Abstract
There is increasing evidence that hippocampal learning correlates strongly with neurogenesis in the adult brain. Increases in neurogenesis after brain injury also correlate with improved outcomes. With aging the capacity to generate new neurons decreases dramatically, both under normal conditions and after injury. How this decrease occurs is not fully understood, but we hypothesized that transforming growth factor (TGF)-β1, a cell cycle regulator that rapidly increases after injury and with age, might play a role. We found that chronic overproduction of TGF-β1 from astrocytes almost completely blocked the generation of new neurons in aged transgenic mice. Even young adult TGF-β1 mice had 60% fewer immature, doublecortin-positive, hippocampal neurons than wild-type littermate controls. Bromodeoxyuridine labeling of dividing cells in 2-month-old TGF-β1 mice confirmed this decrease in neuro-genesis and revealed a similar decrease in astrogenesis. Treatment of early neural progenitor cells with TGF-β1 inhibited their proliferation. This strongly suggests that TGF-β1 directly affects these cells before their differentiation into neurons and astrocytes. Together, these data show that TGF-β1 is a potent inhibitor of hippocampal neural progenitor cell proliferation in adult mice and suggest that it plays a key role in limiting injury and age-related neurogenesis.
New neurons are continually produced in the adult hippocampus from neural progenitor cells that proliferate in close proximity to blood vessels1 and produce neurons, astrocytes, and oligodendrocytes. There is increasing evidence that hippocampal learning correlates strongly with neurogenesis in the adult brain.2–5 Injury stimulates neurogenesis,6 whereas certain inflammatory states inhibit it.7–9 Increases in neurogenesis after brain injury also correlate with improved outcomes.10–15 Basal and injury-induced production of new hippocampal neurons dramatically decreases with age, as does the ability of neural progenitor cells to proliferate.16–18 This is likely in part because of declining levels of mitogens or growth factors19,20; however, inhibitory signals have not been well characterized. It is not known whether neurogenesis is stimulated by chronic as well as acute neuronal injury, but it is clear that production of new neurons does not keep up with neuronal losses in neurodegenerative diseases, in which neuronal losses increase with age and are associated with inflammation.21
The transforming growth factor (TGF)-β family of proteins includes the TGF-βs, bone morphogenic proteins, activins, growth and differentiation factors (GDF), and nodal. TGF-β family members act in a highly contextual manner and, depending on cell type and environment, may promote cell survival or induce apoptosis, stimulate cell proliferation or induce differentiation, and initiate or resolve inflammation.22–25 Members of the TGF-β superfamily are known to regulate both proliferation and differentiation of neural progenitor cells26–28 and TGF-β1 itself can inhibit proliferation in many cell types,23 including neural crest stem cells27 and subventricular zone cells in embryonic brain slice cultures.29
There are three isoforms of TGF-β; 1, 2, and 3. In the brain, TGF-β2, TGF-β3, and TGF-β receptors are widely distributed,30 whereas TGF-β1 is expressed mostly in response to injury and/or aging.31–33 TGF-β1 is increased acutely after brain injury, including trauma, infection, ischemia, encephalitis, and autoimmune diseases, and is chronically elevated in numerous neurodegenerative diseases.34 In these settings it has been shown to have both beneficial and harmful effects, including neuroprotection,30,35–38 gliosis,34 hydrocephalus,39–41 and vascular fibrosis.42 TGF-β1 can also be both pro- and anti-inflammatory25 and mediates tolerance to lipopolysaccharide.36,43,44
We show here that a chronic increase in TGF-β1 production profoundly inhibits neurogenesis in vivo. Aged transgenic mice overexpressing TGF-β1 produced virtually no new neurons in the hippocampus, and even at 9 weeks of age, TGF-β1 overexpression caused a 60% decrease in the total number of immature neurons, hippocampal bromodeoxyuridine (BrdU) incorporation, and production of neurons and astrocytes. We show that TGF-β1 activates its signaling cascade in neural progenitor cells in culture, decreasing proliferation of these undifferentiated neural progenitor cells by prolonging their cell cycles. Together, these data show that TGF-β1 is a potent inhibitor of hippocampal neural progenitor cell proliferation and suggest that this cytokine may contribute significantly to age- and inflammation-related decreases in adult neurogenesis.
Materials and Methods
Mice
TGF-β1 mice, expressing a constitutively active mutant of porcine TGF-β1 under control of a GFAP promoter, have been described (line 64).39 T64 TGF-β1 mice express transgenic TGF-β1 mRNA twofold greater than endogenous mRNA.42 The mature porcine peptide differs only in one amino acid from its murine counterpart, and the mutated pTGF-β1 cDNA has previously been shown to be bioactive when expressed in transgenic animals.39,45,46 High-level overexpression of TGF-β1 results in the development of communicating hydrocephalus; however, low-expressor mice such as those used here do not develop this complication. All mice are maintained on an inbred, C57BL/6J genetic background (The Jackson Laboratory, Bar Harbor, ME). Before sacrifice, mice were deeply anesthetized with chloral hydrate, transcardially punctured, and saline-perfused. Brains were postfixed 24 hours in 4% paraformaldehyde in 1× phosphate buffer, pH 7.4 and then cryoprotected in 30% sucrose in phosphate-buffered saline. Forty-μm coronal sections were cut using a sliding microtome with a freezing stage. All animal care and use was in accordance with institutional guidelines and approved by the Palo Alto VA Committee on Animal Research.
Immunohistochemistry
Immunohistochemistry was performed using standard techniques. 3,3′-Diaminobenzidine (Sigma, St. Louis, MO) stains were performed using an ABC labeling kit (Vector Laboratories, Burlingame, CA). For fluorescent stains, secondary antibodies were purchased from either Molecular Probes (Eugene, OR) or Jackson Immunoresearch (West Grove, PA). The sources for primary antibodies included rat anti-bromodeoxyuridine (BrdU, 1:500; Accurate Chemical and Scientific Corp., Westbury, NY), goat anti-doublecortin (Dcx, 1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit or mouse anti-glial fibrillary acidic protein [GFAP, 1:1500 (DAKO, Carpinteria, CA) and 1:1000 (Chemicon International, Temecula, CA), respectively], rabbit anti-Ki67 (1:1000; Novocastra, Newcastle on Tyne, UK), rabbit anti-ionized calcium-binding associated protein-1 (Iba-1, 1:2500; Wako Bioproducts, Richmond, VA), rabbit anti-capase-3 (1:500, anti-Asp-175 of cleaved caspase-3; Cell Signaling Technologies, Beverly, MA), mouse anti-nestin (1:8; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), and mouse anti-neuronal nuclear antigen (NeuN, 1:1000; Chemicon International, Temecula, CA). Antigen retrieval with 3 mol/L HCl or sodium citrate was used for BrdU and nestin, respectively.
BrdU-Injected Mice
To study the production of new neurons and glia, we injected 8-week-old female TGF-β1 mice and their C57BL/6J littermates with 50 mg/kg of BrdU intraperitoneally once a day for 6 days, and sacrificed them either 1 or 28 days later. To estimate the number of BrdU-positive cells, we performed immunohistochemistry for BrdU on every sixth section. A blinded observer counted every BrdU-positive cell in the granule cell and subgranular cell layer of the dentate gyrus on one, randomly selected, side of each section. The total number of BrdU-positive cells in these hippocampal regions was then estimated by multiplying the number of cells counted by 12. Overestimation was corrected using the Abercrombie method for nuclei with an empirically determined average diameter of 6.5 μm within a 40-μm section. No significant differences were observed in average nuclear diameter between TGF-β1 mice and their wild-type littermates. As performed, this method is a true unbiased stereological method. The number of BrdU-positive cells that assumed a neuronal fate was estimated by triple staining for BrdU and doublecortin to mark immature neurons, and NeuN to mark mature neurons. Confocal microscopy (MRC 1024ES; Bio-Rad, Hercules, CA) was used to examine 100 to 150 BrdU-positive cells from each mouse for each stain and determine whether they co-labeled with Dcx, NeuN, or both. The estimated total number of BrdU-positive neurons was calculated by multiplying the resultant percentage of double-positive cells by the estimated total number of BrdU-positive cells. Estimates of absolute number of BrdU-positive hippocampal cells that became microglia or astrocytes were performed in the same manner, using GFAP as an astrocytic marker and Iba1 as a microglial marker. A second paradigm was used to examine the effect of TGF-β1 on cell proliferation. Eight-week-old TGF-β1 mice and their littermates were intraperitoneally injected with one 50-mg/kg dose of BrdU and sacrificed 2 hours later. BrdU+ and Ki67+ cells were quantified in every sixth section as above.
Quantification of Immunohistochemistry
To quantify doublecortin staining in young adult TGF-β1 mice and their littermates, we used Metamorph imaging software (version 6.1r1; Universal Imaging Corporation, Downington, PA). We quantified the percentage of pixels above background staining within a region drawn around the dentate granule cell layer and subgranular zone. In 22-month-old mice, doublecortin-positive cells in the same region were counted in every twelfth coronal section through the hippocampus and total numbers estimated by multiplying this number by 12. Only cells with a clearly visible nucleus were counted, not doublecortin-positive processes alone. Counts were corrected using the Abercrombie method for nuclei, as above. Caspase-3 staining was counted in neurogenic regions of the hippocampus (dentate gyrus granule cell layer and subgranular zone) in three sets of every twelfth section through the dentate gyrus obtained from 13-week-old female TGF-β1 and wild-type mice. Nestin staining was quantified by counting stained processes that spanned at least 50% of the dentate granule cell layer in one coronal section per mouse, matched for level. If processes were branched, only the major branch was counted.
To estimate dentate gyrus granule cell layer volume, we stained every sixth coronal section through the hippocampus with cresyl violet and then used Metamorph imaging software to quantify the area on each section. We then roughly estimated total volume by multiplying these areas by 6 and 40 μm. Neuronal density was separately estimated using confocal microscopy. One-μm-thick confocal images were obtained from NeuN-stained sections from at least five areas per dentate gyrus granule cell layer per mouse. The NeuN-positive nuclei in each image were counted, and this number divided by the area of each image containing granule cell layer.
Neural Progenitor Cell Cultures
Rat neural progenitor cells were isolated from Fisher 344 adult rats and propagated in culture using standard monolayer culture methods.8 Cells were transduced with a retrovirus that encodes for GFP8 and used for these experiments at propagation doubling numbers of ∼40 to 50. Primary cultures from C57BL/6J-Act-GFP P0 mice were also used at passages 5 to 7. Rat neural progenitor cells were grown in 1:1 Dulbecco’s modified Eagle’s medium:F12 (Invitrogen, Carlsbad, CA) supplemented with N2 and 20 ng/ml of fibroblast growth factor-2 (FGF-2; Peprotech, Rocky Hill, NJ). N2 components were purchased from Sigma and included 30 nmol/L Na selenite, 20 nmol/L progesterone, 30 μg/ml transferrin, 100 μmol/L putrescine, and 5 μg/ml insulin (all purchased from Sigma). For various assays, insulin and FGF-2 were altered as described. Primary mouse neural progenitor cells were grown in Neurobasal A media supplemented with glutamine, B27 without retinoic acid, penicillin, and streptomycin (all from Invitrogen). For normal growth, cells were also supplemented with 20 ng/ml of both epidermal growth factor (EGF) and FGF-2 (Peprotech).
MTT Assays
For MTT assays, 5 × 103 (rat) or 2 × 104 (mouse) cells were plated in triplicate in 96-well plates and incubated for 40 hours with varying concentrations of recombinant TGF-β1 (R&D Systems, Minneapolis, MD). Thiazolyl blue tetrazolium bromide (methylthiazolyldiphenyl-tetrazolium bromide, or MTT), 0.5 mg/ml, was added to the media for the last 2 hours of incubation. Cells were then lysed with 0.08 N HCl in isopropanol, and incubated 2 hours at 37°C. Absorbance at 570 nm was read using an enzyme-linked immunosorbent assay plate reader (SpectraMax 190; Molecular Devices, Sunnyvale, CA).
Cell Cycle Analysis
Rat neural progenitor cells were plated 5 to 10 × 107 per 10-cm diameter dish and grown 12 to 14 hours in varying amounts of TGF-β1 (R&D Systems) and FGF-2. Neurospheres were gently washed off the plates, then dispersed with Hanks’ balanced salt solution (Invitrogen) and ethanol-fixed. DNA was labeled with propidium iodide (Molecular Probes) and flow cytometry (Facscan or FACScalibur; Becton Dickinson, Mountain View, CA) used to gate on single cells and determine their DNA content. Fluorescence-activated cell sorting results were analyzed using MOD-Fit version 3.0 for Macintosh (Verity Software House Increase, Topsham, ME).
Western Blots
Rat neural progenitor cells were plated at 2 × 106 per 10-cm diameter dish and varying concentrations of TGF-β1 added 16 hours later for an additional 6 hours. Cells were collected off the plates and incubated 30 minutes on ice in cell lysis buffer, containing 0.5 mol/L Tris-HCl, pH 8.0, 5 mol/L NaCl, 10% Nonidet P-40, and protease inhibitor (Roche, Basel, Switzerland). After centrifugation at 10,000 × g, protein concentrations were measured using the BCA protein assay kit, (Pierce, Rockford, IL) and lysates were separated on a 4 to 12% Bis-Tris gel in MOPS sodium dodecyl sulfate running buffer, electroblotted onto nitrocellulose membranes, and analyzed with rabbit anti-phospho-Smad2 (Ser465/467, 1:1000; Upstate Cell Signaling Solutions, Lake Placid, NY) and rabbit anti-Smad2 antibodies (1:1000, Cell Signaling Technology). Signal was detected using ECL Western blotting detection reagents, (Amersham Biosciences, Uppsala, Sweden).
Statistical Analysis
All immunohistochemical experiments were analyzed by an investigator blinded to genotype or age. Statistical analysis was performed using Statview 5.0 software for the MacIntosh. A P value of ≤0.05 was considered significant. Unpaired Student’s t-tests were used to determine whether the results were significantly different between mouse groups. Analysis of variance, followed by Dunnett’s posthoc test was used to compare means from two or more groups against a control group.
Results
TGF-β1 Overexpression in Transgenic Mice Decreases the Total Number of Immature Hippocampal Neurons
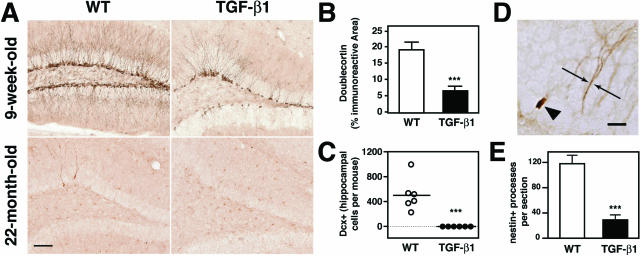
TGF-β1 is an injury-responsive cytokine that often remains chronically elevated after injury and is increased with age.32,33 To determine the effect of chronic TGF-β1 we studied TGF-β1 transgenic mice that secrete constitutively active TGF-β1 from astrocytes (GFAP-TGF-β1 mice, line T64).39 Astrocytes are particularly suitable targets for TGF-β1 expression, because they have been shown to produce TGF-β1 in vivo under pathological conditions.47,48 We found that 9-week-old TGF-β1 mice had many fewer doublecortin-labeled cells than wild-type littermates (Figure 1A). Doublecortin is expressed by proliferating neuroblasts and immature neurons, but not by mature neurons. Quantification of the doublecortin staining in the dentate gyrus granule cell layer and subgranular zone revealed a 60% decrease in the area stained in young TGF-β1 mice (Figure 1B). By 22 months of age, we did not find any doublecortin-positive cells in TGF-β1 transgenic hippocampi, whereas wild-type littermates still had ∼500 such cells on average (Figure 1, A and C). The reduction in doublecortin staining was accompanied by decreased nestin staining (Figure 1, D and E). Nestin-immunoreactive processes that radially span the dentate gyrus granule cell layer are characteristic of individual neural stem/progenitor cells.49 Such profiles were reduced by 75% in TGF-β1 mice (Figure 1E). These data indicate that either the pool of nestin-positive progenitors is reduced or that nestin-positive cells exhibit shorter or less branched processes in mice overexpressing TGF-β1.
Figure 1.
TGF-β1 overexpression dramatically decreases the number of immature neurons and nestin-positive processes. A: Light microscopy images of coronal brain sections stained for the immature neuronal marker doublecortin from 9-week-old and 22-month-old female TGF-β1 mice and their wild-type littermates. B: Quantification of doublecortin immunoreactivity in 9-week-old dentate gyrus, n = 3 mice per genotype. C: Quantification of the total number of hippocampal doublecortin cells in 22-month-old mice, n = 6 per genotype. Each circle represents one mouse. D: Nestin immunohistochemistry on a coronal section from a 9-week-old TGF-β1 mouse. Arrows indicate a nestin-positive process; the arrowhead, a blood vessel. E: Quantification of the total number of nestin-positive processes per hippocampal section. n = 5 mice per genotype. Dcx, doublecortin. Bars are mean ± SEM. **P ≤ 0.005, ***P ≤ 0.0005; Student’s t-test. Scale bars = 100 μm (A); 10 μm (D).
TGF-β1 Mice Have a 60% Reduction in Hippocampal Neurogenesis
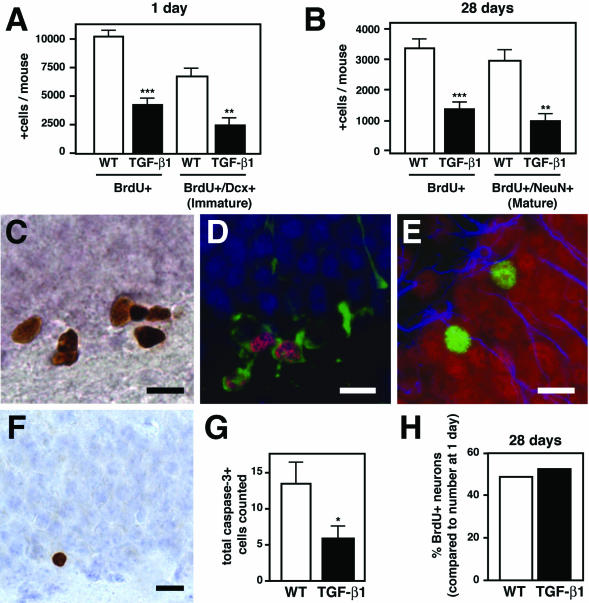
To confirm that TGF-β1 overexpression decreased hippocampal neurogenesis, we used BrdU and neuron-specific markers. Two-month-old mice were sacrificed 1 or 28 days after BrdU administration. Under both conditions, the overall number of dividing, BrdU-incorporating cells was 60% lower in TGF-β1 mice compared with nontransgenic controls (Figure 2, A–C). Newly generated immature neurons, measured in the 1-day groups by immunohistochemistry for BrdU and doublecortin, were decreased by 65% in TGF-β1 mice (Figure 2, A and D). Similarly, TGF-β1 mice had a prominent reduction in hippocampal BrdU-positive cells expressing the mature neuronal marker NeuN 1 month after BrdU labeling (Figure 2, B and E).
Figure 2.
TGF-β1 mice have 60% fewer BrdU-positive cells and BrdU-positive neurons in the hippocampus 1 and 28 days after BrdU administration. TGF-β1 transgenic (n = 5 mice per time point) and nontransgenic littermate controls (n = 5 to 8 mice) were injected at 8 weeks of age with BrdU to label dividing cells and analyzed 1 day or 28 days later for the presence of BrdU-positive cells and BrdU-positive neurons. Doublecortin was used to label immature neurons, and NeuN to label mature neurons. A: Quantification of the number of BrdU-positive cells and the number of BrdU-positive cells co-labeling for doublecortin (BrdU+/Dcx+) 1 day after BrdU administration. Bars are mean ± SEM. B: Quantification of total BrdU-positive cells and BrdU-positive mature neurons (BrdU+/NeuN+ cells) 28 days after BrdU. Bars are mean ± SEM. C: Example of BrdU-positive cells in the subgranular zone of the dentate gyrus, counterstained with hematoxylin. D: Confocal image of brain section containing BrdU+/Dcx+ cells. BrdU, magenta; Dcx, green; and NeuN, blue. E: Confocal image of brain section containing BrdU+/NeuN+ cells. BrdU, green; NeuN, red; and GFAP, blue. F: Example of a cell in the granule cell layer immunostained with caspase-3, counterstained with cresyl violet. G: Quantification of the number of caspase-3-positive cells counted in dentate gyrus subgranular zone and granule cell layer in 13-week-old TGF-β1 mice and their wild-type littermates, n = 5 mice per genotype. H: Relative percentage of surviving neurons calculated as the fraction of new mature neurons (BrdU+/NeuN+) present at 28 days after BrdU compared with the number of new immature neurons (BrdU+/Dcx+) present 1 day after BrdU. Bars represent the percent difference between the means in A and B. *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.0005; Student’s t-test. Scale bars = 10 μm.
To determine whether decreased neurogenesis in TGF-β1 mice resulted from decreased proliferation of neural progenitor cells or from increased cell death, we next evaluated markers of apoptosis and proliferation in dentate granule cell layer cell and subgranular zone. Instead of an increase in apoptosis in TGF-β1 mice, stereological evaluation of cleaved caspase-3 immunostaining demonstrated a significant decrease in apoptotic cells (Figure 2, F and G). Therefore, increased apoptosis is not responsible for the decrease in neurogenesis in TGF-β1 mice. Furthermore, TGF-β1 mice have a decrease in the number of apoptotic cells. We next wanted to determine whether there was less proliferation in TGF-β1 hippocampus. We measured a significant decrease in the proliferation marker Ki67 (1682 ± 161 in TGF-β1 mice versus 2331 ± 141 in wild-type mice, n = 4 per group, P = 0.0229, Student’s t-test). BrdU labeling 2 hours after a single injection of BrdU in 8-week-old mice also showed a strong trend toward a reduction in S phase cells in TGF-β1 mice compared to wild-type littermates (1347 ± 205 versus 1995 ± 199, n = 4 mice per group, P = 0.0639, Student’s t-test). Thus, given the significant decrease in both Ki-67-positive cells and caspase-3 immunostaining, TGF-β1 likely inhibits neurogenesis by decreasing neural progenitor cell proliferation rather than by increasing apoptosis of newborn cells.
Chronic TGF-β1 Does Not Alter the Total Number of Mature Dentate Gyrus Granule Cell Neurons in Aged Mice
We next evaluated whether the sharply reduced production of new hippocampal neurons in TGF-β1 transgenic mice results in a decreased number of mature granule cell layer neurons with age. We did consistently note an increased hilar size in TGF-β1 mice as well as irregularities in the thickness of the dorsal blade of the dentate granule cell layer (Figure 1A). There was no significant difference in the volume of the granule cell layer in 22-month-old TGF-β1 mice compared to their wild-type littermates. We estimated a volume of 4.3 ± 0.1 mm3 in TGF-β1 mice compared to 4.1 ± 0.1 mm3 in their wild-type littermates (P = 0.23, unpaired Student’s t-test). We then quantified the density of NeuN+ nuclei in the dentate gyrus granule cell layer in these mice, again finding no difference (5.2 ± 0.5 versus 5.4 ± 0.3 in TGF-β1 and wild-type mice, P = 0.83, units are nuclei per mm2 × 10−3). Thus, chronic TGF-β1 overproduction does not significantly decrease the total number of mature dentate granule cell layer neurons. Interestingly, when we compared the number of BrdU+ neurons 28 days after BrdU to the number 1 day after BrdU in wild-type and TGF-β1 mice, there was no clear difference (Figure 2H). This argues that TGF-β1 does not increase short-term neuronal survival, meaning between 1 to 7 days to 28 to 34 days after neurons’ birth. Instead, TGF-β1 overexpression may be prolonging long-term survival of granule cell layer neurons after they have survived their first month.
TGF-β1 Inhibits Astrogenesis but Not Microglial Proliferation
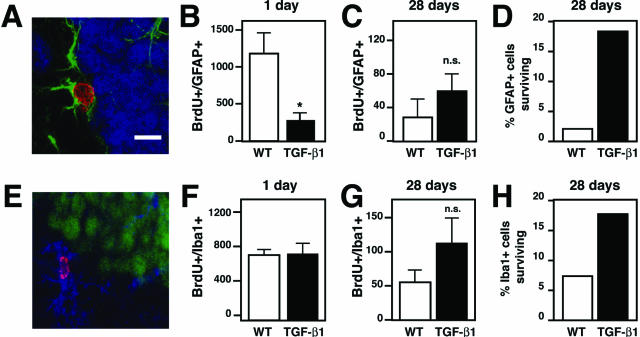
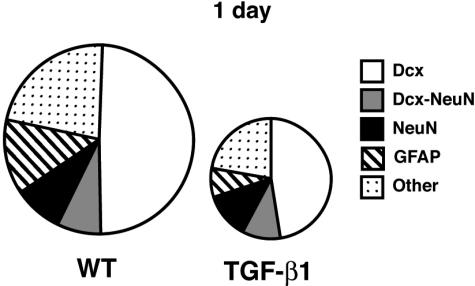
BrdU-positive astrocytes were also sharply reduced in TGF-β1 mice 1 day after BrdU (Figure 3, A and B) but showed a trend toward more cells than wild-type mice 28 days after BrdU labeling (Figure 3C). Thus, although TGF-β1 reduced the generation of new astrocytes, a higher fraction survived relative to wild-type mice (Figure 3D). The number of microglia that were BrdU-positive 1 day after labeling were not different (Figure 3, E and F) but again there was a strong trend toward more BrdU-positive microglia in TGF-β1 mice 28 days after labeling (Figure 3G), implying that TGF-β1 increases the survival of newly born microglia (Figure 3H) as well as astrocytes. These results also demonstrate that TGF-β1 reduces the production of neural progenitor-derived cells (neurons and astrocytes) equally (Figure 4), but not of myeloid lineage cells (microglia; Figure 3H). These data are thus most consistent with TGF-β1 affecting a precursor common to neurons and astrocytes and/or committed progenitor cells for both lineages.
Figure 3.
TGF-β1 mice have decreased astrogenesis, normal microgliogenesis, and prolonged glial survival. Coronal brain sections of TGF-β1 transgenic and nontransgenic littermate controls (n = 5 mice per analysis time point) injected at 8 weeks of age with BrdU to label dividing cells and sacrificed 1 or 28 days later for the presence of BrdU-positive astrocytes and BrdU-positive microglia. GFAP was used to label astrocytes, and Iba1 to label microglia. Bars are mean ± SEM. A: Confocal image of brain section containing a BrdU+/GFAP+ cell. BrdU, red; GFAP, green; and NeuN, blue. B and C: Quantification of the number of BrdU-positive astrocytes (BrdU+/GFAP+) 1 and 28 days after BrdU in TGF-β1 and their wild-type littermates. D: Percentage of the BrdU+/GFAP+ cells that remain 28 days later. E: Confocal image of brain section containing a BrdU+/Iba1+ cell. Iba1, blue; NeuN, green; and BrdU, magenta. F and G: Quantification of the number of BrdU-positive microglia (BrdU+/Iba1+) 1 and 28 days after BrdU in TGF-β1 and their wild-type littermates. H: Percent surviving BrdU+/Iba1+ cells at 28 days. *P ≤ 0.05, Student’s t-test. n.s., not significant. Scale bar = 10 μm.
Figure 4.
Cell fates of newly generated cells 1 day after BrdU labeling reveal that excess TGF-β1 does not alter the proportion of cells becoming neurons versus astrocytes. Microglia were excluded from this analysis because they are not derived from neural progenitor cells. Note the area of each pie graph represents the absolute number of new cells; TGF-β1 mice generate only 40% of the normal number of new cells, but the proportion of cells assuming each phenotype in TGF-β1 mice is not altered compared to wild-type mice.
TGF-β1 Decreases the Number of Proliferating Neural Progenitor Cells in Vitro
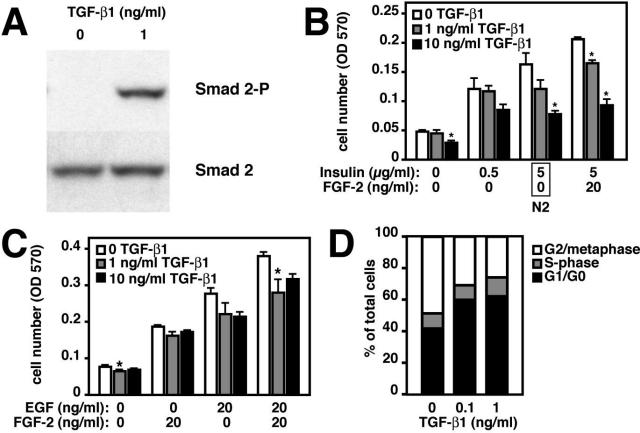
Because TGF-β receptors are widely expressed in the brain,30 and brain overexpression in transgenic mice results in astrogliosis, microgliosis, and vascular fibrosis,34 TGF-β1 could have been inhibiting neurogenesis indirectly in TGF-β1 mice. On the other hand, TGF-β1 could be acting directly on neural progenitor cells. We therefore tested whether TGF-β1 could directly activate its signaling cascade in neural progenitor cells isolated from postnatal brains. We used a rat neural precursor cell line isolated from an adult rat that retains its undifferentiated, neural precursor, phenotype in culture and can be easily propagated under growth conditions, or differentiated into neurons and astrocytes under differentiation conditions.7,8 We found that addition of recombinant TGF-β1 to these neural precursor cells led to phosphorylation of Smad2, a direct downstream mediator of TGF-β signaling (Figure 5A). We next evaluated whether TGF-β1 inhibited neural progenitor cell proliferation. Rat neural progenitor cells were grown in varying amounts of the mitogens they are normally cultured in, insulin and FGF-2, in the presence or absence of TGF-β1. TGF-β1 reduced MTT conversion, an indicator of cell number, in a dose-dependent manner and almost completely antagonized the effect of FGF-2 (Figure 5B). To confirm these results in early passage primary cells, we added varying amounts of TGF-β1 to primary mouse neural progenitor cell cultures, and obtained similar results (Figure 5C). To determine whether these decreases in cell number were caused by apoptosis or decreased growth, we performed flow cytometry on propidium iodide-stained rat cells. There was no evidence of apoptosis using this method, but TGF-β1 increased the percentage of neural progenitor cells in G1/G0 by more than 20% (Figure 5D). Our cell culture data are thus consistent with what we observed in vivo; TGF-β1 decreases the number of proliferating cells either by prolonging the cell cycle or by causing cell cycle exit and does not result in significant cell death.
Figure 5.
TGF-β1 inhibits proliferation and prolongs the cell cycle of neural progenitor cells. Rat (A, B, D) and mouse (C) neural progenitor cells were cultured in varying amounts of TGF-β1, insulin, FGF-2, and EGF. N2, standard media with N2 supplement. *P ≤ 0.05 compared to no TGF-β1, analysis of variance followed by Dunnett’s posthoc test. Bars are mean ± SEM. Data shown are representative of three to five experiments (rat cells) or two experiments (mouse cells). A: Western blot of lysates from neural progenitor cells grown in either 0 or 1 ng/ml of TGF-β1 and probed for phosphorylated (Smad2-P) and total (Smad2) Smad2. B: MTT assay of rat neural progenitor cells grown in varying amounts of TGF-β1, insulin, and FGF-2. The box labeled N2 on the x axis highlights the concentrations of insulin and FGF-2 present in commercially available N2 neuronal culture supplement. C: MTT assay of mouse neural progenitor cells grown in varying amounts of TGF-β1, EGF, and FGF-2. D: Cell cycle analysis of propidium iodide-stained cells showing the proportion of cells in G1/G0, S phase, or G2/metaphase.
Discussion
Adult neurogenesis is increasingly recognized for having a role in recovery after injury and in hippocampal learning.2–5,10–15 TGF-β1 is increased after injury and remains elevated in chronic, neurodegenerative diseases.34 It also increases with aging.32,33 We now report that chronic overproduction of TGF-β1 in mouse brains results in profound decreases in neurogenesis and astrogenesis. Despite this drastic effect on new neurons and astrocytes, both derived from a common neural progenitor cell, TGF-β1 did not affect generation of microglial cells, which are of a different (myeloid) lineage. Importantly, TGF-β1 also did not decrease the total number of granule cells in the dentate granule cell layer, most likely due to a positive effect on long-term neuronal survival. Our cell culture data demonstrate that TGF-β1 directly reduces proliferation of neural progenitor cells in vitro by altering their cell cycles. Our studies suggest that TGF-β1 may be in part responsible for the relative inefficiency of generating new neurons after injury and with aging.
To our knowledge, our study represents the first time that the effects of chronically elevated TGF-β1 on adult neurogenesis have been evaluated. TGF-β1 is a key regulator of the brain’s injury response. As such, it is acutely increased after injury and is chronically increased in the brain in multiple neurodegenerative diseases, including Alzheimer’s disease, vascular dementia, and Parkinson’s disease.34 The primary benefit of increased TGF-β1 in the brain seems to be its ability to promote neuronal survival.35 We now show that when it is chronically increased TGF-β1’s effects on neuronal survival are opposed by its inhibition of neurogenesis and that it affects the cell cycle of neural progenitor cells, by prolonging G1 and/or increasing cell cycle exit (Figure 5). TGF-β1 may actually increase neurogenesis acutely after brain injury50 by causing neural progenitor cells to exit the cell cycle early, which can cause a burst of neurogenesis.51,52 If TGF-β1 remains chronically elevated, it could then inhibit neurogenesis by the same mechanism: early cell cycle exit of neural progenitor cells. An additional alternative explanation is that TGF-β1 may exert different effects on neurogenesis, such as anti-inflammatory ones, in the presence of extensive cell death. Interestingly, there is a growing body of evidence that neuroprotection can also be conferred by inhibiting cell cycle progression or preventing re-entry into the cell cycle.53–56 TGF-β1 has been proposed to use several other molecular mechanisms to confer neuroprotection,30,57–59 and its effect on the cell cycle may be one more.
We observed a significant reduction in astrogenesis at 1 day after the last day of BrdU dosing in TGF-β1 mice compared to their wild-type littermates (Figure 3B). Despite this decrease, we observed a trend toward more new astrocytes 4 weeks after BrdU dosing in TGF-β1 mice (Figure 3, C and D), so TGF-β1 may act as a survival factor for astrocytes. We think that TGF-β1 increases survival of mature granule cell layer neurons as well. We did not observe fewer mature dentate granule cell layer neurons in TGF-β1 mice despite the decrease in neuronal production. Neuronal survival during the first month of individual cells’ lifespans was however normal in TGF-β1 mice (Figure 2E), implying that the effect is on longer term neuronal survival. In wild-type mice, new neurons that are present 4 weeks after they are generated tend to survive at least 11 months,60 so it would be difficult using BrdU-based methodology to directly show that TGF-β1 prolongs the lifespan of granule cell neurons. Despite this, the bulk of the evidence indicates that long-term neuronal loss and replacement do act in concert to maintain dentate granule cell layer size during normal aging.61 Our hypothesis is also consistent with the large body of literature on TGF-β1 as a strong neuroprotectant.30,35–38 Increases in TGF-β1 during aging and in neurodegenerative conditions may therefore have both detrimental and beneficial consequences, producing gliosis by prolonging glial survival, and protecting mature neurons at the expense of generating new ones.
Age-associated decreases in hippocampal neurogenesis likely result from changes in a complex regulatory network that controls neurogenesis, which is influenced by a number of mitogens and neurotrophic factors that decrease with age. Specifically, hippocampal levels of IGF-1, FGF-2, vascular endothelial growth factor, and brain-derived neurotrophic factor have been shown to fall in the aging brain and likely contribute to the age-related drop in neurogenesis.19,20,62 In contrast, few signals that inhibit neurogenesis during aging have been characterized. Glucocorticoids can decrease neurogenesis63 and have been proposed to be a reason why neurogenesis decreases with age.64 However, their major role is probably in stress-induced decreases in neurogenesis because lifelong reduction of glucocorticoid levels does not enhance hippocampal neurogenesis.65 The TGF-β family member GDF11 negatively regulates olfactory neuronal precursor proliferation,28 but whether it plays a role in either hippocampal neurogenesis or changes with age is not known. In addition, radiation and inflammation have been shown to reduce neurogenesis.7–9 We previously reported that endotoxin-induced inflammation decreases adult neurogenesis in rats at least in part by inducing interleukin-6.8 Interestingly, interleukin-6 did not reduce gliogenesis in vitro, implying that it may influence precursor commitment and/or amplification within the neuronal lineage. In contrast, TGF-β1 strikingly decreases the production of new hippocampal astrocytes as well as neurons, which is consistent with what occurs in normal aging,66 and may act in addition to interleukin-6 in the irradiated brain. As TGF-β1 mRNA increases with age32,33 it may be an important inhibitory influence on neurogenesis during aging as well as in neuroinflammation.
We demonstrate that TGF-β1 can activate its signaling cascade in neural progenitor cells and directly decrease their proliferation (Figure 5). Thus, although TGF-β1 is known to affect many cell types in the brain, we found that its effect on neurogenesis can be explained by a direct effect on neural progenitor cells themselves. This does not preclude an additional indirect effect, but in our mouse model TGF-β1’s expression was under GFAP promoter regulation, so it was most likely secreted from GFAP-positive neural progenitor cells67 in the neurogenic niche as well as by traditional, nonprogenitor astrocytes. This location is physiologically relevant because neural progenitor cells cultured from human, mouse, and rat are all known to synthesize TGF-β1 mRNA.68 TGF-β and its family members have also been shown to inhibit proliferation in embryonic neural cell development.26 For example, TGF-β1 blocks proliferation in neural crest stem cells, allowing differentiation.27,69 In embryonic slice cultures, exogenous TGF-β1 increases cell cycle exit and the cell cycle inhibitor p21.29 TGF-β is also necessary for development and survival of dopaminergic neurons.70 The TGF-β-family member GDF11 is critical for the development of olfactory receptor neurons in the olfactory epithelium,71,72 inhibiting proliferation of intermediate neuronal precursors by increasing expression of the cell cycle inhibitor p27.28 Interestingly, although TGF-βs can inhibit proliferation, they are likely to play overlapping roles and/or be only one of several signals that terminate proliferation and or induce differentiation. Mice with a targeted deletion of the intracellular TGF-β signaling component, Smad4, in nestin-positive cells and their progeny have only mild deficits, displaying a decrease in cerebellar granule cell neurogenesis73 and TGF-β1 knockout mice have grossly normal brain structures despite decreased neuronal survival.35
Our data show that TGF-β1 can dramatically decrease adult neurogenesis in mice. It is possible that increased production of TGF-β1 may be responsible, at least in part, for a reduction or inefficiency in generating new neurons and astrocytes in the aged brain or after injury. TGF-β1 increases after brain injury34 and may serve mainly to protect mature neurons from death, but as our studies show here, it can also inhibit the formation of new neurons and astrocytes. Although injury stimulates neurogenesis in young animals, new neurons are produced only rarely outside of typical proliferative regions and neurogenesis after injury is not sufficient to replace injured brain.74,75 Neurogenesis after injury is markedly impaired in aged animals, especially in the hippocampus.17,18 In fact, impaired proliferation, survival, and differentiation of transplanted stem and neural precursor cells in the aged brain represents a significant barrier to using these types of therapies in older patients.76 Several groups have increased neurogenesis or survival of transplanted cells in old animals by adding back growth factors,77–79 but still have not been successful in restoring hippocampal neurogenesis to youthful levels. Because TGF-β1 increases during aging and after injury, strategies that reduce TGF-β1 to youthful levels may potentially improve these results. In particular, targeted strategies that inhibit TGF-β1’s actions on neural progenitor cells while preserving its neuroprotective functions may help to increase neurogenesis and improve brain function.
Acknowledgments
We thank Drs. C.M. Testa and M. Britschgi for helpful discussions.
Footnotes
Address reprint requests to Tony Wyss-Coray, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, SUMC Rm. 343A, Stanford, CA 94305-5235. E-mail: twc@stanford.edu.
Supported in part by the National Institutes of Health (grants NS40994 and AG20603).
References
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Harvey BK, Chang CF, Shen H, Morales M, Wang Y. Neuroregenerative effects of BMP7 after stroke in rats. J Neurol Sci. 2005;240:21–29. doi: 10.1016/j.jns.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Chang YS, Mu D, Wendland M, Sheldon RA, Vexler ZS, McQuillen PS, Ferriero DM. Erythropoietin improves functional and histological outcome in neonatal stroke. Pediatr Res. 2005;58:106–111. doi: 10.1203/01.PDR.0000163616.89767.69. [DOI] [PubMed] [Google Scholar]
- Kleindienst A, McGinn MJ, Harvey HB, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22:645–655. doi: 10.1089/neu.2005.22.645. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita Y, Kitagawa K, Ohtsuki T, Takasawa K, Miyata T, Okano H, Hori M, Matsumoto M. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32:1890–1896. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Xie L, Sun Y, Mao XO, Wang Y, Simon RP, Greenberg DA. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell. 2004;3:373–377. doi: 10.1111/j.1474-9728.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Rao MS, Hattiangady B, Zaman V, Shetty GA. Hippocampal neurotrophin levels after injury: relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520–532. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease: a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Dennler S, Goumans M-J, Dijke PT. Transforming growth factor β signal transduction. J Leukoc Biol. 2002;71:731–740. [PubMed] [Google Scholar]
- Massagué J, Blain SW, Lo RS. TGF-β signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB. Clark RAF, editor. New York: Plenum Press,; Transforming Growth Factor-β. 1996:pp 275–308. [Google Scholar]
- Luethviksson BR, Gunnlaugsdottir B. Transforming growth factor-beta as a regulator of site-specific T-cell inflammatory response. Scand J Immunol. 2003;58:129–138. doi: 10.1046/j.1365-3083.2003.01297.x. [DOI] [PubMed] [Google Scholar]
- Mishra L, Derynck R, Mishra B. Transforming growth factor-beta signaling in stem cells and cancer. Science. 2005;310:68–71. doi: 10.1126/science.1118389. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Hoffmann R, Sieber-Blum M. Mitogenic and anti-proliferative signals for neural crest cells and the neurogenic action of TGF-beta1. Dev Dyn. 1997;208:375–386. doi: 10.1002/(SICI)1097-0177(199703)208:3<375::AID-AJA8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Miller MW. Transforming growth factor beta1 promotes cell cycle exit through the cyclin-dependent kinase inhibitor p21 in the developing cerebral cortex. J Neurosci. 2005;25:8627–8636. doi: 10.1523/JNEUROSCI.1876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsicker K, Flanders KC, Cissel DS, Lafyatis R, Sporn MB. Transforming growth factor beta isoforms in the adult rat central and peripheral nervous system. Neuroscience. 1991;44:613–625. doi: 10.1016/0306-4522(91)90082-y. [DOI] [PubMed] [Google Scholar]
- Finch CE, Laping NJ, Morgan TE, Nichols NR, Pasinetti GM. TGF-β1 is an organizer of responses to neurodegeneration. J Cell Biochem. 1993;53:314–322. doi: 10.1002/jcb.240530408. [DOI] [PubMed] [Google Scholar]
- Bye N, Zieba M, Wreford NG, Nichols NR. Resistance of the dentate gyrus to induced apoptosis during ageing is associated with increases in transforming growth factor-β1 messenger RNA. Neuroscience. 2001;105:853–862. doi: 10.1016/s0306-4522(01)00236-6. [DOI] [PubMed] [Google Scholar]
- Nichols NR. Glial responses to steroids as markers of brain aging. J Neurobiol. 1999;40:585–601. [PubMed] [Google Scholar]
- Buckwalter M, Wyss-Coray T. Modelling neuroinflammatory phenotypes in vivo. J Neuroinflammation. 2004;1:10. doi: 10.1186/1742-2094-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40:1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- Boche D, Cunningham C, Gauldie J, Perry VH. Transforming growth factor-beta 1-mediated neuroprotection against excitotoxic injury in vivo. J Cereb Blood Flow Metab. 2003;23:1174–1182. doi: 10.1097/01.WCB.0000090080.64176.44. [DOI] [PubMed] [Google Scholar]
- Henrich-Noack P, Prehn JHM, Krieglstein J. TGF-β1 protects hippocampal neurons against degeneration caused by transient global ischemia. Dose-response relationship and potential neuroprotective mechanisms. Stroke. 1996;27:1609–1615. doi: 10.1161/01.str.27.9.1609. [DOI] [PubMed] [Google Scholar]
- Gross CE, Bednar MM, Howard DB, Sporn MB. Transforming growth factor-β 1 reduces infarct size after experimental cerebral ischemia in a rabbit model. Stroke. 1993;24:558–562. doi: 10.1161/01.str.24.4.558. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Feng L, Masliah E, Ruppe MD, Lee HS, Toggas SM, Rockenstein EM, Mucke L. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-β1. Am J Pathol. 1995;147:53–67. [PMC free article] [PubMed] [Google Scholar]
- Kitazawa K, Tada T. Elevation of transforming growth factor-beta 1 level in cerebrospinal fluid of patients with communicating hy-drocephalus after subarachnoid hemorrhage. Stroke. 1994;25:1400–1404. doi: 10.1161/01.str.25.7.1400. [DOI] [PubMed] [Google Scholar]
- Tada T, Kanaji M, Kobayashi S. Induction of communicating hydrocephalus in mice by intrathecal injection of human recombinant transforming growth factor-beta 1. J Neuroimmunol. 1994;50:153–158. doi: 10.1016/0165-5728(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Sanan D, Mucke L, Masliah E. Chronic overproduction of TGF-β1 in astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am J Pathol. 2000;156:139–150. doi: 10.1016/s0002-9440(10)64713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly LM, Rauh MJ, Kalesnikoff J, Song CH, Krystal G. LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity. 2004;21:227–239. doi: 10.1016/j.immuni.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk HD. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995;181:1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhappan C, Geiser AG, Kordon EC, Bagheri D, Hennighausen L, Roberts AB, Smith GH, Merlino G. Targeting expression of a transforming growth factor β1 transgene to the pregnant mammary gland inhibits alveolar development and lactation. EMBO J. 1993;12:1835–1845. doi: 10.1002/j.1460-2075.1993.tb05832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellheyer K, Bickenbach JR, Rothangel JA, Bundman D, Longley MA, Krieg T, Roche NS, Roberts AB, Roop DR. Inhibition of skin development by overexpression of transforming growth factor β1 in the epidermis of transgenic mice. Proc Natl Acad Sci USA. 1993;90:5237–5241. doi: 10.1073/pnas.90.11.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SM, Allen JB, McCartney-Francis N, Morganti-Kossmann MC, Kossmann T, Ellingsworth L, Mai UE, Mergenhagen SE, Orenstein JM. Macrophage- and astrocyte-derived transforming growth factor beta as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med. 1991;173:981–991. doi: 10.1084/jem.173.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cunha A, Jefferson JA, Jackson RW, Vitkovic L. Glial cell-specific mechanisms of TGF-β1 induction by IL-1 in cerebral cortex. J Neuroimmunol. 1993;42:71–86. doi: 10.1016/0165-5728(93)90214-j. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci. 2003;116:4947–4955. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- Ohnuma S, Harris WA. Neurogenesis and the cell cycle. Neuron. 2003;40:199–208. doi: 10.1016/s0896-6273(03)00632-9. [DOI] [PubMed] [Google Scholar]
- Copani A, Uberti D, Sortino MA, Bruno V, Nicoletti F, Memo M. Activation of cell-cycle-associated proteins in neuronal death: a mandatory or dispensable path? Trends Neurosci. 2001;24:25–31. doi: 10.1016/s0166-2236(00)01663-5. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard M, Chiaramello A. The basic helix-loop-helix transcription factor Nex-1/Math-2 promotes neuronal survival of PC12 cells by modulating the dynamic expression of anti-apoptotic and cell cycle regulators. J Neurochem. 2005;92:585–596. doi: 10.1111/j.1471-4159.2004.02886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh E, Boutillier AL, Loeffler JP. Regulation of the retinoblastoma-dependent Mdm2 and E2F-1 signaling pathways during neuronal apoptosis. Mol Cell Neurosci. 2001;17:342–353. doi: 10.1006/mcne.2000.0928. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, Faden AI. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci USA. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsicker K, Krieglstein K. Co-activation of TGF-β and cytokine signaling pathways are required for neurotropic functions. Cytokine Growth Factor Rev. 2000;11:97–102. doi: 10.1016/s1359-6101(99)00033-7. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yang G-Y, Ahlemeyer B, Pang L, Che X-M, Culmsee C, Klumpp S, Krieglstein J. Transforming growth factor-β1 increases bad phosphorylation and protects neurons against damage. J Neurosci. 2002;22:3898–3909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn JH, Bindokas VP, Marcuccilli CJ, Krajewski S, Reed JC, Miller RJ. Regulation of neuronal Bcl2 protein expression and calcium homeostasis by transforming growth factor type beta confers wide-ranging protection on rat hippocampal neurons. Proc Natl Acad Sci USA. 1994;91:12599–12603. doi: 10.1073/pnas.91.26.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Turlejski K, Djavadian R. Life-long stability of neurons: a century of research on neurogenesis, neuronal death and neuron quantification in adult CNS. Prog Brain Res. 2002;136:39–65. doi: 10.1016/s0079-6123(02)36006-0. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Baram TZ, Bender RA. Hippocampal neurogenesis is not enhanced by lifelong reduction of glucocorticoid levels. Hippocampus. 2005;15:491–501. doi: 10.1002/hipo.20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen HJ, Imfeld KL, Kirov II, Tai L, Gage FH, Young MJ, Berman MA. Expression of cytokines by multipotent neural progenitor cells. Cytokine. 2003;22:101–106. doi: 10.1016/s1043-4666(03)00120-0. [DOI] [PubMed] [Google Scholar]
- Kim J, Lo L, Dormand E, Anderson DJ. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- Farkas LM, Dunker N, Roussa E, Unsicker K, Krieglstein K. Transforming growth factor-beta(s) are essential for the development of midbrain dopaminergic neurons in vitro and in vivo. J Neurosci. 2003;23:5178–5186. doi: 10.1523/JNEUROSCI.23-12-05178.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MP, Feron F, Mackay-Sim A. Growth factor regulation of neurogenesis in adult olfactory epithelium. Neuroscience. 2000;99:343–350. doi: 10.1016/s0306-4522(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Beites CL, Crocker CE, Wu HH, Bonnin A, Murray R, Calof AL. Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Dev Neurosci. 2004;26:166–180. doi: 10.1159/000082135. [DOI] [PubMed] [Google Scholar]
- Zhou YX, Zhao M, Li D, Shimazu K, Sakata K, Deng CX, Lu B. Cerebellar deficits and hyperactivity in mice lacking Smad4. J Biol Chem. 2003;278:42313–42320. doi: 10.1074/jbc.M308287200. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Recovery and rehabilitation in stroke: stem cells. Stroke. 2004;35:2691–2694. doi: 10.1161/01.STR.0000143323.84008.f4. [DOI] [PubMed] [Google Scholar]
- Felling RJ, Levison SW. Enhanced neurogenesis following stroke. J Neurosci Res. 2003;73:277–283. doi: 10.1002/jnr.10670. [DOI] [PubMed] [Google Scholar]
- Zitnik G, Martin GM. Age-related decline in neurogenesis: old cells or old environment? J Neurosci Res. 2002;70:258–263. doi: 10.1002/jnr.10384. [DOI] [PubMed] [Google Scholar]
- Zaman V, Shetty AK. Survival of fetal hippocampal CA3 cell grafts in the middle-aged and aged hippocampus: effect of host age and deafferentation. J Neurosci Res. 2002;70:190–199. doi: 10.1002/jnr.10401. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neuro-genesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]