Abstract
Transient renal ischemia induces both inflammatory and fibrotic processes and is a major cause of acute and chronic renal insufficiency. Study of ischemia-reperfusion injury in gene-targeted mice has identified multiple factors responsible for inflammation, whereas mechanisms underlying fibrosis remain poorly defined. Here we demonstrate by both gene inactivation and target protein blockade that a single chemokine receptor subtype, the fractalkine receptor CX3CR1, is able to reduce both inflammation and fibrosis after ischemia-reperfusion injury in the mouse, leading to partially preserved renal function after injury. The mechanism involves selective effects in the outer medulla, including reduced accumulation of macrophages and reduced expression of the macrophage and platelet-derived fibrogenic protein platelet-derived growth factor-B. CX3CR1 is the first chemokine receptor shown to contribute to fibrogenesis in renal ischemia-reperfusion injury.
Acute ischemia of the kidney, a major cause of renal failure, occurs most commonly in the setting of renal artery stenosis, renal transplantation, and shock because of hemorrhage or sepsis. Even when a kidney that has been rendered ischemic regains normal perfusion, pathological changes may persist and progress to chronic functional insufficiency resulting in end-stage renal disease.1 Although immunosuppressive drugs have been highly successful in preventing acute kidney allograft rejection, they have not had an impact on late graft failure, which may result in part from early ischemic injury.2,3 Late allograft failure afflicts a growing number of patients, fed by the large increase in patients living with end-stage renal disease since the advent of hemodialysis.4–6 Recently, chronic allograft nephropathy, characterized by progressive renal dysfunction, interstitial inflammation and fibrosis, and vascular occlusion, has been identified as the chief cause of late graft failure.7 All other causes of ischemic renal disease are also characterized by inflammation and fibrosis. Rodent models of renal ischemia-reperfusion injury have been developed in an effort to develop insights into pathogenesis at the molecular level. Recent studies using such models have succeeded in delineating many factors that are involved in inflammation8; however, osteopontin is the only molecular determinant of fibrosis identified to date.9
Transforming growth factor (TGF)-β and platelet-derived growth factor (PDGF) are well-characterized factors that promote fibrosis in many diseases and organs, including the kidney.10,11 PDGF, which stimulates fibroblast proliferation and production of extracellular matrix, is actually a family of four molecules, PDGF-A and -B and the newly discovered PDGF-C and -D.12 PDGF-B has been implicated in renal fibrosis based on the effects of direct injection of the factor into rat kidney in vivo.13 Infiltrating inflammatory cells are rich sources of these and potentially other fibrogenic mediators.14 For this reason we and others have focused on molecular mechanisms responsible for inflammatory leukocyte trafficking to the kidney after ischemia-reperfusion injury. Chemokines, a major class of leukocyte chemoattractants, are particularly relevant in this regard and have been implicated in the pathogenesis of numerous fibrotic disorders; however, none has been linked to fibrosis resulting from renal ischemia-reperfusion injury.7
In the present report we focus on the chemokine fractalkine (CX3CL1 in standard nomenclature) and its 7-transmembrane domain G protein-coupled receptor CX3CR1. Fractalkine is the only known member of the CX3C chemokine subclass, one of the four major structural divisions of the chemokine family. In addition to functioning as a leukocyte chemoattractant, fractalkine is unusual in that it is also able to function as an adhesion molecule. CX3CR1 is expressed on NK cells, monocytes, T cells, mast cells, and platelets.15–18 Recently, patients with systemic sclerosis were reported to have increased serum levels of fractalkine and increased CX3CR1+ macrophages and T cells in fibrotic skin and lung.19 These data have suggested that fractalkine/CX3CR1 may promote tissue fibrosis through inflammatory cell infiltration. Here we test this hypothesis in a mouse model of ischemia-reperfusion injury of the kidney.
Materials and Methods
Animals
Male wild-type and CX3CR1−/− C57BL/6 mice were purchased from Taconic (Germantown, NY). CX3CR1−/− mice, generated as previously described,20 had been backcrossed onto the C57BL/6 background for 10 generations and maintained under specific pathogen-free housing conditions. No significant differences in growth or weight were found between CX3CR1−/− and wild-type C57BL/6 mice. All animals were used at 6 to 8 weeks of age under the auspices of a protocol approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee.
Renal Ischemia-Reperfusion Model
After general anesthesia was established with xylazine, ketamine, and isoflurane, the left renal artery and vein of CX3CR1−/− and wild-type control mice were exposed by flank incision and clamped for 60 minutes. Kidneys that did not completely recover after unclamping, as assessed by restoration of normal color, were not used for analysis. After releasing the clamp the flank incision was closed in two layers with silk sutures. The animals received warm saline instilled into the peritoneal cavity during the procedure and were allowed to recover with free access to food and water. Sham surgery was performed in a similar manner, except that the renal vessels were not clamped. To evaluate function of the injured kidney, the uninjured kidney was removed on day 12 in a subset of mice. Renal tissues were removed for pathological examination 24 hours, 48 hours, 7 days, and 14 days after ischemia-reperfusion from 14 mice at each time point. Blood samples were taken from the abdominal aorta just before sacrifice to evaluate renal function. Purified polyclonal rabbit anti-CX3CR1 antibodies or control rabbit IgG (Torrey Pines Biolabs, San Diego, CA) were used to evaluate the therapeutic effects of CX3CR1 blockade in ischemia-reperfusion injury as described previously.21 Seventy-five μl of anti-CX3CR1 antibodies or control rabbit IgG at a concentration of 20 μg/ml were injected intraperitoneally 1 day before or 1 day after ischemic injury and each day thereafter for a total of 8 or 6 days, respectively. To evaluate effects of anti-CX3CR1 antibodies or control IgG on blood cell counts, we collected whole blood 8 days after injection of the animals (five per group). Effects of anti-CX3CR1 antibodies or control IgG on complement were evaluated by serum C5a concentration by enzyme-linked immunosorbent assay using rat anti-mouse complement component C5a monoclonal antibody (clone 295103), goat biotinylated anti-mouse complement component C5a antibody, and recombinant mouse complement component C5a protein (all from R&D Systems, Minneapolis, MN).
Platelet Labeling
Platelets were prepared and labeled for injection using a previously described method with some modifications.22,23 Briefly, peripheral blood was drawn from anesthetized wild-type mice or CX3CR1-deficient mice by cardiac puncture using citrate and disodium ethylenediaminetetraacetic acid as anti-coagulants. Fresh anti-coagulated blood was centrifuged at 600 × g for 3 minutes, and platelet-rich plasma was then collected. Centrifugation of the platelet-rich plasma at 1300 × g for 10 minutes produced a platelet pellet. Platelets were labeled with PKH26 red fluorescent cell linker mini kit (Sigma, St. Louis, MO) using the method of Michelson and colleagues24 with minor modifications. Platelets were resuspended in Diluent C at 4 × 109/ml to which 10 μmol/L prostaglandin I2 (PGI2) was added. An equal volume of Diluent C containing freshly prepared 4 μmol/L PKH26 was added, and the suspension was mixed and incubated for 8 minutes at room temperature with occasional inversion. An equal volume of citrate-albumin PGE1 buffer (11 mmol/L dextrose, 128 mmol/L NaCl, 4.3 mmol/L NaH2PO4, 7.5 mmol/L Na2HPO4, 4.8 mmol/L trisodium citrate, 2.4 mmol/L citric acid, 0.35% bovine serum albumin, 0.33 μmol/L PGE1, pH 6.5) was added. The mixture was incubated for 1 minute and centrifuged. The pellet was resuspended in 5 ml of citrate-albumin-PGE1 buffer, incubated for 10 minutes, centrifuged, and resuspended in Tyrode’s solution (Sigma) with 0.35% albumin and 3 U/ml apyrase at a platelet count of 2.0 × 109/ml. To evaluate the role of CX3CR1 in the accumulation of platelets in the injured kidney, 2 × 108 PKH26-labeled platelets from wild-type mice or CX3CR1-deficient mice in a total volume of 100 μl were injected in the tail vein of wild-type mice just before ischemia-reperfusion injury.
Immunohistochemistry
One portion of the renal tissue was fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with periodic acid-Schiff reagent, naphthol AS-D chloroacetate esterase, Gomori’s trichrome, or indicated antibodies. Another portion of fresh renal tissue was embedded in OCT compound (Sakura Finetek, Torrance, CA) and snap-frozen on dry ice. Frozen sections were used to detect PKH26-labeled platelets and for immunohistochemistry using antibodies directed against CX3CR1 and F4/80. Deparaffinized sections were treated with Target river solution (DAKO, Carpinteria, CA) before staining of fractalkine and α-smooth muscle actin (α-SMA), with 10 mmol/L Tris buffer and 1 mmol/L ethylenediaminetetraacetic acid for TGF-β staining, or with proteinase K (DAKO) for staining of PDGF-B. Endogenous peroxidase activity and nonspecific binding in the sections was blocked by peroxidase-blocking reagent (DAKO), biotin-blocking system (DAKO) and protein block, serum-free (DAKO). Sections were then incubated with the following primary antibodies and conditions: goat anti-rat fractalkine antiserum (R&D Systems), which cross-reacts with mouse fractalkine, at 1 μg/ml overnight at 4°C; rabbit anti-human PDGF-B antiserum (Calbiochem, San Diego, CA), which cross-reacts with mouse PDGF-B,25 at 10 μg/ml for 2 hours at room temperature; rabbit anti-human TGF-β antiserum (Abcam, Cambridge, MA), which cross-reacts with mouse TGF-β, at 4 μg/ml for 2 hours at room temperature; or rabbit anti-mouse CX3CR1 antiserum (kind gift from Dr. T. Imai, Kyoto University) at 5 μg/ml for 2 hours at room temperature. Normal goat or rabbit IgG was used as a negative control. Thereafter, the sections were incubated with biotinylated secondary antibody, followed by incubation with peroxidase-conjugated streptavidin or the EnVision+/HRP kit (DAKO). For α-SMA, polymer-immunocomplex methods were used. Briefly, a mixture of diluted primary antibody, mouse α-SMA monoclonal antibody clone 1A4, 10 μg/ml (DAKO),26 and EnVision+/HRP for mouse were incubated at room temperature for 60 minutes. After the incubation, normal mouse serum was added, and the mixture was incubated for 60 minutes. Tissues were incubated with the final mixture at room temperature for 60 minutes. Normal mouse IgG was used as a negative control. After washing with phosphate-buffered saline containing 0.01% Tween, the sections were stained with 3,3′-diaminobenzidine solution and then counterstained with hematoxylin. Tubular necrosis was quantitated in a blinded manner as the percentage of tubules in the outer medulla in which epithelial necrosis or necrotic debris was observed in periodic acid-Schiff-stained sections. Interstitial renal fibrosis was quantitated as the area staining blue in sections stained with Gomori’s trichrome. The area of tubular necrosis and interstitial fibrosis in the outer medulla was evaluated by NIH image and expressed as the percentage of total area imaged. Infiltrating leukocytes were quantitated in the outer medulla of the injured kidney, where cell migration was maximal, and the data were expressed as leukocytes/mm2 at magnification ×320. Neutrophils were identified by naphthol AS-D chloroacetate esterase staining (Sigma).27,28 T cells and macrophages were detected immunohistochemically with rabbit anti-CD3 antibody (DAKO)29 and rat anti-mouse F4/80 monoclonal antibody (clone BM8; eBioscience, San Diego, CA), respectively. Rabbit anti-CD3 antibody was detected by EnVision+ System (peroxidase) (DAKO), and rat anti-F4/80 antibody was detected by biotinylated polyclonal rabbit anti-rat immunoglobulin antibodies (DAKO) and the LSAB+ system (peroxidase) (DAKO). To identify F4/80 and CX3CR1 double-positive cells, the sections were first incubated for 2 hours at room temperature with rabbit anti-mouse CX3CR1 antibody and then with Alexa Fluor 568-conjugated donkey anti-rabbit IgG (Molecular Probes, Eugene, OR) at 20 μg/ml for 1 hour at room temperature. For staining of F4/80, sections were incubated with rat anti-mouse F4/80 monoclonal antibody. Subsequently, the samples were incubated with Alexa Fluor-488-conjugated donkey anti-rat IgG (Molecular Probes) at 20 μg/ml for 1 hour at room temperature. Alexa Fluor 568-labeled CX3CR1-positive cells and Alexa Fluor 488-labeled F4/80-positive cells were observed by fluorescent microscopy.
Cytokine Quantitation
Tissue homogenates were used to determine TGF-β and PDGF-B expression using murine Quantikine immunoassay kits (R&D Systems) following the manufacturer’s directions. All enzyme-linked immunosorbent assay samples were run in duplicate. The amount of TGF-β and PDGF-B was standardized by tissue weight. Total RNA was extracted from the kidney using RNAqueous kit (Ambion Inc., Austin, TX). RNA was treated with deoxyribonuclease and purified with DNA-free (Ambion, Inc.). The RNA was quantified by absorbance at 260 nm in a spectrophotometer (BioPhotometer; Eppendorf AG, Hamburg Germany). cDNA was reverse-transcribed from 1 μg of total RNA using a RETROscript kit (Ambion, Inc.). Fractalkine and CX3CR1 mRNA were detected by 28 cycles of polymerase chain reaction (PCR). PCR was found to be linear between 20 and 35 cycles. β-actin was used as internal standard (QuantumRNA β-actin internal standards, Ambion). The following primers were used for fractalkine: forward (5′-TGGTCCAGAGCTGGCAATAA) and reverse (5′-TGGCTTCCTCACTCTCAGGA). Mouse/rat CX3CR1 PCR primer pair (R&D Systems) was used to detect CX3CR1 expression. The mRNA expression of cytokines, chemokines, chemokine receptors, and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was analyzed using an MPCR kit (Maxim Biotech, Inc., San Francisco, CA) according to the manufacturer’s protocol. Images of UV-illuminated agarose gels were captured, and the density of cDNA bands was analyzed using NIH Image software. PCR was found to be linear between 24 and 36 cycles, and 33 cycles were chosen for all results shown. Cytokine, chemokine, and chemokine receptor mRNA levels were standardized by GAPDH signals and are expressed relative to the levels found in sham-operated mice.
Hydroxyproline Assay
Hydroxyproline content was measured as an index of collagen accumulation in the injured kidney, as described previously30 with some modification. Briefly, a portion of the kidney was weighed, dried for 16 hours at 110°C, then hydrolyzed in 6 N HCl at 110°C for 12 hours. Fifty-μl aliquots were added to 1 ml of a solution containing 1.4% chloramine T (Sigma), 10% n-propanol, and 0.5 mol/L sodium acetate, at pH 6.0. After 20 minutes of incubation at room temperature, 1 ml of Erlich’s solution (1 mol/L p-dimethylaminobenzaldehyde in 70% n-propanol, 20% perchloric acid) was added, and the mixture was incubated at 65°C for 15 minutes. Absorbance was measured at 550 nm, and the amount of hydroxyproline was determined against a standard curve generated using known concentrations of reagent hydroxyproline (Sigma), and expressed as the amount (μg) per mg of kidney.
Serum Creatinine Assay
Serum creatinine was measured as a marker of renal function. Blood was collected from each mouse at the time of sacrifice and stored at −80°C until use. Creatinine concentration was measured by colorimetric microplate assay (Oxford Biomedical Research, Oxford, MI).
Statistical Analysis
The mean and SE (SEM) were calculated on all of the parameters determined in this study. Statistical analyses were performed using unpaired Student’s t-test, Kruskal-Wallis test, and analysis of variance test. P < 0.05 was accepted as statistically significant.
Results
Fractalkine and CX3CR1 Expression in the Kidney after Ischemia-Reperfusion Injury
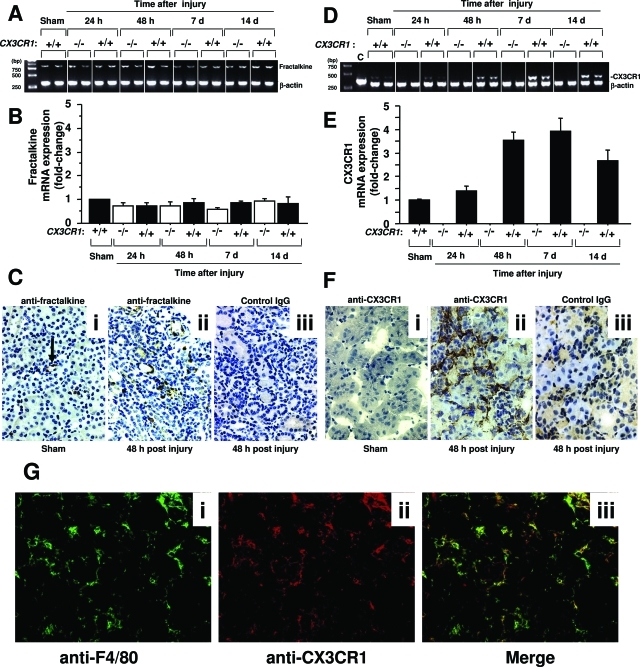
Fractalkine mRNA was constitutively expressed under homeostatic conditions in normal mouse kidney (data not shown); however, as assessed by PCR analysis of total kidney RNA, the level did not increase significantly in sham-operated mice or with time after ischemia-reperfusion injury in either wild-type or CX3CR1-deficient mice (Figure 1, A and B). Nevertheless the protein level, as assessed by immunohistochemistry, and the spatial distribution did change after injury (Figure 1C). Anti-fractal-kine immunoreactivity was detected mainly on endothelial cells throughout the kidney in sham-operated mice (Figure 1C, i) but by 24 hours after ischemia-reperfusion was redistributed to the outer medulla where it could also be detected in association with infiltrating cells and tubular epithelial cells (Figure 1C, ii). Increased expression persisted throughout the course of experiment but began to wane by day 7 after injury (data not shown). The difference between RNA and protein induction in the model may reflect infiltration of inflammatory cells that constitutively express fractalkine.
Figure 1-6944.
Fractalkine and CX3CR1 are expressed in kidney after ischemia-reperfusion injury. A–C: Fractalkine. D–G: CX3CR1. Representative and summary data from PCR analysis of fractalkine mRNA expression is shown in A and B, respectively, and for CX3CR1 in D and E, respectively. −/−, CX3CR1 knockout mice; +/+, wild-type C57BL/6 control mice; sham, sham-operated wild-type C57BL/6 mice tested 1 day after surgery. Summary data are expressed as fold-change, defined as the fractalkine or CX3CR1 PCR signal divided by the corresponding β-actin signal normalized to the same ratio for sham-operated mice, and are the mean ± SEM from three independent experiments with four to five mice in each experiment. Representative results are shown for two mice per condition in A and D. C, F, and G: Immunohistochemical localization of fractalkine and CX3CR1, respectively, in wild-type C57BL/6 mouse kidney 48 hours after surgery. Tissue was counterstained with hematoxylin for each image in C and F. C: i, Sham-operated kidney stained with anti-fractalkine (arrow points to positively stained endothelial cells); ii and iii, ischemia-reperfusion-injured kidney stained with anti-fractalkine and control IgG, respectively. F: i, Sham-operated kidney stained with anti-fractalkine; ii and iii, ischemia-reperfusion-injured kidney stained with anti-CX3CR1 and control IgG, respectively. G: i and ii, Ischemia-reperfusion-injured kidney stained with anti-F4/80 and anti-CX3CR1, respectively; iii, merged image of i and ii. Original magnifications, ×320.
In contrast to fractalkine’s temporal and spatial pattern of expression, constitutive expression of CX3CR1 mRNA was extremely low in sham-operated mice but was markedly increased after ischemia-reperfusion injury, peaking at ∼48 hour in wild-type mice and remaining high throughout the 2-week course of the experiment (Figure 1, D and E). The increase in mRNA corresponded with an increase in anti-CX3CR1 immunoreactivity of wild-type kidney subjected to ischemia-reperfusion injury that was particularly intense in the outer medulla. This co-localized with infiltrating cells, which were mainly macrophages (Figure 1F) as demonstrated by dual staining with anti-F4/80 and anti-CX3CR1 antibodies (Figure 1G). CX3CR1 was expressed by 86.7 ± 1.5% of infiltrating macrophages. This suggested that CX3CR1 might mediate macrophage trafficking to the kidney and promote the inflammatory response after ischemia-reperfusion injury. To test this we examined CX3CR1-deficient mice in greater detail.
CX3CR1 Dependence of Macrophage Infiltration and Fibrosis in the Kidney after Ischemia-Reperfusion Injury
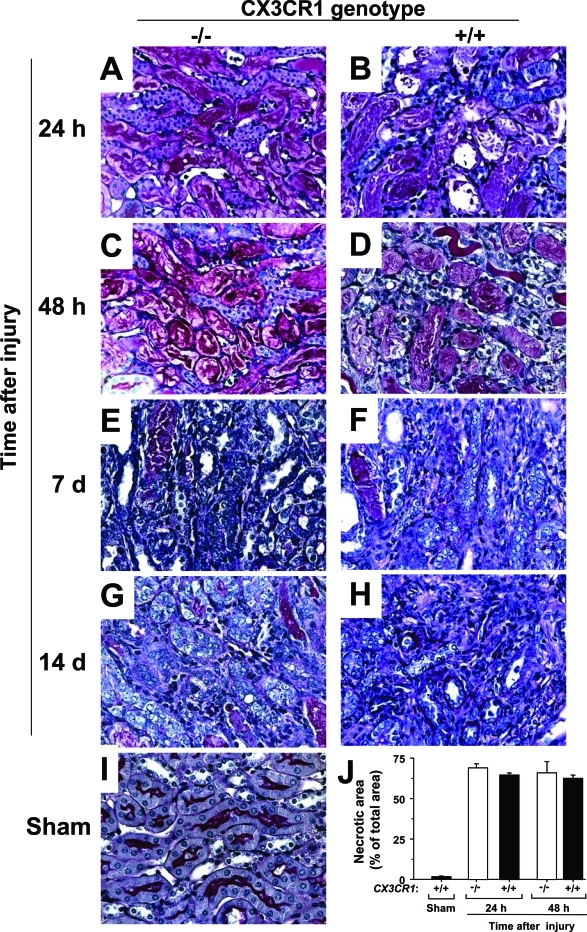
Like fractalkine and CX3CR1, severe acute tubular necrosis was found consistently most intensely in the outer medulla of mouse kidney 24 to 48 hours after ischemia-reperfusion injury (Figure 2, A–D). With time, tubular epithelial cells proliferated, and tubules were regenerated in both wild-type and CX3CR1-deficient mice, as shown for days 7 and 14 after injury in Figure 2, E–H. Little if any evidence of acute tubular necrosis was detectable in the kidneys of sham-operated mice at 24 hours after surgery (Figure 2I). Quantitation of the area of acute tubular necrosis failed to reveal a significant difference between wild-type and CX3CR1-deficient mice at any time point (Figure 2J).
Figure 2-6944.
Kidney reaction to ischemia-reperfusion injury is dependent on CX3CR1: histopathological analysis of CX3CR1 knockout mice. Histopathological examination was performed using periodic acid-Schiff-stained kidney. The outer medulla of the left kidney is shown for CX3CR1-deficient (A, C, E, G) and wild-type mice (B, D, F, H, I) at the times after ischemia-reperfusion injury indicated to the left of each panel (A–H) or 24 hours after sham operation (I). J: Tubular necrosis after ischemia-reperfusion injury is not regulated by CX3CR1. Periodic acid-Schiff-stained sections of the outer medulla were quantitated for necrosis under the conditions shown. −/−, CX3CR1 knockout mice; +/+, wild-type control mice; sham, sham-operated wild-type mice tested 24 hours after surgery. Images represent one experiment representative of three independent experiments with four to five animals in each experiment summarized in J. Original magnifications, ×320.
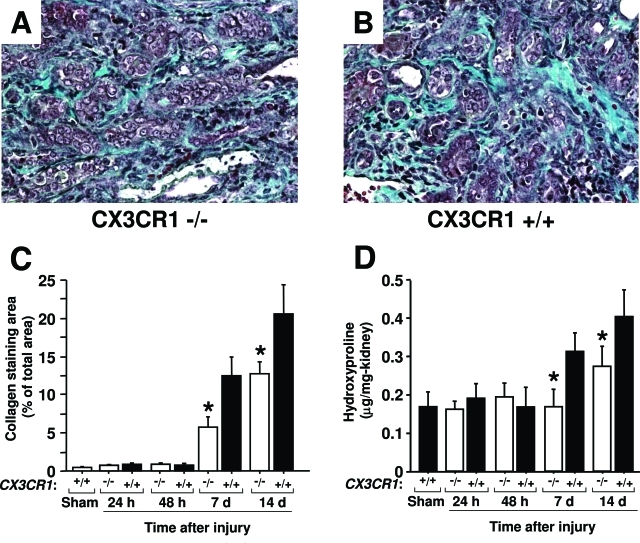
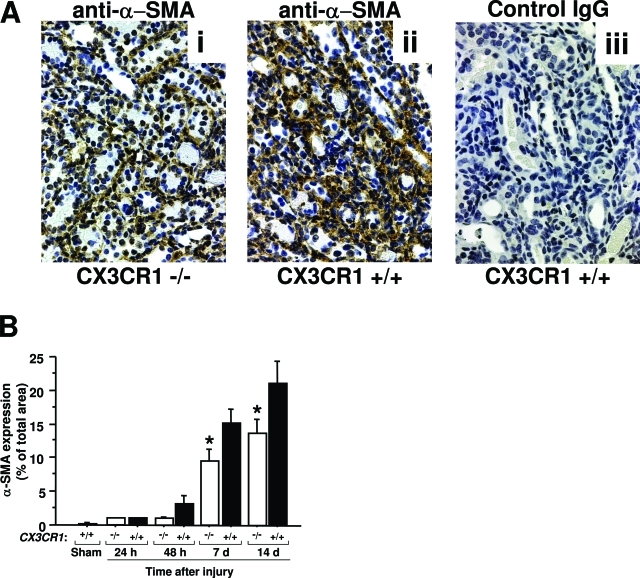
Collagen fibers, which stain blue with trichrome, are normally present at low levels in the renal interstitium and did not increase in the first 48 hours after injury (Figure 3). However, by day 7 large increases were observed in the interstitium of the outer medulla, and this continued to increase through day 14 after injury, the latest time point tested. Unlike acute tubular necrosis, the extent of fibrosis in the outer medulla of the injured kidney was significantly diminished in CX3CR1-deficient mice compared with wild-type mice (Figure 3, A–C). This was confirmed independently and more quantitatively with assays for hydroxyproline content, an index of collagen accumulation in the interstitial space (Figure 3D), and α-SMA, a marker of activated fibroblasts (Figure 4). α-SMA immunoreactivity, which localized with spindle-shaped cells in the interstitium, was also mainly detected in the outer medulla where fibrosis progressed most intensely. Both of these factors were significantly increased in the late phase of injury, eg, 7 and 14 days after reperfusion, but to a significantly lower level in CX3CR1-deficient mice compared to wild-type mice (Figures 3 and 4). In sham-operated mouse kidney, α-SMA expression was detected in the blood vessel wall (data not shown).
Figure 3-6944.
Renal interstitial fibrosis caused by ischemia-reperfusion injury is dependent on CX3CR1. All analyses were performed in three independent experiments with four to five animals in each experiment. A and B: Histopathological analysis. Trichrome-stained outer medulla from CX3CR1-deficient (A) and wild-type (B) mice 14 days after injury. Images are from one representative animal of each genotype. C: Kinetics of fibrotic change: histochemical analysis. Fibrotic change was quantitated in trichrome-stained sections of outer medulla under the conditions shown. Values are mean ± SEM. D: Kinetics of fibrotic change: biochemical analysis. Hydroxyproline content of the left kidney was quantitated for the conditions shown. Values are mean ± SEM. −/−, CX3CR1 knockout mice; +/+, wild-type control mice; sham, sham-operated wild-type mice tested 24 hours after surgery. *P < 0.05 comparing CX3CR1-deficient mice versus wild-type mice at the same time point. Original magnifications, ×320.
Figure 4-6944.
α-SMA is induced during ischemia-reperfusion injury of the kidney in a CX3CR1-dependent manner. A: Histopathological analysis of outer medulla. i: CX3CR1-deficient mice stained with anti-α-SMA; ii and iii: wild-type mice stained with anti-α-SMA and control IgG, respectively. B: Quantitation of histopathological data. −/−, CX3CR1 knockout mice; +/+, wild-type control mice; sham, sham-operated wild-type mice tested 24 hours after surgery. Values are mean ± SEM and represent data from three independent experiments with four to five animals in each experiment. *P < 0.05, comparing CX3CR1-deficient mice versus wild-type mice. Original magnifications, ×320.
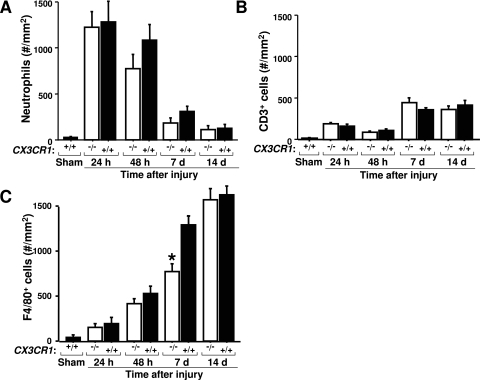
Although fibroblasts are the direct source of collagen in fibrotic lesions, many of the fibrogenic mediators likely to regulate this process come from inflammatory cells. The number of neutrophils increased massively and rapidly after reperfusion, peaking at 31-fold over sham-operated mice 24 hours after injury and then gradually decreasing, but there was no significant difference between CX3CR1-deficient and wild-type mice at any time point (Figure 5A), suggesting that this type of leukocyte may not play a large role in coordinating the CX3CR1-dependent fibrotic response. Lymphocytes also do not appear to be playing a significant role because CD3+ cells were only a small fraction of total infiltrating cells and because there was no significant difference between CX3CR1-deficient and wild-type mice in the number of these cells at any time point (Figure 5B). In contrast, large numbers of F4/80+ macrophages progressively infiltrated the interstitial space throughout the 14-day course of the experiment after reperfusion, resulting in a 35-fold increase at day 14 relative to sham-operated mice, but were ∼40% lower in CX3CR1-deficient mice on day 7 after reperfusion (Figure 5C).
Figure 5-6944.
CX3CR1 specifically regulates macrophage infiltration of the outer medulla after renal ischemia-reperfusion injury. −/−, CX3CR1 knockout mice; +/+, wild-type control mice; sham, sham-operated wild-type mice tested 24 hours after surgery. A–C: Quantitation of density of indicated leukocyte subsets in tissue sections under the conditions shown. Values are mean ± SEM and are from three independent experiments with four to five animals in each experiment. *P < 0.05 comparing CX3CR1-deficient mice versus wild-type mice at the same time point. Original magnifications, ×320.
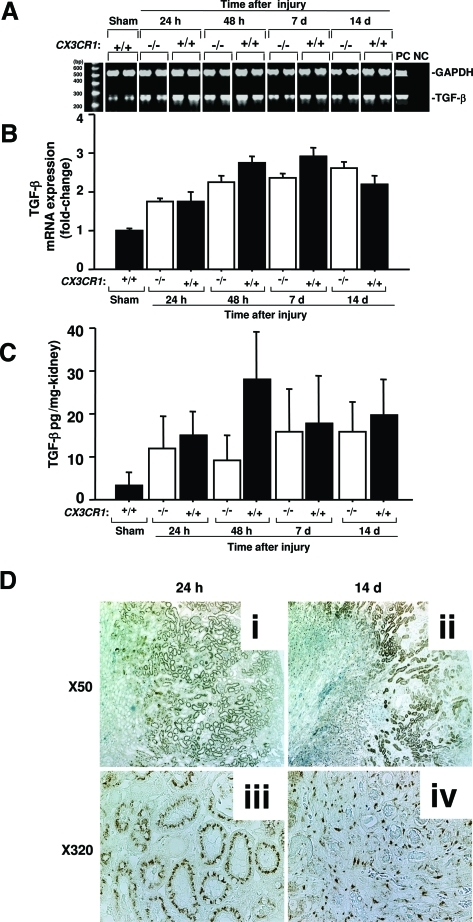
We next investigated specific molecular mediators that might contribute to CX3CR1-dependent fibrosis in renal ischemia-reperfusion injury. TGF-β is a major fibrogenic factor that is clearly relevant in this model because it was significantly up-regulated after ischemia-reperfusion both at the mRNA (Figure 6, A and B) and protein (Figure 6C) levels; however, there was no significant difference in expression in wild-type versus CX3CR1-deficient mice. Immunohistological staining showed that although tubular epithelial cells were mainly positive for TGF-β in the outer medulla at 24 hours after reperfusion (Figure 6D, i and ii), infiltrating cells or interstitial spindle-shaped cells were positive for TGF-β at 14 days after reperfusion (Figure 6D, iii and iv). These data also show that TGF-β from infiltrating cells might play a role in interstitial fibrosis after renal reperfusion in the later phase, but it appeared to contribute mainly, if not exclusively, to CX3CR1-independent fibrosis in the model.
Figure 6-6944.
TGF-β is induced during ischemia-reperfusion injury of the kidney in a CX3CR1-independent manner. −/−, CX3CR1 knockout mice; +/+, wild-type control mice; sham, sham-operated wild-type mice tested 24 hours after surgery. A and B: TGF-β mRNA induction in kidney. A: Representative reverse transcriptase-PCR results are shown for two animals per condition. B: Summary mRNA induction data. Band intensity was quantitated by NIH Image, normalized to GAPDH, and is presented as fold-change relative to GAPDH-normalized TGF-β signal for sham-operated mice. C: TGF-β protein induction in kidney. Data in B and C are presented as the mean ± SEM from three independent experiments with four to five mice in each experiment. Differences comparing −/− and +/+ were not statistically significant at any time point for either RNA or protein analysis. D: Immunohistochemical analysis of TGF-β expression in outer medulla of the kidney after ischemia-reperfusion injury in wild-type mice. The injured kidney is shown 24 hours (i, ii) and 14 days (iii, iv) after ischemia-reperfusion injury. Original magnifications: ×50 (i, iii); ×320 (ii, iv).
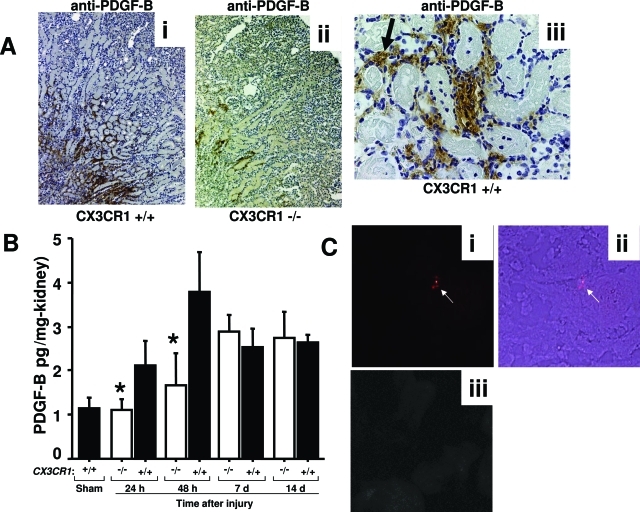
PDGF-B, an important fibrogenic factor produced by macrophages, platelets, and endothelial cells, was significantly increased in the early phase of injury in wild-type mice, peaking at approximately fourfold over sham-operated mice at 48 hours after reperfusion (Figure 7). PDGF-B was detected mainly in the outer medulla of the injured kidney and at lower levels in CX3CR1-deficient than wild-type mice through 48 hours after reperfusion (Figure 7, A and B). Most impressively, levels were reduced by 100% and 80% at 24 and 48 hours after injury, respectively, in CX3CR1−/− mice relative to wild-type controls. When sections were analyzed at high magnification, PDGF-B was observed to co-localize with some tubular epithelial cells as well as with interstitial areas associated with infiltrating cells and peritubular capillaries, the latter possibly reflecting the presence of PDGF-B-laden platelets (Figure 7A, iii). This suggested the possibility that platelets, which express CX3CR1 on the cell surface and are a rich source of PDGF-B, might accumulate at lower levels in the outer medulla of CX3CR1 knockout mice compared with wild-type mice. In mice that underwent ischemia-reperfusion injury, we did not detect any fluorescently labeled platelets in the uninjured right kidney (negative control), whereas we were able to demonstrate accumulation of labeled platelets in the injured left kidney (Figure 7C). However, we did not observe a significant difference in platelet accumulation in the injured kidney between wild-type and CX3CR1 knockout mice in this regard (data not shown).
Figure 7-6944.
PDGF-B is induced at an early stage after ischemia-reperfusion injury of the kidney in a CX3CR1-dependent manner. A: Immunohistochemical analysis of PDGF-B expression in outer medulla of the kidney 48 hours after injury. Tissue was counterstained with hematoxylin. i and ii: Wild-type and CX3CR1-deficient mouse kidney, respectively, stained with anti-PDGF-B. iii: Anti-PDGF-B-stained wild-type kidney. Arrow points to nonnucleated cells, most likely red blood cells. B: Quantitation of PDGF-B protein levels in kidney by enzyme-linked immunosorbent assay. −/−, CX3CR1 knockout mice; +/+, wild-type control mice; sham, sham-operated wild-type mice tested 24 hours after surgery. Values are mean ± SEM of data from three independent experiments with four to five animals in each experiment. *P < 0.05 comparing CX3CR1-deficient mice versus wild-type mice. C: Histological analysis of platelet trafficking into injured kidney. Platelets were fluorescently labeled and infused intravenously immediately before injury as described in the Materials and Methods. i and ii: Left kidney from wild-type mouse 48 hours after injury imaged by fluorescence (i) and combined fluorescence and transmission light microscopy (merged image, ii). Arrows point to labeled platelets. iii: Right kidney from same wild-type mouse as in i and ii, imaged by fluorescence microscopy. Original magnifications: ×5 (A, i and ii); ×320 [A (iii), C].
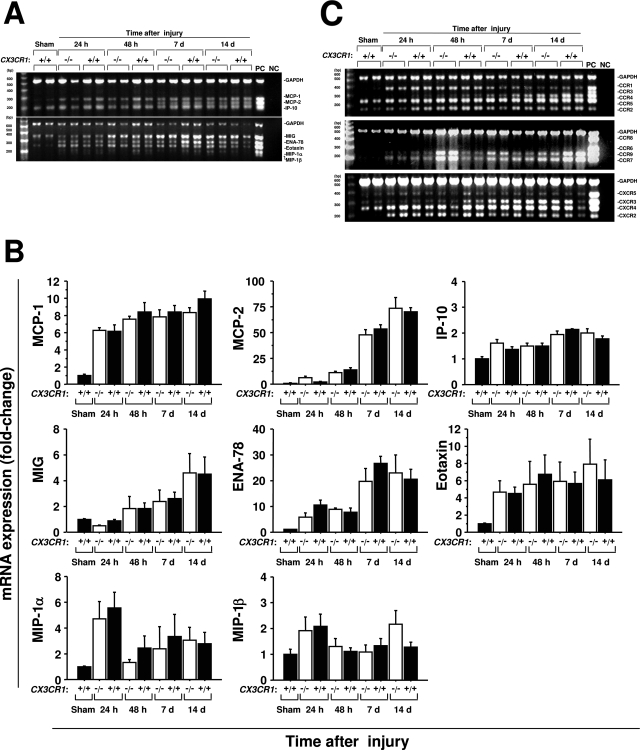
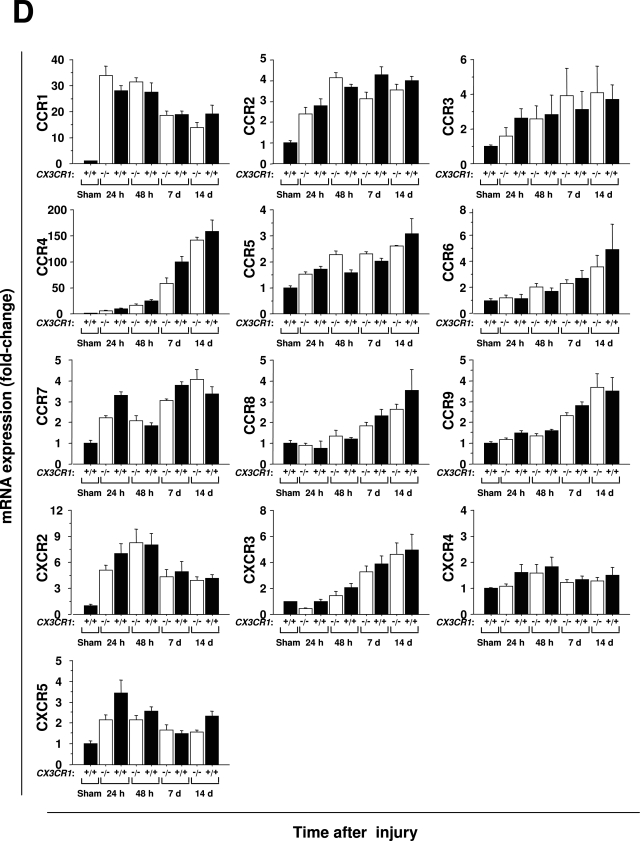
It is possible that reduced levels of macrophages and fibrosis in the kidneys of CX3CR1 knockout mice undergoing ischemia-reperfusion injury are not caused by direct loss of CX3CR1 regulation of these processes but instead result from skewed expression of another factor(s) whose expression is dependent on CX3CR1. To begin to test this possibility we performed a focused survey of mRNA expression levels for other chemokines and chemokine receptors in CX3CR1 knockout mice. As expected many inflammatory chemokines were up-regulated after injury, although the kinetics of induction varied considerably. For example, macrophage inflammatory protein (MIP)-1α(CCL3) peaked 24 hours after injury, monokine induced by interferon-γ (MIG, or CXCL9) and monocyte chemoattractant protein (MCP)-2 (CCL8) were significantly increased in the late phase of injury, and MCP-1 (CCL2) was rapidly induced to high levels that persisted throughout the 14-day period of observation. However, there was no significant difference in expression of any of these chemokines at any time point between wild-type and CX3CR1-deficient mice (Figure 8, A and B). Inflammatory chemokine receptors were also up-regulated after ischemia-reperfusion injury (Figure 8C). CCR1 and CXCR2 peaked in the early phase of the injury, and CCR4 and CXCR3 increased significantly in the late phase. Like its ligand MCP-1, CCR2 was induced early after injury and persisted at high levels throughout the 14-day period of observation. As with all of the chemokine receptor ligands examined, there were no significant differences between wild-type mice and CX3CR1-deficient mice in expression of these chemokine receptors at the times monitored after reperfusion. These results are consistent with a model in which CX3CR1 directly mediates macrophage infiltration and subsequent fibrotic processes after ischemia-reperfusion injury of the kidney.
Figure 8-6944.
Ischemia-reperfusion injury of the kidney induces renal expression of many chemokines and chemokine receptors in a CX3CR1-independent manner. −/−, CX3CR1 knockout mice; +/+, wild-type control mice; sham, sham-operated wild-type mice tested 24 hours after surgery. RNA extracted from kidneys was amplified by multiplex PCR. The band intensity was standardized by GAPDH, and shown as fold-change relative to sham-operated mice. A and C: Representative results from multiplex PCR for the factors indicated to the right of each panel under the conditions shown. PC, positive control for each factor; NC, negative control = PCR in the absence of cDNA. B and D: Quantitation of results from A and C, respectively. Values are the mean ± SEM of data from three experiments with four to five animals in each experiment.
Figure 8a-6944.
continued
CX3CR1-Neutralizing Antibody Reduces Fibrosis in Ischemia-Reperfusion Injury of the Kidney
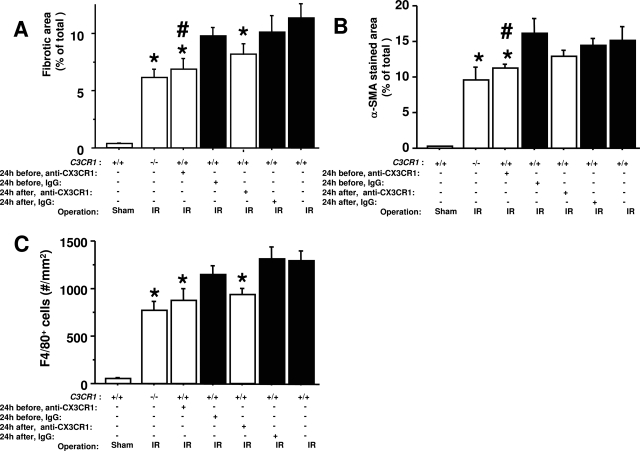
To test this model directly, we injected mice intraperitoneally with CX3CR1-neutralizing antibodies or control IgG 1 day before or 1 day after ischemic injury and each day thereafter for a total of 8 or 6 days, respectively. Although antibody injection may activate complement and reduce peripheral white blood cell and platelet counts, we did not observe a significant difference in these parameters in mice injected with CX3CR1 neutralizing antibodies versus control rabbit IgG after ischemia-reperfusion (C5a levels: 5.1 ± 0.7 ng/ml versus 5.0 ± 0.9 ng/ml; total WBC: 4794 ± 476/μl versus 5092 ± 499/μl; monocytes: 246 ± 20/μl versus 241 ± 53/μl; lymphocytes: 3719 ± 4451/μl versus 4092 ± 371/μl; platelets: 1047 ± 50 × 103/μl versus 985 ± 36 × 103/μl). CX3CR1 neutralization resulted in significant and specific reduction in the area of fibrosis after injury, measured either by trichrome-stained collagen fibers or α-SMA-staining area (Figure 9, A and B).
Figure 9-6944.
Neutralizing CX3CR1 antibodies attenuate renal fibrosis after ischemia-reperfusion injury. Wild-type mice (+/+) were injected intraperitoneally 24 hours before or after ischemia-reperfusion injury (IR) and every 24 hours thereafter for a total of 8 or 6 days, respectively, with anti-CX3CR1 antibodies or control IgG. Uninjected wild-type and CX3CR1 knockout mice underwent ischemia-reperfusion injury in parallel and a set of wild-type mice underwent sham surgery, as indicated in the axis labels. The following parameters were quantitated: area of fibrosis in trichrome-stained sections (A), α-SMA-staining area (B), and density of anti-F4/80-positive cells (C). All analyses were performed on the left kidney 7 days after surgery. Values are the mean ± SEM of data from three independent experiments with four to five animals in each experiment. #P < 0.05 comparing neutralizing CX3CR1 antibody versus control IgG treatment. *P < 0.05 comparing neutralizing CX3CR1 antibody-injected versus uninjected wild-type mice.
CX3CR1 neutralization before and after injury reduced F4/80+ cell recruitment to the outer medulla by 25% (P = 0.087 and 0.077, respectively), whereas genetic inactivation of CX3CR1 reduced F4/80+ cell recruitment to a slightly greater extent (40%, P = 0.0013) (Figure 9C). Thus both experimental approaches reduced recruitment of these cells in the model. The small difference in magnitude of the reduction that was achieved with these two techniques may reflect incomplete receptor coverage by the antibody relative to genetic ablation, which by definition affects 100% of receptors. The lack of statistical significance for the neutralization experiments could indicate a chance effect but more likely represents a power issue, because the P values were quite close to the 0.05 cutoff.
Renal Function of the Injured Kidney Was Significantly Diminished after Ischemia-Reperfusion Injury
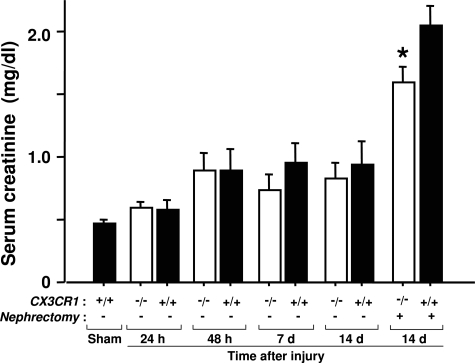
Renal function, measured as serum creatinine, decreased by ∼50% from baseline by 48 hours after injury in both wild-type mice and CX3CR1-deficient mice (Figure 10). To test injured kidney function directly, we analyzed serum creatinine at day 14 after injury in mice that underwent right nephrectomy at day 12 after injury. The results showed an ∼400% increase in serum creatinine of injured wild-type mice relative to sham-operated wild-type mice. Relative to this, in CX3CR1-deficient mice creatinine was reduced by ∼25% at this time point (Figure 10).
Figure 10-6944.
Renal ischemia-reperfusion injury results in impaired renal function through mechanisms partially dependent on CX3CR1. Serum creatinine levels were measured at the indicated times after injury in wild-type and CX3CR1-deficient mice. Right uninjured kidney of mice in nephrectomy group was removed on day 12, and samples collected on day 14. Values are the mean ± SEM of data from three independent experiments with four to five animals in each experiment. IR, ischemia-reperfusion; *P < 0.05 comparing injured CX3CR1-deficient mice versus sham-operated mice.
Discussion
In the present study, we have demonstrated a role for the chemokine receptor CX3CR1 in the pathogenesis of renal ischemia-reperfusion injury, based on both CX3CR1 blockade and gene inactivation experiments in a robust mouse model of disease. Taken together, the data support further evaluation of CX3CR1 in the pathogenesis of specific conditions associated with renal ischemia, such as renal transplantation, renal arterial stenosis, or shock because of hemorrhage or sepsis.
Our findings confirm previous work identifying acute tubular necrosis and fibrosis as major pathological sequelae of injury in this model and demonstrate for the first time that fibrosis (but not acute tubular necrosis) results in part from CX3CR1-dependent processes. CX3CR1 is the first chemokine receptor and only the second molecule, with osteopontin, shown to have a major effect on the fibrosis stage of pathogenesis in this model. On a quantitative level, ∼40% of renal fibrosis 7 days after injury could be attributed to CX3CR1 when assessed in either CX3CR1−/− mice or mice injected with neutralizing anti-CX3CR1 antibodies before injury. This resulted in relatively preserved renal function on day 14 in the injured kidney relative to wild-type mice. The mechanism appears to involve in part CX3CR1-dependent accumulation of infiltrating macrophages, which was also reduced by ∼40% by either CX3CR1 deletion or blockade. Macrophages are thought to be important in this model for the fibrotic response, acting through TGF-β.10,31–34 Although TGF-β expression was clearly induced in the model and interstitial infiltrating cells expressed TGF-β, we found no evidence that expression depended on CX3CR1 function. Additional work will be needed to test whether this result is a false negative, which might occur if for example CX3CR1 affected regional localization of TGF-β.
In contrast, CX3CR1 did regulate production of the potent fibrogenic macrophage and platelet product PDGF-B, one of the four known PDGF subtypes12 considered to be an important endogenous fibrogenic mediator in renal disease, both for initiation and progression stages. This pathological function of PDGF-B is thought to result from dysregulation and exaggeration of its normal activity as a mitogenic and chemotactic factor for fibroblasts, which is important in restorative processes such as wound healing.35
These findings are consistent with expression of CX3CR1 on platelets, monocytes, and macrophages,36,37 but its mechanism of action could differ on these cell types. CX3CR1-dependent macrophage infiltration is most likely to involve the known adhesive and chemotactic functions of CX3CR1. In addition, there is precedent for a role for platelets in progression of ischemia-reperfusion injury, both in brain and in coronary artery disease, by facilitating neutrophil infiltration in both cases.38,39 We demonstrated in the present study that platelets aggregated in the tubulointerstitial space of the outer medulla and that the blood vessel lumen was positive for PDGF-B. Moreover, CX3CR1 on platelets has been reported to regulate platelet adhesion to collagen and fibrinogen.37 Nevertheless, we found no evidence from adoptive transfer studies that CX3CR1 is required for platelet infiltration in the kidney after injury in our model (data not shown). If platelets are a significant source of PDGF-B in the model, CX3CR1 could still regulate this at the level of PDGF-B release. This possibility is supported by previous work indicating that CX3CR1 is capable of regulating cell responses unrelated to cell motility, such as neuronal apoptosis and perforin release from cytotoxic T cells. Additional work will be needed to test this hypothesis.
Our study also defined the spatial and temporal relationships among CX3CR1-dependent events in this model. With regard to location, all of the molecular and cellular changes that we monitored after injury, whether they were dependent on CX3CR1 or not, co-localized mainly in the outer medulla, which is consistent with previously published descriptions of this model. At the cellular level, F4/80+ macrophages accounted for the great majority of CX3CR1+ cells in situ in the damaged kidney. Geissmann and colleagues40 have described two populations of mouse monocytes distinguished by expression of CX3CR1. In this regard it appears that ischemia-reperfusion injury of the kidney induces factors, perhaps fractalkine itself, that may selectively recruit the CX3CR1+ subset.
With regard to temporal aspects of pathogenesis, CX3CR1-dependent fibrosis was a late event (between 2 to 7 days after injury) that clearly followed induction of fractalkine, CX3CR1, and PDGF-B in the outer medulla of the kidney, consistent with a causal relationship. Interestingly, fractalkine was constitutively expressed at low levels throughout the kidney but after injury was selectively up-regulated in the outer medulla, co-localizing with inflammatory and fibrotic changes. Up-regulation also appeared to result from an expansion of the cell types expressing fractalkine in the kidney from endothelial cells in sham-operated mice to include infiltrating cells and tubular epithelial cells after injury. Additional studies will be needed to precisely define the cell types expressing fractalkine in the model; however, the data are consistent with its role in pathogenesis.
Injection of exogenous PDGF-B into rat has demonstrated the importance of PDGF-B in renal tubulointerstitial cell proliferation and fibrosis.13 Peak accumulation of type III collagen occurred at 5 to 7 days after PDGF-B injection. These kinetics match favorably the kinetics we observed in our model for both CX3CR1-dependent and -independent PDGF-B expression and the evolution of fibrosis. Moreover, differentiation of fibroblasts to myofibroblasts, as indicated by an increase in α-SMA, is a key step of interstitial fibrosis.41,42 Consistent with this, the α-SMA-positive area was diminished in CX3CR1-deficient mice to the same degree as for fibrosis, ∼40% 7 and 14 days after reperfusion.
Taken together, these results demonstrate a cascade of CX3CR1-dependent and -independent molecular and cellular events, involving TGF-β, PDGF-B, macrophages, fibroblasts, and fibrosis, localized mainly to the fragile outer medulla in this model. Molecular regulation of CX3CR1-independent fibrosis is likely to be complex and could include one or more chemokines and chemokine receptors. As we showed in Figure 8, many chemokines were rapidly induced to high levels, suggesting a role in the acute neutrophil-mediated inflammatory phase of injury. However, the kinetics of expression of several such as Mig and MCP-2 and the receptors CCR4, CCR9, and CXCR3 were delayed in the model suggesting that they may be particularly good candidates for future studies of additional chemokine regulation of renal interstitial fibrosis.
Recently, chronic allograft nephropathy has been identified as the major factor leading to long-term graft loss after renal transplantation, and progressive tubulointerstitial fibrosis is characteristic of chronic allograft nephropathy.43 Whether CX3CR1 affects outcome in renal allograft transplantation and potentially other clinical settings in which transient renal ischemia occurs is unknown but could possibly be tested by gene association studies of appropriate cohorts using the defective variant of CX3CR1 known as M280, which has been reported to be a risk factor in HIV/AIDS, adult macular degeneration, and atherosclerosis in humans.44–46 Fractalkine/CX3CR1 has also been reported to play a protective role in certain settings, eg, lipopolysaccharide or interferon-γ challenge in the central nervous system,47,48 and in a model of cardiac allograft tolerance induced by donor-specific blood transfusion.49 This serves as a safety caution for attempts to develop CX3CR1 blocking agents.
In summary, our data show that CX3CR1 deletion or blockade attenuates macrophage infiltration and early PDGF-B expression as well as late-phase interstitial fibrosis and renal dysfunction after ischemia-reperfusion injury in a mouse model. These results identify both CX3CR1-dependent and CX3CR1-independent mechanisms of pathogenesis in this model. The results further suggest that CX3CR1 and its cognate ligand, fractalkine, could play roles in the molecular pathogenesis of interstitial fibrosis after renal ischemia-reperfusion injury in humans. Further research is needed to evaluate the clinical relevance of these findings and the potential utility of CX3CR1 as a therapeutic target in relevant clinical settings, particularly in renal transplantation when the time of injury is precisely known and is under exact medical control.
Acknowledgments
We thank Dr. Takashi Wada (Kanazawa University) for his excellent technical advice.
Footnotes
Address reprint requests to Philip M. Murphy, M.D., Laboratory of Molecular Immunology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Building 10, Room 11N113, Bethesda, MD 20892-9000. E-mail: pmm@nih.gov.
Supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health.
References
- Forbes JM, Hewitson TD, Becker GJ, Jones CL. Ischemic acute renal failure: long-term histology of cell and matrix changes in the rat. Kidney Int. 2000;57:2375–2385. doi: 10.1046/j.1523-1755.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346:580–590. doi: 10.1056/NEJMra011295. [DOI] [PubMed] [Google Scholar]
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases,; Bethesda: US Renal Data System, Reference Section A: Incidence of reported ESRD USRDS 2003 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. 2003:pp 231–264. [Google Scholar]
- Wakai K, Nakai S, Kikuchi K, Iseki K, Miwa N, Masakane I, Wada A, Shinzato T, Nagura Y, Akiba T. Trends in incidence of end-stage renal disease in Japan, 1983–2000: age-adjusted and age-specific rates by gender and cause. Nephrol Dial Transplant. 2004;19:2044–2052. doi: 10.1093/ndt/gfh317. [DOI] [PubMed] [Google Scholar]
- Stengel B, Billon S, Van Dijk PC, Jager KJ, Dekker FW, Simpson K, Briggs JD. Trends in the incidence of renal replacement therapy for end-stage renal disease in Europe, 1990–1999. Nephrol Dial Transplant. 2003;18:1824–1833. doi: 10.1093/ndt/gfg233. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Vielhauer V, Schlondorff D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 2003;63:401–415. doi: 10.1046/j.1523-1755.2003.00750.x. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- Persy VP, Verhulst A, Ysebaert DK, De Greef KE, De Broe ME. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney Int. 2003;63:543–553. doi: 10.1046/j.1523-1755.2003.00767.x. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Hugo C. The thrombospondin 1-TGF-beta axis in fibrotic renal disease. Nephrol Dial Transplant. 2003;18:1241–1245. doi: 10.1093/ndt/gfg159. [DOI] [PubMed] [Google Scholar]
- Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Tang WW, Ulich TR, Lacey DL, Hill DC, Qi M, Kaufman SA, Van GY, Tarpley JE, Yee JS. Platelet-derived growth factor-BB induces renal tubulointerstitial myofibroblast formation and tubulointerstitial fibrosis. Am J Pathol. 1996;148:1169–1180. [PMC free article] [PubMed] [Google Scholar]
- Hirschberg R, Wang S. Proteinuria and growth factors in the development of tubulointerstitial injury and scarring in kidney disease. Curr Opin Nephrol Hypertens. 2005;14:43–52. doi: 10.1097/00041552-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, Patel DD. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell CA, Cleary MD, Charo IF. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J Biol Chem. 1999;274:10053–10058. doi: 10.1074/jbc.274.15.10053. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Umehara H, Nakayama T, Yoneda O, Hieshima K, Kakizaki M, Dohmae N, Yoshie O, Imai T. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002;168:6173–6180. doi: 10.4049/jimmunol.168.12.6173. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Sato S, Echigo T, Hamaguchi Y, Yasui M, Takehara K. Up regulated expression of fractalkine/CX3CL1 and CX3CR1 in patients with systemic sclerosis. Ann Rheum Dis. 2005;64:21–28. doi: 10.1136/ard.2003.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debre P, Tedgui A, Murphy PM, Mallat Z. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- Robinson LA, Nataraj C, Thomas DW, Howell DN, Griffiths R, Bautch V, Patel DD, Feng L, Coffman TM. A role for fractalkine and its receptor (CX3CR1) in cardiac allograft rejection. J Immunol. 2000;165:6067–6072. doi: 10.4049/jimmunol.165.11.6067. [DOI] [PubMed] [Google Scholar]
- Rand ML, Wang H, Mody M, Chu I, Treutiger I, Nguyen A, Packham MA, Freedman J. Concurrent measurement of the survival of two populations of rabbit platelets labeled with either two PKH lipophilic dyes or two concentrations of biotin. Cytometry. 2002;47:111–117. doi: 10.1002/cyto.10055. [DOI] [PubMed] [Google Scholar]
- Baker GR, Sullam PM, Levin J. A simple, fluorescent method to internally label platelets suitable for physiological measurements. Am J Hematol. 1997;56:17–25. doi: 10.1002/(sici)1096-8652(199709)56:1<17::aid-ajh4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci USA. 1996;93:11877–11882. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneda S, Hudkins KL, Topouzis S, Gilbertson DG, Ophascharoensuk V, Truong L, Johnson RJ, Alpers CE. Obstructive uropathy in mice and humans: potential role for PDGF-D in the progression of tubulointerstitial injury. J Am Soc Nephrol. 2003;14:2544–2555. doi: 10.1097/01.asn.0000089828.73014.c8. [DOI] [PubMed] [Google Scholar]
- Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaji T, Hu X, Star RA. Alpha-melanocyte-simulating hormone and interleukin-10 do not protect the kidney against mercuric chloride-induced injury. Am J Physiol. 2002;282:F795–F801. doi: 10.1152/ajprenal.00203.2001. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Medina A, Santos F, Carbajo E, Rodriguez J, Alvarez J, Cobo A. Exacerbated inflammatory response induced by insulin-like growth factor I treatment in rats with ischemic acute renal failure. J Am Soc Nephrol. 2001;12:1900–1907. doi: 10.1681/ASN.V1291900. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996;97:232–237. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7:2495–2508. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, Border WA. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2:418–423. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- Akagi Y, Isaka Y, Arai M, Kaneko T, Takenaka M, Moriyama T, Kaneda Y, Ando A, Orita Y, Kamada T, Ueda N, Imai E. Inhibition of TGF-beta 1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int. 1996;50:148–155. doi: 10.1038/ki.1996.297. [DOI] [PubMed] [Google Scholar]
- Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR. Platelet-derived growth factor is chemotactic for fibroblasts. J Cell Biol. 1982;92:584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inngjerdingen M, Damaj B, Maghazachi AA. Expression and regulation of chemokine receptors in human natural killer cells. Blood. 2001;97:367–375. doi: 10.1182/blood.v97.2.367. [DOI] [PubMed] [Google Scholar]
- Schafer A, Schulz C, Eigenthaler M, Fraccarollo D, Kobsar A, Gawaz M, Ertl G, Walter U, Bauersachs J. Novel role of the membrane-bound chemokine fractalkine in platelet activation and adhesion. Blood. 2004;103:407–412. doi: 10.1182/blood-2002-10-3260. [DOI] [PubMed] [Google Scholar]
- Singbartl K, Green SA, Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J. 2000;14:48–54. doi: 10.1096/fasebj.14.1.48. [DOI] [PubMed] [Google Scholar]
- Nijm J, Wikby A, Tompa A, Olsson AG, Jonasson L. Circulating levels of proinflammatory cytokines and neutrophil-platelet aggregates in patients with coronary artery disease. Am J Cardiol. 2005;95:452–456. doi: 10.1016/j.amjcard.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Alpers CE, Hudkins KL, Floege J, Johnson RJ. Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. J Am Soc Nephrol. 1994;5:201–209. doi: 10.1681/ASN.V52201. [DOI] [PubMed] [Google Scholar]
- Tang WW, Van GY, Qi M. Myofibroblast and alpha 1 (III) collagen expression in experimental tubulointerstitial nephritis. Kidney Int. 1997;51:926–931. doi: 10.1038/ki.1997.131. [DOI] [PubMed] [Google Scholar]
- Paul LC. Chronic allograft nephropathy: an update. Kidney Int. 1999;56:783–793. doi: 10.1046/j.1523-1755.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- Faure S, Meyer L, Costagliola D, Vaneensberghe C, Genin E, Autran B, Delfraissy JF, McDermott DH, Murphy PM, Debre P, Theodorou I, Combadiere C. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287:2274–2277. doi: 10.1126/science.287.5461.2274. [DOI] [PubMed] [Google Scholar]
- Tuo J, Smith BC, Bojanowski CM, Meleth AD, Gery I, Csaky KG, Chew EY, Chan CC. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moatti D, Faure S, Fumeron F, Amara Mel W, Seknadji P, McDermott DH, Debre P, Aumont MC, Murphy PM, de Prost D, Combadiere C. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97:1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci USA. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Kawanokuchi J, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979:65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- Louvet C, Heslan JM, Merieau E, Soulillou JP, Cuturi MC, Chiffoleau E. Induction of fractalkine and CX3CR1 mediated by host CD8+ T cells in allograft tolerance induced by donor specific blood transfusion. Transplantation. 2004;78:1259–1266. doi: 10.1097/01.tp.0000140482.20336.77. [DOI] [PubMed] [Google Scholar]