Abstract
Although the roles of survivin in control of cancer cell division and apoptosis as well as targeting survivin for cancer therapeutics have been extensively explored and reviewed, the pathophysiological role of survivin in normal human cells/organs has not been deeply investigated or sufficiently reviewed. Studies in the latter area, however, appear to be important for the identification of different mechanisms of regulation and function of survivin in normal versus abnormal cells and tissues (including cancer), which might ultimately provide the basis for novel approaches for disease treatment with low toxicity. This Review is intended to summarize current observations in the literature related to the physiological and/or pathological roles for survivin in various normal human cells or organs. Our view of potential future research directions for survivin pertinent to potential therapeutic applications will also be discussed.
Increasing evidence indicates that the unique member of the inhibitor of apoptosis (IAP) protein family, survivin,1 is not only an essential protein molecule for the regulation of progression of mitosis, apoptosis inhibition, and drug/radiation resistance in various cancer cells,2 but it also plays a role in certain physiological processes as well as in pathological conditions in many human organs/cells. Based on observations from the current literature, this includes roles of survivin in embryonic development, hematopoietic cell survival/proliferation, T-cell development/maturation, and the pathophysiology of brain, liver, pancreas, gastrointestinal tract, testis, endometrium, and placenta. The pathophysiological roles for survivin in normal human organs or cells to be presented in this Review are summarized in Table 1. The potential role for survivin in normal tissues or cells raises the critical question as to whether targeting survivin for cancer treatment would be toxic to normal human cells/tissues. Evidence derived from studies of in vitro normal/cancerous human cells and in vivo xenograft animal models with human cancer cells suggests that interference of the expression and/or function of survivin in human cancer cells induced apoptosis and inhibited tumor growth with little toxicity to normal cells/organs.1–3 Specifically, adenoviral delivery of the survivin dominant-negative mutant in cancer cells induced apoptotic cell death but did not affect cell viability of proliferating normal human cells, including fibroblasts, endothelial cells, or smooth muscle cells.4 Importantly, in vivo studies of a xenograft mouse model revealed that interference of survivin expression and/or functions by therapeutic inhibitors showed no toxicity to mice.5,6 Recently, Plescia et al7 reported that a cell-permeable peptidomimetic of survivin (designated shepherdin), which functionally disrupts the interaction of heat shock protein 90 with survivin, Akt, and CDK6, effectively induces cancer cell death in vitro regardless of cell cycle status, p53 status, or Bcl-2 overexpression. However, this molecule did not affect colony formation of purified hematopoietic progenitor cells, and systematic administration of this molecule in mice effectively inhibited human xenograft tumor growth without observable toxicity.7 In addition, immunotherapy with a survivinDNA vaccine activated immune responses without effects on wound healing and fertility of mice,8 and treatment of melanoma patients was not associated with toxicity.9
Table 1.
Summary of the Pathophysiological Role for Survivin in Normal Human Organs or Cells
| Organs or cells with survivin positivity | Physiological roles of survivin | Pathological roles of survivin |
|---|---|---|
| Embryos and oocytes | Development/morphogenesis: cell cycle/mitosis,14–17 apoptosis14,17,18 | Loss of survivin: mitotic defect/embryonic lethality,15,16 apoptosis17,18 |
| Hematopoietic cells (bone marrow) | Cell cycle entry/proliferation,22,24 maturation,25,26 survival22,25,26 | Loss of survivin: impairment of proliferation, maturation, and apoptosis22–24; excessive survivin: inflammatory diseases,19 leukemia1–3 |
| T lymphocytes (thymus) | Maturation,31,32 survival,33,34 proliferation,32,35 expansion37 | Loss of survivin: T-cell maturation/proliferation defects,28,32 mitosis defect28; excessive survivin: hyperproliferation,35 lymphoma1–3 |
| Brain | Brain development/maturation,35,42,44 neural cell survival upon injury46,48 | Excessive survivin: brain-related tumor1–3 |
| Liver | Liver regeneration and cell survival49,16 | Excessive survivin: liver-related cancer1–3 |
| Pancreas | Fetal islet development/maturation52 | Excessive survivin: pancreatitis?53 pancreatic cancer1–3,54 |
| Gastrointestinal tract | Epithelial cell renewal,55 cell survival56,62 | Excessive survivin: gastrointestinal tumorigenesis1–3,19 |
| Testis | Spermatogenesis63,64 | Loss of survivin: spermatogenic failure64–66; excessive survivin: tumorigenesis67 |
| Endometrium | Endometrial homeostasis?69 | Excessive survivin: endometriosis,70 endometriotic cysts,73 or cancer1–3,71,72 |
| Placenta | Placenta homeostasis?74 cell survival?76 | Excessive survivin: hydatidiform mole75 |
Observations that targeting survivin for cancer therapy appears to cause little toxicity to normal tissues is obviously not the final answer to the question of toxicity raised above; these results, however, do indicate the possibility that the regulation and/or the signaling interactions of survivin may show differences between cancer cells and normal cells. Consistent with this possibility, it has been shown that survivin antibodies are readily detectable in sera from cancer patients but not in normal human sera (reviewed in Ref. 1). Moreover, another difference, which may contribute to an effective therapeutic index in cancer cells, is that cancer cells have a mitochondrial pool of survivin, whereas normal cells do not. The mitochondrial pool of survivin has been shown to be critical for mediating inhibition of cancer cell apoptosis.10 In addition, it is known that many critical molecules in cell survival pathways such as Akt, Erk, and EGFR are highly active in cancer cells compared with that in normal cells. These molecules have been shown to promote the expression of survivin,1,11,12 thus providing another difference from normal cells that may permit for therapeutic efficacy of targeting survivin with acceptable toxicities. We have previously shown that DNA-protein interactions in the survivinpromoter region differ between nuclear proteins isolated from cancer cells and those from normal cells.1 Collectively, these observations provide new perspectives on survivin as a valid therapeutic target for treatment of cancer and other diseases and suggest new research directions at two levels: 1) investigation of the differential regulation and/or signaling interactions of survivin between cancer and normal cells; and 2) determination of the underlying molecular mechanisms of these differences. These studies would certainly be important because the results derived from them might enable novel strategies to treat different diseases without toxicity to normal human tissues.
The current available information about the role for survivin in the pathophysiology of various human normal cells and/or organs has been collected in this Review (see Table 1 for an overview) in the hope of facilitating potential studies for optimizing low-toxicity disease treatments by targeting survivin. In addition, we have also provided our opinions regarding future studies of the potential value based on the highlighted data from the references.
Survivin and Embryonic Development
Survivin is expressed in a strictly regulated manner during embryonic development13,14 and plays an important role in the control of embryonic cell mitosis/cytokinesis14–17 and apoptosis.14,17,18 Adida et al13 initially investigated the expression of survivin during mouse embryonic development and showed that survivin is strongly expressed in several apoptosis-regulated fetal tissues with a pattern that did not overlap with Bcl-2, indicating a unique role for survivin in organ generation during de-velopment. Using mouse preimplantation embryos, Kawamura et al17 showed that the transcripts for survivin and its splice variant that lacks exon 2 were expressed in unfertilized oocytes up to the hatched blastocyst stage, and survivin protein was detected at all stages of early embryos, suggesting a role of survivin in both unfertilized egg maturation and embryo development. Murphy et al14 examined the regulation of survivin during critical transitions associated with oogenesis and early embryogenesis in Xenopus laevis. These investigators showed that survivin mRNA is present in the earliest stages of Xenopus oocytes and accumulates during oogenesis. After the onset of zygotic transcription, survivin mRNA declined rapidly to undetectable levels, which correlates temporally with decreased cell cycle and increased embryonic apoptosis.14 Although this indicates a role of survivin in cell cycle regulation and apoptosis control for morphogenesis during development, it would be important in the future to determine whether the role for survivin in early stages of embryogenesis in both mouse17 and X. laevis14 reflects/mimics the role of survivin in the process of tumorigenesis. Nevertheless, it is rational to hypothesize that deregulated expression of survivin in individual cell types, resulting, for example, from p53 mutation1 or virus infection,19 may represent an important aberration of survivin expression leading to carcinogenesis, cancer progression, or other diseases.
Interestingly, Murphy et al14 showed that progesterone-induced maturation of Xenopus oocytes leads to polyadenylation of the survivintranscript. This is consistent with survivin mRNA stabilization and accumulation during this process. However, a previous report showed that progesterone down-regulates survivin expression in breast cancer cells.20 This suggests that the regulation of survivin expression could be different in normal embryonic cells versus malignant cells.
Given the dual role for survivin in cytokinesis and apoptosis, the strictly regulated expression profile of survivin may reflect an important role for survivin in morphogenesis and tissue homeostasis during development. Consistent with this notion, deletion of the survivin gene by homologous recombination in mice revealed an important role of survivin in mitosis.15 Null embryos showed disrupted microtubule formation, became polyploid, and failed to survive beyond 4.5 days post coitum.15,16 Early mouse embryos treated with survivin antisense oligonucleotides were arrested at the morula or early blastocyst stage with disruption of tubulin polymerization and abnormal nuclei.17 Survivin was localized on the centromere of chromosomes, and its location and knockout phenotype closely resembled INCENP,15 an important chromosome passenger protein involved in chromosome segregation and cytokinesis. Although this suggests a role for survivin in mitosis/cytokinesis, comparison of the role of survivin in mitosis during development with that in cancer would be important in the future. This may identify unique approaches for disease treatment. On the other hand, treatment of early mouse embryos with survivin antisense oligonucleotides not only disrupted mitosis but also induced apoptosis.17 Apoptosis induced by survivin antisense was inhibited by caspase-3 and -9 inhibitors, suggesting caspase involvements. Similarly, Jiang et al18 reported that during early mouse brain development, survivin also plays an essential role in control of apoptosis in neurons of the central nervous system. Using Cre-loxP mice, conditional deletion of survivinin neuronal precursor cells from embryonic day 10.5 resulted in striking apoptosis in neuronal precursor cells.18 Although these mice were born at the expected Mendelian ratios, they died shortly after birth. The newborn mutant mice showed much smaller brain with severe apoptosis in the cerebrum, cerebellum, brainstem, spinal cord, and retina, which are associated with a significant increase in caspase-3 and -9 activities.18 Together, these results indicate that survivin plays essential roles in both mitosis and apoptosis during embryonic development.
As mentioned above, a critical question is whether the role of survivin in cancer2 recapitulates its physiological function during development. It has been shown that survivin interacts with not only INCENP and Aurora B (another important chromosome passenger protein) but also with many other proteins important for regulation of cell death in cancer cells (reviewed in Refs. 1,2,21). Survivin localizes not only on the centromere but also on the mitotic spindle1 and mitochondria10 in cancer cells. Deregulation of survivin expression or function appears to be an important change during tumorigenesis.19 Thus, unraveling of the molecular mechanisms for the regulation and function of survivin during development could have significant consequences and is, therefore, a potentially important direction for future research.
Survivin and Hematopoietic Cell Survival/Proliferation
Survivin plays an important role in cell cycle entry/progression,22–24 maturation,25,26 and inhibition of apoptosis or increasing survival22,25,26 of hematopoietic stem/progenitor cells. Survivin expression was found in CD34+ hematopoietic stem and progenitor cells newly isolated from human umbilical cord blood and adult bone marrow22 and was up-regulated within 24 hours after treatment by a combination of treatment with thrombopoietin, Flt3 ligand, and stem cell factor. Withdrawal of these factors resulted in decreased survivin expression,22,23 suggesting a role in the survival and proliferation of these cells. Cell-cycle fractionation of cord blood CD34+ cells revealed that survivin mRNA was undetectable in freshly isolated G0 cells but present in G1 cells, and after cytokine treatment, the expression of survivin was detected in G0 and elevated in G1 CD34+ cells and in cells that had progressed to S and G2/M phase.22,23 These observations suggest that induction of survivin by cytokines and growth factors is likely an early event and required for hematopoietic cell cycle progression. Growth factor-mediated up-regulation of survivin was associated with inhibition of apoptosis, and decreased survivin resulting from depletion of growth factors was coincident with increased apoptosis.22 Moreover, up-regulation of survivin by thrombopoietin, Flt3 ligand, and stem cell factor was also detected in CD34+ cells with hypophosphorylated Rb protein and lack of Ki-67 and cyclin D expression.24 This further indicates the expression of survivin before cell cycle entry. Importantly, although treatment with thrombopoietin, Flt3 ligand, and stem cell factor up-regulated survivin, these treatments had no effect on the expression of XIAP, c-IAP-1, c-IAP-2, apollon, livin, and NIAP,24 revealing a unique role for survivin in hematopoietic cell survival and proliferation. Inhibition of the phosphatidylinositol 3-kinase/AKT and Erk1/2 (p42/44) pathways by pharmacological inhibitors blocked growth factor-mediated survivin up-regulation before cell cycle arrest,24 indicating the involvement of these pathways in survivin induction and a potential role for survivin in cell cycle promotion. In addition, retrovirus-mediated expression of the survivin-internal ribosome entry site-enhanced green fluorescence protein expression vector in primary mouse marrow cells increased granulocyte macrophage-colony forming units (CFU-GM) by 1.7- to 6.2-fold compared with internal ribosome entry site-EGFP control, whereas the survivin antisense construct decreased CFU-GM.24 Together, these studies indicated that cytokine/growth factor-mediated induction of survivin is not a consequence of cell cycle progression but is an important early event for promoting CD34+ cell cycle entry. Recent studies also revealed a role of survivin in G1/S transition in cancer cells. Overexpression of survivin in MCF-7E breast cancer cells blocked vitamin D-mediated G1 cell arrest and increased S and G2/M cell populations,27 suggesting that vitamin D-mediated down-regulation of survivin is required for vitamin D-mediated G1 arrest in cancer cells. In contrast, it was recently shown that lentivirus-mediated transfection of CD34+ cells with human T-cell lymphotropic virus type 1 Tax (Tax1) induced G0/G1 cell cycle arrest without down-regulation of endogenous survivin expression and concomitantly resulted in the suppression of multilineage hematopoiesis and the protection of cells from serum withdrawal-mediated apoptosis in vitro.28 Thus, it would be important to determine whether the underlying molecular mechanism for the promotion of G1/S transition by survivin is actually different in cancer cells from that in normal hematopoietic cells. This information would be important for identifying more cancer-specific treatment approaches.
Using bone marrow c-kit+/Lin− cells isolated from wild-type and p21WAF1/Cip1 knockout mice, it was demonstrated that retrovirus-mediated expression of survivin promotes p21+/+ cell CFU-GM formation and proliferation, whereas the expression of survivin has none of these effects in p21−/− cells.29 This indicates an important role for p21 in survivin-mediated hematopoietic progenitor cell proliferation. Overexpression of survivin inhibited apoptosis in c-kit+/Lin− cells from p21+/+ mice but not from p21−/− mice,29 suggesting that inhibition of apoptosis by survivin in hematopoietic progenitor cells requires the presence of p21 protein. However, survivin increased CFU-GM S-phase cells in both p21+/+ and p21−/− cells, indicating that this effect is p21 independent. The authors proposed that survivin in concert with p21 plays an important role in the promotion of normal hematopoietic cell proliferation.29 The important role for p21 in survivin-mediated hematopoietic cell proliferation is consistent with a positive role of p21 in myeloid progenitor cell colony formation reported previously.30 However, in contrast to the positive role for p21 in survivin-mediated hematopoietic proliferation,29,30 a positive role for p21 in survivin-mediated cancer cell proliferation has not been demonstrated. This may reflect the different function and/or signaling interactions of survivin between normal and cancerous cells. Studies characterizing these differences may provide novel approaches for increasing the therapeutic index of survivin-targeted cancer therapy.
In studies of bone marrow neutrophil maturation, it was shown that immature neutrophils express high levels of survivin with little or no expression of NAIP, c-IAP-1, c-IAP-2, and XIAP. However, survivin expression was decreased to a level comparable with other IAPs during the transition/differentiation from immature to mature neutrophils,25 suggesting a role of survivin in neutrophil maturation. Survivin expression was markedly increased in mature neutrophils after exposure to survival factors (GM-CSF or G-CSF) in vitro, but decreased survivin expression was associated with caspase-3 activation and shortened mature neutrophil lifespan compared with the corresponding controls.25 This indicates a role for survivin in maintaining neutrophil viability and delay of apoptosis during neutrophil differentiation. Interestingly, elevated survivin expression was also found in mature neutrophils from patients with active inflammatory diseases (cystic fibrosis or appendicitis),25 suggesting a potential role for survivin in certain pathological conditions as well. Thus, determination of the molecular mechanisms for the regulation and function of survivin in normal physiology versus pathological conditions might lead to novel approaches for treatment of disease associated with these observations.
Gurbuxani et al26 showed that survivin is differentially expressed during erythroid versus megakaryocyte maturation. Erythroid cells expressed survivin throughout their maturation, whereas megakaryocytes expressed approximately fourfold lower levels of survivin mRNA without detectable protein. Overexpression of survivin in mouse bone marrow cells had no effect on erythroid development but antagonized megakaryocyte polyploidization,26 suggesting that a low-level survivin is required for megakaryocyte differentiation. In contrast, decreased survivin expression interfered with the formation of erythroid cells but not megakaryocytes, and survivin-deficient hematopoietic progenitors were unable to produce either erythroid or megakaryocytic colonies,26 suggesting a role for survivin in the maturation of these cells. In sum, these data support a notion that the mechanisms for survivin regulation and/or function may also be different among different types of normal cells. Further studies of these phenomena may provide important information for disease treatment.
Survivin and T-Cell Development
Survivin expression is strictly regulated31 and plays important roles in T lymphocyte maturation,31,32 survival,33,34 and proliferation.32,35 Analysis of survivin expression before and after CD3+ lymphocyte activation in vitro indicated that survivin was undetectable before activation but readily detected after activation by interleukin-2 and OKT-3 with peak expression at 2 to 4 days.33 The apoptotic cell fraction was inversely associated with the level of survivin.33 In addition, CD45RO+ memory T cells showed a higher expression of survivin than CD45RA+ naive T cells after activation.33 These observations provided initial evidence suggesting that survivin plays a role in lymphocyte activation and survival during T-cell maturation as well as in memory T-cell responses. Expression of survivin in thymocytes was developmentally regulated; its expression was low in the CD4/CD8 double negative thymocytes, was up-regulated in double positive thymocytes, and was highest in the late double positive thymocytes with high levels of T-cell receptors.31 Survivin was then down-regulated in the single positive thymocytes and was undetectable in the peripheral blood T cells.31 Although these observations suggest a role for survivin in T-cell development, the mechanisms for the regulation and function of survivin during T-cell development are unclear. Determination of the mechanism of action of survivin in T-cell development would be important for rational disease treatment.
Transgenic overexpression of the mouse survivin gene in thymus indicated that although T-cell development and apoptosis of thymocytes were largely unaffected in lck-survivin mice, thymocytes displayed hyperproliferation in response to phorbol-12-myristate-13-acetate and ionomycin compared with the corresponding control.35 Xing et al32 used the Cre-loxP system to conditionally delete the survivingene in T cells at different developmental stages to study the role of survivin in T-cell maturation. Early deletion of survivinin lck-survivin mice resulted in about one-eighth of thymic cellularities and 50% smaller lymph nodes in comparison with controls, whereas deletion of survivinin CD4-negative survivin mice at a later stage resulted in a drastic reduction of peripheral CD4/CD8 double positive T-cell numbers with an immature phenotype, while thymic development appeared to be normal.32 These observations suggest a different role for survivin in different stages of T-cell development. Interestingly, loss of survivin did not lead to increased apoptosis in vivo,32 indicating a unique role for survivin in normal T-cell development compared with the important role of survivin in both mitosis and apoptosis in cancer cells.2 Nevertheless, survivin-deficient T cells showed greatly impaired thymocyte homeostasis and mitogen-induced proliferation in newborn mice.32 Together, the authors proposed that survivin is not essential for T-cell apoptosis control but is crucial for T-cell maturation and proliferation at multiple stages. Using a similar Cre-loxP system, however, Okada et al34 reported that early deletion of survivinin lck-survivin mice blocked the transition from CD4/CD8 double negative to CD4/CD8 double positive thymocytes. Survivin-deficient thymocytes were unable to respond to pre-T-cell receptor signals although the signals are intact in these cells.34 In response to proliferative stimuli, CD4/CD8 double negative cells lacking survivin exhibited cell cycle arrest, spindle formation defect, and increased cell death, although these authors could not find any evidence that survivin directly regulates apoptotic pathways.34 This may reflect a different mechanism for the regulation of cell death by survivin in normal T cells versus malignant cells. In addition, it was shown that survivin down-regulates the expression and inhibits the function of the p53 tumor suppressor gene in MCF-7 breast cancer cells,36 which is likely to be important for survivin function in promoting cancer cell proliferation. However, although loss of survivin activates p53 during T-cell development, the developmental defects caused by survivin deficiency could not be rescued by either p53 inactivation or ectopic Bcl-2 expression.34 These observations may also reflect different signaling interactions or mechanisms for survivin function in association with other proteins in normal T-cell development versus uncontrolled cancer cell proliferation.
The importance of sustained survivin expression in T-cell clonal expansion has recently been demonstrated. Song et al37 showed that survivin is the downstream target for activated CD134/OX40 through phosphatidylinositol 3-kinase/Akt pathway signaling, which is critical for peripheral T-cell expansion. CD134PX40-induced survivin expression is independent of mitosis in late G1, and inhibition of survivin blocks S-phase transition and division of T cells and leads to apoptosis.37 Importantly, survivin expression alone is sufficient to restore proliferation and to antagonize apoptosis in CD134/OX40-deficient T cells and can rescue T-cell expansion in vivo.37 Thus, it will be important to determine whether the CD134/OX40 pathway plays a role in control of survivin expression and T-cell pathology including lymphoma and T cell-associated leukemia. This may provide important information for the development of novel approaches for disease treatment.
In sum, a significant amount of information on the regulation and function of survivin in T-cell maturation, development, and expansion has been generated. The identification of detailed molecular mechanisms by which these processes are executed should be the next focus of research in this area. As mentioned above, it is also important to compare the signaling interaction associated with survivin in T-cell development with that in the corresponding diseased cells such as lymphoma and T-cell leukemia.
Survivin and Multiple Sclerosis (MS)
MS is a chronic, inflammatory condition of the nervous system and is the most common nontraumatic neurological disease in young adults. Failure to eliminate potentially pathogenic autoreactive lymphocytes by apoptosis appears to be its major etiology. Abnormal up-regulation of survivin expression has been shown to be involved in the inability to eliminate autoreactive lymphocytes.38,39
Studies of survivin expression in lymphocytes from patients with active relapsing-remitting MS indicated that survivin expression was detected in intrathecal lymphocytes but not in peripheral lymphocytes in MS patients,38 suggesting a potential role for survivin in MS pathology. Expression of survivin but not Bcl-2 was strongly induced in mitogen-stimulated intrathecal and peripheral T lymphocytes from MS patients compared with various controls.38,40 This correlated with T lymphocyte resistance to apoptosis in a Fas-independent manner38,40 as well as with features of disease activity, including disease duration and the number of enhanced lesions.40 Although these findings suggest an association between abnormal induction of survivin expression and disease pathology, determination of the molecular basis for the initial survivin increase in intrathecal lymphocytes might be important for disease treatment.
It is known that interferon-β treatment of MS patients reduces clinical exacerbations by augmentation of apoptosis in peripheral T lymphocytes. Studies from 26 patients with MS showed that treatment with interferon-β1 reduced the expression of survivin in the in vitro-stimulated T lymphocytes without significant alteration of the expression of Bcl-2 and Fas.39 Reduction of survivin expression correlated with augmented T-cell susceptibility to apoptosis and with clinical response to treatment.39 Interestingly, studies from 19 MS patients who received long-term interferon-β1 treatment showed that interferon-β1-mediated down-regulation of survivin expression was maintained after long-term therapy.39 These observations suggest that the favorable clinical effect of interferon-β1 on MS patients is at least in part due to interferon-β1-mediated inhibition of survivin expression.
Survivin and Brain Pathology
Expression of survivin was shown in fetal brain tissues41 and the polysialylated NCAM-expressing immature neurons42 but was undetectable in adult human and mouse brain tissues.41,43 It was also reported that normal ependyma and choroid plexus in tissue sections of perinatal and pediatric brain autopsies express a high level of survivin in cell nuclei as determined by immunohistochemistry.44 Although the high survivin positivity in perinatal and pediatric brain cell nuclei remains to be confirmed,45 further investigation of the role for survivin in perinatal brain maturation would be important because unique mechanisms for survivin regulation and/or function different from those in brain pathology may be identified that could be of potential therapeutic use.
Brain injury induces survivin expression. Traumatic brain injury in rats showed a remarkable and sustained induction of survivin in the ipsilateral cortex and hippocampus, peaking at 5 days post injury, whereas survivin was virtually undetectable in craniotomy control brains.46 Brain injury also increases apoptosis. A time-dependent activation of caspase-3 between 5 and 14 days postinjury was observed, and DNA fragmentation was significantly greater in survivin-negative cells than in survivin-positive cells.47 These observations indicate a role for survivin in enhancing neural cell survival after traumatic brain injury, and induction of survivin expression may be used to block brain cell death for apoptosis-generating treatments associated with disease. Using a mouse stroke model to evaluate the in vivo role of survivin in brain in response to hypoxia/ischemia, Conway et al48 showed that permanent occlusion of middle cerebral arteries for 2 days induced survivin expression by microvessels that form in the peri-infarct and infarct regions. Vessel density was significantly reduced in mice with heterozygous deficiency of the survivingene (survivin+/− mice), even though infarct sizes were not different.48 Thus, these findings not only suggest a role for survivin in the extent of vascularization of the infarct but also support our notion for treatment of brain injury by up-regulation of survivin.
Survivin and Liver Pathophysiology
Survivin appears to be involved in liver regeneration and cell survival. Partial hepatectomy or intraperitoneal carbon tetrachloride injection in a murine liver model strikingly elevated the expression of survivin associated with the onset of DNA synthesis.49 Ectopic expression of survivin in a normal liver cell line resulted in a decreased G0/G1 cell fraction with increased numbers of S and G2/M cells in conjunction with Rb phosphorylation.49 Although these observations imply a role for survivin in normal liver tissue regeneration by driving liver cells temporarily into the cell cycle, an important point that should be addressed in the future is to identify the potential differences and similarities for survivin control of G1/S transition in normal liver cells versus abnormal/cancerous liver cells. On the other hand, homologous knockouts of the survivingene induced liver cells into a proapoptotic state in the survivin+/− mouse liver, evidenced by activation of caspases, accumulation of Bax, and release of cytochrome c relative to that in survivin+/+ mouse liver cells.16 This implies a role for survivin in normal liver cell survival. Interestingly, intraperitoneal injection of the Fas agonistic antibody Jo2 into survivin+/− mice caused liver hemorrhagic necrosis 2 hours after injection, which was associated with prominent activation of the apoptotic pathway via the mitochondria and up-regulation of survivin in the cytosol, nuclei, and mitochondria, whereas this treatment had no effect on survivin+/+ mice at 2 hours.16 These observations imply that liver cells from survivin+/− mice showed decreased survival capacity, and increased survivin may reflect a compensatory mechanism to counter against insult-induced liver cell death. However, it was reported that survivin and XIAP were down-regulated during liver ischemia determined by cDNA microarray analyses.50 This result may require confirmation by alternative approaches such as Northern and/or Western blots because inappropriate time points, such as after significant cell death occurs, may miss the real story. In any case, future studies should focus on delineating the function and the mechanism of regulation behind these modulations of survivin in the liver. Using isolated normal rat hepatocytes to study apoptosis induced by the toxic bile acid glycochenodeoxycholate, Wang et al51 reported that survivin, cIAP1, cIAP2, and XIAP were down-regulated by pan-caspase inhibitor Z-VAD-fmk in addition to inhibition of caspase activity, cytochrome c release, and nuclear factor-κB activation. Because the role of the pan-caspase inhibitor Z-VAD-fmk in the down-regulation of IAPs has not been reported in cancer cells, this observation may imply that Z-VAD-fmk may differentially affect signaling pathways in normal versus cancer cells.
Survivin and Pancreas Pathophysiology
Expression of survivin was found in human fetal pancreas islets,13 and recent studies reported that survivin was expressed in α- and β-cells of the fetal pancreas islet but not in δ-cells.52 Its expression was subsequently lost in β-cells but remained in the α-cells of newborns and adult subjects, whereas δ-cells showed a sustained lack of survivin in neonate and adult issues.52 The expression profile of survivin in different subtypes of islet cells during the fetal to newborn transition not only implies a strictly regulated expression of survivin in the pancreas but also suggests that the potential role for survivin in pancreas development is not restricted to cell proliferation. Tashiro et al showed that after induction of acute necrohemorrhagic pancreatitis by intraductal infusion of 4% sodium taurocholate in male Wistar rats, survivin mRNA was significantly increased by 36 hours and peaked at 48 hours, whereas survivin protein was found in the cytoplasm and/or nuclei of acinar and ductal cells and in infiltrating cells at 48 to 72 hours.53 This implies transcriptional and posttranscriptional control of survivin expression in pancreas pathology and further suggests a potential role for survivin in pathological conditions of the pancreas. Importantly, it has been shown that normal human adult pancreata do not express survivin, but survivin is overexpressed in pancreatic cancer, and its expression is correlated with disease stages,54 thus suggesting an important role for survivin in pancreatic cancer development and progression. As we know, the etiology for pancreatic cancer is largely unknown. The disease has very high mortality rates and currently lacks effective approaches for treatment. Although pancreatic cancer only accounts for 2% of new cases of cancer, it is the fourth leading cause of all cancer deaths. Thus, it would be important to elucidate the molecular mechanisms for the regulation and function of survivin in pancreas development versus pathological conditions including cancer. This may define survivin as an important new target for pancreatic cancer treatment. In addition, these studies may lead to the development of novel approaches for the treatment of pancreatic cancer as well as other diseases.
Survivin and Gastrointestinal Pathophysiology
Gastric mucosa undergoes continuous surface epithelial cell renewal with a rapid turnover rate of 3 to 5 days. It was shown that survivin is expressed in normal gastric mucosa of adult humans and rats predominantly in the nuclei of mucosal surface epithelial cells,55 although the significance for survivin expression in the epithelial cells remains to be investigated. The function of survivin in epithelial cell renewal appears to be important. The data derived from this model may provide new perspectives and lead to novel approaches for treatment of various gastrointestinal-associated diseases including cancer and ulcers. Although some progress has been made, efforts exploring and comparing the molecular mechanisms for survivin function and regulation in normal epithelial cell renewal versus pathological conditions including cancer should be emphasized in the future. It was found that indomethacin, a nonsteroidal anti-inflammatory drug, reduced survivin expression before severe injury of gastric mucosa and RGM-1 gastric epithelial cells and that silencing of survivin by siRNA in RGM-1 cells induced cell damage and increased susceptibility to injury by indomethacin.56 NS-398, another Cox-2 inhibitor, however, neither altered survivin expression nor produced injury in vivo or in vitro.56 These observations suggest that drug-mediated inhibition of survivin expression was associated with injury of the gastric mucosa. On the other hand, several reports showed that inhibition of Cox-2 down-regulates survivin expression and sensitizes cells to apoptosis in many types of cancer cells.57–59 Therefore, application of Cox-2 inhibitors for cancer therapeutics may have potential toxicities to normal gastrointestinal structures. One possible way to alleviate the side effect of Cox-2 inhibitors may be to combine a Cox-2 inhibitor with a drug that has the side effect of elevating survivin expression (a drug- resistant factor) to neutralize the side effects and to increase cancer cell death with less injury to normal tissues. A recent report showed that the highly selective Cox-2 inhibitor CAY10404 induced apoptosis in neuroblastoma cells without down-regulation of survivin and XIAP,60 suggesting survivin/XIAP-independent induction of apoptosis by CAY10404. Thus, it would be interesting to determine whether CAY10404 is less toxic to normal gastrointestinal tissues without sacrifice of the effectiveness of the drug in killing cancer cells. As mentioned above, mechanisms for the regulation and/or function of survivin could be different in different pathophysiological conditions. This would provide therapeutic opportunities for disease treatment when using survivin as a target. As an example, rebamipide, a free radical-scavenging drug, is known to accelerate healing of gastric ulcers and gastritis. However, rebamipide significantly down-regulated survivin expression and inhibited AGS gastric cancer cell growth.61 Finally, the expression of survivin in normal gastrointestinal epithelium was shown to be strictly regulated,62 and abnormal expression of survivin in epithelial cells appears to be involved in tumorigenesis.19 Thus, deregulation of survivin expression in individual epithelial cells may represent an early change toward gastrointestinal carcino-genesis. Intuitively, identification of these molecular pathways may provide improved cancer therapeutic approaches.
Survivin and Spermatogenic Pathophysiology
Survivin appears to play an important role in spermatogenesis63,64 because deregulation of survivin expression could result in either spermatogenic failure64–66 or tumorigenesis.67 Survivin was highly expressed in the testes of mice43 and humans (D. Pan, A. Ghadersohi, G. Nielsen, S. Jensen, F. Gellert, X. Ling, A. Black, F. Li, unpublished data). Analysis of survivin expression throughout the seminiferous epithelial cycle of the rat showed a strictly regulated expression of survivin, and its expression was induced by stem-cell factors in vitro.63 Excessive apoptosis has been shown to be associated with male infertility. Analysis of testicular biopsy specimens from normal and azoospermic men showed that survivin expression decreased in specimens isolated from patients with spermatogenesis disorders compared with those from normal individuals.64–66 The level of survivin decrease correlated with the degree of spermatogenic failure,65 and its expression was lost in most specimens with severe spermatogenic failure.66 These observations suggest an important role for survivin in the generation of healthy sperm.
Our recent study revealed that human testis is the only human adult tissue expressing survivin at high levels with 60 to 70% positivity in the nuclei of spermatogonia (B. Spaulding, D. Pan, A. Ghadersohi, G. Nielsen, S. Jensen, F. Gellert, X. Ling, A. Black, F. Li, unpublished observation). Thus, a challenging but important question is why high expression of survivin in testis does not show tumorigenesis. This would seem to be attributable to different mechanisms for survivin regulation and function in spermatogenesis versus tumorigenesis. For example, male germ cell generation involves mitosis, meiosis, and apoptotic culling, and survivin also plays important roles in the regulation of mitosis and apoptosis in cancer cells.1,2 It is likely that the signaling transduction and protein-protein interactions associated with the regulation and function of survivin may vary in different pathophysiological conditions. As an example, survivin up-regulated the expression of human telomerase reverse transcriptase in both colon cancer tissues and cancer cell lines.68 Loss of survivin expression in specimens with severe spermatogenic failure, however, did not correlate with the loss of human telomerase reverse transcriptase expression in these cells.66 In sum, characterization of the molecular mechanisms for the regulation and function of survivin during spermatogenesis versus those associated with tumor cell proliferation should potentially generate valuable information for the development of novel approaches for targeted treatment of cancer and possibly other diseases.1,2
Survivin and Endometrial Pathophysiology
Survivin is strictly regulated during endometrial formation,69 and its abnormal increase may contribute to endometriosis70 or cancer progression.71,72 Survivin protein was strongest in the nuclei of glandular epithelial cells during the late secretory phase but not detected in the proliferative phase, whereas Bcl-2 was strongest in the proliferative phase.69 This suggests that a role for survivin in the homeostasis of normal endometrium that is likely different from Bcl-2. In addition, the lack of survivin expression in the endometrial proliferative phase may reflect roles other than promoting cell proliferation. It was postulated that survivin increase might be due to the concurrent rise in progesterone concentrations during the normal menstrual cycle.69 Consistent with this notion, progesterone treatment led to polyadenylation and accumulation of the survivintranscript during maturation of Xenopus oocytes.14 However, progesterone was shown to down-regulate survivin in breast cancer cells.20 Thus, the different response of survivin to progesterone may reflect fundamental differences in the regulation of survivin expression in normal versus at least certain types of abnormal tissues.
Studies of 63 tissues from 35 women with endometriosis revealed that the expression of survivin in clinically aggressive pigmented lesions was significantly higher than those in normal eutopic endometrium from 12 women without endometriosis and those with nonpigmented lesions,70 suggesting that deregulated increases of survivin may be involved in the etiology of this disease. Survivin expression was closely correlated with the expression of matrix metalloproteinase (MMP)-2, MMP-9, and MT1-MMP as well as with apoptosis inhibition in these endometriotic tissues,70 suggesting a possibility that both survivin and MMPs contribute to survival and invasion of endometriosis. In addition, it was also reported that survivin expression in endothelial cells is involved in the growth of ovarian endometriotic cysts.73 Importantly, increased survivin expression was found in endometrial carcinoma compared with normal endometrium and was significantly associated with proliferating cell nuclear antigen-labeling index, clinical stage, histological grade, invasion, and poor patient survival,71 suggesting an important role for survivin in endometrial cancer. Moreover, tissue microarray analysis revealed that up-regulation of survivin in endometrial carcinoma is associated with the activation of Stat3 and Akt.72 This finding may have important therapeutic values to treat endometrial cancer because normal tissues have no or little activation of Stat3 and Akt. As we emphasized above and from a therapeutic point of view, further studies should focus on the delineation of the underlying molecular mechanisms for the regulation and function of survivin in normal endometrium versus pathological conditions including cancer.
Survivin and Placenta Pathophysiology
Survivin expression was strictly regulated during placenta homeostasis74 and significantly higher in hydatidiform mole than in non-neoplastic placentas,75 suggesting a role for survivin in placental homeostasis as well as in certain pathological conditions. Ka and Hunt76 studied the expression of various IAP proteins, including c-IAP-1, c-IAP-2, XIAP, NAIP, survivin, and livin in placenta, and found that the expression of survivin showed an opposite pattern to that of NAIP and livin, suggesting a unique role for survivin in placenta homeostasis. Overall, various IAP proteins were expressed in various kinds of placenta cells.76 Thus, the specific role for survivin in placenta homeostasis is not clear based on this study. Nevertheless, these authors concluded that the results are consistent with the postulate that IAP proteins have critical roles in placental cell survival and suggest that specific apoptosis inhibitors may protect normal and transformed trophoblast cells from death.76 In any case, whether survivin plays a critical role that is different from these for other IAPs in placenta homeostasis remains to be determined.
Concluding Remarks
It is clear that the studies using mouse transgenic or knockout models such as the ones for studying survivin in T-cell development are comprehensive and have provided tremendous insights into the function of survivin in normal cell proliferation. Many other studies of survivin in normal tissues or cells, however, are largely restricted to descriptions of the expression and regulation of survivin during various pathophysiological conditions. This is partially due to the lack of sufficient recognition for the potential of survivin functions in normal tissues as well as a lack of appropriate in vitro and in vivo model systems compared with various model options for cancer research. Thus, development of appropriate in vitro and in vivo model systems would be important for bringing studies of survivin in normal tissues or cells to the next level.
Although the role for survivin in normal cells (Figure 1) significantly overlaps the role of survivin in cancer cells,2 the fact that targeting survivin for cancer therapeutics shows little toxicity to normal tissues/cells indicates that the mechanisms for the regulation and function of survivin in normal tissues/cells versus a significant number of types of abnormal cells, including cancer, have to be somehow different. Characterization of these differences at the molecular level would certainly provide opportunities to enhance the therapeutic index of targeting survivin in cancer and possibly in other diseases. Growing evidence points to differential interactions, regulation, and function for survivin in normal versus abnormal/cancerous tissues/cells.1,2,21 Survivin plays an essential role in the regulation of apoptosis and control of cell division in cancer cells.1,2 However, many signal interactions associated with survivin regulation and function are lacking or significantly weaker in normal cells compared with those in cancer cells.2,21 This makes survivin a potentially superior target not only for the treatment of cancer and possible other diseases but also for diagnosis and prevention of these diseases.1,2,21 As we emphasized above, future studies should focus on determining the potential differences of survivin in normal versus various pathological conditions including cancer. This would provide a base for the rational exploitation of survivin as a therapeutic target for disease treatment.
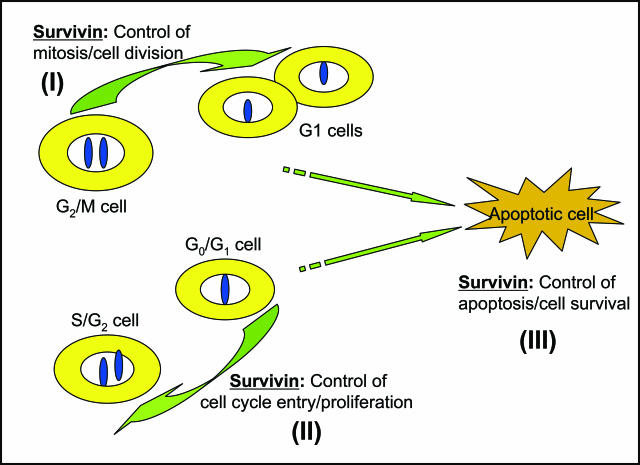
Figure 1.
Three basic roles for survivin in normal cells. I, survivin controls mitosis and cell division. II, survivin promotes cell cycle entry and proliferation. III, survivin maintains cell survival and inhibits apoptosis. As shown, during the periods when survivin performs the first two roles, cells could enter into apoptosis due to a cellular default setting or drug induction, etc. Thus, it is likely that the third role of survivin is required for completion of the first and second roles. Given that there are similar roles for survivin in cancer cells, a critical issue is whether the underlying mechanism for these functions in normal cells is quantitatively and/or qualitatively different from these in cancer cells. This is one of the most important research directions in the future.
Acknowledgments
We thank Dr. Audra Cox for her many constructive and helpful comments and suggestions during the editing of this manuscript. We apologize that some relevant reports may not have been cited because of space limitations.
Footnotes
Address reprint requests to Fengzhi Li, Ph.D., Department of Pharmacology and Therapeutics, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263. E-mail: fengzhi.li@roswellpark.org.
Supported by the grants from National Institutes of Health/National Cancer Institute (R01, CA109481) and Concern Foundation (Beverly Hill, CA) (to F.L.) and by grants from National Institutes of Health/National Cancer Institute (R01, CA034432, CA054807, and CA072001; and R37, CA038173 to M.G.B.).
References
- Li F. Survivin study: what is the next wave? J Cell Physiol. 2003;197:8–29. doi: 10.1002/jcp.10327. [DOI] [PubMed] [Google Scholar]
- Li F, Ling X: Survivin study: an update of “What is the next wave?” J Cell Physiol 2006, [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Mesri M, Wall NR, Li J, Kim RW, Altieri DC. Cancer gene therapy using a survivin mutant adenovirus. J Clin Invest. 2001;108:981–990. doi: 10.1172/JCI12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DS, Wall NR, Porter AC, Altieri DC. A p34(cdc2) survival checkpoint in cancer. Cancer Cell. 2002;2:43–54. doi: 10.1016/s1535-6108(02)00084-3. [DOI] [PubMed] [Google Scholar]
- Wall NR, O’Connor DS, Plescia J, Pommier Y, Altieri DC. Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer Res. 2003;63:230–235. [PubMed] [Google Scholar]
- Plescia J, Salz W, Xia F, Pennati M, Zaffaroni N, Daidone MG, Meli M, Dohi T, Fortugno P, Nefedova Y, Gabrilovich DI, Colombo G, Altieri DC. Rational design of shepherdin, a novel anticancer agent. Cancer Cell. 2005;7:457–468. doi: 10.1016/j.ccr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Xiang R, Mizutani N, Luo Y, Chiodoni C, Zhou H, Mizutani M, Ba Y, Becker JC, Reisfeld RA. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res. 2005;65:553–561. [PubMed] [Google Scholar]
- Otto K, Andersen MH, Eggert A, Keikavoussi P, Pedersen LO, Rath JC, Bock M, Brocker EB, Straten PT, Kampgen E, Becker JC. Lack of toxicity of therapy-induced T cell responses against the universal tumour antigen survivin. Vaccine. 2005;23:884–889. doi: 10.1016/j.vaccine.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Greene MI. EGFR enhances survivin expression through the phosphoinositide 3 (PI-3) kinase signaling pathway. Exp Mol Pathol. 2005;79:100–107. doi: 10.1016/j.yexmp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Qiu L, Wang Q, Di W, Jiang Q, Schefeller E, Derby S, Wanebo H, Yan B, Wan Y. Transient activation of EGFR/AKT cell survival pathway and expression of survivin contribute to reduced sensitivity of human melanoma cells to betulinic acid. Int J Oncol. 2005;27:823–830. [PubMed] [Google Scholar]
- Adida C, Crotty PL, McGrath J, Berrebi D, Diebold J, Altieri DC. Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol. 1998;152:43–49. [PMC free article] [PubMed] [Google Scholar]
- Murphy CR, Sabel JL, Sandler AD, Dagle JM. Survivin mRNA is down-regulated during early Xenopus laevis embryogenesis. Dev Dyn. 2002;225:597–601. doi: 10.1002/dvdy.10194. [DOI] [PubMed] [Google Scholar]
- Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ, Vaux DL, Choo KH. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol. 2000;10:1319–1328. doi: 10.1016/s0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- Conway EM, Pollefeyt S, Steiner-Mosonyi M, Luo W, Devriese A, Lupu F, Bono F, Leducq N, Dol F, Schaeffer P, Collen D, Herbert JM. Deficiency of survivin in transgenic mice exacerbates Fas-induced apoptosis via mitochondrial pathways. Gastroenterology. 2002;123:619–631. doi: 10.1053/gast.2002.34753. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Sato N, Fukuda J, Kodama H, Kumagai J, Tanikawa H, Shimizu Y, Tanaka T. Survivin acts as an antiapoptotic factor during the development of mouse preimplantation embryos. Dev Biol. 2003;256:331–341. doi: 10.1016/s0012-1606(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Jiang Y, de Bruin A, Caldas H, Fangusaro J, Hayes J, Conway EM, Robinson ML, Altura RA. Essential role for survivin in early brain development. J Neurosci. 2005;25:6962–6970. doi: 10.1523/JNEUROSCI.1446-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Role of survivin and its splice variants in tumorigenesis. Br J Cancer. 2005;92:212–216. doi: 10.1038/sj.bjc.6602340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formby B, Wiley TS. Bcl-2, survivin and variant CD44 v7–v10 are downregulated and p53 is upregulated in breast cancer cells by progesterone: inhibition of cell growth and induction of apoptosis. Mol Cell Biochem. 1999;202:53–61. doi: 10.1023/a:1007081021483. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yang J, Li F: Transcriptional and posttranscriptional controls of survivin in cancer cells: essential interfaces for developing novel approaches for cancer treatment. J Exp Clin Cancer Res 2006, in press [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Pelus LM. Regulation of the inhibitor-of-apoptosis family member survivin in normal cord blood and bone marrow CD34(+) cells by hematopoietic growth factors: implication of survivin expression in normal hematopoiesis. Blood. 2001;98:2091–2100. doi: 10.1182/blood.v98.7.2091. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Pelus LM. Elevation of survivin levels by hematopoietic growth factors occurs in quiescent CD34(+) hematopoietic stem and progenitor cells before cell cycle entry. Cell Cycle. 2002;1:322–326. [PubMed] [Google Scholar]
- Fukuda S, Foster RG, Porter SB, Pelus LM. The antiapoptosis protein survivin is associated with cell cycle entry of normal cord blood CD34(+) cells and modulates cell cycle and proliferation of mouse hematopoietic progenitor cells. Blood. 2002;100:2463–2471. doi: 10.1182/blood.V100.7.2463. [DOI] [PubMed] [Google Scholar]
- Altznauer F, Martinelli S, Yousefi S, Thurig C, Schmid I, Conway EM, Schoni MH, Vogt P, Mueller C, Fey MF, Zangemeister-Wittke U, Simon HU. Inflammation-associated cell cycle-independent block of apoptosis by survivin in terminally differentiated neutrophils. J Exp Med. 2004;199:1343–1354. doi: 10.1084/jem.20032033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurbuxani S, Xu Y, Keerthivasan G, Wickrema A, Crispino JD. Differential requirements for survivin in hematopoietic cell development. Proc Natl Acad Sci USA. 2005;102:11480–11485. doi: 10.1073/pnas.0500303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Ling X, Huang H, Brattain L, Apontes P, Wu J, Binderup L, Brattain MG. Differential regulation of survivin expression and apoptosis by vitamin D(3) compounds in two isogenic MCF-7 breast cancer cell sublines. Oncogene. 2005;24:1385–1395. doi: 10.1038/sj.onc.1208330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Banerjee P, Sieburg M, Planelles V, Li F, Feuer G. Induction of cell cycle arrest by human T-cell lymphotropic virus type 1 Tax in hematopoietic progenitor (CD34+) cells: modulation of p21cip1/waf1 and p27kip1 expression. J Virol. 2005;79:14069–14078. doi: 10.1128/JVI.79.22.14069-14078.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Mantel CR, Pelus LM. Survivin regulates hematopoietic progenitor cell proliferation through p21WAF1/Cip1-dependent and -independent pathways. Blood. 2004;103:120–127. doi: 10.1182/blood-2003-05-1756. [DOI] [PubMed] [Google Scholar]
- Braun SE, Mantel C, Rosenthal M, Cooper S, Liu L, Robertson KA, Hromas R, Broxmeyer HE. A positive effect of p21cip1/waf1 in the colony formation from murine myeloid progenitor cells as assessed by retroviral-mediated gene transfer. Blood Cells Mol Dis. 1998;24:138–148. doi: 10.1006/bcmd.1998.0181. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Yukiue H, Sasaki H, Fukai I, Yokoyama T, Kiriyama M, Yamakawa Y, Maeda M, Fujii Y. Developmentally regulated expression of survivin in the human thymus. Hum Immunol. 2002;63:101–107. doi: 10.1016/s0198-8859(01)00369-x. [DOI] [PubMed] [Google Scholar]
- Xing Z, Conway EM, Kang C, Winoto A. Essential role of survivin, an inhibitor of apoptosis protein, in T cell development, maturation, and homeostasis. J Exp Med. 2004;199:69–80. doi: 10.1084/jem.20031588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornacker M, Verneris MR, Kornacker B, Scheffold C, Negrin RS. Survivin expression correlates with apoptosis resistance after lymphocyte activation and is found preferentially in memory T cells. Immunol Lett. 2001;76:169–173. doi: 10.1016/s0165-2478(01)00186-9. [DOI] [PubMed] [Google Scholar]
- Okada H, Bakal C, Shahinian A, Elia A, Wakeham A, Suh WK, Duncan GS, Ciofani M, Rottapel R, Zuniga-Pflucker JC, Mak TW. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J Exp Med. 2004;199:399–410. doi: 10.1084/jem.20032092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikita S, Hatano M, Inoue A, Sekita N, Kobayashi K, Otaki M, Ogasawara T, Okada S, Hirasawa H, Tokuhisa T. Overexpression of TIAP/m-survivin in thymocytes enhances cell proliferation. Mol Immunol. 2002;39:289–298. doi: 10.1016/s0161-5890(02)00111-6. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fukuda S, Pelus LM. Survivin regulates the p53 tumor suppressor gene family. Oncogene. 2004;23:8146–8153. doi: 10.1038/sj.onc.1207992. [DOI] [PubMed] [Google Scholar]
- Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Sharief MK, Semra YK. Heightened expression of survivin in activated T lymphocytes from patients with multiple sclerosis. J Neuroimmunol. 2001;119:358–364. doi: 10.1016/s0165-5728(01)00389-7. [DOI] [PubMed] [Google Scholar]
- Sharief MK, Semra YK. Down-regulation of survivin expression in T lymphocytes after interferon beta-1a treatment in patients with multiple sclerosis. Arch Neurol. 2002;59:1115–1121. doi: 10.1001/archneur.59.7.1115. [DOI] [PubMed] [Google Scholar]
- Sharief MK, Noori MA, Douglas MR, Semra YK. Upregulated survivin expression in activated T lymphocytes correlates with disease activity in multiple sclerosis. Eur J Neurol. 2002;9:503–510. doi: 10.1046/j.1468-1331.2002.00454.x. [DOI] [PubMed] [Google Scholar]
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- Pennartz S, Belvindrah R, Tomiuk S, Zimmer C, Hofmann K, Conradt M, Bosio A, Cremer H. Purification of neuronal precursors from the adult mouse brain: comprehensive gene expression analysis provides new insights into the control of cell migration, differentiation, and homeostasis. Mol Cell Neurosci. 2004;25:692–706. doi: 10.1016/j.mcn.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hatano M, Otaki M, Ogasawara T, Tokuhisa T. Expression of a murine homologue of the inhibitor of apoptosis protein is related to cell proliferation. Proc Natl Acad Sci USA. 1999;96:1457–1462. doi: 10.1073/pnas.96.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura RA, Olshefski RS, Jiang Y, Boue DR. Nuclear expression of survivin in paediatric ependymomas and choroid plexus tumours correlates with morphologic tumour grade. Br J Cancer. 2003;89:1743–1749. doi: 10.1038/sj.bjc.6601334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yang J, Ramnath N, Javle MM, Tan D. Nuclear or cytoplasmic expression of survivin: what is the significance? Int J Cancer. 2005;114:509–512. doi: 10.1002/ijc.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EA, Svetlov SI, Pike BR, Tolentino PJ, Shaw G, Wang KK, Hayes RL, Pineda JA. Cell-specific upregulation of survivin after experimental traumatic brain injury in rats. J Neurotrauma. 2004;21:1183–1195. doi: 10.1089/neu.2004.21.1183. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Svetlov SI, Wang KK, Hayes RL, Pineda JA. Cell-specific DNA fragmentation may be attenuated by a survivin-dependent mechanism after traumatic brain injury in rats. Exp Brain Res. 2005;167:17–26. doi: 10.1007/s00221-005-2362-2. [DOI] [PubMed] [Google Scholar]
- Conway EM, Zwerts F, Van Eygen V, DeVriese A, Nagai N, Luo W, Collen D. Survivin-dependent angiogenesis in ischemic brain: molecular mechanisms of hypoxia-induced up-regulation. Am J Pathol. 2003;163:935–946. doi: 10.1016/S0002-9440(10)63453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi M, Shiraki K, Inoue H, Okano H, Ito T, Yamanaka T, Sugimoto K, Sakai T, Ohmori S, Murata K, Furusaka A, Hisatomi H, Nakano T. Expression of survivin during liver regeneration. Biochem Biophys Res Commun. 2002;297:59–64. doi: 10.1016/s0006-291x(02)02128-9. [DOI] [PubMed] [Google Scholar]
- Lu QP, Cao TJ, Zhang ZY, Liu W. Multiple gene differential expression patterns in human ischemic liver: safe limit of warm ischemic time. World J Gastroenterol. 2004;10:2130–2133. doi: 10.3748/wjg.v10.i14.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Brems JJ, Gamelli RL, Ding J. Reversibility of caspase activation and its role during glycochenodeoxycholate-induced hepatocyte apoptosis. J Biol Chem. 2005;280:23490–23495. doi: 10.1074/jbc.M411607200. [DOI] [PubMed] [Google Scholar]
- Liggins C, Orlicky DJ, Bloomquist LA, Gianani R. Developmentally regulated expression of survivin in human pancreatic islets. Pediatr Dev Pathol. 2003;6:392–397. doi: 10.1007/s10024-003-2014-0. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Nakamura H, Taguchi M, Yoshikawa H, Otsuki M. Expression of survivin after acute necrohemorrhagic pancreatitis in rats. Pancreas. 2003;26:160–165. doi: 10.1097/00006676-200303000-00012. [DOI] [PubMed] [Google Scholar]
- Qiao JG, Zhang YQ, Yin YC, Tan Z. Expression of survivin in pancreatic cancer and its correlation to expression of Bcl-2. World J Gastroenterol. 2004;10:2759–2761. doi: 10.3748/wjg.v10.i18.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SK, Moon WS, Jones MK, Tarnawski AS. Survivin expression in the stomach: implications for mucosal integrity and protection. Biochem Biophys Res Commun. 2003;305:374–379. doi: 10.1016/s0006-291x(03)00724-1. [DOI] [PubMed] [Google Scholar]
- Chiou SK, Tanigawa T, Akahoshi T, Abdelkarim B, Jones MK, Tarnawski AS. Survivin: a novel target for indomethacin-induced gastric injury. Gastroenterology. 2005;128:63–73. doi: 10.1053/j.gastro.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Konturek PC, Konturek SJ, Bielanski W, Kania J, Zuchowicz M, Hartwich A, Rehfeld JF, Hahn EG. Influence of COX-2 inhibition by rofecoxib on serum and tumor progastrin and gastrin levels and expression of PPARgamma and apoptosis-related proteins in gastric cancer patients. Dig Dis Sci. 2003;48:2005–2017. doi: 10.1023/a:1026387908165. [DOI] [PubMed] [Google Scholar]
- Krysan K, Merchant FH, Zhu L, Dohadwala M, Luo J, Lin Y, Heuze-Vourc’h N, Pold M, Seligson D, Chia D, Goodglick L, Wang H, Strieter R, Sharma S, Dubinett S. COX-2-dependent stabilization of survivin in non-small cell lung cancer. FASEB J. 2004;18:206–208. doi: 10.1096/fj.03-0369fje. [DOI] [PubMed] [Google Scholar]
- Krysan K, Dalwadi H, Sharma S, Pold M, Dubinett S. Cyclooxygenase 2-dependent expression of survivin is critical for apoptosis resistance in non-small cell lung cancer. Cancer Res. 2004;64:6359–6362. doi: 10.1158/0008-5472.CAN-04-1681. [DOI] [PubMed] [Google Scholar]
- Parashar B, Latha Shankar S, O’Guin K, Butler J, Vikram B, Shafit-Zagardo B. Inhibition of human neuroblastoma cell growth by CAY10404, a highly selective Cox-2 inhibitor. J Neurooncol. 2005;71:141–148. doi: 10.1007/s11060-004-1721-3. [DOI] [PubMed] [Google Scholar]
- Tarnawski A, Pai R, Chiou SK, Chai J, Chu EC. Rebamipide inhibits gastric cancer growth by targeting survivin and Aurora-B. Biochem Biophys Res Commun. 2005;334:207–212. doi: 10.1016/j.bbrc.2005.05.204. [DOI] [PubMed] [Google Scholar]
- Zhang T, Otevrel T, Gao Z, Ehrlich SM, Fields JZ, Boman BM. Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61:8664–8667. [PubMed] [Google Scholar]
- Wang Y, Suominen JS, Hakovirta H, Parvinen M, Martinand-Mari C, Toppari J, Robbins I. Survivin expression in rat testis is upregulated by stem-cell factor. Mol Cell Endocrinol. 2004;218:165–174. doi: 10.1016/j.mce.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Weikert S, Schrader M, Muller M, Krause H, Miller K. Expression of the apoptosis inhibitor survivin in testicular tissue of infertile patients. Int J Androl. 2004;27:161–165. doi: 10.1111/j.1365-2605.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- Weikert S, Schrader M, Muller M, Schulze W, Krause H, Miller K. Expression levels of the inhibitor of apoptosis survivin in testes of patients with normal spermatogenesis and spermatogenic failure. Fertil Steril. 2005;83(Suppl 1):1100–1105. doi: 10.1016/j.fertnstert.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Weikert S, Christoph F, Schulze W, Krause H, Kempkensteffen C, Schostak M, Miller K, Schrader M. Testicular expression of survivin and human telomerase reverse transcriptase (hTERT) associated with spermatogenic function in infertile patients. Asian J Androl. 2006;8:95–100. doi: 10.1111/j.1745-7262.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- Weikert S, Schrader M, Krause H, Schulze W, Muller M, Miller K. The inhibitor of apoptosis (IAP) survivin is expressed in human testicular germ cell tumors and normal testes. Cancer Lett. 2005;223:331–337. doi: 10.1016/j.canlet.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Endoh T, Tsuji N, Asanuma K, Yagihashi A, Watanabe N. Survivin enhances telomerase activity via up-regulation of specificity protein 1- and c-Myc-mediated human telomerase reverse transcriptase gene transcription. Exp Cell Res. 2005;305:300–311. doi: 10.1016/j.yexcr.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Konno R, Yamakawa H, Utsunomiya H, Ito K, Sato S, Yajima A. Expression of survivin and Bcl-2 in the normal human endometrium. Mol Hum Reprod. 2000;6:529–534. doi: 10.1093/molehr/6.6.529. [DOI] [PubMed] [Google Scholar]
- Ueda M, Yamashita Y, Takehara M, Terai Y, Kumagai K, Ueki K, Kanda K, Yamaguchi H, Akise D, Hung YC, Ueki M. Survivin gene expression in endometriosis. J Clin Endocrinol Metab. 2002;87:3452–3459. doi: 10.1210/jcem.87.7.8682. [DOI] [PubMed] [Google Scholar]
- Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I. Survivin expression correlates with clinical stage, histological grade, invasive behavior and survival rate in endometrial carcinoma. Cancer Lett. 2002;184:105–116. doi: 10.1016/s0304-3835(02)00190-8. [DOI] [PubMed] [Google Scholar]
- Pallares J, Martinez-Guitarte JL, Dolcet X, Llobet D, Rue M, Palacios J, Prat J, Matias-Guiu X. Survivin expression in endometrial carcinoma: a tissue microarray study with correlation with PTEN and STAT-3. Int J Gynecol Pathol. 2005;24:247–253. doi: 10.1097/01.pgp.0000163849.37129.d4. [DOI] [PubMed] [Google Scholar]
- Goteri G, Lucarini G, Pieramici T, Filosa A, Pugnaloni A, Montik N, Biagini G, Tranquilli AL, Fabris G, Ciavattini A, Lo Muzio L. Endothelial cell survivin is involved in the growth of ovarian endometriotic cysts. Anticancer Res. 2005;25:4313–4318. [PubMed] [Google Scholar]
- Shiozaki A, Kataoka K, Fujimura M, Yuki H, Sakai M, Saito S. Survivin inhibits apoptosis in cytotrophoblasts. Placenta. 2003;24:65–76. doi: 10.1053/plac.2002.0860. [DOI] [PubMed] [Google Scholar]
- Lehner R, Bobak J, Kim NW, Shroyer AL, Shroyer KR. Localization of telomerase hTERT protein and survivin in placenta: relation to placental development and hydatidiform mole. Obstet Gynecol. 2001;97:965–970. doi: 10.1016/s0029-7844(01)01131-0. [DOI] [PubMed] [Google Scholar]
- Ka H, Hunt JS. Temporal and spatial patterns of expression of in-hibitors of apoptosis in human placentas. Am J Pathol. 2003;163:413–422. doi: 10.1016/S0002-9440(10)63671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]