Abstract
We examined the role of phagocyte-derived oxygen radicals in tumor cell acquisition of metastatic phenotype by comparing gp91phox−/− mice and C57BL/6J wild-type (WT) mice. The gp91phox−/− mouse is deficient in the gp91phox gene, an essential subunit of the phagocyte nicotinamide adenine dinucleotide phosphate oxidase that generates superoxide anion. QR-32 fibrosarcoma cells are nonmetastatic but are converted into metastatic tumors once in contact with foreign body (gelatin sponge)-induced phagocytes in vivo. Compared to QR-32 cells co-implanted with the foreign body in WT mice, those in gp91phox−/− mice exhibited reduced metastasis. There was no difference in the incidence of primary tumors after injection of B16BL6 melanoma cells in WT and gp91phox−/− mice. However, after resection of the primary tumors, metastases were reduced in gp91phox−/− mice. Thymosin β4 gene expression and cell motility/invasion were seen in the tumors from WT mice but not in those from gp91phox−/− mice. Adoptive transfer of phagocytes from WT mice, but not those from gp91phox−/− mice, restored the metastatic ability of tumors grown in gp91phox−/− mice. These findings show that tumor metastatic behavior can primarily be endowed by phagocyte-derived superoxide anion and its oxidative metabolites, which are generated through activation of nicotinamide adenine dinucleotide phosphate oxidase.
Metastasis has a significant impact on the mortality in cancer patients, as shown by the overall 5-year survival rate being less than 20% because of metastases.1 Metastatic disease is generally difficult to treat with currently available therapies, and thus an improved understanding of the underlying molecular and cellular mechanisms of tumor metastasis is needed. A tumor undergoes a complex series of processes to achieve metastasis,2,3 which is termed “multistep carcinogenesis.” Tumor metastasis is promoted by genetic alterations in each step,4 and epigenetic changes of crucial genes related to organ-specific colonization have been elucidated to some extent.5–7
Although little is known about the causative factors that convert benign tumors into malignant ones, it has been proposed that metastatic tumor cells originate in a pre-existing or dynamic heterogeneity of tumor cells with metastatic potential that reside in the primary tumor8,9 and that expand to clinically distinguishable metastasis in the environment of the host (selection). In the current study, we aimed to identify the endogenous factors that can promote tumor metastasis.
Among endogenous factors potentially related to tumor development and progression, oxygen radicals are thought to have a major influence. Epidemiological studies have indicated that genotoxic reactive oxygen species (ROS) are implicated in one-fourth to one-third of all cancers.10,11 In vitro studies have revealed that oxygen radicals may be associated with tumor development.12 However, in general, research approaches have been indirect, ie, they are performed using in vitro systems or by using antioxidative enzymes,13,14 and thus interpretation of the results remains ambiguous. To verify the involvement of ROS in tumor development and progression, we used mice that have targeted disruption of a gene related to ROS production in inflammatory phagocytes.
The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a membrane-bound cytochrome b558 composed of two subunits (gp91phox and p22 phox) that coordinate flavin adenine dinucleotide and two heme moieties.15 With inflammatory stimulation, the cytosolic subunits p40phox, p47phox, p67phox, and the small GTP-binding protein Rac1/Rac2 translocate to the membrane and interact with cytochrome b558.16–18 The assembled complex mediates electron transfer to extracellular molecular oxygen (O2), resulting in the generation of superoxide anion (O2−) during the phagocyte respiratory burst.19,20 The physiological significance of the phagocyte NADPH oxidase in host defense is indicated by the severe, recurrent bacterial or fungal infections that occur in patients with chronic granulomatous disease. Phagocytes in those patients are unable to generate O2− because of various mutations in four of the oxidase proteins (p22phox, p47phox, p67phox, and gp91phox),21 especially a glycosylated 91-kd glycoprotein (gp91phox).19 gp91phox−/− mice generated by disruption of the X-linked murine cytochrome b558 lack a functional NADPH oxidase, and their phagocytes are unable to generate superoxide anion, thus providing a murine model of chronic granulomatous disease.22
In this study, we demonstrated the contribution of NADPH oxidase-derived superoxide and its oxidative metabolites to acquisition of the metastatic phenotype, using two different models in which we were able to observe the natural transition in vivo of nonmetastatic (or metastatic) tumor cells to metastatic (or highly metastatic) ones. We also present evidence that inflammatory phagocytes can serve as an endogenous factor to irreversibly convert tumor cells into metastatic ones (ie, genetic alteration) by generating ROS in vivo.
Materials and Methods
Tumor Cell Lines and Culture Conditions
The origin and characteristics of the tumor cell lines used in this study have been described previously.23 Briefly, the QR-32 tumor is a clone obtained from 3-methyl-cholanthrene-induced fibrosarcoma in a C57BL/6J mouse that spontaneously regressed when injected into normal syngeneic mice.24 QR-32 and its derivative metastatic cell line (QRsP-11) were maintained in Eagle’s minimum essential medium (MEM; Nissui Pharm., Tokyo, Japan) supplemented with 8% (v/v) fetal bovine serum (Filtron Pty., Ltd., Brookyln, Australia). B16BL6 melanoma cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma, Tokyo, Japan) with 10% fetal bovine serum.
Mice
gp91phox−/− mice, established as previously described,22 lack the membrane gp91phox subunit of the NADPH oxidase multicomponent system. The knockout mice were backcrossed with C57BL6J mice for more than 20 generations. C57BL/6J mice (introduced from the Jackson Laboratory, Bar Harbor, ME) were obtained from Nippon SLC (Hamamatsu, Japan) and used as WT mice. Age- and sex-matched mice were used for each set of experiments. All experiments were approved by the Committee of Institute for Animal Experimentation, Hokkaido University Graduate School of Medicine (no. 02048 and no. 03085).
Model of Inflammation-Based Tumor Progression
Inflammation was induced by insertion of a foreign body, gelatin sponge, as described previously.23 Sterile gelatin sponge (Yamanouchi Pharm., Tokyo, Japan) was cut into 10 × 5 × 3-mm pieces and inserted into a subcutaneous pocket in the right flank of the pelvic region of a mouse. Then QR-32 tumor cells (1 × 105 cells/0.1 ml) were immediately injected into the preinserted gelatin sponge. On day 25, the arising tumors were aseptically removed, and individual culture cell lines were established for evaluation of their metastatic potential in normal mice (1 × 106 cells injected intravenously). On day 25, the mice were sacrificed, and metastatic nodules on the surface of the lungs or other organs were counted macroscopically.
Model of Spontaneous Metastasis of B16BL6 Melanoma Cells
B16BL6 cells (4 × 105 in 0.05 ml) were injected into the hind footpad. On day 25, the primary tumors were surgically removed under anesthesia. The mice were sacrificed when they were in a moribund state, followed by necropsy.
Subcutaneous Tumor Growth and Experimental Metastasis
For evaluation of subcutaneous tumor growth, QRsP-11 tumor cells (2 × 105) or B16BL6 cells (1 × 106) were injected, and the tumor diameters were measured twice a week. For experimental metastasis assay, tumor cells (QRsP-11, 1 × 106 cells; B16BL6, 1 × 105 cells) were injected intravenously. The mice were maintained for 3 and 2 weeks, respectively. After euthanasia, the lungs and other organs were removed and weighed, and surface metastatic lesions were counted macroscopically.
Adoptive Transfer of Inflammatory Phagocytes
In the recipient gp91phox−/− mice, QR-32 cells with the foreign body had been implanted or B16BL6 cells had been injected into the footpad. Inflammatory phagocytes (2 × 106 cells), prepared as described below, were then transferred adoptively to the growing site of QR-32 cells daily from day 0 (simultaneously) to day 5 and to the growing site of B16BL6 cells three times each week for 4 weeks.
The inflammatory phagocytes were collected by lavage of the peritoneal cavity of WT and gp91phox−/− mice in which a sterile gelatin sponge had been inserted 5 days before. The peritoneal exudate cells were then purified for phagocytes by using Mono-Poly resolving medium (Dainippon Pharmaceutical Co. Ltd., Tokyo, Japan). Histological examination revealed that the purified cellular population was composed of ∼70% neutrophils and 30% macrophages/lymphocytes.
Determination of the Total Number and the Types of the Cells Infiltrated into Gelatin Sponge
The gelatin sponge pieces subcutaneously injected into WT and gp91phox−/− mice were removed and digested with 0.2% collagenase in serum-free minimum essential medium for a few minutes at 37°C. After collecting all of the infiltrated cells by centrifugation, we counted the total number of the cells per piece of gelatin sponge. We also performed differential counts of more than 200 cells in smear preparations of the collected cells stained with May-Grünwald’s and Giemsa solution (Wako Pure Chemical Inc., Osaka, Japan). Mean percentages of differential cells were obtained from the mean values of independent counts by two pathologists.
Assay for Determining Scavenging Capacity of Plasma Antioxidants
The aim of this assay was to determine scavenging capacity of plasma antioxidants by evaluation of Cu+ derived from Cu++ by the combined action of all antioxidants in plasma (TA01; Oxford Biomedical Research, Oxford, MI). Generation of Cu+ was detected after stable complex formation between Cu+ and bathocuproine as monitored at 490 nm wavelength on a microplate reader (model 550; Bio-Rad, Tokyo, Japan). The decrease in absorbance after addition of the plasma was plotted on a calibration curve estimated by application of known concentrations of uric acid as standard. Results were expressed as μmol/L uric acid equivalents.
In Vitro Cell Motility Assay (Phagokinetic Track Assay)
Uniform carpets of gold particles were prepared on glass coverslips (22 × 22 mm). The coverslips were placed in 35-mm culture dishes (Greiner Labortechnik, Tokyo, Japan) and 2 × 103 tumor cells were placed in each dish. After 48 hours, phagokinetic tracks of 40 cells were traced under a microscope. The area cleared of gold particles by cells was quantified by using a microscope analyzer (Cosmozone R500; Nikon, Japan).
In Vitro Cell Invasion Assay
A nucleopore filter (8-μm-pore size) was coated with Matrigel matrix (156 μg/cm2; Becton, Dickinson and Company, Tokyo, Japan) and placed between blind-well chemotactic chamber compartments (Costar Transwell, Tokyo, Japan), and then 5 × 103 tumor cells were placed in the upper compartment. Fibronectin (25 μg/ml) was used as chemoattractant. After 6 hours of incubation, the filters were removed, fixed in 5% glutaraldehyde, and stained with May-Grünwald Giemsa stain. The number of cells that had penetrated into the filter was counted under a microscope.
Reverse Transcriptase-Polymerase Chain Reaction (RT- PCR) Analysis
The detail of RT-PCR for gene amplification of thymosin β4 or GAPDH has been described previously.25
Statistical Analysis
The significance of the differences in tumor and metastatic incidences was evaluated by the χ2 test, and the differences in organ weight, number of lung colonies, and antioxidative capacity were calculated by Student’s t-test.
Results
Decreased Acquisition of Spontaneous Metastatic Phenotype in gp91phox−/− Mice
The QR-32 fibrosarcoma cells did not produce tumors or form metastases after subcutaneous (2 × 105 cells) or intravenous (1 × 106 cells) injection into C57BL/6J mice.24 However, they were converted to grow lethally after being co-implanted with a piece of gelatin sponge, which induced inflammation at the site of implantation, and the tumors arising from them acquired a metastatic phenotype.23 Previous studies indicate that infiltrated neutrophils are predominantly involved in the progression process, and oxygen radicals derived from neutrophils play a role in acquisition of metastases.26,27
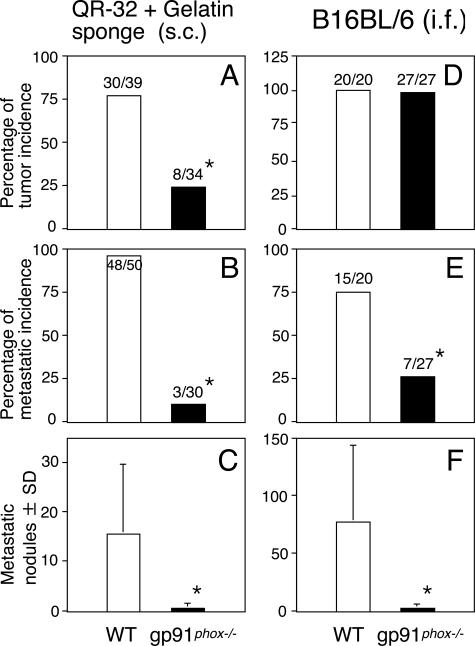
Figure 1A shows that lethal growth of QR-32 cells co-implanted with gelatin sponge was observed in 30 of 39 wild-type (WT) mice in contrast to 8 of 34 gp91phox gene knockout mice (gp91phox−/−) that lack a functional NADPH oxidase. In contrast to the tumor cells grown in WT mice, tumors arising in gp91phox−/− mice acquired a significantly weaker metastatic phenotype, both in the metastases of the lung (Figure 1, B and C; P < 0.001) and other organs (data not shown).
Figure 1.
Decreased acquisition of metastatic ability of tumor cells grown in gp91phox−/− mice. QR-32 tumor cells (A–C, 1 × 105) were subcutaneously (s.c.) co-implanted with gelatin sponge, and B16BL6 cells (D–F, 4 × 105 in 0.05 ml) were injected into the hind footpad (i.f.), respectively, of WT mice and gp91phox−/− mice (A and D). The metastasis incidence (B and E) and number of colonies per lung (C and F) were counted. *P < 0.001 versus WT. The actual number of incidences is indicated above each bar (number of mice with tumor or metastasis/number of mice tested).
The subcutaneously injected gelatin sponge pieces were removed from WT and gp91phox−/− mice, and the exact number of infiltrated cells was counted per gelatin sponge. Table 1 shows that a greater number of cells infiltrated into sponge pieces in gp91phox−/− mice. When we stained the infiltrated cells and determined their cell types by histological examination, there were no differences in the types of cells infiltrated into gelatin sponge between WT and gp91phox−/− mice.
Table 1.
Differential Leukocyte Counts and the Numbers of Cells Infiltrated into Gelatin Sponge in gp91phox−/− and WT Mice
| Mice* | Number of mice examined | Total number of gelatin sponge-infiltrated cells (×106) | Percentage of differential leukocytes per gelatin sponge-infiltrated cells
|
||||
|---|---|---|---|---|---|---|---|
| Mø/MO† | PMN‡ | LC§ | EOS¶ | Others | |||
| WT | 6 | 16.4 ± 1.5∥ | 12.5 ± 7.1 | 57.8 ± 7.7 | 26.9 ± 3.5 | 1.0 ± 1.1 | 2.0 ± 1.2 |
| gp91phox−/− | 6 | 22.6 ± 2.7∥ | 9.8 ± 4.3 | 61.5 ± 5.1 | 26.9 ± 2.3 | 0.8 ± 1.0 | 1.4 ± 0.5 |
A piece of gelatin sponge was implanted into the subcutaneous space of gp91phox−/− and WT mice.
Mø/MO, macrophages/monocytes.
PMN, polymorphonuclear neutrophils.
LC, lymphocytes.
EOS, eosinophils.
p < 0.001.
We next examined spontaneous metastasis of B16BL6 melanoma, another in vivo murine tumor model. B16BL6 cells (4 × 105 cells) were injected into the right footpad of mice, and the arising tumors were surgically resected on day 25. No significant difference was observed between the mice in the incidence of primary tumor growth (Figure 1D), which was also evaluated by tumor thickness in proportion to the left footpad (Figure 2C) and by the weight of amputated legs (data not shown). However, local tumor invasion in vivo was evident in the WT mice compared to gp91phox−/− mice when the primary footpad tumors were removed. Grossly, in 12 of 20 WT mice, tumor invaded the underlying calf muscle and could not be removed completely; in contrast, tumors invaded calf muscle in only 4 of 27 gp91phox−/− mice and were easily removed with minimal damage to the surrounding tissues. Figure 1E shows that the incidence of lung metastasis was significantly higher in WT mice than in gp91phox−/− mice (P < 0.001). Furthermore, the number of metastatic nodules on the lung surface was significantly higher in WT mice than in gp91phox−/− mice (Figure 1F, P < 0.001).
Figure 2.
Growth of primary tumors in gp91phox−/− mice and WT mice after subcutaneous or intrafootpad injections of tumor cells. Growth curves of QR-32 cells (1 × 105 cells) co-implanted with gelatin sponge (A), QRsP-11 tumor cells (1 × 106 cells) injected subcutaneously (B), B16BL6 cells (4 × 105 cells) injected into hind footpad (C), and B16BL6 cells (1 × 106 cells) injected subcutaneously (D) into WT and gp91phox−/− mice, respectively. ○, WT mice; •, gp91phox−/− mice; ▵, gp91phox−/− mice with adoptively transferred WT phagocytes; ▴, gp91phox−/− mice with adoptively transferred gp91phox−/− phagocytes. *P < 0.01; gp91phox−/− versus WT mice, and gp91phox−/− mice with adoptively transferred WT phagocytes versus gp91phox−/− mice with adoptively transferred gp91phox−/− phagocytes. Data are mean diameters of the arising tumors at each injection and are representative results of at least two separate experiments producing similar results.
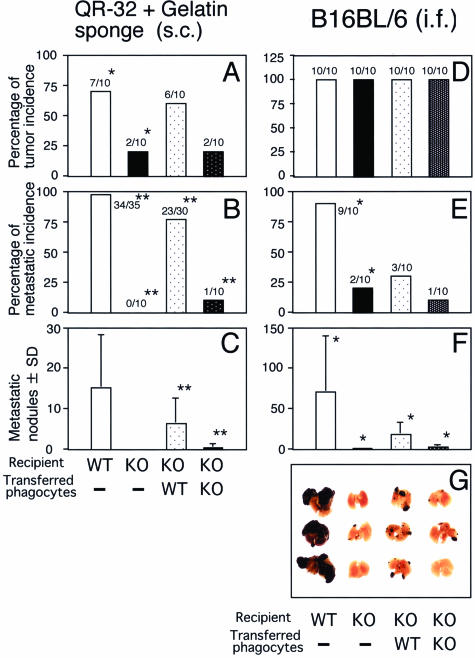
To confirm that ROS derived from NADPH oxidase in phagocytes actually contributed to the acquisition of metastatic properties of tumor cells, we tested whether the reduced metastatic ability of tumor cells in gp91phox−/− mice could be restored by adoptive transfer of inflammatory phagocytes. Since we had found that QR-32 cell progression was promoted by the early-phase inflammation in this model,26 we adoptively transferred phagocytes into the gelatin sponge co-implantation site from day 0 to day 5. Figure 3A shows that tumor growth occurred in 7 of 10 WT mice and 2 of 10 gp91phox−/− mice without adoptive transfer, 6 of 10 gp91phox−/− mice with adoptively transferred WT mice-derived phagocytes and 2 of 10 gp91phox−/− mice with gp91phox−/− mouse-derived phagocytes. Figure 2A shows their growth curves. More interestingly, tumors grown in the gp91phox−/− mice with WT mouse-derived phagocytes acquired a malignant phenotype, as evidenced by the development of lung metastasis; in contrast, those in gp91phox−/− mice with gp91phox−/− mouse-derived phagocytes did not (Figure 3, B and C).
Figure 3.
Restored metastatic ability of tumor cells by adoptive transfer of inflammatory phagocytes obtained from WT mice. Adoptively transferred inflammatory phagocytes obtained from WT mice recovered acquisition of metastatic potential of tumor cells of both tumor cell lines. *P < 0.05 and **P < 0.001 versus adjacent group.
Next, we confirmed recovery of metastatic potential in the B16BL6 model. Phagocytes isolated from WT or gp91phox−/− mice were injected into the B16BL6 tumor bed in gp91phox−/− mice. As shown in Figure 3D, tumor incidence did not differ between the mice; however, adoptive transfer of phagocytes from WT mice significantly enhanced metastatic ability, whereas transfer of phagocytes of gp91phox−/− mice did not (Figure 3, F and G).
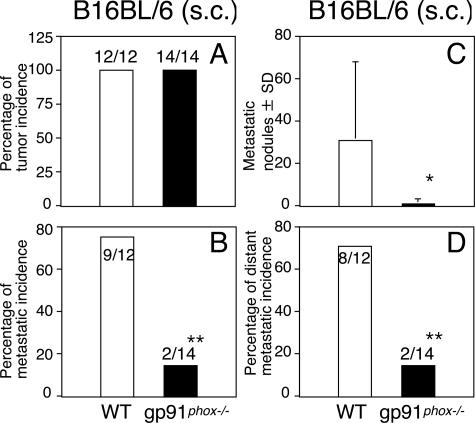
We also examined the effects of the wild-type versus gp91phox−/− background on growth of the primary tumor. For subcutaneous injection, we used highly tumorigenic and metastatic QRsP-11 tumor cells derived from QR-32 tumor cells in contact with inflammation.23 There were no differences in the growth incidence and growth rate between gp91phox−/− mice and WT mice (Figure 2B). In addition, we subcutaneously injected B16BL6 cells, finding no difference either in the growth rate (Figure 2D) or in the growth incidence (Figure 4A) between WT and gp91phox−/− mice. However, we found dramatic differences in the incidence of spontaneous distant metastases. Of the 12 WT mice, 9 mice had prominent lung metastasis at necropsy, in contrast to 2 of the 14 gp91phox−/− mice (Figure 4B, P < 0.01). The number of metastatic nodules on the lung surface was significantly higher in WT mice than in gp91phox−/− mice (Figure 4C, P < 0.05). Furthermore, the majority of B16BL6-bearing WT mice developed extrapulmonary metastases, frequently accompanied by metastases to regional lymph nodes (inguinal and axilla), whereas in gp91phox−/− mice metastases were significantly less frequent (Figure 4D). We then used an experimental metastasis assay to evaluate the primary growth properties in the lungs after intravenous injection of QRsP-11 cells or B16BL6 cells. The number and size of lung metastases of the respective tumor lines did not differ between WT mice and gp91phox−/− mice (Table 2). Oxidative stress is balanced on the match of oxidant stimuli and various antioxidants in vivo. We next confirmed the scavenging capacity of plasma antioxidants in WT and gp91phox−/− mice. There was no significant difference between the mice either at 8 or 16 weeks of age (Table 3).
Figure 4.
Decreased acquisition of spontaneous metastatic ability of B16BL6 melanoma cells after subcutaneous injection into gp91phox−/− mice. B16BL6 melanoma cells (1 × 106) were injected subcutaneously into WT mice and gp91phox−/− mice. The mice were sacrificed for necropsy when they were in a moribund state. *P < 0.05 and **P < 0.01 versus WT mice.
Table 2.
Experimental Metastatic Abilities of Tumor Cells Intravenously Injected into gp91phox−/− and WT Mice
| Mice | Cell line | Number of lung metastatic nodules | Lung weight (g) | Metastatic incidence (%) |
|---|---|---|---|---|
| WT | QRsP-11 | 144.1 ± 11.8 | 1.2 ± 0.3 | 8/8 (100) |
| gp91phox−/− | QRsP-11 | 138.0 ± 19.1 | 1.0 ± 0.2 | 9/9 (100) |
| WT | B16BL6 | 111.5 ± 49.7 | 0.4 ± 0.1 | 10/10 (100) |
| gp91phox−/− | B16BL6 | 97.8 ± 43.2 | 0.4 ± 0.2 | 13/13 (100) |
QRsP-11 fibrosarcoma (1 × 106 cells) and B16BL6 melanoma (4 × 105 cells) were injected via the tail vein. They were sacrificed for necropsy on days 21 and 14, respectively. Data represent the mean ± SD.
Table 3.
Scavenging Capacity of Plasma Antioxidants in gp91phox−/− and WT Mice
| Mice | Antioxidative capacity (μmol/L uric acid equivalent)
|
|
|---|---|---|
| 8 weeks old | 16 weeks old | |
| WT | 47.1 ± 36.0 | 264.3 ± 389.1 |
| gp91phox−/− | 47.8 ± 28.9 | 225.5 ± 315.8 |
Each group consisted of more than 12 mice.
Thymosin β4 Gene Expression and Corresponding Cell Motility/Invasion Were Augmented in Tumor Cells Grown in WT Mice but Not Those Grown in gp91phox−/− Mice
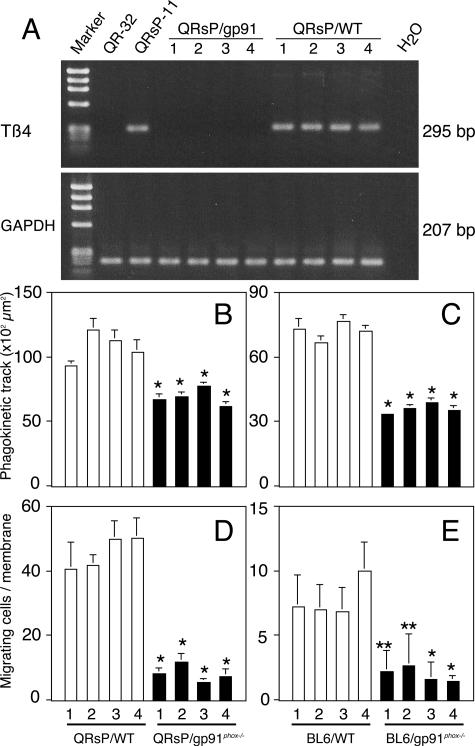
Because we had demonstrated that thymosin β4 gene expression was responsible for the acquisition of a metastatic phenotype through regulating cell motility,25 the levels of thymosin β4 expression of the primary tumors from WT mice were tested and found to be high, whereas those from gp91phox−/− mice had no expression of thymosin β4 (Figure 5A). A phagokinetic track assay, which reveals the motile potential of a single tumor cell, showed that motile potential was significantly higher in both QRsP tumors and B16BL6 tumors in WT mice, as compared to those in gp91phox−/− mice (Figure 5, B and C). Penetration of tumor cells into a Nucleopore membrane coated with Matrigel matrix was evaluated as one aspect of invasion in vitro. Both QRsP tumors and B16BL6 tumors grown in WT mice exhibited significant invasion of the membrane compared to those grown in gp91phox−/− mice (Figure 5, D and E).
Figure 5.
Thymosin β4 gene expression and corresponding cell motility/invasion were augmented in tumor cells grown in WT mice but not those grown in gp91phox−/− mice. Tumor cells were established from QR-32 cells co-implanted with gelatin sponge in gp91phox−/− mice (QRsP/gp91phox−/−) and those in WT mice (QRsP/WT). B16BL6 cells were implanted into footpad in gp91phox−/− mice and in WT mice and established cell lines BL6/gp91phox−/− and BL6/WT, respectively. RT-PCR analysis for thymosin β4 and GAPDH (A), phagokinetic track patterns (B and C) and invasion through transwell (D and E) are shown. Each bar represents the mean ± SD of at least two independent experiments.*P < 0.001 and **P < 0.05, compared with tumors in WT with lowest motility or invasion.
Discussion
In this study, we used two different murine tumor models to establish that tumor cells do not acquire a metastatic phenotype in the gp91phox−/− mice, indicating that NADPH oxidase-derived oxygen radicals are essential for generating metastatic variants in primary tumors. We confirmed this finding by adoptively transferring phagocytes obtained from WT mice with intact respiratory burst oxidase activity. The results of this study demonstrate the impact of phagocyte-derived superoxide and its oxidative metabolites as an endogenous factor to endow tumor cells with metastatic ability in vivo.
Metastatic tumor cells are a specialized subset of cells capable of completing the multistep metastasis cascade.2,3,25,28 The process is divided into two stages. First, tumor cells detach from the primary lesion, migrate and degrade the surrounding extracellular matrix, and intravasate into blood and/or lymph vessels. Second, in the circulation where they escape immunological attack, tumor cells adhere to endothelial cells and then extravasate from blood and/or lymph vessels through the endothelium. Then, tumor cells begin to proliferate at sites distant from the primary tumor. The difference in the incidence of spontaneous metastasis between gp91phox−/− mice and WT mice appears to reflect events occurring during the first stage of metastasis, ie, from detachment from the primary lesion to intravasation into vessels, because there was no difference in the incidence of experimental metastasis induced by directly injecting tumor cells into the circulation by intravenous injection, which mimicked the second stage of metastasis.29 During the first stage of metastasis, the tumors grown in gp91phox−/− mice had a reduced capacity to acquire cell motility and invasiveness compared to those in WT mice. In vitro motility and invasion phenotype are closely correlated with the degree of invasiveness of tumor cells in vivo. Hence we suggest that the genes for motility/invasion can be regulated by ROS. Among those genes, we previously identified thymosin β4 expression as a regulator of tumor metastasis phenotype through controlling tumor cell motility.25
Thymosin β4 is known to regulate re-organization of actin network that may affect the dynamics of focal adhesion assembly and lead to modulation of cell-substrate interactions because thymosin β4 controls both actin-based cytoskeletal system (myosin IIA, α-actinin, and tropomyosin) and cell-cell adhesions (vinculin, talin, α5-integrin, and focal adhesion kinase).30–32 Therefore, the levels of thymosin β4 expression affect the dynamics of cellular shape (including epithelial-to-mesenchymal transition-like morphological changes), cell motility, cell survival, and consequently metastatic ability of tumor cells.25 The correlation between acquisition of metastatic phenotype and thymosin β4 gene overexpression seems to be a universal phenomenon as Clark and colleagues33 have indicated; by using human and mouse melanoma cells, they demonstrated that thymosin β4 gene expression was commonly up-regulated by repeated selection of highly metastatic variants in vivo. Therefore, thymosin β4 gene and/or its upstream regulatory gene(s) are likely to be one of the candidates for ROS-mediated acquisition of tumor metastasis. We noticed that the inflammatory environment, especially with a large amount of long-lasting active nitrogen oxides, modifies tumor malignant phenotypes in primarily growing tumors. Matrix metalloproteases (MMPs) are considered to be directly involved in the tumor-associated angiogenesis, invasion, and metastasis. Several lines of evidence revealed that peroxynitrite that was a reactive substance of inflammatory cell-derived superoxide and nitric oxide mediate activation of latent (inactive)-form MMPs to active ones.34,35 In the gp91phox−/− mice that are thought to maintain MMPs in less active forms because the mice lack NADPH-derived oxygen radicals. Such oxidative modification of MMPs in the primary tumors may be a possible mechanism to reduce acquisition of malignant phenotypes in gp91phox−/− mice.
Six possible metastatic cascades have been proposed in studies of metastatic animal models.9 1) Subpopulations of tumor cells acquire metastatic capacity late in tumorigenesis.8 2) All tumor cells have the capacity to develop a metastasis.36–39 DNA microarrays revealed that metastases and primary tumors (including those even in the premalignant stage) in individual patients show similar gene expression profiles.40–42 3) Heterogeneous tumor subpopulations containing metastatic tumors arises during the growth of the primary tumors (dynamic heterogeneity).43,44 4) Metastatic subclones within a primary tumor can overgrow and dominate the tumor mass itself (clonal dominance theory).45,46 5) Metastasis occurs through transfection of suitable cells in distant organs with circulating oncogenes derived from the primary tumors (genometastasis hypothesis).47,48 6) Metastatic variants derive from cancer stem cells.49,50 Five of the six metastasis theories indicate that metastatic variants derive from a primary tumor population by undergoing genetic alteration(s). We considered that emergence of metastatic tumor is likely due to metastasis-related gene alterations. In this study, we used thymosin β4 gene expression as a genetic marker for acquisition of a metastatic phenotype. We found that up-regulation of thymosin β4 was seen in the primary tumors grown in WT but not in gp91phox−/− mice. We therefore propose that phagocyte NADPH oxidase-derived superoxide and its oxidative metabolites correlate with expression of thymosin β4 and are likely to be microenvironmental factors that spawn highly metastatic variants in primary tumors.
Ames and colleagues51,52 have proposed that release of oxidative mutagens from phagocytic cells is a major contributor to cancer. They described that, although phagocyte-derived oxidants protect us from immediate death from infection, they also cause oxidative damage to DNA and cells, which simultaneously increases compensatory cell division, and mutations, if induced, ultimately become fixed in the proliferated cells53,54; thus, the oxidants contribute to the carcinogenic process. In our models, we have verified that QR-32 cells acquire metastatic ability once they contact infiltrated neutrophils.26 We observed somatic mutations at a high frequency when tumor cells were co-cultured with neutrophils, and the frequency was reduced by addition of an ROS scavenger.27 In cases in which phagocytes at the primary tumor site contribute to a mutagenic environment, tumor cells can be converted to varying degrees of malignancy by genotoxic events, such as those caused by ROS. In fact, in studies in which tumorigenic tumor cells are exposed to mutagenic agents in vitro, their response falls into three categories: 1) conversion to benign (or regressive) tumors, 2) conversion to malignant tumors (metastatic), and 3) no change.24 The same categories are likely to occur at the site of the primary growth due to ROS generated by phagocytes. However, in the in vivo situation, benign tumors and low-grade malignant tumor cells will evade clinical observation, whereas highly malignant tumors will grow and spread to secondary sites. Such mutagenic conditions seem to be an important intrinsic factor for generation of metastatic tumor cells.
Several associations of carcinogenesis and endogenous/exogenous factors have been postulated, eg, inflammation/infection, chemical carcinogens, and radiation. All of them are believed to involve free radicals.55 The source of endogenous ROS can be inflammatory phagocytes, tumor-surrounding stroma,56 hypoxic conditions,57 ischemia-reperfusion,58–61 trauma,62,63 and tumor cells themselves.64 Those underlying common factors cause tumors and tumor progression. Thus, oxygen radicals are considered as a major and universal contributor to acceleration of tumor malignancy in vivo.
Acknowledgments
We thank Dr. Junichi Fujii for invaluable advice and Ms. Masako Yanome for her help in English revision of this manuscript.
Footnotes
Address reprint requests to Futoshi Okada, Ph.D., Department of Biomolecular Function, Graduate School of Medical Science, Yamagata University, 2-2-2, Iidanishi, Yamagata 990-9585 Japan. E-mail: fuokada@med.id.yamagata-u.ac.jp.
Supported in part by the Japanese Ministry of Health, Labor, and Welfare (grants-in-aid for cancer research 16-1 and 14-11 to F.O.); and the Japan Society for the Promotion of Science (grants-in-aid 17016007 and 17590334 to F.O.).
References
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Critical determinants of cancer metastasis: rationale for therapy. Cancer Chemother Pharmacol. 1999;43:S3–S10. doi: 10.1007/s002800051091. [DOI] [PubMed] [Google Scholar]
- Hart IR, Saini A. Biology of tumour metastasis. Lancet. 1992;339:1453–1457. doi: 10.1016/0140-6736(92)92039-i. [DOI] [PubMed] [Google Scholar]
- Welch DR, Steeg PS, Rinker-Schaeffer CW. Molecular biology of breast cancer metastasis. Genetic regulation of human breast carcinoma metastasis. Breast Cancer Res. 2000;2:408–416. doi: 10.1186/bcr87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson GL. Cancer metastasis: tumor cell and host organ properties important in metastasis to secondary sites. Biochem Biophys Acta. 1988;948:175–224. doi: 10.1016/0304-419x(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Zetter BR. Cellular basis of site-specific tumor metastasis. N Engl J Med. 1990;322:605–612. doi: 10.1056/NEJM199003013220907. [DOI] [PubMed] [Google Scholar]
- Radinsky R. Modulation of tumor cell gene expression and phenotype by organ specific metastatic environment. Cancer Metastasis Rev. 1995;14:323–338. doi: 10.1007/BF00690601. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto RJ. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Park SJ, Yoon SY, Yun CH, Chung AS. Sustained production of H2O2 activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-kappaB pathway. J Biol Chem. 2002;277:30271–30282. doi: 10.1074/jbc.M202647200. [DOI] [PubMed] [Google Scholar]
- Yoshizaki N, Mogi Y, Muramatsu H, Koike K, Kogawa K, Niitsu Y. Suppressive effect of recombinant human Cu, Zn-superoxide dismutase on lung metastasis of murine tumor cells. Int J Cancer. 1994;57:287–292. doi: 10.1002/ijc.2910570226. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kogawa K, Nishihori Y, Kuribayashi K, Nakamura K, Muramatsu H, Koike K, Sakamaki S, Niitsu Y. Suppression of intracellular Cu-Zn SOD results in enhanced motility and metastasis of Meth A sarcoma cells. Int J Cancer. 1997;73:187–192. doi: 10.1002/(sici)1097-0215(19971009)73:2<187::aid-ijc4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Yu L, Zhen L, Dinauer MC. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. Role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J Biol Chem. 1997;272:27288–27294. doi: 10.1074/jbc.272.43.27288. [DOI] [PubMed] [Google Scholar]
- DeLeo FR, Burritt JB, Yu L, Jesaitis AJ, Dinauer MC, Nauseef WM. Processing and maturation of flavocytochrome b588 include incorporation of heme as a prerequisite for heterodimer assembly. J Biol Chem. 2000;275:13986–13993. doi: 10.1074/jbc.275.18.13986. [DOI] [PubMed] [Google Scholar]
- Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Rotrosen D, Yeung CL, Leto TL, Malech HL, Kwong CH. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science. 1992;256:1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Yu L, Quinn MT, Cross AR, Dinauer CM. Gp91phox is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci USA. 1998;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. The respiratory burst oxidase and the molecular genetics of chronic granulomatous disease. Crit Rev Clin Lab Sci. 1993;30:329–369. doi: 10.3109/10408369309082591. [DOI] [PubMed] [Google Scholar]
- Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fishman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Okada F, Hosokawa M, Hamada J, Hasegawa J, Kato M, Mizutani M, Ren J, Takeichi N, Kobayashi H. Malignant progression of a mouse fibrosarcoma by host cells reactive to a foreign body (gelatin sponge). Br J Cancer. 1992;66:635–639. doi: 10.1038/bjc.1992.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Okada F, Hamada J, Hosokawa M, Kobayashi H. Changes in the tumorigenic and metastatic properties of tumor cells treated with quercetin or 5-azacytidine. Int J Cancer. 1987;39:338–342. doi: 10.1002/ijc.2910390312. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Okada F, Fujii N, Tomita N, Ito S, Tazawa H, Aoyama T, Choi SK, Shibata T, Fujita H, Hosokawa M. Thymosin-β4 regulates motility and metastasis of malignant mouse fibrosarcoma cells. Am J Pathol. 2002;160:869–882. doi: 10.1016/s0002-9440(10)64910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells. Implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2221–2232. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada F, Nakai K, Kobayashi T, Shibata T, Tagami S, Kawakami Y, Kitazawa T, Kominami R, Yoshimura S, Suzuki K, Taniguchi N, Inanami O, Kuwabara M, Kishida H, Nakae D, Konishi Y, Moriuchi T, Hosokawa M. Inflammatory cell-mediated tumour progression and minisatellite mutation correlate with the decrease of antioxidative enzymes in murine fibrosarcoma cells. Br J Cancer. 1999;79:377–385. doi: 10.1038/sj.bjc.6690060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JT, Bonovich MT, Kohn EC. The biochemistry of cancer dissemination. Mol Biol. 1997;32:175–253. doi: 10.3109/10409239709082573. [DOI] [PubMed] [Google Scholar]
- Li D, Yee JA, Thompson LU, Yan L. Dietary supplementation with secoisolariciresinol diglycoside (SDG) reduces experimental metastasis of melanoma cells in mice. Cancer Lett. 1999;142:91–96. doi: 10.1016/s0304-3835(99)00158-5. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Craig SW. F-Actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature. 1995;373:261–264. doi: 10.1038/373261a0. [DOI] [PubMed] [Google Scholar]
- Golla R, Philp N, Safer D, Chintapalli J, Hoffman R, Collins L, Nachmias VT. Co-ordinate regulation of the cytoskeleton in 3T3 cells overexpressing thymosin-4. Cell Motil Cytoskeleton. 1997;38:187–200. doi: 10.1002/(SICI)1097-0169(1997)38:2<187::AID-CM7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphtidyl-inositol-4–5-bisphosphate. Nature. 1996;381:531–535. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for Rho C. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- Wu J, Akaike T, Hayashida K, Okamoto T, Okuyama A, Maeda H. Enhanced vascular permeability in solid tumor involving peroxynitrite and matrix metalloproteinases. Jpn J Cancer Res. 2001;92:439–451. doi: 10.1111/j.1349-7006.2001.tb01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Akuta T, Tamura F, van Der Vliet A, Akaike T. Molecular mechanism for activation and regulation of matrix metalloproteinases during bacterial infections and respiratory inflammation. Biol Chem. 2004;385:997–1006. doi: 10.1515/BC.2004.130. [DOI] [PubMed] [Google Scholar]
- Milas L, Peters LJ, Ito H. Spontaneous metastasis: random or selective? Clin Exp Metastasis. 1983;1:309–315. doi: 10.1007/BF00121193. [DOI] [PubMed] [Google Scholar]
- Giavazzi R, Alessandri G, Spreafico F, Garattini S, Mantovani A. Metastasizing capacity of tumor cells from spontaneous metastases of transplanted murine tumours. Br J Cancer. 1980;42:462–472. doi: 10.1038/bjc.1980.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Giavazzi R, Alessandri G, Spreafico F, Garattini S. Characterization of tumour lines derived from spontaneous metastases of a transplanted murine sarcoma. Eur J Cancer. 1981;17:71–76. doi: 10.1016/0014-2964(81)90213-9. [DOI] [PubMed] [Google Scholar]
- Vaage J. Metastasizing potentials of mouse mammary tumors and their metastases. Int J Cancer. 1988;41:855–858. doi: 10.1002/ijc.2910410614. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P, Payette T, Pisyone M, Stecker K, Zhang BM, Zhou YX, Varnholt H, Smith B, Gadd M, Chatfield E, Kessler J, Baer TM, Erlander MG, Sgroi DC. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D, Lahti-Domenici J, Keshaviah A, Bae YK, Argani P, Marks J, Richardson A, Cooper A, Strausberg R, Riggins GJ, Schnitt S, Gabrielson E, Gelman R, Polyak K. Molecular markers in ductal carcinoma in situ of the breast. Mol Cancer Res. 2003;1:362–375. [PubMed] [Google Scholar]
- Hill RP, Chambers AF, Ling V, Harris JF. Dynamic heterogeneity: rapid generation of metastatic variants in mouse B16 melanoma cells. Science. 1984;224:998–1001. doi: 10.1126/science.6719130. [DOI] [PubMed] [Google Scholar]
- Ling V, Chambers AF, Harris JF, Hill RP. Quantitative genetic analysis of tumour progression. Cancer Metastasis Rev. 1985;4:173–192. doi: 10.1007/BF00050694. [DOI] [PubMed] [Google Scholar]
- Kerbel RS, Waghome C, Man MS, Elliott B, Breitman ML. Alteration of the tumorigenic and metastatic properties of neoplastic cells is associated with the process of calcium phosphate-mediated DNA transfection. Proc Natl Acad Sci USA. 1987;84:1263–1267. doi: 10.1073/pnas.84.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS, Waghorne C, Korczak B, Lagarde A, Breitman ML. Clonal dominance of primary tumours by metastatic cells: genetic analysis and biological implications. Cancer Surv. 1988;7:597–629. [PubMed] [Google Scholar]
- Garcia-Olmo D, Garcia-Olmo DC, Ontanon J, Martinez E, Vallejo M. Tumor DNA circulating in the plasma might play a role in metastasis. The hypothesis of the genometastasis. Histol Histopathol. 1999;14:1159–1164. doi: 10.14670/HH-14.1159. [DOI] [PubMed] [Google Scholar]
- Garcia-Olmo D, Garcia-Olmo DC. Functionality of circulating DNA: the hypothesis of genometastasis. Ann NY Acad Sci. 2001;945:265–275. doi: 10.1111/j.1749-6632.2001.tb03895.x. [DOI] [PubMed] [Google Scholar]
- Wiedswang G, Borgen E, Karesen R, Kvalheim G, Nesland JM, Qvist H, Schlichting E, Sauer T, Janbu J, Harbitz T, Naume B. Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol. 2003;21:3469–3478. doi: 10.1200/JCO.2003.02.009. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc Natl Acad Sci USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN, Gold LS. Environmental pollution, pesticides, and the prevention of cancer: misconceptions. FASEB J. 1997;11:1041–1052. doi: 10.1096/fasebj.11.13.9367339. [DOI] [PubMed] [Google Scholar]
- Shacter E, Beecharm EJ, Covey JM, Kohn KW, Potter M. Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis. 1988;9:2297–2304. doi: 10.1093/carcin/9.12.2297. [DOI] [PubMed] [Google Scholar]
- Yamashina K, Miller BE, Heppner GH. Macrophage-mediated induction of drug-resistant variants in a mouse mammary tumor cell line. Cancer Res. 1986;46:2396–2401. [PubMed] [Google Scholar]
- Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8:583–599. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- Shaughnessy SG, Buchman MR, Turple S, Richardson M, Orr FW. Walker carcinoma cells damage endothelial cells by the generation of reactive oxygen species. Am J Pathol. 1989;134:787–796. [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikumar P, Dong Z, Weinberg JM, Venkatachalam MA. Mechanisms of cell death in hypoxia/reoxygeneration injury. Oncogene. 1998;17:3341–3349. doi: 10.1038/sj.onc.1202579. [DOI] [PubMed] [Google Scholar]
- Parks DA, Granger DN. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Pathol. 1983;245:285–289. doi: 10.1152/ajpgi.1983.245.2.G285. [DOI] [PubMed] [Google Scholar]
- Kirschner RE, Fantini GA. Role of iron oxygen-derived free radicals in ischemia-reperfusion injury. J Am Coll Surg. 1994;179:103–117. [PubMed] [Google Scholar]
- Bulkley GB. The role of oxygen free radicals in human disease processes. Surgery. 1983;94:407–411. [PubMed] [Google Scholar]
- Billing AG, Jochum M, Frohlich D, Cheronis JC, Fritz H. Oxidative autoaggression by phagocytes in human peritonitis. Eur J Clin Invest. 1997;27:1030–1037. doi: 10.1046/j.1365-2362.1997.2280786.x. [DOI] [PubMed] [Google Scholar]
- van Rossen ME, Sluiter W, Bonthuis F, Jeekel H, Marquet RL, van Eijck CH. Scavenging of reactive oxygen species leads to diminished peritoneal tumor recurrence. Cancer Res. 2000;60:5625–5629. [PubMed] [Google Scholar]
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]