Abstract
Selectins mediate the initial adhesion of leukocytes to endothelial cells in many contexts of inflammation-dependent leukocyte recruitment. The glycans that contribute to P- and E-selectin counterreceptor activity arise through glycosylation reactions in which the terminal steps are catalyzed by α(1,3) fucosyltransferases (FTs). We examined how selectin ligand activities are controlled in eosinophils by characterizing FT expression profiles and regulatory mechanisms in eosinophils isolated from human blood. We found that FT-IV and FT-VII mRNAs were up-regulated by transforming growth factor-β1, but the FT-IV transcript consistently predominated in eosinophils. To further define the physiological role of FT-IV and FT-VII in expression of eosinophil selectin ligand, we characterized models of dermal eosinophilia in FT-IV- and/or FT-VII-deficient mice in vivo. FT-IV deficiency yielded a significant decrease in eosinophil recruitment to the skin. Likewise, deficiency of FT-VII also yielded a decrease in eosinophil recruitment. Eosinophil recruitment that remained in the absence of FT-VII was further inhibited by blocking P- or E-selectin and was essentially absent in mice deficient in both enzymes. These observations indicate that FT-IV and FT-VII are both important contributors to selectin-dependent eosinophil recruitment to the skin and may represent therapeutic targets for treating diseases in which eosinophil recruitment contributes to pathophysiology.
P- and E-selectins expressed by endothelial cells interact with leukocyte counterreceptors and mediate initial adhesion of leukocytes and their subsequent rolling along endothelial surfaces. One of the major ligands for leukocyte P-selectin is P-selectin glycoprotein-1 (PSGL-1). To become functional, PSGL-1 requires posttranslational modifications such as the sulfation of at least one tyrosine residue in its NH2-terminal tyrosine sulfation motif, modifications of O-linked glycans by core 2 β1, 6-N-acetyl-glucosaminyl transferase (C2GnT)2, the addition of α2,3-linked sialic acid, and the addition of fucose in an α1,3-linkage to generate sialyl-Lewis x (sLex)-related structures.1 E-selectin also interacts with leukocytes through surface sLex-related structures.2 In general, the lineage-specific expression of the α(1,3) fucosyltransferases (FTs) responsible for fucose addition to the α1,3-linkage is critically involved in the expression of selectin counterreceptor activities.
Of six known FTs, FT-IV and FT-VII are expressed in leukocytes. These two enzymes have distinct substrate specificities in Lex and sLex synthesis. FT-IV primarily fucosylates nonsialylated N-acetyllactosamine (LN), which results in Lex. FT-IV also utilizes α2,3-sialylated LN units, but synthesizes only a small amount of sLex under most circumstances.3,4 On the one hand, FT-VII has reduced activity with nonsialylated LN units,3,4 but significant activity in fucosylation of the α2,3-sialylated LN units to generate the HECA452, 2H5, and CSLEX1 epitopes. The HECA-452-reactive epitope also enables PSGL-1 to bind to E-selectin.5
The critical participation of FT-VII in the construction of selectin ligands has been demonstrated by severely impaired P- and E-selectin-dependent leukocyte adhesion/rolling in FT-VII(−/−) mice.6 On the other hand, the importance of FT-IV in selectin ligand synthesis has been less certain. Whereas CHO cells transfected with PSGL1, C2GnT, and FT-IV bind P-selectin,1 constitutive or transfection-enhanced levels of FT-IV do not generate active P-selectin ligand in MOLT-4 cells.7 However, recent analyses of mice deficient in the FT-IV gene have demonstrated that FT-IV involvement is subtle when FT-VII is expressed, providing a significant contribution to selectin-dependent neutrophil and lymphocyte recruitment in vivo only when the FT-VII gene is disrupted.6,8
Eosinophils are thought to contribute to the inflammation associated with several diseases including bronchial asthma, allergic rhinitis, and hypereosinophilic syndrome. Blood and tissue eosinophilia are also characteristic of atopic dermatitis, bullous pemphigoid, drug-induced skin eruptions, eosinophilic cellulitis, and eosinophilic pustular folliculitis, and eosinophil accumulation is oftentimes associated with certain parasitic infestations. Previous reports have indicated that eosinophils exhibit a marked avidity for P-selectin.9–13 In mice, P-selectin deficiency resulted in reduced eosinophil rolling and tissue eosinophilia in ragweed-induced peritonitis.12,13 Although an inhibitor of selectin-mediated eosinophil-endothelial interaction might have immense potential for treating diseases in which eosinophils contribute to tissue damage and pathogenesis, little is known about the glycosylation requirement for eosinophil selectin counterreceptor activity. Our previous work disclosed that eosinophils from human blood express nonsialylated Lex and sialyl-dimeric Lex (FH6 epitope), but not other sLex-related structures, such as HECA-452 epitope.14,15 Considering the substrate specificities of FT-IV and FT-VII in Lex and sLex synthesis, these findings suggested that FT-IV rather than FT-VII may be a major regulatory enzyme in the synthesis of Lex-related structures and selectin ligands in eosinophils, because FT-IV alone can generate some FH6 epitope.16
To begin to understand how FT-IV and FT-VII are regulated in eosinophils, we have examined the expression regulation of FT-IV and FT-VII mRNA in human eosinophils. We find that FT-IV is the predominant transcript in eosinophils, even in patients with atopic dermatitis, and observe that transforming growth factor (TGF)-β1 up-regulates FT-IV mRNA. By contrast, steady state accumulation of FT-VII mRNA is relatively less than FT-IV mRNA, although TGF-β1 increases FT-VII mRNA accumulation to some extent. To further define the roles of FT-IV and FT-VII in selectin ligand activities, we assessed selectin binding and recruitment to the skin of eosinophils from mice deficient in FT-IV and/or FT-VII. We observe that FT-IV deficiency significantly reduces eosinophil recruitment to the dermis even in the presence of FT-VII. We also observe a modest degree of eosinophil recruitment in the absence of FT-VII, which is reflective of FT-IV-dependent selectin ligand expression because such recruitment is suppressed by blocking E- or P-selectin, and is essentially absent in mice deficient in both FT-IV and FT-VII. These observations assign an important role to FT-IV in the synthesis of selectin ligand activities in eosinophils that enable the recruitment of these cells into the skin.
Materials and Methods
Cell Preparation
We isolated granulocytes by 6% dextran sedimentation from peripheral blood anti-coagulated with ethylenediamine tetraacetic acid obtained from healthy donors and from patients with atopic dermatitis. Eosinophils were prepared by separating granulocytes on Percoll (density 1.087 g/ml) and by hypotonic lysis of contaminating erythrocytes. Eosinophils were then purified from neutrophils by negative selection in the presence of magnetic beads conjugated to a monoclonal antibody to CD16 (Miltenyi Biotechnology, Sunnyvale, CA). Neutrophils were purified by Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation. Purity determined by Diff-Quick staining (International Reagents Corp., Kobe, Japan) was >99.9% for eosinophils and >96% for neutrophils.
Mice
The generation of FT-IV(−/−) mice, FT-VII(−/−) mice, and FT-IV(−/−)/VII(−/−) mice has been described in detail.6,17 The mice had been backcrossed nine or more generations to the C57BL/6J strain, bred, and housed under strict specified pathogen-free conditions. All experiments were conducted according to protocols approved by the animal care and use committee at the Tokyo Medical and Dental University.
Induction of Blood Eosinophilia in Mice
Blood eosinophilia was induced as described18 by injecting mice subcutaneously with cyclophosphamide (150 mg/kg) (Shionogi Co., Osaka, Japan) on day 2. On day 0, mice were immunized subcutaneously with keyhole limpet hemocyanin (150 μg/mice) (Wako Pure Chemical Industries Ltd., Osaka, Japan) in complete Freund’s adjuvant (Chemicon Int., Temecula, CA). On day 12, peripheral blood was collected from the retro-orbital plexus.
IgE-Mediated Late-Phase Reaction (LPR)
The dorsal site of the mouse ear lobe was injected subcutaneously with 1.25 μg of anti-dinitrophenyl-specific IgE (MP Biomedicals, Inc., Aurora, OH) and challenged 24 hours later with 20 μl of 0.2% 2,4-dinitrofluorobenzene (Nacalai Tesque, Kyoto, Japan) in acetone:olive oil (4:1). Ear swelling responses were measured 24 hours thereafter. Ear thickness measured using a dial thickness gauge (Peacock, Tokyo, Japan) before and after challenge is expressed as mean increments in thickness greater than the basal control value.
Induction of Irritation Dermatitis with Eosinophilia
Tissue eosinophilia can be enhanced by the systemic administration of interleukin (IL)-5.19 Mice ear lobes were painted with 20 μl of 1% croton oil (Nacalai Tesque) in acetone, and then 10 pmol/kg of IL-5 (Techne Corp., Minneapolis, MN) was administered subcutaneously into the nape of the neck 16 hours later. Ear swelling responses were measured after 24 hours. The dose and the time of IL-5 administration were optimized in a preliminary experiment.
Histological Assessment
Excised specimens of skin tissue were fixed in 10% formalin and embedded in paraffin. Each specimen was stained with Giemsa or carbolchromotrope solution.
Binding Assay of Soluble Form of Selectin by Whole-Blood Flow Cytometry
Eosinophil surface selectin ligands were analyzed by indirect immunofluorescence using whole-blood flow cytometry as described14,20–22 with some modification. Platelet-rich plasma was removed from mouse peripheral blood anti-coagulated with ethylenediamine tetraacetic acid, then the blood cells were washed with phosphate-buffered saline (PBS) (−). The cells were suspended in PBS containing 0.1% NaN3, 3% fetal calf serum, 1 mmol/L Ca2+, and 1 mmol/L Mg2+, then incubated with murine P-, E-selectin human IgG Fc chimera (Techne Corp.) or with control human IgG (10 μg/ml) (ICN Pharmaceuticals, Inc., Aurora, OH) for 30 minutes on ice. The cells were washed and incubated with phycoerythrin-conjugated F(ab′)2 goat anti-human IgG Fc antibody (Rockland, Gilbertsville, PA) for 30 minutes on ice. After an additional wash, red blood cells were hemolyzed with 1.5 ml of FACS lysing solution (Becton Dickinson, San Jose, CA), then fixed with 0.4% parabenzoquinone (Wako Pure Chemical Industries). Cells were examined by flow cytometry using a FACScalibur (Becton Dickinson, Mountain View, CA) and analyzed using Cell Quest software (Becton Dickinson). Fixation with parabenzoquinone permitted gating of eosinophils without prior puri-fication as determined by CD49d/VLA-4 (Southern Biotechnology Associates, Inc., Birmingham, AL) and anti-mouse neutrophil antibody (7/4 clone; Cedarlane, Ontario, Canada).23
Measurement of Eosinophil Peroxidase (EPO) in Skin
The dorsal skin including epidermis and entire dermis or whole ear lobe (8 mm in diameter) was excised and frozen at −70°C. EPO activity was measured as described24,25 with some modification. Briefly, frozen skin specimens were homogenized with 1 ml of PBS containing 0.5% hexadecyltrimethylammonium bromide (Wako Pure Chemical Industries Ltd.) and sonicated for 20 seconds. Serially diluted supernatants were placed in wells (50 μl per well) in 96-well flat-bottomed microtiter plates and then 100 μl of substrate (1 mmol/L O-phenylenediamine dihydrochloride and 0.5 mmol/L hydrogen peroxide in 50 mmol/L Tris-HCl, pH 8.0) was added. After 30 minutes at room temperature, the reaction was stopped by adding 50 μl of 2 N sulfuric acid and the absorbance was read at 492 nm. Interference by myeloperoxidase released from contaminated neutrophils to the reaction was monitored by adding 2 mmol/L 3-amino-1,2,4 triazole (Sigma-Aldrich Japan KK, Tokyo, Japan).26
Eotaxin-Induced Dermal Eosinophilia
Dermal eosinophilia was induced by the protocol reported previously.27 Briefly, 10 pmol/site recombinant mouse eotaxin (Techne Corp.) was intradermally injected into the dorsal skin. After 4 hours, 8 mm (diameter) sections of the skin were excised and processed for EPO measurements.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) to Detect α(1,3)-Fucosyltransferase (FT)-IV and -VII and Core 2 β1,6-N-Acetylglucosaminyl Transferase (C2GnT)
Total cellular RNA isolated using RNAzol B (Tel-Test Inc., Friendswood, TX) was digested with DNase I (Takara Biomedicals, Tokyo, Japan). Twenty μl of RT mix consisted of 8 μl of 5× buffer (250 mmol/L Tris-HCl, pH 8.3, 375 mmol/L KCl, 50 mmol/L dithiothreitol, 15 mmol/L MgCl2), 4 μl of hexanucleotide mixture (62.5 A260 U/ml; Boehringer Mannheim, Mannheim, Germany), 2 μl of dNTP (2.5 mmol/L each), 4 μl of 20 U/μl of human placenta ribonuclease inhibitor (Takara Biomedicals), and 2 μl of 200 U/μl reverse transcriptase (Moloney murine leukemia virus, Takara Biomedicals). The RT mixture was dispensed at 20 μl/tube with 20 μl of 40 ng/μl total RNA, vortex-mixed, and then incubated at 37°C for 60 minutes. Reverse transcriptase was inactivated at 70°C for 10 minutes, then the samples were stored at −70°C.
The PCR mixture contained 5 μl of reverse-transcribed RNA (100 ng total RNA), 5 μl of 10× buffer (100 mmol/L Tris-HCl, pH 8.3, 500 mmol/L KCl, 15 mmol/L MgCl2), 4 μl of dNTP (2.5 mmol/L each), 2.5 μl of 20 μmol/L sense primer, 2.5 μl of 20 μmol/L anti-sense-primer, 0.5 μl of 1 U/μl Perfect Match (Stratagene, La Jolla, CA), 0.4 μl of 5 U/μl TaqDNA polymerase (Takara Biomedicals), and 30.1 μl of water. The tubes were transferred to a thermal cycler (DNA amplifier PC-700; Astec, Fukuoka, Japan). The reaction was started at 94°C for 3 minutes, followed by repeated 1-minute cycles of 94°C, 60°C, and 72°C.
The primers were: β-actin mRNA, 5′-CGCGAGAAGATGACCCAGATC-3′ and 5′-ATCACGATGCCAGTGGTACGG-3′;28 FucT-IV, 5′-CGGGTGTGCCAGGCTGTA CAGAGG-3′ and 5′-TCGGGAACAGTTGTGTATGAGATT-3′;29 for FucT-VII, 5′-CC CACCGTGGCCCAGTACCGCTTCT-3′ and 5′-CTGACCTCTGTGCCCAGCCTCCC GT-3′;29 for C2GnT, 5′-TTTTCTGGCAGTGCCTACTTCGTGGTC-3′ and 5′-ATGCTCATCCAAACACTGGATGGCAAA-3′.30 Aliquots from each sample were resolved by electrophoresis on 3.3% agarose in glycine buffer and stained with ethidium bromide. The reactions were stopped after various numbers of cycles to assess the region of linear response. Experiments were repeated three times.
Real-Time Quantitative PCR
Quantitative RT-PCR was performed by monitoring in real time the increase in fluorescence of the SYBR Green dye (Brilliant SYBR Green QPCR Master Mix) (Stratagene) with the Mx3000P real-time PCR system (Stratagene). The primers for C2GnT, FT-IV, and FT-VII were purchased from Takara Biomedicals.
Statistical Analyses
Student’s t-test determined statistical differences between means. Bonferroni’s multiple comparison test was performed for analysis of more than two groups. A P value of <0.05 was taken as the criteria for statistical significance.
Results
Profiles of FT-IV and FT-VII mRNA Levels in Eosinophils from Human Blood
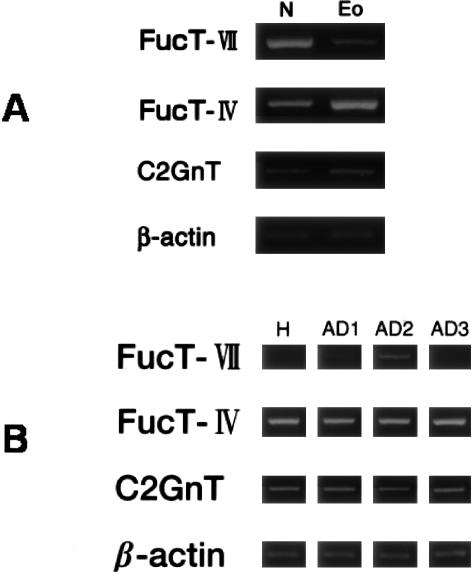
We previously reported that eosinophils from human blood express the FH6 epitope (sialyl-dimeric Lex), but not other sLex-related structures.14 Sialyl-dimeric sLex may be a major E-selectin ligand on the surface of eosinophils.30 Conversely, sLex-structures are primarily expressed in neutrophils, but the FH6 epitope is representative of only a minor proportion of these.14,30 These observations implied that the nature and/or expression levels of FTs in eosinophils (or their bone marrow precursors) may differ from the FTs in neutrophils (and neutrophil precursors in the bone marrow). Indeed, the level of FT-VII mRNA was low in eosinophils (Figure 1A). On the other hand, the high levels of FT-IV mRNA are in striking contrast to neutrophils, where large amounts of both FT-IV and FT-VII mRNA are expressed. FT-VII mRNA expression was consistently low in eosinophils even from patients with atopic dermatitis (Figure 1B). These data confirmed that FT-IV is the predominant transcript in human blood eosinophils compared with FT-VII.14
Figure 1.
Profiles of mRNA levels of FT-IV, FT-VII, and C2GnT in human eosinophils and neutrophils. mRNA from isolated eosinophils and neutrophils was analyzed by RT-PCR as described in Materials and Methods. Amplification was continued for up to 36 cycles for FT-VII and FT-IV, 30 cycles for C2GnT, and 23 cycles for β-actin. A: Whereas FT-IV is the predominant transcript in eosinophils (Eo), neutrophils (N) expressed a large amount of FT-VII as well as FT-IV mRNA. Expression of C2GnT did not significantly differ between eosinophils and neutrophils. Results are representative of two healthy donors and two patients with atopic dermatitis. Eosinophils and neutrophils were isolated from the same donor. B: In eosinophils, FT-VII mRNA level was consistently low or below detection limits at 36 cycles. H, healthy donor; AD, atopic dermatitis.
Regulation of FT-IV, FT-VII, and C2GnT mRNA in Human Blood Eosinophils
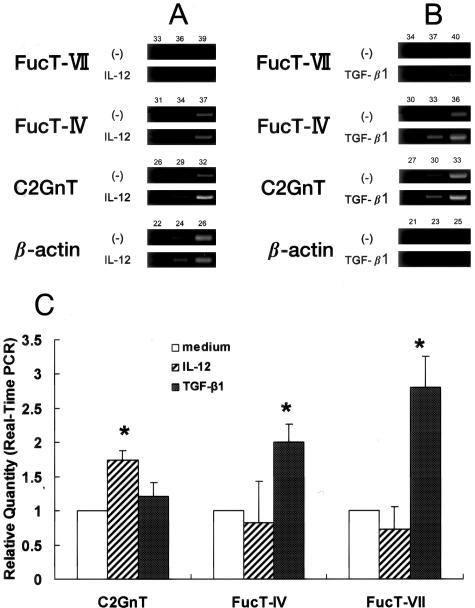
The regulatory mechanisms of FTs and C2GnT mRNA expression in CD4+ T cells have been analyzed in detail,31–35 but little is understood about these processes in granulocytes. To assess regulation of these genes in mature blood eosinophils, we quantified mRNA levels for FT-IV, FT-VII, and C2GnT in eosinophils subjected to an 18-hour incubation with several different human recombinant cytokines known to regulate these genes in lymphocytes. Cytokines included IL-4 (10 ng/ml) (Techne Corp.), IL-5 (10 ng/ml) (Techne Corp.), IL-12 (10 ng/ml) (Wako Pure Chemical Industries Ltd.), and TGF-β1 (5 ng/ml) (PeproTech EC, Ltd., UK). We determined the concentration of cytokines, which induced maximal responses, in preliminary experiments. Incubating the cultures for more than 24 hours caused cell viability to significantly decrease (data not shown). IL-12 significantly enhanced the steady state accumulation of C2GnT mRNA, but did not appreciably alter FT-IV or FT-VII mRNA levels (Figure 2A). On the other hand, the mRNA levels of FT-IV and FT-VII were enhanced by TGF-β1 (Figure 2B). These were further confirmed by real-time PCR analysis (Figure 2C). The level of FT-VII mRNA expression enhanced by TGF-β1, however, was weaker than that in neutrophils (Figure 1A), and FT-IV was still predominant in eosinophils. Neither IL-5 nor IL-4 affected FT and C2GnT mRNA expression (data not shown).
Figure 2.
Effect of IL-12 and TGF-β1 on FT-IV, FT-VII, and C2GnT mRNA in eosinophils. Isolated human eosinophils were stimulated with IL-12 (10 ng/ml) or TGF-β1 (5 ng/ml) for 18 hours. A and B: Gel electrophoresis for PCR products at various PCR cycles. C: Real-time PCR analysis. Relative quantity indicates the relative amount of mRNA of target genes compared with that of β-actin mRNA (n = 3). Relative quantity of genes in unstimulated cells (medium alone) was regarded as 1.0. IL-12 enhances C2GnT mRNA expression in eosinophils, whereas FT-IV and FT-VII mRNA levels were up-regulated by TGF-β1. C2GnT mRNA was weakly enhanced by TGF-β1, but this was not statistically significant. *P < 0.05 compared with corresponding sample incubated in medium alone.
Contribution of FTs to Selectin Ligand Generation in Eosinophils
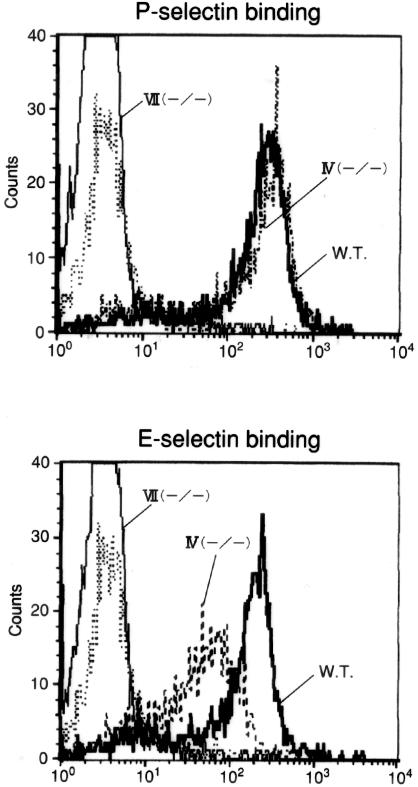
Evidence indicates that soluble P-selectin binds to eosinophils although these cells express extremely low levels of FT-VII.14 Thus, FT-IV but not FT-VII might be a key contributor to the control of P-selectin ligand activity in eosinophils. To determine the role of FT-IV and FT-VII in eosinophil selectin ligand synthesis, we used whole-blood flow cytometry to examine the in vitro binding activity of soluble selectin-human IgG chimeras to blood eosinophils isolated from mice deficient in FT-IV and/or FT-VII. Initially, however, it was difficult to analyze peripheral blood from untreated mice because blood eosinophils were less than 1 × 104 cells/ml as determined by the staining with Discombe’s solution.36 When mice were treated with cyclophosphamide/KLH, eosinophil counts from wild-type (WT), FT-IV(−/−), FT-VII(−/−), and FT-IV(−/−)/VII(−/−) mice were elevated to ∼2.5 ± 0.45, 3.3 ± 0.67, 7.5 ± 2.12, and 8.9 ± 1.25 × 105cells/ml, respectively. These procedures enabled us to analyze whole populations of circulating eosinophils regardless of cell density without the risk of missing subpopulations such as light density eosinophils. P-selectin bound equivalently to wild-type (WT) and FT-IV(−/−) eosinophils (Figure 3), but did not bind to FT-VII(−/−) eosinophils. Soluble E-selectin also bound to wild-type murine eosinophils. We observed a small, but significant decrease in E-selectin binding to FT-IV-deficient eosinophils and no binding to eosinophils from FT-VII(−/−) mice. Neither P- nor E-selectin bound eosinophils from FT-IV(−/−)/VII(−/−) mice.
Figure 3.
Soluble selectin binding to blood eosinophils from FT-IV- or FT-VII-deficient mice. Binding of mouse selectin-human IgG chimera to eosinophils was assessed by whole-blood flow cytometry. Whereas P-selectin bound identically to eosinophils from FT-IV(−/−) and WT mice, E-selectin binding was partially reduced in FT-IV-deficient eosinophils. Binding of P- and E-selectin to eosinophils from FT-VII(−/−) mice was entirely abolished. Results are from a single representative of three separate experiments.
FT-IV Contributes to Eosinophil Recruitment and Skin Inflammation in Vivo
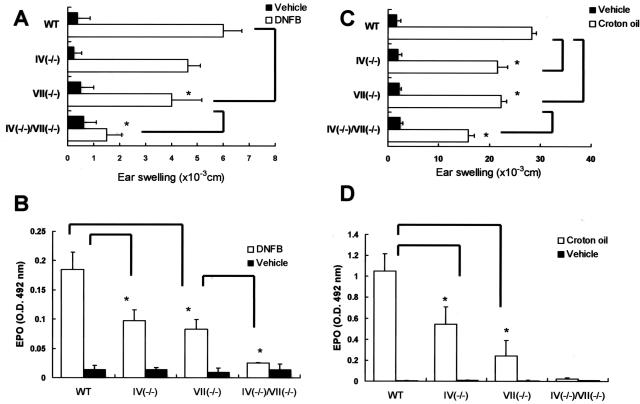
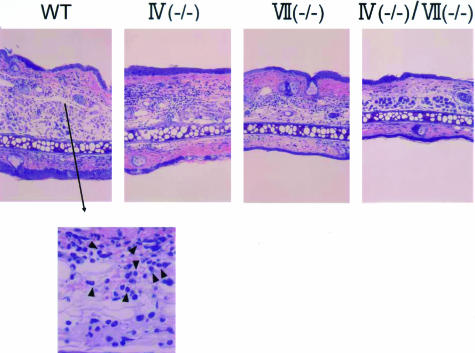
Binding assays with soluble selectins by flow cytometry do not necessarily allow an accurate prediction of selectin ligand activity in vivo.6,37 To further investigate the physiological participation of FTs in selectin ligands generated by eosinophils, we subjected wild-type and FT-deficient mice to a model of allergic skin inflammation as follows. Mice were passively sensitized with dinitrophenyl-specific IgE and challenged with 2,4-dinitrofluorobenzene. This model does not require an afferent limb, which may be impaired in FT-deficient mice.8 In the immediate-type reaction detected at 3 hours after challenge, the ear swelling response of FT-IV(−/−) and FT-VII(−/−) mice was comparable to WT mice, and the response of FT-IV(−/−)/VII(−/−) was minimally decreased compared with that of WT mice, although this did not achieve statistical significance (data not shown). The ear swelling responses of both FT-VII(−/−) and FT-IV(−/−) mice were also weakly reduced in late-phase reactions (LPRs) at 24 hours (Figure 4A). In addition, these responses were remarkably reduced in mice deficient in both enzymes. Corresponding histological analyses disclose a decrease in the amount of inflammatory cells that infiltrated the skin in all three strains of FT-deficient mice (Figure 5). To define the contribution of FTs to eosinophil recruitment, we measured EPO activity in challenged skin. Deficiency of either FT-IV or FT-VII yielded a partial yet significant reduction of EPO activity, whereas EPO activity was almost totally abrogated in the doubly-deficient mice (Figure 4B). Similar results were obtained by counting dermal eosinophils in tissue specimens stained with carbolchromotrope solution under light microscopy (data not shown).
Figure 4.
Allergic and irritant dermatitis in FT-deficient mice. Allergic mouse models of dermatitis (LPR) were induced by sensitization with dinitrophenyl-specific IgE and challenge with 2,4-dinitrofluorobenzene. Croton oil was painted on ear lobes to create irritant dermatitis, and then IL-5 was administered 16 hours later to enhance local eosinophil accumulation (see Materials and Methods). Ear swelling responses were evaluated 24 hours later and excised ear specimens were processed for EPO measurements. Ear swelling responses of mice deficient in FT-VII were reduced (A and C). Whereas ear swelling responses were remarkably suppressed in LPR of doubly-deficient mice, inhibition in croton oil-induced dermatitis was moderate. Activities of EPO in skin of FT-IV- and FT-VII-deficient mice were significantly reduced (B and D). In addition, inhibition was almost complete when both FT-IV and FT-VII genes were knocked out. Each group consisted of at least four mice. Error bars indicate SD. The assay samples for B and D were diluted 8× and undiluted, respectively. *P < 0.05.
Figure 5.
Histopathological features of IgE-mediated LPR in FT-deficient mice. Giemsa stain. Original magnifications: ×40; ×200 (arrowhead, eosinophils).
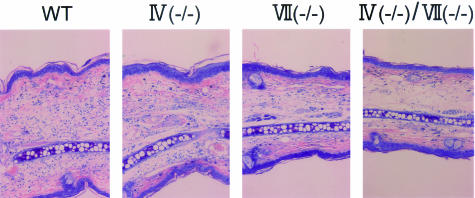
The reduced eosinophil recruitment in the skin of FT-deficient mice might have been due to the impaired extravasation of other effector cells such as lymphocytes that can prime and chemoattract eosinophils.38 To resolve this issue, we assessed skin responses in a model of irritant dermatitis39 induced by croton oil (P- and E-, but not L-selectin-dependent) (Figure 4C). Ear swelling responses were weakly reduced in FT-IV(−/−) and FT-VII(−/−) mice. The decrease in ear swelling responses was more obvious in mice with a double versus a single deficiency. However, the swelling was suppressed by only 47%, in contrast to that of IgE-mediated LPR, which was 80%. Histologically, dermal edema was still obvious in doubly-deficient mice, although inflammatory cell infiltrates were scarce (Figure 6). The EPO activity in the skin was obviously reduced in FT-IV-deficient mice (Figure 4D), which was in striking contrast to the previous finding that neutrophil recruitment as assessed by MPO (myeloperoxidase activity) was not reduced in FT-IV-deficient mice with dermatitis induced by croton oil.6
Figure 6.
Histopathological features of croton oil dermatitis in FT-deficient mice. Giemsa stain. Original magnifications, ×40.
FT-IV-Dependent Eosinophil Accumulation in Response to Eotaxin
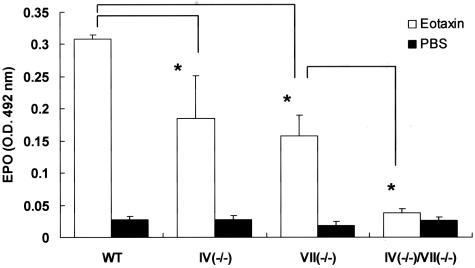
Eotaxin when injected intradermally induces selectin-dependent eosinophil accumulation.27 To further assess the direct effect of a FT deficiency on selectin-dependent eosinophil recruitment, we intradermally injected eotaxin into the dorsal skin of mice. After 4 hours, the skin was excised and processed for EPO activity assays. Eosinophil recruitment in FT-IV- or FT-VII-deficient mice was reduced to equivalent degrees (Figure 7). Eosinophils in the dermis were abolished in doubly-deficient mice. These findings showed that FT-IV plays an important role in eosinophil recruitment in vivo.
Figure 7.
Effect of FT deficiency on eotaxin-induced eosinophil accumulation in skin. Eotaxin (10 pmol/kg) was injected intradermally to dorsal skin and EPO activity in skin was measured 4 hours later. Activities of EPO in FT-IV- and FT-VII-deficient mice were similarly reduced. Eosinophil recruitment was virtually absent in doubly-deficient mice. Results are from a single representative of three separate experiments. Each group consisted of at least four mice. Error bars indicate SD. *P < 0.05.
FT-IV Directs Expression of E- and P-Selectin Ligand Activities in Eosinophils
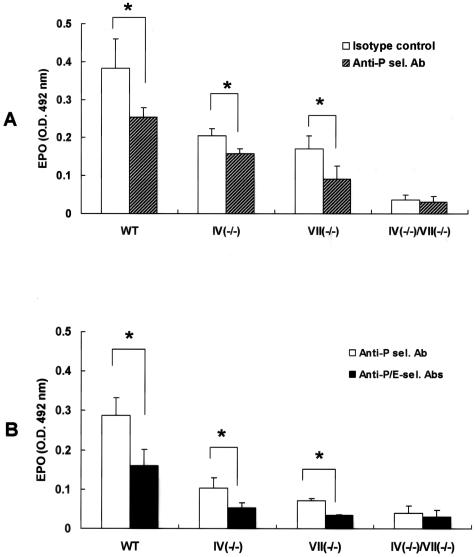
An FT-IV deficiency reduced eosinophil binding to soluble E-, but not to P-selectin in vitro (Figure 3). We therefore postulated that eosinophil accumulation in FT-IV(−/−) mice in vivo was due to the remaining P-selectin ligand activities. FT-VII(−/−) mice, in which eosinophil recruitment is entirely FT-IV-dependent, were injected intravenously with anti-P-selectin antibody (RB40.34; BD Biosciences, San Jose, CA) or anti-E-selectin antibody (10E9.6, BD Biosciences) to determine the relative contributions of P- or E-selectin-mediated, FT-IV-dependent eosinophil accumulation in response to intradermal eotaxin. Anti-P-selectin yielded a substantial but partial suppression of eosinophil recruitment to the skin (Figure 8A). E-selectin antibody alone did not affect EPO activity (data not shown). However, the partial suppression of eosinophil recruitment induced by blocking P-selectin was further inhibited by concomitantly blocking E-selectin (Figure 8B). These observations imply that FT-IV apparently contributes to the synthesis of ligands that mediate both P-selectin-dependent and E-selectin-dependent eosinophil recruitment.
Figure 8.
Effect of E- and/or P-selectin blocking antibodies on in vivo eosinophil recruitment. Mice were administered intravenously with anti-P- and/or anti-E-selectin antibodies (100 μg/mouse) 2 hours before eotaxin dermal injection. A: P-selectin blocking significantly inhibited eosinophil accumulation. B: Although E-selectin blocking alone did not exhibit an inhibitory effect (data not shown), combined E- and P-selectin blocking remarkably suppressed eosinophil recruitment compared with P-selectin blocking alone. EPO activity in doubly-deficient mice was barely detectable and blocking of P- and/or E-selectins failed to exhibit any effect. EPO activity of normal skin from WT, FT-IV(−/−), FT-VII(−/−), and FT-IV(−/−)/VII(−/−) mice was 0.04 ± 0.003, 0.03 ± 0.003, 0.03 ± 0.004, and 0.02 ± 0.008, respectively. Results are from a single representative of three separate experiments. Each group consisted of at least three mice. Error bars indicate SD. *P < 0.05.
Discussion
The α(1,3) fucosyltransferases (FTs) can regulate the construction of sialylated, fucosylated molecules that can contribute to the activities of P- and E-selectin ligands. Recent findings indicate that C2GnT glycosylation can also increase tether bond strength to P-selectin.40 The regulatory mechanisms of these modifying enzymes in granulocytes remain poorly defined. We demonstrated here that IL-12 enhanced C2GnT mRNA expression in eosinophils (Figure 2A). In CD4+ T cells, the IL-12/STAT4 signaling pathway is required to induce C2GnT, but not FT-VII mRNA.33 The levels of FT-VII mRNA in eosinophils were up-regulated by TGF-β1 (Figure 2B). This is consistent with the induction of TGF-β1-dependent FT-VII through p38 mitogen-activated protein kinase in activated CD4+ T cells.35 However, the level of FT-VII mRNA up-regulated by TGF-β1 in eosinophils was still considerably lower than that in neutrophils. On the other hand, FT-IV mRNA was also up-regulated by TGF-β1 (Figure 2B). In eosinophils, unlike neutrophils and T cells, FT-IV seemed consistently predominant compared with FT-VII. The absence of HECA-452-reactive epitope, a FT-VII-dependent sLex structure,41 also implies that FT-VII activity is low in TGF-β1-stimulated eosinophils (data not shown). Eosinophils even from the blood of atopic patients express extremely low levels of FT-VII mRNA (Figure 1B) and do not express the HECA-452-reactive epitope.14 Thus, an unknown mechanism may be responsible for switching FT-VII mRNA off in eosinophils where FT-IV transcripts are relatively abundant.
We used FT-IV- and/or FT-VII-deficient mice to examine the roles of FT-IV and FT-VII in the generation of eosinophil selectin ligands. In three different in vivo mouse models with either a FT-IV and/or a FT-VII deficiency, we demonstrated that both FT-IV and FT-VII are required for effective and optimal eosinophil recruitment to the skin. The single deficiency caused only a partial reduction in eosinophil recruitment, whereas the double deficiency abrogated eosinophil accumulation. These findings indicated that FT-IV plays a FT-VII-independent role in eosinophil recruitment. In FT-VII-deficient mice in which leukocyte tethering/rolling was entirely dependent on FT-IV, P-selectin blocking remarkably inhibited eosinophil recruitment. This indicated that FT-IV is involved in generating functional P-selectin ligand in murine eosinophils, although the molecules responsible for P-selectin ligand activities retained in FT-VII-deficient mice have not been clarified. These data from FT-IV- and/or FT-VII-deficient mice suggest that the large amount of FT-IV predominantly expressed in human eosinophils can collaborate with or compensate for limited levels of FT-VII in the synthesis of P-selectin ligand. This supposition was further supported by observations of polymorphonuclear leukocytes from humans carrying a missense mutation of the FT-VII gene.37 Leukocytes from individuals deficient in FT-VII activity bound to and rolled on P-selectin to a similar degree to cells from those without the mutation. Individuals carrying the mutation have elevated levels of FT-IV activity that may compensate for the FT-VII deficiency to generate P-selectin ligands.
Flow cytometric analysis did not reveal an essential or even substantive role for FT-IV in P-selectin binding in the context of wild-type FT-VII genotype because the degrees of WT and FT-IV-deficient eosinophil binding to P-selectin were identical (Figure 3). P-selectin binding was not retained in the absence of FT-VII. The discrepancy in the flow cytometric data concerning FT involvement in selectin binding in vitro and in models of eosinophilia in vivo probably reflects the fact that FACS-based analyses do not accurately assess the flow-dependent adhesion that characterizes interactions between selectin ligands and the selectins in vivo, as unveiled by the binding assays under flow conditions at a specific selectin density.6,37
Soluble E-selectin binding to murine eosinophils (Figure 3) was observed, although surface expression of HECA-452, CSLEX-1, or FH6 was not detected (data not shown). Previous evidence also revealed possible expression of a counter receptor for E-selectin on murine eosinophils in eotaxin-induced eosinophil accumulation and active cutaneous anaphylaxis in vivo.27 Murine eosinophils might contain extremely low levels of FT-IV and FT-VII, but even the very limited fucosylation of specific glycans may be sufficient to confer binding of P- and E-selectin as has been reported in other murine leukocytes.42 Conversely, somewhat conflicting data has been reported for the binding capacity of human eosinophils to E-selectin. Whereas human eosinophils have been reported to bind E-selectin in static adhesion assays,30,43 E-selectin did not support eosinophil rolling under conditions of physiological shear stress.9,44 We have also demonstrated that soluble E-selectin did not bind to human eosinophils even from patients with atopic dermatitis.14 Although an explanation for this discrepancy is unclear, the adhesive interaction between human eosinophils and endothelial E-selectin may not be physiologically significant.
A FT-IV deficiency partially but significantly reduced binding to E-selectin, possibly because of the abolished activity of ESL-1, as FT-IV preferentially but not exclusively directs ESL-1 more than PSGL-1, compared with FT-VII.45 In our study, administration of E-selectin antibody (clone 10E9.6) alone did not inhibit eosinophil accumulation in response to intradermal eotaxin. Similarly, in LTB4-, eotaxin-, and MIP-1α-induced eosinophil accumulation in the skin, blocking with only anti-E-selectin (10E6) antibody did not exhibit an inhibitory effect.27 However, co-administration of 10E6 antibody with anti-P-selectin antibody (5H1) resulted in further inhibition of eosinophil recruitment compared with P-selectin blocking alone,27 as was observed in our experiments (Figure 8B). The actual role of E-selectin in murine eosinophils may exert its function effectively where P-selectin is acting.
The blocking activity of anti-E-selectin antibody (clone 10E9.6) used in our study in C57BL/6 mice is controversial. Whereas 10E9.6 antibody has been demonstrated to be ineffective in C57BL/6 mice, but effective in BALB/c mice,46 concomitant administration of anti-P-selectin and anti-E-selectin antibodies (RB40.34 antibody and 10E9.6 antibody, respectively) unveiled blocking activity of 10E9.6 antibody.47 It is clear from our experiments that 10E9.6 antibody definitely exhibited an inhibitory effect when co-administered with anti-P-selectin antibody (RB40.34) (Figure 8B).
FT-VII plays predominant roles for neutrophil recruitment in irritant cutaneous inflammation and thioglycollate-induced peritonitis.6 The enzyme also provides a major and critical contribution to the control of selectin ligand activities for optimal lymphocyte recruitment in contact sensitivity.8 The contributions of FT-IV in neutrophils and in T cells are subtle when FT-VII expression is normal and appear only in the absence of FT-VII. On the other hand, this study discovered that in eosinophils, a FT-IV deficiency caused significant reduction in dermal infiltration even when FT-VII was normal. Based on these findings together with the fact that FT-IV expression is consistently predominant in human eosinophils, we conclude that the dependence of eosinophils on FT-IV in P-selectin ligand synthesis is more prominent than that of other leukocytes. FT-IV can be a potent therapeutic target for yielding the selective inhibition of eosinophil recruitment in allergic diseases.
Acknowledgments
We thank Ms. Motoko Sekiya for her excellent technical assistance.
Footnotes
Address reprint requests to Dr. Takahiro Satoh, Department of Dermatology, Graduate School, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113-8519, Japan. E-mail: tasa-1688.derm@tmd.ac.jp.
Supported by the Ministry of Education, Japan (grant no. 16591091).
References
- Li F, Wilkins PP, Crawley S, Weinstein J, Cummings RD, McEver RP. Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J Biol Chem. 1996;271:3255–3264. [PubMed] [Google Scholar]
- Polley MJ, Philips ML, Wayner E, Nudelman E, Singhal AK, Hakomori S, Paulson JC. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis X. Proc Natl Acad Sci USA. 1991;88:6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemela R, Natunen J, Majuri M-L, Maaheimo H, Helin J, Lowe JB, Renkonen O, Renkonen R. Complementary acceptor and site specificities of Fuc-TIV and Fuc-TVII allow effective biosynthesis of sialyl-triLex and related polylactosamines present on glycoprotein counterreceptors of selectins. J Biol Chem. 1998;273:4021–4026. doi: 10.1074/jbc.273.7.4021. [DOI] [PubMed] [Google Scholar]
- Britten CJ, van den Eijnden DH, McDowell W, Kelly VA, Witham SJ, Edbrooke MR, Bird MI, de Vries T, Smithers N. Acceptor specificity of the human leukocyte α3 fucosyltransferase: role of FucT-VII in the generation of selectin ligands. Glyobiology. 1998;8:321–327. doi: 10.1093/glycob/8.4.321. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge RC, King SL, Dimitroff CJ, Kupper TS, Sackstein R. Direct real-time observation of E- and P-selectin-mediated rolling on cutaneous lymphocyte-associated antigen immobilized on Western blots. J Immunol. 2002;68:5645–5651. doi: 10.4049/jimmunol.168.11.5645. [DOI] [PubMed] [Google Scholar]
- Homeister JW, Thall AD, Petryniak B, Maly P, Rogers CE, Smith PL, Kelly RJ, Gersten KM, Askari SW, Cheng G, Smithson G, Marks RM, Misra AK, Hindsgual O, von Andrian UH, Lowe JB. The α(1,3)fucosyltransferase FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- Knibbs RN, Craig RA, Maly P, Smith PL, Wolber FM, Faulkner NE, Lowe JB, Stoolman LM. α(1,3)-Fucosyltransferase VII-dependent synthesis of P- and E-selectin ligands on cultured T lymphoblasts. J Immunol. 1998;161:6305–6315. [PubMed] [Google Scholar]
- Smithson G, Rogers CE, Smith PL, Scheidegger EP, Petryniak B, Myers JT, Kim DSL, Homeister JW, Lowe JB. Fuc-TVII is required for helper 1 and T cytotoxic 1 lymphocyte selectin ligand expression and requirement in inflammation, and together with Fuc-TIV regulates naïve T cell trafficking to lymph nodes. J Exp Med. 2001;194:601–614. doi: 10.1084/jem.194.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama J, Fuhlbrigge RC, Puri KD, Springer TA. P-selectin, L-selectin, and α4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cells under physiological flow conditions. J Immunol. 1997;159:3929–3939. [PubMed] [Google Scholar]
- Patel KD, McEver RP. Comparison of tethering and rolling of eosinophils and neutrophils through selectins and P-selectin glycoprotein ligand-1. J Immunol. 1997;159:4555–4565. [PubMed] [Google Scholar]
- Edwards BS, Curry MS, Tsuji H, Brown D, Larson RS, Sklar LA. Expression of P-selectin at low site density promotes selective attachment of eosinophils over neutrophils. J Immunol. 2000;165:404–410. doi: 10.4049/jimmunol.165.1.404. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Frenette PS, Rayburn H, Cummiskey M, Ullman-Cullere M, Wagner DD, Hynes RO. Multiple targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment. Proc Natl Acad Sci USA. 1999;96:11452–11457. doi: 10.1073/pnas.96.20.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broide DH, Humber D, Sriramarao P. Inhibition of eosinophil rolling and recruitment in P-selectin- and intercellular adhesion molecule-1-deficient mice. Blood. 1998;91:2847–2856. [PubMed] [Google Scholar]
- Satoh T, Kaneko M, Wu M-H, Yokozeki H, Nishioka K. Contribution of selectin ligands to eosinophil recruitment into the skin of patients with atopic dermatitis. Eur J Immunol. 2002;32:1274–1281. doi: 10.1002/1521-4141(200205)32:5<1274::AID-IMMU1274>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Satoh T, Knowles MS, Li M-S, Sun L, Tooze JA, Zabucchi G, Spry CJF. Expression of lacto-N-fucopentaose III (CD15)- and sialyl-Lewis X-bearing molecules and their functional properties in eosinophils from patients with the idiopathic hypereosinophilic syndrome. Immunology. 1994;83:313–318. [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Shinya N, Nishihara S, Kaneko M, Irimura T, Narimatsu H. Distinct substrate specificities of five human α-1,3-fucosyltransferases for in vivo synthesis of the sialyl Lewis x and Lewis x epitopes. Biochem Biophys Res Commun. 1997;237:131–137. doi: 10.1006/bbrc.1997.7100. [DOI] [PubMed] [Google Scholar]
- Weninger W, Ulfman LH, Cheng G, Souchkova N, Quackenbush EJ, Lowe JB, von Andrian UH. Specialized contributions by α(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity. 2000;12:665–676. doi: 10.1016/s1074-7613(00)80217-4. [DOI] [PubMed] [Google Scholar]
- Vadas MA. Cyclophosphamide pretreatment induces eosinophilia to nonparasite antigens. J Immunol. 1981;127:2083–2086. [PubMed] [Google Scholar]
- Satoh T, Yokozeki H, Nishioka K. Pathogenic roles of eosinophils in guinea-pig contact sensitivity: regulation of dermal eosinophilia with remotely administered IL-5. Clin Exp Immunol. 2000;122:300–307. doi: 10.1046/j.1365-2249.2000.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug N, Thurau AM, Lackie P, Baier J, Schultze-Werninghaus G, Rieger CHL, Schauer U. A flow cytometric method for the detection of intracellular basic proteins in unseparated peripheral blood and bone marrow eosinophils. J Immunol Methods. 1996;190:245–254. doi: 10.1016/0022-1759(95)00272-3. [DOI] [PubMed] [Google Scholar]
- Krug N, Napp U, Enander I, Eklund E, Rieger CHL, Schauer U. Intracellular expression and serum levels of eosinophil peroxidase (EPO) and eosinophil cationic protein in asthmatic children. Clin Exp Allergy. 1999;29:1507–1515. doi: 10.1046/j.1365-2222.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Chihara J, Kayaba H, Kamata Y, Oyamada H, Saito N, Shioya T, Sasaki M, Kagaya M, Tsuda A. Whole-blood flow-cytometric analysis of eosinophil EG2 expression as a marker of the pathological conditions of asthma. Int Arch Allergy Immunol. 1998;117(Suppl 1):77–80. doi: 10.1159/000053578. [DOI] [PubMed] [Google Scholar]
- Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- Strath M, Warren DJ, Sanderson CJ. Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods. 1985;83:209–215. doi: 10.1016/0022-1759(85)90242-x. [DOI] [PubMed] [Google Scholar]
- Collins PD, Marleau S, Griffith-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer R, Soranzo MR, Dri P, Menegazzi R, Pitotti A, Zabucchi G, Patriarca P. A simple reliable assay for myeloperoxidase activity in mixed neutrophil-eosinophil cell suspensions: application to detection of myeloperoxidase deficiency. J Immunol Methods. 1984;70:119–125. doi: 10.1016/0022-1759(84)90396-x. [DOI] [PubMed] [Google Scholar]
- Teixeira MM, Hellewell PG. Contribution of endothelial selectins and α4 integrins to eosinophil trafficking in allergic and nonallergic inflammatory reactions in skin. J Immunol. 1998;161:2516–2523. [PubMed] [Google Scholar]
- Satoh T, Sun L, Li M-S, Spry CJF. Interleukin-5 mRNA levels in blood and bone marrow mononuclear cells from patients with the idiopathic hypereosinophilic syndrome. Immunology. 1994;83:308–312. [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Stoolman LM, Craig R, Knibbs RN, Kansas GS. An sLex-deficient variant of HL60 cells exhibits high levels of adhesion to vascular selectins: further evidence that HECA-452 and CSLEX1 monoclonal antibody epitopes are not essential for high avidity binding to vascular selectins. J Immunol. 1998;160:5122–5129. [PubMed] [Google Scholar]
- Bochner BS, Sterbinsky SA, Bickel CA, Werfel S, Wein M, Newman W. Differences between human eosinophils and neutrophils in the function and expression of sialic acid-containing counterligands for E-selectin. J Immunol. 1994;152:774–782. [PubMed] [Google Scholar]
- Wagers AJ, Waters CM, Stoolman LM, Kansas GS. Interleukin 12 and interleukin 4 control T cell adhesion to endothelial selectins through opposite effects on α1,3-fucosyltransferase VII gene expression. J Exp Med. 1998;188:2225–2231. doi: 10.1084/jem.188.12.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y-C, Henault L, Wagers AJ, Kansas GS, Luscinskas FW, Lichtman AH. Expression of functional selectin ligands on Th cells is differentially regulated by IL-12 and IL-4. J Immunol. 1999;162:3193–3201. [PubMed] [Google Scholar]
- White SJ, Underhill GH, Kaplan MH, Kansas GS. Differential requirements for Stat4 in expression of glucosyltransferases responsible for selectin ligand formation in Th1 cells. J Immunol. 2001;167:628–631. doi: 10.4049/jimmunol.167.2.628. [DOI] [PubMed] [Google Scholar]
- Lim Y-C, Vie H, Come CE, Alexander SI, Grusby MJ, Lichtman AH, Luscinskas FW. IL-12, STAT4-dependent up-regulation of CD4+ T cell core 2 β-1,6-n-acetylglucosaminyltransferase, an enzyme essential for biosynthesis of P-selectin ligands. J Immunol. 2001;167:4476–4484. doi: 10.4049/jimmunol.167.8.4476. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Kansas GS. Potent induction of α(1,3)-fucosyltransferase VII in activated CD4+ T cells by TGF-β1 through a p38 mitogen-activated protein kinase-dependent pathway. J Immunol. 2000;165:5011–5016. doi: 10.4049/jimmunol.165.9.5011. [DOI] [PubMed] [Google Scholar]
- Satoh T, Chen Q-J, Sasaki G, Yokozeki H, Katayama I, Nishioka K. Cyclophosphamide-induced blood and tissue eosinophilia in contact sensitivity: mechanism of hapten-induced eosinophil recruitment into the skin. Eur J Immunol. 1997;27:85–91. doi: 10.1002/eji.1830270113. [DOI] [PubMed] [Google Scholar]
- Bengtson P, Lundbald A, Larson G, Pahlsson P. Polymorphonuclear leukocytes from individuals carrying the G329A mutation in the α1,3-fucosyltransferase VII gene (FUT7) roll on E- and P-selectins. J Immunol. 2002;169:3940–3946. doi: 10.4049/jimmunol.169.7.3940. [DOI] [PubMed] [Google Scholar]
- Simon D, Braathen LR, Simon H-U. Eosinophils and atopic dermatitis. Allergy. 2004;59:561–570. doi: 10.1111/j.1398-9995.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Catalina MD, Estess P, Siegleman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999;93:580–589. [PubMed] [Google Scholar]
- Smith MJ, Smith BRE, Lawrence MB, Snapp KR. Functional analysis of the combined role of the O-linked branching enzyme core 2 β1–6-N-glucosaminyltransferase and dimerization of P-selectin glyco-protein ligand-1 in rolling on P-selectin. J Biol Chem. 2004;279:21984–21991. doi: 10.1074/jbc.M402731200. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Stoolman LM, Kannagi R, Craig R, Kansas GS. Expression of leukocyte fucosyltransferases regulates binding to E-selectin. J Immunol. 1997;159:1917–1929. [PubMed] [Google Scholar]
- Kobzdej MMA, Leppanen A, Ramachandran V, Cummings RD, McEver RP. Discordant expression of selectin ligands and sialyl Lewis x-related epitopes on murine myeloid cells. Blood. 2002;100:4485–4494. doi: 10.1182/blood-2002-06-1799. [DOI] [PubMed] [Google Scholar]
- Kim M-K, Brandley BK, Anderson MB, Bochner BS. Antagonism of selectin-dependent adhesion of human eosinophils and neutrophils by glycomimetics and oligosaccharide compounds. Am J Respir Cell Mol Biol. 1998;19:836–841. doi: 10.1165/ajrcmb.19.5.3032. [DOI] [PubMed] [Google Scholar]
- Sriramarao P, Norton CR, Borgstrom P, DiScipio RG, Wolitzky BA, Broide DH. E-selectin preferentially supports neutrophil but not eosinophil rolling under conditions of flow in vitro and in vivo. J Immunol. 1996;157:4672–4680. [PubMed] [Google Scholar]
- Huang M-C, Zollner O, Moll T, Maly P, Thall A, Lowe JB, Vestweber D. P-selectin glycoprotein ligand-1 and E-selectin ligand-1 are differentially modified by fucosyltransferases Fuc-TIV and Fuc-TVII in mouse neutrophils. J Biol Chem. 2000;275:31353–31360. doi: 10.1074/jbc.M005449200. [DOI] [PubMed] [Google Scholar]
- Ramos CL, Kunkel EJ, Lawrence MB, Jung U, Vestweber D, Bosse R, McIntyre KW, Gillooly KM, Norton CR, Wolitzky BA, Ley K. Differential effect of E-selectin antibodies on neutrophil rolling and recruitment to inflammatory sites. Blood. 1997;89:3009–3018. [PubMed] [Google Scholar]
- Yanaba K, Kaburagi Y, Takehara K, Steeber DA, Tedder TF, Sato S. Relative contributions of selectins and intercellular adhesion molecule-1 to tissue injury induced by immune complex deposition. Am J Pathol. 2003;162:1463–1473. doi: 10.1016/S0002-9440(10)64279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]