Abstract
Excessive wall stretch of distensible hollow organs in cardiovascular and urinary systems can activate matrix metalloproteinases (MMPs), thereby releasing matrix neoepitopes and growth factor ligands, leading to ERK1/2 activation. However, the role of MMPs in mechanotransduction of ERK1/2 signaling in the bladder is unknown. We examined bladders undergoing sustained distension over time, which provides a novel platform for smooth muscle mechanotransduction studies. Bladder distension ex vivo caused increased proliferation and MMP activity. Conditioned medium from distended compared with undistended bladders induced proliferation in bladder smooth muscle cells (BSMCs). When conditioned medium from distended bladders was used to proteolyze collagen type I matrices, matrices augmented BSMC proliferation, which was inhibited if bladders were distended in presence of broad-spectrum MMP inhibitors. Distension of ex vivo bladders also induced ERK1/2 phosphorylation in situ, which was dependent on MMP activity in the intact bladder. Similarly, stretching BSMCs in vitro induced increases in ERK1/2 activation and ERK1/2-dependent proliferation under discrete mechanical conditions, and distension conditioned medium itself induced MMP-dependent ERK1/2 activation in BSMCs. Overall, stretch-induced proliferation and ERK1/2 signaling in bladder tissue and BSMCs likely depend on secreted MMP activity. Identification of intermediaries between MMPs and ERK1/2 may elaborate novel mechanisms underlying mechanotransduction in bladder smooth muscle.
The mechanical design of distensible hollow organs such as the heart, vessels, and urinary bladder allows for stretching the wall of the organ to permit filling and contraction to facilitate accommodation and propulsion of fluid. Muscle cells in these organs are responsive to stretch in their microenvironment. Mechanotransduction in the heart and vessels involves growth factor release and activation of a number of signaling cascades. In particular, stretch activation of the mitogen-activated protein (MAP) kinase (MAPK) family can modulate cell proliferation, apoptosis, integrity of the extracellular matrix (ECM), muscle wall development, and homeostasis. As in the heart, partial obstruction and distension models that create excessive bladder wall stretch are used to mimic clinical pathological conditions. These models have shown increased muscle growth, accumulation of ECM structural components such as fibrillar collagen types I and III,1 and increased matrix metalloproteinase (MMP) activity.2
Appreciation of MMP function has evolved significantly since their description as interstitial collagenases. MMPs exert pleiotropic influences by virtue of their ability to cleave diverse substrates, including not only structural ECM proteins but also growth-factor receptors and precursors, receptor tyrosine kinases, cell-adhesion molecules, and other proteinases. In response to wall tension in hollow organs, remodeling of the ECM correlates with alterations in levels and activities of the matrix metalloproteinases and in tissue inhibitors of metalloproteinases (TIMPs). The fibroproliferative response to stretch may involve dysregulation of MMPs. In the human heart, progressive increases in tissue levels of MMP-1, -2, -3, -9, -13, and -14 and net gelatinolytic activity are linked to worsening clinical left ventricular failure.3 During heart failure, the activity of TIMP-1, -2, and -4 appears inadequate to inhibit the transition from compensation to decompensation.3 Conversely, a preponderance of MMP-2 activity characterizes the progression to excess fibrosis, wall stiffness, and ventricular failure in spontaneously hypertensive rats.4 Similarly, in adult and fetal bladder models, obstructive lesions are also characterized by wall hypertrophy associated with augmented tissue levels of MMP-2 and -95 or by an increased ratio of MMP-1 to TIMP-2 activity.6
Although the mechanisms that mediate fibroproliferative responses through MMPs include direct proteolysis of ECM proteins, their precise role in bladder tissue hypertrophy is unknown but may be associated with specific signaling cascades. In a fibrosis model, reduction in osteoarthritic lesions through inhibition of extracellular-signal regulated kinases 1and 2 (ERK1/2) MAPK activity was accompanied by reduced MMP-1 activity in chondrocytes.7 In the heart, protein glycation products that promote fibrosis activate p38, ERK1/2, and c-Jun N-terminal kinase (JNK) MAP kinases and concurrently stimulate the activity of gelatinases MMP-2, -9, and -13.8 More specifically, MAPK cascades can regulate MMP activity in response to mechanical strain. In a study of MMP-2 activity in endothelial cells, ERK1/2 or p38 inhibition results in reduction of strain-induced MMP-2 expression and activity.9 In cultured osteoblasts, activation of ERK1/2 but not p38 or JNK increased MMP-13 transcription and zymographic activity when subjected to biaxial strain on type I collagen.10 Cyclical strain of bladder smooth muscle cells (BSMCs) on type I collagen has been shown to increase activation of MAP kinases11 and enhance transcription of MMP-1.12 Although stretch appears to alter metalloproteinase activities via activation of discrete kinase cascades, how ECM remodeling and MMP activity participate in MAPK signaling remains to be fully explored.
We recently observed that a heat-denatured type I collagen matrix creates a powerful mitogenic milieu for growth of primary culture BSMCs.13 Moreover, the BSMC growth response on denatured collagen is concordant with intact ERK1/2 MAP kinase activation.13 The present study shows that ERK1/2s are activated by stretch under discrete mechanical and ECM conditions, both in distended whole bladder ex vivo and in BSMCs stretched on deformable membranes. Although this appears contradictory to previous work,11 the particular conditions in each model system clearly explain the results and shed new light on how different signaling cascades integrate matrix and mechanical stimuli. Moreover, the induction of ERK1/2 by distension of the intact bladder implies a physiological relevance for the present in vitro and ex vivo signaling findings. Furthermore, ERK1/2 activation appears to be dependent on active MMPs secreted by the bladder during distension. The MMPs in the conditioned medium of distended bladders have the ability to modify the matrix and to alter cell signaling more directly, leading to proliferative responses in BSMCs. Although previous reports suggest that MMP activation is dependent on activity of specific kinase pathways, the present novel findings suggest that this regulation may be bi-directional, whereby MMPs are themselves sufficiently able to effect rapid signaling. Finally, the mechanical characteristics of the bladder differ greatly from the rapid cycling of the heart and vasculature. As such, the bladder smooth muscle compartment provides a novel platform for potentially informative smooth muscle mechanotrans-duction studies. Finally, we propose a model whereby distension-stimulated MMP activity influences ERK1/2-dependent BSMC growth.
Materials and Methods
Ex Vivo Bladder Culture and Distension
Throughout the text, we refer to mechanical stimulation of the ex vivo whole bladder as “distension” and of cultured BSMCs (see below) as “stretch.” Bladders were stretch-injured by distension with culture medium as previously reported1 with some modifications. Briefly, after anesthesia, bladders of female Sprague-Dawley rats (100–120 g) were surgically exposed, and the ureters were ligated. Bladders were then catheterized per urethra and removed after firmly securing the catheter in the urethra with sutures (Figure 1A). For blocking, 1-hour pre-incubations were performed using the relevant inhibitors (20 μmol/L doxycycline, GM6001) or vehicle (ethanol or DMSO). After blocking, bladders were distended by filling the bladder with culture minimal essential medium (MEM; MultiCell Technologies, Lincoln, RI) (with 1 μg/ml insulin, 5 μg/ml transferrin, and 0.1 μg/ml biotin) to 40 cm hydrostatic pressure using water manometry at 37°C/95%O2/5%CO2 or were undistended. For inhibitor samples, medium used for the distension in vesico also contained inhibitors. Sham controls constituted ligated, catheterized, but undistended bladders.
Figure 1-6940.
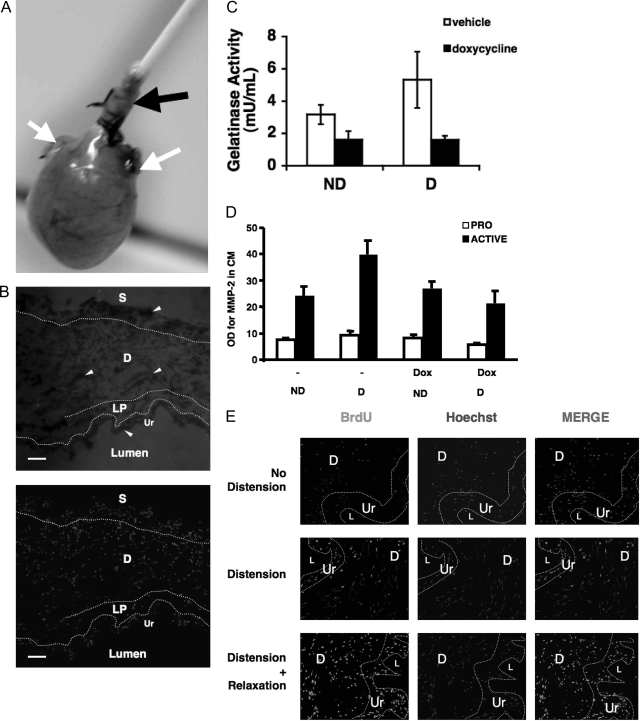
A:Ex vivo model of bladder distension. Bladders of female Sprague-Dawley rats (100–120 g) were surgically exposed, and ureters were ligated. Bladders were catheterized per urethra and removed with sutures that firmly held the catheters in the urethra. For blocking, 1-hour pre-incubations were performed in medium containing the relevant inhibitors. After blocking, bladders were distended by filling the bladder with culture medium to 40 cm of hydrostatic pressure using water manometry or were not distended. Sham controls included ligated, catheterized, but uninflated bladders. White arrows indicate the ligated ureters. Black arrows point to the catheterized urethra, which has been sutured to hold the catheter in place during distension. B: Bladder hyperdistension stimulates in situ gelatinase activity. Ex vivo bladders were examined by in situ zymography. FITC-gelatin layered onto cryosections of ex vivo bladders is digested by MMP activity. Nonfluorescent dark zones (arrowheads) represent digestion of the FITC-gelatin, localizing regions of gelatinase A/B activity. Bottom panel: Hoechst stain of serial section to show nuclei. Ur, urothelium; LP, lamina propria; D, detrusor; S, serosa. Original magnification, ×125. C: Net gelatinase activity of bladders is increased during distension ex vivo. Gelatinases in the conditioned medium released the quenched activity of FITC in the DQ-gelatin. Doxycycline inhibited the increase in gelatinase A/B activity from distended bladders, P = 0.0126; n = 3. D: Secretion of active MMP-2 is increased in distended bladders and inhibited by doxycycline. Pro- and active-MMP-2 are analyzed by Western blotting in the conditioned medium of distended (D) and nondistended (ND) bladders +/− Doxycycline (Dox) pretreatment for 1 hour. E: Bladder distension stimulates in situ BSMC proliferation. BSMC proliferation was assessed by BrdU incorporation and detected using anti-BrdU and anti-mouse-Alexa 488 antibody on cryosections. Bladders were distended by hydrodistension in serum-free medium with BrdU or were not distended. Distended bladders showed increased BrdU incorporation compared with undistended. Ur, uroepithelium; D, detrusor smooth muscle layer; L, lumen. n = 3. Original magnification, ×125.
BSMC Culture
As reported by Herz and colleagues,13,14 bladders from neonatal Sprague-Dawley rats were minced into 0.5-mm2 explants and cultured in MEM containing 10% fetal calf serum (Sigma, St. Louis, MO) and antibiotic/antimycotic (MultiCell) at 37°C in 95%O2/5%CO2 on 100-mm tissue culture-coated dishes. Cultures reached confluency in 2 to 3 weeks. Medium with 10% fetal calf serum (FCS) was used for regular maintenance. Cells between passages 2 and 5 were used for experiments. For plating and passaging, BSMCs were incubated in 0.25% trypsin and 0.53 mmol/L ethylenediamine tetraacetic acid (MultiCell), washed, and resuspended at 5 × 104 cells/ml. For proliferation assays, 2 × 104 BSMCs/well were seeded onto 6-well plates or BioFlex plates. For all experiments, cells were serum-deprived for 2 days before treatment. Staining with α-SMA antibody (Sigma) was performed periodically to assure purity of the cell populations.
Cell Stretching
Experiments were performed on a cell stretching device (Flexcell 2000 and 4000; Flexcell International Corporation, Hillsborough, NC) as previously described.1 BSMCs (1 × 104 or 5 × 104/ml) were plated onto Flexcell I or Bioflex stretch plates and incubated overnight for 2 days. Low passage number (2 to 5) BSMCs were made quiescent by culture in MEM (0.5% FCS) for 48 hours. Cells were stretched at frequencies, amplitudes, and durations indicated in the figure legends.
5-Bromo-2′-Deoxyuridine (BrdU) Incorporation
Serial cryosections (5 μm) from ex vivo bladders were assessed for proliferation by localizing incorporated BrdU (Roche, Mannheim, Germany). Bladder stretch was performed by hydrodistension of bladders in medium containing 10 μmol/L BrdU. After 23 hours of distension, bladders were washed in phosphate-buffered saline (PBS), incubated 60 minutes in BrdU-free medium, embedded in OCT, and then flash-frozen in liquid nitrogen. Negative control tissue was not treated with BrdU. Cryosections (5 μm) were treated with 2 mol/L HCl followed by 0.1 mol/L borate. After blocking, anti-BrdU antibody (5 μg/ml, Sigma) was applied overnight at 4°C, washed, and subsequently exposed to anti-mouse Alexa-488 antibody (Molecular Probes, Eugene, OR). Counterstaining was performed with Hoechst dye. A Zeiss Deconvolution Axiovert 200M microscope (Jena, Germany) equipped with a mercury lamp was used to visualize fluorescence at 488 nm, and representative images were recorded with OpenLab 4.0.3 software.
In Situ Zymography
In situ zymography was performed as by Galis et al.15 Cryosections were layered with 0.1 mg/ml fluorescein isothiocyanate (FITC)-gelatin (Molecular Probes) in developing solution (50 mmol/L Tris-Base, 40 mmol/L HCl, 1 mmol/L CaCl2, 50 μmol/L phenylmethylsulfonyl fluoride, and 0.05% [w/v] Brij35) with or without 20 μmol/L 1,10-phenanthroline as a negative control. Sections were incubated at 37°C for 48 hours in a humidified chamber. MMP activity caused digestion of the FITC-gelatin, which was visualized as dark zones without fluorescence, using deconvolution microscopy as in BrdU staining.
Western Blotting
Protein extraction and blotting were performed as reported by Herz et al13 with modifications. Whole-bladder lysates were extracted by crushing under N(2)liq and then ground with a polytron homogenizer in lysis buffer (20 mmol/L Tris, 20 mmol/L β-glycerophosphate, 150 mmol/L NaCl, 3 mmol/L ethylenediamine tetraacetic acid, 3 mmol/L EGTA, 1 mmol/L Na3VO4, 0.5% Nonidet P-40, 1 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 4 μg/ml aprotinin, and 1 μg/ml pepstatin A). The following primary antibodies were used: phospho-ERK1/2 antibodies against phosphorylated p44/ERK-1 and p42/ERK-2 (9106; Cell Signaling, Beverly, MA), total ERK1/2 antibody (9102; Cell Signaling), actin (conserved c-terminal region) antibody (Sigma), and MMP-2 antibody (Chemicon, Temecula, CA). Total protein lysates were initially probed for phospho-ERK1/2 and reprobed for total ERK1/2 and actin after stripping blots in Tris-sodium dodecyl sulfate-β-mercaptoethanol. Normalization of phospho-ERK1/2 to actin or total ERK1/2 (Sigma) was performed by densitometry of protein bands, using a BioRad Gel-Doc system and the BioRad MultiAnalyst (1.0) program (BioRad, Hercules, CA).
Immunostaining
Bladders were washed in PBS and passed through isotonic sucrose (0.25 mol/L) and then sucrose-OCT before embedding in OCT and flash freezing. Cryosections (5 μm) were fixed in formalin, washed in PBS, permeabilized in 0.1% Triton X-100, and washed in Tris-buffered saline-Triton X-100. After blocking with 5% goat serum, cells were stained with anti-phospho-ERK1/217 (Cell Signaling), anti-ERK1/2 (rabbit; Sigma), anti-mouse Alexa-488 and anti-rabbit Cy3 (both Molecular Probes) and counterstained in Hoechst dye (Sigma). A Zeiss Deconvolution Axiovert 200M microscope equipped with a mercury lamp was used to visualize epifluorescence at 488 and 565 nm, and representative images were recorded with OpenLab 4.0.3 software.
Thymidine Incorporation
Proliferation was assessed and analyzed as previously described,13,16 by incorporating [3H]thymidine into the DNA of dividing smooth muscle cells. MEK inhibitor (25 μmol/L PD98059) was added 30 minutes before beginning the experiments (stretching or addition of condition medium).
Collagen Gels and Proteolysis of Matrix
Substrates were prepared based on our previous report.13 Briefly, normal intact type I collagen gels (native collagen) were prepared using neutralized collagen (2.48 mg/ml) prepared from Vitrogen (Cohesion Technologies, Inc., Palo Alto, CA). For proteolysis of type I collagen, gels were treated with conditioned medium from distended and undistended bladders or FCS (proteolyzed collagen in Figure 3). Briefly, the type I collagen gels were incubated overnight at 37°C with undiluted conditioned medium from undistended bladders or bladders previously distended in 5 ml of culture medium +/−MMP inhibitors. Remodeled gels were thoroughly washed three times with serum-free medium to remove any residual MMP activity. Negative controls included treatment of gels with serum-free medium. BSMC (passages 2 to 4) proliferation on collagen substrates was assayed for 24 hours in [3H]thymidine-containing medium, as by Herz et al.13 BSMCs were plated at 50% confluency initially in 2% heat-treated FCS and then washed three times before incubation in 0%FCS MEM containing 2 μCi [3H]thymidine/ml. For the cell-counting experiment (Figure 3), quiescent BSMCs were allowed to attach to native collagen or proteolyzed collagen gels for 2 hours and then PD98059 or vehicle was added. After additional incubation on gels for 22 hours, BSMCs excluding trypan blue were counted in 10 fields at ×10 power.
Figure 3-6940.
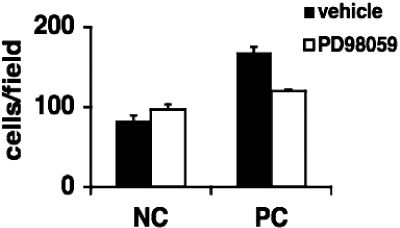
Proliferation of BSMCs on proteolyzed matrix is ERK1/2 dependent. Native type I collagen gels (NC) were treated with MEM (NC) or proteolyzed with FCS (PC) overnight and then washed extensively in MEM. BSMCs (n = 3) were plated on matrices. After 24 hours of culture, trypan blue-excluding cells were counted. Proliferation was induced by FCS-proteolyzed gels and was blocked by addition of PD98059.
Gelatinase Assays
Net gelatinase activity was assayed using the EnzCheck gelatinase assay kit (Molecular Probes). DQ-gelatin fluorescein conjugate (0.1 mg/ml; Molecular Probes) was incubated in Tris buffer (50 mmol/L, pH 7.6) with conditioned medium for 48 hours; gelatinases release the quenched activity of FITC from the FITC-gelatin. Released FITC-gelatin was measured on a fluorescent microplate reader, with absorption at 495 nm and emission at 525 nm. Type IV collagen was serially diluted and added to the DQ-gelatin to create a standard curve. Negative controls were performed with 20 μmol/L 1,10-phenanthroline to inhibit collagenases/gelatinase activity. Controls for background activity used nonconditioned medium.
Statistical Analysis
Differences between groups were evaluated by analysis of variance and post hoc Student’s t-test with commercially available statistical software. A P value of <0.05 was considered statistically significant. For Western blots, data are representative of n = 3.
Results
Distension of Intact Bladder Induces MMP Activity and Proliferation
Previous studies have described increased gelatinase activity in the bladders of partially obstructed animals and some models of stretched BSMCs.5 Because mechanically induced ECM perturbations and remodeling in vivo involve MMPs, we wished to assess whether mechanical distension per se in the intact ex vivo bladder increases MMP activity.
In situ zymography revealed MMP activity in the muscle and urothelial compartments of the bladder wall in response to 24 hours of distension (Figure 1B) as dark zones where the FITC-gelatin has been proteolyzed by MMPs. We also quantitatively evaluated gelatinase activity of conditioned medium from bladders distended for 15 minutes by using DQ-gelatin, a heavily fluoresceinated form of FITC-gelatin, the activity of which is quenched unless digested by gelatinases. After bladder distension, gelatinase activity in bladder conditioned medium was increased (Figure 1C). Pre-incubation of the bladder with doxycycline, a general inhibitor of MMPs, including principal gelatinases MMP-2 and -9, significantly reduced stretch-induced conditioned medium gelatinase activity. We also found that the level of secreted active MMP-2 by Western blotting was increased in distended bladders and was decreased by MMP inhibition (Figure 1D), suggesting that the increased gelatinase activity was partly due to increased secretion of MMPs into the conditioned medium.
We also wished to see whether proliferation in ex vivo bladders was increased during isolated stretch injury in the bladder. Bladders were distended for 0 or 15 minutes or 24 hours under 40 cm hydrostatic pressure and then relaxed to a total of 24 hours. In situ localization of proliferation by detection of BrdU incorporation showed greater amounts of BrdU staining in distended (either 15-minute or 24-hour stretch) when compared with undistended bladders (Figure 1E).
Distension of Intact Bladder Induces Secretion of MMP-Dependent Factors Mitogenic to BSMCs
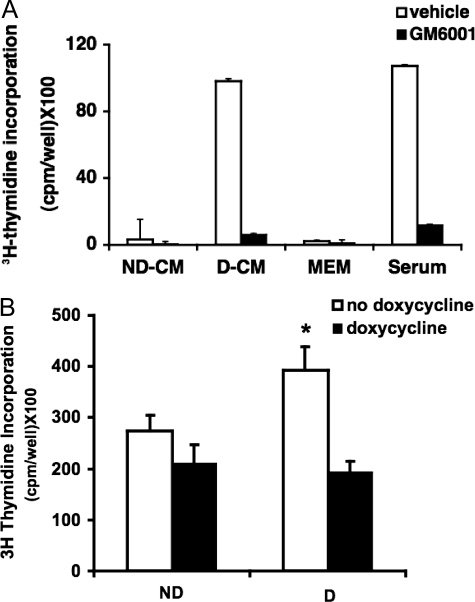
We were interested in the role of the secreted gelatinase activity found in distended bladders. To probe the physiological nature of the BSMC mitogenic response to proteolytically remodeled matrix, we assessed BSMC growth on type I collagen matrices subjected to proteolysis rather than heat denaturation as we reported previously.13 When collagen matrices were pre-incubated in conditioned medium from whole intact bladders that had been distended ex vivo (versus undistended bladders), subsequently seeded BSMCs again showed significant proliferation (Figure 2A). When the broad-spectrum MMP inhibitor GM-6001 was added to the matrix pre-incubation step, the subsequent BSMC growth advantage was abolished (Figure 2A). As a positive control, BSMCs cultured on type I collagen that had been previously pre-incubated with fetal calf serum, which is rich in endogenous metalloproteinase activity, showed significantly accelerated growth (Figure 2A). That this positive control response was dependent on MMP activity was demonstrated by pre-inhibition of MMP activity with GM6001 as above. The growth response was also lost if the conditioned medium was derived from distended bladders that had been pretreated with GM6001. Thus, BSMC proliferation appears to be linked to MMP activity stimulated by bladder wall distension.
Figure 2-6940.
Bioactivity of conditioned medium (CM) from ex vivo distended bladders is dependent on MMP activity. A: BSMC proliferation is induced by CM-remodeled matrix and is blocked when MMPs are inhibited with GM6001. CM from bladders distended (D-CM) or not (ND-CM) for 24 hours were allowed to proteolyze collagen type I gels in the presence or absence of GM6001. Proliferation was assayed by thymidine incorporation of BSMCs incubated on proteolyzed matrices for 24 hours. FCS and MEM were used as positive and negative controls, respectively; n = 3. B: Bladders were pre-incubated in the MMP inhibitor doxycycline, or vehicle, for 1 hour and then distended for 15 minutes or undistended. BSMCs were incubated in bladder CM plus [3H]thymidine for 72 hours to assess proliferative responses. Inhibition of MMP activity before bladder distension abolished increased proliferative responses of BSMCs; n = 3. *P < 0.05.
To examine whether the BSMC response is due to conditioned medium containing mitogenic factors in the absence of a defined ECM, BSMCs were incubated with conditioned medium from distended bladders with or without MMP inhibition. Conditioned medium from distended bladders increased MMP-dependent proliferation of BSMCs. In Figure 2, A and B, the MMP inhibitors worked well, with GM6001 showing a dramatic inhibition of the proliferative response (Figure 2A). After collection of conditioned medium in Figure 2A, inhibition of MMPs was achieved by exogenous addition of inhibitors that act on formerly secreted factors in the bladder conditioned medium. In contrast, in Figure 2B, MMP inhibitors were included in the medium during distension of the bladders. Thus, both MMP inhibition during the secretion phase (Figure 2B) or afterward (Figure 2A) prevented proliferation of BSMCs in response to conditioned medium itself, as well as to conditioned medium-treated matrix.
Proteolyzed Matrix Induces ERK1/2-Dependent Proliferation
Our previous work examined ERK1/2-dependent proliferation in response to heat-denatured matrices.13 We wished to determine whether ERK1/2 also plays a similar role in the growth response to a proteolyzed matrix as well. FCS-proteolyzed collagen type I matrices induced a proliferative response in BSMCs compared with native collagen (Figure 3). This proliferative response was inhibited using the MEK inhibitor PD98059, which acts directly upstream of ERK1/2.
Mechanical Stretch in the Intact Bladder and in Bladder Smooth Muscle Cells (SMCs) in Vitro Induces ERK1/2 Signaling
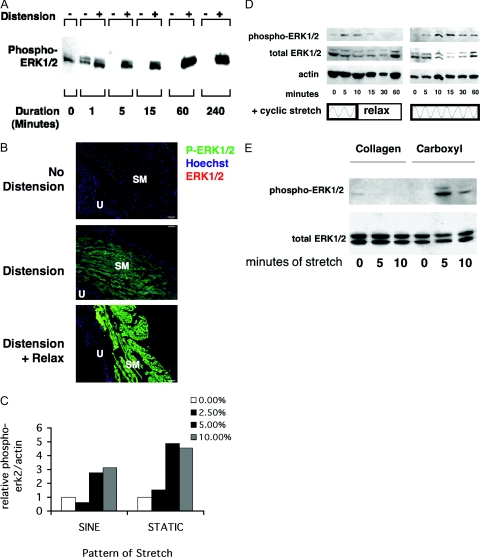
Although we have established a role here and elsewhere13 for ERK1/2 in response to proteolyzed matrix as well as a role for proteolytic enzymes in the response to bladder distension, the relationship between bladder distension and ERK1/2 activation is unknown. ERK1/2 activation was assessed by Western blotting and immunofluorescence staining for dually phosphorylated ERK1/2. In whole bladder ex vivo, distension rapidly induced phosphorylation of ERK1/2 (Figure 4A). A small amount of ERK1/2 was phosphorylated in the undistended bladders at 1 minute, likely due to the manipulation of the bladder during harvesting. However, ERK1/2 activation remained elevated until 24 hours, as revealed by immunostaining (Figure 4B). Immunostaining after 24 hours of distension as well as distension followed by a period of relaxation (deflation) induced considerable ERK1/2 activation, predominantly in the smooth muscle compartment. In contrast, total ERK1/2 expression (Cy3 channel) assessed by an antibody to total ERK1/2, was localized to all compartments of the bladder wall, although the intense staining of phospho-ERK1/2 overshadowed the total ERK1/2 staining, in the stretched samples.
Figure 4-6940.
Distension activates ERK1/2 in whole intact bladder as well as BSMCs. A: ERK1/2 activation in whole distended bladder over a time course from 1 to 240 minutes. Western blotting of bladder tissue protein lysates with phospho-ERK1/2 antibody shows increased P-ERK1/2 after distension; n = 3. B: Localization of ERK1/2 phosphorylation was determined by immunostaining using anti-phospho-ERK1/2 and anti-mouse-Alexa-488 (green). Total ERK1/2 was localized using anti-ERK1/2 and anti-rabbit-Cy3 (red). Nuclei were counterstained with Hoechst (blue). Bladders were cultured ex vivo for 24 hours including 0 minutes (n = 3), 10 minutes, or 24 hours of distension (n = 3). The 10-minute distended bladders were relaxed (deflated) for the rest of the 24-hour period (stretch + relax). Phospho-ERK1/2 appears most strongly in the distension plus relax group in the smooth muscle compartment (SM). Total ERK1/2 can be seen best in the urothelium (U) in these merged images, although it appears throughout when seen on the red channel only. Original magnification, ×125. C–E: Stretch parameters alter ERK1/2 activation in vitro. After adherence, BSMCs were serum starved and mechanically stretched on Flexcell plates under various conditions, and their protein was analyzed by Western blotting for phospho- and total ERK1/2 and actin. C: BSMCs were stretched using a cyclic sine pattern or a static pattern at indicated percentages of elongation (n = 3). D: Cells were stretched using a cyclic stretch program (5 seconds on, 5 seconds off) with a sinusoidal pattern on carboxyl-coated plates either transiently (5-minute cyclic stretch + 55-minute relaxation) or continuously (60-minute cyclic stretch) on carboxyl plates (n = 2). E: Cells were stretched on collagen or carboxyl plates with a cyclic stretch pattern as above (n = 3).
Given that in vitro responses can differ from those in whole tissue, we questioned whether BSMCs stretch in vitro also induces ERK1/2 activation. In a previous survey of MAPK signaling elicited during cyclic mechanical stretch of BSMCs, Nguyen et al11 noted that cyclic stretching for 30 minutes or more on native type I collagen substrates in vitro activated MAP kinase-dependent proliferation via p38 and JNK but not ERK1/2. To resolve this apparent inconsistency, we examined whether particular stretch parameters (pattern, amplitude, duration, and matrix) may activate ERK1/2. When BSMCs were stretched in vitro, ERK1/2 MAP kinase was more highly activated in cells stretched statically compared with those stretched cyclically with a sine pattern (Figure 4C). This rapid stretch-induced ERK1/2 activation (within 5 minutes) is consistent with reports in other cell types.18 Because transient and continuous ERK1/2 activation has been found to induce different effects on cell growth,19 we also examined whether transient and continuous stretch differentially activates ERK1/2. Transient stretch resulted in only transient ERK1/2 phosphorylation, whereas continuous stretch led to increased duration and magnitude of activation of ERK1/2 above controls levels, even after 1 hour (Figure 4D). Finally, the ECM effects on ERK1/2 activation were examined. Stretching on collagen type I plates did not show any large increases in phosphorylation of ERK1/2 as observed previously by Nguyen et al,11 whereas BSMCs stretched on carboxyl plates showed prompt ERK1/2 activation within 5 minutes (Figure 4E).
ERK1/2-Dependent Growth Responses
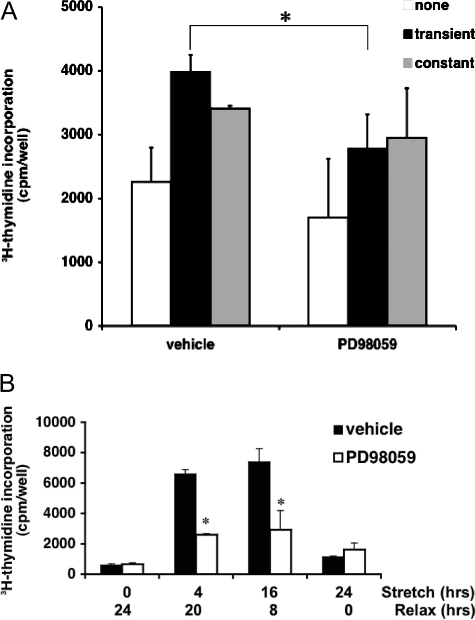
To assess the relevance of stretch-induced ERK1/2 activation to proliferation, BSMCs were cyclically stretched on carboxyl surfaces with or without a nonstretch recovery (relaxation) phase. Over 4 hours—whether cells were stretched for 0.25 hour (15 minutes) followed by a nonstretch recovery phase for 3.75 hours or stretched for the entire 4 hours—thymidine incorporation was increased compared with unstretched controls (Figure 5A), with the relaxation period promoting a higher level of proliferation. Interestingly, in the presence of MEK inhibition upstream of ERK1/2, proliferation in the stretch plus relaxation setting was reduced (Figure 5A), whereas MEK inhibitor did not significantly block proliferation induced by continuous stretch over the entire 4 hours.
Figure 5-6940.
Stretch plus a relaxation period is favored by ERK1/2-mediated BSMC proliferation. BSMCs were stretched and then relaxed over defined short- and long-term time periods, in the presence and absence of the MEK inhibitor PD98059. Proliferation was assayed by tritiated thymidine incorporation. A: Over 4 hours (versus unstretched controls), proliferation was preferentially stimulated (up to ∼75%; white versus black bars; P < 0.05) by stretch + relaxation. Constant stretch produced less proliferation (∼40%; white versus gray bars) (n = 3). B: Over 24 hours (versus unstretched controls), proliferation was enhanced nearly 800% during stretch + relaxation (P < 0.05) versus 24-hour continuous stretch (up to ∼50%) or no stretch (0-hour stretch) controls. Proliferation stimulated by a stretch + relaxation pattern was significantly inhibited by blocking ERK1/2 with PD98059 (*P < 0.05 in A; *P < 0.005 in B). PD98059 did not reduce proliferation in continuously stretched cells; n = 3.
To confirm that this mechanism was not restricted to short-term (4 hour) assays and to further assess whether stretch plus relaxation (versus stretch alone) induced similar responses in a mechanical milieu more appropriate to the bladder, we assessed this phenomenon over more chronic periods of stretching using static (ie, noncyclical as opposed to cyclical) stretch pattern over 24 hours with or without a recovery phase. Again, introduction of a relaxation phase provoked a significant proliferative response not observed in cells stretched without a relaxation phase. Furthermore, inhibiting ERK1/2 during the stretch plus recovery setting significantly reduced proliferation, whereas proliferation was not significantly impaired by ERK1/2 inhibition if cells were stretched over 24 hours without a recovery period (Figure 5B).
Stretch-Induced ERK1/2 Activation Is Dependent on MMP Activity
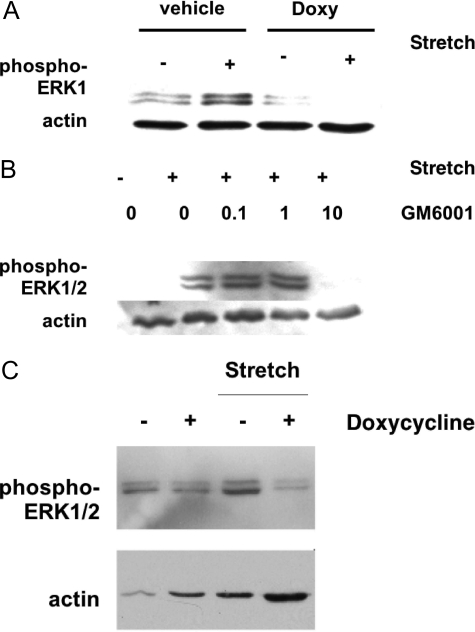
Because the modification of type I collagen by endogenous bladder metalloproteinases produced an appropriate ECM milieu for MMP- and ERK1/2-dependent BSMC proliferation in vitro, we assessed whether MMPs were required for stretch-induced ERK1/2 signaling in the intact bladder. Distension of ex vivo bladders for 15 minutes activated ERK1/2 in situ(Figure 6, A and B). Pre-incubation in medium containing MMP inhibitors doxycycline (Figure 6A) or GM6001 (Figure 6B) followed by 15-minute stretch of the whole bladder was sufficient to abolish distension-induced ERK1/2 phosphorylation. It appears, therefore, that in situ ERK1/2 activation requires intact MMP activity.
Figure 6-6940.
MMPs mediate stretch-induced ERK1/2 activation. A: ERK1/2 activation is seen in response to stretch injury and is blocked by addition of doxycycline. Bladders (n = 3) were stretched 15 minutes ex vivo after a 1-hour incubation in doxycycline (doxy) or vehicle control (EtOH). B: Similarly, GM6001 treatment decreases ERK1/2 activation in response to stretch injury. Bladders (n = 3) were stretched for 15 minutes ex vivo after a 1-hour incubation in increasing concentrations of GM6001 or vehicle control (NS, 0). C: Conditioned medium from distended bladders also induced MMP-dependent ERK1/2 phosphorylation in BSMCs. MMP inhibition by doxycycline prevented ERK1/2 activation in BSMC response to distension-conditioned medium. Bladders (n = 3) were distended ex vivo in the presence of doxycycline or were undistended. Conditioned medium was added in vitro to BSMCs for 5 minutes, and BSMC protein was analyzed by Western blotting.
To further clarify the nature of the requirement for MMP activity in ERK1/2 activation, we next determined whether distension-induced MMPs (Figure 1C) can activate ERK1/2 in BSMCs. We observed that briefly exposing BSMCs in vitro to conditioned medium obtained by bladder distension (5 minutes) was sufficient to achieve increased phosphorylation of ERK1/2 when compared with exposure to conditioned medium from the nondistended bladder (Figure 6C). Pre-incubation of bladders with doxycycline before distension abrogated the ability of conditioned medium to induce activation of phospho-ERK1/2 in BSMCs in vitro.
Gelatinase Activity Is Regulated by ERK1/2 Signaling
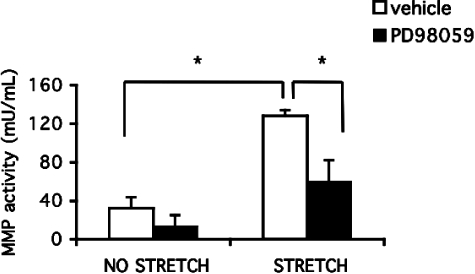
Although MMPs are required for ERK1/2 activation during distension, it is unknown whether stretch-induced ERK1/2 regulates MMPs. Thus, we examined whether stretched BSMCs secrete MMPs in an ERK1/2-dependent manner by adding the MEK inhibitor (PD98059). Conditioned medium from BSMCs stretched for 24 hours were assessed for MMP activity by gelatinase assay. MMP activity was increased in the conditioned medium of stretched versus unstretched BSMCs. Pre-incubation with PD98059 abolished the increase in stretch-induced gelatinase activity in the conditioned medium (Figure 7).
Figure 7-6940.
Stretch-induced gelatinase activity is dependent on ERK1/2 activity. Net MMP activity was assayed using the Enzcheck gelatinase activity kit (Molecular Probes). MMP activity in stretched BSMCs was decreased when ERK1/2 was blocked by an MEK inhibitor. BSMCs (n = 3) were stretched with and without PD98059 (25 μmol/L) for 24 hours or were unstretched, and conditioned medium was collected. Gelatinases in the conditioned medium released the quenched activity of FITC in DQ-gelatin, which was compared with a standard curve (*P < 0.01).
Discussion
Despite recognition and treatment of mechanical factors inciting pressure or distension-induced injury in the early stages of life and even in utero, these measures remain insufficient to mitigate histological changes in the bladder wall and its functional deterioration. Bladder obstruction leading to excessive distension can induce fibroproliferative changes,20 which cannot be completely reversed by decompressive or pharmacological treatment. These alterations include deposition of fibrillar ECM proteins and BSMC hypertrophy and hyperplasia, all of which inevitably lead to maladaptive changes in the mechanical properties of the bladder wall with subsequent high pressure storage, failure to empty, and deterioration of renal function.2,21 Although urological treatment for obstructive bladder lesions has greatly improved, it is largely directed at symptoms; still one-third of obstructed patients will develop some form of renal impairment or failure and another two-thirds will suffer from incontinence, urinary tract infection, and vesico-ureteral reflux due to bladder malfunction.22,23 Understanding the molecular pathways involved in this pathological response may lead to better treatment options.
In BSMCs, mechanotransduction of ERK1/2 activation takes place at both the cellular and extracellular level. At the extracellular level, the present results demonstrate that conditioned medium from distended bladders can induce ERK1/2 activation and proliferation in cultured BSMCs. Furthermore, the mitogenic effects of proteolyzed collagen (Figure 3) are likely due to decryption of epitopes able to induce ERK1/2-dependent proliferation, supporting the widely reported role of ERK1/2 in proliferative responses to matrix alterations.18,24 Although we observe that cryptic epitopes from heat-denatured ma-trices13 and also FCS MMP proteolyzed matrices increase ERK1/2-dependent proliferation of BSMCs cultured on these matrices, we also show that matrices proteolyzed by 24 hour-stretched bladder conditioned medium induce proliferation of BSMCs. In addition, the present data show that distension of the bladder also triggers gelatinolytic activity in situ and that distension-induced ERK1/2 activation in the bladder ex vivo depends on intact MMP activity. Taken together, it appears likely that bladder ERK1/2 mechanotransduction and downstream proliferative responses in BSMCs involve some response to in situ processing of the bladder ECM.
At the cellular level, conditioned medium from bladders stretched for only 15 minutes also had a rapid and direct effect on BSMC proliferation. This reveals that mitogenic factors are rapidly released in the hyperdistended bladder. Furthermore, the observed dependence of ERK1/2 activation on MMP activity in distended bladder tissue suggests that soluble factors present in conditioned medium affect signaling in BSMCs cultured in vitro. Whether these factors are also involved in remodeling of the native ECM or, rather, represent rapid growth factor ligand-receptor interaction is part of our ongoing studies. Nevertheless, ERK1/2 mechanotransduction at 15 minutes of stretch in the whole bladder is dependent on MMPs. Moreover, the conditioned medium from 15-minute stretched bladders causes ERK1/2 activation in cultured BSMCs in an MMP-dependent manner, suggesting that MMPs may be facilitating putative growth factor activation. These rapid aspects of ERK1/2 mechanotransduction may likely involve MMP-induced transactivation of growth factor signaling or modulation of receptors and ligands on the cell surface. It is important to note however, that the MMPs could also be affecting ECM receptors that interact quickly with the matrix. One candidate for this response is αvβ3 integrin processing.25 We have previously reported that signaling through β3 integrin is important for stretch-induced proliferation.16 Also, stretch-sensitive adrenoreceptors can induce autophosphorylation of epidermal growth factor (EGF) receptors in the presence of MMPs.1,26 Growth factor signaling facilitated by MMP-3 and -7 processing of pro-heparin-binding (HB)-EGF27,28 and insulin-like growth factor binding protein29 is of particular interest, given that heparin-binding (HB)-EGF is a potent BSMC mitogen.12 Finally, MMP activity itself may be regulated in part by downstream events after ERK1/2 activation30,31 because ERK1/2 inhibition reduced stretch-induced gelatinase activity in vitro.
Regardless of how this extracellular-mediated stretch response is occurring in the whole bladder, it is evident in vitro that stretch-induced ERK1/2 proliferation depends on the combined parameters of elongation, duration, frequency, and matrix culture surface. Previous methodological approaches to study bladder stretch responses have largely relied on the vascular literature in which SMCs respond physiologically to rapid cyclical stretch. In contrast, although BSMCs can respond to such patterns of stretch, they may have little physiological basis in the bladder itself. Long durations of noncyclic stretch followed by relaxation are fundamentally more relevant to bladder physiology, because the periodic filling and emptying of this organ define more static or sustained patterns of cell stretch. This observation also appears to be relevant in myometrial cells, in which static versus cyclic stretch has been suggested to induce differing downstream signaling effects.32 In prior studies of BSMC stretch, limited parameters (cyclic sinusoidal pattern, 30 minutes minimum duration, and collagen culture surfaces) failed to reveal ERK1/2 signaling.11 Limiting assessment to time points only greater than 30 minutes11 can result in missing relevant signaling activation. In the present study, brief ERK1/2 phosphorylation within 30 minutes of cell stretch can mediate cell proliferation hours later (see below).
Matrix composition also modulated ERK1/2 activation by stretch in BSMCs, a response also seen in vascular SMCs.18 These discrete variations in the stretch milieu have dramatic downstream effects on ERK1/2 activation and ERK1/2-dependent proliferation. Additional non-ERK1/2 signaling pathways, such as p38 and JNK, may be recruited to support proliferation during mechanical stretching under different matrix and mechanical conditions, as previously reported in BSMCs.11 However, the present data confirm that ERK1/2 is activated by stretch in vitro and more importantly in the whole bladder during distension, raising the possibility that the interplay between extracellular distensive forces that occur during obstruction and the subsequent remodeling of the ECM provide at least two discrete levels of control of MAPK signaling in the bladder.
Interestingly, we found that brief distension is sufficient to support subsequent proliferation in the whole bladder despite the withdrawal of the mechanical stimulus. Similarly, in vitro, transient stretch triggers ERK1/2 signaling in BSMCs, leading to proliferation during the subsequent relaxation period. Thus, sustained ERK1/2 signaling may not be required for the downstream proliferative events to occur.33 Rather, transient signaling through ERK1/2 is necessary to initiate the process. Our group has observed in vitro that only 20 minutes of hypoxia-induced ERK1/2 activation is needed to effect BSMC growth 18 hours later.14 Short-term distension of the intact bladder also augmented ERK1/2 activity and proliferation. These findings support the possibility that a brief burst of ERK1/2 activity coupled to a late growth response is a phenotype unique to bladder smooth muscle physiology. Future comparative studies of pulmonary, vascular, and cardiac SMCs will be required to address this question.
Signaling duration may also represent an additional regulator of downstream responses to ERK1/2 activation. For example, it has been shown in PC12 cells that transient stimulation of ERK1/2 results in proliferation, whereas more sustained ERK1/2 activation supports neuronal differentiation.19 In the present study, brief ERK1/2 activation in the context of a single isolated mechanical event is sufficient to trigger late proliferation in this system. However, the duration of bladder ERK1/2 activation is also coupled to the duration of distension (Figure 4A). This observation may also be clinically relevant because the bladder is subject to excessive degree and duration of distension due to overfilling or delayed emptying, respectively. It is therefore possible that prolonged ERK1/2 signaling may occur in vivo, which may incite distinct cellular responses that remain to be elucidated. Nevertheless, to our knowledge, this is the first observation that ERK1/2 activation is coupled to the duration of mechanical strain in smooth muscle cells.
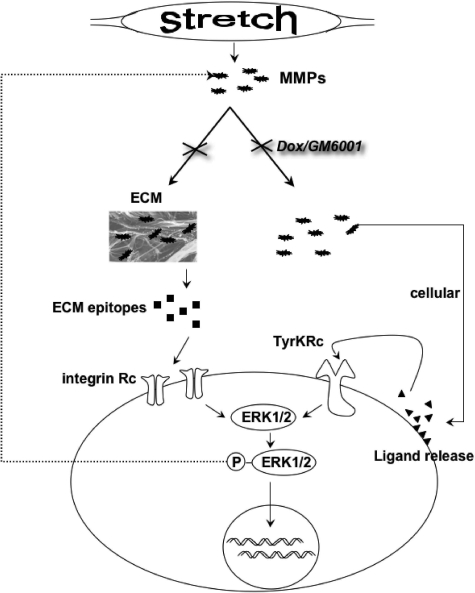
We have shown that MMPs act on BSMC ERK1/2 activation, likely through both paracrine factors and matrix alterations. Our previous work showed that heat-denatured matrix induces ERK1/2 activation, whereas here, distension-activated MMPs from the intact bladder initiate rapid signaling in BSMCs. Further studies assessing the relative interdependency between MMPs and the ECM on ERK1/2 activation and the dynamic reciprocity of these relationships are in progress. Classically, the concept of dynamic reciprocity has suggested that neoepitopes in the ECM can elicit reciprocal cellular responses.34,35 However, because rapid activation of ERK1/2, after just 5 minutes of exposure to conditioned medium from distended bladders, was dependent on MMPs, it raises the possibility that stretch-induced growth responses may also be supported by growth factor-receptor interactions. Thus, MMPs not only alter the ECM but also act in a direct manner on the bladder to induce BSMC ERK1/2 signaling, suggesting a pivotal pathway in stretch-induced proliferation (Figure 8). Future experiments should focus on identification of specific gelatinases, integrin heterodimers, and growth factor receptors and receptor-tyrosine kinases within the unique context of bladder pathomechanics to clarify the intermediates that support MMP-dependent ERK1/2 signaling.
Figure 8-6940.
Schematic of stretch-induced ERK1/2 activation in BSMCs. Both extracellular and cellular mechanisms of mechanotransduction involving MMPs are induced during stretch injury, which may involve integrins and tyrosine kinase receptors, respectively.
Footnotes
Address reprint requests to Darius J. Bägli, MDCM, Division of Urology, 555 University Ave., Toronto, Ontario, Canada, M5G 1X8. E-mail: dbagli@sickkids.ca.
Supported in part by The Canadian Institutes of Health Research (grant 53266 to D.J.B., and training grant in regenerative medicine to K.J.A.), by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grant P50DK065298-01 to D.J.B.), and by an American Urological Association Foundation Fellowship (to A.L.).
References
- Capolicchio G, Aitken KJ, Gu JX, Reddy P, Bagli DJ. Extracellular matrix gene responses in a novel ex vivo model of bladder stretch injury. J Urol. 2001;165:2235–2240. doi: 10.1016/S0022-5347(05)66173-3. [DOI] [PubMed] [Google Scholar]
- Levin R, Levin S, Zhao Y, Buttyan R. Cellular and molecular aspects of bladder hypertrophy. Eur Urol. 1997;32(Suppl 1):15–21. [PubMed] [Google Scholar]
- Polyakova V, Hein S, Kostin S, Ziegelhoeffer T, Schaper J. Matrix metalloproteinases and their tissue inhibitors in pressure-overloaded human myocardium during heart failure progression. J Am Coll Cardiol. 2004;44:1609–1618. doi: 10.1016/j.jacc.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y, Yoneda T, Sasayama S. Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol. 2002;39:1384–1391. doi: 10.1016/s0735-1097(02)01756-4. [DOI] [PubMed] [Google Scholar]
- Sutherland RS, Baskin LS, Elfman F, Hayward SW, Cunha GR. The role of type IV collagenases in rat bladder development and obstruction. Pediatr Res. 1997;41:430–434. doi: 10.1203/00006450-199703000-00021. [DOI] [PubMed] [Google Scholar]
- Peters CA, Freeman MR, Fernandez CA, Shepard J, Wiederschain DG, Moses MA. Dysregulated proteolytic balance as the basis of excess extracellular matrix in fibrotic disease. Am J Physiol. 1997;272:R1960–R1965. doi: 10.1152/ajpregu.1997.272.6.R1960. [DOI] [PubMed] [Google Scholar]
- Pelletier JP, Fernandes JC, Brunet J, Moldovan F, Schrier D, Flory C, Martel-Pelletier J. In vivo selective inhibition of mitogen-activated protein kinase kinase 1/2 in rabbit experimental osteoarthritis is associated with a reduction in the development of structural changes. Arthritis Rheum. 2003;48:1582–1593. doi: 10.1002/art.11014. [DOI] [PubMed] [Google Scholar]
- Daoud S, Schinzel R, Neumann A, Loske C, Fraccarollo D, Diez C, Simm A. Advanced glycation endproducts: activators of cardiac remodeling in primary fibroblasts from adult rat hearts. Mol Med. 2001;7:543–551. [PMC free article] [PubMed] [Google Scholar]
- von Offenberg Sweeney N, Cummins PM, Birney YA, Cullen JP, Redmond EM, Cahill PA. Cyclic strain-mediated regulation of endothelial matrix metalloproteinase-2 expression and activity. Cardiovasc Res. 2004;63:625–634. doi: 10.1016/j.cardiores.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Yang CM, Chien CS, Yao CC, Hsiao LD, Huang YC, Wu CB. Mechanical strain induces collagenase-3 (MMP-13) expression in MC3T3–E1 osteoblastic cells. J Biol Chem. 2004;279:22158–22165. doi: 10.1074/jbc.M401343200. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Adam R, Bride S, Park J, Peters C, Freeman M. Cyclic stretch activates p38 SAPK2-, ErbB2-, and AT1-dependent signaling in bladder smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C1155–C1167. doi: 10.1152/ajpcell.2000.279.4.C1155. [DOI] [PubMed] [Google Scholar]
- Park JM, Adam RM, Peters CA, Guthrie PD, Sun Z, Klagsbrun M, Freeman MR. AP-1 mediates stretch-induced expression of HB-EGF in bladder smooth muscle cells. Am J Physiol. 1999;277:C294–C301. doi: 10.1152/ajpcell.1999.277.2.C294. [DOI] [PubMed] [Google Scholar]
- Herz DB, Aitken K, Bagli DJ. Collagen directly stimulates bladder smooth muscle cell growth in vitro: regulation by extracellular regulated mitogen activated protein kinase. J Urol. 2003;170:2072–2076. doi: 10.1097/01.ju.0000091810.33953.13. [DOI] [PubMed] [Google Scholar]
- Sabha N, Aitken K, Lorenzo A, Szybowska M, Jairath A, Bägli DJ: MMP-7 and EGF receptor mediate hypoxia-induced Erk1/2 MAPK activation and subsequent proliferation in bladder smooth muscle cells. In Vitro Cell Dev Biol Anim 2006, (in press) [DOI] [PubMed] [Google Scholar]
- Galis ZS, Sukhova GK, Libby P. Microscopic localization of active proteases by in situ zymography: detection of matrix metalloproteinase activity in vascular tissue. FASEB J. 1995;9:974–980. doi: 10.1096/fasebj.9.10.7615167. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Aitken KJ, Damdar C, Bolduc S, Bagli DJ. Integrins expressed with bladder extracellular matrix after stretch injury in vivo mediate bladder smooth muscle cell growth in vitro. J Urol. 2003;169:750–755. doi: 10.1097/01.ju.0000051682.61041.a5. [DOI] [PubMed] [Google Scholar]
- Xu H, Goldfarb M. Multiple effector domains within SNT1 coordinate ERK activation and neuronal differentiation of PC12 cells. J Biol Chem. 2001;276:13049–13056. doi: 10.1074/jbc.M009925200. [DOI] [PubMed] [Google Scholar]
- Reusch HP, Chan G, Ives HE, Nemenoff RA. Activation of JNK/SAPK and ERK by mechanical strain in vascular smooth muscle cells depends on extracellular matrix composition. Biochem Biophys Res Commun. 1997;237:239–244. doi: 10.1006/bbrc.1997.7121. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Levin RM, Monson FC, Haugaard N, Buttyan R, Hudson A, Roelofs M, Sartore S, Wein AJ. Genetic and cellular characteristics of bladder outlet obstruction. Urol Clin N Am. 1995;22:263–283. [PubMed] [Google Scholar]
- Jaureguizar E, Lopez-Pereira P, Martinez-Urrutia MJ. The valve bladder: etiology and outcome. Curr Urol Rep. 2002;3:115–120. doi: 10.1007/s11934-002-0021-8. [DOI] [PubMed] [Google Scholar]
- Close CE. Urethral valves and potential for healing in very young patients. Am Urol Assoc Update Ser. 2000;19:106–111. [Google Scholar]
- Mitchell MEaCEC. Early primary valve ablation for posterior urethral valves. Semin Pediatr Surg. 1996;5:66–71. [PubMed] [Google Scholar]
- Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol. 1997;139:279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Jones FS, Zhou B, Rabinovitch M. Induction of vascular smooth muscle cell tenascin-C gene expression by denatured type I collagen is dependent upon a beta3 integrin-mediated mitogen-activated protein kinase pathway and a 122-base pair promoter element. J Cell Sci. 1999;112:435–445. doi: 10.1242/jcs.112.4.435. [DOI] [PubMed] [Google Scholar]
- Miura S, Ohno I, Suzuki J, Suzuki K, Okada S, Okuyama A, Nawata J, Ikeda J, Shirato K. Inhibition of matrix metalloproteinases prevents cardiac hypertrophy induced by beta-adrenergic stimulation in rats. J Cardiovasc Pharmacol. 2003;42:174–181. doi: 10.1097/00005344-200308000-00004. [DOI] [PubMed] [Google Scholar]
- Shah BH, Yesilkaya A, Olivares-Reyes JA, Chen HD, Hunyady L, Catt KJ. Differential pathways of angiotensin II-induced extracellularly regulated kinase 1/2 phosphorylation in specific cell types: role of heparin-binding epidermal growth factor. Mol Endocrinol. 2004;18:2035–2048. doi: 10.1210/me.2003-0476. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997;272:31730–31737. doi: 10.1074/jbc.272.50.31730. [DOI] [PubMed] [Google Scholar]
- Hemers E, Duval C, McCaig C, Handley M, Dockray GJ, Varro A. Insulin-like growth factor binding protein-5 is a target of matrix metalloproteinase-7: implications for epithelial-mesenchymal signaling. Cancer Res. 2005;65:7363–7369. doi: 10.1158/0008-5472.CAN-05-0157. [DOI] [PubMed] [Google Scholar]
- Lakka SS, Jasti SL, Gondi C, Boyd D, Chandrasekar N, Dinh DH, Olivero WC, Gujrati M, Rao JS. Downregulation of MMP-9 in ERK-mutated stable transfectants inhibits glioma invasion in vitro. Oncogene. 2002;21:5601–5608. doi: 10.1038/sj.onc.1205646. [DOI] [PubMed] [Google Scholar]
- Tan X, Egami H, Abe M, Nozawa F, Hirota M, Ogawa M. Involvement of MMP-7 in invasion of pancreatic cancer cells through activation of the EGFR mediated MEK-ERK signal transduction pathway. J Clin Pathol. 2005;58:1242–1248. doi: 10.1136/jcp.2004.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooranna SR, Engineer N, Loudon JA, Terzidou V, Bennett PR, Johnson MR. The mitogen-activated protein kinase dependent expression of prostaglandin H synthase-2 and interleukin-8 messenger ribonucleic acid by myometrial cells: the differential effect of stretch and interleukin-1{beta}. J Clin Endocrinol Metab. 2005;90:3517–3527. doi: 10.1210/jc.2004-1390. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol. 2002;64:755–763. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–262. [PubMed] [Google Scholar]
- Bissell MJ, Barcellos-Hoff MH. The influence of extracellular matrix on gene expression: is structure the message? J Cell Sci Suppl. 1987;8:327–343. doi: 10.1242/jcs.1987.supplement_8.18. [DOI] [PubMed] [Google Scholar]