Abstract
Natural killer (NK) cells play a key role in inflammation and tumor regression through their ability to migrate into tissues. CXCL12 is a chemokine that promotes lymphocyte invasion and migration into tissues; however, the mechanism for this process remains incompletely understood. In this study, we show that CXCL12 significantly enhanced CD16+CD56+ human peripheral NK-cell invasion into type I collagen by the catalytic activity of matrix metalloproteinase-1 (MMP-1). Confocal immunofluorescence and co-immunoprecipitation studies suggest that MMP-1 colocalized with α2β1 integrin on CXCL-12-stimulated NK-cell surface. The binding of pro-MMP-1 with α2β1 integrin required activation of Gi-coupled pathway. However, the production of MMP-1 from CXCL12-stimulated NK cells was mediated by p38 and mitogen-activated or extracellular signal-regulation protein kinase kinase 1/2 in a manner independent of the Gi-coupled pathway. These results suggest that CXCL12/CXCR4 interaction transduces the two signaling pathways to promote NK-cell invasion, which stimulates pericellular degradation of extracellular matrix proteins by membrane-associated MMP-1. The mechanisms would thus play a role in facilitating lymphocyte trafficking and accumulation in tissues during physiological and pathological processes.
Natural killer (NK) cells were first discovered based on their cytolytic ability against tumor cells and are considered to be a subset of immune cells responsible for tumor regression and inhibition of tumor metastasis.1 The ability of NK cells to migrate appears to be tightly regulated by various molecules, such as integrins, chemokines, and proteinases. Chemokines regulate lymphocyte trafficking in the body and also selectively induce the migration of lymphocytes into inflammatory sites. Stromal cell-derived factor 1α (CXCL12) is the only known ligand for the chemokine receptor CXCR4.2–4 CXCL12 stimulates migration of specific types of lymphocytes including CD34+ hematopoietic progenitor cells; T, B, and NK cells; and monocytes5–8 but not neutrophils.5–7,9,10 Although the significance of lymphocyte invasion into tissues has been described, the molecular mechanisms of chemokine-stimulated invasion of lymphocytes, such as NK cells, remain poorly understood.
Remodeling and degradation of the extracellular matrix (ECM) are vital components of physiological and pathological processes such as differentiation, proliferation, cell migration, and invasion. During cellular invasion, cells must degrade pericellular collagens such as type I collagen. The matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that play a primary role in the degradation of ECM proteins.11 So far, there are several MMPs categorized as collagenases: MMP-1, MMP-8, MMP-13, MMP-14, and to some extent MMP-2.12 These enzymes hydrolyze native collagens to generate one-fourth and three-fourths fragments, which can then act as gelatinases and stromelysins.13 Because gelatinases and stromelysins fail to degrade native collagens,13 it is plausible that the catalytic activity of collagenases plays a key role not only in disease status (ie, inflammation and tumor invasion) but also in the normal development of organogenesis.
Previous studies showed that cytokines such as interleukin-2 (IL-2) stimulate expression of multiple MMPs from lymphocytes,14 suggesting that the MMPs serve to degrade ECM proteins as lymphocytes extravasate from blood vessels and accumulate in the target sites. However, the mechanism by which lymphocytes invade tissues is not clear. In this study, we characterized the invasion of CD16+CD56+ human peripheral NK cells into type I collagen. Our results indicate that MMP-1 plays a key role in promoting NK-cell invasion in response to CXCL12. The association of MMP-1 with α2β1 integrin on CXCL-12-stimulated NK cells suggests that this integrin is important not only to promote cell adhesion to type I collagen but also to concentrate MMP-1 in the pericellular spaces to facilitate matrix protein degradation. These results suggest that the selective regulation of MMP-1 production and/or localization in NK cells may lead to effective strategies in controlling inflammation and tumor elimination.
Materials and Methods
Regents and Antibodies
Collagen type I was obtained from Nitta Gelatin Inc. (Osaka, Japan). Recombinant human tissue inhibitor of metalloproteinase-2 (TIMP-2) was purchased from Wako Pure Chemical Industries (Osaka, Japan). Anti-CXCR4 (12G5) antibody, a neutralizing anti-MMP-1 antibody (MAB901), and human recombinant CXCL12 were purchased from R&D Systems (Minneapolis, MN). SP600125, U0126, SB203580, GM6001, anti-MMP14 (Ab-2) polyclonal antibody, and anti-MMP-1 polyclonal antibody were purchased from Calbiochem (Darmstadt, Germany). Anti-α2β1-integrin antibody (MAB1998Z), anti-α2-integrin antibody (AB1936), and a neutralizing anti-MMP-1 (MAB13402)15 antibody were purchased from Chemicon (Temecula, CA). Anti-α2 (P1H5) and anti-TIMP-1 (H-150) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Streptavidin PerCP, fluorescein isothiocyanate (FITC)-conjugated anti-CD16, anti-CD49b, anti-CD29, and phycoerythrine-conjugated anti-CD56 antibodies were obtained from BD Biosciences (San Jose, CA). Biotinylated horse anti-mouse IgG was obtained from Vector Laboratories (Burlingame, CA). Mouse IgG1κ (MOPC) was purchased from Sigma Chemical Co. (St. Louis, MO). Alexa Fluor 488 goat anti-mouse IgG (H+L), Alexa Fluor 546 goat anti-rabbit IgG (H+L), and DQ-collagen I were purchased from Molecular Probes (Eugene, OR). Recombinant pro-MMP-1 was purchased from Oncogene (Boston, MA).
Isolation of Human NK Cells
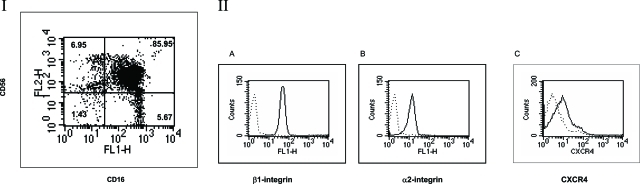
The following studies using human donors were performed in accordance with the Osaka Dental University (Osaka, Japan) (protocol no. 040522). Peripheral blood mononuclear cells were isolated from samples of venous blood from consenting healthy volunteers by Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) density-gradient centrifugation. For NK-cell isolation, cells were depleted of T lymphocytes, B lymphocytes, and macrophages/monocytes using an NK isolation kit II (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Using a MACS magnetic separator (MiniMacs MS+/RS; Miltenyi Biotec GmbH), more than 85% of NK cells were CD16+CD56+ as shown in Figure 1.
Figure 1-6938.
Expression of CXCR4 on freshly isolated CD16+CD56+ human peripheral NK cells. This figure shows the results of purified NK cells using a magnetic separator as described in Materials and Methods. Panel I shows the purified CD16+CD56+ NK cells; more than 85% of NK cells were CD16+CD56+. Panel II: The CD16+CD56+ NK cells were further purified and stained with anti-CD29 (β1 integrin) (A), anti-CD49b (α2 integrin) (B), or anti-CXCR4 antibodies (C) as described in Materials and Methods. The dotted lines represent the patterns of staining with the secondary antibody only. Results shown are for one donor (the same donor shown in other assay) but are representative of 12 donors tested.
Fluorescence-Activated Cell Sorting (FACS) Analysis
Expression of surface antigens was measured by FACS analyses. The purified NK cells were incubated at 4°C for 30 minutes with FITC-conjugated anti-CD16 and PE-conjugated anti-CD56 antibodies and washed extensively to remove excess amounts of the antibodies. The purified CD16+CD56+ NK cells were incubated with FITC-conjugated anti-CD49b (α2 integrin), or FITC-conjugated anti-CD29 (β1 integrin) antibodies. For detecting CXCR4 on CD16+CD56+ NK cells, cells were incubated with anti-CXCR4 antibody, washed, and incubated with biontinylated horse anti-mouse IgG, followed by streptavidin PerCP. Cells were washed extensively and analyzed using a FACS Caliber (BD Biosciences, Mountain View, CA).
Invasion Assays
CD16+CD56+ NK-cell invasion was evaluated using type I collagen gels cast in 24-well transwell culture inserts with 3-μm pore size (Corning Incorporated, Corning, NY). Type I collagen was diluted in serum-free RPMI 1640 (RPMI-SF) to a final concentration of 200 μg/ml. One hundred microliters of the diluted type I collagen solution was allowed to form a gel in the upper chamber of a 24-well transwell by incubating at 37°C for 4 to 5 hours. Gels were washed twice with prewarmed RPMI-SF. Peripheral NK cells were harvested, washed three times with RPMI 1640 to 10% fetal calf serum, and then resuspended in RPMI 1640 to 1% fetal calf serum at a concentration of 1 × 106 cells/ml to ensure the viability of NK cells during the assay period. The cell suspension (100 μl) was placed in the upper chamber of each transwell. The lower chamber was filled with 600 μl of RPMI-SF containing 10 μg/ml human plasma fibronectin as an adhesive matrix. CXCL12 was added to the bottom chambers (200 ng/ml). MMP inhibitors (20 μmol/L GM6001, 200 ng/ml TIMP-2, or 2 and 20 μg/ml neutralizing anti-MMP-1 monoclonal antibodies) and anti-α2 integrin antibody (P1E6) (20 μg/ml) were added to both the cell suspension and the collagen gel. Isotype-matched control antibody was used at a concentration of 20 μg/ml. NK cells were preincubated with 5 μmol/L U0126, 10 μmol/L SB203580, and 100 μmol/L SP600125 for 30 minutes at 37°C, and these inhibitors were included in cell suspension and collagen gel during invasion assays for 20 hours. The polycarbonate membranes were stained with Diff-Quick (Baxter, Miami, FL), and noninvaded cells were removed by scraping from the upper side of the membranes. Invaded cells were visualized microscopically (50×) and counted from three randomly selected areas from one membrane. Each assay condition was performed in triplicate, and the results were expressed as means ± SD per mm2 from one representative experiment. In each experiment, we have checked bottom chambers to ensure that there were no floating cells that had failed to attach to the membrane.
Migration Assays
NK cells were serum-starved in migration medium (RPMI 1640 containing 1% bovine serum albumin [BSA] and 10 mmol/L HEPES buffer, pH 6.9) for 4 hours. Migration assays were performed in transwell chambers with 3-μm polycarbonate membrane (Corning Incorporated) precoated with 100 μg/ml type I collagen or BSA on both sides of the filter. Human CXCL12 (100 ng/ml) was diluted to appropriate concentrations in migration medium and added to the lower chamber of the transwell chambers. Medium alone was added to wells left unstimulated. The cells were allowed to migrate for 4 hours at 37°C in 5% CO2. Filters were stained with Diff-Quick. Each sample was assayed in triplicate, and migrated cells were counted in five randomly selected high power fields (×400) per well. Results were expressed as mean ± SD per mm2 from one representative experiment.
DQ-Collagen Degradation Assay
Precoated glass slides were coated with 25 μg/ml quenched fluorescent substrate DQ-collagen I (Molecular Probes). Fresh NK cells were incubated with 20 ng/ml CXCL12 for 20 hours followed by incubating on DQ-collagen I-coated plates for a period of 4 hours. Cells were gently resuspended and placed on glass slides and fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS). The slides were mounted with coverslips using glycerol/PBS and examined with 488 nm (excitation) and 533 nm (emission) using an Olympus LSM-GB200 (Olympus, Tokyo, Japan) with an oil immersion lens. Differential interference contrast (DIC) was shown to visualize cells cultured on the matrix.
Western Blot Analysis
CD16+CD56+ NK (1 × 106) cells were incubated in serum-free RPMI 1640 with 20 ng/ml CXCL12 or 500 U/ml IL-2 for 24 hours. Conditioned media were collected, centrifuged to remove debris, and concentrated in Amicon Centriprep concentrators (Millipore Corporation, Bedford, MA) up to 10-fold, to visualize proteins by Western blotting and zymography analysis. Total cell lysates were prepared by dissolving cells in sodium dodecyl sulfate (SDS)-sample buffer and briefly sonicated to sheer DNA. In some studies, freshly isolated NK cells were preincubated with 100 ng/ml pertussis toxin, 5 μmol/L U0126, 10 μmol/L SB203580, or 100 μmol/L SP600125 for 30 minutes at 37°C before incubation with CXCL12. Samples were separated on 10% SDS polyacrylamide gels (SDS-PAGE) under reducing conditions. Proteins were transferred to polyvinylidene difluoride (Immobilon-P) membranes (Sigma Chemical Co.). Membranes were incubated for 3 hours with primary antibodies in PBS containing 0.05% Tween 20 and 10% Blockace (Dainippon Pharm Co., Osaka, Japan). Peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ) was used at a 1:1000 dilution, and immunoreactive bands were visualized using Super Signal West Pico chemiluminescent substrate (Pierce Biotechnology Inc., Rockford, IL). Signals on each membrane were analyzed with VersaDoc 5000 (Bio-Rad, Hercules, CA).
Immunoprecipitation
CD16+CD56+ NK (1.5 × 107) cells were preincubated with 100 ng/ml PTX, 5 μmol/L U0126, or 10 μmol/L SB203580 for 30 minutes at 37°C, and then NK cells were placed in serum-free RPMI 1640 with 20 ng/ml CXCL12 overnight and lysed by adding 200 μl of lysis buffer (50 mmol/L Tris-HCl [pH 7.4], 150 mmol/L NaCl, 0.1% SDS, 1% Igepal, 1 mmol/L ethylenediamine tetraacetic acid, 2 mmol/L phenylmethylsulfonyl fluoride [PMSF], 2 mmol/L sodium vanadate, 20 μg/ml leupeptin, and 20 μg/ml aprotinin). Lysates were clarified by centrifugation at 12,000 rpm for 10 minutes at 4°C. Immunoprecipitations were performed as previously described using anti-α2β1 integrin antibody (MAB1998Z) and protein G beads (Amersham Biosciences) for 2 hours at 4°C as previously described.16,17 After washing with lysis buffer, proteins were released from the beads by heating at 95°C for 5 minutes. Samples were separated on 8% SDS polyacrylamide gels (SDS-PAGE) under reducing conditions. Proteins were transferred and detected as described above.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from human NK cells by RNeasy kit (Qiagen, Valencia, CA). Fresh NK cells were placed in serum-free RPMI 1640 with 50 ng/ml CXCL12 in suspension at indicated periods. After denaturation of total RNA at 70°C for 10 minutes, cDNA was synthesized with oligo-dT primer by incubating with reverse transcriptase (Qiagen) at 50°C for 30 minutes. The primers for MMP-1 were 5′-AAT GGA AAA CAC ATG GTG TGA GTC C-3′ (forward) and 5′-TAT CTA GGG TGA CAC CAG TGA CTG-3′ (reverse). The primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were 5′-ACC ACA GTC CAT GCC ATC AC-3′ (forward) and 5′-TCC ACC ACC TTG CTG CTG TA-3′ (reverse). Polymerase chain reactions were performed with Pfu polymerase (Qiagen) initiated by 1 cycle at 95°C for 15 minutes followed by 30 cycles at 94°C for 45 seconds, 55°C for 45 seconds, 72°C for 1 minute, and then 1 cycle at 72°C for 10 minutes for final extension. PCR products were loaded onto agarose gel and stained with ethidium bromide. The bands were analyzed using AlphaImager IS-3400 software (Alpha Innotech, San Leandro, CA). Briefly, integrated density value (IDV) was measured as the sum of all of the pixel values after background correction in each band. The values (AVG) of each band were calculated as IDV/area, where area is the size of the region that was measured. The results are shown the values of AVGMMP1/AVGGAPDH at each time point.
Gelatin Zymography
The ability of NK cells to produce MMP-2 and MMP-9 was determined by gelatin zymography as described previously.18 In brief, cells were incubated in serum-free medium containing 20 ng/ml CXCL12 or 500 U/ml IL-2. After a 24-hour incubation at 37°C, the supernatants were collected, concentrated, and resolved under nonreducing conditions on 10% polyacrylamide gels containing 1 mg/ml gelatin (Sigma Chemical Co.). Gels were rinsed once in 2.5% Triton X-100 for 30 minutes at room temperature and then incubated in developing solution (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, and 1 μmol/L ZnCl2, pH 7.6) for 8 to 16 hours at 37°C. Gels were stained with Coomassie blue, and areas of gelatinolytic activity were detected as transparent bands. As a standard control, the conditioned medium prepared from HT1080 cells stimulated with ConA was used to show the pro-, intermediate, and active forms of MMP-2.
Subcellular Fractionation
Cellular fractionation was adapted from previously described methods for isolating cytosolic and membrane fraction.19 NK cells (1 × 107) were stimulated with 20 ng/ml CXCL12 for 3 minutes. Cells were resuspended in 1 ml of hypotonic buffer (10 mmol/L Tris, pH 8.0, 1 mmol/L MgCl2, 1 mmol/L NaOVO4, 1 mmol/L PMSF, 1 μg/ml aprotinin, and 1 μg/ml leupeptin) and sonicated for 1 minute. Thirty microliters of 5 mol/L NaCl was added, and the lysate was spun down at 200 × g for 10 minutes. The pellet containing membrane proteins was resuspended in 1 ml of 2-(N-morpholino)ethanesulfonic acid-4-morpholineethanesulfonic acid (MES)-buffered saline (25 mmol/L MES, 150 mmol/L NaCl, pH 6.5, 2 mmol/L ethylenediamine tetraacetic acid, 0.5% Triton X-100, 1 mmol/L NaOVO4, 1 mmol/L PMSF, 1 μg/ml aprotinin, and 1 μg/ml leupeptin) and sonicated for 1 minute. Protein concentrations were determined, and then the same amounts of proteins were separated on SDS-PAGE and blotted with anti-MMP-1 antibody as described above.
Laser Scanning Confocal Microscopy
Peripheral blood NK cells were stimulated with 20 ng/ml CXCL12 for 24 hours. Cells were washed twice and incubated with anti-MMP-1 antibody and anti-α2 antibody (P1H5) for 30 minutes on ice, washed twice in ice-cold PBS containing 0.2% BSA (PBS/BSA), and then incubated with the Alexa Fluor 488-goat anti-mouse IgG (H+L) secondary antibody and the Alexa Fluor 546-goat anti-rabbit IgG (H+L) secondary antibody for 30 minutes on ice. Cells were gently resuspended and placed on glass slides and fixed with 2% paraformaldehyde in PBS. The slides were mounted with coverslips using glycerol/PBS and examined using an Olympus LSM-GB200 (Olympus) with an oil immersion lens. Appropriate excitation and barrier filters were used to observe fluorescence. For determining the distribution of antigens, approximately 200 cells were evaluated per slide. DIC was shown to indicate the presence of cells. Photographs of cells shown in figures are representative of the majority of cells displaying cell surface staining patterns observed in these experiments.
Pro-MMP-1 Binding Assay
Peripheral blood NK cells were incubated with 1 ng/ml recombinant pro-MMP-1 and 20 ng/ml CXCL12 in the presence or absence of PTX (100 ng/ml) for 10 minutes in RPMI serum-free medium. Cells were washed twice and incubated with anti-MMP-1 antibody for 30 minutes on ice, washed twice in ice-cold PBS containing 0.2% BSA (PBS/BSA), and then incubated with the Alexa Fluor 546-goat anti-rabbit IgG (H+L) secondary antibody for 30 minutes on ice. Cells were gently resuspended and placed on glass slides and fixed with 2% paraformaldehyde in PBS. The slides were mounted with coverslips using glycerol/PBS and examined using an Olympus LSM-GB200 (Olympus) with an oil immersion lens.
Statistical Analysis
Values shown are mean ± SD. All data were analyzed by analysis of variance followed by a paired Tukey-Kramer multiple comparison method. Statistically significant differences are shown with asterisks.
Results
CXCL12 Enhanced CD16+CD56+ NK-Cell Invasion into Type I Collagen in an MMP-Dependent Manner
Although CD16+CD56+ NK cells have been demonstrated to play a key role in tumor elimination by traversing from blood vessels to tumor mass,1 the molecular mechanisms for invading into tissues are not clear. The purified NK cells (Figure 1I) demonstrated that this population consisted of more than 85% of CD16+CD56+ cells. The double positive population was further purified and stained with anti-α2(Figure 1I, A), anti-β1(Figure 1I, B), and anti-CXCR4 (Figure 1I, C) antibodies with appropriate secondary antibodies (Figure 1). The purified CD16+CD56+ NK cells uniformly expressed type I collagen receptor α2β1 integrin, and the majority of them express CXCR4 chemokine receptor, consistent with the predominance of this population in CXCL12 (SDF-1α/PBSF)-attracted populations9(Figure 1). Stimulation of purified CD16+CD56+ NK cells with CXCL12 significantly enhanced migration to type I collagen or fibronectin (not shown), suggesting that CXCR4 expressed on CD16+CD56+NK cells can functions to induce signals. When CD16+CD56+ NK cells were cultured on type I collagen and stimulated with CXCL12 in the bottom chamber, a significant increase in the NK-cell invasion was observed (Figure 2A). Because type I collagen is a substrate for MMPs, we used GM6001, a broad synthetic MMP inhibitor, to inhibit the increased invasion. GM6001 significantly inhibited CXCL12-stimulated enhanced invasion (Figure 2A). Furthermore, recombinant tissue inhibitor of metalloproteinases, TIMP-2, a well-characterized, naturally occurring MMP inhibitor, also significantly inhibited CXCL12-stimulated invasion into type I collagen (Figure 2A). These results indicate that MMPs play a key role in CXCL12-stimulated NK-cell invasion into type I collagen.
Figure 2-6938.
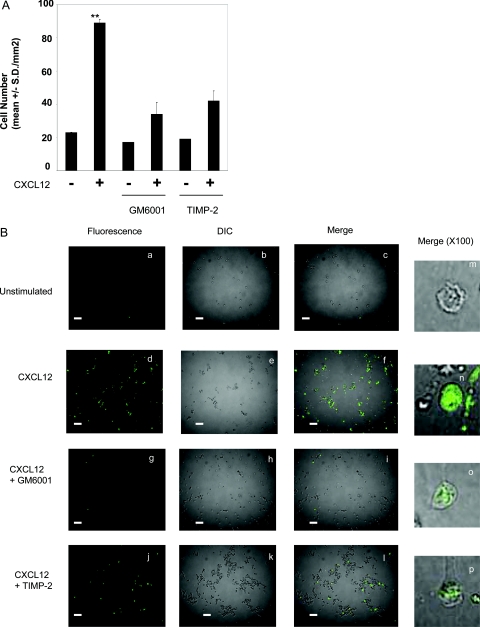
CXCL12 enhances NK-cell invasion and degradation of type I collagen. A: NK cells were assayed for invasive activity in transwell chambers precoated with type I collagen gel (200 μg/ml). CXCL12 (200 ng/ml) was added to the bottom chambers. For inhibiting NK-cell invasion, MMP inhibitors GM6001 (20 μmol/L) or TIMP-2 (200 ng/ml) were added to both the cell suspension and the collagen gel during invasion assays for 20 hours. Results are shown as the mean number of invasive cells in 10 randomly selected fields in a single representative experiment of four performed. **P < 0.001 compared with unstimulated NK cells. B: Glass slides were coated with 25 μg/ml of quenched fluorescent substrate DQ-collagen I. Fresh NK cells were incubated in the presence or absence of 20 ng/ml CXCL12 for 20 hours. Cells were cultured on DQ-collagen I-coated plates for an additional 4 hours. Unstimulated NK cells (a, b, c, and m), CXCL12-stimulated NK cells (d, e, f, and n), and CXCL12-stimulated NK cells with MMP inhibitors GM6001 (20 μmol/L) (g, h, i, and o) or TIMP-2 (200 ng/ml) (j, k, l, and p) are shown. Degradation of type I collagen (green fluorescence) was detected by confocal microscopy (excitation, 488 nm; emission, 530 nm). Pictures were taken at ×40 magnitude (a–l). DIC images are shown. Bar represents 500 μm. The images in the right column (m–p) are shown of the representative degradation of type I collagen by one NK cell taken at ×100 magnitude.
To show that CXCL12 stimulation of NK cells enhanced type I collagen degradation, NK cells were incubated on intramolecular-quenched fluorescent protein substrate, DQ-type I collagen (Figure 2B). Fresh NK cells were incubated in the presence or absence of CXCL12 and plated on the DQ-collagen I-coated plates as described in Materials and Methods. Degradation of DQ-collagen I was visualized in optical section as green fluorescent signal. Whereas unstimulated NK cells gave minimal visible signals (Figure 2B, a), CXCL12-stimulated cells significantly enhanced degradation of type I collagen as visualized by the increased green fluorescence signals (Figure 2B, d). This enhanced degradation was significantly inhibited by GM6001 or TIMP-2, although there was residual degradation of type I collagen (Figure 2B, g and j). The degradation was semiquantitatively determined in more than 100 cells by calculating green fluorescence-positive cells in randomly chosen fields. Although unstimulated NK cells that degrade type I collagen represented less than 0.1% (Figure 2B, a), CXCL12 stimulated type I collagen degradation by up to 80% (Figure 2B, d). Conversely, treatment with GM6001 (Figure 2B, g) or TIMP-2 (Figure 2B, j) reduced CXCL12-stimulated degradation back to 0.8 and 10%, respectively. The incomplete inhibition of degradation of type I collagen can account for the residual invasion of CXCL12-stimulated NK cells in the presence of these inhibitors (Figure 2A). More importantly, the type I collagen degradation by CXCL12-stimulated NK cells was localized to the cell surface (Figure 2B, n), and the pericellular degradation was significantly inhibited by GM6001 (Figure 2B, o) or TIMP-2 (Figure 2B, p), suggesting that CXCL12 enhances NK-cell surface proteolysis of type I collagen in an MMP-dependent manner, thereby promoting NK-cell invasion into the matrix.
Characterization of MMPs and TIMPs Produced from CXCL12-Stimulated NK Cells
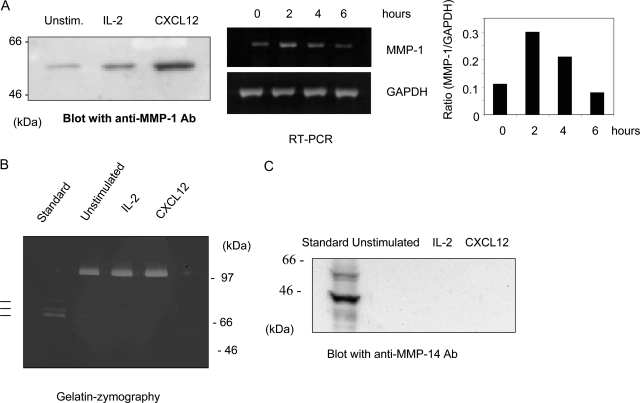
Because the type I collagen used in this study was not digested with pepsin, the triple helical structure of type I collagen would be native. Thus, we hypothesize that CXCL12-stimulated NK-cell invasion into type I collagen could be mediated by enhanced production or activity of collagenases. We characterized MMP-1, -2, -8, -13, and -14 expression in CXCL12-stimulated NK cells. When NK cells were cultured in the presence of CXCL12, the production of MMP-1 in the serum-free conditioned medium was significantly enhanced compared with the unstimulated NK cells (Figure 3A). These results were confirmed by RT-PCR studies demonstrating that expression of MMP-1 mRNA was enhanced 2 hours after CXCL12 treatment and gradually decreased over the time course (Figure 3A). In contrast, the production of MMP-1 from IL-2-stimulated NK cells was enhanced but was not as significant as that from CXCL12-stimulated NK cells (Figure 3A), which could account for the inability of IL-2 to enhance NK invasion into collagen I (not shown). When NK cells were cultured with CXCL12 in the presence or absence of type I collagen matrix, the expression of MMP-1 was not affected, as evaluated by RT-PCR and Western blotting analysis (not shown), suggesting that the induction of MMP-1 from NK cells was enhanced by CXCL12 but not by the matrix. The same samples prepared in Figure 3A were separated on gelatin zymography to visualize MMP-2 and MMP-9. Unstimulated NK cells spontaneously produced the latent form of MMP-9, and the amount was not altered by stimulation with CXCL12 or IL-2, thus serving as a loading control for Figure 3A(Figure 3B). These stimulations failed to induce the production of MMP-2 (Figure 3B). MMP-14 is a membrane-associated collagenase and has been demonstrated to promote cell invasion into type I collagen.20 The cell lysates prepared from CXCL12 or IL-2-stimulated NK cells were tested for the production of MMP-14. Neither IL-2 nor CXCL12 stimulated MMP-14 expression (Figure 3C). Neither MMP-8 nor MMP-13 expression was detected in CXCL12-stimulated NK cells (not shown).
Figure 3-6938.
Expression of MMPs from CXCL12-stimulated NK cells. NK cells were incubated in serum-free RPMI 1640 containing 500 U/ml IL-2 or 20 ng/ml CXCL12 for 24 hours, and the conditioned medium and total cell lysates were prepared as described in Materials and Methods. A: The concentrated conditioned medium was separated on SDS-PAGE and blotted with anti-MMP-1 antibody and visualized with Super Signal West Pico chemiluminescent substrate. Molecular markers (kd) are shown in the left column. Total RNA was isolated from NK cells cultured in the presence of CXCL12 for 0, 2, 4, or 6 hours and subjected to RT-PCR experiments by using specific primers for MMP-1 (top) or GAPDH (bottom). The RT-PCR results were subjected to densitometry analysis as described in Materials and Methods. The result is shown as the ratio of MMP-1 to GAPDH at each time point. B: The concentrated conditioned medium was analyzed by gelatin-zymography as described in Materials and Methods. As a standard, conditioned medium prepared from HT1080 cells stimulated with ConA was used to localize pro- (top), intermediate (middle), and active (bottom) forms of MMP-2 are shown as lines. Molecular markers (kd) are shown in the left column. C: Total cell lysates were separated on SDS-PAGE, blotted with anti-MMP-14 antibody, and visualized with Super Signal West Pico chemiluminescent substrate. As a standard, HT1080 cells lysates stimulated with ConA were used. Note that the MT1-MMP proteins were detected as three species. The 56- and 54-kd bands represent zymogen and active form, respectively.47 The 45-kd band is a degraded MT1-MMP.47 Molecular markers (kd) are shown in the left column.
Because IL-2-activated mouse NK cells produce TIMPs,14,21 we also tested whether CXCL12-stimulated NK cells produce TIMP-1 and -2. Using Western blotting analysis, we did not detect these inhibitors in the conditioned medium of CXCL12-activated NK cells (not shown). Taken together, these results suggest that CXCL12 specifically stimulates the production of MMP-1 from NK cells without altering the production of other collagenases and TIMPs.
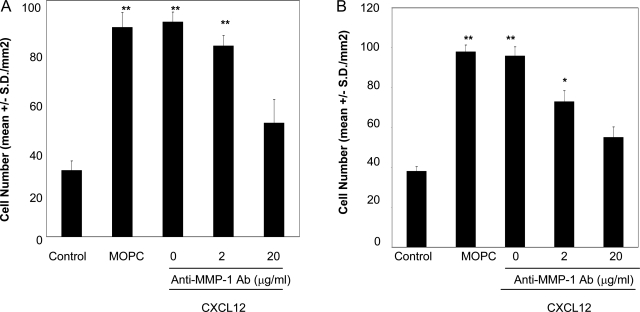
To evaluate the role of MMP-1 activity in CXCL12-stimulated NK-cell invasion into type I collagen, we tested two neutralizing antibodies against MMP-1 for their ability to inhibit invasion into type I collagen. In both experiments, treatment with the inhibitory antibodies significantly (more than 80%) inhibited CXCL12-stimulated NK-cell invasion at a concentration of 20 μg/ml, whereas the isotype-matched control antibody (MOPC) did not affect the cell invasion at this concentration. (Figure 4, A and B). The concentrations of the antibodies that inhibited the invasion were similar to those that inhibited epithelial cell migration.15 These results indicate that the catalytic activity of MMP-1, as a major matrix-degrading enzyme, is important in promoting invasion into type I collagen. The incomplete inhibition observed by these antibodies would be because of the insufficient accessibility of the antibodies on NK-cell surface. It is also possible that other MMPs are involved in the process.
Figure 4-6938.
MMP-1 plays a key role in CXCL12-stimulated NK-cell invasion into type I collagen. NK cells were assayed for invasive activity in transwell chambers precoated with type I collagen gel (200 μg/ml). CXCL12 (200 ng/ml) was added to the bottom chambers. Anti-MMP-1 antibody (MAB13402) (A) or anti-MMP-1 antibody (MAB901) (B) was added to both cell suspension and the collagen gel. The concentrations of the inhibitors are shown in the figure. Isotype-matched control antibody (MOPC) was used at a concentration of 20 μg/ml. Results are shown as the mean number of invasive cells in 10 randomly selected fields in a single representative experiment of four performed. **P < 0.001, *P < 0.05 (compared with unstimulated NK cells).
Cell Surface Association of MMP-1 in CXCL-12-Stimulated NK Cells
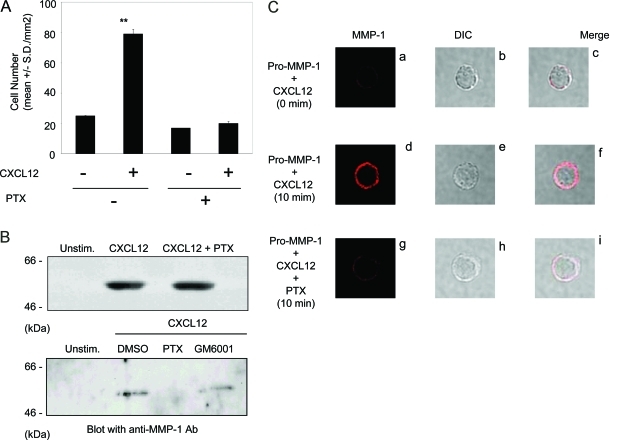
To test whether Gi-coupled signaling pathways were involved in the CXCL12-stimulated NK-cell invasion into type I collagen, NK cells were incubated with CXCL12 in the presence or absence of PTX. PTX significantly inhibited CXCL12-stimulated NK-cell invasion (Figure 5A), indicating the involvement of Gi-coupled pathway in the process of invasion. Although PTX did not affect CXCL12-stimulated MMP-1 production in the conditioned medium (Figure 5B, top panel), PTX significantly reduced the amount of MMP-1 associated with plasma membrane fractions of CXCL12-stimulated NK cells (Figure 5B, bottom panel). GM6001 did not affect the membrane association of MMP-1 (Figure 5B, bottom panel). These results indicate that membrane association of MMP-1 is regulated by Gi-coupled pathway but not by the enzymatic activity of MMP-1 in CXCL12-stimulated NK cells.
Figure 5-6938.
PTX inhibits CXCL12-stimulated NK-cell invasion. A: NK cells were pretreated with PTX (100 ng/ml) at 37°C for 30 minutes and assayed for the invasive ability into type I collagen in the continuous presence of PTX (100 ng/ml) in both the cell suspension and the collagen gel. CXCL12 (200 ng/ml) was added to the bottom chambers and gel during invasion assays for 20 hours. Results are shown as the mean number of invasive cells in 10 randomly selected fields in a single representative experiment of four performed. **P < 0.001 compared with unstimulated NK cells. B: NK cells were pretreated with PTX (100 ng/ml) for 30 minutes and incubated in serum-free RPMI 1640 containing 20 ng/ml CXCL12 for 24 hours. Medium supernatants were concentrated and blotted with anti-MMP-1 antibody (top panel). Membrane fraction was purified from CXCL12-stimulated NK cells cultured in the presence or absence of PTX (100 ng/ml) or GM6001 (20 μmol/L) for 24 hours and separated on SDS-PAGE. Membranes were blocked and incubated with anti-MMP-1 antibody followed by Super Signal West Pico chemiluminescent substrate. Molecular weight markers (kd) are shown in the left column (bottom panel). C: Fresh NK cells were incubated for 10 minutes with CXCL12 (20 ng/ml) and recombinant pro-MMP-1 (1 ng/ml) in the absence (d–f) or presence (g–i) of PTX (100 ng/ml). Cells were incubated with anti-MMP-1 antibody and then incubated with the Alexa Fluor 546-goat anti-rabbit IgG (H+L) secondary antibody for 30 minutes on ice (a, d, and g). A representative cell from the 200 examined in each experimental condition is shown. The top panel (a–c) shows an NK cell immediately after incubation with CXCL12 and exogenous pro-MMP-1. DIC (b, e, and h); merged images (c, f, and i) of the same cells are shown.
To further characterize the Gi-coupled pathway in MMP-1 associated with NK-cell surface, NK cells were incubated with CXCL12 in the presence of recombinant pro-MMP-1 for a short period (10 minutes) and stained with anti-MMP-1 antibody with a fluorescence-conjugated secondary antibody (Figure 5C). In the following studies, we observed more than 200 cells in each experiment and showed one NK-cell representing the localization of MMP-1 on cell surface. Whereas exogenous pro-MMP-1 bound minimally to CXCL12-stimulated NK cells immediately after the incubation (Figure 5C, a and c), it efficiently bound to CXCL12-stimulated NK cells at the 10-minute time point (Figure 5C, d and f). The binding was significantly decreased when CXCL12-stimulated NK cells were incubated in the presence of PTX (Figure 5C, g and i). Endogenous production of MMP-1 from CXCL12-stimulated NK cells was not detected at the 10-minute incubation period (not shown), indicating that the MMP-1-associated NK-cell surface was derived from the exogenous pro-MMP-1. Taken together, these results suggest that the MMP-1 association with CXCL12- stimulated NK cells requires the activation of the Gi-coupled pathway.
α2β1-Integrin Plays a Major Role for Promoting Invasion and Association with MMP-1
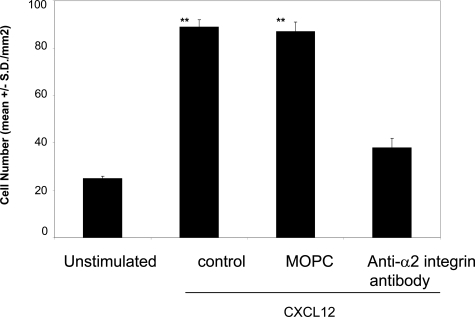
There is substantial evidence demonstrating that α2β1 integrin promotes cell adhesion to type I collagen.22 CXCL12-stimulated NK-cell invasion into type I collagen gel was significantly inhibited by a function-blocking anti-α2 integrin antibody but not by an isotype-matched control antibody (MOPC) (Figure 6). Thus, these results suggest that both the catalytic activity of MMP-1 and the function of α2β1 integrin are responsible for promoting CXCL12-stimulated NK-cell invasion into type I collagen, and the two proteins may form a complex on CXCL-12-stimulated NK cells.
Figure 6-6938.
α2β1 integrin mediates CXCL12-stimulated NK-cell invasion. NK cells were assayed for invasive activity in transwell chambers precoated with type I collagen gel (200 μg/ml). CXCL12 (200 ng/ml) was added to the bottom chambers. For inhibiting NK-cell invasion, anti-α2 integrin antibody (P1E6) (20 μg/ml) was added to both the cell suspension and the collagen gel during invasion assays for 20 hours. MOPC was used as an isotype-matched control antibody at a concentration of 20 μg/ml. Results are shown as the mean number of invasive cells in 10 randomly selected fields in a single representative experiment of four performed. **P < 0.001 compared with unstimulated NK cells.
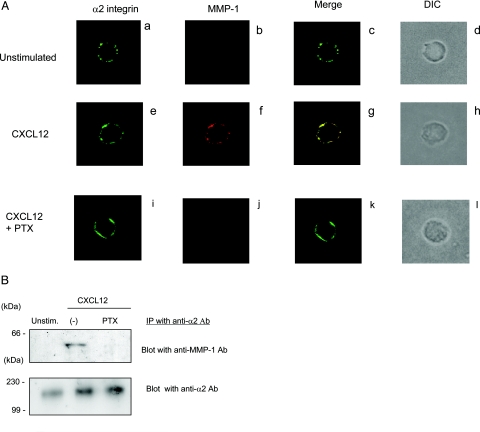
To localize MMP-1 and α2 integrin subunit on NK-cell surface, NK cells cultured in the presence or absence of CXCL12 were stained with anti-MMP-1 antibody (red) and anti-α2 integrin antibody (green). Although unstimulated NK cells did not express any detectable MMP-1 on cell surface (Figure 7A, b), stimulation with CXCL12 enhanced MMP-1 expression on NK-cell surface (Figure 7A, f). More importantly, the majority of the MMP-1 colocalized with α2 integrin subunit (Figure 7A, g). When NK cells were stimulated by CXCL12 in the presence of PTX, there was significantly less MMP-1 on the cell surface (Figure 7A, j). The results were confirmed by co-immunoprecipitation studies (Figure 7B). Although MMP-1 was not detected in the immunoprecipitates with anti-α2 integrin antibody in unstimulated NK cells, CXCL12 stimulated the production and association of MMP-1 with α2 integrin subunit (Figure 7B). Treatment of CXCL12-stimulated NK cells with PTX abolished the association of MMP-1 with α2β1 integrin (Figure 7B). These results suggest that α2β1 integrin expressed on CXCL12-stimulated NK cells associates with MMP-1, and the association requires the activation of Gi-coupled pathway.
Figure 7-6938.
PTX inhibits α2β1 integrin associate with MMP-1 on CXCL12-stimulated NK cells. A: NK cells were incubated with CXCL12 (20 ng/ml) in the absence (e–h) or presence (i–l) of PTX (100 ng/ml) for 24 hours. Unstimulated NK cells are shown in a–d. Cells were incubated with anti-MMP-1 (b, f, and j) and anti-α2 antibodies (a, e, and i) and then incubated with the Alexa Fluor 488-goat anti-mouse IgG (H+L) secondary antibody and the Alexa Fluor 546-goat anti-rabbit IgG (H+L) secondary antibodies. Confocal images of the localization of α2 integrin (green) and MMP-1 (red) are shown. Merged images (yellow) are shown (c, g, and k). DIC of the same cells are also shown (d, h, and i). B: Cell lysates prepared from unstimulated, CXCL12-stimulated, or CXCL12-stimulated in the presence of PTX were immunoprecipitated with anti-α2β1 integrin antibody (MAB1998Z). Immunoprecipitates were separated on SDS-PAGE and blotted with anti-MMP-1 antibody. Membranes were subsequently stripped and reprobed with anti-α2 integrin antibody (AB1936). Proteins were detected with Super Signal West Pico chemiluminescent substrate. Molecular weight markers (kd) are shown in the left column.
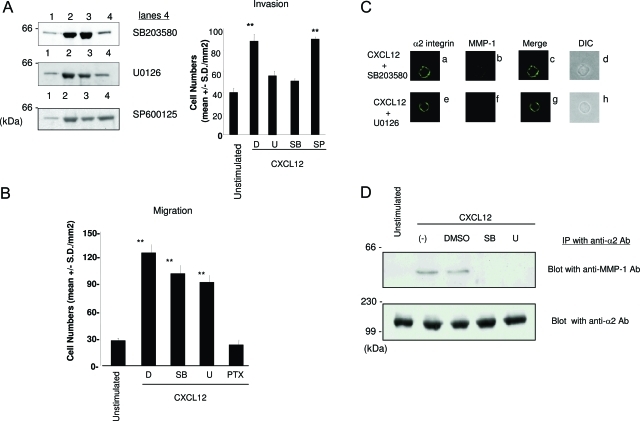
p38 and MEK1/2 Are Involved in CXCL12-Stimulated MMP-1 Production and Invasion into Type I Collagen on NK Cells
Previous studies demonstrated that CXCL12/CXCR4 interaction stimulates both Gi-coupled pathway-dependent and -independent signaling in T cells.23 We demonstrated that MMP-1 production from CXCL12-stimulated NK cells is independent of the Gi-coupled pathway (Figure 5B). Because mitogen-activated protein (MAP) kinases such as p38, MEK1/2, and JNK are involved in MMP production in various cell types,24,25 we tested specific inhibitors of these MAP kinases for the ability to inhibit MMP-1 production from CXCL12-stimulated NK cells. The production of MMP-1 was significantly inhibited by SB203580 (p38 inhibitor) and U0126 (MEK1/2 inhibitor) but not by SP600125 (JNK inhibitor) (Figure 8A), indicating that p38 and MEK1/2 play a key role in Gi-coupled pathway-independent MMP-1 production from CXCL12-stimulated NK cells. To test whether inhibitors for p38 and MEK1/2 inhibit CXCL12-stimulated NK-cell invasion into type I collagen, invasion assays were performed in the presence of SB203580, U0126, or SP600125 (Figure 8A). Under these experimental conditions, SB203580 and U0126 but not SP600125 significantly inhibited the enhanced NK-cell invasion. In contrast, neither SB203580 nor U0126 inhibited CXCL12-stimulated migration to type I collagen (Figure 8B) as previously reported in T cells.26,27 These results suggest that production of MMP-1 is responsible for promoting CXCL12-stimulated NK-cell invasion, which is independent of the ability of the stimulated cells to migrate.
Figure 8-6938.
MAP kinase inhibitors reduce CXCL12-stimulated production of MMP-1 and NK-cell invasion. A: The concentrated conditioned medium prepared from unstimulated NK cells (lane 1), CXCL12-stimulated NK cells (lane 2), CXCL12-stimulated NK cells cultured in the presence of dimethylsulfoxide (DMSO; solvent control) (lane 3), SB203580 (10 μmol/L) (lane 4 in the top panel), U0126 (5 μmol/L) (lane 4 in the middle panel), or SP600125 (100 μmol/L) (lane 4 in the bottom panel) were separated on SDS-PAGE. The membranes were blotted with anti-MMP-1 antibody and visualized with Super Signal West Pico chemiluminescent substrate. Molecular weight markers (kd) are shown in the left column. NK cells were pretreated with DMSO (D), MAP kinase inhibitors U0126 (5 μmol/L) (U), SB203580 (10 μmol/L) (SB), or SP600125 (100 μmol/L) (SP) at 37°C for 30 minutes and assayed for the invasive ability into type I collagen in the continuous presence of these inhibitors in both the cell suspension and the collagen gel. CXCL12 (200 ng/ml) was added to the bottom chamber gel during invasion assays for 20 hours. **P < 0.001 compared with unstimulated NK cells. B: The ability of migration of NK cells by CXCL12 toward type I collagen was evaluated in the transwell chambers. NK cells were treated with DMSO (D), SB203580 (SB), or U0126 (U) at the same concentrations as described above. PTX was used at a concentration of 100 ng/ml. Migration assays were performed for 4 hours. Results are shown as the mean number of invasive cells in 10 randomly selected fields in a single representative experiment of four performed. **P < 0.001 compared with unstimulated NK cells. C: NK cells that were prepared as described for A were stained with anti-MMP-1 and anti-α2 integrin antibodies followed by secondary antibodies as described for Figure 7A. Confocal immunofluorescence images of the localization of α2 integrin (green) (a and e), MMP-1 (red) (b and f) and their merged images (yellow) (c and g) are shown. DIC (d and h) of the same cells are shown. D: Cell lysates were prepared from unstimulated, CXCL12 (20 ng/ml)-stimulated, and CXCL12 (20 ng/ml)-stimulated cells in the presence of DMSO, SB203580 (10 μmol/L) (SB), or U0126 (5 μmol/L) (U) for 20 hours. Cell lysates were immunoprecipitated with anti-α2β1 integrin antibody (MAB1998Z). Immunoprecipitates were separated on SDS-PAGE and blotted with anti-MMP-1 antibody. Membranes subsequently stripped and reprobed with anti-α2 integrin antibody (AB1936). The proteins were detected by Super Signal West Pico chemiluminescent substrate. Molecular weight markers (kd) are shown in the left column.
To test whether p38 and MEK1/2 are involved in the association of MMP-1 with CXCL12-stimulated NK cells, we conducted confocal immunostaining and co-immunoprecipitation assays. When NK cells were pretreated with SB203580 or U0126 before stimulation with CXCL12, membrane-associated MMP-1 was almost totally abolished (Figure 8C, b and f). Further studies with co-immunoprecipitation indicate that both SB203580 and U0126 abrogated association of MMP-1 with α2β1 integrin in CXCL12-stimulated NK cells without affecting the expression level of α2 integrin (Figure 8D). These results suggest that the CXCL12-stimulated NK-cell invasion and migration are regulated by the distinct mechanisms involving MAP kinases including p38 and ERK1/2 and confirmed the critical role for membrane-associated MMP-1 in promoting CXCL12-stimulated NK-cell invasion.
Discussion
The migration of lymphocytes through the tissues in response to chemokines is a multistep process that requires cell adhesion coupled with mechanisms to overcome the physical tissue barrier. MMPs have been demonstrated to play a key role in the degradation of ECM and hence are important in cellular invasion and migration into tissues.28 In this study, we demonstrate that CXCL12-stimulated CD16+CD56+NK cells efficiently invade into type I collagen gel in an MMP-1-dependent manner. Confocal immunofluorescence and co-immunoprecipitation studies indicate that MMP-1 is produced and associates with α2β1 integrin in CXCL12-stimulated NK cells. These results suggest that integrins play a key role in regulating lymphocyte migration into tissues not only by recognizing ECM proteins but also by immobilizing matrix-degrading enzymes on cell surface to facilitate pericellular degradation of ECM. Thus, CXCL12 would be important for promoting invasion and accumulation of NK cells through the production of MMP-1 and activation of α2β1 integrin in physiological and disease status.
The cell surface expression and secretion of MMPs are important for invasion in lymphocytes. We demonstrate that CXCL12 significantly enhanced type I collagen degradation on the NK-cell surface. The degradation was dependent on the catalytic activity of MMPs expressed on cell surface. Similarly, CXCL12 stimulated NK-cell invasion in an MMP-dependent manner. These results suggest that the pericellular degradation of type I collagen on the NK-cell surface by MMPs is a critical step for promoting invasion. However, the enhanced invasion of NK cells by CXCL12 was not completely inhibited by GM6001 or TIMP2. This can be accounted for by the fact that these inhibitors failed to completely inhibit the degradation of type I collagen. It is also possible that MMPs and other proteases such as serine proteases cooperatively enhance CXCL12-stimulated NK-cell invasion. Previous studies have shown that NK cells produce both types of enzymes, and the addition of inhibitors against both types exhibited a more potent inhibitory effect on NK-cell invasion than either inhibitor alone.29 Although the exact roles for these matrix-degrading enzymes in NK-cell invasion into tissues is incompletely understood, chemokines such as CXCL12 produced in inflammation or disease status may facilitate lymphocyte migration and accumulation by inducing the expression of matrix-degrading enzymes on the cell surface.
For lymphocytes to extravasate from the blood stream to tissues, cells must degrade ECM proteins in the basement membrane and interstitial stroma. MMP-2 and MMP-9 have been demonstrated to promote cell invasion into basement membrane,21 because of their ability to degrade type IV collagen. However, cellular extravasation from the bloodstream also requires lymphocytes to traverse the interstitial stroma, which is made up of collagens I and III. Of significance is the fact that this degradation is accomplished most efficiently by the interstitial collagenases such as MMP-1, MMP-8, MMP-13, and MMP-14.30 Previous studies showed that CXCL12 stimulates production of MMP-9 from monocytes and megakaryocytes.31,32 Our results, however, indicate that CXCL12/CXCR4 ligation specifically induces MMP-1 from NK cells, suggesting that CXCL12 plays a role in regulating immune cell functions by inducing MMPs in a cell type-specific manner. Ishida et al33 reported that IL-18 stimulates the production of MMP-14 from CD16+CD56+ NK cells and promotes invasion into Matrigel, further supporting a pivotal role for collagenases in promoting lymphocyte invasion into tissues. Given the potent collagenolytic activity of MMP-1 and its association with NK-cell surface, it is possible that MMP-1 promotes NK-cell invasion by degrading matrix proteins on cell surface by similar mechanisms as previously reported in tumor cells.34 Recent studies suggest that MMPs degrade not only ECM proteins but also non-ECM substrates such as chemokines to alter their biological functions.35 For example, MMP-1 has been shown to degrade chemokines including MCP-1, -2, and -3 and to process them to act as antagonists to their cognate receptors.36 Although the exact details of in vivo functions of MMP-1 produced from NK cells still need to be studied, these results suggest that MMP-1 produced from CD16+CD56+ NK cells would play a role in traversing of tissues by promoting degradation of matrix collagens as well as processing of chemokines.
We demonstrate that MMP-1 is associated with CXCL12-stimulated NK cells through its interaction with α2β1 integrin. These results are consistent with the previous studies showing the association of MMP-1 with α2β1 integrin in keratinocytes.37 Despite the fact that blocking antibodies against MMP-1 can significantly inhibit NK-cell invasion into type I collagen, we could not detect the active form of this enzyme from either serum-free conditioned medium or cell surface. The critical step in the activation of pro-MMPs is disruption of the interaction of the conserved cysteine residue in the pro-domain with the zinc ion of the catalytic center, and this process does not necessarily require proteolysis.38 Our results indicate that a Gi-coupled pathway activated by CXCL12/CXCR4 interaction enhances the association of MMP-1 with α2β1 integrin in NK cells. Thus, these results suggest that MMP-1 that binds to activated α2β1 integrin would be in the catalytically active state to degrade substrates on NK-cell surface.37 The binding of MMPs to integrins would be a critical step for promoting lymphocyte migration and would likely be dependent on cell type, because pro-MMP-9 and pro-MMP-9 directly associates with β2 integrins on lymphocytes, and the disruption of this association decreased migratory abilities of the cells.39 To further characterize the mechanisms of MMP-1-mediated NK-cell invasion, we are now identifying the domains of MMP-1 that directly bind to α2β1 integrin on NK cells including the hinge and hemopexin domains as reported previously.37
Our results indicate that Gi-coupled pathway-independent signaling is important for promoting CXCL12-stimulated NK-cell invasion, demonstrated by the results in which the production of MMP-1 is not affected by PTX but significantly decreased in the presence of inhibitors for p38 and MEK1/2. Conversely, NK-cell migration stimulated with CXCL12 is mediated by Gi-coupled pathway but not by these MAP kinases, as previously reported in T cells,26,27 indicating that CXCL12/CXCR4 stimulates distinct mechanisms for promoting invasion and migration in NK cells. Earlier studies showed that CXCL12 stimulates Gi-coupled pathway-independent signaling involving activation of p38 and JANUS-kinase family members such as JAK2 and JAK3 in T cells.23,40 Although the biological functions of the pathway are not well understood, it is possible that the activation of Gi-coupled pathway-independent signaling would play a key role in mandating cell fate in tissues. The activation of p38 through the ligation of CXCL12/CXCR4 induces cell death, whereas Gi-coupled pathway induces cell survival.23 In this regard, the production of MMP-1 from CXCL12-stimulated NK cells provide an additional function of the Gi-coupled pathway-independent signaling that directly regulates NK-cell invasion into tissues on stimulation with CXCL12, mechanisms of which may regulate T cell invasion as described previously.41 The CXCL12/CXCR4 pathway was first identified to be functionally important in the homing or immobilization of hematopoietic stem cells.42 Previous studies showed that CXCL12 stimulates homing of hematopietic cells to the target organs (ie, liver), and this process was enhanced by the production of MMPs in liver.43 Although the details of the mechanisms of homing of hematopietic cells should be studied in detail, our results suggest that CXCL12 could induce homing of hematopoietic cells to target organs by inducing MMP production and activating integrins to immobilize MMPs on cell surface. CXCL12 stimulates MMP productions from peripheral hematopoietic cells and promotes cell invasion.44 Thus, further characterization of the downstream signaling pathways on CXCL12/CXCR4 ligation in lymphocytes would provide important information for mechanisms for lymphocyte invasion, migration, and survival in the target organs.
In conclusion, our studies suggest a pivotal role of MMP-1 in promoting CD16+CD56+ NK-cell invasion into tissues. This subset of NK cells has been characterized as cytotoxic NK cells,45 and they need to traverse from blood vessels to the tumor mass. Recent studies demonstrate that the expression of CXCL12 by melanoma cells can stimulate CTL migration toward tumors, resulting in tumor cell apoptosis.46 Thus, further characterization of lymphocyte-trafficking mechanisms involving the production of matrix-degrading enzymes and their mechanisms of cell surface association can provide targets for tumor growth inhibition by using immune cells such as CD16+CD56+NK cells and CTL.
Acknowledgments
We thank Drs. Yamashita, Nakai, Yakushiji, and Sawai for the insightful discussion and Mr. Christopher Wilson (Laboratory Medicine and Pathology, University of Minnesota, Minneapolis) for densitometry analysis of RT-PCR.
Footnotes
Address reprint requests to Dr. Seiji Goda, Department of Biochemistry, Osaka Dental University, 8-1 Kuzuhahanazono-cho, Hirakata-shi, Osaka 573-1121, Japan. E-mail: goda@cc.osaka-dent.ac.jp.
Supported by Osaka Dental Joint Research Funds (grants B02-01 and B03-01), by Grant-in-Aid for Young Scientists (B) (16791150 and 70351476), and partially by the National Institutes of Health (grant CA92222).
S.G. and J.I. contributed equally to this work.
References
- Vujanovic NL, Herberman RB, Maghazachi AA, Hiserodt JC. Lymphokine-activated killer cells in rats: III A simple method for the purification of large granular lymphocytes and their rapid expansion and conversion into lymphokine-activated killer cells. J Exp Med. 1988;167:15–29. doi: 10.1084/jem.167.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- Inngjerdingen M, Damaj B, Maghazachi AA. Expression and regulation of chemokine receptors in human natural killer cells. Blood. 2001;97:367–375. doi: 10.1182/blood.v97.2.367. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE. Janus kinase 2 is involved in stromal cell-derived factor-1alpha-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood. 2001;97:3342–3348. doi: 10.1182/blood.v97.11.3342. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghazachi AA. Role of the heterotrimeric G proteins in stromal-derived factor-1alpha-induced natural killer cell chemotaxis and calcium mobilization. Biochem Biophys Res Commun. 1997;236:270–274. doi: 10.1006/bbrc.1997.6937. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Kim MH, Kitson RP, Albertsson P, Nannmark U, Basse PH, Kuppen PJ, Hokland ME, Goldfarb RH. Secreted and membrane-associated matrix metalloproteinases of IL-2-activated NK cells and their inhibitors. J Immunol. 2000;164:5883–5889. doi: 10.4049/jimmunol.164.11.5883. [DOI] [PubMed] [Google Scholar]
- Daniels JT, Limb GA, Saarialho-Kere U, Murphy G, Khaw PT. Human corneal epithelial cells require MMP-1 for HGF-mediated migration on collagen I. Invest Ophthalmol Vis Sci. 2003;44:1048–1055. doi: 10.1167/iovs.02-0442. [DOI] [PubMed] [Google Scholar]
- Tabassam FH, Umehara H, Huang JY, Gouda S, Kono T, Okazaki T, van Seventer JM, Domae N. Beta2-integrin, LFA-1, and TCR/CD3 synergistically induce tyrosine phosphorylation of focal adhesion kinase (pp125(FAK)) in PHA-activated T cells. Cell Immunol. 1999;193:179–184. doi: 10.1006/cimm.1999.1472. [DOI] [PubMed] [Google Scholar]
- Goda S, Quale AC, Woods ML, Felthauser A, Shimizu Y. Control of TCR-mediated activation of beta(1) integrins by the ZAP-70 tyrosine kinase interdomain B region and the linker for activation of T cells adapter protein. J Immunol. 2004;172:5379–5387. doi: 10.4049/jimmunol.172.9.5379. [DOI] [PubMed] [Google Scholar]
- Iida J, Pei D, Kang T, Simpson MA, Herlyn M, Furcht LT, McCarthy JB. Melanoma chondroitin sulfate proteoglycan regulates matrix metalloproteinase-dependent human melanoma invasion into type I collagen. J Biol Chem. 2001;276:18786–18794. doi: 10.1074/jbc.M010053200. [DOI] [PubMed] [Google Scholar]
- Inoue H, Miyaji M, Kosugi A, Nagafuku M, Okazaki T, Mimori T, Amakawa R, Fukuhara S, Domae N, Bloom ET, Umehara H. Lipid rafts as the signaling scaffold for NK cell activation: tyrosine phosphorylation and association of LAT with phosphatidylinositol 3-kinase and phospholipase C-gamma following CD2 stimulation. Eur J Immunol. 2002;32:2188–2198. doi: 10.1002/1521-4141(200208)32:8<2188::AID-IMMU2188>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett. 2003;194:1–11. doi: 10.1016/s0304-3835(02)00699-7. [DOI] [PubMed] [Google Scholar]
- Kitson RP, Appasamy PM, Nannmark U, Albertsson P, Gabauer MK, Goldfarb RH. Matrix metalloproteinases produced by rat IL-2-activated NK cells. J Immunol. 1998;160:4248–4253. [PubMed] [Google Scholar]
- Wayner EA, Carter WG. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987;105:1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahakis SR, Villasis-Keever A, Gomez T, Vanegas M, Vlahakis N, Paya CV. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169:5546–5554. doi: 10.4049/jimmunol.169.10.5546. [DOI] [PubMed] [Google Scholar]
- Sugioka Y, Watanabe T, Inagaki Y, Kushida M, Niioka M, Endo H, Higashiyama R, Okazaki I. c-Jun NH2-terminal kinase pathway is involved in constitutive matrix metalloproteinase-1 expression in a hepatocellular carcinoma-derived cell line. Int J Cancer. 2004;109:867–874. doi: 10.1002/ijc.20095. [DOI] [PubMed] [Google Scholar]
- Brauchle M, Gluck D, Di Padova F, Han J, Gram H. Independent role of p38 and ERK1/2 mitogen-activated kinases in the upregulation of matrix metalloproteinase-1. Exp Cell Res. 2000;258:135–144. doi: 10.1006/excr.2000.4913. [DOI] [PubMed] [Google Scholar]
- Stupack DG, Cho SY, Klemke RL. Molecular signaling mechanisms of cell migration and invasion. Immunol Res. 2000;21:83–88. doi: 10.1385/IR:21:2-3:83. [DOI] [PubMed] [Google Scholar]
- Cherla RP, Ganju RK. Stromal cell-derived factor 1 alpha-induced chemotaxis in T cells is mediated by nitric oxide signaling pathways. J Immunol. 2001;166:3067–3074. doi: 10.4049/jimmunol.166.5.3067. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today. 2000;6:149–156. doi: 10.1016/s1357-4310(00)01686-5. [DOI] [PubMed] [Google Scholar]
- Al-Atrash G, Kitson RP, Xue Y, Mazar AP, Kim MH, Goldfarb RH. uPA and uPAR contribute to NK cell invasion through the extracellular matrix. Anticancer Res. 2001;21:1697–1704. [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Klier CM, Nelson EL, Cohen CD, Horuk R, Schlondorff D, Nelson PJ. Chemokine-induced secretion of gelatinase B in primary human monocytes. Biol Chem. 2001;382:1405–1410. doi: 10.1515/BC.2001.173. [DOI] [PubMed] [Google Scholar]
- Lane WJ, Dias S, Hattori K, Heissig B, Choy M, Rabbany SY, Wood J, Moore MA, Rafii S. Stromal-derived factor 1-induced megakaryocyte migration and platelet production is dependent on matrix metalloproteinases. Blood. 2000;96:4152–4159. [PubMed] [Google Scholar]
- Ishida Y, Migita K, Izumi Y, Nakao K, Ida H, Kawakami A, Abiru S, Ishibashi H, Eguchi K, Ishii N. The role of IL-18 in the modulation of matrix metalloproteinases and migration of human natural killer (NK) cells. FEBS Lett. 2004;569:156–160. doi: 10.1016/j.febslet.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6:4823–4830. [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- Dumin JA, Dickeson SK, Stricker TP, Bhattacharyya-Pakrasi M, Roby JD, Santoro SA, Parks WC. Pro-collagenase-1 (matrix metalloproteinase-1) binds the alpha(2)beta(1) integrin upon release from keratinocytes migrating on type I collagen. J Biol Chem. 2001;276:29368–29374. doi: 10.1074/jbc.M104179200. [DOI] [PubMed] [Google Scholar]
- Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidakis M, Bjorklund M, Ihanus E, Gahmberg CG, Koivunen E. Identification of a negatively charged peptide motif within the catalytic domain of progelatinases that mediates binding to leukocyte beta 2 integrins. J Biol Chem. 2003;278:34674–34684. doi: 10.1074/jbc.M302288200. [DOI] [PubMed] [Google Scholar]
- Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez AC, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999;13:1699–1710. [PubMed] [Google Scholar]
- Soede RD, Zeelenberg IS, Wijnands YM, Kamp M, Roos E. Stromal cell-derived factor-1-induced LFA-1 activation during in vivo migration of T cell hybridoma cells requires Gq/11, RhoA, and myosin, as well as Gi and Cdc42. J Immunol. 2001;166:4293–4301. doi: 10.4049/jimmunol.166.7.4293. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, Goichberg P, Kalinkovich A, Arenzana-Seisdedos F, Nagler A, Hardan I, Revel M, Shafritz DA, Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML. Differential MMP and TIMP production by human marrow and peripheral blood CD34(+) cells in response to chemokines. Exp Hematol. 2000;28:1274–1285. doi: 10.1016/s0301-472x(00)00532-4. [DOI] [PubMed] [Google Scholar]
- Yoneda O, Imai T, Goda S, Inoue H, Yamauchi A, Okazaki T, Imai H, Yoshie O, Bloom ET, Domae N, Umehara H. Fractalkine-mediated endothelial cell injury by NK cells. J Immunol. 2000;164:4055–4062. doi: 10.4049/jimmunol.164.8.4055. [DOI] [PubMed] [Google Scholar]
- Zhang T, Somasundaram R, Berencsi K, Caputo L, Rani P, Guerry D, Furth E, Rollins BJ, Putt M, Gimotty P, Swoboda R, Herlyn M, Herlyn D. CXC chemokine ligand 12 (stromal cell-derived factor 1 alpha) and CXCR4-dependent migration of CTLs toward melanoma cells in organotypic culture. J Immunol. 2005;174:5856–5863. doi: 10.4049/jimmunol.174.9.5856. [DOI] [PubMed] [Google Scholar]
- Iida J, Wilhelmson KL, Price MA, Wilson CM, Pei D, Furcht LT, McCarthy JB. Membrane type-1 matrix metalloproteinase promotes human melanoma invasion and growth. J Invest Dermatol. 2004;122:167–176. doi: 10.1046/j.0022-202X.2003.22114.x. [DOI] [PubMed] [Google Scholar]