Abstract
The abnormal megakaryocytopoiesis associated with idiopathic myelofibrosis (IM) plays a role in its pathogenesis. Because mice with defective expression of transcription factor GATA-1 (GATA-1low mutants) eventually develop myelofibrosis, we investigated the occurrence of GATA-1 abnormalities in IM patients. CD34+ cells were purified from 12 IM patients and 8 controls; erythroblasts and megakaryocytes were then obtained from unilineage cultures of CD34+ cells. Purified CD61+, GPA+, and CD34+ cells from IM patients contained levels of GATA-1, GATA-2, and FOG-1 mRNA, as well as of GATA-2 protein, that were similar to controls. In contrast, CD61+ cells from IM patients contained significantly reduced GATA-1 protein. Furthermore, 45% of megakaryocytes in biopsies from IM patients did not stain with anti-GATA-1 antibody, as compared to controls (2%), essential thrombocythemia (4%), or polycythemia vera (11%) patients. Abnormalities in immunoreactivity for FOG-1 were not found, and no mutations in GATA-1 coding sequences were found. The presence of GATA-1neg megakaryocytes in bone marrow biopsies was independent of the Val617Phe JAK2 mutation, making it unlikely that a downstream functional relationship exists. We conclude that megakaryocytes from IM patients have reduced GATA-1 content, possibly contributing to disease pathogenesis as in the GATA-1low mice and also representing a novel IM-associated marker.
Chronic idiopathic myelofibrosis (IM; ICD-O9961/3), according to the World Health Organization classification of tumors of hematopoietic and lymphoid tissues,1 is the least frequent among chronic myeloproliferative disorders (CMPDs), with an estimated incidence of ∼0.5 to 1.5 new cases per 100,000 individuals per year. It is a clonal disorder of the multipotent hematopoietic stem cell, characterized by accumulation of abnormal megakaryocytes in the marrow and progressive structural changes of the microenvironment, which include extensive collagen and reticulin deposition, osteosclerosis, and angiogenesis.2,3 Foci of extramedullary hematopoiesis may develop in several organs, mostly the spleen and the liver. The blood film of these patients is characterized by a leuko-erythroblastic picture, with immature myeloid and erythroid cells, and by tear-drop erythrocytes.4 The CD34+ hematopoietic progenitor cells are constitutively mobilized in the peripheral blood,5,6 and their number may be related to the severity of the disease and the risk of leukemic transformation;6,7 the latter occurs in ∼5 to 20% of patients,8,9 with a dismal outcome.8 The changes in bone marrow (BM) stroma are the result of a response of local fibroblasts, that remain polyclonal and do not derive from the neoplastic clone,10 to cytokines, such as transforming growth factor-β1, released by the abnormal megakaryocytes.11,12 Clinical course and overall survival are greatly variable, depending on prognostic scores.13 At present, the only curative approach is represented by allogeneic hematopoietic stem cell transplantation,14 either conventional or reduced intensity, that can be sadly offered to a minority of younger patients. Cytogenetic abnormalities occur in almost 50% of patients at diagnosis and increase further in the follow-up,15 but they are not recurrent nor have they been clearly associated with the pathogenesis of disease, leaving its primary molecular defect still unknown. However, the recent description of a Val617Phe mutation in the exon 12 of JAK2 gene may represent the first reliable molecular marker of IM16–18 although, unlike in polycythemia vera (PV) in which 74 to 97% of patients presented the mutation, only 35 to 57% of those with IM were Val617Phepositive, a figure comparable to the finding in essential thrombocythemia (ET) (32 to 50%); intriguingly, this suggests that other molecular pathway(s) in addition to those depending on Jak2, and/or individual genetic characteristics, might be responsible for the unique clinical phenotype of IM in respect to either PV or ET.
Insights into the pathogenesis of IM derived from the observation that mice with induced alterations in the regulation of megakaryocytopoiesis develop a myelofibrosis-like syndrome.19 Mice chronically exposed to high levels of the megakaryocytic-specific growth factor thrombopoietin (TPO) after transplantation with BM cells infected with a retrovirus containing the TPO gene20–22 show thrombocytosis and develop within a few months a myelofibrosis-like syndrome, terminating in acute leukemia in some animals.20 The development of a phenotype resembling human IM has been documented also in mice genetically modified at the GATA-1 locus (GATA-1low mutation).23,24 GATA-1 is the founder of a six-member family of transcription factors that, by binding to the consensus sequences (A/T)GATA(A/G) in the promoter and enhancer regions of target genes, regulates proliferation and differentiation of erythroid, megakaryocytic, eosinophilic, and mast cells.25 In erythroid and megakaryocytic cells, GATA-1 binds to its cognate sequences as an obligatory dimer of FOG-1 (for friend-of-GATA-1)25 and both the FOG-1null and the GATA-1null mutation in mice cause embryonic lethality because of fatal anemia.26,27 The GATA-1low mice were developed in Dr. S. Orkin’s laboratory (Children’s Hospital, Boston, MA)23 through the targeted deletion of regulatory sequences in the GATA-1 locus, that include the DNase hypersensitivity site I (HS I). The mutation totally abolished GATA-1 expression in megakaryocytes and strongly reduced it in erythroid28 and mast cells.29 GATA-1low mice suffer from a life-long severe thrombocytopenia due to complex defects in megakaryocyte maturation, that under several respects resemble those observed in IM patients.30,31 These include abnormal P-selectin localization on the demarcation membrane system, increased neutrophil emperipolesis with the subsequent release in the microenvironment of neutrophil proteases as well as of transforming growth factor-β1 produced by megakaryocytes, and death by para-apoptosis.32 Old (15 months on) GATA-1low mice show features resembling human IM, that include: severe marrow fibrosis, osteosclerosis, thrombocytopenia and tear-drop poikilocytes on blood films, an increased number of circulating hematopoietic progenitors, splenomegaly, and extramedullary hematopoiesis.24
Although alterations of the TPO receptor or of TPO regulatory pathways have not been documented in a well-characterized series of IM patients,33 it has not been addressed thoroughly whether GATA-1 is mutated or abnormally expressed in IM.34 We show here that megakaryocytes from IM patients, either examined in vitro or ex vivo in core biopsies, contain reduced levels of GATA-1 protein when compared to control cells, as it has been reported in the GATA-1low mice.24 Because this deficiency does not involve two other megakaryocytopoietic transcription factors, GATA-2 and FOG-1, we suggest that defective expression of GATA-1 represents a selective abnormality of IM megakaryocytes eventually contributing to the pathogenesis of the disease, and also constitutes a novel marker with potential diagnostic implications.
Patients and Methods
Study Participants
IM patients12 were diagnosed according to the following consensus criteria:35 as obligatory criteria, diffuse marrow fibrosis and absence of BCR-ABL rearrangement; as accessory criteria, splenomegaly, teardrop erythrocytes, circulating immature myeloid cells and erythroblasts, and clusters of abnormal megakaryocytes in BM biopsy. Their clinical features at the time of blood sampling are summarized in Table 1. They were all chemotherapy-free and 7 of 12 received supportive treatment or low-dose prednisone. They all presented high numbers of circulating CD34+ cells (median, 76.5; range, 27 to 800 × 106/L); in three of them (patients 2, 4, 9), CD34+ count was more than the 300 × 106/L value that has been associated with greater risk of leukemic transformation,6 yet at 6 to 9 months after blood sampling for this study their disease was still in chronic phase. On histopathological evaluation, all patients were in a typical fibrotic stage of disease.1 The study included as controls 8 healthy BM donors, 10 patients with solid tumors without BM involvement, and patients with two other Philadelphia-negative CMPDs, PV (10 patients) and ET (10 patients), diagnosed according to the World Health Organization criteria.1 Samples from PV and ET patients were obtained at the time of diagnosis. The study had received the approval from the local Ethical Committee; an informed consent was obtained from each one of the study participants involved at the time of sample collection.
Table 1.
Patients’ Characteristics at the Time of Blood Sampling for This Study
| Patient no./sex | Lillie score13 | Time since diagnosis (years) | RBC transfusion requirement | Circulating CD34+ cells (×106/L) | White blood cells (×109/L) | Hemoglobin (g/L) | Platelets (×109/L) |
|---|---|---|---|---|---|---|---|
| 1/M | 1 | 0.2 | No | 38 | 7.5 | 128 | 213 |
| 2/F | 1 | 5 | Yes | 468 | 2.2 | 58 | 7 |
| 3/F | 0 | 2 | Yes | 98 | 4.7 | 105 | 81 |
| 4/M | 0 | 3 | No | 379 | 9.4 | 113 | 350 |
| 5/M | 1 | 2 | Yes | 55 | 17.7 | 85 | 226 |
| 6/F | 1 | 1 | No | 45 | 37.7 | 121 | 472 |
| 7/M | 0 | 1 | Yes | 38 | 6.7 | 118 | 305 |
| 8/M | 0 | 1.5 | No | 27 | 23.5 | 135 | 198 |
| 9/M | 1 | 2 | Yes | 800 | 11.8 | 95 | 120 |
| 10/M | 2 | 3 | Yes | 180 | 32.2 | 87 | 200 |
| 11/F | 0 | 1 | No | 50 | 13.3 | 128 | 43 |
| 12/M | 2 | 0.5 | Yes | 285 | 2.2 | 79 | 253 |
| Median | 76.5 | 10.6 | 109 | 206 | |||
| (range) | (27 to 800) | (2.2 to 37.7) | (58 to 135) | (7 to 472) |
Cell Purification and Culture
CD34+ cells were purified from 30 to 50 ml of peripheral blood collected from IM patients in ethylenediamine tetraacetic acid tubes or from BM aspirates from healthy donors in preservative-free heparin. In both cases, mononuclear cells were separated over a Ficoll gradient (Lymphoprep; Nycomed Pharma, Oslo, Norway), and processed through two sequential steps of immunomagnetic CD34+ selection (Miltenyi Biotech, Gladbach, Germany). The purity of the CD34+ cell population isolated with this procedure was assessed by flow cytometry (FACScan flow cytometer; Becton Dickinson, San Diego, CA) after labeling with a phycoerythrin-conjugated anti-CD34 monoclonal antibody (PE-HPCA2, Becton-Dickinson). Aliquots of CD34+ cells were either immediately resuspended in lysis buffer for RNA purification (see below) or placed in culture. Liquid cultures were established in six-well plates by culturing 105 CD34+ cells/ml in serum-free medium (StemSpan; StemCell Technologies; Vancouver, Canada) supplemented with either human recombinant TPO (50 ng/ml), for unilineage megakaryocyte differentiation, or human recombinant erythropoietin (3 U/ml), stem cell factor (2.5 ng/ml), and interleukin-3 (5 ng/ml; all from Sigma-Aldrich, St. Louis, MO), for erythroid differentiation. The cultures were supplemented with fresh cytokines every other day. After 14 to 16 days of culture, ≥70% or ≥80% of the cells in the two systems expressed megakaryocytic (CD41/CD61) or erythroid (glycophorin-A; GPA) markers, respectively, by fluorescence-activated cell sorting analysis. Erythroid and megakaryocytic cells were further purified by immunomagnetic selection with anti-GPA- or anti-CD61-coated magnetic microbeads (Miltenyi Biotech).
mRNA Analysis
Total RNA was isolated from purified cells with a guanidine thiocyanate/phenol solution (Trizol; Gibco BRL, Paisley, UK) and quantified by UV absorbance at 280/260 nm. The expression level of genes of interest was determined by quantitative real-time polymerase chain reaction (PCR). Total RNA (1 μg) was reverse-transcribed with random hexamers and the resulting cDNA (50 ng/tube) was amplified in a ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA) using the following parameters: 40 cycles of a two-step PCR program at 95°C for 15 seconds and 60°C for 60 seconds, after an initial denaturation/activation step at 95°C for 10 minutes. The sequence of primers for GATA-1, GATA-2, and FOG-1, and of corresponding probes labeled with TAMRA-FAM, is reported in Table 2. To normalize expression levels in different cell samples, mRNA of the housekeeping GAPDH gene was concurrently amplified (Pre-Developed TaqMan Assay Reagent, Applied Biosystem). The SDS software was used to analyze fluorescent signals and to calculate the cycle threshold (CT); quantitative normalization of cDNA in each sample was obtained by the ΔCT method (target gene CT − GAPDH CT). Each sample was assayed in triplicate, and both a negative (mock cDNA) and a positive (cDNA from cord blood mononuclear cells) control were used in each assay for reproducibility accuracy.
Table 2.
Primers Used for PCR Analysis
| GATA-1 forward primer: 5′ CAAGAAGCGCCTGATTGTCAG 3′ |
| GATA-1 reverse primer: 5′ AGTGTCGTGGTGGTCGTCTG 3′ |
| GATA-1 probe: 5′ AAACGGGCAGGTACTCAGTGCACCAACT 3′ |
| Amplified fragment size: 71 bp |
| GATA-2 forward primer: 5′ ATCCAGACTCGGAACCGGA 3′ |
| GATA-2 reverse primer: 5′ GCACTTTGACAGCTCCTCGAA 3′ |
| GATA-2 probe: 5′ ATGTCCAACAAGTCCAAGAAGAGCAAGAAAGG 3′ |
| Amplified fragment size: 83 bp |
| FOG-1 forward primer: 5′ TTGCCACCGCAGTGATCA 3′ |
| FOG-1 reverse primer: 5′ GCTCGCTGCGGTACCAGAT 3′ |
| FOG-1 probe: 5′ AGACGTCTTCCCCTGCAAGGACTGTG 3′ |
| Amplified fragment size: 68 bp |
| GATA-1 alternative transcripts36 |
| Exon 1 forward primer: 5′ AGGACACCCCCTGGGATC 3′ |
| Exon 3 reverse primer: 5′ CTCCATACAGTTGAGCAATGGG 3′ |
| Amplified fragment size (full-length cDNA): 384 bp |
| Amplified fragment size (alternative splice variant cDNA): 109 bp |
| JAK2 mutation analysis16 |
| Reverse primer: 5′ CTGAATAGTCCTACAGTGTTTTCAGTTTCA 3′ |
| Forward (specific): 5′ AGCATTTGGTTTTAAATTATGGAGTATATT 3′ |
| Forward (internal control): 5′ ATCTATAGTCATGCTGAAAGTAGGAGAAAG 3′ |
The expression of the two human GATA-1 mRNA transcripts was assessed by reverse transcriptase (RT)-PCR, starting from 200 ng of cDNA, using a forward primer from exon 1 and a reverse primer from exon 3 (Table 2).36 Amplification was performed at 63°C in a GeneAmp 9700 Perkin-Elmer thermocycler and samples were analyzed after 25, 28, 31, and 33 cycles to be in the linear range of amplification. Positive and negative controls were included in each experiment. Amplified products were resolved by 2.5% agarose gel electrophoresis and stained with ethidium bromide; UV-light images were acquired and analyzed with the Kodak EDAS290 analyzer (Eastman Kodak Company, New Haven, CT).
Protein Analysis
To cope with the limiting number of purified cells obtained from a single individual, an equal number of cells was pooled from each of three (IM patients) to four (control) patients. Proteins were extracted from CD34+, GPA+, and CD61+ cells in the presence of protease inhibitor cocktail (Sigma Aldrich). Aliquots containing ≃50 μg of proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Hybond ECL-nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ). After blocking, the blots were incubated with either the rat monoclonal anti-GATA-1 (0.8 μg/ml, N6) or anti-GATA-2 antibody (H-116; both from Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with peroxidase-conjugated rabbit anti-rat secondary antibody. In some experiments, blots were also reprobed with a goat polyclonal antibody (C20) that recognizes the C-terminus of GATA-1 with the aim to identify the shorter isoform. The blots were developed with enhanced chemiluminescence (West Pico; Pierce, Rockford, IL), and exposed to ECL-hyperfilms (Amersham) for 1 to 5 minutes. Protein loading was checked by reprobing stripped blots with goat anti-actin antibody (sc-1615, Santa Cruz); relative quantification of GATA-1/2 versus actin levels was obtained by scanning the films with the Kodak EDAS290 analyzer.
GATA-1 Sequencing
DNA was extracted from purified CD34+; 100 ng of DNA were amplified using experimental conditions and primers described.37 PCR products were prepared with the QIAquick PCR purification kit (Qiagen, GmbH, Germany) and sequenced directly using the BigDye terminator system and the ABI 310 analyzer (Applied Biosystem).
JAK2 Mutation Analysis
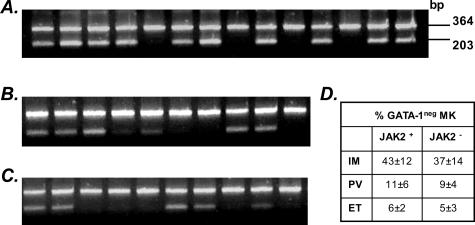
This was performed essentially as described.16 Briefly, 100 ng of DNA purified from granulocytes was amplified at 58°C for 38 cycles in an allele-specific PCR (the sequence of primers is reported in Table 2); amplified fragments were resolved in a 2.5% agarose gel and visualized under ethidium-bromide staining. A 364-bp amplified product was derived from both normal and mutant allele whereas the 203-bp product specifically originated from the mutant allele.
Immunohistochemistry on BM Biopsies
BM biopsies were obtained by standard procedures and embedded in paraffin. After antigen retrieval, slides were incubated with monoclonal rat anti-GATA-1 or polyclonal rabbit anti-FOG-1 (kindly donated by Dr. K. Freson, University of Leuven, Leuven, Belgium)38 antibody. Immunoreactivity was developed with the Ultrastain polyvalent peroxidase immunostaining kit (Ylem, Rome, Italy). Slides not incubated with the primary antibody served as negative controls. Sections were analyzed with a Leica Light Microscope (Leica LTD, Heidelberg, Germany) equipped with a Coolsnap video camera for computerized images (RS Photometrics, Tucson, AZ). To minimize staining variability, slides from both controls and IM patients were concurrently processed and independently evaluated by two observers who were blinded of clinical diagnosis (observer intervariability <10%). GATA-1/FOG-1 staining was determined by analyzing all of the megakaryocytes found in randomly selected fields at ×40 up to at least 100 megakaryocytes/sample. Megakaryocytes were classified as GATA-1pos if they showed clearly stained nuclei ± cytoplasm, and GATA-1neg if both nucleus and cytoplasm exhibited no to very faint immunoreactivity.
Statistical Analysis
Statistical evaluations of data were performed using the SPSS statistical software package (release 11.5.1; SPSS, Chicago, IL). Analysis of variance, Mann-Whitney U-test, or Fisher exact test were used for direct comparison, as appropriate. For all of the comparisons, the P value was two-tailed, and a P < 0.05 was exhibited as statistically significant.
Results
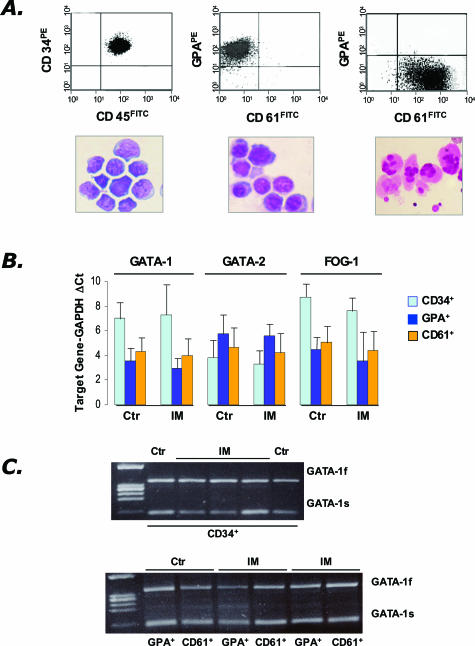
Due to the high number of circulating CD34+ cells, in all of the IM patients included in this study it was feasible to isolate to high purity (97 ± 2%) a number of CD34+ cells (range, 2.1 to 20 × 106) sufficient for RNA analysis and for seeding unilineage cultures. At the end of the culture, purified erythroid (GPA+, purity 94 ± 3%) and megakaryocytic (CD61+, purity 93 ± 4%) cells in number sufficient for further analysis (range, 0.9 to 3.3 × 106 cells starting from 0.2 to 4 × 106 CD34+ cells) were obtained from all of the normal donors (n = 8) and from 11 of 12 and 10 of 12 IM patients, respectively. Representative images of fluorescence-activated cell sorting histograms and morphology of the purified cell populations analyzed are shown in Figure 1A.
Figure 1.
Analysis of GATA-1 mRNA in CD34+ cells purified from the peripheral blood of IM patients or the BM of controls, and in their erythroid (GPA+) or megakaryocyte (CD61+) progeny obtained in vitro. A: Purity of the isolated cell fractions (CD34+, GPA+, CD61+, from the left) was re-evaluated by fluorescence-activated cell sorting analysis (top), whereas morphological appearance was assessed on May-Grümwald/Giemsa-stained cytosmears (bottom). B: Quantification of GATA-1, GATA-2, and FOG-1 mRNA levels by real-time RT-PCR analysis. A total of 8 controls (for all different cell populations), and 12, 11, and 10 IM patients, respectively, for CD34+, GPA+, and CD61+ cells were analyzed. Results are expressed as mean (±SD; each sample assayed in triplicate) ΔCt for each target gene after normalization for the amplification level of the housekeeping GAPDH gene; lower ΔCt values indicate higher target gene expression levels. C: RT-PCR amplification of the full-length (GATA-1f) and the shorter (GATA-1s) GATA-1 transcript starting from mRNA of CD34+, GPA+, and CD61+ cells. The results are representative of those obtained with cells purified from a total of four controls and seven IM patients. Amplicons were analyzed during the exponential phase of PCR (cycles = 31). The lane on the left contains molecular weight markers (marker IX).
The results of real-time PCR analysis for the quantitative determination of GATA-1, FOG-1, and GATA-2 mRNA levels are presented in Figure 1B. GATA-1 was expressed at significantly higher levels (P = 0.013) in GPA+ and CD61+ cells than in CD34+ cells in both controls (ΔCt: 3.6 ± 0.4, 4.3 ± 1.1, and 7.0 ± 0.9, respectively) and IM patients (ΔCt: 3.0 ± 0.8, 4.0 ± 0.7, and 7.2 ± 2.3, respectively), with no difference between the two groups of subjects. GATA-2 mRNA levels were slightly higher in the CD34+ cells than in their differentiated progeny, and behaved similarly in controls and IM patients: ΔCt was 3.8 ± 0.8, 5.7 ± 1.0, 4.6 ± 0.9, respectively, in the CD34+, GPA+, and CD61+ cells of controls, and 3.3 ± 0.8, 5.6 ± 1.6, 4.2 ± 1.1, respectively, in IM patients. Finally, approximately comparable FOG-1 mRNA levels were found in GPA+ and CD61+ cells, which were both significantly higher than in CD34+ cells (P = 0.035): ΔCt was 4.4 ± 0.9, 5.1 ± 1.0, 8.6 ± 1.7, respectively, in the controls and 3.5 ± 0.3, 4.4 ± 0.9, and 7.6 ± 1.4, respectively, in IM patients.
The human GATA-1 mRNA exists as a full-length and a short-length transcript, whose relative ratio might be relevant for the control of megakaryocyte proliferation and differentiation.39 To evaluate whether both isoforms were transcribed in IM patients, we performed a RT-PCR using primers that allow the contemporary amplification of both transcripts. Results presented in Figure 1C show that no obvious differences in the amplification profile of the two GATA-1 mRNAs could be demonstrated in cells isolated from IM patients as compared to controls. Finally, to check for possible mutations in the coding sequence of the GATA-1 gene, GATA-1 exons 1 to 6 were amplified and directly sequenced using DNA isolated from CD34+ cells from seven IM patients and four normal controls. In all cases, the resulting sequences were identical to those published (National Cancer Institute; accession number, NM 002049).
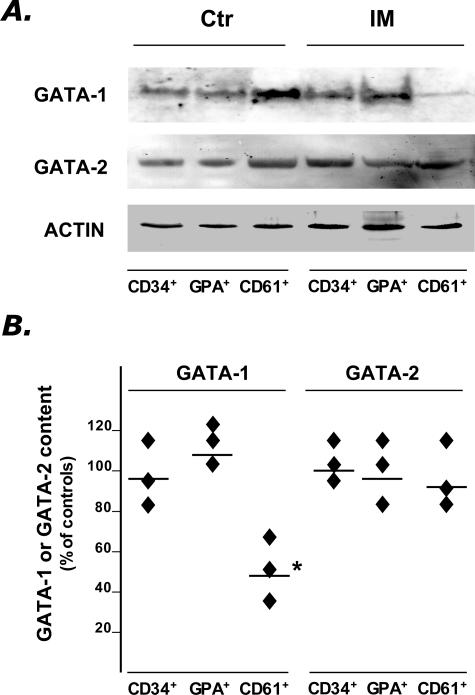
Because the above results indicated that the transcriptional activity of neither GATA-1, GATA-2, or FOG-1 is impaired in any of the cellular populations examined, we searched for possible alterations in the posttranscriptional processing of GATA-1 (and of GATA-2, as control) by analyzing content of these proteins by Western blot analysis. A band of the expected molecular weight for the full-length GATA-1 (≃ 46 kd) was detected in all purified cell populations obtained from IM patients and normal donors (Figure 2A), unlike the shorter transcript that was hardly discernible using an anti-C-terminus antibody (not shown in detail). However, the amount of GATA-1 in megakaryocytes from IM patients was significantly lower (by 44 ± 12%, P = 0.02) than in the corresponding cells from normal donors, while the content of the protein in CD34+ and GPA+ cells was approximately comparable to control (Figure 2B). The GATA-2 content was also evaluated in the same cell preparations, and found similar in cells from IM patients and healthy subjects (Figure 2, A and B), suggesting that the reduced GATA-1 content in IM megakaryocytes might reflect a selective alteration in its posttranscriptional processing in these cells.
Figure 2.
Western blot analysis of GATA-1 and GATA-2 protein in purified CD34+, GPA+, and CD61+ cells obtained from IM patients and controls. A: Due to the low amount of protein recovered, pools of three IM patients or four controls were prepared by mixing equal numbers of cells purified from each patient. A representative blot from one normal and one IM pool is shown in A, while the percentage changes of GATA-1 and GATA-2 protein content in the three IM pools (as compared to the two control pools), after normalization for the actin content, is shown in B. Horizontal lines indicate the mean values. *P = 0.02. Films in A were exposed for different times (5 minutes for GATA-1 and GATA-2, 45 seconds for actin).
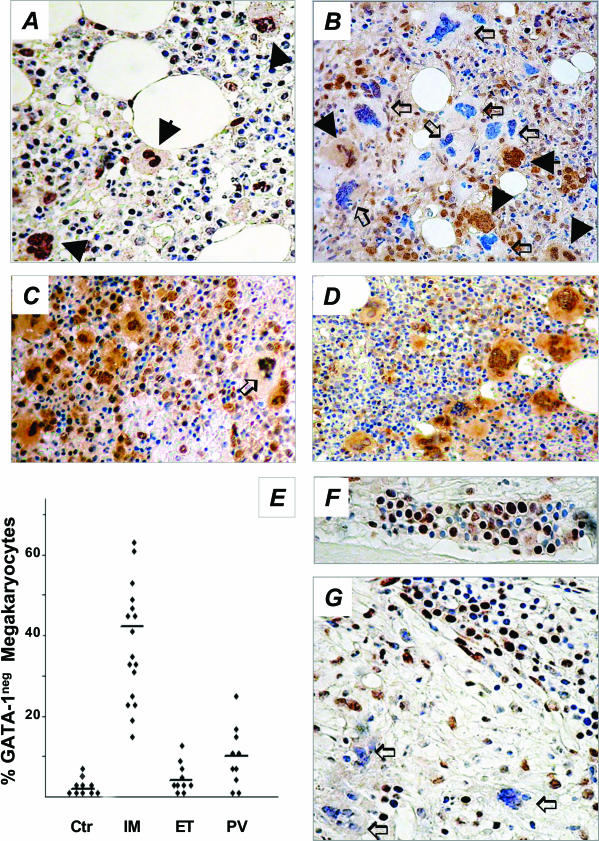
To rule out that the reduced protein levels in IM megakaryocytes might be limited to cells obtained in culture, the GATA-1 content in ex vivo BM megakaryocytes was evaluated by immunohistochemistry. We used core biopsies obtained from 17 IM patients (patients 1 to 8 of Table 1, plus an additional 9 randomly selected patients’ biopsies from the repository of our institutions) and 10 patients with solid neoplasia but no evidence of BM involvement (normal controls). Because megakaryocyte hyperproliferation, a hallmark of IM, can also be found in two other Philadelphia-negative CMPDs, PV and ET, BM biopsies from 10 patients with either of these diseases were analyzed as well.
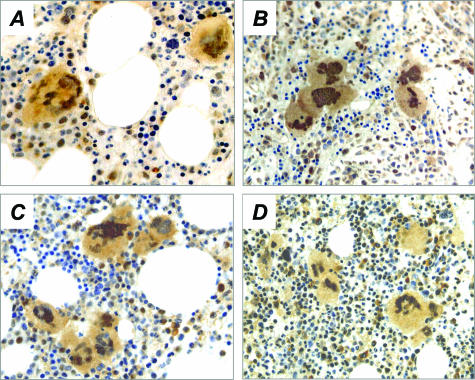
In control samples, GATA-1 immunoreactivity was reproducibly appreciated in the nucleus of all morphologically recognizable megakaryocytes (Figure 3A); a faint stain could also be observed in the cytoplasm, irrespective of the degree of cell maturity. On the contrary, the staining pattern of megakaryocytes from IM patients was clearly less homogeneous, because 44.5% of megakaryocytes (range, 18 to 67%) failed to stain with GATA-1 antibody, or showed very faint nuclear/cytoplasm staining (Figure 3; B, E, and G), as compared to 2.1% (range, 0 to 5) found in normal subjects (P = 0.01) (Figure 3, A and E). These GATA-1neg megakaryocytes could be found scattered in the BM, and were often quite close to strongly stained megakaryocytes within the same cluster (Figure 3B). On the other hand, early erythroid cells from both normal donors and IM patients showed strong staining (Figure 3, F and G). GATA-1neg megakaryocytes were detected only occasionally in ET patients (4.0%, range, 1 to 13), whereas, interestingly, they were increased, although not significantly, in some patients with PV (11.0%, range, 1 to 25) (Figure 3; C to E). Finally, the staining pattern of FOG-1 in the same BM samples used for GATA-1 staining was also evaluated. Representative images are presented in Figure 4, and show that virtually all IM megakaryocytes (Figure 4B) as well as those from controls (Figure 4A) and PV or ET patients (Figure 4, C and D), had a strong uniform staining for FOG-1 both in the nucleus and the cytoplasm.
Figure 3.
Immunohistochemistry analysis of GATA-1 in BM biopsies from IM patients (B, G) or controls (A, F). In A, all morphologically recognizable megakaryocytes (filled arrows) are strongly stained in the nucleus, with some cytoplasmic staining. On the other hand, the heterogeneity typically observed in IM samples, with a combination of megakaryocytes normally stained (filled arrows) or showing no to very faint nucleus/cytoplasmic staining (open arrows) (GATA-1neg megakaryocytes) can be appreciated in B. The staining pattern in PV or ET patients is shown in C and D, respectively. The percentage of GATA-1neg megakaryocytes in controls (n = 10) and in patients with either IM (n = 17), ET, or PV (n = 10 for both) is shown in E. Early erythroid cells in a typical erythroid island of a normal patient (F) are heavily stained, whereas more mature erythroblasts show weaker staining; the same pattern can be appreciated in the section from an IM patient (G), in which GATA-1pos erythroblasts appear surrounded by GATA-1neg megakaryocytes (open arrows). Original magnifications, ×200.
Figure 4.
Immunohistochemistry analysis of FOG-1 in BM biopsies from controls (A) or patients with IM (B), PV (C), or ET (D). Megakaryocytes and rare erythroid cells appear strongly and similarly stained with FOG-1 antibody. Original magnifications, ×400.
We finally evaluated the JAK2 mutational status in 14 of the 17 patients with IM whose biopsies were available for the study, and in each of the 10 patients with either PV or ET, with the aim to search for any correlation with GATA-1 expression. As shown in Figure 5, 10 of 14 IM patients presented the JAK2 mutation using an allele-specific PCR approach, as compared to 6 of 10 and 5 of 10 for PV or ET, respectively;16 none of 20 normal subjects used as controls had the JAK2 mutation. However, there was no correlation between the presence of JAK2 mutation and the percentage of GATA-1neg megakaryocytes in BM biopsies (Figure 5D).
Figure 5.
JAK2 mutation analysis in patients with IM (A), PV (B), or ET (C). The presence of the Val617Phe JAK2 mutation was analyzed by an allele-specific PCR; the 203-bp product is specifically amplified from the mutant allele, while the 364-bp product serves as an internal control for both mutant and normal allele. The percentage of GATA-1neg megakaryocytes according to the presence or not of JAK2 mutation is reported in D.
Discussion
The mechanisms underlying the aberrant proliferation of hematopoietic cells in Philadelphia-negative CMPDs, including PV, ET, and IM, have remained primarily unknown, notwithstanding a number of cellular abnormalities have been well characterized. However, the recent description of an acquired mutation of the JAK2 gene,16–18 which has been found in a variable proportion of patients with the different clinical forms of CMPD, will probably permit a more focused approach to disease pathogenesis in the near future. Among CMPDs, IM is characterized by well-defined and specific alterations in the proliferation and differentiation of megakaryocytes. Mutations in the GATA-1 gene have recently been associated with inherited40 and acquired41 human pathologies that present defective megakaryocytopoiesis; the latter include the transient myeloproliferative disorder of the newborn, the megakaryoblastic leukemia of Down syndrome, and at least a sporadic case of megakaryoblastic leukemia.42 These diseases are also characterized by a conspicuous BM fibrosis that accompanies the proliferation of abnormal megakaryocyte precursors. Interestingly, a role for defective GATA-1 expression in the pathogenesis of IM has been advocated following the observation that the GATA-1low mutation induces in mice a syndrome that recapitulates several aspects of the human disease.
Our aim was to clarify whether megakaryocytes from IM patients would present abnormalities in GATA-1. To this end, and owing the difficulties in obtaining ex vivo megakaryocytes from the BM that is almost invariably inaspiratable in the fibrotic stage of disease, CD34+ cells were purified from the peripheral blood and induced to differentiate toward either the erythroid (as control) or the megakaryocytic lineage in vitro. Levels of GATA-1 mRNA and protein were analyzed in highly purified early hematopoietic progenitors cells (CD34+), as wells as in their erythroid (GPA+) and megakaryocytic (CD61+) progeny. The results were compared with those obtained with the corresponding cell populations from healthy donors. The levels of GATA-1 mRNA, as measured by a sensitive real-time PCR assay, resulted similar to controls in any of the cell populations analyzed, as well as it was the content of both GATA-2 and FOG-1 mRNA. Although no data concerning GATA-2 expression in IM has yet been published to the best of our knowledge, our results differ from those of Martyrè and colleagues34 who reported increased expression of FOG-1 in peripheral blood CD34+ cells using a semiquantitative PCR technique unlike a real-time RT-PCR assay as we did; these technical differences might at least partially help to explain these discrepancies. On the contrary, GATA-1 protein content by Western blot analysis was significantly reduced in megakaryocytes obtained from IM patients, but not in their CD34+ or GPA+ cells. Most significantly, the reduction in GATA-1 content in IM megakaryocytes was not dependent on in vitro culture conditions, because the levels of both mRNA and protein for GATA-2 were otherwise normal. Furthermore, immunohistochemical analyses on core biopsies confirmed that also ex vivo megakaryocytes from IM patients contain reduced levels of GATA-1 protein, while they normally express FOG-1. These data support the contention that megakaryocytes from IM patients show defects in term of low GATA-1 content similar to the megakaryocytes from GATA-1low mice, and reinforce the view that this abnormality might represent a key event involved in the pathogenesis of the disease. However, although in GATA-1low mice defective GATA-1 expression is the consequence of the deletion of megakaryocytic-specific regulatory sequences, the mechanism(s) responsible for the reduced levels of GATA-1 protein in IM megakaryocytes are less clear, owing to the normal mRNA levels found in these cells. Because mutations were not detected in the coding region of GATA-1, we can predict a normal primary and secondary structure of the protein, making unlikely changes in the phosphorylation or acetylation status that may affect its half-life. Furthermore, because the full-length and the short-length GATA-1 mRNA isoforms were both transcribed in normal ratio, but only the full-length GATA-1 protein was detectable by Western blots in both IM and controls also using an antibody that recognizes the C-terminus of the protein, the mechanisms leading to abnormal megakaryocytopoiesis in IM are clearly different from those implicated in the Down-associated megakaryoblastic leukemia, in which the shorter transcript is prevalent or absolute. These considerations point to a defect in trans to the GATA-1 gene as the most likely mechanism underlying the deficiency of GATA-1 protein in IM megakaryocytes; this might involve a GATA-1 partner still to be identified, that would prevent GATA-1 degradation by the proteosome machinery of the megakaryocytes. These aspects are being addressed in ongoing studies.
The observation that, among CMPD patients, defective GATA-1 staining was consistently associated with megakaryocytes from IM patients, and not with those from either PV or ET patients, not only stresses the specificity of the observation, but also suggests that GATA-1 staining of core biopsies might represent a diagnostic tool to discriminate IM, and eventually the prefibrotic stages of disease, from the other CMPDs. In this regard, it is interesting that some of PV patients showed increased frequencies of GATA-1neg megakaryocytes; although this difference was not statistically significant, it might point to a subgroup of PV patients that would eventually evolve to IM. Prospective studies are required to confirm this hypothesis.
Recently, a point mutation in exon 12 of JAK2 kinase has been described as a molecular lesion shared by the different clinical entities of Philadelphia-negative CMPDs.16–18 We found that 10 of 14 IM patients (76%) evaluated carried this mutation, as well as 60% and 50% of 10 patients with either PV or ET, respectively. The Val617Phe mutation results in constitutive tyrosine phosphorylation activity because of the disruption of the autoinhibitory activity of JAK2,17 thereby promoting cytokine hypersensitivity. Because GATA-1 may be a downstream target of JAK2 after the binding of TPO to its receptor c-MPL, we aimed at correlating the presence or not of the JAK2 mutation with the percentage of GATA-1neg megakaryocytes in BM biopsies. Results obtained do not actually support a downstream pathogenetic relationship between JAK2 mutation and the abnormalities in GATA-1 content in megakaryocytes.
In summary, the data presented demonstrate that megakaryocytes from patients with IM contain reduced levels of GATA-1 protein, and suggest that this abnormality is specifically disease-associated and might play a role in its pathogenesis.
Acknowledgments
We thank all colleagues who referred patients for this study, Dr. G. Longo for collaboration in the statistical analysis of data, Dr. C. Doglioni (Scientific Institute San Raffaele, Milano, Italy) for performing confirmatory GATA-1 staining on three additional IM patients not included in this study, and Dr. K. Freson for kindly providing the rabbit anti-human FOG-1 antibody.
Footnotes
Address reprint requests to Prof. Alessandro M. Vannucchi, Department of Hematology, Careggi Hospital, University of Florence, 50134 Florence, Italy. E-mail: amvannucchi@unifi.it.
Supported by the Italian Ministry of Health (Progetti di Ricerca di Interesse Nazionale 2002, no. 06103241; 2003, no. 06488803); the Associazione Italiana per la Ricerca sul Cancro, Milano; the National Program on Stem Cells; Associazione Italiana Leucemie, Firenze (fellowship to A.P.); and the Fondazione Italiana per la Ricerca sul Cancro, Milano (fellowship to L.B.).
References
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- Tefferi A. The forgotten myeloproliferative disorder: myeloid metaplasia. Oncologist. 2003;8:225–231. doi: 10.1634/theoncologist.8-3-225. [DOI] [PubMed] [Google Scholar]
- O’Brien S, Tefferi A, Valent P. Chronic myelogenous leukemia and myeloproliferative disease. Hematology (Am Soc Hematol Educ Program) 2004:146–162. doi: 10.1182/asheducation-2004.1.146. [DOI] [PubMed] [Google Scholar]
- Hoffman R. Agnogenic myeloid metaplasia. Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen H, Silberstein LE, McGlave P, editors. New York: Churchill Livingstone,; Hematology. Basic Principles and Practice. 2000:pp 1172–1188. [Google Scholar]
- Xu M, Bruno E, Chao J, Ni H, Lindgren V, Nunez R, Mahmud N, Finazzi G, Fruchtman SM, Popat U, Liu E, Prchal JT, Rondelli D, Barosi G, Hoffman R. The constitutive mobilization of bone marrow repopulating cells into the peripheral blood in idiopathic myelofibrosis. Blood. 2005;105:4508–4515. doi: 10.1182/blood-2004-06-2485. [DOI] [PubMed] [Google Scholar]
- Barosi G, Viarengo G, Pecci A, Rosti V, Piaggio G, Marchetti M, Frassoni F. Diagnostic and clinical relevance of the number of circulating CD34(+) cells in myelofibrosis with myeloid metaplasia. Blood. 2001;98:3249–3255. doi: 10.1182/blood.v98.12.3249. [DOI] [PubMed] [Google Scholar]
- Arora BSS, Hoyer JD, Mesa RA, Tefferi A. Peripheral blood CD34 count in myelofibrosis with myeloid metaplasia: a prospective evaluation of prognostic value in 94 patients. Br J Haematol. 2004;128:42–48. doi: 10.1111/j.1365-2141.2004.05280.x. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single institution experience with 91 cases. Blood. 2005;105:973–977. doi: 10.1182/blood-2004-07-2864. [DOI] [PubMed] [Google Scholar]
- Cervantes F, Tassies D, Salgado C, Rovira M, Pereira A, Rozman C. Acute transformation in nonleukemic chronic myeloproliferative disorders: actuarial probability and main characteristics in a series of 218 patients. Acta Haematol. 1991;85:124–127. doi: 10.1159/000204873. [DOI] [PubMed] [Google Scholar]
- Castro-Malaspina H, Jhanwar SC. Properties of myelofibrosis-derived fibroblasts. Prog Clin Biol Res. 1984;154:307–322. [PubMed] [Google Scholar]
- Martyre MC, Romquin N, Le Bousse-Kerdiles MC, Chevillard S, Benyahia B, Dupriez B, Demory JL, Bauters F. Transforming growth factor-beta and megakaryocytes in the pathogenesis of idiopathic myelofibrosis. Br J Haematol. 1994;88:9–16. doi: 10.1111/j.1365-2141.1994.tb04970.x. [DOI] [PubMed] [Google Scholar]
- Chagraoui H, Komura E, Tulliez M, Giraudier S, Vainchenker W, Wendling F. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood. 2002;100:3495–3503. doi: 10.1182/blood-2002-04-1133. [DOI] [PubMed] [Google Scholar]
- Dupriez B, Morel P, Demory JL, Lai JL, Simon M, Plantier I, Bauters F. Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system. Blood. 1996;88:1013–1018. [PubMed] [Google Scholar]
- Fruchtman SM. Transplant decision-making strategies in the myeloproliferative disorders. Semin Hematol. 2003;40:30–33. doi: 10.1053/shem.2003.50032. [DOI] [PubMed] [Google Scholar]
- Reilly JT. Cytogenetic and molecular genetic aspects of idiopathic myelofibrosis. Acta Haematol. 2002;108:113–119. doi: 10.1159/000064708. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D’Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Migliaccio AR, Paoletti F, Chagraoui H, Wendling F: Pathogenesis of myelofibrosis with myeloid metaplasia: lessons from mouse models of the disease. Semin Oncol (in press) [DOI] [PubMed] [Google Scholar]
- Villeval JL, Cohen-Solal K, Tulliez M, Giraudier S, Guichard J, Burstein SA, Cramer EM, Vainchenker W, Wendling F. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood. 1997;90:4369–4383. [PubMed] [Google Scholar]
- Yan XQ, Lacey D, Fletcher F, Hartley C, McElroy P, Sun Y, Xia M, Mu S, Saris C, Hill D, Hawley RG, McNeice IK. Chronic exposure to retroviral vector encoded MGDF (mpl-ligand) induces lineage-specific growth and differentiation of megakaryocytes in mice. Blood. 1995;86:4025–4033. [PubMed] [Google Scholar]
- Yan XQ, Lacey D, Hill D, Chen Y, Fletcher F, Hawley RG, McNiece IK. A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood. 1996;88:402–409. [PubMed] [Google Scholar]
- McDevitt MA, Fujiwara Y, Shivdasani RA, Orkin SH. An upstream, DNase I hypersensitive region of the hematopoietic-expressed transcription factor GATA-1 gene confers developmental specificity in transgenic mice. Proc Natl Acad Sci USA. 1997;94:7976–7981. doi: 10.1073/pnas.94.15.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi AM, Bianchi L, Cellai C, Paoletti F, Rana RA, Lorenzini R, Migliaccio G, Migliaccio AR. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice). Blood. 2002;100:1123–1132. doi: 10.1182/blood-2002-06-1913. [DOI] [PubMed] [Google Scholar]
- Cantor AB, Orkin SH. Hematopoietic development: a balancing act. Curr Opin Genet Dev. 2001;11:513–519. doi: 10.1016/s0959-437x(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Tsang AP, Fujiwara Y, Hom DB, Orkin SH. Failure of megakaryopoiesis and arrested erythropoiesis in mice lacking the GATA-1 transcriptional cofactor FOG. Genes Dev. 1998;12:1176–1188. doi: 10.1101/gad.12.8.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MJ, Keller G, Orkin SH. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 1994;8:1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Shivdasani RA, Fujiwara Y, Yang H, Orkin SH. A “knockdown” mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc Natl Acad Sci USA. 1997;94:6781–6785. doi: 10.1073/pnas.94.13.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AR, Rana RA, Sanchez M, Lorenzini R, Centurione L, Bianchi L, Vannucchi AM, Migliaccio G, Orkin SH. GATA-1 as a regulator of mast cell differentiation revealed by the phenotype of the GATA-1low mouse mutant. J Exp Med. 2003;197:281–296. doi: 10.1084/jem.20021149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Jouault H, Guichard J, Wendling F, Drouin A, Cramer EM. Pathologic interaction between megakaryocytes and polymorphonuclear leukocytes in myelofibrosis. Blood. 2000;96:1342–1347. [PubMed] [Google Scholar]
- Thiele J, Kuemmel T, Sander C, Fischer R. Ultrastructure of bone marrow tissue in so-called primary (idiopathic) myelofibrosis-osteomyelosclerosis (agnogenic myeloid metaplasia). I. Abnormalities of megakaryopoiesis and thrombocytes. J Submicrosc Cytol Pathol. 1991;23:93–107. [PubMed] [Google Scholar]
- Centurione L, Di Baldassarre A, Zingariello M, Bosco D, Gatta V, Rana RA, Langella V, Di Virgilio A, Vannucchi AM, Migliaccio AR. Increased and pathological emperipolesis of neutrophils within megakaryocytes associated with marrow fibrosis in GATA-1low mice. Blood. 2004;104:3573–3580. doi: 10.1182/blood-2004-01-0193. [DOI] [PubMed] [Google Scholar]
- Taksin AL, Couedic JP, Dusanter-Fourt I, Masse A, Giraudier S, Katz A, Wendling F, Vainchenker W, Casadevall N, Debili N. Autonomous megakaryocyte growth in essential thrombocythemia and idiopathic myelofibrosis is not related to a c-mpl mutation or to an autocrine stimulation by Mpl-L. Blood. 1999;93:125–139. [PubMed] [Google Scholar]
- Martyre MC, Steunou V, LeBousse-Kerdilès MC, Wietzerbin J, Vannucchi AM, Migliaccio AR. Lack of alterations in GATA-1 expression in CD34+ hematopoietic progenitors from patients with idiopathic myelofibrosis. Blood. 2003;101:5087–5089. doi: 10.1182/blood-2002-11-3366. [DOI] [PubMed] [Google Scholar]
- Barosi G, Ambrosetti A, Finelli C, Grossi A, Leoni P, Liberato NL, Petti MC, Pogliani E, Ricetti M, Rupoli S, Visani G, Tura S. The Italian Consensus Conference on Diagnostic Criteria for Myelofibrosis with Myeloid Metaplasia. Br J Haematol. 1999;104:730–737. doi: 10.1046/j.1365-2141.1999.01262.x. [DOI] [PubMed] [Google Scholar]
- Rainis L, Bercovich D, Strehl S, Teigler-Schlegel A, Stark B, Trka J, Amariglio N, Biondi A, Muler I, Rechavi G, Kempski H, Haas OA, Izraeli S. Mutations in exon 2 of GATA1 are early events in megakaryocytic malignancies associated with trisomy 21. Blood. 2003;102:981–986. doi: 10.1182/blood-2002-11-3599. [DOI] [PubMed] [Google Scholar]
- Nichols KE, Crispino JD, Poncz M, White JG, Orkin SH, Maris JM, Weiss MJ. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat Genet. 2000;24:266–270. doi: 10.1038/73480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freson K, Thys C, Wittewrongel C, Vermylen J, Hoylaerts MF, Van Geet C. Molecular cloning and characterization of the GATA1 cofactor human FOG1 and assessment of its binding to GATA1 proteins carrying D218 substitutions. Hum Genet. 2003;112:42–49. doi: 10.1007/s00439-002-0832-1. [DOI] [PubMed] [Google Scholar]
- Calligaris R, Bottardi S, Cogoi S, Apezteguia I, Santoro C. Alternative translation initiation site usage results in two functionally distinct forms of the GATA-1 transcription factor. Proc Natl Acad Sci USA. 1995;92:11598–11602. doi: 10.1073/pnas.92.25.11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman JG. Inherited thrombocytopenia: when a low platelet count does not mean ITP. Blood. 2004;103:390–398. doi: 10.1182/blood-2003-05-1742. [DOI] [PubMed] [Google Scholar]
- Gurbuxani S, Vyas P, Crispino JD. Recent insights into the mechanisms of myeloid leukemogenesis in Down syndrome. Blood. 2004;103:399–406. doi: 10.1182/blood-2003-05-1556. [DOI] [PubMed] [Google Scholar]
- Harigae HXG, Sugawara T, Ishikawa I, Toki T, Ito E. The GATA1 mutation in an adult patient with acute megakaryoblastic leukemia not accompanying Down syndrome. Blood. 2004;103:3242–3243. doi: 10.1182/blood-2004-01-0016. [DOI] [PubMed] [Google Scholar]