Abstract
Trophic interactions between pulmonary epithelial and mesenchymal cell types, known as the epithelial-mesenchymal trophic unit (EMTU), are crucial in lung development and lung disease. Transforming growth factor (TGF)-β is a key factor in mediating these interactions, but it is expressed in a latent form that requires activation to be functional. Using intact fetal tracheal tissue and primary cultures of fetal tracheal epithelial cells and fibroblasts, we demonstrate that a subset of integrins, αvβ6 and αvβ8, are responsible for almost all of the TGF-β activation in the EMTU. Both αvβ8 and αvβ6 contribute to fetal tracheal epithelial activation of TGF-β, whereas only αvβ8 contributes to fetal tracheal fibroblast activation of TGF-β. Interestingly, fetal tracheal epithelial αvβ8-mediated TGF-β activation can be enhanced by phorbol esters, likely because of the increased activity of MT1-MMP, an essential co-factor in αvβ8-mediated activation of TGF-β. Autocrine αvβ8-mediated TGF-β activation by fetal tracheal fibroblasts results in suppression of both transcription and secretion of hepatocyte growth factor, which is sufficient to affect phosphorylation of the airway epithelial hepatocyte growth factor receptor, c-Met, as well as airway epithelial proliferation in a co-culture model of the EMTU. These findings elucidate the function and complex regulation of integrin-mediated activation of TGF-β within the EMTU.
The lung develops from tightly orchestrated interactions between opposing layers of epithelial and mesenchymal cells separated by a basement membrane, the components of the epithelial-mesenchymal trophic unit (EMTU).1 During development these reciprocal interactions are driven by paracrine factors that determine the differentiation state of airway epithelial cells and fibroblasts.2,3 In the adult, reactivation of the EMTU has been proposed to occur in response to injury contributing either to the maintenance of homeostasis or to lung pathology, depending on the nature and duration of the insult.1,4,5
The multifunctional cytokine transforming growth factor (TGF)-β has been widely implicated as a master regulatory cytokine, acting as both an autocrine and paracrine factor in lung development and in the regulation of homeostasis of the EMTU.5,6 Thus, mice genetically engineered to be deficient in TGF-β1 develop pulmonary inflammation, airway epithelial hyperplasia, and dysplasia.7–9 In addition, mice that are haploinsufficient in TGF-β1 are more susceptible to chemically induced pulmonary carcinoma, whereas overexpression of TGF-β in the mouse lung results in pulmonary fibrosis.10,11 Mice deficient in TGF-β3 show defective airway branching.6 These results highlight the importance of TGF-β in the homeostatic regulation of pulmonary immunity, epithelial growth, and extracellular matrix deposition as well as in lung development.
TGF-β is ubiquitously expressed in multiple cell types in the lung in three isoforms (TGF-β1 to TGF-β3), but almost entirely in an inactive (latent) form.12 Latent-TGF-β is further covalently linked to the extracellular matrix by binding to latent TGF-β-binding proteins.13 Therefore, the activation of TGF-β from these latent stores is a major point of regulation of TGF-β function. TGF-β is activated by diverse mechanisms in the lung, ultimately involving either proteolysis or conformational alteration of the latency-associated peptide (LAP) of TGF-β.12 Recently, it has become evident that certain integrins are able to mediate activation of TGF-β and may be essential components of the TGF-β activation apparatus.14,15 Thus, integrins have the potential to sequester latent TGF-β to the cell surface, where activation can be tightly coupled to cellular responses to environmental stress to maintain homeostasis.
The LAP of TGF-β1 and -3 contains the RGD tri-peptide motif, which binds to a subset of integrins.14–18 Of the six different integrins that bind to LAP-β1, only two appear to be able to activate TGF-β, αvβ6, and αvβ8 efficiently.14,15 The αvβ6 integrin mediates activation of TGF-β through a mechanism involving the conformational alteration of TGF-β; the αvβ8 integrin mediates activation of TGF-β through the transmembrane matrix-metalloprotease-1 (MT1-MMP)-dependent cleavage of LAP.14,15 These different mechanisms of integrin-mediated activation may have evolved to serve cell-type-specific roles in particular physiological settings.
In the airway and alveolar epithelium, the integrin αvβ6 is normally expressed at low levels but can be induced by inflammatory stimuli; the integrin αvβ8 is normally expressed at high levels by normal airway epithelium.11,19 The implications of these patterns of integrin expression are that the αvβ6-dependent conformational mechanism of TGF-β activation would be biased toward TGF-β activation at injured sites after the local induction of αvβ6 expression; the αvβ8-dependent mechanism of TGF-β activation involving the metalloprotease-dependent liberation of active TGF-β would support homeostatic paracrine interactions between normal cells.
We have recently developed a system based on the use of intact human bronchial tissue to evaluate the role of integrin-mediated activation of TGF-β in the wounded adult human airway. Thus, we found that the integrin αvβ8 is expressed at high levels in fragments of intact bronchial tissues and that it mediates activation of TGF-β, ultimately inhibiting epithelial cell growth.20 These findings suggest a possible role for TGF-β during the reactivation of the EMTU in the injured adult airway. However, the EMTU has only been rigorously experimentally defined in nonhuman lung systems, which have significant differences in anatomy and cell-type distribution from human lungs.3,21 For instance, rodent airways are relatively lacking in basal cells, the cell type in which αvβ8 and αvβ6 are expressed in human airway.11,19 Therefore, we sought to develop a model of the human EMTU to define the role that integrin-mediated activation of TGF-β plays in epithelial-mesenchymal interactions because these same interactions are likely to contribute to airway disease in the adult.
Here, we describe the individual roles that fetal tracheal epithelial cells and fetal tracheal fibroblasts play in integrin-mediated activation of TGF-β and then demonstrate the cell-type-dependent role of TGF-β activation in autocrine and paracrine interactions within the human EMTU. Surprisingly, we found that despite expressing robust levels of αvβ8, fetal tracheal epithelial cells fail to efficiently activate TGF-β unless stimulated with phorbol esters, an effect most likely attributable to the increased activity of MT1-MMP. In contrast, we find that fetal tracheal fibroblasts express relatively low levels of αvβ8 but efficiently support αvβ8-dependent activation of TGF-β. Furthermore, autocrine activation of TGF-β by fetal tracheal fibroblasts leads to the suppression of transcription and secretion of hepatocyte growth factor (HGF), a potent airway epithelial cell mitogen. This suppression of HGF is sufficient to negatively impact both phosphorylation of the HGF receptor c-Met, expressed by fetal tracheal epithelial cells, as well as proliferation of fetal tracheal epithelial cells. Taken together, our results suggest that integrins, in particular the integrin αvβ8, are central regulators of homeostatic interactions within the human pulmonary EMTU.
Materials and Methods
Cell Culture, Antibodies, and Reagents
Fetal tracheas were obtained at the time of elective termination of intrauterine pregnancy (18 to 22 weeks gestation) from otherwise healthy females. Informed consent was obtained from all participants as part of an approved ongoing research protocol by the University of California, San Francisco, Committee on Human Research. Tracheal fragments were microdissected into 0.2- to 0.5-mm fragments and cultured in an individual well of an agar-coated 96-well plate using the liquid overlay culture technique as described.20 Fetal tracheal epithelial cells were isolated as previously described.22 Freshly isolated fetal tracheal epithelial cells were plated onto rat-tail collagen type I (10 μg/ml)-coated dishes and incubated overnight, and then the medium was changed to bronchial epithelial growth medium (Clonetics, San Diego, CA). Fetal tracheal fibroblasts were cultured from the tracheal tissues remaining after epithelial cell isolation by the explant technique.22 Briefly, fibroblasts outgrown from tracheal fragments were cultured in fibroblast growth media (Dulbecco’s modified Eagle’s medium with 10% fetal calf serum and penicillin-streptomycin; UCSF Cell Culture Facility). Confluent cells were passaged by trypsin treatment (0.025%; UCSF Cell Culture Facility) and used for the experiments at the initial passage (P1). Wild-type SW480 (American Type Culture Collection, Rockville, MD) and SW480 cells stably transduced with integrin β8 were prepared and passaged as described.19 All cells were incubated at 37°C in a humidified incubator in 7.5% CO2. Cultures were characterized immunohistochemically using anti-prolyl-4-hydroxylase (DAKO, Carpinteria, CA), anti-vimentin (Sigma, St. Louis, MO), and anti-cyto-keratin antibodies (Lu-5; BioCare Medical, Concord, CA). Fetal tracheal epithelial cells showed >95% positive staining with anti-cytokeratin and <5% positive staining with the anti-vimentin antibody. Fetal tracheal fibroblasts demonstrated >95% positive staining with anti-prolyl-4-hydroxylase and anti-vimentin antibodies and <5% positive staining with the anti-cytokeratin antibody (data not shown).
Additional antibodies used were mouse anti-β8 (clones 14E5 and 37e115); rabbit-anti-β823 (generous gift of Joseph McCarty, Massachusetts Institute of Technology, Cambridge, MA); anti-αv (8B815, clone L230; American Tissue Type Collection, Manassas, VA); anti-β1 (clone P5D2; Developmental Studies Hybridoma Bank, Iowa City, IA); anti-β3 [clone AP3 (American Type Culture Collection) and LM609 (Chemicon, Temecula, CA)]; anti-β5 (clone P1F6, Chemicon); anti-β6 (10D524; gift of Dean Sheppard, University of California at San Francisco, San Francisco, CA); anti-pan-TGF-β, anti-TGF-β1, anti-TGF-β3, anti-HGF (R&D Systems, Minneapolis, MN); anti-phosphorylated SMAD-225 (gift of Dr. Matsuzaki, Kansai Medical University, Osaka, Japan); anti-phosphorylated c-Met and anti-c-Met (Cell Signaling Technology, Beverly, MA); and anti-smooth muscle actin (SMA, Sigma). Recombinant active TGF-β1 and HGF (R&D Systems), gelatin (Sigma), pro-MMP-2 (Chemicon), and the pan-metalloprotease inhibitor GM6001 (Ryss Lab, Union City, CA) were purchased. Rat-tail collagen was isolated as previously described.26 The GST-β8 cytoplasmic domain fusion protein was prepared as previously described.27
Immunocytochemistry, Immunohistochemistry, and Flow Cytometry
Immunohistochemistry was performed as previously described with minor modification.19 Briefly, the fetal tracheal tissue was formalin-fixed and paraffin-embedded or flash-frozen in embedding medium (VWR, Westchester, PA). The anti-β8 primary antibody was applied after preabsorption onto frozen sections of fetal liver. Flow cytometry was performed as previously described.19
RNA Isolation and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
RNA isolation, reverse transcription, and polymerase chain reaction were performed as previously described.15 The primers used were β8 integrin sense primer, 5′-CATCTGAAAAACAACGTCTACG-3′; β8 integrin anti-sense primer, 5′-ATCTGGACAGATGGCGGTAAT-3′; HGF sense primer 5′-CAGAGGGACAAAGGAAAAGAA-3′, HGF anti-sense primer 5′-GCAAGTGAATGGAAGTCCTTTA-3′; β-actin sense primer 5′-TGACGGGGTCACCCACACTGTGCC-3′, β-actin anti-sense primer 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′. These primer sets yielded PCR products of 306, 167, and 662 bp for β8 integrin, HGF,28 and β-actin, respectively.
Immunoprecipitation Analysis, Western Blotting, and Zymography
Confluent dishes of cells were surface-labeled with 0.1 mg/ml NHS-LC-biotin (Pierce Corp., Rockford, IL) and lysed in Tris-buffered saline with 1% Triton X-100, 1 mmol/L phenylmethyl sulfonyl fluoride, and protease inhibitor cocktail (Calbiochem, San Diego, CA). Immunoprecipitations and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were performed as previously described.27 After transfer to polyvinylidene difluoride membrane (Immobilon-P; Amersham, Arlington Heights, IL), biotinylated proteins were detected by horseradish peroxidase-streptavidin conjugate (Amersham) followed by enhanced chemiluminescence (Amersham). Western blotting was performed as described with minor modification.19 Briefly, fibroblast-conditioned medium was used to treat fetal tracheal epithelial cells for 1 hour, followed by lysis in Tris-buffered saline with 1% Triton X-100, 1 mmol/L phenylmethyl sulfonyl fluoride, 5 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L sodium orthovanadate (Sigma), and protease inhibitor cocktail (Calbiochem). Zymography was performed as previously described.15
TGF-β Bioassay
The TGF-β bioassays were performed as previously described.15,20 TMLC cells (the generous gift of John Munger and Daniel Rifkin, New York University, New York, NY)29 were cultured with fetal tracheal fragments, epithelial cells, or fibroblasts pretreated (3 hours) or not with 1 or 10 nmol/L phorbol 12-myristate-13-acetate (PMA, Sigma), anti-pan-TGF-β-blocking antibody, or isoform-specific TGF-β antibodies, anti-β8-specific antibody (clone 37E1), or pan-metalloprotease inhibitor GM6001 (Ryss Lab, Inc., Union City, CA). TGF-β standard curves were generated with a 1:2 dilution series of recombinant TGF-β added to the TMLC bioassay.
HGF Enzyme-Linked Immunosorbent Assay
Fetal tracheal fibroblasts were grown to confluence in 12-well tissue culture plates, washed two times with PBS, and then incubated in serum-free Dulbecco’s modified Eagle’s medium in the presence or absence of anti-pan-TGF-β-blocking antibody or anti-β8-specific antibody with or without recombinant active TGF-β1 for 24 hours. HGF was measured in conditioned media with an HGF Quantikine ELISA kit (R&D Systems).
Co-Culture Model of the EMTU
Fetal tracheal fibroblasts (10 × 104) were cultured for 12 to 16 hours on culture inserts (1 μm pore size; Fisher Scientific, Pittsburgh, PA) in a 24-well plate in fibroblast growth media. Fetal tracheal epithelial cells (5 × 104) were cultured for 12 to 16 hours on rat-tail collagen (10 μg/ml coating concentration)-coated wells of a 12-well tissue culture plate (Fisher Scientific, Pittsburg, PA) in 1 ml of bronchial epithelial growth medium (Clonetics). The culture inserts containing the fetal tracheal fibroblasts were placed into wells containing fetal tracheal epithelial cells, and the media in the upper and lower chambers was changed to Dulbecco’s modified Eagle’s medium with 0.1% fetal calf serum and penicillin and streptomycin in the presence or absence of neutralizing anti-pan-TGF-β, anti-β8, anti-HGF, anti-β8 plus anti-TGF-β, or a control nonfunction-blocking anti-αv integrin subunit antibody (8b815). Cell proliferation was determined by cell counting, as described.19 Cell adhesion assays were performed as described.27
Collagen Gel Contraction Assay
Collagen gels were prepared as previously described with slight modification.30 A collagen solution was prepared from 1.5 ml of Vitrogen (Cohesion, Palo Alto, CA), 0.3 ml of 10× α-minimal essential medium, 0.3 ml of 0.26 mol/L NaHCO3, 0.3 ml of fetal calf serum, 0.12 ml of 0.1 mol/L NaOH, and 0.5 ml of fibroblast suspension (6 × 105/ml of gel) in the presence or absence of recombinant active TGF-β1 or neutralizing antibodies against TGF-β or β8. Five hundred μl of the gel solution was then cast in each well of a 24-well plate, and the plates were incubated at 37°C to induce gelation. A spatula was used to loosen the edges of formed gel from the well, and the maximal gel diameter was measured at 0 and 72 hours.
Statistics
Student’s t-test was used for comparison of two data sets. Tukey’s or Dunn’s test were used for parametric and nonparametric data, respectively, to find where the difference lay. Significance was defined as P < 0.05. Statistical software was Prism version 3cx (GraphPad Software, Inc., San Diego, CA).
Results
Integrin αvβ8 Is Expressed by Fetal Tracheal Epithelial Cells and Fibroblasts
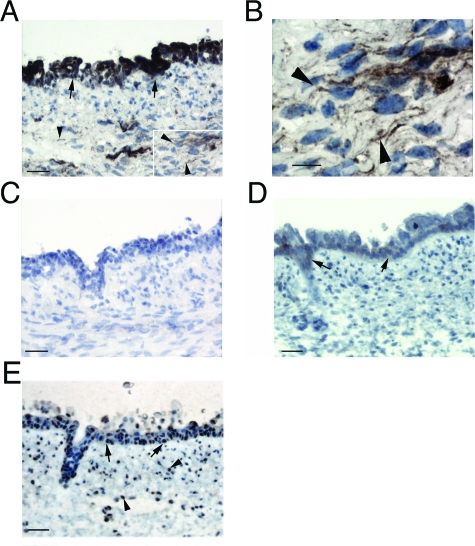
We used immunohistochemistry of fetal trachea to determine the relative expression levels and cell-type distribution of the two integrins, αvβ8 and αvβ6, thought to account for the majority of TGF-β activation in the adult airway.20 Strong β8 immunoreactivity of the fetal tracheal epithelial cells and weak staining of the subepithelial mesenchymal cells, which are a heterogeneous population of fibroblasts and myofibroblasts, was demonstrated (Figure 1, A and B). The staining was specific because no staining was seen in control sections preabsorbed with the immunogenic antigen (Figure 1C). The integrin β6 was expressed at very low levels in the fetal tracheal epithelium, concentrated in the necks of glands, and in small clusters of cells; no staining was found in cells of the mesenchymal layer (Figure 1D). The intracellular TGF-β signaling mediator SMAD-2 was activated in the majority of fetal tracheal epithelial and mesenchymal cells because an antibody recognizing phosphorylated SMAD-2 demonstrated nuclear localization in these cell types (Figure 1E).
Figure 1-6929.
Integrin αvβ8 is expressed by fetal tracheal epithelial cells and fibroblasts. Immunohistochemistry of fetal tracheas was performed using integrin β8 (A–C) and β6 (D) subunit-specific or phosphorylated SMAD-2 antibodies (E). In A, inset indicates area magnified in B. In C, the primary anti-β8 antibody was preincubated with a GST-β8 cytoplasmic domain fusion protein. Epithelial and mesenchymal staining is indicated by arrows and arrowheads, respectively. Scale bars: 50 μm (A, C–E); 10 μm (B).
Fetal Tracheal Epithelial Cells and Fibroblasts Express the Integrin αvβ8
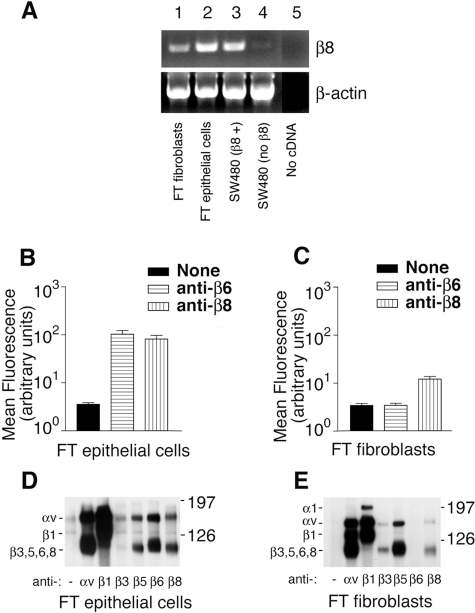
To confirm our immunohistochemical data, we determined the integrin expression profiles of fetal tracheal fibroblasts and fetal tracheal epithelial cells. A robust amplification product for the β8 integrin subunit transcript was easily detected from fetal tracheal fibroblasts (Figure 2A, lane 1; top), but the band was less intense than from fetal tracheal epithelial cells (Figure 2A, lane 2; top). The amplified band from the fetal tracheal epithelial cells was equally as intense as that from SW480 cells stably transfected with a CMV promoter-driven β8 expression construct (Figure 2A, lane 3; top). No β8 amplification product was detected from nontransfected SW480 cells (Figure 2A, lane 4; top) or from a control amplification mixture without added cDNA (Figure 2A, lane 5; top). Amplification of β-actin confirmed similar levels of input cDNA (Figure 2A, lanes 1 to 4; bottom).
Figure 2-6929.
The integrin αvβ8 is expressed by fetal tracheal epithelial cells and fibroblasts. A: RT-PCR for the β8 integrin subunit (top) and β-actin (bottom) was performed from total RNA harvested from fetal tracheal fibroblasts (lane 1), fetal tracheal epithelial cells (lane 2), wild-type SW480 cells stably transfected with a β8 expression construct (lane 3), SW480 cells (lane 4), and a control with no added cDNA (lane 5). Shown are bands migrating at the appropriate sizes for the amplification products. Flow cytometry of passage 1 fetal tracheal epithelial cells (B) and fetal tracheal fibroblasts (C) was performed using either no primary antibody (black bars), anti-β6 (horizontal hatched bars), or anti-β8 (vertical hatched bars). Results are expressed as the mean fluorescence intensity in arbitrary fluorescence units (log10). Immunoprecipitation of biotin surface-labeled fetal tracheal (FT) epithelial cells (D) or fetal tracheal fibroblasts (E) using no primary antibody or anti-integrin subunit and complex specific antibodies against αv (L230), β1 (P5D2), β3 (AP3), αvβ5 (P1F6), αvβ6 (E7P6), or αvβ8 (14E5). Antibodies used are indicated below each lane. The migration of the molecular size markers is indicated on the right, and the subunits corresponding to each band are indicated on the left. The experiments shown are representative of at least three independent experiments giving similar results.
We next performed flow cytometry of cultured fetal tracheal epithelial cells (Figure 2B) and fetal tracheal fibroblasts (Figure 2C) to confirm the surface expression of αvβ8 in fetal tracheal fibroblasts. The integrin αvβ8 was expressed at similar levels as the αvβ6 integrin by passage 1 fetal tracheal epithelial cells (Figure 2B). The integrin αvβ8 was expressed at easily detectable levels by fetal tracheal fibroblasts, albeit at 10-fold lower levels than in fetal tracheal epithelial cells (Figure 2C). The integrin αvβ6 was not expressed by fetal tracheal fibroblasts (Figure 2C).
To determine whether the integrin αvβ8 was expressed on the fetal tracheal epithelial cells and fibroblasts as a proper integrin heterodimer, we performed immunoprecipitation of surface-labeled cell lysates using integrin subunit-specific antibodies (Figure 2, D and E). The integrin αvβ8 was easily detected as an integrin heterodimer on fetal tracheal epithelial cells (Figure 2D) and fibroblasts (Figure 2E) because it could be immunoprecipitated by two separate integrin β8 antibodies, both of which immunoprecipitated two bands that co-migrated with the α and β bands from an αv immunoprecipitation and migrated at the expected molecular weights previously reported for the αv and β8 subunits.27 Consistent with flow cytometry data, fetal tracheal epithelial cells expressed both the αvβ6 and αvβ8 integrins (Figure 2D) whereas fetal tracheal fibroblasts expressed αvβ8 and no αvβ6(Figure 2E). Taken together these results demonstrate that αvβ8 is expressed on the cell surface of cultured fetal tracheal fibroblasts at lower levels than on fetal tracheal epithelial cells, as predicted by the relative intensities of β8 staining seen on fetal tracheal epithelial cells and fibroblasts using immunohistochemistry (Figure 1A).
The αvβ6 and αvβ8 Integrins Mediate the Majority of TGF-β Activation in Intact Fragments of the Fetal Trachea
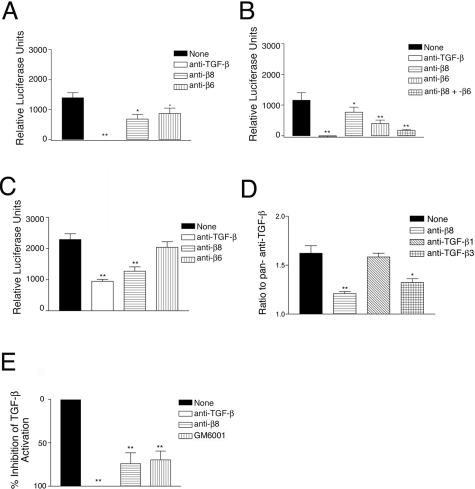
We next determined the total TGF-β activation and integrin-dependent TGF-β activation using cultured fetal tracheal fragments (Figure 3A). Most TGF-β produced by the fragments was latent because only 9.9 ± 1.6% of the total TGF-β was active, as determined by comparing heat-activated versus nonheat-activated conditioned media from fetal tracheal fragments. The active TGF-β produced by fetal tracheal fragments was almost completely dependent on the αvβ8 and αvβ6 integrins, with the αvβ8 integrin responsible for a greater fraction of TGF-β activation than αvβ6(Figure 3A). These data mirror results obtained previously using human adult bronchial fragments.20
Figure 3-6929.
Integrin-mediated activation of TGF-β by fetal tracheal fragments, fetal tracheal epithelial cells, and fibroblasts. TGF-β bioassay of active TGF-β produced by fetal tracheal fragments (A), fetal tracheal epithelial cells (B), and fetal tracheal fibroblasts (C–E). Fetal tracheal fragments were co-cultured with TGF-β reporter cells (TMLC), which stably express a portion of the plasminogen activator inhibitor-1 promoter driving the luciferase minigene, in the presence of no inhibitor (filled bar), anti-pan-TGF-β (open bar), anti-β8 (horizontal hatched bars), or anti-β6 (vertical hatched bars). The results are expressed in arbitrary luciferase units with the TMLC background subtracted. *P < 0.05, **P < 0.001. D: αvβ8-dependent activation of TGF-β3 in fetal tracheal fibroblasts. The assay was performed in the presence of no inhibitor (filled bar), anti-β8 (horizontal hatched bar), anti-TGF-β1 (diagonal hatched bars), or anti-TGF-β3 (crosshatched bars). E: Inhibition of TGF-β activation by the metalloprotease inhibitor GM6001, a pan-metalloprotease inhibitor, in the TGF-β bioassay. The assay was performed in the presence of no inhibitor (filled bar), anti-pan-TGF-β (open bar), anti-β8 (horizontal hatched bars), or GM6001 (vertical hatched bars). The results are presented as the percent inhibition of total TGF-β activation. *P < 0.05, **P < 0.001.
The αvβ8 Integrin Mediates TGF-β3 Activation in Fetal Tracheal Fibroblasts through a Metalloprotease-Dependent Mechanism
To elucidate which cellular compartments of the fetal trachea were responsible for the integrin-mediated TGF-β activation, we used cultures of isolated fetal tracheal epithelial cells or fetal tracheal fibroblasts. At the first passage, fetal tracheal epithelial cells expressed similar levels of αvβ6 and αvβ8 integrins (Figure 2B). However, they activated TGF-β mainly via the αvβ6 integrin (Figure 3B). Simultaneous use of both β6- and β8-specific antibodies showed an additive blocking effect on TGF-β activation, blocking TGF-β activation almost as well as a pan-TGF-β antibody (Figure 3B).
Isolated fetal tracheal fibroblasts activated TGF-β in the TMLC assay, and this activation was almost entirely αvβ8-dependent because a β8-specific neutralizing antibody decreased TGF-β activation almost as well as pan-TGF-β neutralizing antibody. A control antibody to αvβ6, which is not expressed by fetal tracheal fibroblasts, had no effect (Figure 3C). The amount of fibroblastic αvβ8-dependent TGF-β activation in this assay was ∼20 pg/ml (data not shown).
Although αvβ6 and αvβ8 appear to account for ∼90% of the TGF-β activated by fetal tracheal epithelial cells, and αvβ8 accounts for ∼80% of the TGF-β activated by fetal tracheal fibroblasts, we cannot exclude a small contribution from other TGF-β activation mechanisms such as thrombospondin, plasmin, or the integrins αvβ3 and αvβ5, which have recently been implicated in TGF-β activation in scleroderma fibroblasts.7,12,31,32 However, neutralizing antibodies to αvβ3 or αvβ5 had no effect on fetal tracheal epithelial cell or fibroblast TGF-β activation (data not shown). Thus, despite expressing the αvβ3 integrin at similar levels and the αvβ5 integrin at 10-fold higher levels compared to αvβ8, fetal tracheal fibroblasts failed to show any αvβ3- or αvβ5-dependent activation of TGF-β. This result is consistent with previous studies failing to show αvβ3- or αvβ5-dependent activation of TGF-β, despite the integrin αvβ3 being highly expressed by many tumor cell lines and primary cells in culture and αvβ5 being nearly ubiquitously expressed in culture.14,15,18,20,33 This suggests that αvβ3- and αvβ5-dependent activation of TGF-β is likely confined to specific cell types, such as scleroderma fibroblasts, but does not play a major role in the cell types used in this study.
To determine the TGF-β isoform(s) activated by αvβ8 in fetal tracheal fibroblasts, we used TGF-β1 and -β3 isoform-specific neutralizing antibodies (the LAP of TGF-β2 does not contain an RGD site, which is required for integrin-mediated activation of TGF-β14,15). TGF-β3 accounted for the majority of αvβ8-mediated activation of TGF-β because neutralizing antibodies against TGF-β3 blocked fibroblastic activation of TGF-β almost as well as anti-β8(Figure 3D). These findings are consistent with our previous results using adult human bronchial fragments in which we found that the integrin β8 subunit and TGF-β3 have overlapping patterns of distribution and the transcript for the TGF-β3 isoform was expressed in 200-fold excess to the TGF-β1 isoform.20
We have recently reported that αvβ8 integrin-mediated TGF-β activation is dependent on the presence of the membrane type 1 matrix metalloprotease (MT1-MMP), which facilitates the release of active TGF-β into the extracellular space.15 Indeed, the metalloprotease inhibitor GM6001 significantly blocked fetal tracheal fibroblast αvβ8 integrin-mediated TGF-β activation almost as efficiently as an anti-β8-specific antibody (Figure 3E), confirming the involvement of a metalloprotease in TGF-β activation by fetal tracheal fibroblasts.
Fetal Tracheal Epithelial Cells Regulate αvβ8-Mediated Activation of TGF-β through Induction of MT1-MMP Activity
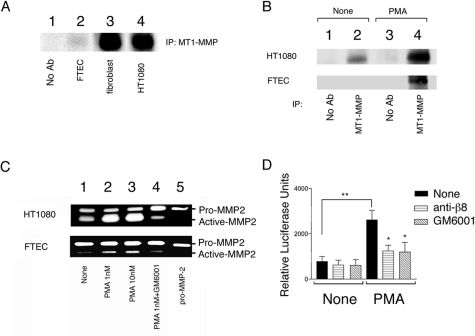
Fetal tracheal epithelial cells do not efficiently support αvβ8-mediated activation of TGF-β despite expressing 10-fold higher levels of αvβ8 than fetal tracheal fibroblasts (Figure 2C), suggesting that MT1-MMP activity might be reduced in fetal tracheal epithelial cells. MT1-MMP activity has been shown to be dependent on levels of both surface expression and activation state.15,34 Indeed, low levels of cell surface MT1-MMP were found in fetal tracheal epithelial cells, whereas high levels of cell surface MT1-MMP were found in fetal tracheal fibroblasts and HT1080 cells, correlating with the relative ability of these cell types to support αvβ8-dependent TGF-β activation15(Figure 4A). PMA has been shown to increase both the expression and activation state of MT1-MMP.34,35 PMA treatment markedly increased both the surface expression and activity of MT1-MMP, as determined by immunoprecipitation of surface labeled cells (Figure 4B) and cleavage of the MT1-MMP substrate pro-MMP2 (Figure 4C) by fetal tracheal epithelial cells. These changes were similar to those in a control cell line, HT1080, known to increase its surface expression and activity of MT1-MMP in response to PMA35(Figure 4, B and C). PMA treatment markedly increased the total and αvβ8-dependent activation of TGF-β by fetal tracheal epithelial cells (Figure 4D). Finally, this increase in αvβ8-mediated activation of TGF-β was metalloprotease-dependent because GM6001 could block TGF-β activation to a similar extent as the neutralizing β8 antibody (Figure 4D).
Figure 4-6929.
αvβ8-mediated activation of TGF-β is regulated by PMA inducible metalloprotease activity. A: The level of surface MT1-MMP correlates with the relative abilities of fetal tracheal epithelial cells and fibroblasts to active TGF-β. Immunoprecipitations of cell surface biotinylated cell lysates using either no primary antibody (lane 1) or anti-MT1-MMP (lanes 2 to 4) were resolved by 10% SDS-PAGE under reducing conditions. Lanes 1 and 2: fetal tracheal epithelial cells; lane 3: fetal tracheal fibroblasts; lane 4: HT1080 fibrosarcoma cells. Shown is the immunoprecipitation product migrating at the expected kd for MT1-MMP. B: PMA markedly up-regulates the surface expression of MT1-MMP by fetal tracheal epithelial cells. Immunoprecipitations of cell surface biotinylated cell lysates of HT1080 fibrosarcoma cells (top) or fetal tracheal epithelial cells (FTEC, bottom) using either no primary antibody (control, lanes 1 and 3) or anti-MT1-MMP (lanes 2 and 4) were resolved by 10% SDS-PAGE under reducing conditions. The bands shown migrated at the size expected for MT1-MMP. C: PMA treatment of fetal tracheal epithelial cells leads to increased activity of MT1-MMP. Gelatin zymography was performed using conditioned media harvested from HT1080 cells (top) or fetal tracheal epithelial cells (FTEC, bottom) treated with pro-MMP with no inhibitor (none, lane 1), 1 nmol/L PMA (lane 2), 10 nmol/L PMA (lane 3), or PMA with GM6001 (lane 4). The migration of recombinant pro-MMP-2 is shown (pro-MMP-2, lane 5). A–C show representative experiments of at least three independent experiments giving similar results. D: Fetal tracheal epithelial cell αvβ8-dependent activation of TGF-β is markedly enhanced by PMA treatment. Fetal tracheal epithelial cells (n = 5) either not treated or treated with PMA (1 nmol/L) were co-cultured with TMLC TGF-β reporter cells in the presence of nothing (filled bar), neutralizing anti-β8 (horizontal crosshatched bar), or the metalloprotease inhibitor GM6001 (diagonal hatched bars). *P < 0.05, **P < 0.001.
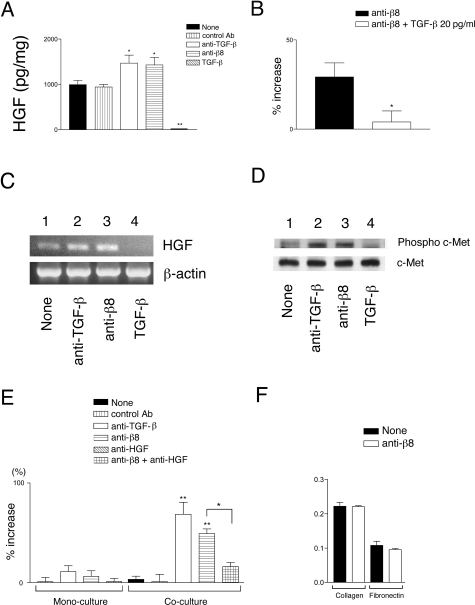
Autocrine αvβ8-Mediated TGF-β Activation Regulates HGF Secretion by Fetal Tracheal Fibroblasts, Which Is Sufficient to Impact Fetal Tracheal Epithelial Proliferation
HGF is a mesenchymal-derived growth factor that is a potent lung epithelial mitogen and morphogen.36,37 The release of HGF from fibroblasts is regulated by autocrine TGF-β signaling.38 However, little is known of the source of active TGF-β produced by fibroblasts. αvβ8-Mediated activation of TGF-β regulated the autocrine secretion of HGF by fetal tracheal fibroblasts because the amount of HGF secreted into conditioned medium was significantly increased by a pan-TGF-β-neutralizing antibody or a β8-neutralizing antibody (Figure 5A). However, the αvβ8-dependent autocrine suppression of HGF was incomplete because high concentrations (2 ng) of exogenous TGF-β could almost completely suppress HGF secretion (Figure 5A). The effect of the αvβ8 antibody was specific to TGF-β activation because 20 pg/ml of recombinant TGF-β, the amount of TGF-β activated by fibroblastic αvβ8 in this system, was able to almost completely block the αvβ8 antibody-mediated increase in fibroblastic HGF secretion (Figure 5B). The autocrine αvβ8-dependent suppression of HGF was attributable to the transcriptional suppression of HGF, as demonstrated by an increase in the HGF amplification product after treatment with neutralizing β8 or TGF-β antibodies (Figure 5C). The amount of HGF suppressed by αvβ8-mediated activation of TGF-β was physiologically significant because the phosphorylation of the HGF receptor, c-Met, expressed by fetal tracheal epithelial cells increased after exposure to the conditioned medium from fetal tracheal fibroblasts treated with neutralizing anti-β8 or anti-TGF-β (Figure 5D, top). These αvβ8-dependent changes in c-Met phosphorylation status were attributable to increased phosphorylation of c-Met and not because of an overall increase in total c-Met because treatment of fetal tracheal fibroblasts by anti-β8 or anti-TGF-β did not change total protein levels of c-Met (Figure 5D, bottom). Conditioned media from fetal tracheal fibroblasts treated with exogenous active TGF-β did not efficiently induce c-Met phosphorylation by fetal tracheal epithelial cells (Figure 5D), consistent with a TGF-β-dependent reduction of HGF secretion (Figure 5, A–C).
Figure 5-6929.
Autocrine αvβ8-mediated activation of TGF-β by fetal tracheal fibroblasts negatively regulates fetal tracheal epithelial proliferation through the TGF-β-dependent suppression of HGF secretion by fetal tracheal fibroblasts. A: Supernatants of fetal tracheal fibroblasts (n = 3) incubated for 24 hours in the presence of no treatment (filled bar), control function blocking anti-β6 antibody (10D5, vertical hatched bar), anti-pan-TGF-β (open bar), anti-β8 (horizontal hatched bar), and recombinant active-TGF-β1 (diagonal hatched bar) were assayed for HGF secretion using an HGF Quantikine ELISA kit (R&D Systems). HGF concentration is expressed as pg of HGF/μg total cellular protein. *P <0.05, **P < 0.001. B: The anti-β8-mediated increase in HGF secretion by fibroblasts is attributable to TGF-β activation. Fibroblasts were treated with blocking anti-β8 (filled bar) with or without recombinant TGF-β (20 pg/ml, open bar). Shown is percent increase in HGF secretion, by treated fibroblasts relative to nontreated fibroblasts, as measured by an HGF Quantikine ELISA kit (R&D Systems). *P < 0.05. C: HGF transcription was monitored by RT-PCR after a 16-hour incubation (top) in the presence of no treatment (lane 1), anti-pan-TGF-β (lane 2), anti-β8 (lane 3), and recombinant active-TGF-β1 (lane 4). The level of input cDNA was monitored by β-actin amplification (bottom). D: Western blotting of fetal tracheal epithelial cells treated for 1 hour with fetal tracheal fibroblast-conditioned medium treated with no treatment (lane 1), anti-pan-TGF-β (lane 2), anti-β8 (lane 3), and recombinant active-TGF-β1 (lane 4). Phosphorylation status was compared to total receptor levels using an antibody recognizing phosphorylated and nonphosphorylated c-Met (bottom). Shown in C and D are representative experiments from at least three independent experiments. E: Fetal tracheal epithelial proliferation in a transwell fetal tracheal epithelial-fetal tracheal fibroblast co-culture model. The assay (n = 3) was performed in the presence of no antibody (solid bar), control nonfunction blocking anti-αv antibody (vertical hatched bar), neutralizing antibodies to pan-TGF-β (open bar), to αvβ8 (horizontal hatched bar), or a combination of neutralizing antibodies to β8 and HGF (cross-hatched bar). As controls, the effects of the same antibodies on fetal tracheal epithelial proliferation in monoculture are shown on the left. E–F: The effect of the blocking anti-β8 antibodies on fetal tracheal cell proliferation are specific to the fibroblasts in the co-culture model because the antibodies do not significantly effect proliferation (E) and do not effect adhesion (F) of the fetal tracheal epithelial cells to the coating substrate of the co-culture assay (collagen I, Col I) or to a control substrate (fibronectin, FN). *P < 0.05, **P < 0.001. Shown is SE.
In the EMTU, the airway epithelial cells are physically separated from fibroblasts by a porous basement membrane.4 To test the physiological significance of αvβ8-mediated autocrine TGF-β in fetal tracheal fibroblastic regulation of HGF secretion, we devised a co-culture model mimicking the in vivo situation by separating fetal tracheal epithelial cells from fetal tracheal fibroblasts by a porous transwell filter. We found that fetal tracheal epithelial proliferation was not significantly increased by co-culture with fetal tracheal fibroblasts under control conditions (control antibody or no primary antibody), relative to fetal tracheal epithelial cells cultured alone (Figure 5E; monoculture versus co-culture, filled bar and vertical hatched bars). In contrast, fetal tracheal epithelial proliferation markedly increased in co-cultures treated with neutralizing antibodies to TGF-β or anti-β8 (Figure 5E; open bar and horizontal hatched bar). The increase in fetal tracheal epithelial proliferation was mostly attributable to HGF because a neutralizing antibody to HGF blocked the majority of increase in fetal tracheal epithelial proliferation induced in co-cultures by anti-αvβ8 (Figure 5E, crosshatched bar). The effect of the anti-β8 antibody on fetal tracheal epithelial cell proliferation was not because of an effect of the anti-β8, TGF-β, or HGF antibody directly on fetal tracheal epithelial cells because the antibodies had an insignificant effect on proliferation (Figure 5E; monoculture, open, horizontal hatchmark, and crosshatched bars), or adhesion to the coating substrate collagen I or to fibronectin, a major pericellular matrix protein (Figure 5F). The source of the HGF in this system was solely from fetal tracheal fibroblasts because HGF was not detected in the fetal tracheal epithelial conditioned media (data not shown). These experiments demonstrate that autocrine αvβ8 integrin-mediated activation of TGF-β by fetal tracheal fibroblasts regulates the physiologically relevant secretion of HGF.
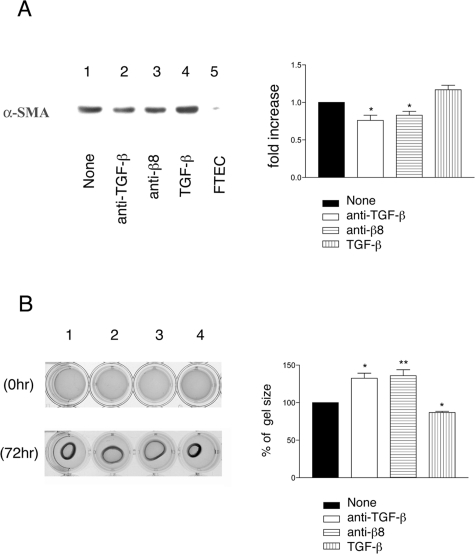
Autocrine αvβ8-Mediated Activation of TGF-β by Fetal Tracheal Fibroblasts Regulates Fibroblast Differentiation
TGF-β plays a well-established role in controlling the differentiation of fibroblasts into myofibroblasts, regulating the expression of crucial components of the contractile apparatus such as SMA.39 Fetal tracheal fibroblasts display a myofibroblastic contractile phenotype as determined by expression of SMA and contraction of collagen gels (Figure 6, A and B). αvβ8-Dependent autocrine activation of TGF-β by fetal tracheal fibroblasts contributes to SMA expression and the contractile phenotype because neutralizing antibodies to αvβ8 or TGF-β produce a modest but significant reduction in SMA expression and the contraction of collagen gels (Figure 6, A and B). These results demonstrate that αvβ8-dependent autocrine activation of TGF-β by fetal tracheal fibroblasts contributes to the myofibroblast phenotype.
Figure 6-6929.
Autocrine αvβ8-mediated activation of TGF-β activation contributes to the regulation of the myofibroblast phenotype. A: Western blot for α-SMA of fetal tracheal fibroblast cell lysates that had been treated for 24 hours with no treatment (lane 1), anti-pan TGF-β (lane 2), anti-β8 (lane 3), or recombinant active TGF-β1 (lane 4). A lysate of fetal tracheal epithelial cells is added as a negative control (lane 5). Shown at the left is a representative experiment and at the right is densitometry performed from three independent experiments. Filled bar is no treatment, open bar is anti-TGF-β, horizontal crosshatched bar is anti-β8, and vertical hatched bar is recombinant active TGF-β. *P < 0.05. B: Collagen gel contraction assay of fetal tracheal fibroblasts treated with no treatment (1), anti-TGF-β (2), anti-β8 (3), or recombinant active TGF-β (4). On the left photomicrographs depict the well containing the collagen gels at 0 (top) and 72 hours (bottom). On the right is the average maximum gel diameter taken from three independent experiments shown as percent increase in size compared to the no treatment control. Filled bar is no treatment, open bar is anti-TGF-β, horizontal crosshatched bar is anti-β8, and vertical hatched bar is recombinant active TGF-β. *P < 0.05, **P < 0.001. Shown is SE.
Discussion
The findings of this study elucidate the complex regulation of TGF-β activation and role of TGF-β in autocrine and paracrine interactions within the human EMTU. The major findings are as follow: the integrins αvβ6 and αvβ8 account for the majority of TGF-β activated within the human EMTU as demonstrated using fragments of intact fetal tracheal tissue; isolated fetal tracheal epithelial cells constitutively activate TGF-β mainly through an αvβ6-dependent mechanism whereas fetal tracheal epithelial cell αvβ8-dependent activation of TGF-β is regulated by the induction of metalloprotease activity; in contrast, isolated fetal tracheal fibroblasts constitutively activate TGF-β mainly through an αvβ8-dependent mechanism; and integrin αvβ8-mediated autocrine activation of TGF-β suppresses the paracrine secretion of HGF by fetal tracheal fibroblasts, which is sufficient to negatively regulate fetal tracheal epithelial proliferation in a co-culture model of the EMTU.
We developed a unique system based on human intact fragments of fetal tracheal tissues and isolated fetal cells to demonstrate the central role that integrin-mediated TGF-β activation plays in maintaining homeostasis in the EMTU. Such an ex vivo system provides a reasonably close approximation to in vivo events and closely models the complex interactions in play during development of the human fetal airway. We have previously developed a similar system based on the use of intact adult human bronchial tissues to show that αvβ8-mediated activation of TGF-β regulates adult airway epithelial proliferation.20 In this previous study, we did not determine the cellular source of the active TGF-β but found that β8 was highly expressed in the airway epithelium but not in the airway mesenchymal cells.20 In the current study, we find β8 expressed in cell processes in the subepithelial fibroblasts. The discrepancy between these two studies likely represents several methodological differences. In this study, we have used frozen tissues instead of paraffin-embedded tissues, which is likely to have improved the sensitivity of detection. In addition, we have used a recently developed β8-polyclonal antibody, which has been tested for staining specificity using frozen sections from mice deficient in all αv integrins.23 Using this new antibody, it is apparent that αvβ8 is more widely distributed than initially thought and is expressed in both epithelial and mesenchymal cells of the lung.
The majority of TGF-β in vivo is latent and therefore nonfunctional, and active TGF-β is difficult to detect in vivo, presumably because of its short half-life.40–42 Yet, we and others find evidence of widespread TGF-β signaling by most fetal airway epithelial and mesenchymal cells in tissue sections.43 Thus, in vivo, fetal tracheal cells are likely to be exposed to activated TGF-β at low but sufficient levels to induce continuous signaling. Our in vitro data suggests that the integrin αvβ6, expressed by epithelial cells, and αvβ8, expressed by fibroblasts, play major roles in this local activation of TGF-β.
There are several possible roles for integrin-mediated activation of TGF-β in fetal lung development. TGF-β1 and TGF-β3, respectively, have been shown to either inhibit or stimulate lung-branching morphogenesis, possibly reflecting distinct TGF-β isoform-specific spatiotemporal localization patterns.6,44–46 Thus, the integrins αvβ6 and αvβ8 could play a role in lung branching morphogenesis because both αvβ6 and αvβ8 mediate activation of TGF-β1 and TGF-β3 and have distinct spatiotemporal expression patterns. In addition, αvβ8-dependent TGF-β activation regulates the fetal tracheal fibroblastic autocrine secretion of HGF, which has also been shown to influence lung epithelial morphogenesis.36
Immunohistochemisty suggests that the integrin αvβ8 is expressed at high levels and the integrin β6 at low levels in the airway epithelium. However, airway epithelial cells appear normally and efficiently to support only αvβ6-mediated TGF-β activation. This suggests that integrin αvβ6-mediated activation is regulated by expression levels of αvβ6, and αvβ8-mediated activation of TGF-β is regulated by some other mechanism. Interestingly, airway fibroblasts express relatively low levels of αvβ8 yet efficiently support αvβ8-mediated activation of TGF-β. This demonstrates that surface expression of αvβ8 does not necessarily correlate with the magnitude of TGF-β activation, which is most likely attributable to the contribution of the metalloproteolytic co-factor MT1-MMP, a co-factor required for αvβ8-mediated activation of TGF-β in lung carcinoma cells.15 Indeed, we found that airway epithelial cells have reduced surface levels and activity of MT1-MMP compared to airway fibroblasts.
MT1-MMP plays a major role in pericellular proteolysis and has been shown to regulate cell migration, invasion, proliferation, and differentiation through diverse mechanisms.34,47 For instance, MT1-MMP has been shown to modify the platelet-derived growth factor signaling of vascular smooth muscle cells, which in turn regulates vessel wall architecture.47 Here, our data suggests that induction of MT1-MMP activity may play a similar role in the regulation of TGF-β signaling through the regulation of the αvβ8-dependent activation of TGF-β.
In the adult airway, aberrant reactivation of the EMTU is likely to occur in airway remodeling in asthma and smoking-related airways disease, and amplification of TGF-β activation and metalloprotease activity are likely to be important in this reactivation.4 In this regard, integrin αvβ8-mediated activation of TGF-β could be a crucial determinant in aberrant reactivation of the EMTU because airway epithelial cells express high levels of αvβ8 and both MT1-MMP activity and the ability of αvβ8 to activate TGF-β can be markedly enhanced by PMA, a potent agonist of protein kinase C (PKC).48 Oxidative stress has been shown to increase both PKC and MT1-MMP activity, providing a possible physiological link between environmental stress and αvβ8-mediated activation of TGF-β.49,50 Finally, increased integrin-mediated activation of TGF-β could result in delayed epithelial wound closure and stimulation of subepithelial fibroblastic extracellular matrix production, ultimately contributing to airway remodeling.
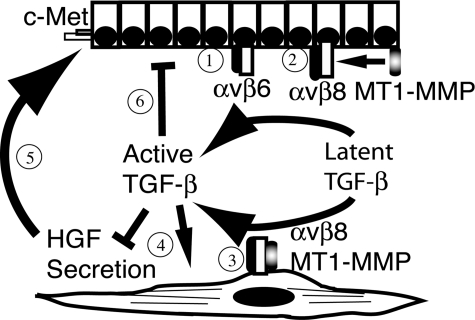
In summary, we have determined a novel pathway of autocrine integrin-dependent TGF-β activation within the EMTU of the lung, which ultimately regulates fibroblast-epithelial interactions through the paracrine secretion of TGF-β of the epithelial mitogen HGF (Figure 7). These findings provoke further investigation into the cell-type-specific regulation of TGF-β activation in lung fibroblasts in diverse pulmonary pathological processes.
Figure 7-6929.
Model of integrin αvβ8-mediated activation of TGF-β as a central homeostatic mechanism within the EMTU. 1: The airway epithelial integrin αvβ6 is expressed by airway epithelial cells and activates TGF-β. 2: On stimulation of airway epithelial cells, MT1-MMP is activated and recruited to αvβ8-latent TGF-β complexes, cleaving the latency-associated peptide and liberating active TGF-β. Active TGF-β released by airway epithelial cells is potentially available to act on neighboring fibroblasts as a paracrine factor. 3: The fibroblast integrin αvβ8 activates TGF-β3 through a metalloprotease-dependent mechanism leading to the next step. 4: Autocrine TGF-β signaling by fibroblasts suppressing HGF secretion and inducing myofibroblast differentiation, which 5: negatively influences the phosphorylation status of c-Met and proliferation of airway epithelial cells. 6: Finally, integrin αvβ8-dependent paracrine secretion of TGF-β by fibroblasts or autocrine integrin-dependent activation of TGF-β by epithelial cells negatively influences airway epithelial cell proliferation.
Acknowledgments
We thank Joseph McCarty, Richard Hynes, Dean Sheppard, Amha Atakilit, John Munger, Dr. Matsuzaki, and Daniel Rifkin for valuable reagents.
Footnotes
Address reprint requests to Stephen L. Nishimura, M.D., Department of Pathology, Bldg. 3, Rm 207, 1001 Potrero Ave., San Francisco, CA 94110. E-mail: stephen.nishimura@ucsf.edu.
Supported by the National Institutes of Health (grants HL63993, HL70622, and NS44655 to S.N.).
References
- Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105:193–204. doi: 10.1016/s0091-6749(00)90066-6. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Demayo F, Minoo P, Plopper CG, Schuger L, Shannon J, Torday JS. Mesenchymal-epithelial interactions in lung development and repair: are modeling and remodeling the same process? Am J Physiol. 2002;283:L510–L517. doi: 10.1152/ajplung.00144.2002. [DOI] [PubMed] [Google Scholar]
- Knight DA, Lane CL, Stick SM. Does aberrant activation of the epithelial-mesenchymal trophic unit play a key role in asthma or is it an unimportant sideshow? Curr Opin Pharmacol. 2004;4:251–256. doi: 10.1016/j.coph.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Holgate ST. Epithelial damage and response. Clin Exp Allergy. 2000;30(Suppl 1):37–41. doi: 10.1046/j.1365-2222.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Bottinger EP, Letterio JJ, Roberts AB. Biology of TGF-β in knockout and transgenic mouse models. Kidney Int. 1997;51:1355–1360. doi: 10.1038/ki.1997.185. [DOI] [PubMed] [Google Scholar]
- Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T. Early-onset multifocal inflammation in the transforming growth factor β1-null mouse is lymphocyte mediated. Proc Natl Acad Sci USA. 1995;92:12215–12219. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Yokosaki Y, Ferrando R, Balmes J, Sheppard D. Differential regulation of airway epithelial integrins by growth factors. Am J Respir Cell Mol Biol. 1996;15:664–672. doi: 10.1165/ajrcmb.15.5.8918373. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF-β activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Rifkin DB. Latent transforming growth factor-β (TGF-β) binding proteins: orchestrators of TGF-β availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin αvβ6 binds and activates latent TGF β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludbrook SB, Barry ST, Delves CJ, Horgan CM. The integrin αvβ3 is a receptor for the latency-associated peptides of transforming growth factors β1 and β3. Biochem J. 2003;369:311–318. doi: 10.1042/BJ20020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Munger JS, Steadele M, Busald C, Tellier M, Schnapp LM. Integrin α8β1 mediates adhesion to LAP-TGF-β1. J Cell Sci. 2002;115:4641–4648. doi: 10.1242/jcs.00145. [DOI] [PubMed] [Google Scholar]
- Munger JS, Harpel JG, Giancotti FG, Rifkin DB. Interactions between growth factors and integrins: latent forms of transforming growth factor-β are ligands for the integrin αvβ1. Mol Biol Cell. 1998;9:2627–2638. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier S, Mu D, O’Connell D, Boylen K, Travis W, Liu W, Broaddus VC, Nishimura SL. The integrin αvβ8 negatively regulates epithelial cell growth. Cancer Res. 2000;60:7084–7093. [PubMed] [Google Scholar]
- Fjellbirkeland L, Cambier S, Broaddus VC, Hill A, Brunetta P, Dolganov G, Jablons D, Nishimura SL. Integrin αvβ8-mediated activation of transforming growth factor-β inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol. 2003;163:533–542. doi: 10.1016/s0002-9440(10)63681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema JR, Mariassy A, George JS, Hyde DM, Plopper CG. Epithelial cells of the conducting airways: a species comparison. Farmer SG, Hay DWP, editors. Marcel Decker; New York: The Airway Epithelium. 1991:pp 3–39. [Google Scholar]
- Finkbeiner WE. Respiratory cell culture. Crystal RG, editor. Lippincott-Raven Publishers,; Philadelphia: The Lung: Scientific Foundations. 1997:pp 415–433. [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of αv integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- Weinacker A, Chen A, Agrez M, Cone RI, Nishimura S, Wayner E, Pytela R, Sheppard D. Role of the integrin αvβ6 in cell attachment to fibronectin Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994;269:6940–6948. [PubMed] [Google Scholar]
- Yamagata H, Matsuzaki K, Mori S, Yoshida K, Tahashi Y, Furukawa F, Sekimoto G, Watanabe T, Uemura Y, Sakaida N, Yoshioka K, Kamiyama Y, Seki T, Okazaki K. Acceleration of Smad2 and Smad3 phosphorylation via c-Jun NH(2)-terminal kinase during human colorectal carcinogenesis. Cancer Res. 2005;65:157–165. [PubMed] [Google Scholar]
- Montesano R, Mouron P, Amherdt M, Orci L. Collagen matrix promotes reorganization of pancreatic endocrine cell monolayers into islet-like organoids. J Cell Biol. 1983;97:935–939. doi: 10.1083/jcb.97.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura SL, Sheppard D, Pytela R. Integrin αvβ8 Interaction with vitronectin and functional divergence of the β8 cytoplasmic domain. J Biol Chem. 1994;269:28708–28715. [PubMed] [Google Scholar]
- Plantier L, Marchand-Adam S, Marchal-Somme J, Leseche G, Fournier M, Dehoux M, Aubier M, Crestani B. Defect of hepatocyte growth factor production by fibroblasts in human pulmonary emphysema. Am J Physiol. 2005;288:L641–L647. doi: 10.1152/ajplung.00249.2004. [DOI] [PubMed] [Google Scholar]
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-β induction of α-smooth muscle actin in fibroblasts. Am J Pathol. 1999;154:871–882. doi: 10.1016/s0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin-mediated activation of latent transforming growth factor β1 in autocrine transforming growth factor β signaling in systemic sclerosis fibroblasts. Arthritis Rheum. 2005;52:2897–2905. doi: 10.1002/art.21246. [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J Immunol. 2005;175:7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin αvβ8-mediated activation of transforming growth factor-β by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez BG, Matias-Roman S, Albar JP, Sanchez-Madrid F, Arroyo AG. Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J Biol Chem. 2001;276:37491–37500. doi: 10.1074/jbc.M104094200. [DOI] [PubMed] [Google Scholar]
- Zucker S, Hymowitz M, Conner CE, DiYanni EA, Cao J. Rapid trafficking of membrane type 1-matrix metalloproteinase to the cell surface regulates progelatinase a activation. Lab Invest. 2002;82:1673–1684. doi: 10.1097/01.lab.0000041713.74852.2a. [DOI] [PubMed] [Google Scholar]
- Ohmichi H, Koshimizu U, Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) acts as a mesenchyme-derived morphogenic factor during fetal lung development. Development. 1998;125:1315–1324. doi: 10.1242/dev.125.7.1315. [DOI] [PubMed] [Google Scholar]
- Taipale J, Keski-Oja J. Hepatocyte growth factor releases epithelial and endothelial cells from growth arrest induced by transforming growth factor-β1. J Biol Chem. 1996;271:4342–4348. doi: 10.1074/jbc.271.8.4342. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Tanigaki T, Heimer D, Wang W, Ross WG, Murphy GA, Sakai A, Sussman HH, Vu TH, Raffin TA. TGF-β1 causes increased endothelial ICAM-1 expression and lung injury. J Appl Physiol. 1994;77:1281–1287. doi: 10.1152/jappl.1994.77.3.1281. [DOI] [PubMed] [Google Scholar]
- LaMarre J, Hayes MA, Wollenberg GK, Hussaini I, Hall SW, Gonias SL. An α2-macroglobulin receptor-dependent mechanism for the plasma clearance of transforming growth factor-β1 in mice. J Clin Invest. 1991;87:39–44. doi: 10.1172/JCI114998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn MB. Recombinant latent transforming growth factor β1 has a longer plasma half-life in rats than active transforming growth factor β1, and a different tissue distribution. J Clin Invest. 1990;86:1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders KC, Kim ES, Roberts AB. Immunohistochemical expression of Smads 1–6 in the 15-day gestation mouse embryo: signaling by BMPs and TGF-β. Dev Dyn. 2001;220:141–154. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1096>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Zhao J, Bu D, Lee M, Slavkin HC, Hall FL, Warburton D. Abrogation of transforming growth factor-β type II receptor stimulates embryonic mouse lung branching morphogenesis in culture. Dev Biol. 1996;180:242–257. doi: 10.1006/dbio.1996.0298. [DOI] [PubMed] [Google Scholar]
- Schmid P, Cox D, Bilbe G, Maier R, McMaster GK. Differential expression of TGF β1, β2 and β3 genes during mouse embryogenesis. Development. 1991;111:117–130. doi: 10.1242/dev.111.1.117. [DOI] [PubMed] [Google Scholar]
- Millan FA, Denhez F, Kondaiah P, Akhurst RJ. Embryonic gene expression patterns of TGF β1, β2 and β3 suggest different developmental functions in vivo. Development. 1991;111:131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- Lehti K, Allen E, Birkedal-Hansen H, Holmbeck K, Miyake Y, Chun TH, Weiss SJ. An MT1-MMP-PDGF receptor-β axis regulates mural cell investment of the microvasculature. Genes Dev. 2005;19:979–991. doi: 10.1101/gad.1294605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Park SJ, Yoon SY, Yun CH, Chung AS. Sustained production of H2O2 activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-kappa B pathway. J Biol Chem. 2002;277:30271–30282. doi: 10.1074/jbc.M202647200. [DOI] [PubMed] [Google Scholar]