Abstract
The presence of mucus obstruction and neutrophil-predominant inflammation in several lung disorders, such as cystic fibrosis, suggests a relationship between neutrophils and excess mucus production. Mechanisms of human neutrophil elastase (HNE)-induced mucin secretion by well-differentiated normal human bronchial epithelial (NHBE) cells maintained in air/liquid interface culture were investigated. HNE increased mucin secretion in a concentration-dependent manner, with maximal stimulation (more than twofold) occurring within a short (15 minutes) time period. Mucins MUC5AC and MUC5B, but not MUC2, were released in response to HNE. Stimulation of mucin secretion required partial elastase enzymatic activity and did not appear to involve a soluble product released by the cells. HNE-stimulated secretion involved activation of protein kinase C (PKC), as HNE exposure rapidly provoked PKC enzymatic activity that was attenuated by the general PKC inhibitors calphostin C and bisindoylmaleimide I. Of the different isoforms, PKCα, δ, ζ, λ, ι, and ε were constitutively expressed in NHBE cells while PKCβ, η, and μ were PMA-inducible. PKCδ was the only isoform to translocate from cytoplasm to membrane in response to HNE. Inhibition of PKCδ attenuated HNE-mediated mucin secretion. The results suggest HNE stimulation of mucin release by human airway epithelial cells involves intracellular activation of PKC, specifically the δ isoform.
Neutrophils are involved in a variety of inflammatory lung disorders including chronic bronchitis, bronchiectasis, cystic fibrosis, and probably asthma. In these diseases, the pathological findings of mucus obstruction and neutrophil-predominant inflammation in airways1–6 suggest a relationship between neutrophil recruitment/infiltration and excess mucus production and secretion. Neutrophils store three proteases that have been implicated in airway mucin secretion: elastase,7–9 cathepsin G,10 and proteinase-3.11,12 Of these, human neutrophil elastase (HNE), a major component of primary or azurophilic granules,13 is the most widely studied with regard to enhanced mucus secretion. Levels of HNE are elevated in airways of patients with chronic bronchitis and cystic fibrosis,14 and levels in patients’ sputum may exceed 100 μg/ml (3.3 × 10−6 mol/L).15–17 Purified HNE has been shown to provoke secretion of mucin by isolated airway epithelial cells and glands from several species.7,8,10,18 Although there have been suggestions that interactions between HNE and epithelial cell surfaces may be involved in the response,9,19 intracellular mechanisms and signaling pathways associated with HNE-induced mucin hypersecretion have not been elucidated.
In this study, well-differentiated primary normal human tracheobronchial epithelial (NHBE) cells maintained in vitro in air/liquid interface were exposed to HNE, and the secretory response assessed. Elastase proved to be a potent mucin secretagogue for NHBE cells, eliciting a robust (greater than twofold) increase in mucin secretion within 15 minutes. The mucin gene products released included those of MUC5AC and MUC5B, but not of MUC2. The mechanism appeared to involve activation of protein kinase C (PKC), as HNE exposure rapidly provoked phosphorylation of MARCKS (myristoylated alanine-rich C kinase substrate) protein, a cellular substrate of PKC, and the mucin secretory response to HNE was attenuated by two different PKC inhibitors. Additional studies provided compelling evidence that PKCδ is the specific PKC isoform involved in the secretory pathway.
Materials and Methods
Materials
All chemicals were of analytical grade or higher. NHBE cells, bronchial epithelial basal medium, and supplements for air/liquid interface cell cultures were purchased from Cambrex (San Diego, CA). Endotoxin-free HNE purified from human sputum was purchased from Elastin Products Company (EPC, Owensville, MO). Cytotoxicity was evaluated with CytoTox 96 nonradioactive cytotoxicity assay kits obtained from Promega Corp. (Madison, WI). A specific HNE substrate, MeO-SUC-AL-AL-PRO-VAL-PNA, and an HNE inhibitor, chloromethyl ketone-modified tetrapeptide (CMK), also were purchased from EPC and the HNE inhibitor elastatinal was obtained from Calbiochem (La Jolla, CA). 17Q2 pan mucin antibody was purchased from Babco (Richmond, CA) and anti-MUC5AC (45M1) was purchased from Neomarkers (Fremont, CA). A monoclonal antibody (11C1) against human MUC5B was generously provided by Dr. Reen Wu, University of California at Davis, Davis, CA. The epitope for this antibody, which was generated from the secreted mucin of well-differentiated airway epithelial cells, is not known, but by immunohistochemical staining and Western blot analysis, it appears to recognize the MUC5B peptide. A monoclonal antibody that cross reacts with human MUC2, raised against the guinea pig 522-bp gene sequence analogous to the human D4 domain located in the carboxy-terminal region of the Muc2 gene sequence established previously in our laboratory, was used to detect MUC2 mucins.20 An ImmunoPure (G) IgG purification kit used for purification of antibodies for enzyme-linked immunosorbent assay (ELISA) was from Pierce (Rockford, IL). For Western blot analysis of PKC isoforms expressed in NHBE cells, a PKC sampler kit and E-cadherin antibody were obtained from BD Biosciences (San Jose, CA). Goat anti-PKCζ and mouse anti-α-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phosphorylated (ser) PKC substrate and phosphorylated MARCKS were from Cell Signaling Technology (Beverly, MA). Horseradish peroxidase-conjugated goat anti-mouse IgG and donkey anti-goat IgG also were purchased from Santa Cruz Biotechnology. Horseradish peroxidase-conjugated goat anti-rabbit IgG was purchased from Upstate Biotechnology (Lake Placid, NY). Enhanced chemiluminescence development kits and Hyperfilm were from Amersham Pharmacia Biotech (Piscataway, NJ). All PKC-related inhibitors (ie, calphostin C, bisindoylmaleimide, PKC epsilon and zeta inhibitor peptides, rottlerin) were purchased from Calbiochem. A PepTag assay for nonradioactive detection of PKC activity was purchased from Promega. Other chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO). Transwell-Clear culture inserts and high-binding 96-well assay plates were purchased from Corning Inc. (Corning, NY).
Epithelial Cell Culture
Primary cultures of NHBE cells were established using an air/liquid interface cell culture system described previously.21 Briefly, NHBE cells were expanded once and cells collected and frozen in liquid nitrogen (referred to as passage-2 cells). Air/liquid interface cultures of NHBE cells were established on Transwell-Clear culture inserts thin-coated with rat-tail type I collagen. The basic medium used for NHBE cells was a 1:1 mixture of bronchial epithelial basal medium and high glucose (4.5 g/L) Dulbecco’s modified Eagle’s medium. The complete medium was composed of basic medium containing a final concentration of 0.5 ng/ml human recombinant epidermal growth factor, 0.5 μg/ml hydrocortisone, 5 μg/ml insulin, 10 μg/ml transferrin, 0.5 μg/ml epinephrine, 6.5 ng/ml triiodothyronine, 50 μg/ml gentamicin, and 50 ng/ml amphotericin-B. In addition, the media contained 0.13 mg/ml bovine pituitary extract made according to the protocol of Bertolero and colleagues,22 5 × 10−8 mol/L all-trans retinoic acid, 1.5 μg/ml bovine serum albumin, and 20 U/ml nystatin.
Frozen NHBE cells were recovered and seeded at a density of ∼2 × 104 cells/cm2 onto the apical surface of the inserts. Media were changed the next day, then every other day until the cells reached ∼90% confluence. At this point, the air/liquid interface was established by removing the apical media, whereas basolateral media were changed daily for up to 21 days. A mucin phenotype was observed at ∼14 days in culture (∼7 days in air-liquid interface culture) and cilia were apparent by 18 days in culture. Mucin secretion reached maximal levels at ∼18 days in culture, so cells cultured for ∼18 to 21 days were used for the experiments described below.
Exposure of Cells to HNE
HNE stock was made as 10 mg/ml (339 μmol/L) in a 1:1 mixture of glycerol and 0.02 mol/L NaOAc, pH 5.0. The stock was diluted into the culture medium to the final concentration indicated. In all studies, the above solvent appropriately diluted was used as a negative control.
Quantification of Mucin Secretion
NHBE cells were exposed to HNE from both apical and basolateral sides for 15 minutes (unless otherwise indicated). At the end of each treatment, apical medium containing the secreted mucin was collected and quantified. Briefly, 0.25 ml of media containing secreted mucin was collected, 0.5 ml of 1 mmol/L dithiothreitol in phosphate-buffered saline (PBS) was added into each well, and the plates were gently agitated and allowed to stand for 3 minutes before the dithiothreitol/PBS plus mucin was collected in the same tube. Finally, 0.5 ml of 10 μmol/L CMK in PBS was added and collected the same way. Approximately 1.25 ml of the collected mucin mixture with dithiothreitol and CMK was centrifuged at 8000 rpm for 5 minutes to remove cell debris, and then collected in a fresh tube. Phenylmethyl sulfonyl fluoride was added to a final concentration of 1 mmol/L.
Baseline and treatment mucin secretions were collected from each culture plate. Baseline mucin secretion was collected to normalize variations from well to well, and to control for possible release of mucin in response to the stress of media change or washing. After the baseline mucin secretion sample was collected, the cells were rested overnight and exposed to test agents the next day for indicated periods of time. Mucin samples were quantified using specific ELISA methods. Firstly, total mucin was quantified by a double-sandwich ELISA using a pan-mucin antibody, 17Q2, that cross reacts with a carbohydrate epitope on human mucins, as described previously.21 Additional studies were performed using ELISAs for secreted protein products of the mucin genes MUC5AC, MUC5B, and MUC2 to determine which mucin gene products were being released on exposure to HNE. MUC5AC was measured via ELISA as described by Takeyama and colleagues23 using the 45M1 antibody. MUC5B protein was assayed via a standard double-sandwich ELISA method using the 11C1 monoclonal antibody against MUC5B provided by Dr. Reen Wu, University of California, Davis, Davis, CA, as described previously.24,25 The MUC2 gene product was quantified by modification of an ELISA as described previously.20
Assay of HNE Activity
HNE activity assays were performed following the manufacturer’s protocol (EPC). HNE substrate was prepared in substrate buffer (Tris-NaCl buffer: 0.1 mol/L Tris, pH 7.5, containing 0.5 mol/L NaCl and 0.01% Na3N). Briefly, 3 ml of substrate solution at 25°C was added to test tubes, 1.0 μg of HNE then was added, and the developed color was read immediately and continuously thereafter at 1 minute intervals. Elastase activity was reflected by the rate increase in absorbance in time units (minutes). Color development was read at 410 nm on a spectrophotometer UV160U (Shimadzu, Kyoto, Japan). The specific activity of HNE was expressed as U/mg, and results expressed as percentage of activity of native HNE for each treatment.
Effects of HNE Enzymatic Inhibition
Effects of enzymatic inhibition of HNE were investigated using three different elastase inhibitors: 1) elastatinal, a natural HNE inhibitor produced by Actinomycetes;26 2) CMK, a synthetic tetrapeptide;27 and 3) α1-antitrypsin (α1-AT), a physiological HNE inhibitor.28 The inhibitors were added directly to HNE, incubated for 15 minutes at 37°C, and then added directly to the cells for another 15 minutes. At the end of this exposure, secreted mucin was collected and quantified as described above.
To determine whether HNE enzymatic activity was directly required for stimulated mucin secretion, or if a secondary product(s) released by NHBE cells after exposure to HNE could be involved in the secretory response, NHBE cells were exposed to HNE (or vehicle) for 5 minutes. After exposure, the conditioned medium was collected and treated with 5 μmol/L of the HNE enzymatic inhibitor, α1-AT, for 15 minutes, at which time this α1-AT-treated medium was added to a new set of NHBE cells and effects on mucin secretion quantified as described above.
Effects of PKC Inhibition
The PKC inhibitors, bisindolylmaleimide I (10, 100, 1000 nmol/L)29 or calphostin C (5, 50, 500 nmol/L)30 were used to determine PKC involvement in HNE-induced mucin secretion. NHBE cells were preincubated with these agents (or vehicle control) for 15 minutes, then HNE was added for another 15 minutes before mucin secretion was quantified as described above.
PKC Activity Assay
PKC activity in NHBE cells after exposure to HNE was assessed using a PepTag assay for nonradioactive detection of PKC (following the manufacturer’s protocol). Briefly, 10 μg of protein extracted from each treatment of NHBE cells was added into the PKC reaction buffer (20 mmol/L HEPES, pH 7.4, 1.3 mmol/L CaCl2, 1 mmol/L dithiothreitol, 10 mmol/L MgCl2, 1 mmol/L ATP) containing 1 mg/ml phosphatidylserine and PepTag C1 PKC substrate peptide (P-L-S-R-T-L-S-V-A-A-K) conjugated with fluorescent dye, and incubated for 30 minutes at 30°C. The reaction was stopped by boiling at 100°C for 10 minutes. Reaction mixtures were separated on 0.8% agarose gels and proteins quantified by Labworks image acquisition and analysis software (UVP Bioimaging System, Upland, CA). Phosphorylation of MARCKS was detected by Western blot using an antibody against phophospecific-MARCKS.
PKC Isoform Analysis
After treatments, NHBE cells were washed with ice-cold PBS twice and then scraped into lysis buffer (50 mmol/L Tris, pH 7.5, 1 mmol/L ethylenediamine tetraacetic acid, 100 mmol/L NaCl, 1 mmol/L phenylmethyl sulfonyl fluoride) using a rubber policemen. The collected cells were lysed by sonication. For separation of cytosolic and membrane fractions, the lysates were spun at 400,000 × g in a Sorvall Discovery 100S ultracentrifuge (Sorvall, Inc. Newtown, CT) for 1 hour. The supernatant was reserved as the cytosolic sample. The pellet was resuspended in the same lysis buffer containing 0.05% Triton-100, dissolved by sonication, and incubated on ice for 30 minutes. After incubation, the same ultracentrifugation as described above was performed on the pellet mixture, and the supernatant separated from the pellet mixture was reserved as the membrane fraction. For preparation of whole cell crude lysates, the disrupted cellular mixture was centrifuged at 15,000 rpm in an Eppendorf 5417R centrifuge (Eppendorf Corp., Hamburg, Germany) for 1 hour at 4°C. The supernatant was collected as the whole crude NHBE cell lysate.
The protein concentration of cell lysate samples was quantified by a Bradford assay (Bio-Rad Laboratories, Hercules, CA). Each sample was boiled in 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer for 10 minutes, loaded on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, and transferred to a polyvinylidene difluoride membrane (Micron Separation Inc., Westborough, MA). After blocking with 5% skim milk, the antigen was captured by the specific PKC antibody and further amplified by binding to horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies. Anti-α-tubulin and E-cadherin antibodies were used for cytosolic and membrane controls, respectively, for each sample. Final development was accomplished by the enhanced chemiluminescence method. The amount of each PKC isoform was analyzed by Labworks image acquisition and analysis software.
Effects of PKC Isoform-Specific Inhibitors on HNE-Induced PKC Activation and Mucin Secretion
Because the studies above indicated that PKCδ was the only isoform to translocate to membranes in response to HNE, additional studies were performed with rottlerin, an inhibitor of PKCδ and θ.31 (Because PKCθ was not expressed in NHBE cells under basal or stimulated conditions, rottlerin is referred to below as a specific inhibitor of PKCδ). Rottlerin has the following potency against PKC isoforms: PKC δ (IC50 = 3 to 6 μmol/L); PKCθ (IC50 = 50 μmol/L); PKCα, PKCβ, and PKC γ (IC50 = 30 to 42 μmol/L); PKCε, PKCη, and PKCζ (IC50 = 80 to 100 μmol/L). It also can inhibit CaM kinase III (IC50 = 5.3 μmol/L).31,32
Cells were preincubated with rottlerin (1.5 μmol/L; IC50 = 3 to 6 μmol/L) for 20 minutes before exposure to HNE, and effects on PKC activity [using detection of phosphorylated (ser) PKC substrate] and on HNE-induced mucin secretion were assessed. As additional controls, the potential role of other PKC isoforms present in these cells was assessed: cells were exposed to the following specific inhibitors for 15 minutes before exposure to HNE and assay for mucin secretion: The PKCα/β inhibitor, Gö 6976 (10 nmol/L; IC50 = 2 ∼ 6 nmol/L);29 a PKCζ peptide inhibitor (50 μmol/L; Ser-Ile-Tyr-Arg-Arg-Gly-Ala-Arg-Arg-Trp-Arg-Lys-Leu; IC50 = 10 μmol/L);33 or a PKCε peptide inhibitor (3 ∼ 300 μmol/L; Glu-Ala-Val-Ser-Leu-Lys-Pro-Thr; IC50 = 80.3 μmol/L).34,35
Statistical Analysis
Data were expressed as the ratio of treatment to the corresponding vehicle control. Results were evaluated using one-way analysis of variance with Bonferroni posttest correction for multiple comparisons.36 A P value of <0.05 was considered significant.
Results
Cytotoxicity
All reagents used were tested for cytotoxicity using a Promega Cytotox 96 nonradioactive cytotoxicity assay kit according to the manufacturer’s instructions. The data were expressed as the ratio of released lactate dehydrogenase to total lactate dehydrogenase. Released lactate dehydrogenase never exceeded 10% of total lactate dehydrogenase (data not shown) in any of the experiments below.
Effects of HNE on Mucin Secretion
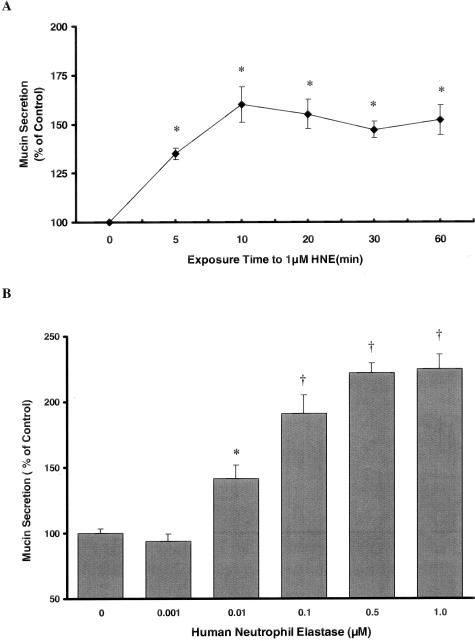
As illustrated in Figure 1, HNE stimulated mucin secretion by NHBE cells. Maximal mucin secretion was elicited after 15 minutes exposure to HNE (Figure 1A) so this time point was chosen for additional experiments. HNE increased mucin secretion in a concentration-dependent manner, with 0.01 to 1.0 μmol/L HNE increasing secretion significantly over vehicle control (Figure 1B).
Figure 1.
NHBE cells were exposed to HNE throughout a range of times and concentrations. A: NHBE cells were exposed to 1 μmol/L HNE or vehicle control for 5, 10, 20, 30, or 60 minutes, and mucin secretion was quantified by ELISA as described in the text using the 17Q2 antibody. Mucin secretion was rapidly increased after exposure to HNE and appeared to plateau at 10 to 15 minutes. B: HNE stimulates mucin secretion from NHBE cells in a concentration-dependent manner after 15 minutes of exposure. Cells were exposed to HNE for 15 minutes and mucin secretion was quantified by ELISA as described in text using 17Q2 antibody. *, Significantly different from vehicle control (P < 0.005). †, Significantly different from vehicle control (P < 0.001). Data are presented as mean ± SEM (n = 6 at each point in A, 12 at each point in B).
Mucin Gene Products Released by NHBE Cells in Response to HNE
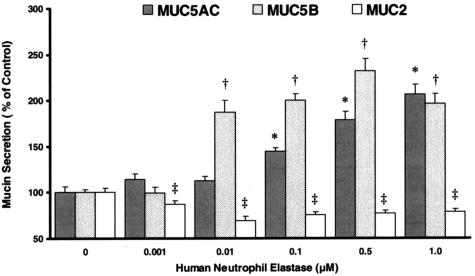
Secretion of major gel-forming mucins, including MUC2, MUC5AC, and MUC5B, was investigated after exposure to HNE. As illustrated in Figure 2, HNE enhanced release of both MUC5AC and MUC5B mucins from NHBE cells in a concentration-dependent manner. Secretion of MUC2 mucin was significantly decreased by HNE.
Figure 2.
Differential effect of HNE on secretion of mucin gene products by NHBE cells. Cells were exposed to HNE for 15 minutes and secretion of MUC5AC, MUC5B, and MUC2 protein quantified by ELISA as described in text. *, Significantly greater than vehicle control (P < 0.005); †, significantly greater than vehicle control (P < 0.001); ‡, significantly less than vehicle control (P < 0.05). Data are presented as mean ± SEM (n = 6 at each point).
HNE Enzymatic Activity and HNE-Stimulated Mucin Secretion
Elastatinal appeared to be the weakest of the three HNE inhibitors used in this study because the highest concentration used, 100 μmol/L, blocked only 50% of HNE enzymatic activity and did not affect HNE-stimulated mucin secretion (Figure 3A). CMK proved to be a more potent HNE enzymatic inhibitor because 50 μmol/L CMK completely blocked the enzymatic activity of 1 μmol/L HNE, whereas lower concentrations partially inhibited HNE activity in a concentration-dependent manner. CMK also showed an inhibitory effect on HNE-stimulated mucin secretion in a concentration-dependent manner with 50 μmol/L CMK almost completely blocking the secretory effect of HNE (Figure 3B). AT was the most potent HNE inhibitor among the three tested because 5 μmol/L AT blocked 90% of HNE enzymatic activity and completely inhibited its mucin-secretory effect (Figure 3C). Elastatinal (100 μmol/L), CMK (50 μmol/L), or AT (5 μmol/L) by themselves did not affect constitutive mucin secretion (data not shown). As illustrated in Figure 3D, boiling HNE inactivated the enzyme rapidly: boiling for 5 minutes inhibited ∼70 to 80% HNE activity, while boiling for 15 minutes inhibited HNE activity. Interestingly, HNE boiled for 5 minutes still was able to induce mucin secretion, but boiling for 15 minutes abolished the ability of HNE to provoke mucin secretion.
Figure 3.
Effect of elastase enzymatic inhibitors on HNE activity and HNE-induced mucin secretion from NHBE cells. A: Elastatinal, 1 to 100 μmol/L; B: CMK, 0.5 to 50 μmol/L; C: α1-AT, 0.5 to 5 μmol/L; and D: boiling HNE for indicated times. HNE activity: 1 μmol/L HNE was incubated with or without the indicated inhibitor for 15 minutes at 37°C or boiled for the indicated time. HNE activity in each mixture then was measured as described in text. †, Significantly different from HNE alone (P < 0.001). Data are presented as mean ± SEM (n = 6 at each point). Mucin secretion: HNE was incubated with or without the indicated inhibitor for 15 minutes at 37°C (or boiled), and the mixture then added to NHBE cells for another 15 minutes at 37°C. §, Significantly different from vehicle control (P < 0.001). , Significantly different from HNE alone (P < 0.05). Data are presented as mean ± SEM (n = 12 at each point).
Finally, as illustrated in Figure 4, media collected from NHBE cell cultures exposed to HNE, called conditioned medium, was capable of stimulating mucin secretion when applied to a different set of NHBE cells. However, this effect was inhibited by AT, indicating that the secretory response was only due to the presence of HNE in the conditioned medium. Thus, effects of HNE on mucin secretion do not appear to involve a soluble secondary product released by NHBE cells.
Figure 4.
α1-Antitrypsin (AT) blocks mucin secretion stimulated by medium from cells exposed to HNE. NHBE cells were exposed to medium alone or medium plus 1.0 μmol/L HNE for 5 minutes. Medium from cells was then collected and incubated under cell-free conditions with or without AT (5 μmol/L) for an additional 15 minutes at 37°C. At the end of this incubation, a new set of NHBE cells was exposed to the different mixtures for 15 minutes at 37°C, and secreted mucin quantified as described in text. AT added to medium from cells exposed previously to HNE attenuated the stimulatory effect of that medium on new NHBE cells, indicating that a secondary soluble product is not involved in the secretory response to HNE. *, Significantly different from vehicle control (P < 0.05). †, Significantly different from HNE alone (P < 0.05). Data are presented as mean ± SEM (n = 12).
Effects of Kinase Inhibitors on HNE-Induced Mucin Secretion
As illustrated in Figure 5, A and B, both PKC inhibitors, bisindolylmaleimide I and calphostin C, attenuated HNE-stimulated mucin secretion in a concentration-dependent manner.
Figure 5.
HNE appears to stimulate mucin secretion by a PKC-dependent mechanism. A and B: PKC inhibitors attenuate HNE-induced mucin secretion by NHBE cells. NHBE cells were pre-exposed to PKC inhibitors or solvent alone for 15 minutes, after which HNE or vehicle control was added to cells at indicated concentrations for an additional 15 minutes, and secreted mucin was then quantified as described in text. Both PKC inhibitors: bisindolylmaleimide I (Bis I) (A) and calphostin C (Cal C) (B) attenuated HNE-stimulated mucin secretion in a concentration-dependent manner. (Bis V is bisindolylmaleimide V, a functionally inactive control for Bis I). *, Significantly different from vehicle control (P < 0.05); †, significantly different from HNE alone (P < 0.05). Data are presented as mean ± SEM (n = 12). C: Addition of 1 μmol/L HNE to NHBE cells results in a rapid (within 1.5 minutes) activation of PKC, as reflected by phosphorylation of the PKC substrate MARCKS protein at that time point. MARCKS appears to be rapidly dephosphorylated within the next 10 to 15 minutes. Blot is representative of three replicate experiments. D: Phosphorylated MARCKS and MARCKS protein from blot C were quantified by densitometry. Phosphorylated MARCKS was normalized to total MARCKS protein. A clear increase in phosphorylated MARCKS is apparent.
Effects of HNE on PKC Activation
As illustrated in Figure 5C, exposure of NHBE cells to HNE caused a rapid (within 2 minutes) phosphorylation of MARCKS protein, reflecting activation of PKC.
PKC Isoforms in HNE-Induced Mucin Secretion
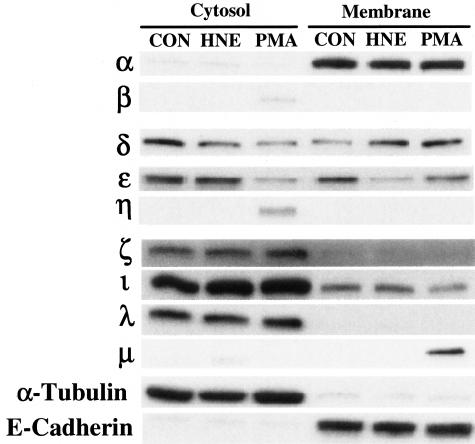
Western blot studies on cytosolic and membrane fractions of NHBE cells were performed under constitutive conditions and after stimulation with HNE (0.5 μmol/L) or a positive control, PMA (100 nmol/L). As illustrated in Figure 6, PKCα, δ, ε, λ, ζ, and ι were expressed constitutively in nonstimulated NHBE cells, while PKCβ and η were induced in the cytosol and PKCμ in the membrane only after exposure to PMA. PKCγ or θ were not detected under any conditions. Of these isoforms, only PKCδ translocated from cytosol to membrane after HNE exposure (Figure 6). Preincubation of cells with rottlerin, a specific PKCδ inhibitor, for 20 minutes before HNE exposure resulted in reduction of total PKC activity and attenuation of HNE-induced mucin secretion (Figure 7). None of the other PKC isoform-specific inhibitors affected HNE-induced mucin secretion (data not shown).
Figure 6.
Several PKC isoforms are expressed by NHBE cells in vitro, but only PKCδ translocates from cytosol to membrane in response to HNE. All proteins were detected by Western blot from both cytosolic and membrane fractions. All PKC isoforms were detected using the same original gel. Under nonstimulated conditions, cells contain PKC α, δ, ε, ζ, ι, and λ in the cytosol, and α, δ, and ι in membrane fractions. On stimulation with PMA (100 nmol/L; 15 minutes), PKC β, η, and μ appear induced in the cytosol or membrane fraction, and only PKC δ and ε translocate to the membrane. In response to HNE (0.5 μmol/L; 15 minutes), however, only PKCδ translocates to the membrane. α-Tubulin and E-cadherin are used as controls to show cytosolic and membrane protein expression, respectively. Blots are representative of experiments repeated at least three times.
Figure 7.
Effects of the PKCδ inhibitor, rottlerin, on HNE-induced PKC activity and mucin secretion in NHBE cells. Cells were preincubated with rottlerin or vehicle control for 20 minutes, and then exposed to HNE (0.5 μmol/L) for an additional 15 minutes, at which time PKC activity and mucin secretion were assessed as described. A: Rottlerin attenuates HNE-induced PKC activity in a concentration-dependent manner. Data are representative densitometric scans averaged from two replicate experiments. B: Rottlerin inhibits, in a concentration-dependent manner, secretion of mucin (MUC5AC) in NHBE cells exposed to HNE. Rottlerin also significantly decreased constitutive mucin secretion. *, Significantly different from vehicle control (P < 0.05); †, significantly different from HNE alone (P < 0.05). Data are presented as mean ± SEM (n = 4).
Discussion
The results of the studies reported here indicate that elastase causes rapid release of preformed mucins from primary cultures of human bronchial epithelial cells maintained in air-liquid interface, a cell culture system that maintains characteristics of well-differentiated airway epithelium in vitro, including mucin synthesis and secretion.21,37 The concentrations and time course for HNE-induced mucin release from NHBE cells were similar to those reported for HNE in studies using the MM39 human submucosal gland cell line38,39 and hamster goblet cells in vitro.9 The concentrations of HNE (0.01 to 1 μmol/L) that elicited a significant secretory response in NHBE cells in the present study appear to be achievable in inflamed airways. For example, it has been reported that HNE concentrations in sputum of cystic fibrosis patients may exceed 3.3 μmol/L (∼100 μg/ml).15,16 Interestingly, in studies related to mucin production in response to HNE, Voynow and colleagues40 showed that a much lower HNE concentration (25 nmol/L) can increase MUC5AC mRNA and protein expression in human airway epithelial cells.
The majority of the secretion measurements in our study were done via an ELISA using the pan-mucin antibody, 17Q2, which is directed toward a carbohydrate epitope and therefore recognizes mucins derived from different MUC genes.41 It has been reported previously that HNE has an ability to release mucins from airway goblet cells and then to degrade the released mucins.9 However, because we measured pan-mucins using the 17Q2 antibody that recognizes carbohydrate epitope(s), it is very likely that the amount of mucins detected would be virtually the same regardless of the intactness of mucins. Therefore, degradation of mucins by the hydrolytic effects of HNE would not affect the results or the conclusion of this study.
The mucin proteins secreted in response to elastase appear to be a combination of the gene products of MUC5AC and MUC5B, as ELISA-based analysis revealed significant secretion of both mucins in response to HNE. MUC5AC mucin is usually considered to be the major mucin protein produced and secreted by airway epithelial cells, with MUC5B restricted mostly to submucosal glands.42,43 However, Chen and colleagues44 and Holmen and colleagues45 independently reported recently that cultured human airway epithelial cells express MUC5B, as does epithelium in situ in both human bronchitis and mouse models of asthma.44 Thus, MUC5B might also be a relevant mucin gene to investigate in relation to the pathogenesis of mucus overproduction and hypersecretion in inflammatory airway disease. MUC2 is not produced in large amounts by human airway epithelium under normal conditions, and the absolute amounts released in control or HNE-exposed cells were miniscule compared to MUC5AC or MUC5B. The statistically significant decrease in response to HNE could be the result of degradation, because the antibody is against the peptide core. Although it is possible that some degradation of MUC5AC and MUC5B also occurred in these experiments, it would be masked by the high levels of release of these proteins, whereas the effects on MUC2, already at low levels, would be observable. The story has become even more complicated with the recent description of a newly discovered respiratory mucin, MUC19, that also could be involved in inflammation-associated responses of the secretory apparatus in airways.46 The exact contribution(s) of each of these mucin genes to human airway mucin secretions in health and disease remains to be determined.
In the studies reported here, we found that the secretagogue activity of HNE was linked to its enzymatic activity. However, although complete inhibition of HNE activity also blocked HNE-induced mucin secretion (Figure 3), inhibition of HNE enzymatic activity by ∼50% [as caused by addition of 100 μmol/L elastatinal (Figure 3A), 5 μmol/L CMK (Figure 3B), or 0.5 μmol/L AT (Figure 3C)] did not alter the secretory response, as the increase in mucin secretion by cultures in response to HNE that had been treated with any of these agents, and thus contained only approximately half of its original enzymatic activity, still reached the same levels as that induced by intact HNE. However, as indicated in Figure 1B, 0.1 μmol/L of HNE had essentially the same effect on mucin secretion as 1.0 μmol/L, so the relationship between enzymatic activity and secretory stimulation does not appear direct, at least at the higher concentrations. It is possible that attenuation of enzymatic activity at lower concentrations of HNE could have had more of an effect on secretory activity. In any case, given that concentrations of HNE in airways of patients with airway inflammation can reach micromolar levels,15–17 potential anti-elastase therapy in such diseases might need to significantly diminish the enzymatic activity of HNE before it could become effective against its secretory action.
There is a possibility that a potential HNE-related product that acts as a secondary stimulus, rather than HNE directly, could be responsible for HNE-stimulated secretion. If this were true and the potential product(s) were soluble, then the product might be found in the conditioned medium from cells exposed to HNE, and this conditioned medium would induce mucin secretion if added to other cells in a manner such that an elastase inhibitor, such as AT, would not block the enhanced secretion if added directly to the conditioned medium. As illustrated in Figure 4, AT did in fact inhibit conditioned medium-induced mucin secretion, suggesting that a soluble product released on HNE exposure was not involved. Of course, a soluble product could be released and bind rapidly to the NHBE cells, so these results do not preclude the existence of secondary mediators in the secretory response to HNE. These findings also do not preclude either an HNE substrate or HNE cleavage product that could influence secretion being present on epithelial cell membranes (or even intracellularly). In this regard, Takeyama and colleagues19 have reported that contact between neutrophils and epithelial cells enhances neutrophil chemoattractant-stimulated goblet cell degranulation, and Kim and colleagues9 suggested that HNE binding to epithelial cell surfaces maximized its stimulatory activity.
Whatever the actual stimulatory product, be it HNE directly or a secondary product, the secretory response appears to be mediated by PKC. The PKC family contains three types of isoforms: classical (cPKCs: α, β1, β2, γ), novel (nPKCs: δ, ε, η, θ, μ), and atypical (aPKCs: ζ, ι/λ). The classical isoforms are calcium- and phorbol ester-activated, the novel are calcium-insensitive but activated by phorbol esters, and the atypical isoforms are both calcium- and phorbol ester-insensitive, with all isoforms activated by phosphatidylserine.46 Our studies demonstrating that human tracheal epithelial cells express α, β1, β2, δ, ε, and ζ isoforms of PKC are in general agreement with previous reports.47–49
Involvement of PKC in secretion of airway mucin in response to various stimuli has been indicated previously.50–55 The specific PKC isoenzyme(s) (singly or in combination) that contribute to PKC-induced mucin secretion have not been determined, although PKCζ and PKCδ have been suggested as potential candidates.50,53,54 In our studies with HNE, both the DAG-binding site inhibitor of PKC (calphostin C) and the ATP-binding site inhibitor (bisindolylmaleimide I) blocked mucin release in concentration-dependent manners, suggesting that a cPKC or nPKC isoform(s) is involved in the secretory response. When we examined translocation of PKC isoforms in response to HNE, the only isoform that moved from cytoplasm to membrane in NHBE cells exposed to stimulatory concentrations of HNE was PKCδ (Figure 6). When NHBE cells then were exposed to PKC isoform-specific pharmacological inhibitors, the only inhibitor that attenuated secretion was rottlerin, which also appeared to attenuate enhancement of PKC activity in cells exposed to HNE (Figure 7). Further verification of the involvement of the PKCδ isoform in the secretory process using molecular constructs, such as dominant-negative PKCδ, siRNA, and so forth, would be valuable, but at this time problems with transfection of these constructs into primary cells and the presence of a heterogeneous cell population in NHBE cultures make these kinds of studies difficult to perform.
As illustrated in Figure 6, there were effects of PMA and HNE on expression of other isoforms of PKC. For example, in response to PMA, PKCβ appeared in the cytosol and PKCμ appeared in the membrane fraction, whereas neither isoform appeared to be expressed constitutively. PMA also caused an apparent decrease of protein expression of PKCε in the cytosolic fraction. Since these blots were performed within 15 minutes of exposure, there is little if any probability that transcription could have occurred. There are (at least) two possible explanations for these responses. Firstly, they could reflect rapid posttranscriptional and/or translational events. Secondly, PMA is known to bind to PKCs in the C1 regulatory domain of the enzyme, and it is possible that such binding could induce conformational changes that could expose previously masked epitopes, or, in the case of PKCε, block access of an antibody to such epitopes. Relatedly, PKCε expression in the membrane fraction appeared to be decreased after exposure to HNE, and it could be that HNE effects on membranes, alluded to previously, could affect expression of several membrane proteins, including some PKC isoforms. In any case, these alterations in expression of several isoforms require further study to determine the mechanisms of action of PMA and/or HNE.
The involvement of PKCδ in the secretory response to HNE is interesting in light of recent findings that this particular PKC isoform has a number of potentially important functions in airway epithelial cell pathophysiology. These include regulation of nuclear factor-κB-dependent expression of proinflammatory genes in a human airway epithelial cell line,47 control of both NKCC1 function and Na-K-2Cl co-transport in airway epithelial cells,49,56 and regulation of asbestos-induced apoptosis in alveolar epithelial cells.57 Interestingly, Abdullah and colleagues50 reported that mucin secretion in response to purinergic stimulation in SPOC1 cells, a rat airway cell line, also may involve activation of PKCδ. In addition, a recent report implicated PKCδ in HNE-induced mucin gene expression in airway epithelium.58
A role for PKCδ in mucin secretion fits in nicely with previous studies from our laboratory showing that MARCKS protein, a widely expressed PKC phosphorylation target, is a key molecule regulating mucin secretion in airway epithelium,21,59 as PKCδ is activated by PMA and can phosphorylate MARCKS protein. Interestingly, as illustrated in Figure 5C, MARCKS is rapidly phosphorylated when HNE is added to NHBE cells, but is quickly dephosphorylated. This pattern of phosphorylation-dephosphorylation of MARCKS was shown in a previous report from this laboratory to be a critical step in the mucin secretory pathway after stimulation21 so these results are consistent with previous studies implicating MARCKS protein in airway mucin secretion. Whether or not the secretagogue action of HNE depends on MARCKS, as shown with other stimuli that enhance mucin secretion, presently is under study, and if so would provide additional information supporting the existence of a common intracellular secretory pathway involving MARCKS present in goblet cells in response to a variety of pathophysiological stimuli in the airways.
Footnotes
Address reprint requests to Dr. Kenneth B. Adler, Department of Molecular Biomedical Sciences, College of Veterinary Medicine, North Carolina State University, 4700 Hillsborough St., Raleigh, NC 27606. E-mail: kenneth_adler@ncsu.edu.
Supported by the National Institutes of Health (grant R37 HL36982 to K.B.A.).
Present address of F.H.: Allergan, Inc., P.O. Box 19534, Irvine CA 92623; present address of Y.L.: Tanox, Inc., 10301 Stella Link, Ste. 110, Houston, TX 77025.
References
- Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95:843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- Stockley RA. Role of inflammation in respiratory tract infections. Am J Med. 1995;99:8S–13S. doi: 10.1016/s0002-9343(99)80304-0. [DOI] [PubMed] [Google Scholar]
- Welsh MD, Adair BM, Foster JC. Effect of BVD virus infection on alveolar macrophage functions. Vet Immunol Immunopathol. 1995;46:195–210. doi: 10.1016/0165-2427(94)05366-z. [DOI] [PubMed] [Google Scholar]
- Mohapatra NK, Cheng PW, Parker JC, Paradiso AM, Yankaskas JR, Boucher RC, Boat TF. Alteration of sulfation of glycoconjugates, but not sulfate transport and intracellular inorganic sulfate content in cystic fibrosis airway epithelial cells. Pediatr Res. 1995;38:42–48. doi: 10.1203/00006450-199507000-00008. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Schuster A, Ueki I, Boushey HA, Nadel JA. Mucus hypersecretion in bronchiectasis. The role of neutrophil proteases. Am Rev Respir Dis. 1992;146:1430–1433. doi: 10.1164/ajrccm/146.6.1430. [DOI] [PubMed] [Google Scholar]
- Stockley RA, Hill SL, Morrison HM, Starkie CM. Elastolytic activity of sputum and its relation to purulence and to lung function in patients with bronchiectasis. Thorax. 1984;39:408–413. doi: 10.1136/thx.39.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer R, Christensen TG, Lucey EC, Stone PJ, Snider GL. An ultrastructural morphometric analysis of elastase-treated hamster bronchi shows discharge followed by progressive accumulation of secretory granules. Am Rev Respir Dis. 1987;136:698–703. doi: 10.1164/ajrccm/136.3.698. [DOI] [PubMed] [Google Scholar]
- Nadel JA. Protease actions on airway secretions. Relevance to cystic fibrosis. Ann NY Acad Sci. 1991;624:286–296. doi: 10.1111/j.1749-6632.1991.tb17027.x. [DOI] [PubMed] [Google Scholar]
- Kim KC, Wasano K, Niles RM, Schuster JE, Stone PJ, Brody JS. Human neutrophil elastase releases cell surface mucins from primary cultures of hamster tracheal epithelial cells. Proc Natl Acad Sci USA. 1987;84:9304–9308. doi: 10.1073/pnas.84.24.9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerhoff CP, Nadel JA, Basbaum CB, Caughey GH. Neutrophil elastase and cathepsin G stimulate secretion from cultured bovine airway gland serous cells. J Clin Invest. 1990;85:682–689. doi: 10.1172/JCI114492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NV, Marshall BC, Gray BH, Hoidal JR. Interaction of secretory leukocyte protease inhibitor with proteinase-3. Am J Respir Cell Mol Biol. 1993;8:612–616. doi: 10.1165/ajrcmb/8.6.612. [DOI] [PubMed] [Google Scholar]
- Renesto P, Halbwachs-Mecarelli L, Nusbaum P, Lesavre P, Chignard M. Proteinase 3. A neutrophil proteinase with activity on platelets. J Immunol. 1994;152:4612–4617. [PubMed] [Google Scholar]
- Bainton DF, Ullyot JL, Farquhar MG. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971;134:907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick RB, Jr, Naegel GP, Squier SU, Wood RE, Gee JB, Reynolds HY. Proteins of the cystic fibrosis respiratory tract. Fragmented immunoglobulin G opsonic antibody causing defective opsonophagocytosis. J Clin Invest. 1984;74:236–248. doi: 10.1172/JCI111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring G, Goldstein W, Botzenhart K, Kharazmi A, Schiotz PO, Hoiby N, Dasgupta M. Elastase from polymorphonuclear leucocytes: a regulatory enzyme in immune complex disease. Clin Exp Immunol. 1986;64:597–605. [PMC free article] [PubMed] [Google Scholar]
- Goldstein W, Doring G. Lysosomal enzymes from polymorphonuclear leukocytes and proteinase inhibitors in patients with cystic fibrosis. Am Rev Respir Dis. 1986;134:49–56. doi: 10.1164/arrd.1986.134.1.49. [DOI] [PubMed] [Google Scholar]
- Suter S, Schaad UB, Tegner H, Ohlsson K, Desgrandchamps D, Waldvogel FA. Levels of free granulocyte elastase in bronchial secretions from patients with cystic fibrosis: effect of antimicrobial treatment against Pseudomonas aeruginosa. J Infect Dis. 1986;153:902–909. doi: 10.1093/infdis/153.5.902. [DOI] [PubMed] [Google Scholar]
- Kim KC, Nassiri J, Brody JS. Mechanisms of airway goblet cell mucin release: studies with cultured tracheal surface epithelial cells. Am J Respir Cell Mol Biol. 1989;1:137–143. doi: 10.1165/ajrcmb/1.2.137. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Agusti C, Ueki I, Lausier J, Cardell LO, Nadel JA. Neutrophil-dependent goblet cell degranulation: role of membrane-bound elastase and adhesion molecules. Am J Physiol. 1998;275:L294–L302. doi: 10.1152/ajplung.1998.275.2.L294. [DOI] [PubMed] [Google Scholar]
- Li Y, Martin LD, Minnicozzi M, Greenfeder S, Fine J, Pettersen CA, Chorley B, Adler KB. Enhanced expression of mucin genes in a guinea pig model of allergic asthma. Am J Respir Cell Mol Biol. 2001;25:644–651. doi: 10.1165/ajrcmb.25.5.4485. [DOI] [PubMed] [Google Scholar]
- Li Y, Martin LD, Spizz G, Adler KB. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem. 2001;276:40982–40990. doi: 10.1074/jbc.M105614200. [DOI] [PubMed] [Google Scholar]
- Bertolero F, Kaighn ME, Gonda MA, Saffiotti U. Mouse epidermal keratinocytes. Clonal proliferation and response to hormones and growth factors in serum-free medium. Exp Cell Res. 1984;155:64–80. doi: 10.1016/0014-4827(84)90768-7. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci USA. 1999;96:3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, Carlstedt L, Nicholson AG, Chung KF. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir Med. 2002;96:81–86. doi: 10.1053/rmed.2001.1221. [DOI] [PubMed] [Google Scholar]
- Crowther JR. ELISA. Theory and practice. Methods Mol Biol. 1995;42:1–218. doi: 10.1385/0-89603-279-5:1. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Structures and activities of protease inhibitors of microbial origin. Methods Enzymol. 1976;45:678–695. doi: 10.1016/s0076-6879(76)45058-9. [DOI] [PubMed] [Google Scholar]
- Rees DD, Brain JD, Wohl ME, Humes JL, Mumford RA. Inhibition of neutrophil elastase in CF sputum by L-658,758. J Pharmacol Exp Ther. 1997;283:1201–1206. [PubMed] [Google Scholar]
- Gadek JE, Fells GA, Zimmerman RL, Rennard SI, Crystal RG. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981;68:889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- Takahashi I, Saitoh Y, Yoshida M, Sano H, Nakano H, Morimoto M, Tamaoki T. UCN-01 and UCN-02, new selective inhibitors of protein kinase C. II. Purification, physico-chemical properties, structural determination and biological activities. J Antibiot (Tokyo) 1989;42:571–576. doi: 10.7164/antibiotics.42.571. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- Villalba M, Kasibhatla S, Genestier L, Mahboubi A, Green DR, Altman A. Protein kinase C cooperates with calcineurin to induce fas ligand expression during activation-induced T cell death. J Immunol. 1999;163:5813–5819. [PubMed] [Google Scholar]
- Bandyopadhyay G, Standaert ML, Galloway L, Moscat J, Farese RV. Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997;138:4721–4731. doi: 10.1210/endo.138.11.5473. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Gray MO, Chen CH, Mochly-Rosen D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J Biol Chem. 1996;271:24962–24966. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- Mendez CF, Leibiger IB, Leibiger B, Hoy M, Gromada J, Berggren PO, Bertorello AM. Rapid association of protein kinase C-epsilon with insulin granules is essential for insulin exocytosis. J Biol Chem. 2003;278:44753–44757. doi: 10.1074/jbc.M308664200. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE. Applied Regression Analysis and Other Multivariable Methods. Boston: PWS-Kent Pub. Co.; 1988:pp 341–386. [Google Scholar]
- Krunkosky TM, Fischer BM, Martin LD, Jones N, Akley NJ, Adler KB. Effects of TNF-alpha on expression of ICAM-1 in human airway epithelial cells in vitro. Signaling pathways controlling surface and gene expression. Am J Respir Cell Mol Biol. 2000;22:685–692. doi: 10.1165/ajrcmb.22.6.3925. [DOI] [PubMed] [Google Scholar]
- Jacquot J, Merten M, Millot JM, Sebille S, Menager M, Figarella C, Manfait M. Asynchronous dynamic changes of intracellular free Ca2+ and possible exocytosis in human tracheal gland cells induced by neutrophil elastase. Biochem Biophys Res Commun. 1995;212:307–316. doi: 10.1006/bbrc.1995.1971. [DOI] [PubMed] [Google Scholar]
- Maizieres M, Kaplan H, Millot JM, Bonnet N, Manfait M, Puchelle E, Jacquot J. Neutrophil elastase promotes rapid exocytosis in human airway gland cells by producing cytosolic Ca2+ oscillations. Am J Respir Cell Mol Biol. 1998;18:32–42. doi: 10.1165/ajrcmb.18.1.2841. [DOI] [PubMed] [Google Scholar]
- Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am J Physiol. 1999;276:L835–L843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- Lin H, Carlson DM, St. George JA, Plopper CG, Wu R. An ELISA method for the quantitation of tracheal mucins from human and nonhuman primates. Am J Respir Cell Mol Biol. 1989;1:41–48. doi: 10.1165/ajrcmb/1.1.41. [DOI] [PubMed] [Google Scholar]
- Davies JR, Svitacheva N, Lannefors L, Kornfalt R, Carlstedt I. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem J. 1999;344:321–330. [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Dudus L, Nielsen PA, Clausen H, Yankaskas JR, Hollingsworth MA, Engelhardt JF. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am J Respir Cell Mol Biol. 1998;19:30–37. doi: 10.1165/ajrcmb.19.1.3054. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhao YH, Wu R. In silico cloning of mouse Muc5b gene and upregulation of its expression in mouse asthma model. Am J Respir Crit Care Med. 2001;164:1059–1066. doi: 10.1164/ajrccm.164.6.2012114. [DOI] [PubMed] [Google Scholar]
- Holmen JM, Karlsson NG, Abdullah LH, Randell SH, Sheehan JK, Hansson GC, Davis CW. Mucins and their O-glycans from human bronchial epithelial cell cultures. Am J Physiol. 2004;287:L824–L834. doi: 10.1152/ajplung.00108.2004. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhao YH, Kalaslavadi TB, Hamati E, Nehrke K, Le AD, Ann DK, Wu R. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol. 2004;30:155–165. doi: 10.1165/rcmb.2003-0103OC. [DOI] [PubMed] [Google Scholar]
- Page K, Li J, Zhou L, Iasvovskaia S, Corbit KC, Soh JW, Weinstein IB, Brasier AR, Lin A, Hershenson MB, Iasvoyskaia S. Regulation of airway epithelial cell NF-kappa B-dependent gene expression by protein kinase C delta. J Immunol. 2003;170:5681–5689. doi: 10.4049/jimmunol.170.11.5681. [DOI] [PubMed] [Google Scholar]
- Alpert SE, Walenga RW, Mandal A, Bourbon N, Kester M. 15-HETE-substituted diglycerides selectively regulate PKC isotypes in human tracheal epithelial cells. Am J Physiol. 1999;277:L457–L464. doi: 10.1152/ajplung.1999.277.3.L457. [DOI] [PubMed] [Google Scholar]
- Liedtke CM, Cole T, Ikebe M. Differential activation of PKC-delta and -zeta by alpha 1-adrenergic stimulation in human airway epithelial cells. Am J Physiol. 1997;273:C937–C943. doi: 10.1152/ajpcell.1997.273.3.C937. [DOI] [PubMed] [Google Scholar]
- Abdullah LH, Bundy JT, Ehre C, Davis CW. Mucin secretion and PKC isoforms in SPOC1 goblet cells: differential activation by purinergic agonist and PMA. Am J Physiol. 2003;285:L149–L160. doi: 10.1152/ajplung.00359.2002. [DOI] [PubMed] [Google Scholar]
- Abdullah LH, Conway JD, Cohn JA, Davis CW. Protein kinase C and Ca2+ activation of mucin secretion in airway goblet cells. Am J Physiol. 1997;273:L201–L210. doi: 10.1152/ajplung.1997.273.1.L201. [DOI] [PubMed] [Google Scholar]
- Fischer BM, Rochelle LG, Voynow JA, Akley NJ, Adler KB. Tumor necrosis factor-alpha stimulates mucin secretion and cyclic GMP production by guinea pig tracheal epithelial cells in vitro. Am J Respir Cell Mol Biol. 1999;20:413–422. doi: 10.1165/ajrcmb.20.3.3393. [DOI] [PubMed] [Google Scholar]
- Larivee P, Levine SJ, Martinez A, Wu T, Logun C, Shelhamer JH. Platelet-activating factor induces airway mucin release via activation of protein kinase C: evidence for translocation of protein kinase C to membranes. Am J Respir Cell Mol Biol. 1994;11:199–205. doi: 10.1165/ajrcmb.11.2.8049080. [DOI] [PubMed] [Google Scholar]
- Scott CE, Abdullah LH, Davis CW. Ca2+ and protein kinase C activation of mucin granule exocytosis in permeabilized SPOC1 cells. Am J Physiol. 1998;275:C285–C292. doi: 10.1152/ajpcell.1998.275.1.C285. [DOI] [PubMed] [Google Scholar]
- Ko KH, Jo M, McCracken K, Kim KC. ATP-induced mucin release from cultured airway goblet cells involves, in part, activation of protein kinase C. Am J Respir Cell Mol Biol. 1997;16:194–198. doi: 10.1165/ajrcmb.16.2.9032127. [DOI] [PubMed] [Google Scholar]
- Liedtke CM, Hubbard M, Wang X. Stability of actin cytoskeleton and PKC-delta binding to actin regulate NKCC1 function in airway epithelial cells. Am J Physiol. 2003;284:C487–C496. doi: 10.1152/ajpcell.00357.2002. [DOI] [PubMed] [Google Scholar]
- 57. Shukla A, Stern M, Lounsbury KM, Flanders T, Mossman BT. Asbestos-induced apoptosis is protein kinase C delta-dependent. Am J Respir Cell Mol Biol. 2003;29:198–205. doi: 10.1165/rcmb.2002-0248OC. [DOI] [PubMed] [Google Scholar]
- Shao MXG, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Nat Acad Sci. 2005;102:767–772. doi: 10.1073/pnas.0408932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]