007890-200112270-00016. [DOI] [PubMed] [Google Scholar]
- Arai K, Liu ZX, Lane T, Dennert G. IP-10 and Mig facilitate accumulation of T cells in the virus-infected liver. Cell Immunol. 2002;219:48–56. doi: 10.1016/s0008-8749(02)00584-1. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Morita A, Nishioji K, Narumi S, Toyama T, Daimon Y, Nakamura H, Kirishima T, Okanoue T. Clinical significance of elevated serum interferon-inducible protein-10 levels in hepatitis C virus carriers with persistently normal serum transaminase levels. J Viral Hepatol. 2001;8:341–348. doi: 10.1046/j.1365-2893.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- Harvey CE, Post JJ, Palladinetti P, Freeman AJ, French RA, Kumar RK, Marinos G, Lloyd AR. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003;74:360–369. doi: 10.1189/jlb.0303093. [DOI] [PubMed] [Google Scholar]
- Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, Itoh Y, Okanoue T. Expression of IFN-inducible protein-10 in chronic hepatitis. J Immunol. 1997;158:5536–5544. [PubMed] [Google Scholar]
- Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002;169:983–992. doi: 10.4049/jimmunol.169.2.983. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Clements JM, Pigott R, Humphries MJ, Spragg JH, Nash GB. Association between receptor density, cellular activation, and transformation of adhesive behavior of flowing lymphocytes binding to VCAM-1. Eur J Immunol. 1997;27:1422–1426. doi: 10.1002/eji.1830270619. [DOI] [PubMed] [Google Scholar]
- Joplin R. Isolation and culture of biliary epithelial cells. Gut. 1994;35:875–878. doi: 10.1136/gut.35.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick MD, Kunkel SL, Lincoln PM, Wilke CA, Strieter RM. Specific ELISAs for the detection of human macrophage inflammatory protein-1 alpha and beta. Immunol Invest. 1993;22:441–449. doi: 10.3109/08820139309063422. [DOI] [PubMed] [Google Scholar]
- Chomsczynzki P, Sacchi N. Single-step method of RNA isolation by acid guadinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Grant AJ, Goddard S, Ahmed-Choudhury J, Reynolds G, Jackson DG, Briskin M, Wu L, Hubscher SG, Adams DH. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160:1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DG, Prevo R, Clasper S, Banerji S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–321. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Boyles J, Mahley RW, Bissell DM. Uptake of chemically modified low-density lipoproteins in vivo is mediated by specific endothelial cells. J Cell Biol. 1985;100:103–117. doi: 10.1083/jcb.100.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H, Murphy M, Liao F, Farber J, Cassatella MA. Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol. 1999;162:4928–4937. [PubMed] [Google Scholar]
- Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol. 2003;38:67–75. doi: 10.1016/s0168-8278(02)00328-8. [DOI] [PubMed] [Google Scholar]
- Leroy V, Vigan I, Mosnier JF, Dufeu-Duchesne T, Pernollet M, Zarski JP, Marche PN, Jouvin-Marche E. Phenotypic and functional characterization of intrahepatic T lymphocytes during chronic hepatitis C. Hepatology. 2003;38:829–841. doi: 10.1053/jhep.2003.50410. [DOI] [PubMed] [Google Scholar]
- Hu H, Aizenstein BD, Puchalski A, Burmania JA, Hamawy MM, Knechtle SJ. Elevation of CXCR3-binding chemokines in urine indicates acute renal-allograft dysfunction. Am J Transplant. 2004;4:432–437. doi: 10.1111/j.1600-6143.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- Segerer S, Banas B, Wornle M, Schmid H, Cohen CD, Kretzler M, Mack M, Kiss E, Nelson PJ, Schlondorff D, Grone HJ. CXCR3 is involved in tubulointerstitial injury in human glomerulonephritis. Am J Pathol. 2004;164:635–649. doi: 10.1016/S0002-9440(10)63152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolinario A, Majano PL, Alvarez-Perez E, Saez A, Lozano C, Vargas J, Garcia-Monzon C. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002;97:2861–2870. doi: 10.1111/j.1572-0241.2002.07054.x. [DOI] [PubMed] [Google Scholar]
- Helbig KJ, Ruszkiewicz A, Semendric L, Harley HA, McColl SR, Beard MR. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology. 2004;39:1220–1229. doi: 10.1002/hep.20167. [DOI] [PubMed] [Google Scholar]
- Piali L, Weber C, Larosa G, Mackay CR, Springer TA, Clark-Lewis I, Moser B. The chemokine receptor CXCR3 mediates rapid and shear-resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur J Immunol. 1998;28:961–972. doi: 10.1002/(SICI)1521-4141(199803)28:03<961::AID-IMMU961>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio D, Albanesi C, Lang R, Girolomoni G, Sinigaglia F, Laudanna C. Quantitative differences in chemokine receptor engagement generate diversity in integrin-dependent lymphocyte adhesion. J Immunol. 2002;169:2303–2312. doi: 10.4049/jimmunol.169.5.2303. [DOI] [PubMed] [Google Scholar]
- Faint JM, Annels NE, Curnow SJ, Shields P, Pilling D, Hislop AD, Wu L, Akbar AN, Buckley CD, Moss PA, Adams DH, Rickinson AB, Salmon M. Memory T cells constitute a subset of the human CD8+CD45RA+ pool with distinct phenotypic and migratory characteristics. J Immunol. 2001;167:212–220. doi: 10.4049/jimmunol.167.1.212. [DOI] [PubMed] [Google Scholar]
- Faveeuw C, Di Mauro ME, Price AA, Ager A. Roles of alpha(4) integrins/VCAM-1 and LFA-1/ICAM-1 in the binding and transendothelial migration of T lymphocytes and T lymphoblasts across high endothelial venules. Int Immunol. 2000;12:241–251. doi: 10.1093/intimm/12.3.241. [DOI] [PubMed] [Google Scholar]
- Andrew DP, Spellberg JP, Takimoto H, Schmits R, Mak TW, Zukowski MM. Transendothelial migration and trafficking of leukocytes in LFA-1-deficient mice. Eur J Immunol. 1998;28:1959–1969. doi: 10.1002/(SICI)1521-4141(199806)28:06<1959::AID-IMMU1959>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Masuyama J, Yoshio T, Suzuki K, Kitagawa S, Iwamoto M, Kamimura T, Hirata D, Takeda A, Kano S, Minota S. Characterization of the 4C8 antigen involved in transendothelial migration of CD26(hi) T cells after tight adhesion to human umbilical vein endothelial cell monolayers. J Exp Med. 1999;189:979–990. doi: 10.1084/jem.189.6.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- Cinamon G, Grabovsky V, Winter E, Franitza S, Feigelson S, Shamri R, Dwir O, Alon R. Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow. J Leukoc Biol. 2001;69:860–866. [PubMed] [Google Scholar]
- Cinamon G, Shinder V, Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat Immunol. 2001;2:515–522. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- Morland CM, Fear J, Joplin R, Adams DH. Inflammatory cytokines stimulate human biliary epithelial cells to express interleukin-8 and monocyte chemotactic protein-1. Biochem Soc Trans. 1997;25:232S. doi: 10.1042/bst025232s. [DOI] [PubMed] [Google Scholar]
- Marra F. Hepatic stellate cells and the regulation of liver inflammation. J Hepatol. 1999;31:1120–1130. doi: 10.1016/s0168-8278(99)80327-4. [DOI] [PubMed] [Google Scholar]