Abstract
The pro form of neurotrophic growth factor (pro-NGF), purified by chromatography from human Alzheimer’s disease (AD)-affected brains (ADhbi-pro-NGF), has been shown to induce apoptotic cell death in neuronal cell cultures through its interaction with the p75 neurotrophin receptor (p75NTR). In the present work, we report that ADhbi-pro-NGF stimulates processing of p75NTR with α- and γ-secretases, yielding a 20-kd intracellular domain (p75ICD) that translocates to the nucleus. This process was accompanied by delayed apoptosis. In AD, p75ICD was significantly increased in human entorhinal cortex. Although human frontal cortex has been described as showing a higher pro-NGF increase in AD, the increase in the entorhinal cortex paralleled p75NTR processing in its intracellular domain. In addition, pro-NGF isolated from AD-affected brains differed functionally from pro-NGF isolated from comparably aged control brains, with pro-NGF isolated from control brains being unstable and undergoing degradation to NGF when added to cell culture. As p75ICD and pro-NGF are both mediators of apoptosis and are both found in increased levels in the cerebral cortex in AD, the present data have implications for understanding neuronal degeneration in AD.
The highly multifunctional neurotrophin receptor p75NTR (p75 neurotrophin receptor) belongs to the family of tumor necrosis factor receptors,1 which share a repeated cysteine domain in the extracellular domain. p75NTR direct ligands have been referred to as the neurotrophins, including neurotrophic growth factor (NGF), brain-derived neurotrophic factor, neurothrophin 3, neurothrophin 4/5, and the pro-neurotrophins pro-brain-derived neurotrophic factor and pro-NGF. Of these, pro-NGF is the most specific ligand and the one with the highest affinity.2 Rather than NGF, it has been shown to be the predominant form of this neurotrophin in the human brain.3
Recent studies have shown that in Alzheimer’s disease (AD), brain levels of pro-NGF are increased in a stage-dependent manner. It is expressed particularly in glial cells as well as in cortical and hippocampal neurons.3–5 AD is characterized by the presence of senile plaques composed of β-amyloid (Aβ) and neurofibrillary tangles containing hyperphosphorylated tau, which are further accompanied by degeneration of synapses and dendrites and by cell death and loss of neurons.6
Several pieces of evidence support the idea that neurotrophin binding to p75NTR can mediate cell death in different neuronal models.7–9 Under NGF activation, the ability of p75NTR to induce cell death depends on the ratio of p75NTR/high affinity NGF receptor (TrkA) expression.10 However, under pro-NGF activation, the receptor complex composed of p75NTR and Sortilin mediates apoptotic signals.11 Sortilin has been shown to be widely expressed in rat cerebral neurons,12 although information for other models is limited. The ratios of NGF:pro-NGF and p75NTR:Sortilin:TrkA therefore emerge as critical regulatory events in maintaining the balance between survival and death for a given cellular model. Several works support the idea that p75NTR is induced in a number of pathological situations, such as multiple sclerosis, amyotrophic lateral sclerosis, osmotic stress, and AD.13 For example, spinal cord injury induces an increase in both p75NTR expression as well as NGF and pro-NGF levels in some neuronal and glial cells.14,15
The finding that Aβ directly binds p75NTR, inducing apoptosis in several cell culture lines, is especially relevant in neuronal apoptosis in AD.16–18 Furthermore, p75NTR can be processed on both transmembrane domain sides by α-secretase and γ-secretase consecutively by a regulated intramembrane proteolysis. This has been shown to be activated by phorbol esters (such as phorbol 12-myristate 13-acetate)19,20 or by neurotrophin binding to the receptor.21 α-Secretase activity yields p75NTR C-terminal fragments (CTF) of 25 to 30 kd (p75CTF), containing the trans-membrane and intracellular domains, whereas γ-secretase activity yields p75NTR intracellular domain (p75ICD), which has been described as translocating to the nucleus in some cell models.21 Other similar processing by γ-secretase is known to occur in Aβ precursor peptide, yielding Aβ42 amylogenic peptide.22 All these studies give γ-secretase a central role in AD.
In the present work we report that in AD p75ICD is significantly increased in human entorhinal cortex. We also report that pro-NGF isolated from AD-affected brains (ADhbi-pro-NGF) differs functionally from pro-NGF isolated from comparably aged control brains (Chbi-pro-NGF). ADhbi-pro-NGF stimulates processing of p75NTR with α- and γ-secretases yielding p75ICD, which translocates to the nucleus. This process is needed for the apoptosis caused by ADhbi-pro-NGF binding to p75NTR. As both p75ICD and pro-NGF are increased in AD-affected human brain cortex, the data we present here could be relevant to the understanding of neuronal degeneration in AD.
Materials and Methods
Cell Cultures and Treatments
Cells of the 3T3 cell line, stably transfected to express human p75NTR (3T3-p75st; kindly provided by M.V. Chao, Skirball, New York University, NY), were grown in 24-well plates at 15,000 cells/cm2 with Dulbecco’s modified Eagle’s medium (Gibco-BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS). Before treatment, cells were washed twice with serum-free medium, and treatments were performed in 0.5% FBS medium. Rat pheochromocytoma cell line (PC12 cells) was grown in 24-well plates in Dulbecco’s modified Eagle’s medium supplemented with 6% FBS, 6% horse serum, 2 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid and antibiotics. Before treatment, cells were washed twice with serum-free medium, and treatments were performed in the absence of FBS medium. In all experiments, controls were included with the elution buffer from chromatography purification of hbi-pro-NGF. For γ-secretase inhibition, pretreatment with 1 μmol/L N-[N-(3,5-difluorophenacetyl)-l-alanyl]-(S)-phenylglycine t-butyl ester (DAPT; Calbiochem, San Diego, CA) was performed.
Human Samples
Human cases are summarized in Table 1. Human brain samples were provided by the Institute of Neuropathology and Brain Bank of the Institut d’Investigació Biomèdica de Bellvitge-Hospital Universitari de Bellvitge (Barcelona, Spain) following the approval of the corresponding Ethics Committee. At autopsy, half of the brain was fixed in 10% formalin for no less than 3 weeks, whereas the other half was cut in coronal sections 1-cm thick, frozen on dry ice, and stored at −80°C until use. The neuropathological study was performed in formalin-fixed, paraffin-embedded sections of the frontal, primary motor, primary somatosensory, posterior parietal, primary and association visual, temporal superior, temporal inferior, anterior insular, anterior cingulate, and entorhinal cortices; subiculum and anterior and posterior levels of the hippocampus; the caudate and putamen, nucleus pallidus, amygdala, Meynert nucleus, and medial and posterior levels of the thalamus; midbrain (two levels); pons (two levels, including the locus ceruleus) and medulla oblongata (two levels); and upper vermis, lateral hemisphere, and dentate nucleus of the cerebellum. Dewaxed sections were stained with hematoxylin and eosin, Luxol Fast Blue-Klüver Barrera, or processed for immunohistochemistry following the streptavidin-peroxidase method (Supersensitive Kit, Menarini, Florence, Italy) or with the EnVision System Peroxidase (diaminobenzidine) procedure (Dako, Carpinteria, CA) for Aβ (M. Sarasa, Zaragoza, Spain, Boehringer-Mannheim, Mannheim, Germany), tau (Sigma-Aldrich, St. Louis, MO), phospho-tau (phospho-specific antibodies Thr-181, Ser-202, Ser-214, Ser-262, Ser-396, and Ser-422; Calbiochem), B-crystallin (Novocastra, New Castle Upon Tyne, UK), α-synuclein (Chemicon, Temecula, CA), ubiquitin (Dako), phosphorylated neurofilament epitopes (Boehringer, Ingelheim, Germany), and α- and β-tubulin (Sigma-Aldrich). Neuropathological diagnosis and staging were performed following Braak and Braak classification.23 A summary of the cases examined in the present study is shown in Table 1. Control and diseased cases were processed in parallel. The frontal cortex (area 8), along with the entorhinal cortex and anterior hippocampus, were used for further immunohistochemical and biochemical studies. Rabies virus infection was excluded by immunohistochemistry using a mouse monoclonal anti-rabies antibody (4G4; Abcam, Cambridge, UK).
Table 1.
Summary of Cases Examined in the Present Study
| No. | Age (years) | Gender* | Diagnostic | Braak stage, according to Aβ† | Braak stage, according to taupathology‡ | Post mortem delay (hours) | Used for Hbi-pro-NGF purification§ |
|---|---|---|---|---|---|---|---|
| 1 | 82 | F | No lesions | A | 0 | 11 | + |
| 2 | 63 | M | No lesions | A | 0 | 17 | |
| 3 | 49 | F | No lesions | A | 0 | 7 | + |
| 4 | 80 | F | No lesions | A | 0 | 21 | + |
| 5 | 74 | M | No lesions | A | 0 | 24 | + |
| 6 | 86 | F | AD | C | V | 10 | + |
| 7 | 82 | F | AD | C | V | 10 | + |
| 8 | 69 | M | AD | C | V | 6 | + |
| 9 | 82 | F | AD | C | IV | 5 | + |
| 10 | 69 | M | AD | C | V | 20 |
F, female; M, male.
A, B, and C, Braak and Braak’s classification of AD stages depending on amyloid plaques.
0–V, Braak and Braak’s classification of AD stages depending on the distribution and amount of neurofibrillary tangles.
Samples labeled as ′+′ were used for independent hbi-pro-NGF purifications.
Antibodies
Anti-human ICDp75 was either from Promega (G3231; Madison, WI) or 9992 kindly provided by M.V. Chao. Anti-p75NTR antibody (REX; directed against the extracellular domain) was kindly provided by L.F. Reichardt (Howard Hughes Medical Institute, University of California). Anti-β-actin antibody (AC-15) was obtained from Sigma-Aldrich. Anti-NRH2 antibody was kindly provided by P.A. Barker (Montreal Neurological Institute, McGill University, Canada). Secondary anti-rabbit IgG-horseradish peroxidase was obtained from Amersham. The following antibodies were used in the neuropathological classification of the human brain samples: Aβ amyloid (M. Sarasa, Boehringer-Mannheim), tau (Sigma-Aldrich) phospho-tau (phospho-specific antibodies Thr-181, Ser-202, Ser-214, Ser-262, Ser-396, and Ser-422; Calbiochem), B-crystallin (Novocastra), α-synuclein (Chemicon), ubiquitin (Dako), phosphorylated neurofilament epitopes (Boehringer), and α- and β-tubulin (Sigma-Aldrich).
Immunocytochemistry and Confocal Imaging
The cells were grown and treated on precoated p-l-lysine coverslips. After the indicated times of treatment, the cells were fixed with 4% paraformaldehyde in phosphate-buffered saline for 1 hour at room temperature and permeabilized with methanol for 10 minutes at room temperature. The incubation with corresponding primary antibody was done overnight at 4°C. Anti-human p75 (Promega) and anti-p75NTR (REX) antibody (kindly provided by L.F. Reichardt) were used to detect intra- and extracellular domains of p75NTR, respectively. The secondary antibody incubation with anti-rabbit Alexa Fluor 488 (Promega) was for 1 hour at room temperature. After washing with phosphate-buffered saline, the coverslips were mounted with Mowiol, and fluorescence images were acquired using a confocal microscopy setup (Model IX81; Olympus, Tokyo, Japan, ×60 PlanApo using Fluoview version 4.3) or an inverted epifluorescence microscope (Olympus IX71, ×20 LCPlanFl). To use an objective criterion to determine whether a 3T3-p75st cell was positive for ICD nuclear translocation, we performed intensity profiles of confocal images. We selected cells as positive if they show higher nuclear values than cellular membrane values.
Western Blotting
Protein extracts from the frontal and entorhinal cortex were prepared as described. Pieces weighing from 0.3 to 1.0 g were sonicated in 1% sodium dodecyl sulfate, 10 mmol/L Tris, pH 6.8 (Scharlab, Barcelona, Spain). Homogenates were centrifuged at 12,000 × g for 10 minutes, and protein concentration in the supernatant was determined by DC-Protein Assay (Bio-Rad, Hercules, CA). 30 μg of total protein was resolved in 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to Immobilon-P membranes (Millipore, Billerica, MA), and blocked for 1 hour at room temperature in TBS-T (50 mmol/L Tris, pH 8.0, 133 mmol/L NaCl, 0.2% Tween 20) with 5% skimmed milk. For immunodetection of the p75NTR forms, membranes were incubated with 9992 anti-p75NTR antibody (1:2500 in TBS-T) or with anti-p75NTR antibody (Promega, 1:1000 in TBS-T) at 4°C overnight. After washing in TBS-T, membranes were incubated with horseradish peroxidase-conjugated anti-rabbit antibody (1:5000 in TBS-T) at room temperature for 1 hour. For detection, an ECL chemiluminescence system (Amersham Biosciences, Piscataway, NJ) was used in accordance with the manufacturer’s instructions. Membranes were stripped and re-blotted with anti-β-actin antibody (Sigma-Aldrich, 1:5000 in TBS-T) to assess correct protein loading.
Isolation of Pro-NGF from Human Brain
The methodology was based on the protocol for isolation of mature NGF from mouse submaxillar gland described by Longo et al.24 Briefly, frozen human brain tissue from AD-affected or control (see Table 1) frontal cortex (8 to 10 g) was homogenized in 20 ml of sterile water using a Potter on ice. After centrifugation of homogenates (2500 × g for 1 hour at 4°C), supernatants were dialyzed against 20 mmol/L Na2HPO4/NaH2PO4 (pH 6.8) overnight using a 10-kd molecular weight cut-off membrane (Sigma-Aldrich). The samples were loaded on a DEAE-Sepharose FF column (Amersham Biosciences) pre-equilibrated in the same buffer. Eluted fractions having absorbance A280 > 0.5 were equilibrated by a second dialysis against 20 mmol/L Na2HPO4/NaH2PO4 (pH 6.8) overnight. Salt concentration was adjusted to 0.4 mol/L NaCl in 50 mmol/L CH3COONa (pH 4.0). The sample was centrifuged at 2500 × g for 30 minutes, and the supernatant was loaded on a DEAE-Sepharose FF column previously equilibrated with the same buffer. All of the procedures were performed at 4°C. Eluted fractions with absorbance A280 > 0.1 were collected, concentrated, and analyzed by Western blot using antibodies against either NGF (H20, Santa Cruz Biotechnology, Santa Cruz, CA) or pro-NGF antibody.4 NGF protein was undetectable in all of the fractions obtained using H20 anti-NGF antibody. Absence of β-amyloid peptide or derived aggregates in the eluted fractions was excluded by Western blot using rabbit polyclonal anti-Aβ1–40 and Aβ1–42 antibodies (Dr. M. Sarasa) or anti-Aβ1 (Boehringer) used at a dilution of 1:50.
Detection of Apoptosis in the Cultures
Cells were fixed with 4% paraformaldehyde. Nuclei were stained with Hoechst 33258 (Sigma-Aldrich). The fluorescence was analyzed using an Olympus ×20 LCPlan objective and documented with an Olympus DP70 camera. The number of pyknotic nuclei was established, and the percentage of apoptosis was determined. At least 500 nuclei in random, nonoverlapping fields per condition were counted in each experiment. Apoptotic cells were also detected by using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL; in situ cell death detection kit, POD; Roche Applied Science, Mannheim, Germany), following the manufacturer’s instructions.
Densitometry and Statistical Analysis
The density of the immunoreactive bands was determined by densitometry of the films using a Bio-Rad image analysis system. Statistical significance between groups was calculated using Student’s t-test.
Results
Increased Levels of p75ICD from p75NTR Processing Observed in Vivo and in Vitro: AD Cerebral Cortex and p75NTR-Expressing Cells Stimulated with Pro-NGF or NGF
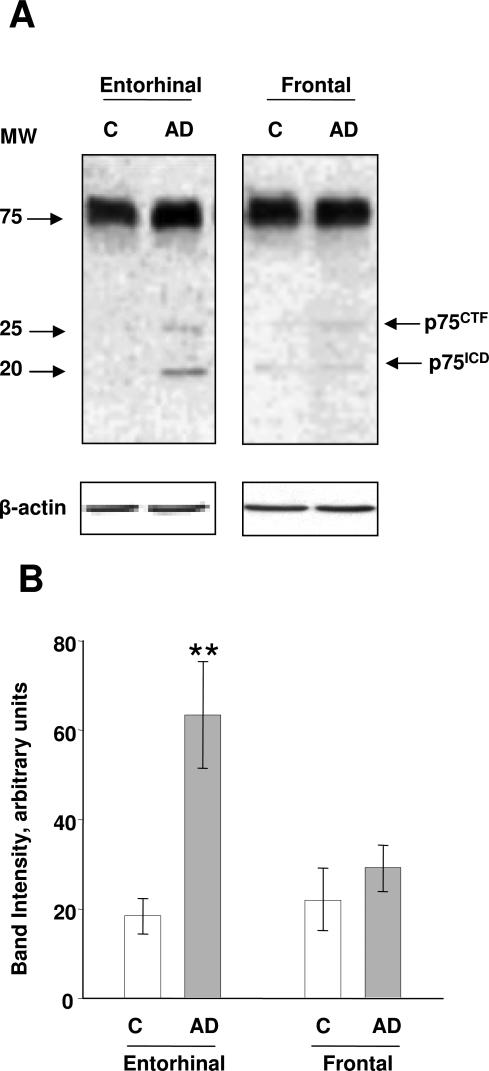
p75ICD 20-kd product from γ-secretase has recently been suggested to be involved in p75NTR signaling.21,25,26 γ-Secretase is active in AD-affected human brain and has an important role in the etiopathogenesis of this disease.27 The increase of p75NTR in AD-affected human brain is not fully accepted in the literature. To further investigate the pathophysiological role of p75, we first wanted to verify a possible increase in the 20-kd p75ICD levels in AD. We used an antibody raised against the p75ICD as described in the Materials and Methods to analyze samples from AD and control human frontal and entorhinal cortex at an advanced stage of the disease (stage C, following the staging of Aβ amyloid burden of Braak and Braak23) (Figure 1, A and B). The use of this antibody showed the presence of 25- and 20-kd bands corresponding to p75CTF and p75ICD as previously reported,25 respectively, as sequential products of α- and γ-secretases. Both fragments appeared to increase in AD entorhinal cortex (Figure 1A). Densitometry analysis of the 20-kd band revealed significant differences between the control and AD brains in entorhinal cortex (Figure 1B). However, no differences between control and AD with respect to the amount of 75-kd p75NTR in frontal and entorhinal cortex were observed.
Figure 1.
p75ICD is increased in the frontal and entorhinal cortex in Alzheimer’s disease. A: The p75ICD and p75CTF are detected as 20- and 25-kd immunoreactive bands, respectively, with an antibody directed against the intracellular domain of p75NTR (Promega). The content of p75ICD is clearly increased in stage C (according to Braak and Braak, see Table 1 and Materials and Methods) in AD entorhinal cortex (line AD) compared to controls (line C). β-Actin is a control of protein loading. B: Densitometric analysis of anti-p75ICD immunodetected bands shows a significant increase of p75ICD 20-kd band in AD-affected entorhinal cortex. Bars represent the mean of five samples of stage C (AD) and five samples of stage A (C). **P < 0.01; Student’s t-test.
In previous works, an increase in pro-NGF levels in human AD cerebral cortex was reported.3–5 Here we show a significant increase of both pro-NGF and p75NTR processing to p75ICD in the same brain samples. The pro-NGF increase in the entorhinal cortex in AD, as shown in our previous publication,4 is lower than in frontal cortex. Yet, p75NTR processing and pro-NGF levels increased in parallel in the entorhinal cortex.
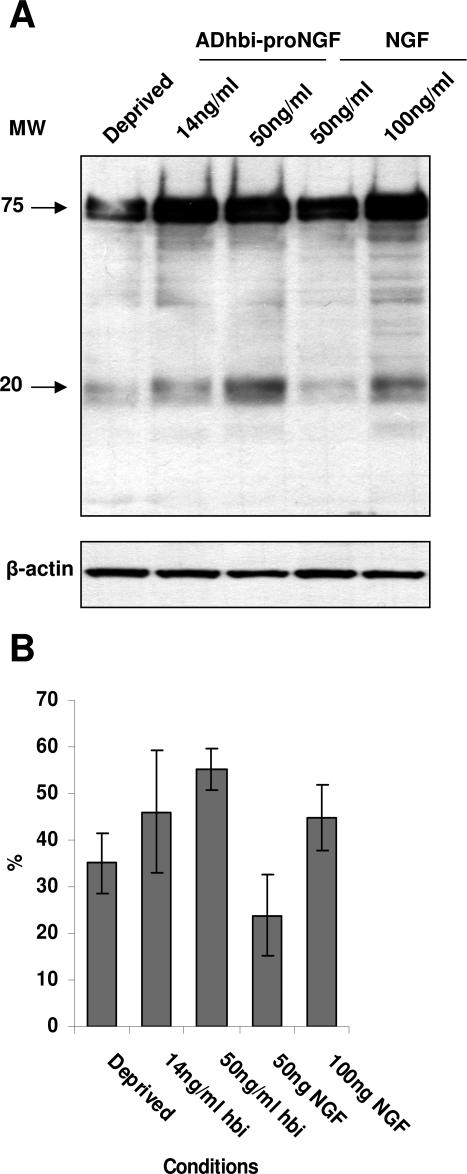
As it is has been noted that NGF is able to induce γ-secretase-dependent p75ICD nuclear translocation,21 we wanted to assess if this effect could also be observed following ADhbi-pro-NGF p75NTR stimulation. As can be seen in Figure 2, ADhbi-pro-NGF was able to induce higher p75ICD levels than NGF.
Figure 2.
p75NTR shedding yielding ICD is induced by ADhbi-pro-NGF and by NGF treatment. 3T3-p75st cells were serum-deprived (0.5% FBS) and left untreated or treated, with increasing concentrations of ADhbi-pro-NGF and NGF, for 12 hours. 30 μg of total protein was used per lane. A: Western blot using anti-p75ICD antibody (Promega) shows induction of the 20-kd band. β-Actin was used as a control of protein loading. B: The experiment was repeated three times with similar results. Bands were scanned, and 20-kd band density units are expressed as percentage of 75-kd corresponding units. Bars represent the mean ± SD of three independent experiments using Promega antibody (two experiments) and 9992 antibody (one experiment).
Pro-NGF Activation of p75NTR Induces Nuclear Translocation of p75ICD but Not of the Full-Length p75NTR
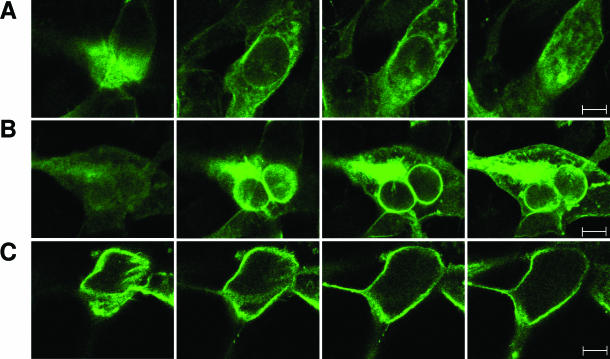
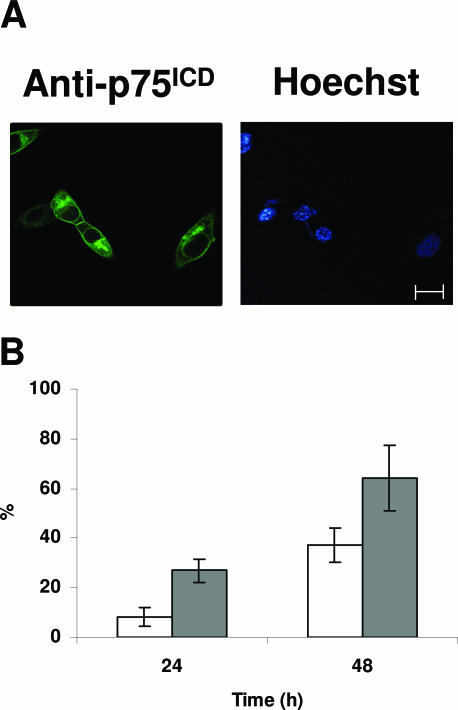
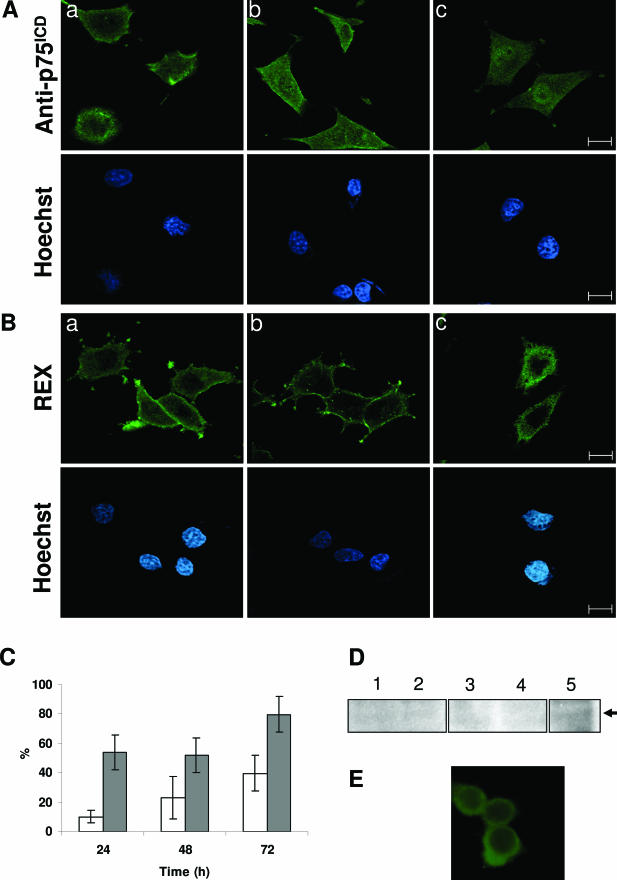
It has been reported that p75ICD translocates to the nucleus on neurotrophin stimulation in RN22-SC cell line and primary Schwann cells.21 Therefore, we wanted to assess whether ADhbi-pro-NGF was responsible for p75ICD yield and whether this was followed by nuclear translocation. For this purpose, we first used a cell model expressing high levels of p75NTR, such as 3T3-p75st, which has a good dose response to this pro-neurotrophin in terms of a high degree of apoptosis.4 Confocal images of sequential sections of 3T3-p75st cells immunostained with an antibody raised against p75ICD (see Materials and Methods) showed increased perinuclear distribution in ADhbi-pro-NGF-treated cells (Figure 3, A and B). Hoescht staining of the same cells showed the localization of the nuclei (Figure 4A). As shown in Figure 4B, the percentage of apoptosis in ADhbi-pro-NGF-treated cells was low within the first 24 hours but increased significantly at 48 hours. This may explain the observation that positive cells for p75ICD perinuclear translocation show non-apoptotic nuclei. The criteria used to quantify percentage of cells positive for perinuclear ICDp75 distribution are detailed in the Materials and Methods. To distinguish between the internalization of full-length p75NTR and the translocation of p75ICD, we used an antibody raised against the extracellular domain of p75NTR (REX). As shown in Figure 3C, immunoreactive signal remained mainly in the cell membrane even in ADhbi-pro-NGF treated cells.
Figure 3.
ADhbi-pro-NGF induces internalization of p75ICD but not of the entire p75NTR in 3T3-p75st cells. 3T3-p75st cells were serum-deprived (0.5% FBS) and left untreated (A) or treated with 25 ng/ml of ADhbi-pro-NGF (B and C) for 12 hours. Cells were washed, fixed, permeabilized, and immunostained using anti-p75ICD (A and B) or REX antibodies (C). Representative confocal sequential optical sections of 10 μm from the same cell are shown. Scale bars = 40 μm.
Figure 4.
Induction of translocation of p75ICD after ADhbi-pro-NGF treatment as a previous event to induction of apoptosis. A: 3T3-p75st cells were serum-deprived (0.5% FBS), treated with 25 ng/ml ADhbi-pro-NGF for 24 hours, and immunostained for p75ICD. Scale bar = 90 μm. B: At time points 24 and 48 hours, the nuclear translocation (white bars) and the induction of apoptosis (gray bars) were assayed. Percentages are corrected for serum-deprived cells (0.5% FBS). Bars represent the mean ± SD of three independent experiments.
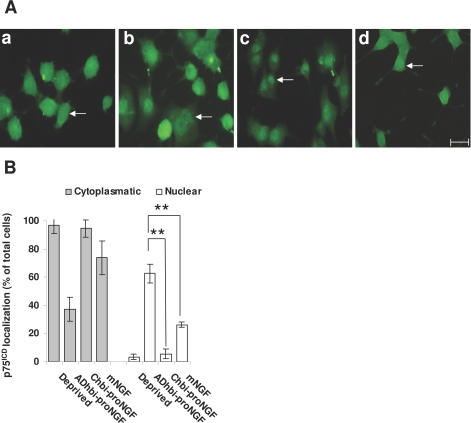
Because the pattern of localization may depend on many factors, such as the level of expression of p75NTR, the presence or absence of other membrane proteins or intracellular interacting proteins, we performed parallel experiments with PC12 cells. This model expresses more physiological levels of p75NTR than 3T3-p75st and undergoes apoptosis following ADhbi-pro-NGF treatment. Figure 5A shows that ADhbi-pro-NGF induced nuclear translocation of a proportion of p75ICD present in the cell, with immunofluorescence being more or less uniformly distributed throughout the nucleus (Figure 5A, panel c). It colocalized with Hoechst staining, even though there were remaining traces of the signal in the cytoplasm. We observed a lesser degree of nuclear p75ICD translocation with NGF (Figure 5A, panel b) and in deprived cells (Figure 5A, panel a). Following a similar approach, to make sure that this nuclear localization did not correspond to internalization of the full-length p75NTR, we also used REX antibody (Figure 5B). Unprocessed p75NTR remained mainly in the membrane in untreated and NGF-treated cells (Figure 5B, panels a and b) and showed a degree of internalization without nuclear translocation as a consequence of the addition of ADhbi-pro-NGF (Figure 5B, panel c).
Figure 5.
ADhbi-pro-NGF induces nuclear translocation of p75ICD but not of the entire p75NTR in PC12 cells. PC12 cells were serum-deprived (a), serum-deprived and treated with 100 ng/ml NGF (b), or serum-deprived and treated with 25 ng/ml ADhbi-pro-NGF (c). Cells were washed, fixed, permeabilized, immunostained with anti-p75ICD antibody (A) or with REX antibody (B) and visualized with confocal microscopy. Nuclei were stained with Hoechst. C: Apoptosis (white bars) and nuclear translocation of p75ICD (gray bars) were quantified at 24, 48, and 72 hours. Bars represent the mean ± SD of three independent experiments. D: Western blot using anti-NRH2 antibody shows a band in PC12 total cell lysate (lane 5), which is barely detectable in total lysates from human entorhinal cortex (lanes 1 and 2) and frontal cortex (lanes 3 and 4). The arrow indicates apparent molecular weight of 24 kd. E: Epifluorescence NRH2 immunostaining image of PC12 cells treated with 25 ng/ml ADhbi-pro-NGF for 24 hours shows the absence of NRH2 nuclear translocation. Scale bars = 50 μm.
The time course of ICD nuclear translocation and apoptosis of treated PC12 cells (Figure 5C) showed an initial effect of ICD translocation (at 24 hours) and a delayed increase in the number of apoptotic cells. At the time chosen to best observe nuclear translocation (24 hours), the percentage of apoptotic cells was still very low. Cells shown in the figures to be positive for translocation were still not positive for apoptosis. To exclude the possible cross-reaction between the anti ICDp75NTR antibodies used and NRH2, we performed western blotting with an anti-NRH2 antibody. As can be observed (Figure 5D), the 24-kd positive band in PC12 lysates was not present in any of the control or AD-affected human brain samples. Furthermore, ICD nuclear translocation is clearly visible in an epifluorescence NRH2 immunostaining image of PC12 cells treated with ADhbi-pro-NGF in the same conditions as Figures 7 and 8. Further, the patterns of NRH2 and ICD distribution were completely different. NRH2 labeling was not visible in the nucleus.
Figure 7.
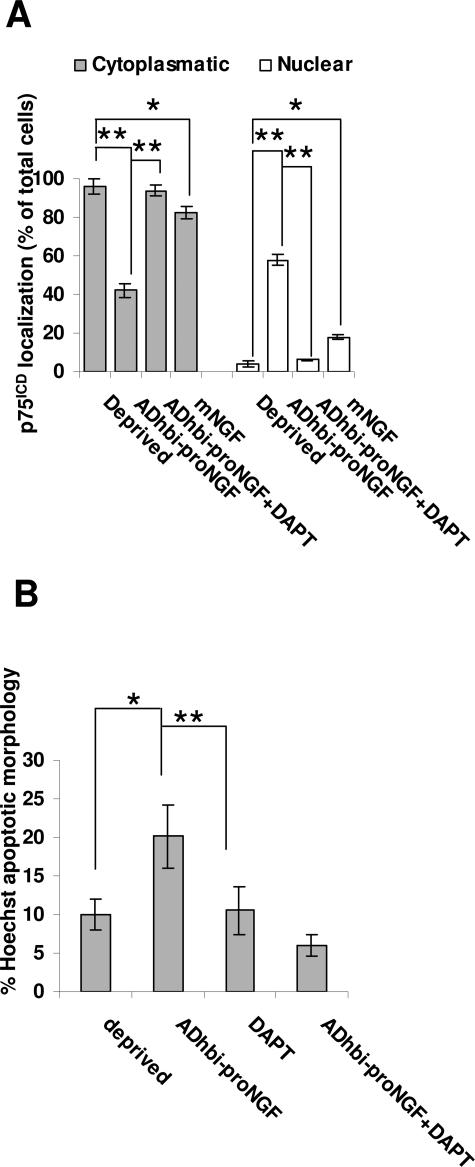
A: ADhbi-pro-NGF but not Chbi-pro-NGF induces nuclear translocation of p75ICD in PC12 cells. PC12 cells were serum-deprived and left untreated (a) or treated for 24 hours with 100 ng/ml of NGF (b), 25 ng/ml ADhbi-pro-NGF (c), or 25 ng/ml Chbi-pro-NGF (d). Cells were washed, fixed, permeabilized, and immunostained with anti-p75ICD antibody. Arrows in a and d are representative of the cytoplasmic distribution quantified in B. Arrows in b and c are representative of the nuclear distribution quantified in B. Cytoplasmic and nuclear p75ICD was examined with the help of an inverted epifluorescence microscope. Bars represent the mean of percentages of at least 500 cells per condition (B). *P < 0.05; **P < 0.01; Student’s t-test. Values result from three independent experiments. Scale bar = 80 μm.
Figure 8.
Inhibition of γ-secretase in PC12 cells involves loss of nuclear translocation and decreased apoptosis caused by ADhbi-pro-NGF. PC12 cells were serum-deprived and pre-treated, or not, with 1 μmol/L DAPT for 30 minutes. Cells were then treated, or not, with 25 ng/ml ADhbi-pro-NGF. After 12 hours, cells were washed, fixed, permeabilized, and immunostained with anti-p75ICD antibody. Localization was scored using an inverted epifluorescence microscope (A). Apoptotic morphology, as visualized with Hoechst staining, was quantified at 24 hours and expressed as a percentage of the total number of cells (B). Bars represent the mean of percentages of at least 500 cells per condition. *P < 0.05; **P < 0.01; Student’s t-test. Values are the result of three independent experiments.
Control Human Cerebral Cortex Contains a Pro-NGF That Is Not Actively Involved in Inducing Apoptosis or Nuclear p75ICD Translocation
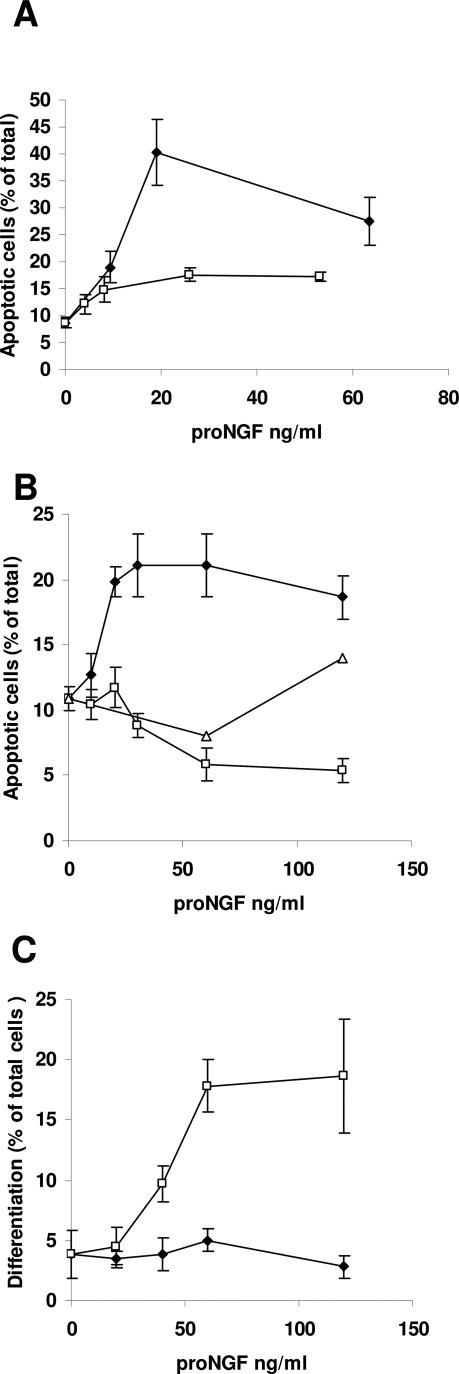
A relevant question is whether there were any differences in the potential pathogenicity of pro-NGF obtained from AD when compared with pro-NGF obtained from age-matched controls. Western blot in cerebral cortex lysates showed a similar molecular weight pattern in AD and controls, although the total amount of pro-NGF changed.4 However, it remains to be established whether ADhbi-pro-NGF and Chbi-pro-NGF were equally efficient in inducing apoptosis. Using the same purification procedure (see Materials and Methods), we obtained pro-NGF from both sources in similar yields. We performed four parallel purifications from each source and assayed induction of apoptosis in 3T3-p75st (Figure 6A) and in PC12 cells (Figure 6B). As previously reported,4 the percentage of apoptosis was higher in 3T3-75st cells than in PC12 cells. Surprisingly, Chbi-pro-NGF was not able to induce apoptosis in 3T3-p75st or PC12 cells, even at high concentrations. Because 50 ng/ml NGF is able to maintain survival and is very specific in inducing differentiation in PC12 cells, we used this cell line to further study whether protection from death following Chbi-pro-NGF treatment (Figure 6B) was due to pro-NGF degradation to NGF. Moreover, at the incubation time used for the treatment (24 hours), even 100 ng/ml of mature NGF was not able to induce a significant increase of apoptosis in PC12 cells. Figure 6C shows that Chbi-pro-NGF, but not ADhbi-pro-NGF, was able to differentiate PC12 cells to a degree similar to that of NGF. This is an indication that Chbi-pro-NGF is degraded in the cell culture to NGF. In contrast, ADhbi-pro-NGF was especially stable under these conditions.
Figure 6.
ADhbi-pro-NGF but not Chbi-pro-NGF induces apoptosis in p75NTR-expressing cells. Cultured 3T3-p75st cells (A) and PC12 cells (B and C) were serum-deprived (0.5% FBS) and left untreated or treated for 24 hours with increasing concentrations of either ADhbi-pro-NGF (⧫) or Chbi-pro-NGF (□) or with NGF (100 ng/ml) (▵). Apoptosis was evidenced by nuclear morphology after Hoechst staining. Apoptosis increases in a dose-dependent manner with ADhbi-pro-NGF (A and B). In contrast, Chbi-pro-NGF treatment resulted in cell differentiation. Differentiated PC12 cells are those with neurite extensions longer than the cell body (C). Values represent the mean ± SD of three independent experiments.
To determine whether Chbi-pro-NGF had the capacity to induce p75ICD nuclear translocation, PC12 cells were deprived of or treated with Chbi-pro-NGF, ADhbi-pro-NGF, or NGF. Nuclear or cytoplasmic localizations were counted with the help of an inverted epifluorescence microscope using anti-p75ICD antibody (Figure 7A). Arrows in Figure 7A (panels a and d) represent the cytoplasmic distribution quantified in Figure 7B. Arrows in Figure 7A (panels b and c) represent the nuclear distribution quantified in Figure 7B. As can be observed in Figure 7, Chbi-pro-NGF did not induce nuclear p75ICD localization in PC12 cells treated with the same concentration of ADhbi-pro-NGF (25 ng/ml). NGF was used at the concentration shown to be effective for inducing survival and differentiation in this cell line (100 ng/ml). At this concentration, NGF was more active than Chbi-pro-NGF but less effective than ADhbi-pro-NGF. In sum, our results strongly suggest a role for ADhbi-pro-NGF in neurodegeneration.
Inhibition of γ-Secretase Processing of p75NTR Blocks Both p75ICD Nuclear Translocation and Apoptosis Mediated by ADhbi-Pro-NGF
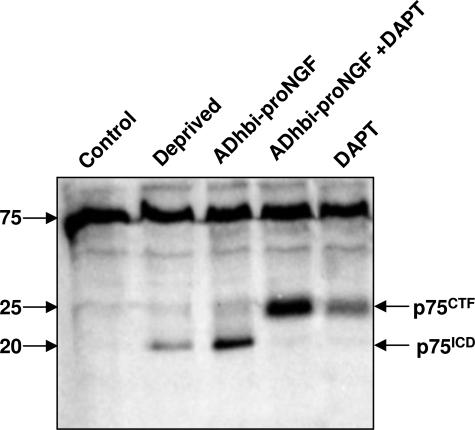
An increase in pro-NGF has been reported in AD.3,4 Because we have shown p75ICD to be present in the same regions as pro-NGF, we were interested in studying whether the nuclear translocation of p75ICD induced by ADhbi-pro-NGF (Figure 8A) could be relevant for the apoptosis caused by this pro-neurotrophin. To assess whether γ-secretase processing of p75ICD could be involved in the induction of apoptosis, we used DAPT to specifically inhibit this enzyme. Nuclei were stained with Hoechst, and the percentage of pyknotic nuclei among the total number of nuclei was measured as described in Materials and Methods (Figure 8B). TUNEL yielded similar results as those obtained by morphological criteria (data not shown). When stimulated with pro-NGF, PC12 cells showed both apoptosis and p75ICD nuclear translocation. Pretreatment of these cells with DAPT completely blocked p75ICD nuclear translocation, as well as apoptosis induced by ADhbi-pro-NGF (Figure 8). The apoptosis level under these conditions was even lower than with serum deprivation, suggesting that p75NTR processing could also be, at least partially, involved in PC12 apoptosis under conditions of deprivation (Figure 8B). To confirm further that α- and γ-secretase activities were involved in p75NTR processing under ADhbi-pro-NGF binding, we treated 3T3-p75st cells with ADhbi-pro-NGF in both the presence and absence of DAPT (Figure 9), under the same conditions shown to block p75ICD nuclear translocation in PC12 cells (Figure 8). As seen in Figure 9, the use of a γ-secretase inhibitor completely blocked the yield of p75ICD, proportionally increasing the amount of p75CTF. As previously described in different cell models for p75NTR,25 and also for different membrane-associated proteins such as Aβ precursor peptide and Notch,27 cleavage by γ-secretase preceded α-secretase activity. Here, we report (Figure 9) that DAPT blocked the yield of p75ICD producing an accumulation in p75CTF, which was also sensitive to ADhbi-pro-NGF p75NTR activation; p75CTF levels were also increased following pro-neurotrophin treatment.
Figure 9.
Inhibition of γ-secretase in 3T3-p75st cells results in loss of the 20 kd p75ICD band together with accumulation of the 25kDa p75CTF fragment. 3T3-p75st cells were serum-deprived (0.5% FBS) and pretreated for 30 minutes with DAPT. Subsequently, the cells were treated with 25 ng/ml ADhbi-pro-NGF or with 100 ng/ml NGF for 12 hours in Dulbecco’s modified Eagle’s medium containing 0.5% serum, or they were maintained in media containing 10% serum as controls. 30 μg of total protein was used per lane. Western blots (30 μg of total protein per lane) using 9992 anti-p75ICD antibody showed characteristic 20-kd p75ICD and 25-kd p75CTF bands. The experiment was repeated three times with similar results using 9992 (two experiments) and Promega (one experiment) anti-p75ICD antibodies.
Taken together these results suggest that pro-NGF from AD brains, but not from controls, can induce p75NTR processing by secretases, which are involved in Aβ precursor peptide and Notch processing. Structural or modification differences between Chbi- and ADhbi-pro-NGF and molecular mechanisms involved in p75ICD apoptosis induction have yet to be elucidated.
Discussion
Our results indicate that p75ICD levels significantly increase in AD in human entorhinal cortex in parallel with pro-NGF levels. We have previously shown4 that pro-NGF from AD-affected cortex can induce apoptotic death mediated by its interaction with p75NTR. In the present work, our results suggest that pro-NGF treatment induces α- and γ-secretase processing of p75NTR, which yields a p75ICD that is subsequently translocated to the nucleus. This process is accompanied by a delayed induction of apoptosis. More importantly, in this work we also demonstrate that pro-NGF from human control cortex is unable to induce p75NTR processing with p75ICD nuclear translocation or apoptosis.
ADhbi-Pro-NGF Stimulation of p75NTR Causes p75ICD Nuclear Translocation and Apoptotic Cell Death
p75NTR is known to be involved in signaling apoptosis in a number of cell models of NGF binding.7–9 However, marked variations have been reported, depending on the plethora of intracellular adaptors and on the coreceptor pattern in a given cellular context.13 In 2001, new insights were provided when it was shown that pro-NGF binds more strongly than NGF to p75NTR, inducing a higher degree of apoptosis.2 Furthermore, the death induced by ADhbi-pro-NGF through p75NTR is independent of the presence of TrkA, because the pro-neurotrophin is not a TrkA ligand. It has been reported that p75NTR is subjected to shedding in a sequential process of regulated intramembrane proteolysis. This process occurs by a mechanism involving α- and γ-secretase processing on both sides near its trans-membrane domain, in a protein kinase C-dependent manner.25The product of α-secretase is p75CTF, comprising the transmembrane domain with an apparent molecular weight of 25 kd.25,26 The product of γ-secretase processing is p75ICD with an apparent molecular weight of 20 kd.25,26 It has been described that α-secretase activity is essential for the activity of γ-secretase.25 p75ICD is difficult to be detected, because it is described as being very unstable.19 The proportion of p75ICD:p75CTF observed can present variations between models or may be the consequence of different γ/α-secretases activity and other processing mechanisms. The data that we present here show that the 20-kd band is increased in AD-affected entorhinal cortex. Only a faint 25-kd band can be observed, with relatively constant levels. NRH2, a closely related homologue of p75NTR, lacks the extracellular domain and does not bind directly to NGF.28 Several studies support the idea that NRH2 is not detected by the anti-p75ICD used in the present study.21,28 We have used the anti-NRH2 antibody to confirm that the p75ICD present in human cortex brain samples, and increased in AD, is not NRH2. Only positive controls of PC12 lysates, and not of human brain samples, showed a band of 24 kd corresponding to NRH2.
It has been shown recently that in a Schwannoma cell line21 the yield of p75ICD and its nuclear localization are dependent on neurotrophin binding to the receptor. Other authors have shown no p75NTR processing20 or only a weak p75ICD yield under NGF stimulation.25 In the present work, we have shown that in deprived 3T3-p75st cells both p75ICD and p75CTF are only barely detected. Treatment with NGF or with ADhbi-pro-NGF showed that a clear induction of p75ICD with a parallel p75ICD nuclear translocation was stronger with ADhbi-pro-NGF.
Immunocytochemistry with an antibody raised against the extracellular domain of p75NTR (REX) does not show nuclear translocation under confocal microscopy, indicating that p75ICD follows a different trafficking pathway and localization than internalization of the whole p75NTR. As described by Saxena et al,29 NGF activation of p75NTR causes an internalization that is activated by TrkA phosphorylation. This occurs through sequential internalization of both receptors in clathrin-coated vesicles and early endosome vesicles, recycling of p75NTR, and lysosomal degradation of TrkA. 3T3-p75st does not show internalization of the whole receptor in NGF- or in pro-NGF-stimulated cells. This can be explained by the lack of TrkA expression in these cells. Comparing localization of p75NTR using the REX antibody in pro-NGF-stimulated 3T3-p75st and PC12 cells, a degree of cytoplasmic internalization in PC12 not visible in 3T3-p75st can be observed, where immunolabeling is restricted to the plasmatic membrane. The fact that perinuclear or nuclear localization in 3T3-p75st and PC12, respectively, stimulated by pro-NGF, is only detected with anti-p75ICD and not with REX reinforces the idea that nuclear or perinuclear translocation only occurs with p75ICD from p75NTR processing by α- and γ-secretases. Inhibitors of these secretases completely abolished the process.
Until now, no information regarding p75NTR processing or nuclear translocation of p75ICD in apoptosis was available. Recently, a first physiological role was noticed for p75ICD yield under myelin-associated glycoprotein activation in cerebellar neurons.26 In this model, α- and γ-secretase cleavage of p75NTR was necessary for activation of Rho and inhibition of neurite outgrowth.
It is widely reported that p75NTR can interact with different sets of receptors, depending on their respective proportions and on the ligand availability. Sortilin receptor has been described as being essential in p75NTR-induced apoptosis activated by pro-NGF.11 The binding of the pro-domain to Sortilin and of the mature NGF domain to p75NTR starts a poorly understood cascade of events leading to apoptosis. Future studies will be needed to ascertain if shedding of p75NTR with the yield and nuclear translocation of p75ICD is part of the process. Initial interaction of receptors and ligands could recruit a set of intracellular proteins that might orient the physiological role of p75ICD in one direction or another.
Pro-NGF from AD-Affected Cortex Is Not Only Present at Higher Levels but Is Also More Active in Inducing Apoptosis through p75NTR Processing
As previously reported, pro-NGF is increased in human cortex brain in a manner that is dependent on the progress of the disease, reaching maximum levels in advanced stages.4 We have also shown that pro-NGF isolated from AD cerebral cortex (ADhbi-pro-NGF) can induce apoptosis in neuronal cell cultures through its interaction with the p75NTR receptor in a mechanism that is dependent on γ-secretase shedding of the receptor. As control brains also present some basal levels of pro-NGF,4 it was interesting to explore whether there were any differences in the process of apoptosis induction by pro-NGF between AD-affected brains and controls. We first wanted to assess whether pro-NGF isolated from the two kinds of sources gave rise to similar physiological effects, or whether the only difference was due to the increased amount of the pro-neurotrophin in AD. Surprisingly, pro-NGF isolated from four different control samples was not able to induce apoptosis in any of the cell models used. Previous studies performed with the bacterially expressed wild-type pro-NGF showed the functionality of the protein by partially hydrolyzing the pro-domain with trypsin, demonstrating that the NGF resulting from the subsequent hydrolysis could induce the survival of dorsal root ganglia neurons at a similar rate as seen with the same concentration of NGF.30 NGF is the only neurotrophin reported as inducing neuronal differentiation in PC12 cells through its interaction with TrkA. This interaction also activates strong survival signals that are able to block apoptosis acting on a number of molecular targets. This would make it difficult to detect apoptosis caused by stimulation of p75NTR under conditions of phosphatidylinositol 3-kinase/protein kinase A activation. The study of interactions between these two pathways, pro-NGF/p75NTR and phosphatidylinositol 3-kinase/protein kinase A, is the aim of future work.
As we previously reported,4 ADhbi-pro-NGF could not sustain the survival and/or the differentiation of PC12 cells. However, when ADhbi-pro-NGF was partially digested by trypsin, PC12 survival and differentiation were observed. Moreover, partially digested ADhbi-pro-NGF protected PC12 cells from death caused by deprivation, to the same extent as NGF, thus indicating that NGF is obtained as a product of trypsin digestion. Based on these findings, we wanted to assess whether Chbi-pro-NGF was either inducing apoptosis, as does ADhbi-pro-NGF, or survival and differentiation, as does NGF. The assay on PC12 cells shows that Chbi-pro-NGF was degraded to NGF when it was added to the culture, inducing neuritogenesis. As we described previously, the 53-kd pro-NGF isoform is at least partially deglycosylated by N-glycanase treatment.4 In that work, we did not exclude the possibility that the other isoforms as well as the 53-kd isoform were modified by different glycosylations or by other post-translational modifications such as glycation. Moreover, we cannot exclude the possibility that differences in glycosylation or any other kind of modifications between control and AD brain samples may not be distinguished by Western blot and that these modifications might account for the differences in stability or in protease degradation. Glycosylation and glycation are common modifications of proteins in AD. Among other proteins, glycosylation of acetylcholinesterase has been used as a marker for the disease.31 Further work needs to be done to test the hypothesis that post-translational modifications occurring in AD could be responsible for protecting ADhbi-pro-NGF from its processing, thus switching its function from insuring survival and differentiation to causing neuronal.
Taken together, the present results indicate that a cascade of events relevant to the etiopathogenesis of AD could begin with an increase of pro-NGF. The activation of p75NTR by the pro-neurotrophin would lead to the processing of the receptor by α- and γ-secretases. We found that this processing can be increased in AD to yield p75ICD, which tends to translocate to the nucleus. The relationship between this process and the induction of neuronal apoptosis is still not fully understood.
Acknowledgments
We acknowledge the generous donations of REX antibody by L.F. Reichardt, anti-p75NTR 9992 by M.V. Chao, and anti-NRH2 by P.A. Barker. We thank J. Ros and his team for the help in proteomics and J.C. Arevalo, J. Esquerda, and J. Frade for helpful suggestions.
Footnotes
Address reprint requests to Carme Espinet, Ph.D., Laboratori de Neuropatología Molecular, Departament de Ciències Mèdiques Bàsiques, Universitat de Lleida, C/Montserrat Roig 2, 25008 Lleida, Spain. E-mail: carme.espinet@cmb.udl.es.
Supported by grants from Foudo Investigación Sanitarie (FIS PI020128 to C.E.; FIS 06 to M.P.; and FIS PI051570 to I.F.).; and the Fundació “La Caixa” (Programa de Recerca Biomèdica) and the Fundació Roviralta (to C.E.). P.P. is the recipient of predoctoral fellowships from the Universitat de Lleida and Ministerio de Educación y Ciencie; A.K. is the recipient of a predoctoral fellowship from Department Universitats, Recerca i Societat de la Informació.
P.P. and K.A. contributed equally to this work.
References
- Chao MV. The p75 neurotrophin receptor. J Neurobiol. 1994;25:1373–1385. doi: 10.1002/neu.480251106. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Pedraza CE, Podlesniy P, Vidal N, Arévalo JC, Lee R, Hempstead B, Ferrer I, Iglesias M, Espinet C. Pro-NGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. Am J Pathol. 2005;166:533–543. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased pro-NGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63:641–649. doi: 10.1093/jnen/63.6.641. [DOI] [PubMed] [Google Scholar]
- Duychaerts CH, Dickson DW. Neuropathology of Alzheimer’s disease: Neurodegeneration: the molecular pathology of dementia and movement disorders. Basel: ISN Neuropathology Press,; 2003:pp 47–65. [Google Scholar]
- Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann T, Casademunt E, Hollerbach E, Hofmann J, Dechant G, Frotscher M, Barde YA. Complete deletion of the neurotrophin receptor p75NTR leads to long-lasting increases in the number of basal forebrain cholinergic neurons. J Neurosci. 2002;22:2409–2418. doi: 10.1523/JNEUROSCI.22-07-02409.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Genetic evidence for cell death mediated by nerve growth factor and the neurotrophin receptor p75 in the developing mouse retina and spinal cord. Development. 1999;126:683–690. doi: 10.1242/dev.126.4.683. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen M, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for pro-NGF-induced neuronal cell death. Nature. 2004;26:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Sarret P, Krzywkowski P, Segal L, Nielsen MS, Petersen CM, Mazella J, Stroh T, Beaudet A. Distribution of NTS3 receptor/sortilin mRNA and protein in the rat central nervous system. J Comp Neurol. 2003;461:483–505. doi: 10.1002/cne.10708. [DOI] [PubMed] [Google Scholar]
- Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33:9–12. doi: 10.1016/s0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. Pro-NGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Mörl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted pro-NGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci USA. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M, Zhai S, Pilch PF, Doyle SM, Eisenhauer PB, Fine RE, Gilchrest BA. Binding of beta-amyloid to the p75 neurotrophin receptor induces apoptosis. A possible mechanism for Alzheimer’s disease. J Clin Invest. 1997;100:2333–2340. doi: 10.1172/JCI119772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M, Zhai S, Fine RE, Eisenhauer PB, Arble BL, Stewart KB, Gilchrest BA. Amyloid beta binds trimers as well as monomers of the 75-kDa neurotrophin receptor and activates receptor signaling. J Biol Chem. 2002;277:7720–7725. doi: 10.1074/jbc.M110929200. [DOI] [PubMed] [Google Scholar]
- Tsukamoto E, Hashimoto Y, Kanekura K, Niikura T, Aiso S, Nishimoto I. Characterization of the toxic mechanism triggered by Alzheimer’s amyloid-beta peptides via p75 neurotrophin receptor in neuronal hybrid cells. J Neurosci Res. 2003;73:627–636. doi: 10.1002/jnr.10703. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Scecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci. 2003;23:5425–5436. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Tan S, Landman N, Petrova K, Murray S, Lewis R, Kim PK, Kim DS, Ryu SH, Chao Kim TW. Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J Biol Chem. 2003;43:42161–42169. doi: 10.1074/jbc.M306028200. [DOI] [PubMed] [Google Scholar]
- Frade J. Nuclear translocation of the p75 neurotrophin receptor cytoplasmic domain in response to neurotrophic binding. J Neurosci. 2005;25:1407–1411. doi: 10.1523/JNEUROSCI.3798-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Sequence of Alzheimer’s disease-related pathology. Peters A, Morrison JH, editors. New York: Kluwer Academic/Plenum Press Publishers,; Cerebral Cortex, vol. 14. Neurodegenerative and age-related changes in structure and function of cerebral cortex. 1999:pp 475–512. [Google Scholar]
- Longo FM, Woo JE, Mobley WC. Rush RA, editor. Chicago: John Wiley and Sons Ltd.,; Purification of nerve growth factorNerve growth factors. 1989:pp 3–30. [Google Scholar]
- Zampieri N, Xu CF, Neubert TA, Chao MV. Cleavage of p75 neurotrophin receptor by α-secretase and γ-secretase requires specific receptor domains. J Biol Chem. 2005;280:14563–14571. doi: 10.1074/jbc.M412957200. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Zampieri N, Spencer T, Hilaire M, Mellado W, Chao MV, Filbin MT. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron. 2005;46:849–855. doi: 10.1016/j.neuron.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- Murray SS, Perez P, Lee R, Hempstead BL, Chao MV. A novel p75 neurotrophin receptor-related protein, NRH2, regulates nerve growth factor binding to the TrkA receptor. J Neurosci. 2004;24:2742–2749. doi: 10.1523/JNEUROSCI.3960-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Howe CL, Cosgaya JM, Steiner P, Hirling H, Chan JR, Weis J, Kruttgen A. Differential endocytic sorting of p75NTR and TrkA in response to NGF: a role for late endosomes in TrkA trafficking. Mol Cell Neurosci. 2005;28:571–587. doi: 10.1016/j.mcn.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Rattenholl A, Lilie H, Grossmann A, Stern A, Schwarz E, Rudolph R. The pro-sequence facilitates folding of human nerve growth factor from Escherichia coli inclusion bodies. Eur J Biochem. 2001;268:3296–3303. doi: 10.1046/j.1432-1327.2001.02232.x. [DOI] [PubMed] [Google Scholar]
- Saez-Valero J, Small DH. Acetylcholinesterase and butyrylcholinesterase glycoforms are biomarkers of Alzheimer’s desease. J Alzheimers Dis. 2001;3:323–328. doi: 10.3233/jad-2001-3307. [DOI] [PubMed] [Google Scholar]