Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is the causative agent of not only adult T-cell leukemia but also HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Among the rat strains infected with HTLV-1, chronic progressive myelopathy, named HAM rat disease, occurs exclusively in WKAH rats. In the present study, we found that HTLV-1 infection induces interferon (IFN)-γ production in the spinal cords of HAM-resistant strains but not in those of WKAH rats. Neurons were the major cells that produced IFN-γ in HTLV-1-infected, HAM-resistant strains. Administration of IFN-γ suppressed expression of pX, the gene critically involved in the onset of HAM rat disease, in an HTLV-1-immortalized rat T-cell line, indicating that IFN-γ protects against the development of HAM rat disease. The inability of WKAH spinal cord neurons to produce IFN-γ after infection appeared to stem from defects in signaling through the interleukin (IL)-12 receptor. Specifically, WKAH-derived spinal cord cells were unable to up-regulate the IL-12 receptor β2 gene in response to IL-12 stimulation. We suggest that the failure of spinal cord neurons to produce IFN-γ through the IL-12 pathway is involved in the development of HAM rat disease.
Human T-cell leukemia virus type 1 (HTLV-1) is the causative agent of adult T-cell leukemia (ATL)1,2 and so-called HTLV-1-associated diseases such as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP),3,4 HTLV-1 uveitis (HU),5 HTLV-1-associated arthropathy (HAAP),6 T-cell alveolitis,7 Sjögren’s syndrome,8 polymyositis,9 and infective dermatitis.10 Only a small proportion (<5%) of HTLV-1-infected individuals develop ATL or HTLV-1-associated diseases, whereas more than 95% of carriers remain asymptomatic for life.11 Little is known about the factors that govern susceptibility to diseases caused by HTLV-1.
We previously established a rat model of HAM/TSP in which chronic progressive myelopathy with paraparesis of lower limbs occurred in WKAH rats 15 to 22 months after HTLV-1 infection.12 Although the provirus was detected in the systemic organs of all HTLV-1-infected strains examined, myelopathy, hereafter referred to as HAM rat disease, occurred exclusively in WKAH rats. Histopathological alterations were limited to the thoracic spinal cord in HAM rat disease. The most crucial finding was apoptotic cell death of oligodendrocytes in the anterior and lateral funiculi of the upper thoracic cord, which became manifest 7 months after inoculation with HTLV-1.13 Subsequently, demyelination occurred with infiltration of activated macrophages, and at the end stage of the disease, proliferation of astrocytes was observed in the affected region.14 Interestingly, lymphocytic infiltration into the spinal cord, which is characteristic of human HAM/TSP,15 was absent throughout the disease process in the HAM rat model. Although the significance of this finding is not clear, lymphocytic infiltration in human HAM/TSP may represents a cellular response to tissue damage.
The HTLV-1 provirus, which predominantly localizes in microglia and macrophages, becomes detectable in the spinal cord of both HAM-resistant and -susceptible rats 3 months after infection.16 Selective expression of the HTLV-1 pX gene peaks 7 months after infection, accompanied by an increase in tumor necrosis factor-α levels in the spinal cord and down-regulation of the anti-apoptotic bcl-2 gene in oligodendrocytes.17,18 Thus, we reasoned that the most crucial molecular events occurred ∼7 months after HTLV-1 infection in our rat model.
The proinflammatory cytokine interferon (IFN)-γ, secreted from activated T and NK cells, increases MHC class I and II expression on a wide variety of cells and then induces a Th1-type immune response. Until recently, IFN-γ had been considered a deleterious factor for central nervous system (CNS) disorders such as multiple sclerosis and experimental autoimmune encephalomyelitis.19,20 However, several lines of evidence indicate that, in some instances, IFN-γ exerts protective effects against CNS disorders. First, inactivation of the IFN-γ gene by gene knockout converts an otherwise experimental autoimmune encephalomyelitis-resistant mouse strain to experimental autoimmune encephalomyelitis-susceptible.21 Second, a low level of IFN-γ expression in the CNS plays a protective role in cuprizone-induced demyelination.22 Third, BALB/c mice treated with an anti-IFN-γ antibody become susceptible to measles virus encephalitis, and viral clearance from the CNS is impaired.23 Fourth, treatment with IFN-γ results in inhibition of viral replication in primary cultured nerve cells infected with measles virus.24 Fifth, IFN-γ protects neurons from apoptosis during destructive encephalitis induced by herpes simplex virus type 1.25 In these reports,21–25 the authors assumed that IFN-γ was derived from mononuclear cells, including T and NK cells, infiltrating into the CNS.
We show here that IFN-γ levels in the spinal cord are significantly increased in HAM-resistant ACI and LEW rats 7 months after HTLV-1 infection, whereas no such increase occurs in HAM-susceptible WKAH rats. Infiltration of mononuclear cells was never seen in the CNS of HTLV-1-infected rats, indicating that IFN-γ was produced by resident cells of the CNS. By confocal laser-scanning microscopy, we identified IFN-γ-producing cells in the spinal cord of HAM-resistant rats as neurons. We suggest that IFN-γ produced by neurons in response to HTLV-1 infection has a protective role against the development of myelopathy.
Materials and Methods
Rats and HTLV-1 Infection
Inbred ACI, LEW, and WKAH rats were obtained from the Institute for Animal Experimentation, Hokkaido University Graduate School of Medicine. HTLV-1 infection was achieved as described.12 Briefly, HTLV-1-immortalized MT-226 was injected into the peritoneal cavity of newborn rats (1 × 107 cells/rat). All HTLV-1-infected rats were maintained in the P3 room. At least three rats were used in each experiment. All rats used in this study were anesthetized with sodium pentobarbital and then intravascularly perfused with ice-cold saline. All animal experiments were done in accordance with the Guide for Care and Use of Laboratory Animals in Hokkaido University Graduate School of Medicine.
Tissue Sampling for mRNA Extraction
After perfusion with ice-cold saline, the spinal cord, cerebrum, and spleen were harvested, flash-frozen in liquid nitrogen, and served as samples for mRNA extraction. Microglia- and neuron-rich populations were prepared from the spinal cord as follows: the harvested spinal cord was dissected and then incubated in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO) containing 0.25% collagenase (Worthington Biochemical Corp., Freehold, NJ) and 700 U DNase I (Takara, Otsu, Japan) for 30 minutes at 37°C. Microglia-rich populations were separated from the solution by Percoll-gradient centrifugation as described by Tomaru and colleagues17 and Jiang and colleagues.18 For separation of neuron-rich populations, myelin residues were removed from the solution by centrifugation at 6 × g for 1 minute, and then neurons in the supernatant were collected by centrifugation at 36 × g for 7 minutes. All samples were stored at −80°C until use.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) and Quantitative Real-Time RT-PCR
Total RNAs were extracted using Isogen (Nippon Gene, Tokyo, Japan) and purified using the RNeasy mini kit (Qiagen, Alameda, CA). The purified total RNAs were reverse-transcribed using the Super Script III first-strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA). Quantitative real-time RT-PCR was done with the cDNAs, SYBR Green I dye (SYBR Green PCR Master Mix; Qiagen), and the primer set for IFN-γ (sense: 5′-GATCCA-GCACAAAGCTGTCA-3′, anti-sense: 5′-GACTCCTTT-TCCGCTTCCTT-3′), interferon regulatory factor 1 (IRF-1) (sense: 5′-TGAAGCTGCAACAGATGAGG-3′, anti-sense: 5′-AGCAAGTATCCCTTGCCATC-3′), IL-12p40 (sense: 5′-AGGTGCGTTCCTCGTAGAGA-3′, anti-sense: 5′-CC-ATTTGCTGCATGATGAAT-3′), IL-12 receptor β1 (IL-12Rβ1) (sense: 5′-AGGTGCAGATTTCCCGTTTA-3′, anti-sense: 5′-CAGCCCTGTTTAAGCCAATG-3′), IL-12 receptor β2 (IL-12Rβ2) (sense: 5′-TGCCACCAATCCACAAACTA-3′, anti-sense: 5′-CCTGCTTCCTAGCACCTTGT-3′), IL-23p19 (sense: 5′-CACCACTGGGAGACTCAACA-3′, anti-sense: 5′-AGGATCTTGGAACGGAGA-AGA-3′), IL-23 receptor (IL-23R) (sense: 5′-TTGATG-AATTGTGCCTCGTT-3′, anti-sense: 5′-GTCTGCGCTG-GGATAGTTTC-3′), IL-27 (sense: 5′-ACTCTGCTTCCT-CGCTACCA-3′, anti-sense: 5′-GGAGATCCAGCCTCA-TTGC-3′), IL-27 receptor (IL-27R, WSX-1) (sense: 5′-AGCCCAGGGATAAAGGTGAC-3′, anti-sense: 5′-AGA-CGGGTCCAGTTGAGCTT-3′), or GAPDH (sense: 5′-ATGGGAGTTGCTGTTGAAGTCA-3′, anti-sense: 5′-CC-GAGGGCCCACTAAAGG-3′). PCR was performed in a two-step reaction (95°C for 30 seconds, 60°C for 30 seconds) for 45 cycles after initial denaturation (95°C, 15 minutes), using the ABI Prism 7000 sequence detector system (Applied Biosystems, Foster City, CA). Relative expression of target genes was analyzed using the ΔΔCT-method.27 The amount of specific mRNA was quantified at the point where the system detected uptake in the exponential phase of PCR accumulation and normalized to GAPDH mRNA levels.
Emzyme-Linked Immunosorbent Assay (ELISA)
ELISA for rat IFN-γ was performed using a kit (BioSource, Camarillo, CA). In brief, after perfusion with ice-cold saline, harvested spinal cords were homogenized with 1 ml of phosphate-buffered saline (PBS) containing 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml phenylmethyl sulfonyl fluoride. Duplicate samples (100 μl) of spinal cord homogenates were subjected to ELISA according to the manufacturer’s instructions. The detection limit of the kit was 13 pg/ml.
Primary Culture of Rat Spinal Cord Cells
Saline-perfused spinal cords were obtained from rats 7 months after HTLV-1 infection and from age-matched control rats. Harvested spinal cords were dissected and digested as described above. Cell suspensions were centrifuged, and then the pellet was resuspended in 30% Percoll (Amersham Biosciences, Uppsala, Sweden) diluted with Hanks’ balanced salt solution (Invitrogen, Carlsbad, CA). The cell suspension was laid gently on 80% Percoll solution. The gradient solution was centrifuged at 1800 × g for 40 minutes. Cells in the 30% Percoll layer were dissociated, washed, and then plated sparsely on poly-l-lysine-coated dishes in Dulbecco’s modified Eagle’s medium/Ham’s F12 medium (Invitrogen) supplemented with 10% fetal calf serum and 50 ng/ml of nerve growth factor 2.5S (Invitrogen) at 37°C in an atmosphere of 5% CO2.
Recombinant Cytokines
Recombinant rat IFN-γ was purchased from PeproTech EC (London, UK). Recombinant mouse interleukin (IL)-12, previously shown to function in rats,28 was purchased from R&D Systems (Minneapolis, MN).
Immunofluorescent Staining
Cells cultured on poly-l-lysine/laminin-coated glasses for 5 days were fixed with 4% paraformaldehyde for 15 minutes. For intracellular staining, cells were treated with PBS containing 0.1% Triton-X and 0.05% bovine serum albumin for 4 minutes and then fixed with ice-cold 70% methanol for 4 minutes. Nonspecific binding was blocked with PBT (0.05% Tween-20/0.1% bovine serum albumin in PBS) containing 0.1% goat serum for 10 minutes. Primary antibodies used were mouse monoclonal anti-rat IFN-γ (DB1; PBL Biomedical Laboratories, Piscataway, NJ), mouse monoclonal anti-rat CD68 (ED-1; Serotec, Oxford, UK), rabbit polyclonal anti-neurofilament (NF) 150-kd molecule (AB1981; Chemicon International, Temecula, CA), and rabbit polyclonal anti-glial fibrillary acidic protein (GFAP) (Dakocytomation, Glostrup, Denmark). For double staining, cells were labeled with DB1 and AB1981, DB1 and anti-GFAP, or ED-1 and AB1981 followed by labeling with Alexa Fluor 488-conjugated goat polyclonal antibody to mouse IgG and Alexa Fluor 568-conjugated goat polyclonal antibody to rabbit IgG. Confocal images were acquired with a laser-scanning microscope (MRC-1024; Bio-Rad Laboratories, Hercules, CA).
Effects of IFN-γ on HTLV-1 Gene Expression
LEW-S1,12 an HTLV-1-immortalized rat T-cell line, was incubated with 100 or 1000 U/ml recombinant rat IFN-γ for 3 hours, and the relative expression of the HTLV-1 pX gene to the structural gag gene was calculated using the quantitative real-time RT-PCR method. Primer sets used were 5′-ATCCCGTGGAGACTCCTCAA-3′ (sense) and 5′-CCAAACACGTAGACTGGGTATCC-3′ (anti-sense) for pX and 5′-CCAATGCAAACAAAGAATGC-3′ (sense) and 5′-AGCCCGCAACATATCTCCTA-3′ (anti-sense) for gag.
Sequencing of the 5′-Flanking Region of the Rat IL-12Rβ2 Gene
Genomic DNA was extracted from the tails of ACI, LEW, and WKAH rats using the DNeasy tissue kit (Qiagen). The 5′-flanking region of the IL-12Rβ2 gene (1.8 kb) was amplified by nested PCR (outer primers, 5′-ACCACA-CCTCTTGCCATTTT-3′ and 5′-CGAATCGGAGTACACTGCTG-3′; inner primers, 5′-CCCAGAGGCACTTTAAG-CA-3′ and 5′-ACCGATGGACAATGGGTATC-3′). After gel electrophoresis, the PCR products were purified with Freeze ’N Squeeze DNA gel extraction spin columns (Bio-Rad Laboratories) and subjected to direct sequencing with the CEQ 2000XL DNA analysis system (Beckman-Coulter, Fullerton, CA). Sequences were aligned using the online ClustalW service (http://www.ddbj.nig.ac.jp/search/clustalw-j.html) and potential binding sites of transcription factors were identified using the Transfec database (http://motif.genome.jp/).
Statistical Analysis
Data were analyzed with either Student’s t-test or repeated measures analysis of variance, appropriately. P values less than 0.05 were considered to be significant.
Results
HTLV-1 Infection Induces IFN-γ Production in the Spinal Cord of HAM-Resistant but Not HAM-Susceptible Rats
In view of the fact that IFN-γ exerts protective effects against CNS disease,21–25 we compared the expression levels of IFN-γ mRNA between HAM-susceptible and -resistant rats 7 months after inoculation with HTLV-1 (Figure 1). Expression of IFN-γ was quantified by real-time RT-PCR in the spinal cord, cerebrum, and spleen. We used tissue samples obtained 7 months after infection because our previous work indicated that critical molecular events leading to the development of HAM rat disease occurred at this time period.17,18,29
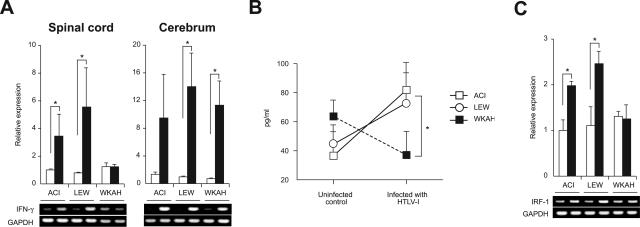
Figure 1.
A: The amount of IFN-γ mRNA in the spinal cord and cerebrum was quantified by real-time RT-PCR. Samples were obtained from rats 7 months after HTLV-1 infection (black columns) and from age-matched uninfected controls (white columns). Results of experiments done in triplicate were evaluated as relative expression levels to the GAPDH gene. Data are represented as mean ± SD values of experiments done independently three times. Representative photos of gel electrophoresis of RT-PCR products are shown beneath the graph. B: The amount of IFN-γ protein in the spinal cord was quantified using the ELISA kit. Samples were obtained from rats 7 months after HTLV-1 infection and from age-matched uninfected controls. Data are represented as mean ± SD values of experiments done independently three times. C: The amount of IRF-1 mRNA in the spinal cord was quantified by real-time RT-PCR. Samples were obtained from rats 7 months after HTLV-1 infection (black columns) and from age-matched uninfected controls (white columns). Results of experiments done in triplicate were evaluated as relative expression levels to the GAPDH gene. Data are represented as mean ± SD values of experiments done independently three times. Representative photos of gel electrophoresis of RT-PCR products are shown beneath the graph. In each group of all experiments, at least three rats were used. *P < 0.05.
The expression of IFN-γ in the spinal cord, an organ affected in HAM rat disease, was significantly elevated in HAM-resistant ACI and LEW rats compared with age-matched, uninfected controls, whereas the expression levels of IFN-γ in the spinal cord of HAM-susceptible WKAH rats were almost the same as those in uninfected controls (Figure 1A, left). The expression of IFN-γ in the cerebrum, an organ never affected in HAM rat disease, was remarkably increased in infected rats regardless of whether they were HAM-resistant or -susceptible (Figure 1A, right). In spleen cells, the mRNA level of IFN-γ did not change by infection; however, even in the absence of infection, IFN-γ was expressed more abundantly than in the cerebrum of infected rats (data not shown). Increased expression of IFN-γ mRNA was not evident in the spinal cord of HAM-resistant rats 3 months after infection, when the provirus was barely detected (data not shown).
To evaluate expression of IFN-γ at the protein level, spinal cords were harvested from infected and age-matched uninfected rats and the tissue extracts subjected to assay using an ELISA kit. Consistent with the results obtained at the mRNA level, the amount of IFN-γ proteins in the spinal cord was increased in HAM-resistant ACI and LEW rats but not in HAM-susceptible WKAH rats when measured 7 months after HTLV-1 infection (Figure 1B).
We next examined expression of the IRF-1 gene. IRF-1 is known as a downstream molecule induced by IFN-γ.30 Like IFN-γ, expression of IRF-1 was significantly increased in the spinal cord of ACI and LEW rats 7 months after infection, whereas no such increase was seen in the spinal cord of WKAH rats (Figure 1C). Thus, collective evidence clearly indicated that IFN-γ was induced by HTLV-1 infection only in the spinal cords of HAM-resistant strains.
IFN-γ Suppresses pX Gene Expression in Cultured HTLV-1-Immortalized Rat Cells
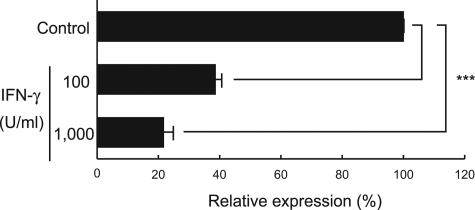
IFN-γ was recently shown to have a negative regulatory role against HTLV-1 gene expression.31 To examine whether IFN-γ can suppress expression of the pX gene, previously shown to play a critical role in the onset of HAM rat disease,17,18 we treated the HTLV-1-immortalized rat T-cell line LEW-S112 with IFN-γ for 3 hours in vitro and then monitored expression of the pX gene by real-time RT-PCR (Figure 2). Expression of the pX gene relative to that of the structural gag gene was decreased in response to IFN-γ in a dose-dependent manner. These results suggest that IFN-γ is likely to protect against the development of HAM rat disease by down-regulating pX gene expression. Thus, WKAH rats presumably develop HAM rat disease because their spinal cord cells cannot produce IFN-γ in response to HTLV-1 infection.
Figure 2.
The HTLV-1-immortalized rat T-cell line, LEW-S1, was incubated with 100 or 1000 U/ml of recombinant rat IFN-γ for 3 hours, and then expression of the pX gene was quantified using the real-time RT-PCR method. Results of experiments done in triplicate were evaluated as relative expression levels to the structural gag gene. Data are represented as mean ± SD values of experiments done independently three times. ***P < 0.0001.
Cells that Produce IFN-γ in the Spinal Cord of HTLV-1-Infected HAM-Resistant Rats Are Neurons
To identify IFN-γ-producing cells, we established primary cultures of rat spinal cord cells from both LEW and WKAH strains (Figure 3A). These cells were observed under a confocal laser-scanning microscope after immunofluorescent double staining for NF and ED-1. The cultured cells prepared from both strains, infected and uninfected, contained a nearly equivalent proportion of NF+ neurons, ED-1+ microglia, and other glial cells (Figure 3B), thus ensuring that the samples subjected to comparison were not substantially different in terms of cell populations.
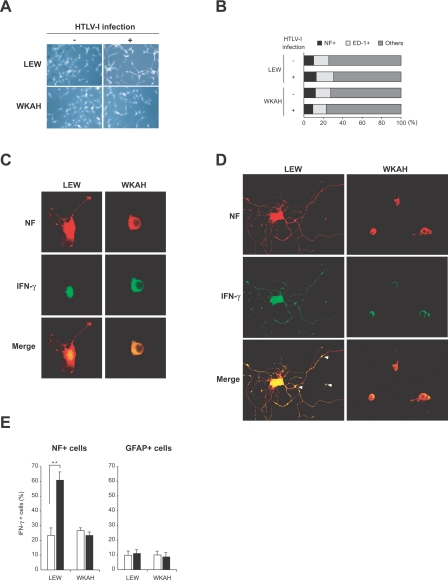
Figure 3.
Spinal cord cells were isolated from HTLV-1-infected rats and from age-matched uninfected rats. Infected rats were sacrificed 7 months after inoculation with HTLV-1. A: Phase-contrast photos of cultured cells. B: Cells cultured on poly-l-lysine/laminin-coated glasses for 5 days were fixed and then used for double-immunofluorescent staining for NF (a marker for neurons) and ED-1 (a marker for microglia). The proportion of each cell population in the culture was calculated. C and D: Spinal cord cells derived from uninfected (C) and HTLV-1-infected rats (D) were stained for NF (red) and IFN-γ (green). Arrowheads indicate the expression of IFN-γ in synapses. E: Ratios of IFN-γ+ cells in neurons (NF+ cells) and astrocytes (GFAP+ cells) in the spinal cord. Cells were isolated from the spinal cord of rats 7 months after HTLV-1 infection (black columns) and from age-matched uninfected controls (white columns). After double staining for IFN-γ and NF, or IFN-γ and GFAP, 100 cells were counted in three independent high-power views, respectively, under a fluorescence microscope. In each group, at least three rats were used. Results are represented as mean ± SD values of experiments done independently three times. **P < 0.001. Original magnifications: ×200 (A); ×620 (C, D).
We stained the cells with antibodies for NF and IFN-γ, or for GFAP and IFN-γ, and focused our analysis on neurons and astrocytes because they are known to express IFN-γ in the CNS.32–34 Weak expression of IFN-γ was seen in the perinuclear cytoplasm of NF+ neurons derived from uninfected LEW and WKAH rats (Figure 3C). The neurons obtained from LEW rats infected with HTLV-1 showed intense immunoreactivity to IFN-γ not only in the perinuclear cytoplasm but also in the dendrites, whereas HTLV-1 infection did not cause any significant alteration in the staining pattern or morphology in the neurons of WKAH rats as compared with those of uninfected control rats (Figure 3D). Neurite outgrowth was markedly induced by HTLV-1 infection in LEW but not WKAH rats. IFN-γ treatment is known to induce differentiation of neurons and an outgrowth of dendrites.35 Thus, the morphological alterations seen in Figure 3, C and D, are consistent with the observation that HTLV-1 infection induced IFN-γ expression in the spinal cord of HAM-resistant but not HAM-susceptible rats (Figure 1). We presume that IFN-γ produced by neurons of LEW rats acts in an autocrine and/or paracrine manner and promotes their differentiation and neurite outgrowth. Interestingly, IFN-γ induced in the neurons of HTLV-1-infected LEW rats appeared to accumulate in synaptic junctions (Figure 3D, arrowheads). This is in line with the observation that the receptors for IFN-γ are expressed at synapses in the superficial dorsal horn and lateral spinal nucleus36 and suggests that IFN-γ produced in neurons might function as a neurotransmitter in the CNS. On the other hand, the expression of IFN-γ in GFAP+ astrocytes was weak regardless of whether they originated from infected or uninfected animals or from HAM-susceptible or -resistant strains (data not shown). Quantitative analysis based on cell counting confirmed that neurons rather than astrocytes were the major IFN-γ-producing cells in the spinal cord of infected rats (Figure 3E).
Spinal Cord Cells of HAM-Susceptible Rats Do Not Produce IFN-γ in Response to IL-12
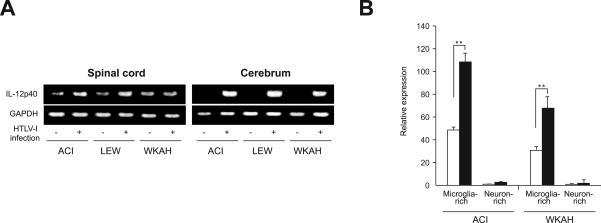
Certain infections induce production of IL-12, which in turn promotes production of IFN-γ.37 Our RT-PCR experiments showed that HTLV-1 infection induced expression of IL-12p40 mRNA in the cerebrum of both HAM-resistant and -susceptible strains (Figure 4A, right). Induction of IL-12p40 was not obvious when the whole spinal cord samples were subjected to analysis (Figure 4A, left); however, when they were fractionated into microglia- and neuron-rich populations, we could clearly see elevated expression of IL-12p40 in the former, but not in the latter, populations (Figure 4B). These findings are consistent with our previous observation that the HTLV-1 provirus was localized to microglia and macrophages.16 Basal IL-12p40 mRNA levels in the microglia-rich population were lower in WKAH than in ACI rats. However, in both strains, HTLV-1 infection almost doubled the expression levels of IL-12p40 in microglia-rich populations (Figure 4B). We thus reasoned that the failure of WKAH spinal cord cells to produce IFN-γ is unlikely to be caused by defective induction of IL-12.
Figure 4.
A: Expression of the IL-12p40 gene in the spinal cord and cerebrum. Samples were obtained from rats 7 months after HTLV-1 infection and from age-matched uninfected controls. Experiments were repeated independently at least three times. Representative photos of gel electrophoresis of RT-PCR products are shown. B: The amount of IL-12p40 mRNA in microglia- or neuron-rich populations prepared from the spinal cord was quantified by real-time RT-PCR. Samples were obtained from rats 7 months after HTLV-1 infection (black columns) and from age-matched uninfected controls (white columns). Data (relative expression levels to the GAPDH gene) are represented as mean ± SE values obtained from experiments performed in triplicate and repeated three times. In each group, at least three rats were used. **P < 0.001.
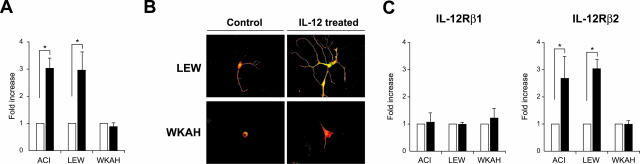
We then tested the possibility that the inability of WKAH rats to produce IFN-γ in their spinal cords is caused by defective response to IL-12. To this end, primary culture cells from the spinal cord of uninfected rats were treated with IL-12 in vitro, and then expression of IFN-γ was examined. By exposure to IL-12 for 18 hours, expression levels of IFN-γ were markedly increased in tissue-cultured spinal cord cells from HAM-resistant ACI and LEW rats, whereas no alteration was seen in the cells from HAM-susceptible WKAH rats (Figure 5A).
Figure 5.
A: The amount of IFN-γ mRNA in the cells isolated from the spinal cord of uninfected rats was quantified by real-time RT-PCR. Samples were obtained from the cells after treatment with recombinant IL-12 (100 ng/ml) for 18 hours (black columns). Results of experiments done in triplicate and repeated three times were evaluated as mean ± SE values of the fold increase to the data without IL-12 treatment (white columns). *P < 0.05. B: Cells isolated from the spinal cord of uninfected rats were cultured on poly-l-lysine/laminin-coated glasses. After incubation with recombinant IL-12 (100 ng/ml) for 5 days, immunofluorescent double staining was performed using anti-IFN-γ (green) and anti-NF (red) antibodies. Representative merged images are shown. Experiments were performed independently three times. C: The amount of IL-12Rβ1 and IL-12Rβ2 mRNAs in the cells isolated from the spinal cord of uninfected rats was quantified by real-time RT-PCR. Samples were obtained from the cells after treatment with recombinant IL-12 (100 ng/ml) for 18 hours (black columns). Results of experiments done in triplicate and repeated three times were evaluated as mean ± SE values of the fold increase to the data without IL-12 treatment (white columns). *P < 0.05. For all experiments, at least three rats were used in each group. Original magnifications, ×620 (B).
We confirmed by immunofluorescent staining that treatment with IL-12 induced IFN-γ only in the neurons of HAM-resistant rats (Figure 5B). IL-12 also induced neurite outgrowth in neurons prepared from LEW rats, presumably through the actions of IFN-γ. By contrast, similar treatment did not induce neurite outgrowth in WKAH rats. Thus, the unresponsiveness of WKAH-derived neurons to IL-12 in vitro closely mirrored the inability of WKAH-derived neurons to produce IFN-γ and undergo neurite outgrowth in response to HTLV-1 infection (Figures 1 and 3).
Spinal Cord Cells from HAM-Susceptible Rats Do Not Show Elevated IL-12Rβ2 Expression in Response to IL-12
To understand why WKAH neurons do not respond to IL-12, we examined expression of IL-12 receptors in tissue-cultured spinal cord cells obtained from HAM-resistant and -susceptible strains. IL-12 receptors are composed of β1 and β2 subunits.38 Although IL-12Rβ1 is expressed constitutively, expression of IL-12Rβ2 is up-regulated by IL-12. Expression levels of IL-12Rβ1 mRNA were not altered by IL-12 treatment in HAM-resistant or -susceptible strains (Figure 5C, left). By contrast, treatment with IL-12 markedly increased IL-12Rβ2 mRNA in spinal cord cells from ACI and LEW but not from WKAH rats (Figure 5C, right). These results indicate that the absence of IFN-γ production in the spinal cord of WKAH rats results from the inability of the IL-12Rβ2 gene to respond to IL-12 signals.
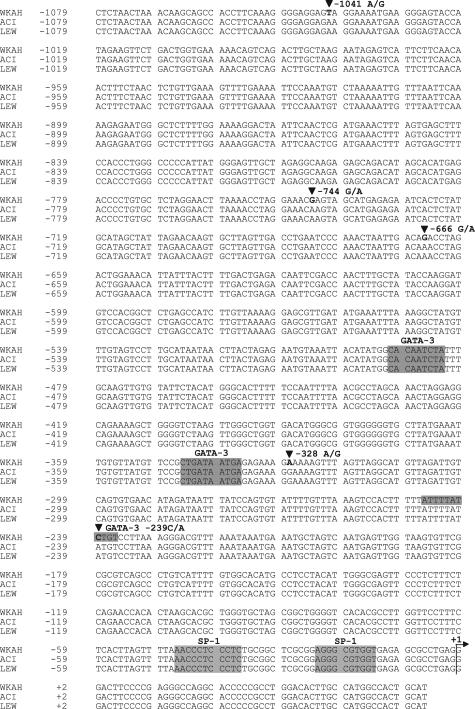
The 5′-Flanking Region of the Rat IL-12Rβ2 Gene Is Polymorphic
To examine whether the defect is in the IL-12Rβ2 gene itself, we compared its 5′-flanking sequence between HAM-resistant and -susceptible strains. Although HAM-resistant ACI and LEW rats had an identical sequence, the sequence of HAM-susceptible WKAH rats differed from that of ACI and LEW rats by 5 bp in the region spanning −1079 to −1 (Figure 6). We focused our analysis on SP-1 and GATA-3 binding sites because expression of the IL-12Rβ2 gene has been shown to be regulated positively and negatively by SP-1 and GATA-3 transcription factors, respectively.39,40 None of the 5-bp substitutions affects potential SP-1 binding sites; however, the substitution from A to C at nucleotide position −239 generates an additional potential GATA-3 binding site in the WKAH sequence. As a result, there are three potential GATA-3 binding sites in WKAH, whereas ACI and LEW have only two such sites. Because GATA-3 is known to repress IL-12Rβ2 gene expression strongly,40 the single nucleotide polymorphism at nucleotide position −239 may be related to the defective induction of IL-12Rβ2 transcription in WKAH rats.
Figure 6.
The 5′-flanking region of the rat IL-12Rβ2 gene. The genomic DNA was extracted from the tail of HAM-susceptible (WKAH) and HAM-resistant (ACI, LEW) rats, and then the 5′-flanking region of the IL-12Rβ2 gene was amplified by nested PCR. The PCR products were purified and subjected to direct sequencing. Shaded sequences represent potential SP-1 or GATA-3 binding sites. +1 represents a tentatively assigned transcription start site deduced from the data available for the mouse IL-12Rβ2 gene.
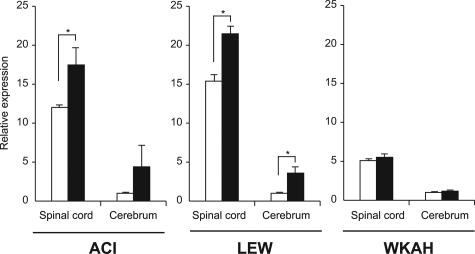
In HAM-Susceptible Rats, HTLV-1 Infection Elevates mRNA Expression of IL-23, IL-27, and Their Receptors in the Cerebrum but Not in the Spinal Cord
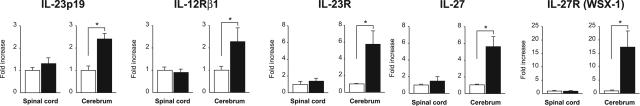
In line with the in vitro studies, HTLV-1 infection did not induce IL-12Rβ2 mRNA in the spinal cord or the cerebrum in WKAH rats (Figure 7). By contrast, HTLV-1 infection elevated IL-12Rβ2 mRNA in both the spinal cord and cerebrum in HAM-resistant ACI and LEW rats. In WKAH rats, defective induction of IL-12Rβ2 mRNA was observed not only in the spinal cord, but also in the cerebrum (Figure 7). This raised the question of why IFN-γ was induced in the cerebrum of WKAH rats 7 months after HTLV-1 infection (Figure 1A). To answer this question, we focused our analysis on IL-23 and IL-27 because these cytokines are known to induce IFN-γ.41 IL-23 is composed of p19 and p40 subunits,42 and the p40 subunit is shared by IL-12. IL-23 receptors are made up of IL-12Rβ1 and the specific subunit, IL-23R. Expression levels of IL-23p19, IL-12Rβ1, IL-23R, IL-27, and IL-27R (WSX-1) genes were significantly increased in the cerebrum but not in the spinal cord of WKAH rats 7 months after HTLV-1 infection (Figure 8). These observations indicate that IFN-γ was induced in the cerebrum of WKAH rats through the IL-23 and/or IL-27 pathways.
Figure 7.
The amount of IL-12Rβ2 mRNA in the spinal cord and cerebrum was quantified by real-time RT-PCR. Samples were obtained from rats 7 months after HTLV-1 infection (black columns) and from age-matched uninfected controls (white columns). Results of experiments performed in triplicate were evaluated as relative expression levels to the GAPDH gene. For each strain, the expression level of IL-12Rβ2 mRNA in the cerebrum of uninfected control rats was set as 1. Relative expression levels (mean ± SD values) were determined from the experiments done independently three times. In each group, at least three rats were used. *P < 0.05.
Figure 8.
The amounts of IL-23p19, IL-12Rβ1, IL-23R, IL-27, and IL-27R (WSX-1) mRNAs in the spinal cord and cerebrum were quantified by real-time RT-PCR. Samples were obtained from WKAH rats 7 months after HTLV-1 infection (black columns) and from age-matched uninfected controls (white columns). In each group, at least three rats were used. Results of experiments done in triplicate were evaluated as relative expression levels to the GAPDH gene. Data from experiments done independently three times are represented as the fold increase (mean ± SD values) to the data without infection (white columns). *P < 0.05.
Discussion
In the present study, we have demonstrated that expression of the proinflammatory cytokine IFN-γ is significantly elevated in the spinal cord of HAM-resistant rats 7 months after HTLV-1 infection (Figure 1). The increased expression of IFN-γ in the spinal cord was seen only in HAM-resistant strains. Importantly, we found that IFN-γ could suppress expression of the pX gene in vitro (Figure 2), the gene previously shown to be critically involved in the development of myelopathy in WKAH rats.17,18 Thus, combined evidence argues strongly that IFN-γ prevents the development of myelopathy by down-regulating pX gene expression in the spinal cord. However, there may be other mechanisms through which IFN-γ exerts protective effects against HAM rat disease. For example, IFN-γ is known to protect cord blood mononuclear cells from HTLV-1 infection when they are co-cultured with an HTLV-1-immortalized T-cell line MT-2, without altering the provirus load in the culture.31 Thus, IFN-γ may protect against the development of myelopathy through multiple mechanisms.
The CNS including the spinal cord has been considered as an absolute immunologically privileged site because of multiple anatomical and biochemical barriers known as blood-brain barriers. However, throughout the past decade, this view has been challenged by a number of observations showing that resident cells of the CNS produce cytokines and that such cytokines affect both proliferation and differentiation of cells in the CNS even under physiological conditions.43 In the 1990s, it was suggested that endogenous IFN-γ exists in the CNS.44 Endogenous IFN-γ was detected in the CNS of mice infected with Theiler’s virus,45 and Kiefer and colleagues46 showed that IFN-γ was produced by rat neurons. Because there was no inflammatory cell infiltration in the CNS of HTLV-1-infected rats, our present study makes a strong case for the production of cytokines by CNS-resident cells. Neurons are not the sole source of IFN-γ in the CNS. Astrocytes in primary culture are known to produce and release IFN-γ after the mechanical and ischemic injuries.47 It is also known that cultured rat astrocytes secrete IFN-γ in response to tumor necrosis factor-α in a dose-dependent manner.48 We therefore asked which population of cells produced IFN-γ in the CNS of HTLV-1-infected, HAM-resistant rats. The observation made under a confocal microscope provided convincing evidence that neurons were the major cells that produced IFN-γ in our rat model of myelopathy (Figure 3). This is the first report demonstrating that HTLV-1 infection induces production of IFN-γ by neurons.
To understand why HTLV-1 infection failed to induce production of neuronal IFN-γ in the spinal cord of WKAH rats (Figures 1 and 3), we initially turned our attention to IL-12, an innate cytokine induced early during certain viral infections and a potent stimulator of IFN-γ.37 This cytokine is secreted mainly from microglia in the CNS,49 and in HTLV-1-infected rats, the provirus is predominantly localized in microglia and macrophages.16 We therefore assumed that infected microglia and/or macrophages in the CNS were the most likely source of IL-12. Consistent with this assumption, we detected IL-12p40 mRNA in microglia-rich populations (Figure 4B). To examine whether poor induction of IL-12 after infection is responsible for the failure of WKAH rats to produce IFN-γ in their spinal cord neurons, we compared induction kinetics of IL-12p40 mRNA between HAM-resistant and -susceptible strains (Figure 4B). In both ACI and WKAH strains, HTLV-1 infection elevated the amount of IL-12p40 mRNA almost twofold in the microglia-rich population. Thus, the ability to produce IL-12 in response to infection is apparently not impaired in WKAH rats.
We then examined the possibility that WKAH rats might have a defect in its ability to respond to IL-12. To this end, cultured spinal cord cells from uninfected rats were treated with IL-12 in vitro, and then expression of IFN-γ was evaluated by real-time RT-PCR and by immunofluorescent staining (Figure 5). These experiments showed that IFN-γ induction by IL-12 occurs only in neurons obtained from the spinal cords of HAM-resistant strains (Figure 5), indicating that signaling through the IL-12 receptor is defective in WKAH.
To examine whether the defect lies in the IL-12Rβ2 gene itself, we compared its 5′-flanking sequence between HAM-resistant and -susceptible strains. The two HAM-resistant strains, ACI and LEW, had an identical sequence; however, the sequence of WKAH rats differed from that of ACI and LEW by 5 bp (Figure 6). Interestingly, the A to C substitution at nucleotide position −239 generates an additional potential GATA-3 binding site in WKAH rats. Although the mechanism regulating the expression of the IL-12Rβ2 gene is only poorly understood, GATA-3 is known to repress its expression strongly.40 Thus, the single nucleotide polymorphism at nucleotide position −239 may be involved in the defective induction of the IL-12Rβ2 gene in WKAH rats. However, functional studies are required to understand whether this polymorphism is biologically significant.
In WKAH rats, HTLV-1 infection did not up-regulate IL-12Rβ2 gene expression in the cerebrum (Figure 7). This observation was initially puzzling because the cerebrum of WKAH rats was able to produce IFN-γ in response to HTLV-1 infection (Figure 1A). A solution to this apparent paradox came from the fact that production of IFN-γ is regulated not only by IL-12 but also by IL-23 and IL-27.41 We observed that HTLV-1 infection increased the amount of mRNA for IL-23, IL-27, and their receptors in the cerebrum but not in the spinal cord of WKAH rats (Figure 8). Thus, alternative pathways of IFN-γ induction are active in the cerebrum of WKAH rats. We suggest that induction of IFN-γ via IL-12-independent pathways explains at least in part why the cerebrum is never affected in HAM rat disease.
In conclusion, this study is the first to indicate that neuronal IFN-γ protects the CNS from tissue damage caused by HTLV-1 infection. Although IL-12Rβ2 is a prime candidate for the gene that controls susceptibility to HAM rat disease, genes involved in the regulation of IL-12Rβ2 are also potential candidates. We envision that IL-12/IL-12 receptor-mediated, neuronal IFN-γ responses are also critically involved in the pathogenesis of human HAM/TSP. Hence, the dissection of the molecular mechanisms leading to the development of HAM rat disease should help us understand the factors that govern susceptibility to human HAM/TSP.
Acknowledgments
We thank the entire staff of the Institute of Animal Experimentation, Hokkaido University Graduate School of Medicine for their skillful maintenance of rats.
Footnotes
Address reprint requests to Akihiro Ishizu, Department of Pathology/Pathophysiology, Division of Pathophysiological Science, Hokkaido University Graduate School of Medicine, Kita-15, Nishi-7, Kita-ku, Sapporo 060-8638, Japan. E-mail: aishizu@med.hokudai.ac.jp.
Supported by the Ministry of Education, Culture, Sports, Science, and Technology; and the Ministry of Health, Labour, and Welfare, of Japan.
Present address of H.I.: Hakodate Central General Hospital, Hakodate, Japan; present address of T.Y.: Genetic Lab Co. Ltd., Sapporo, Japan.
References
- Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Watanabe T, Yamaguchi K, Takatsuki K, Yoshimura K, Shirao M, Nakashima S, Mori S, Araki S, Miyata N. HTLV-I uveitis: a distinct clinical entity caused by HTLV-I. Jpn J Cancer Res. 1992;83:236–239. doi: 10.1111/j.1349-7006.1992.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka K, Maruyama I, Sato K, Kitajima I, Nakajima Y, Osame M. Chronic inflammatory arthropathy associated with HTLV-I. Lancet. 1989;1:441. doi: 10.1016/s0140-6736(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Nakashima H, Watanabe S, Uyama E, Tanaka F, Ando M, Araki S, Kawasaki S. T-lymphocyte alveolitis in HTLV-I-associated myelopathy. Lancet. 1987;2:1220. doi: 10.1016/s0140-6736(87)91362-6. [DOI] [PubMed] [Google Scholar]
- Vernant JC, Buisson G, Magdeleine J, De Thore J, Jouannelle A, Neisson-Vernant C, Monplaisir N. T-lymphocyte alveolitis, tropical spastic paresis, and Sjögren syndrome. Lancet. 1988;1:177. doi: 10.1016/s0140-6736(88)92744-4. [DOI] [PubMed] [Google Scholar]
- Morgan OS, Rodgers-Johnson P, Mora C, Char G. HTLV-1 and polymyositis in Jamaica. Lancet. 1989;2:1184–1187. doi: 10.1016/s0140-6736(89)91793-5. [DOI] [PubMed] [Google Scholar]
- LaGrenade L, Hanchard B, Fletcher V, Cranston B, Blattner W. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet. 1990;336:1345–1347. doi: 10.1016/0140-6736(90)92896-p. [DOI] [PubMed] [Google Scholar]
- Hollsberg P, Hafler DA. Seminars in medicine of the Beth Israel Hospital, Boston. Pathogenesis of diseases induced by human lymphotropic virus type I infection. N Engl J Med. 1993;328:1173–1182. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- Ishiguro N, Abe M, Seto K, Sakurai H, Ikeda H, Wakisaka A, Togashi T, Tateno M, Yoshiki T. A rat model of human T lymphocyte virus type I (HTLV-I) infection. 1. Humoral antibody response, provirus integration, and HTLV-I-associated myelopathy/tropical spastic paraparesis-like myelopathy in seronegative HTLV-I carrier rats. J Exp Med. 1992;176:981–989. doi: 10.1084/jem.176.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto K, Abe M, Ohya O, Itakura O, Ishiguro N, Ikeda H, Wakisaka A, Yoshiki T. A rat model of HTLV-I infection: development of chronic progressive myeloneuropathy in seropositive WKAH rats and related apoptosis. Acta Neuropathol (Berl) 1995;89:483–490. doi: 10.1007/BF00571502. [DOI] [PubMed] [Google Scholar]
- Ohya O, Ikeda H, Tomaru U, Yamashita I, Kasai T, Morita K, Wakisaka A, Yoshiki T. Human T-lymphocyte virus type I (HTLV-I)-induced myeloneuropathy in rats: oligodendrocytes undergo apoptosis in the presence of HTLV-I. APMIS. 2000;108:459–466. doi: 10.1034/j.1600-0463.2000.d01-83.x. [DOI] [PubMed] [Google Scholar]
- Jacobson S. Immunopathogenesis of human T cell lymphotropic virus type I-associated neurologic disease. J Infect Dis. 2002;186(Suppl 2):S187–S192. doi: 10.1086/344269. [DOI] [PubMed] [Google Scholar]
- Kasai T, Ikeda H, Tomaru U, Yamashita I, Ohya O, Morita K, Wakisaka A, Matsuoka E, Moritoyo T, Hashimoto K, Higuchi I, Izumo S, Osame M, Yoshiki T. A rat model of human T lymphocyte virus type I (HTLV-I) infection: in situ detection of HTLV-I provirus DNA in microglia/macrophages in affected spinal cords of rats with HTLV-I-induced chronic progressive myeloneuropathy. Acta Neuropathol (Berl) 1999;97:107–112. doi: 10.1007/s004010050962. [DOI] [PubMed] [Google Scholar]
- Tomaru U, Ikeda H, Ohya O, Abe M, Kasai T, Yamasita I, Morita K, Wakisaka A, Yoshiki T. Human T lymphocyte virus type I-induced myeloneuropathy in rats: implication of local activation of the pX and tumor necrosis factor-alpha genes in pathogenesis. J Infect Dis. 1996;174:318–323. doi: 10.1093/infdis/174.2.318. [DOI] [PubMed] [Google Scholar]
- Jiang X, Ikeda H, Tomaru U, Morita K, Tanaka Y, Yoshiki T. A rat model for human T lymphocyte virus type I-associated myeloneuropathy. Down-regulation of bcl-2 expression and increase in sensitivity to TNF-alpha of the spinal oligodendrocytes. J Neuroimmunol. 2000;106:105–113. doi: 10.1016/s0165-5728(00)00216-2. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- Renno T, Taupin V, Bourbonniere L, Verge G, Tran E, De Simone R, Krakowski M, Rodriguez M, Peterson A, Owens T. Interferon-gamma in progression to chronic demyelination and neurological deficit following acute EAE. Mol Cell Neurosci. 1998;12:376–389. doi: 10.1006/mcne.1998.0725. [DOI] [PubMed] [Google Scholar]
- Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- Gao X, Gillig TA, Ye P, D’Ercole AJ, Matsushima GK, Popko B. Interferon-gamma protects against cuprizone-induced demyelination. Mol Cell Neurosci. 2000;16:338–349. doi: 10.1006/mcne.2000.0883. [DOI] [PubMed] [Google Scholar]
- Finke D, Brinckmann UG, ter Meulen V, Liebert UG. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J Virol. 1995;69:5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CE, Lawrence DM, Echols LA, Rall GF. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol. 2002;76:4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger KD, Nash TC, Sawyer S, Krahl T, Patstone G, Reed JC, Krajewski S, Dalton D, Buchmeier MJ, Sarvetnick N. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology. 1997;238:189–197. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Bloch G, Toma DP, Robinson GE. Behavioral rhythmicity, age, division of labor and period expression in the honey bee brain. J Biol Rhythms. 2001;16:444–456. doi: 10.1177/074873001129002123. [DOI] [PubMed] [Google Scholar]
- Pelidou SH, Zou LP, Deretzi G, Nennesmo I, Wei L, Mix E, Van Der Meide PH, Zhu J. Intranasal administration of recombinant mouse interleukin-12 increases inflammation and demyelination in chronic experimental autoimmune neuritis in Lewis rats. Scand J Immunol. 2000;51:29–35. doi: 10.1046/j.1365-3083.2000.00636.x. [DOI] [PubMed] [Google Scholar]
- Tomaru U, Ikeda H, Jiang X, Ohya O, Yoshiki T. Provirus expansion and deregulation of apoptosis-related genes in the spinal cord of a rat model for human T-lymphocyte virus type I-associated myeloneuropathy. J Neurovirol. 2003;9:530–538. doi: 10.1080/13550280390241160. [DOI] [PubMed] [Google Scholar]
- Kroger A, Koster M, Schroeder K, Hauser H, Mueller PP. Activities of IRF-1. J Interferon Cytokine Res. 2002;22:5–14. doi: 10.1089/107999002753452610. [DOI] [PubMed] [Google Scholar]
- D’Onofrio C, Franzese O, Puglianiello A, Peci E, Lanzilli G, Bonmassar E. Antiviral activity of individual versus combined treatments with interferon alpha, beta and gamma on early infection with HTLV-I in vitro. Int J Immunopharmacol. 1992;14:1069–1079. doi: 10.1016/0192-0561(92)90152-b. [DOI] [PubMed] [Google Scholar]
- Neumann H, Schmidt H, Wilharm E, Behrens L, Wekerle H. Interferon gamma gene expression in sensory neurons: evidence for autocrine gene regulation. J Exp Med. 1997;186:2023–2031. doi: 10.1084/jem.186.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T, Kelic S, Edlund C, Bakhiet M, Hojeberg B, van der Meide PH, Ljungdahl A, Kristensson K. Neuronal interferon-gamma immunoreactive molecule: bioactivities and purification. Eur J Immunol. 1994;24:308–314. doi: 10.1002/eji.1830240205. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Stoll G, Toyka KV, Hartung HP. Rat astrocytes express interferon-gamma immunoreactivity in normal optic nerve and after nerve transection. Brain Res. 1990;515:347–350. doi: 10.1016/0006-8993(90)90621-h. [DOI] [PubMed] [Google Scholar]
- Barish ME, Mansdorf NB, Raissdana SS. Gamma-interferon promotes differentiation of cultured cortical and hippocampal neurons. Dev Biol. 1991;144:412–423. doi: 10.1016/0012-1606(91)90433-4. [DOI] [PubMed] [Google Scholar]
- Vikman K, Robertson B, Grant G, Liljeborg A, Kristensson K. Interferon-gamma receptors are expressed at synapses in the rat superficial dorsal horn and lateral spinal nucleus. J Neurocytol. 1998;27:749–759. doi: 10.1023/a:1006903002044. [DOI] [PubMed] [Google Scholar]
- Frucht DM, Fukao T, Bogdan C, Schindler H, O’Shea JJ, Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556–560. doi: 10.1016/s1471-4906(01)02005-1. [DOI] [PubMed] [Google Scholar]
- Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- van Rietschoten JG, Smits HH, van de Wetering D, Westland R, Verweij CL, den Hartog MT, Wierenga EA. Silencer activity of NFATc2 in the interleukin-12 receptor beta 2 proximal promoter in human T helper cells. J Biol Chem. 2001;276:34509–34516. doi: 10.1074/jbc.M102536200. [DOI] [PubMed] [Google Scholar]
- van Rietschoten JG, Westland R, van den Bogaard R, Nieste-Otter MA, van Veen A, Jonkers RE, van der Pouw Kraan TC, den Hartog MT, Wierenga EA. A novel polymorphic GATA site in the human IL-12Rbeta2 promoter region affects transcriptional activity. Tissue Antigens. 2004;63:538–546. doi: 10.1111/j.0001-2815.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig SD, Holland SM. Defects in the interferon-gamma and interleukin-12 pathways. Immunol Rev. 2005;203:38–47. doi: 10.1111/j.0105-2896.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- Xiao BG, Link H. Immune regulation within the central nervous system. J Neurol Sci. 1998;157:1–12. doi: 10.1016/s0022-510x(98)00049-5. [DOI] [PubMed] [Google Scholar]
- Eneroth A, Andersson T, Olsson T, Orvell C, Norrby E, Kristensson K. Interferon-gamma-like immunoreactivity in sensory neurons may influence the replication of Sendai and mumps viruses. J Neurosci Res. 1992;31:487–493. doi: 10.1002/jnr.490310311. [DOI] [PubMed] [Google Scholar]
- Kohanawa M, Nakane A, Asano M, Minagawa T. Theiler’s virus is eliminated by a gamma-interferon-independent mechanism in the brain. J Neuroimmunol. 1994;52:79–86. doi: 10.1016/0165-5728(94)90165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer R, Haas CA, Kreutzberg GW. Gamma interferon-like immunoreactive material in rat neurons: evidence against a close relationship to gamma interferon. Neuroscience. 1991;45:551–560. doi: 10.1016/0306-4522(91)90270-x. [DOI] [PubMed] [Google Scholar]
- Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18:351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- Xiao BG, Link H. IFN-gamma production of adult rat astrocytes triggered by TNF-alpha. Neuroreport. 1998;9:1487–1490. doi: 10.1097/00001756-199805110-00044. [DOI] [PubMed] [Google Scholar]
- Li J, Gran B, Zhang GX, Ventura ES, Siglienti I, Rostami A, Kamoun M. Differential expression and regulation of IL-23 and IL-12 subunits and receptors in adult mouse microglia. J Neurol Sci. 2003;215:95–103. doi: 10.1016/s0022-510x(03)00203-x. [DOI] [PubMed] [Google Scholar]