Abstract
Alport syndrome is a glomerular basement membrane (GBM) disease caused by mutations in type IV collagen genes. A unique irregular thickening and thinning of the GBM characterizes the progressive glomerular pathology. The metabolic imbalances responsible for these GBM irregularities are not known. Here we show that macrophage metalloelastase (MMP-12) expression is >40-fold induced in glomeruli from Alport mice and is markedly induced in glomeruli of both humans and dogs with Alport syndrome. Treatment of Alport mice with MMI270 (CGS27023A), a broad spectrum MMP inhibitor that blocks MMP-12 activity, results in largely restored GBM ultrastructure and function. Treatment with BAY-129566, a broad spectrum MMP inhibitor that does not inhibit MMP-12, had no effect. We show that inhibition of CC chemokine receptor 2 (CCR2) receptor signaling with propagermanium blocks induction of MMP-12 mRNA and prevents GBM damage. CCR2 receptor is expressed in glomerular podocytes of Alport mice, suggesting MCP-1 activation of CCR2 on podocytes may underlie induction of MMP-12. These data indicate that the irregular GBM that characterizes Alport syndrome may be mediated, in part, by focal degradation of the GBM due to MMP dysregulation, in particular, MMP-12. Thus, MMP-12/CCR2 inhibitors may provide a novel and effective therapeutic strategy for Alport glomerular disease.
Alport syndrome has become a leading model for genetic disorders affecting basement membranes. The gene frequency is about 1 in 5000 people, making it among the more prevalent of known genetic disorders.1,2 It has been determined that X-linked Alport syndrome is caused by any of a series of mutations in the COL4A5gene.3 Hundreds of different mutations in the gene have been identified in families carrying the disease thus far.4,5 The autosomal form of Alport syndrome, which displays the same range of phenotypes as the X-linked form of the disease, is due to mutations in either basement membrane collagen genes COL4A3 or COL4A4.6,7 Alport syndrome is characterized by a juvenile onset of proteinuria. The protein in the urinary space of the glomerulus precedes changes in glomerular cell types, including effaced podocyte foot processes and expansion of the mesangium. These changes culminate in accumulation of extracellular matrix associated with progressive glomerulonephritis. The glomeruli eventually become fibrotic, resulting in a decreased capacity of the kidney to filter the blood. This ultimately results in a fatal uremia. Current therapy is limited to dialysis and transplantation, with a higher risk than normal patients of anti-glomerular basement membrane disease in the transplanted organ due to immune reaction against the type IV collagen chains.
A hallmark and unique characteristic of Alport glomerular disease is an irregular thickening, thinning, and splitting of the glomerular basement membrane (GBM).8 The progressive GBM damage is associated with podocyte foot process effacement. The mechanism underlying this phenotype is unknown; however, it has been suggested that thickened regions might represent areas of matrix deposition.9–11 Alternatively, it has been shown that type IV collagen matrix from Alport kidneys is more susceptible to endoproteolytic cleavage than that from normal kidneys.12 This is presumably due to a significant reduction of interchain disulfide cross-links resulting from differences in collagen chain composition.13
Homeostatic extracellular matrix turnover is a delicately balanced system of coupled biosynthetic and degradative processes. The matrix metalloproteinase (MMP) family consists of over 25 members that collectively can degrade all components of the extracellular matrix. MMP activity is associated with several normal processes of tissue remodeling. Dysregulation of the MMPs may contribute to disease processes. The control and regulation of the extracellular matrix degradation has been shown to be complex, and knowledge of the system in Alport syndrome is rudimentary. Preliminary evidence implicates a role for MMPs in renal pathogenesis associated with Alport syndrome.14–16
MMP-12 (metalloelastase) is a potent protease with broad matrix substrate specificity that has long been associated with macrophages and lung disease.17 To date, expression of MMP-12 has only been demonstrated in macrophages,18,19 hypertrophic osteoclasts,20 vascular smooth muscle cells,21 and some cancer cells.22,23 Here we show MMP-12 is markedly induced in the glomeruli of Alport mice. The degree of induction in glomeruli from Alport mice correlates well with the progression of glomerular disease. We provide evidence to suggest that the cellular mechanism of MMP-12 induction might be linked to monocyte chemoattractive protein-1 (MCP-1)-mediated activation of the CCR2 receptor on glomerular podocytes. Inhibition of MMP-12 with either the small molecule inhibitor MMI270, or the CCR2 receptor antagonist propagermanium, attenuates GBM thickening and preserves the integrity of the glomerular filter. These data suggest that irregular thickening of the GBM in Alport syndrome may be caused, in part, by proteolytic degradation of the GBM due to elevated expression of MMP-12 in glomerular podocytes. The cellular mechanism of MMP-12 induction (MCP-1 activation of CCR2 on glomerular podocytes) is quite unexpected and only previously described in macrophages.
Materials and Methods
Mice and Administration of MMP Inhibitors
The Alport mouse model is in the 129 Sv background and has been described previously.9 Wild-type mice are normal for both collagen α3(IV) alleles and are a product of double heterozygote crosses for the Alport mutation. The use of animals in this study was performed in accordance with an approved Institutional Animal Care and Use Committees protocol. Extreme care was taken to minimize pain and discomfort. MMP inhibitors were administered between 4 and 7 weeks of age. All drugs were freshly prepared before administration. BAY-129566 was emulsified in suspension with 0.5% carboxymethylcellulose, and 4 mg was given once a day by oral gavage. MMI270 was solubilized in 0.9% saline and administered by intraperitoneal injection (10 mg/kg body weight) twice a day.
Type IV Collagen Degradation by MMP-12 in the Presence and Absence of MMP Inhibitors
Mouse Engelbreth-Holm-Swarm tumor type IV collagen was obtained from BD Biosciences Discovery Labware (Two Oak Park, Bedford, MA). Protein substrates were incubated with recombinant mouse MMP-12 (a kind gift from Dr. Y. Kaneko, Nigata, Japan) in 40 μl of assay buffer (0.1 mol/L Tris-HCl, pH 8.0, 0.1 mol/L NaCl, 10 mmol/L CaCl2, 1.0 mmol/L ZnCl2, 0.05% Brij 35, and 0.02% NaN3) at 37°C for 24 hours. The inhibitors used were MMI270 and BAY-129566 at 50 nmol/L. Reactions were stopped with 4× loading buffer containing dithiothreitol and heated in boiling water for 5 minutes. Sodium dodecyl sulfate-PAGE using 5% gels was followed by staining with Coomassie R-250 and destaining.
Glomerular Isolation
Isolation of mouse glomeruli was performed as described previously.24 The procedure involves cardiac perfusion with deactivated 4.5 μmol/L Dynabeads (Dynal Biotech, Oslo, Sweden) followed by collagenase digestion and glomerular isolation using a magnet. We found these preparations to be consistently >97% pure, allowing reliable assessment of glomerular-specific gene expression in mice.
Real-Time PCR Analysis
Total RNA samples were treated with RNase-free DNase I (Gibco-BRL, Gaithersburg, MD) at 37°C for 1 hour to remove any contaminating genomic DNA before reverse transcription (RT). Total RNA was reverse-transcribed by using Superscript II (Gibco-BRL) with oligo(dT) primers. To ensure that the quantitation of MMP transcripts in serial samples was not affected by differences in the amount of RNA added, integrity of RNA, or sample to sample differences in levels of RT-polymerase chain reaction (PCR) inhibition, an internal control reaction targeting the GAPDH gene was run in multiplex with each reaction and used to normalize results for MMP transcripts. Primers and TaqMan probes for murine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Applied Biosystems (catalogue no. 4308313, Foster City, CA) and used as per the manufacturer’s instructions. The data were analyzed using comparative threshold cycle (CT) method. The mRNA quantity for the control is expressed as 1× sample, and all other quantities from Alport samples are expressed as -fold differences relative to the controls. No measurable fluorescence signal was detected in repeated RT-PCR runs in which the reverse transcriptase was omitted from the reaction mixture. Primers were tested by standard endpoint RT-PCR, and the single band obtained was sequence-verified. Real-time RT-PCR was performed on a TaqMan ABI 7000 Sequence Detection System (Applied Biosystems).
PCR was performed with TaqMan Universal PCR Master Mix (Applied Biosystems), which contained AmpliTaq Gold DNA polymerase, AmpErase urasil-N-glycosylate, dNTPs with dUTP, and optimized buffer components. AmpErase urasil-N-glycosylate treatment prevented the possible reamplification of carryover PCR products. Each target molecule was co-amplified with primers and TaqMan probe for GAPDH in the same PCR tube. The total volume of the PCR reaction was 50 μl. The final concentration of each oligonucleotide in the PCR reaction was as follows: GAPDH primers, 100 nmol/L; primers for target molecules, 900 nmol/L; TaqMan probe for GAPDH, 200 nmol/L; and TaqMan probe for the target molecules, 250 nmol/L.
Sequences and Fluorescent Labeling of PCR Primers and TaqMan Probes
The primers and probes are as follows. MMP-2: Sense, 5′-GTT TAT TTG GCG GAC AGT GAC A-3′; Antisense, 5′-AGA ATG TGG CCA CCA GCA A-3′; Probe, 5′-6FAM-CCA CGT GAC AAG CC-MGBNFQ-3′; MMP-9: Sense, 5′-CCA AGG GTA CAG CCT GTT CCT-3′; Antisense, 5′-GCA CGC TGG AAT GAT CTA AGC-3′; Probe, 5′-6FAM-ACT CGT GCG CTG CC-MGBNFQ-3′; MMP-12: Sense, 5′-GCC ACA CTA TCC CAG GAG CAT ATA-3′; Antisense, 5′-AGC TGC ATC AAC CTT CTT CAC A-3′; Probe, 5′-6FAM-ATG CAG AGA AGC CC-MGBNFQ-3′; MMP-14: Sense, 5′-GAG GAG AGA TGT TTG TCT TCA AGG A-3′; Antisense, 5′-GGG TAT CCA TCC ATC ACT TGG TTA-3′; Probe, 5′-6FAM-TCC TCA CCC GCC AGA G-MGBNFQ-3′.
GAPDH
TaqMan Rodent GAPDH Control Reagents (catalogue no. 4308313) containing the primers and VIC-probe were purchased from Applied Biosystems.
PCR Conditions
Thermal cycling was initiated with incubation at 50°C for 2 minutes and 95°C for 10 minutes for optimal EmpErase UNG activity and activation of AmpliTaq Gold DNA polymerase, respectively. After this initial step, 40 cycles were performed. Each PCR cycle consisted of heating at 95°C for 15 seconds for melting and 60°C for 60 seconds for annealing and extension. All controls consisting of ddH2O were negative for target and housekeeping genes.
Conventional PCR
Results of PCR are as follows: CCR2: annealed at 58°C for 35cycles, 199 bp (Forward: 5′-CAC GAA GTA TCC AAG AGC TT-3′; Reverse: 5′-CAT GCT CTT CAG CTT TTT AC-3′); MCP-1: annealed at 60°C for 30 cycles, 519 bp (Forward: 5′-AGA GAG CCA GAC GGA GGA AG-3′; Reverse: 5′-GTC ACA CTG GTC ACT CCT AC-3′).
Data Presentation and Statistical Analysis
Data are expressed as mean ± SD. Differences between means were tested for significance using Student’s t-test. Differences were considered significant at the level of P < 0.05.
Immunohistochemistry
Cryosections (4 μmol/L) of kidneys from 7-week-old wild-type and Alport mice were air-dried, fixed by immersion in ice-cold acetone, and subjected to immunohistochemical staining analysis. Antibodies used were specific for MMP-12 (goat polyclonal antibody against mouse MMP-12; Santa Cruz Biotechnology, Santa Cruz, CA, used at 1:100 dilution), type IV collagen α1/2 chains (rabbit polyclonal against mouse type IV collagen; Biodesign, Inc., Saco, ME, used at 1:200 dilution), fibronectin (rabbit polyclonal against human plasma fibronectin; Sigma-Aldrich, St. Louis, MO, used at 1:200). Anti-CD11b antibodies were directly conjugated to Alexa 568 (Molecular Probes, Eugene, OR) and purchased from Cedarlane Laboratories (Hornby, ON, Canada). For dual immunofluorescence immunostaining, this antibody was added to the mixture containing the secondary antibody. All antibodies were diluted into 7% nonfat dry milk in phosphate-buffered saline (PBS) to reduce nonspecific binding. Primary antibodies were allowed to react for 2 hours at room temperature in a humidified chamber. After three 5-minute washes in PBS, slides were incubated with fluorescein isothiocyanate-conjugated secondary antibodies for 1 hour at room temperature (goat anti-rabbit; Vector Laboratories, Burlingame, MA, used at 1:200). The sections were covered with coverslips, sealed, and imaged. Images were collected using a Cytovision Ultra Image analysis system interfaced with an Olympus BH-2 fluorescence microscope.
Northern Blot Analysis
Northern blots analysis was performed as described previously.9 Ten micrograms of total glomerular RNA was fractionated on 1% agarose formaldehyde gels and transferred to nylon membranes. Probes were either a gel-purified PCR fragment of the MMP-12 transcript using a specific primer set (Sense: 5′-AAG CAA CTG GGC AAC TGG ACA ACT C-3′ and antisense: 5′-TGG TGA CAG AAA GTT GAT GGT GGA C-3′, annealing at 60°C for 30 cycles) or the DECA template for mouse β-actin (Ambion, Inc., Austin TX). Probes were labeled with 32P-dCTP using either random primers or the DECA method provided by the manufacturer. Hybridizations were performed overnight at 50°C using ULTRAhyb hybridization buffer (Ambion), and the membranes were washed according to the manufacturer’s instructions. Membranes were exposed to X-ray film overnight.
In Situ Hybridization
For riboprobe preparation, a 631-bp fragment of the mouse MMP-12 cDNA and a 199-bp fragment of the mouse CCR2 cDNA were amplified from reverse-transcribed RNA using the primers listed above for Northern blot analysis. The resulting fragments were cloned into the pCRII TOPO cloning vector (Invitrogen) and sequence-verified. Fifteen micrograms of the plasmids was linearized using HindIII to provide a 5′ overhang. DNA was isolated using phenol/chloroform extractions. One μg of linear DNA was transcribed as recommended in the Boehringer Mannheim DIG Labeling Kit using T7 polymerase. The yield of labeled probe was estimated by spotting the probe and labeled control onto nylon membrane and developing as recommended in the nonradioactive in situhybridization application manual (Roche Applied Science, Mannheim, Germany). For hybridization, 6-μm paraffin sections were digested in 3 μg/ml proteinase K in 0.1 mol/L Tris, pH 7.5, for 10 minutes at 37°C. They were prehybridized for 1 hour at 45°C in 50% deionized formamide, 2× standard saline citrate, 10 Tween 20, and 1 mg of Escherichia coli tRNA. The hybridization solution consisted of 50 ng heat-denatured riboprobe, 50% deionized formamide, 8% dextran sulfate, 10% Tween 20, 2× standard saline citrate, 20% tRNA, and 10 mg/ml boiled salmon sperm. Slides were hybridized at 45°C overnight. The DIG Wash and Block Buffer Set was used to develop the slides in conjunction with the color substrate solution to which we added 25 mmol/L Levamisole.
Electron Microscopy
Transmission electron microscopy was performed as previously described.9
Immunogold
Ultrastructural localization studies of type IV collagen were performed essentially as previously described.25 Kidneys from 7-week-old wild-type and Alport mice were fixed by heart perfusion with 2% paraformaldehyde in PBS and postfixed in this same solution overnight. Ultrathin (70-nm) sections were reacted for 4 hours at room temperature with goat anti-collagen IV antibodies (Southern Biotechnology, Birmingham, AL). After six 10-minute washes in PBS, specimen were reacted for 2 hours at room temperature with 10-nm gold-conjugated anti-rabbit antibodies (Vector Laboratories). Grids were washed as before, air-dried, counterstained with uranyl acetate, and examined using transmission electron microscopy.
Western Blot Analysis
Isolated glomeruli were lysed in radioimmune precipitation assay lysis buffer (consisting of 0.1% sodium dodecyl sulfate, 0.5% deoxycholate, 1% Nonidet P-40, 100 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.4) containing protease inhibitors (P8340; Sigma-Aldrich). An aliquot was assayed for total protein (Pierce Biochemicals, Rockford, IL). From 20 μg of total protein, CCR2 protein was immunoprecipitated using goat anti-human CCR2 antibody (Santa Cruz Biotechnology) as described previously.14,15 The immunoprecipitated protein was electrophoresed on a 12% sodium dodecyl sulfate-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane that was first incubated with the antibody against CCR2 (1:1000) followed by incubation with a horseradish peroxidase-conjugated secondary antibody (Sigma-Aldrich). The protein bands were visualized using the ECL plus Western Blotting Detection System (Amersham Biosciences, Piscataway, NJ).
Treatment of Mice with the CCR2 Antagonist, Propagermanium
Propagermanium (3-oxygemylpropinic acid polymer; Sanwa Kagaku Kenkyusho Co., Nagoya, Japan) was administered orally (10 mg/kg in 1% gelatin) by gavage once daily starting at 4 weeks of age, and kidneys were harvested at 7 weeks of age. Three animals per group, Alport mice and wild-type littermates, were gavaged with drug or vehicle only and analyzed.
Derivation of Podocyte Cell Cultures
129sv wild-type and 4α3 KO mice were back-crossed into transgenic mice containing a γ-interferon-inducible, temperature-sensitive mutant of the SV-40 large T-antigen.26 Glomeruli were isolated as described above. The decapsulated glomeruli were resuspended in 0.1% trypsin, 0.05% collagenase I in PBS and digested for up to 2 hours at 37°C with moderate shaking.27 The glomeruli were monitored visually every 30 minutes, at which time the suspension was pipetted to alleviate clumping. After the glomeruli were disassociated, media containing FCS was added, and the suspension was spun for 10 minutes at 1500 rpm at 4°C. The pellet was resuspended in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 containing 10% fetal calf serum (FCS), penicillin, streptomycin, gentamicin, and glutamine and plated on a 10-cm tissue culture dish at 37°C. The culture was left undisturbed for several days. The trypsinized culture was then passed through a sieve with 25-μm pore size to remove mesangial and endothelial cells.28 The one or more “sieved” cultures were then resuspended in Renal Basal Media with supplements (BioWhittaker, Inc., San Diego, CA) in the presence of 100 U/ml of mouse recombinant γ-interferon (Calbiochem, La Jolla, CA) and placed at 33°C. The one ore more proliferative cultures were passed and gradually weaned to DMEM/Ham’s F-12 containing 10% FCS and 10 U/ml γ-interferon at 33°C. Cells were placed at 37°C in DMEM/Ham’s F-12 containing 5% FCS without γ-interferon and allowed to differentiate for at least 2 weeks. Observations on proliferative nature and morphology were noted. The culture(s) were then validated after 2 weeks of the “nonpermissive” conditions by markers of differentiated podocytes in vivo.29
Differentiated podocytes grown on slides were characterized using immunofluorescence. Cells were fixed with −20°C acetone for 5 minutes (air-dry for 2 hours) or 25°C 2% paraformaldehyde and 4% sucrose for 10 minutes. Cells were washed once with PBS for 5 minutes, permeabilized with 0.3% Triton X-100 for 10 minutes, washed again, and then blocked with 2% FCS, 2% bovine serum albumin, 0.2% fish gelatin for at least 30 minutes. The slides were rinsed once with PBS and incubated with primary antibodies (1:50) of interest in blocking solution at 4°C overnight. The slides were washed three times with PBS at 25°C and incubated with the appropriate Alexa-labeled secondary antibody (1:150) in blocking solution at 25°C for 2 hours. Slides were washed, mounted, and visualized under an Olympus BH2 fluorescence microscope and captured with a Spot-RT digital camera model 2.3.1. Primary antibodies included CD2AP (H-290), WT-1 (C-19), and VWF (H-300), all from Santa Cruz Biotechnology. Nephrin (extracellular domain) was a gift from R. Kalluri.
Derivation of Primary Mesangial Cell Cultures
Glomeruli were isolated and dissociated as above for podocyte cell cultures using wild-type mice (non-immortomouse). After the glomeruli were disassociated, the suspension was transferred to a 15-ml conical tube containing 10 ml of media plus FCS and spun for 10 minutes at 4°C at 1500 rpm. The pellet was resuspended in 12.5 ml of DMEM/Ham’s F-12 containing 20% FCS, penicillin, streptomycin, gentamicin, glutamine, insulin, and selenium followed by transfer and plating to a 10-cm tissue culture dish. The culture was left undisturbed for several days. Cells were grown to near confluency, trypsinized, and passed 1:3 in growth media. Within 24 hours, the culture media was replaced with d-valine containing DMEM (eliminating fibroblast contamination). Under these conditions the mesangial cells quickly outgrow all other cells in the mixture. The cells were qualified as mesangial based on derivation/culture conditions, morphology, and immunofluorescence. The cultures were very homogeneous in appearance on passage after d-valine treatment. The cells displayed stellate, spindle-shaped morphology, accompanied by “hills and valleys” on confluency. By immunofluorescence, the cells were positive for desmin (Abcam, Cambridge, UK), smooth muscle actin (Sigma-Aldrich), and vimentin (Chemicon, Temecula, CA), all of which are major requisites to assign mesangial origin to the cells.30
Results
MMP-12 Expression Is Markedly Induced in Glomeruli from Alport Mice and Humans
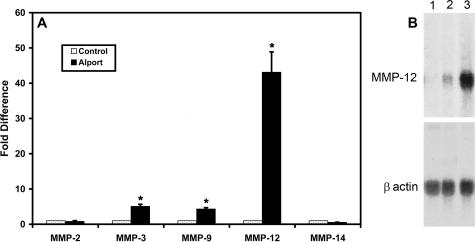
A newly described glomerular isolation technique24 was used to obtain pure glomerular RNA preparations from wild-type mice and Alport mice at 7 weeks of age (7-week-old Alport mice have advanced glomerular disease). Total RNA was prepared and analyzed using real-time RT-PCR for expression of the MMPs shown in Figure 1 using an internally quenched carboxy fluorescein-conjugated primer method. GAPDH was run in multiplex in all reactions as an internal control for RNA loading. Three independent glomerular preparations were analyzed in triplicate. The results in Figure 1A show that mRNAs encoding MMP-2 and MMP-14 are not significantly changed in diseased Alport glomeruli relative to wild-type glomeruli. In contrast, mRNAs encoding MMP-3 and MMP-9 are four- to fivefold higher in Alport glomeruli relative to controls. Remarkably, expression of MMP-12 mRNA is >40-fold higher in Alport mice compared to wild-type mice. MMP-7 was also analyzed; however, no expression was observed in glomeruli (data not shown).
Figure 1.
A: Real-time PCR analysis of MMPs of glomerular RNA from 7-week-old wild-type and Alport mice. Glomerular RNA was analyzed using primers specific for the indicated MMPs as described in Materials and Methods. Data from three independent sets of glomeruli were pooled and analyzed in triplicate. Asterisks denote statistically significant differences in specific MMP expression when comparing wild-type and Alport mice (P > 0.005). B: MMP-12 mRNA is induced as a function of Alport renal disease progression. Glomerular RNA from 7-week-old wild-type, 7-week-old Alport, and 4-week-old Alport mice were analyzed by Northern blot and probed with radiolabeled MMP-12 cDNA. The blot was stripped and reprobed with β-actin cDNA to control for RNA loading.
MMP-12 is a potent metalloproteinase with broad substrate specificity. Thus, we aimed to define whether dysregulation of MMP-12 in the glomeruli of Alport mice might be functionally connected to Alport glomerular disease progression. To determine whether MMP-12 mRNA is inducible as a function of glomerular disease progression, we analyzed glomerular RNA by Northern blot. Glomeruli from three independent preparations were combined, and total RNA was fractionated on MOPS gels, transferred to nylon membranes, and hybridized to a radiolabeled probe specific for MMP-12. The results in Figure 1B show that MMP-12 RNA is inducible, as evidenced by the absence of expression in RNA from wild-type mice and the obvious presence of signal in 4-week-old Alport mice. The signal is markedly intensified by 7 weeks, indicating a progressive induction of MMP-12 RNA during the course of glomerular disease progression.
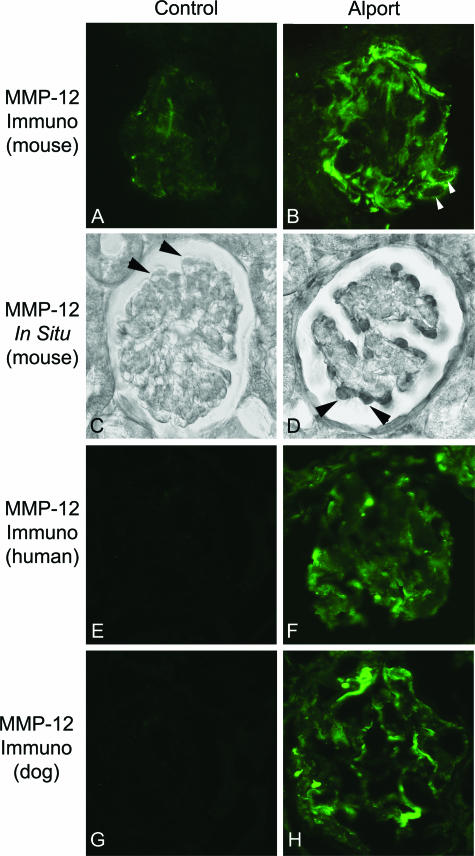
Although induction of MMP-3 and MMP-9 are well documented in glomerular disease,31,32 expression of MMP-12 in normal or diseased glomeruli has not been documented, with the exception of autoimmune glomerulonephritis, where the MMP-12 expression is due to infiltrating macrophages.18 Kidneys from wild-type and 7-week-old Alport mice were immunostained using antibodies specific for MMP-12. The results in Figure 2 (A and B) show that MMP-12 is induced in Alport mice relative to controls. Arrows in panel B denote that the strongest immunostaining for MMP-12 is observed around what appear to be glomerular podocytes. To confirm that induction of MMP-12 was indeed occurring in the glomerular podocytes, we performed in situhybridization analysis. The results in Figure 2D show that strong signal is observed for MMP-12 mRNA in cells lining the outer circumference of the glomerulus (arrows), consistent with the localization of glomerular podocytes. MMP-12 mRNA is not observed in the podocytes of wild-type littermates (Figure 2C, arrows). It is possible that our findings in mice are not true in glomeruli from other species. Figure 2 (F and H) shows that Alport glomeruli from both human (nonsclerotic glomerulus from a human with clinically determined X-linked Alport syndrome) and dog (nonsclerotic glomerulus from a mixed breed dog kindred with genetically confirmed X-linked Alport syndrome)33 express high levels of MMP-12, whereas MMP-12 immunostaining is not observed in glomeruli from normal humans and littermate dogs (Figure 2, E and G).
Figure 2.
MMP 12 is up-regulated in glomerular podocytes. Tissue sections from 7-week-old wild-type and Alport mice were immunostained using antibodies specific for MMP-12 (A and B). Weak and somewhat punctate immunostaining was observed in wild-type mice (A) compared to robust immunostaining in Alport glomeruli (B), which appears to localize primarily to glomerular podocytes (arrowheads). In situhybridization confirms induction of MMP-12 mRNA in glomerular podocytes of Alport mice (D), which appears to be absent in glomeruli from wild-type mice (C). F and H: Positive immunostaining for MMP-12 in glomeruli from both human and dog, respectively. Normal human and dog glomeruli showed no detectable immunostaining (E and G, respectively).
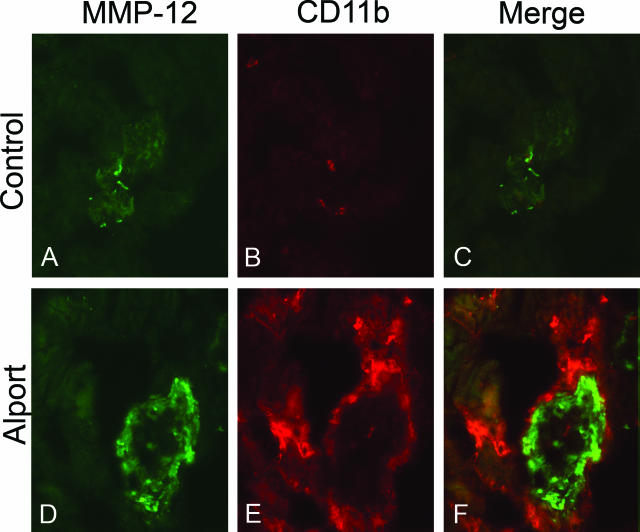
The observed expression of MMP-12 in Alport glomeruli might represent expression by infiltrating macrophages. To address this we performed dual immunofluorescence analysis using antibodies specific for MMP-12 and CD11b (a specific marker for monocytes and macrophages). The results in Figure 3 show that there are no monocytes or macrophages present in Alport glomeruli and that macrophages in the renal interstitium (CD11b positive cells in red) do not express MMP-12.
Figure 3.
Dual immunofluorescence analysis of renal cortex from 7-week-old Alport and wild-type mice. Cryosections from wild-type and Alport mouse kidneys were immunostained with antibodies specific for MMP-12 (A and D) and CD11b (B and E). Dual immunostaining (C and F) illustrates that monocytes (red) are immunonegative for MMP-12 (green) while glomeruli are immunopositive for MMP-12.
Inhibition of MMP-12 activity restores normal GBM architecture and glomerular function in Alport mice. MMP-12 has a broad substrate specificity that includes many of the known basement membrane proteins, including type IV collagen, laminins, entactin, and proteoglycans.17,34 Thus, it is likely that such a significant increase in MMP-12 may influence the functional integrity of the GBM in Alport glomeruli. To test this we used two different small molecule inhibitors for the MMPs. BAY-129566 is a biphenyl nonpeptidic MMP inhibitor with known substrate specificity for MMP-2, -3, and -9 at nanomolar concentrations.35,36 This compound binds the P1′ pocket of MMP enzymes (Tullis et al (37)). MMI270 is a hydroxamate inhibitor that inhibits MMP-2, -3, -9, -14, and -12 at nanomolar concentrations.37,38 This compound binds the S1′ pocket of MMP enzymes,39 affording it broader substrate specificity than BAY-129566. This activity underlies the clinical application of the compound, which is primarily aimed at lung fibrosis where macrophage-derived MMP-12 has been shown to underlie fibrogenesis.
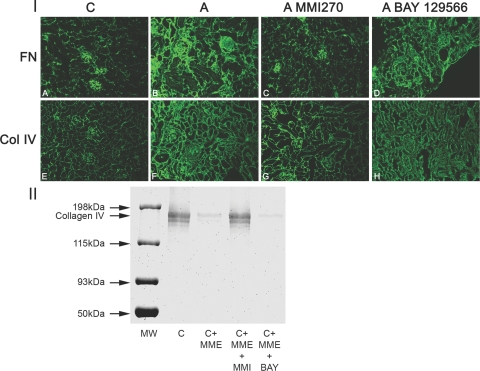
To assess the effect of these two compounds on Alport renal disease progression, Alport mice were administered either MMI270 or BAY-129566 from 4 to 7 weeks of age. The animals were transcardially perfused with PBS, and the kidneys were harvested. Cryosections were analyzed by immunofluorescence microscopy to assess for the degree of glomerular and tubulointerstitial damage using antibodies specific for collagen IV α1 and α2 chains and fibronectin. The results in Figure 4 (panel I) illustrate that the MMI270-treated animals appear to have very little glomerular or interstitial disease compared to the age-matched untreated Alport mice (compare Figure 4, panels C and G with B and F). This observation is in contrast to the BAY-129566-treated animals, which show the same degree of renal pathology as untreated Alport mice (compare Figure 4, panels D and H with B and F). To verify the specificity of the two MMP inhibitors with regard to MMP-12, equal amounts of purified type IV collagen were digested with recombinant MMP-12 in the presence or absence of either MMI270 or BAY-129566. The resulting collagen was fractionated by polyacrylamide gel electrophoresis and stained with Coomassie Blue. The results in Figure 3 (panel II) clearly show that MMI270 is a potent inhibitor of MMP-12, whereas BAY-129566 does not inhibit MMP-12.
Figure 4.
Treatment of Alport mice with a small molecule MMP inhibitor, MMI270, arrests the progression of glomerular and tubulointerstitial pathogenesis associated with the disease. I: Treated mice were injected twice daily from 4 to 7 weeks of age with 10 mg/kg body weight of MMI270, or once daily with 4 mg of BAY-129556 by oral gavage in a 0.5% carboxymethylcellulose carrier. Kidneys were harvested, and frozen sections were subjected to fluorescence immunostaining using antibodies specific for either fibronectin (A–D) or type IV collagen α1 and α2 chains (E–H). ‘C’ represents wild-type mice (A and E), and ‘A’ represents untreated Alport mice (B and F); ‘A MMI270′ represents MMI270-treated Alport mice (C and G); ‘A BAY-129566′: Note the remarkable improvement in both the glomerular and tubulointerstitial compartments of the MMI270-treated Alport mice (C and G) relative to the BAY-129566-treated Alport mice (D and H), which showed no improvement relative to untreated Alport mice (B and F). II: While MMI-270 inhibits collagen IV digestion by MMP-12 (C+MME+MMI), BAY-129566 does not (C+MME+BAY). Lane 1, undigested collagen IV; lane 2, collagen IV plus MMP-12; lane 3, collagen IV plus MMP-12 plus MMI-270; lane 4, collagen IV plus MMP-12 plus BAY-129566.
Electron microscopic analysis of the GBM in MMI270-treated mice revealed near complete restoration of normal glomerular basement membrane architecture in most of the glomeruli examined. Figure 5 shows typical observed improvement of the GBM in a glomerular capillary loop. Normal GBM is shown in panel A. Untreated Alport mice show marked irregular thickening of the GBM (Figure 5B). MMI270-treated mice showed a remarkable restoration of normal glomerular basement membrane architecture (Figure 5C). Restoration of the GBM architecture likely underlies restoration of glomerular function as measured by proteinuria. It has long been suspected that proteolytic degradation of the GBM in Alport syndrome might underlie the progressive irregular GBM damage and podocyte foot process effacement. Type IV collagen from Alport kidneys is more susceptible to proteolytic degradation in vitro than type IV collagen from healthy kidneys,12,40 presumably owing to the reduced number of interchain disulfide cross-links.13 Recombinant MMP-12 will digest native type IV collagen, suggesting the collagen network is a suitable substrate for degradation.34,41
Figure 5.
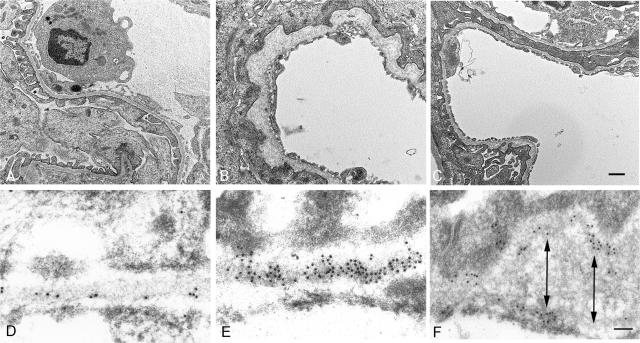
Treatment of Alport mice with MMI270 arrests progressive loss of glomerular structure and function. Treatment with MMI270 from 3 to 7 weeks of age results in markedly improved glomerular basement membrane architecture. Representative glomerular capillary loops are shown from a 7-week-old: A: Wild-type mouse; B: Alport mouse; C: Alport mouse treated from 4 to 7 weeks with MMI270. Scale bar = 1 μm. Immunogold localization of type IV collagen revealed evidence for focal degradation in thickened regions of the GBM. Immunogold localization of type IV collagen was used to examine the integrity of the collagen network in the GBM in Alport mice. D: a sparse subendothelial labeling pattern in the GBM of a wild-type mouse. In Alport mice, areas of the capillary loop that were structurally normal displayed a regular distribution of gold particles along the lamina densa (E). Areas where focal thickening of the GBM was observed showed evidence of focal degradation of the collagen network (F). Arrows denote evidence of a collagen network splitting, where gold deposition is primarily on the epithelial or endothelial aspect of the GBM. There was no significant variation in gold particles per unit length of the GBM in Alport mice, suggesting that collagen is not accumulating in the thickened regions. Scale bar = 50 nm.
If elevated expression of MMP-12 leads to progressive proteolytic destruction of the GBM, the observed GBM irregularities might represent areas of focal degradation. In an attempt to visualize this directly, we used colloidal gold immunocytochemistry using antibodies against type IV collagen α1 and α2 chains. The results in Figure 5D show that immunogold labeling is sparse and localized to the subendothelial aspect of the GBM in wild-type mice, consistent with previous reports.42 In Figure 5, E and F represent two different regions of the GBM in an affected Alport glomerular capillary. Panel E represents a region of GBM in an Alport mouse with normal ultrastructure. Here the immunogold labeling is localized along the lamina densa of the GBM. Panel F illustrates a region of focal thickening in the GBM. In contrast to panel E, here the immunogold shows an irregular localization pattern, with labeling clustering either along the epithelial or endothelial boundaries of the GBM. The arrows in panel E denote a consistent observation. When immunogold clusters were observed on the epithelial boundary, they were relatively absent in the opposing endothelial boundary and vice versa. This might reflect splitting and cleavage of the basement membrane collagen superstructure, which would be expected on endoproteolytic damage.
Cellular Mechanism of MMP-12 Induction in Alport Glomeruli May Involve MCP-1-Mediated Activation of the CCR2 Receptors on Glomerular Podocytes
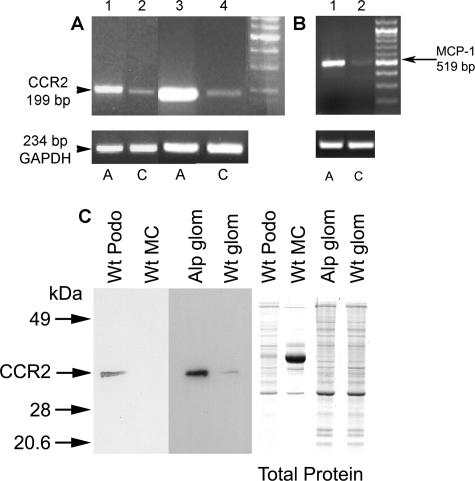
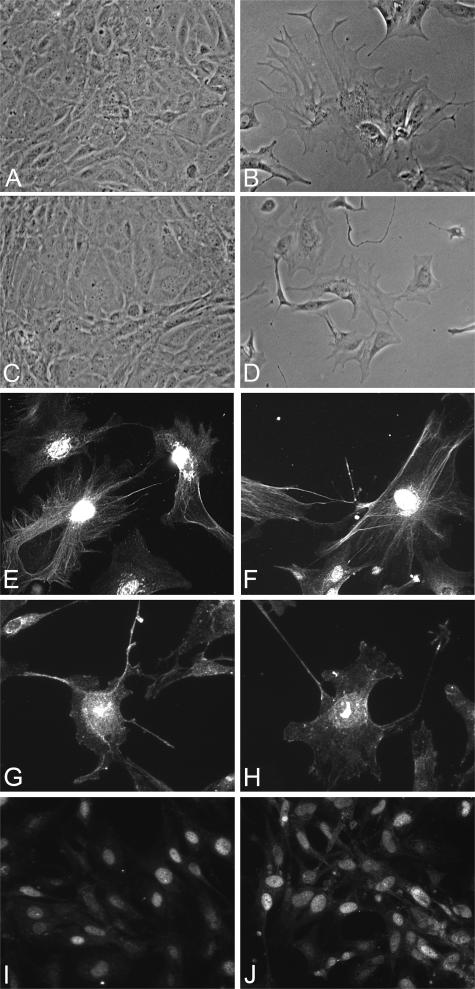
Blocking the CCR2 receptor reduces MMP-12 expression and restores the GBM architecture in Alport mice. The cellular mechanism of MMP-12 induction in macrophages has been linked to granulocyte/monocyte chemoattractive factor, interleukin-1β, and MCP-1.43 We examined glomeruli from wild-type and Alport mice for expression of these regulatory systems. The results in Figure 6A show that CCR2 mRNA is markedly up-regulated in glomeruli from Alport mice relative to wild-type mice (lanes 3 and 4). Cultured glomerular podocytes from Alport mice also show elevated expression of CCR2 mRNA relative to podocytes from wild-type littermates (lanes 1 and 2). Western blot analysis confirmed that CCR2 protein is elevated in Alport glomeruli relative to wild-type glomeruli (Figure 6C). In addition, CCR2 protein was observed in extracts of cultured wild-type podocytes but not detected in extracts from cultured wild-type mesangial cells (Figure 6C). MCP-1 (also called CCL2), the chemokine ligand for CCR2, is also induced in glomeruli from Alport mice relative to glomeruli from wild-type mice (Figure 6B). The conditionally immortalized podocyte and primary mesangial cell cultures were derived in-house as outlined in Materials and Methods. Differentiated podocyte cultures were qualified by their arborized morphology (Figure 7, B and D), expression of the podocyte markers nephrin, CD2AP, and WT-1 by immunofluorescence microscopy (Figure 7, E–J) and lack of von Willebrand factor protein expression (data not shown). We also demonstrated expression of isoforms of vascular endothelial growth factor (VEGF165, VEGF189) in differentiated cultures (data not shown), which is highly specific for podocytes in the glomerulus.44
Figure 6.
Expression of both MCP-1 and CCR2 are induced in glomeruli from Alport mice relative to wild-type mice. A and B: RNA from isolated glomeruli (A, lanes 3 and 4; B, lanes 1 and 2) or cultured glomerular podocytes (A, lanes 1 and 2) was amplified using primers specific for CCR2 (A) or MCP1 (B). GAPDH was amplified as a control. A, Alport; C, wild-type. C: Western blot analysis of protein from cultured wild-type mesangial cells (MC), cultured wild-type podocytes (podo), or isolated glomeruli (glom) from wild-type (Wt) and Alport (Alp) mice probed with antibodies specific for CCR2. One-tenth of the protein was fractionated on a duplicate gel and stained with Coomassie Blue to control for loading (total protein).
Figure 7.
Verification of conditionally immortalized podocyte cell cultures from both wild-type and Alport mice. Cell lines were derived as described in Materials and Methods. A and C: Representative phase-contrast fields of undifferentiated podocyte cell cultures from wild-type and Alport mice, respectively. B and D: Representative fields of differentiated cells from these same mice, respectively. Note the typical arborized phenotype of the differentiated cells. Cultures from wild-type (E, G, and I) and Alport (F, H, and J) were further confirmed by positive immunostaining for both nephrin (E and F), CD2AP (G and H), and WT-1 (I and J) confirming that the cultures are indeed podocytes.
In situhybridization analysis for CCR2 confirms elevated expression of CCR2 mRNA in Alport podocytes compared to wild-type controls (Figure 8). Thus a chemokine/ligand system is present and induced in Alport glomeruli, which may constitute the cellular mechanism of MMP-12 induction in Alport glomerular podocytes.
Figure 8.
CCR2 mRNA is induced in glomerular podocytes. In situhybridization analysis was performed on kidney sections from 7-week-old wild-type (A and B) and Alport (C and D) mice using either sense (A and C) or antisense (B and D) riboprobes specific for the CCR2 transcript. Arrowheads denote representative glomerular podocytes.
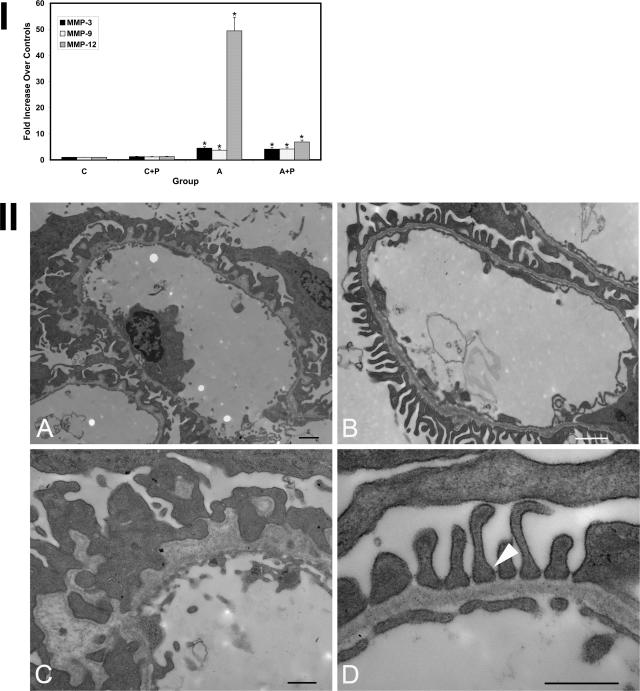
To test whether the MCP-1/CCR2 system is active in Alport glomeruli, we used a specific inhibitor of CCR2, propagermanium. This compound inhibits CCR2 receptor by targeting glycosylphosphatidylinositol-anchored proteins that are closely associated with CCR2.45 The drug was given by oral gavage to three Alport mice and three wild-type mice starting at 4 weeks of age, and the glomeruli were harvested from the kidneys at 7 weeks of age. As controls, both Alport and wild-type mice were gavaged with vehicle only. RNA was isolated from glomeruli and analyzed in triplicate for expression of the MMPs using real-time PCR. The results in Figure 9, panel I, show that MMP-3, MMP-9, and MMP-12 are all induced in glomeruli from Alport mice gavaged with vehicle relative to normal controls, which is both qualitatively and quantitatively consistent with the results in Figure 1. In the propagermanium-treated Alport mice, induction of both MMP-3 and -9 mRNAs was unaffected by the drug, whereas MMP-12 mRNA induction was reduced from 50-fold in glomeruli from vehicle-treated mice to sixfold in glomeruli from propagermanium-treated Alport mice. Renal cortex from these same mice was examined by transmission electron microscopy. Figure 9, panel II, shows that the reduced expression of MMP-12 was sufficient to restore normal GBM architecture. This restoration results in reestablishment of the slit diaphragm (Figure 9II, panel D, arrow) and the reappearance of healthy fenestrated endothelium in the glomerular capillary tuft. These data are consistent with a mechanism where MCP-1 activation of CCR2 on glomerular podocytes underlies MMP-12 induction in Alport glomeruli. Additional experimentation will be required to definitively test this mechanism.
Figure 9.
Treatment of Alport mice with propagermanium knocks down elevated MMP-12 expression and restores normal GBM architecture in Alport glomeruli. I: Mice were administered propagermanium or vehicle alone, as in Materials and Methods, and glomeruli were isolated and analyzed for expression of the indicated MMPs using real-time PCR. Data from three independent sets of glomeruli pooled from three animals were analyzed in triplicate. Asterisks denote statistically significant differences in specific MMP expression when comparing wild-type and Alport mice (P > 0.005). Note that only MMP-12 expression was affected by administration of propagermanium. II: Representative capillary loops from Alport mice treated with vehicle alone (A and C) or with propagermanium (B and D) were analyzed by transmission electron microscopy. Restoration of uniform GBM thickness in the treated mice is observed along with reconstitution of normal podocyte foot processes and restoration of the slit diaphragms (D, arrow). Scale bars = 1 μm (A and B); 50 nm (C and D).
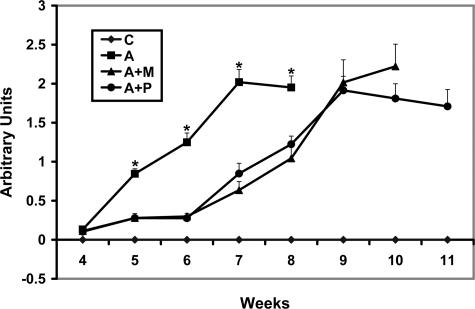
To address the kinetics of the protective effect of MMP-12 blockade on glomerular function, wild-type or Alport mice (three per group) were either treated with MMI270, propagermanium, or vehicle starting at 4 weeks of age. Urine was collected at weekly intervals and analyzed by gel electrophoresis, gel staining, and densitometric quantitation of the albumin bands. The results in Figure 10 show that both drugs provide significant protection from 4 to 6 weeks of age. After 6 weeks of age, however, the protective effect appears to be lost, and proteinuria progresses rapidly. These animals lived to between 11 and 12 weeks of age, compared to between 8 and 8.5 weeks for Alport mice given vehicle alone
Figure 10.
Progression of proteinuria is attenuated in Alport mice treated with either MMI-270 or propagermanium compared to untreated Alport mice. Urine was collected from mice weekly at the indicated time points and frozen. Equivalent volumes were fractionated on acrylamide gels, and the gels were stained with Coomassie Blue. Images were captured using a Viewsonic image analysis system, and the bands were quantified densitometrically using LabWorks software (UVP, Inc., Upland, CA). Data are presented as means with standard deviations for triplicate samples. Animals from both MMI 270- and propagermanium-treated groups survived through approximately 12 weeks of age. Asterisks denote statistically significant differences for proteinuria when comparing Alport mice with either MMI-270 or propagermanium-treated Alport mice (P > 0.005).
Discussion
Alport syndrome is clinically diagnosed based on an irregular thickening and thinning of the renal glomerular basement membrane. No other renal disease presents with similar characteristics, making this observation, when combined with clinically relevant information, a definitive and accepted method for clinical determination of Alport syndrome. The development of animal model systems for Alport syndrome has considerably enhanced our understanding of the molecular processes underlying the associated renal pathogenesis; however, the mechanisms underlying the unique GBM defect has remained obscure. In this report, we have shown that MMP-12 mRNA and protein expression is markedly induced in glomeruli in an autosomal Alport mouse model. Two distinct lines of evidence are provided that support the notion that elevated MMP-12 is linked to GBM destruction. First, comparative studies using two different small molecule inhibitors of the MMPs show that MMI270 prevents or reverses GBM damage, whereas BAY-12–9566 has no effect. Although these two compounds share inhibitory activity for a number of MMPs, MMI270 inhibits MMP-12, whereas BAY-129566 does not (Figure 4, panel II). In addition, we provide a second body of evidence that suggest an unexpected cellular mechanism underlying MMP-12 activation in glomerular podocytes (MCP-1 activation of CCR2 on glomerular podocytes). The influence of these pathways on glomerular pathogenesis is most profound between 4 and 7 weeks of age, a period when disease is rapidly progressing in untreated 129 Sv Alport mice. After 7 weeks of age, however, the therapeutic effect is diminished, and disease progresses more rapidly, suggesting that the influence of other contributing factors assume a dominant role in the progression of renal pathogenesis even when the MMP-12 pathway is blocked (Figure 10). This observation underscores the complexity of the interacting systems involved in renal pathogenesis in Alport syndrome.
We conclude that elevated MMP-12 expression may play a key contributory role in the progression of GBM pathogenesis of Alport syndrome. The irregular “thickening” of the GBM and the associated loss of glomerular filter integrity may reflect proteolytic degradation of the GBM by either MMP-12 alone or MMP-12 in combination with other proteases. In a very recent closely related paper, both genetic models and inhibitor studies were used to show that inhibition of MMP-2, -3, and -9, before the onset of proteinuria, markedly slowed the progression of renal disease in the Alport mouse, whereas inhibition of these same MMPs after the onset of proteinuria actually accelerated disease progression.40 These findings suggest that MMP-2, -3, and -9 contribute to the initiation of GBM pathogenesis in Alport syndrome but then play a protective role, slowing the rate of disease progression. Based on the work presented herein, the role of MMP-12 is distinct from that of MMP-2, -3, and -9, in that beneficial effects are observed even when drug administration begins after the onset of proteinuria. One distinct difference between these two studies is the strain of mouse used. The Zeisberg study used the C57 Bl/6 strain, which displays a very slow disease progression, whereas renal disease progression in the 129 Sv strain is very rapid. Earlier studies show that these strain differences are likely due to variances in quantitative trait loci; however, the specific genes involved have not been elucidated.46 One must consider the possibility that these genetic variations might influence the contributory role of MMPs in renal disease progression in the two strains.
Another recent study from our laboratory shows that MMP-2, -3, -9, and -12 are also overexpressed in the stria vascularis of 129 Sv Alport mice.47 In contrast with the GBM, treatment of mice with MMI 270 exacerbated thickening of the strial capillary basement membranes, suggesting that the influence of MMPs on pathogenic alterations of basement membrane metabolism is likely tissue/organ-specific. This difference might reflect the presence of collagen α(IV)5 networks in the strial capillary basement membrane of Alport mice, which are notably absent from the GBM in these same mice.48,49
The role of MMP-12 in Alport glomerular pathogenesis is unexpected. Previous studies suggest expression of MMP-12 is highly restricted, having only been described in macrophages,18,19 hypertrophic osteoblasts,20 and vascular smooth muscle cells.21 Expression of MMP-12 by a differentiated epithelial cell has not been previously demonstrated. Elevated glomerular expression of MMP-12 has been shown in autoimmune glomerulonephritis; however, the source of MMP-12 was shown to be infiltrating macrophages.18 Nonetheless, this study further supports the notion that overexpression of MMP-12 in glomeruli can lead to pathology.
We show there are no macrophages in Alport glomeruli and that the interstitial monocytes present in Alport kidneys are immunonegative for MMP-12 (Figure 3). Immunofluorescence data show the source of MMP-12 expression in Alport glomeruli is glomerular podocytes (Figure 2D); however, mesangial cells might also contribute to a lesser degree. MMP-12 is highly regulated by cytokines. Known pathways for MMP-12 activation include granulocyte/monocyte chemoattractive factor, MCP-1, and platelet-derived growth factor-BB.21,50,51 All three of these cytokines are known to be induced in various glomerular diseases as well as in mesangial cell culture systems.52,53 Here we show that the cellular mechanism of MMP-12 induction in glomerular podocytes might be linked to MCP-1 activation of the CCR2 receptor. This is the first report of CCR2 expression on glomerular podocytes and reveals that this cytokine system, which has previously only been characterized in macrophages, may play an important role in mesangial/epithelial cell interactions in a progressive glomerular disease.
Propagermanium inhibits CCR2 activation via targeting glycosylphosphatidylinositol-anchored proteins closely associated with CCR2.45 Because CCR2 activation by MCP-1 is associated with acute and chronic inflammatory response mechanisms, animal studies to date using propagermanium have focused on its anti-inflammatory activity and include assessments of atherosclerosis, renal fibrosis, and liver disease.45,54,55 The therapeutic potential has been focused on the pivotal role of CCR2 activation by MCP-1 in monocyte/lymphocyte recruitment to sites of local inflammation.56–58 This is the first report demonstrating a role for this system in a pathobiological system not involving monocyte/lymphocyte recruitment.
The observed 40-fold induction of MMP-12 in Alport glomeruli and the arrested glomerular pathogenesis in animals treated with MMP-12 inhibitor MMI270 support a role for proteolytic degradation underlying the irregular rarification of the GBM. It has been previously shown that the RBM from patients with Alport syndrome appear more susceptible to endoproteolysis.12 Biochemical analysis shows that type IV collagen networks composed of the α3(IV), α4(IV), and α5(IV) chains are more heavily cross-linked than those composed solely of collagen α1(IV) and α2(IV) chains.13 Very recently direct biochemical evidence for this association was provided, where it was shown that renal basement membranes from both Alport patients and Alport mice, compared to normal patients or control littermate mice, were more susceptible to proteolytic degradation by either recombinant MMP-2, -3, or -9, based on in vitro hydroxyproline release measurements.40 This could account for the enhanced resistance of normal GBM to proteolysis. Thus, elevated MMP-12 combined with enhanced susceptibility to endoproteolysis (due to collagen α1(IV) and α2(IV) composition) may both contribute to the observed ultrastructural dysmorphology of the GBM in Alport syndrome.
Alport syndrome is currently treated by dialysis and transplant. Study of the molecular processes underlying Alport renal disease has been significantly enhanced by the development of animal model systems resulting in the evolution of potential treatment modalities that are at varying stages of development. Ramipril, an angiotensin converting enzyme inhibitor currently in use, doubles the lifespan of Alport mice59 and is currently under consideration for human clinical trials. Neutralization of integrin α1β1 also doubles lifespan in the mouse model,10 and a therapeutic approach involving neutralizing antibodies is entering phase II clinical trials. Gene therapy is also being developed for testing in animal models.60 Based on the results provided in our study, small molecule inhibitors of MMP-12 and/or CCR2 antagonists, such as propagermanium, should be considered alone or in combination with other approaches, as new therapeutics for Alport syndrome.
Acknowledgments
We thank John (Skip) Kennedy for help in figure preparation; Caroline Miller for technical assistance with colloidal gold immunolabeling experiments; and Dr. George Lees (Texas A&M University for necropsy specimen from Alport dogs and normal littermates. We also thank the Bayer Corporation for the gift of BAY-129566.
Footnotes
Address reprint requests to Dominic Cosgrove, Ph.D., Boys Town National Research Hospital, 555 No. 30th St., Omaha, NE 68131. E-mail: cosgrove@boystown.org.
Supported by grants from the National Institutes of Health (R01DK55000, R01DC04844, and P30DC04662 to D.C.; and R01DC006442 to M.A.G.) and by the tobacco settlement fund from the State of Nebraska.
References
- Atkin CL, Gregory MC, Border WA. Schrier RW, Gottschalk CW, editors. Boston: Little, Brown,; 1988:617–641. [Google Scholar]
- Pescucci C, Longo I, Bruttini M, Mari F, Renieri A. Type-IV collagen related diseases. J Nephrol. 2003;16:314–316. [PubMed] [Google Scholar]
- Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;348:1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- Kashtan CE. Alport syndrome. An inherited disorder of renal, ocular, and cochlear basement membranes. Medicine (Baltimore) 1999;78:338–360. doi: 10.1097/00005792-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Kashtan CE. Familial hematuria due to type IV collagen mutations: Alport syndrome and thin basement membrane nephropathy. Curr Opin Pediatr. 2004;16:177–181. doi: 10.1097/00008480-200404000-00011. [DOI] [PubMed] [Google Scholar]
- Lemmink HH, Mochizuki T, van den Heuvel LP, Schroder CH, Barrientos A, Monnens LA, van Oost BA, Brunner HG, Reeders ST, Smeets HJ. Mutations in the type IV collagen α3 (COL4A3) gene in autosomal recessive Alport syndrome. Hum Mol Gen. 1994;3:1269–1273. doi: 10.1093/hmg/3.8.1269. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schroder CH, Smeets HJ. Identification of mutations in the α3(IV) and α4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet. 1994;8:77–81. doi: 10.1038/ng0994-77. [DOI] [PubMed] [Google Scholar]
- Rumpelt HJ. Alport’s syndrome: specificity and pathogenesis of glomerular basement membrane alterations. Pediatr Nephrol. 1987;3:422–427. doi: 10.1007/BF00849248. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Meehan DT, Grunkemeyer JA, Kornak JM, Sayers R, Hunter WJ, Samuelson GC. Collagen COL4A3 knockout: a mouse model for autosomal Alport syndrome. Genes Dev V. 1996;10:2981–2992. doi: 10.1101/gad.10.23.2981. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Rodgers K, Meehan D, Miller C, Bovard K, Gilroy A, Gardner H, Kotelianski V, Gotwals P, Amatucci A. Integrin alpha1-beta1 and transforming growth factor-beta1 play distinct roles in Alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am J Pathol. 2000;157:1649–1659. doi: 10.1016/s0002-9440(10)64802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson DR, Prettyman AC, Robert B, St John PL. Laminin-1 reexpression in Alport mouse glomerular basement membranes. Kidney Int. 2003;63:826–834. doi: 10.1046/j.1523-1755.2003.00800.x. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Shield CF, Todd P, Hudson B, Neilson EG. Isoform switching of type IV collagen is developmentally arrested in X-linked Alport syndrome leading to increased susceptibility of renal basement membranes to endoproteolysis. J Clin Invest. 1997;99:2470–2478. doi: 10.1172/JCI119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunwar S, Ballester F, Noelken ME, Sado Y, Ninomiya Y, Hudson BG. Identification of a novel disulfide-cross-linked network of alpha3, alpha4 and alpha5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J Biol Chem. 1998;273:8767–8775. doi: 10.1074/jbc.273.15.8767. [DOI] [PubMed] [Google Scholar]
- Rao VH, Lees GE, Kashtan CE, Nemori R, Singh RK, Meehan DT, Rodgers K, Berridge BR, Bhattacharya G, Cosgrove D. Increased expression of MMP-2, MMP-9 (type IV collagenases/gelatinases) and MT1-MMP in canine X-linked Alport syndrome (XLAS). Kidney Int. 2003;63:1736–1748. doi: 10.1046/j.1523-1755.2003.00939.x. [DOI] [PubMed] [Google Scholar]
- Rao VH, Lees GE, Kashtan CE, Delimont DC, Singh R, Meehan DT, Bhattacharya G, Berridge BR, Cosgrove D. Dysregulation of renal MMP-3 and MMP-7 in canine X-linked alport syndrome. Pediatric Nephrology. 2005;20:732–739. doi: 10.1007/s00467-004-1805-5. [DOI] [PubMed] [Google Scholar]
- Rodgers KD, Rao V, Meehan DT, Fager N, Gotwals P, Ryan ST, Koteliansky V, Nemori R, Cosgrove D. Tissue monocytes may promote myofibroblast accumulation and tubular epithelial cell death in renal fibrosis. Kidney Int. 2003;63:1338–1355. doi: 10.1046/j.1523-1755.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- Chandler S, Cossins J, Lury J, Wells G. Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumour necrosis factor-α fusion protein. Biochem Biophys Res Commun. 1996;228:421–429. doi: 10.1006/bbrc.1996.1677. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Sakatsume M, Xie Y, Kuroda T, Igashima M, Narita I, Gejyo F. Macrophage metalloelastase as a major factor for glomerular injury in anti-glomerular basement membrane nephritis. J Immunol. 2003;170:3377–3385. doi: 10.4049/jimmunol.170.6.3377. [DOI] [PubMed] [Google Scholar]
- Vos CM, van Haastert ES, de Groot CJ, van der Valk P, de Vries HE. Matrix metalloproteinase-12 is expressed in phagocytotic macrophages in active multiple sclerosis lesions. J Neuroimmunol. 2003;138:106–114. doi: 10.1016/s0165-5728(03)00036-5. [DOI] [PubMed] [Google Scholar]
- Hou P, Troen T, Ovejero MC, Kirkegaard T, Andersen TL, Byrjalsen I, Ferreras M, Sato T, Shapiro SD, Foged NT. Matrix metalloproteinase-12 (MMP-12) in osteoclasts: new lesson on the involvement of MMPs in bone resorption. Bone. 2004;34:37–47. doi: 10.1016/j.bone.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Wu L, Fan J, Matsumoto S, Watanabe T. Induction and regulation of matrix metallopro6teinase 12 by cytokines and CD40 CD40 signaling in monocytes/macrophages. Biochem Biophys Res Commun. 2000;269:808–815. doi: 10.1006/bbrc.2000.2368. [DOI] [PubMed] [Google Scholar]
- Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. doi: 10.1023/a:1025867130437. [DOI] [PubMed] [Google Scholar]
- Ding Y, Shimada Y, Gorrin-Rivas MJ, Itami LZ, Hong T, Maeda M, Komoto I, Kawabe A, Kaganoi J. Clinicopathological significance of human macrophage metalloelastase expression in esophageal squamous cell carcinoma. Oncology. 2002;63:378–384. doi: 10.1159/000066231. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya G, Miller C, Kimberling WJ, Jablonski MM, Cosgrove D. Localization and expression of usherin: a novel basement membrane protein defective in people with Usher syndrome type IIa. Hear Res. 2002;3476:1–11. doi: 10.1016/s0378-5955(01)00344-6. [DOI] [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg JI, Hoover RL, Karnovsky MJ. Isolation and characterization of rat glomerular epithelial cells in vitro. Kidney Int. 1978;14:21–30. doi: 10.1038/ki.1978.86. [DOI] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Kriz W. Induction of differentiation on cultured rat and human podocytes. J Am Soc Nephrol. 1997;8:697–705. doi: 10.1681/ASN.V85697. [DOI] [PubMed] [Google Scholar]
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Mené P. Mesangial cell cultures. J Nephrol. 2000;14:198–203. [PubMed] [Google Scholar]
- Suzuki D, Miyazaki M, Jinde K, Koji T, Yagame M, Endoh M, Nomoto Y, Sakai H. In situ hybridization studies of matrix metalloproteinase-3, tissue inhibitor of metalloproteinase-1 and type IV collagen in diabetic nephropathy. Kidney Int. 1997;52:111–119. doi: 10.1038/ki.1997.310. [DOI] [PubMed] [Google Scholar]
- Urushihara M, Kagami S, Kuhara T, Tamaki T, Kuroda Y. Glomerular distribution and gelatinolytic activity of matrix metalloproteinases in human glomerulonephritis. Nephrol Dial Transpl. 2002;17:1189–1196. doi: 10.1093/ndt/17.7.1189. [DOI] [PubMed] [Google Scholar]
- Cox ML, Lees GE, Kashtan CE, Murphy KE. Genetic cause of X-linked Alport syndrome in a family of domestic dogs. Mamm Genome. 2003;14:396–403. doi: 10.1007/s00335-002-2253-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto SI, Kobayshi T, Katoh M, Saito S, Ikeda Y, Kobori M, Masuho Y, Watanabe T. Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits. Am J Pathol. 1998;153:109–119. doi: 10.1016/s0002-9440(10)65551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto C, Rieppi M, Borsotti P, Innocenti S, Ceruti R, Drudis T, Scanziani E, Casazza AM, Taraboletti G, Giavazzi R. BAY 12–9566, a novel inhibitor of matrix metalloproteinases with antiangiogenic activity. Clin Cancer Res. 1999;5:3603–3607. [PubMed] [Google Scholar]
- Hidalgo M, Eckhardt G. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:178–193. doi: 10.1093/jnci/93.3.178. [DOI] [PubMed] [Google Scholar]
- Tullis JS, Laufersweiler MJ, VanRens JC, Matchus MG, Bookland RG, Almstead NG, Pikul S, De B, Hsieh LC, Janusz MJ, Branch TM, Peng SX, Jin YY, Hudlicky T, Oppong K. The development of new carboxylic acid-based MMP inhibitors derived from a cyclohexylglycine scaffold. Bioorg Med Chem Lett. 2001;11:1975–1979. doi: 10.1016/s0960-894x(01)00371-7. [DOI] [PubMed] [Google Scholar]
- Levitt NC, Eskens FALM, O’Byrne KJ, Propper DJ, Denis LJ, Owen SJ, Choi L, Foekens JA, Wilner S, Wood JM, Nakajima M, Talbot DC, Steward WP, Harris AL, Verweij J. Phase I and pharmacological study of the oral matrix metalloproteinase inhibitor, MMI270 (CGS27023A), in patients with advanced solid cancer. Clin Cancer Res. 2001;7:1912–1922. [PubMed] [Google Scholar]
- Nar H, Werle K, Bauer MMT, Dollinger H, Jung Birgit. Crystal structure of human macrophage elastase (MMP-12) in complex with a hydroxamic acid inhibitor. J Mol Biol. 2001;312:743–751. doi: 10.1006/jmbi.2001.4953. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC, Shield CF, III, Werb Z, Kalluri R. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 2006;3:e100. doi: 10.1371/journal.pmed.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler S, Cossins J, Lury J, Wells G. Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumour necrosis factor-α fusion protein. Biochem Biophys Res Commun. 1996;228:421–429. doi: 10.1006/bbrc.1996.1677. [DOI] [PubMed] [Google Scholar]
- Desjardins M, Bendayan M. Ontogenesis of glomerular basement membrane: structural an functional properties. J Cell Biol. 1991;113:689–700. doi: 10.1083/jcb.113.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Fan J, Matsumoto S, Watanabi T. Induction and regulation of matrix metalloproteinase-12 by cytokines and CD40 signaling in monocytes/macrophages. Biochem Biophys Res Commun. 2000:808–815. doi: 10.1006/bbrc.2000.2368. [DOI] [PubMed] [Google Scholar]
- Cui TG, Foster RR, Saleem M, Mathieson PW, Gillatt DA, Bates DO, Harper SJ. Differentiated human podocytes endogenously express an inhibitory isoform of vascular endothelial growth factor (VEGF165b) mRNA and protein. Am J Physiol. 2004;286:F767–F773. doi: 10.1152/ajprenal.00337.2003. [DOI] [PubMed] [Google Scholar]
- Yokochi S, Hashimoto H, Ishiwata Y, Shimokawa H, Haino M, Terashima Y, Matsushima K. An anti-inflammatory drug, propagermanium may target GPI-anchored proteins associated with a MCP-1receptor, CCR2. J Interferon Cytokine Res. 2001;21:389–398. doi: 10.1089/107999001750277862. [DOI] [PubMed] [Google Scholar]
- Andrews KL, Mudd JL, Li C, Miner JH. Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome. Am J Pathol. 2002;160:721–730. doi: 10.1016/S0002-9440(10)64892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton MA, Rao VH, Cosgrove D. Matrix metalloproteinase dysregulation in the stria vascularis of Alport mice: implications for capillary basement membrane pathology. Am J Pathol. 2005;166:1465–1474. doi: 10.1016/S0002-9440(10)62363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DE, Samuelson G, Meehan DT, Miller C, McGee J, Walsh EJ, Siegel M. Ultrastructural, physiological, and molecular defects in the inner ear of a gene-knockout mouse model for autosomal Alport syndrome. Hear Res. 1998;121:84–98. doi: 10.1016/s0378-5955(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Cosgrov DE. Assembly of type IV collagen: insights from α3(IV) collagen-deficient mice. J Biol Chem. 2000;275:12719–12724. doi: 10.1074/jbc.275.17.12719. [DOI] [PubMed] [Google Scholar]
- Jost MM, Ninci E, Meder B, Kempf C, Van Royen N, Hua J, Berger B, Hoefer I, Modolell M, Buschmann I. Divergent effects of GM-CSF and TGFbeta1 on bone marrow deprived macrophage arginase-1 activity, MCP-1 expression, and matrix metalloproteinase-12: a potential role during arteriogenesis. FASEB J. 2003;17:2281–2283. doi: 10.1096/fj.03-0071fje. [DOI] [PubMed] [Google Scholar]
- Feinberg MW, Jain MK, Werner F, Sibinga NE, Wiesel P, Wang H, Topper JN, Perrella MA, Lee ME. Transforming growth factor-β1 inhibits cytokine-mediated induction of human metalloelastase in macrophages. J Biol Chem. 2000;275:25766–25773. doi: 10.1074/jbc.M002664200. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Shirato I, Ishidoh K, Kominami E, Tomino Y. Selective modulation of the secretion of proteinases and their inhibitors by growth factors in cultured differentiated podocytes. Kidney Int. 2002;62:822–831. doi: 10.1046/j.1523-1755.2002.00539.x. [DOI] [PubMed] [Google Scholar]
- Blom IE, van Dijk AJ, Wieten L, Duran K, Ito Y, Kleij L, deNichilo M, Rabelink TJ, Weening JJ, Aten J. In vitro evidence for differential involvement of CTGF, TGFβ, and PDGF-BB in mesangial response to injury. Nephrol Dial Transplant. 2001;16:1139–1148. doi: 10.1093/ndt/16.6.1139. [DOI] [PubMed] [Google Scholar]
- Eto Y, Shimokawa H, Tanaka E, Morishige K, Fuchigami M, Ishiwata Y, Matsushima K, Takeshita A. Long-term treatment with propagermanium suppresses atherosclerosis in WHHL rabbits. J Cardiovasc Pharmacol. 2003;41:171–177. doi: 10.1097/00005344-200302000-00004. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165:237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetamino-phen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- Maus U, Henning S, Wenschuh H, Mayer K, Seeger W, Lohmeyer J. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med. 2002;166:268–273. doi: 10.1164/rccm.2112012. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Weber KS, Erwig LP, Kluth DC, Schroppel B, Rees AJ, Weber C. Combinatorial model of chemokine involvement in glo-merular monocyte recruitment: role of CXC chemokine receptor 2 in infiltration during nephrotoxic nephritis. J Immunol. 2001;166:5755–5762. doi: 10.4049/jimmunol.166.9.5755. [DOI] [PubMed] [Google Scholar]
- Gross O, Beirowski B, Koepke ML, Kuck J, Reiner M, Addicks K, Smyth N, Schulze-Lohoff E, Weber M. Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int. 2003;63:438–446. doi: 10.1046/j.1523-1755.2003.00779.x. [DOI] [PubMed] [Google Scholar]
- Heikkila P, Tibell A, Morita T, Chen Y, Wu G, Sado Y, Ninomiya Y, Pettersson E, Tryggvason K. Adenovirus-mediated transfer of type IV collagen alpha5 chain cDNA into swine kidney in vivo: deposition of the protein into the glomerular basement membrane. Gene Ther. 2001;8:882–890. doi: 10.1038/sj.gt.3301342. [DOI] [PubMed] [Google Scholar]