Abstract
The recent prospective isolation of a wide variety of somatically derived stem cells has affirmed the notion that homeostatic maintenance of most tissues and organs is mediated by tissue-specific stem and progenitor cells and fueled enthusiasm for the use of such cells in strategies aimed at repairing or replacing damaged, diseased, or genetically deficient tissues and organs. Hematopoietic stem cells (HSCs) are arguably the most well-characterized tissue-specific stem cell, with decades of basic research and clinical application providing not only a profound understanding of the principles of stem cell biology, but also of its potential pitfalls. It is our belief that emerging stem cell fields can benefit greatly from an understanding of the lessons learned from the study of HSCs. In this review we discuss some general concepts regarding stem cell biology learned from the study of HSCs with a highlight on recent work pertaining to emerging topics of interest for stem cell biology.
Although primarily comprised of terminally differentiated postmitotic effector cells, many tissues are believed to retain minor populations of stem cells capable of replenishing cells that are lost through wear and tear, injury, and disease. An emerging body of evidence, including the prospective isolation of stem cells from a number of different tissues and organs,1–6 suggests that the homeostatic maintenance of most tissues capable of regeneration and repair is ultimately mediated by such tissue-specific stem cells. Along with embryonic stem cells, tissue-specific stem cells have the ability to self-perpetuate through a process known as self-renewal, in addition to being capable of giving rise to mature effector cell types in a sustained manner through differentiation. The combination of these properties has positioned stem cell biology at the forefront of regenerative medicine, the ultimate goal of which is to harness the potential of stem cells to develop strategies aimed at treating heritable, malignant, or degenerative conditions.
In the hematopoietic system, the properties of differentiation, multipotentiality, and self-renewal were first demonstrated more than 40 years ago through a series of seminal experiments demonstrating the ability of a subset of cells within the bone marrow (BM) to form macroscopic colonies on transplantation into the spleens of lethally irradiated recipient animals.7 Such colonies, termed colony-forming unit spleen (CFU-S), were found to contain differentiated progeny of multiple blood lineages,7 and a subset of these colonies could reform CFU-S when transplanted into secondary hosts.8 Although originally believed to be derived from hematopoietic stem cells (HSCs), it is noteworthy that the CFU-S described by Till and McCulloch7 were later found to be derived from more committed progenitor cells,9 thus providing an important lesson regarding the complexity of stem and progenitor cell biology. The pioneering experiments by Till and McCulloch were nonetheless instrumental in launching the field of adult stem cell biology through their fundamental demonstration of the concepts of self-renewal and multipotentiality, which remain key defining properties of all stem cells. Their work also ignited a flurry of investigations aimed at identifying, functionally characterizing, and purifying HSCs.
HSCs were the first tissue-specific stem cells to be prospectively isolated10 and are the only stem cells in routine clinical use to date, with extensive use of HSC-containing grafts being used in the treatment of a variety of blood cell diseases such as leukemias and autoimmune disorders.11 A number of important experimental breakthroughs underlie the success of HSC biology. These include the development of in vitro and in vivo assay systems making evaluation of lineage potential and self-renewal possible, as well as technological advances involving fluorescence-activated cell sorting and monoclonal antibody technology, together allowing discrimination and functional evaluation of minor cellular subsets at a clonal level. The breadth of studies detailing the fundamental cellular and molecular properties of HSCs, in addition to work aimed at exploiting their clinical potential, provides a framework from which emerging stem cell fields should be able to gain insight. In this review we attempt to highlight some of the conceptual lessons that have been learned through the study of HSCs, which we believe will be fundamentally applicable to the characterization of other stem cells and will expedite their translation to the clinic.
Stem and Progenitor Cell Hierarchy: Proliferation and Protection
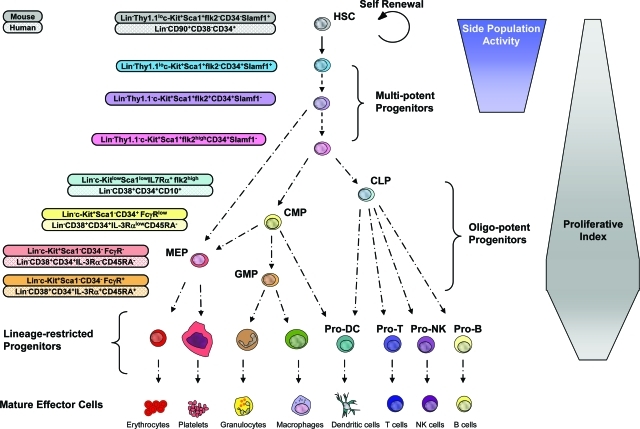
The regenerative potential of all stem cells rests on their ability to generate mature effector cell types through processes of differentiation. In the hematopoietic system, HSCs reside at the top of the hematopoietic hierarchy and give rise to functional effector cells of at least nine distinct types produced from HSCs in successive differentiation processes of increasingly committed progenitor cells (Figure 1). Because of the very short life span of most effector cells, mature blood cell production is an ongoing process with estimates suggesting the production of 1.5 × 106 blood cells every second in an adult human. This high turnover rate necessitates profound homeostatic control mechanisms, the primary level of which resides with the HSCs. However, because of the enormous proliferative and developmental capacity of the more committed multipotent, oligo-potent, and lineage-restricted progenitor cells within the hematopoietic hierarchy, a significant degree of homeostatic control of mature blood cells is also mediated at the level of these progenitors.
Figure 1-6946.
Model of the hematopoietic developmental hierarchy. Self-renewing HSCs reside at the top of the hierarchy, giving rise to a number of multipotent progenitors. Multipotent progenitors give rise to oligo-potent progenitors including the CLP, which gives rise to mature B lymphocytes, T lymphocytes, and natural killer (NK) cells. The common myeloid progenitor (CMP) gives rise to granulocyte-macrophage progenitors, which differentiate into monocytes/macrophages and granulocytes, and megakaryocyte/erythrocyte progenitors, which differentiate into megakaryocytes/platelets and erythrocytes. Both CMPs and CLPs have been proposed to give rise to dendritic cells. The cell surface phenotype of many of these cell types is shown for the murine and human systems. It should be noted that certain markers such as Thy1.1 and ScaI are only informative in some but not all mouse strains. Development from the oligo-potent progenitors to mature blood cells proceeds through a number of intermediate progenitors (not shown). It should be noted that the absolute lineage potential and developmental relationships of some of the subsets indicated have not yet been fully resolved. For example, evidence suggests that the megakaryocyte and erythrocyte lineages may originate from a multipotent progenitor and not pass through a CMP intermediate. The developmental passage of HSCs through multipotent progenitors, oligo-potent progenitors, and lineage-specific progenitors is generally associated with increases in proliferative index although this trend is not absolute and has not been resolved for all stages of development. The capacity to efflux dyes such as Hoechst 33342 or rhodamine-123 (termed side population activity) is restricted to HSCs and its immediate multipotent progenitors. It should be noted that although the different multipotent progenitor subsets in mice have been functionally resolved to a significant degree, this is not true of the human system. We therefore believe that cells defined as human HSCs by the cell surface markers Lin−CD90+CD38−CD34+ are likely to include one or more multipotent progenitor populations.
The immediate progeny of HSCs are multipotent progenitor cells that retain full lineage potential yet have a limited capacity for self-renewal. The therapeutic potential of these transiently reconstituting multipotent progenitors is nonetheless very high because they are capable of more rapidly reconstituting myeloablated recipients than HSCs and are significantly more abundant than HSCs and therefore more readily obtainable. Indeed, evidence suggests that the lymphoid correction of some severe combined immunodeficiency (SCID) patients receiving MHC-matched grafts may be attributable to transiently reconstituting progenitors and not HSCs, based on data showing an absence of CD34+ BM chimerism in otherwise lymphoid reconstituted SCID patients.12 In support of this, we have demonstrated that transiently reconstituting multipotent progenitor subsets are capable of giving sustained functional lymphoid reconstitution sufficient to rescue the lymphoid deficiencies seen in several mouse models of SCID.13 However, as such progenitor cells are incapable of prolonged self-renewal and are therefore only capable of reconstituting myeloid lineages throughout the short term, they do not represent the cell of choice in settings in which permanent reconstitution of all blood lineages is desired.
Multipotent progenitors in turn give rise to oligopotent progenitors, which possess more restricted developmental potential. This appears to represent a branching point in the hematopoietic hierarchy with the common lymphoid progenitor (CLP) giving rise to mature lymphoid effector cells including B, T, dendritic, and natural killer (NK) cells but lacking the potential to form myeloerythroid cells14 and myeloid progenitor subsets capable of giving rise to mature myeloerythroid effector cells yet lacking the capacity to form lymphoid progeny.15 Such oligo-potent progenitors in turn give rise to more lineage-restricted progenitors from which all of the mature blood cells eventually arise. Although questions remain regarding the absolute lineage potential of the different hematopoietic progenitor subsets and their relationship to one another, there is wide consensus that the sequential differentiation of HSCs through progenitors to fully differentiated blood cells is a primarily irreversible process under normal physiological steady-state conditions.
The multitiered differentiation scheme of hematopoietic differentiation has several intrinsic advantages. Firstly it allows for an enormous amplification in the numbers of differentiated cells from a single stem cell. This is achieved by combining different steps in differentiation with increases in proliferative potential (Figure 1).16,17 This feature has the added advantage of being able to be fine-tuned to suit the homeostatic demands of a given effector cell type. For example, granulocyte-macrophage progenitors, which gives rise to mature granulocytes that turn over very rapidly, have a very high proliferative index, whereas the CLP has a lower proliferative index,17 presumably resulting from the fact that the progeny of the CLP are more long-lived B and T cells, which themselves are capable of extensive proliferation during maturation.
The hierarchical differentiation scheme also has the effect of putting very little proliferative pressure on HSCs themselves, which cycle very infrequently and primarily reside in the G0 phase of the cell cycle.18 The minimal proliferative pressure on stem cells has the benefit of not subjecting them to the potentially mutagenic hazards of DNA replication and cell division, and may thus contribute to the integrity and longevity required of these cells. Furthermore, because G0 is a relatively metabolically inactive phase of the cell cycle, it has been suggested that stem cells may therefore be subjected to lower levels of damage-inducing metabolic side products and reactive oxygen species than more metabolically active differentiated cell types.19 The concept that stem cells may be uniquely protected is supported by findings demonstrating that stem cells express high levels of numerous ABC/MDR transporter genes,19,20 whose products are known to have physiological roles in cytoprotection. This property has in fact been extensively exploited as a strategy to enrich for stem cells by virtue of their ability to efflux intravital staining dyes such as rhodamine-12321 or Hoechst 33342.22
An increasing amount of data has suggested that many cancers are derived and maintained by small subsets of cells, commonly referred to as cancer stem cells, which exhibit many of the properties of normal tissue-specific stem cells including the capacity for unlimited self-renewal.23 The extent to which cancer stem cells are derived from tissue-specific stem cells themselves, or are the result of transformation events that imbue other cell types with stem cell-like properties, is currently under intense investigation and is likely to differ in different types of cancers. However, if acquisition or retention of cytoprotective properties is a characteristic feature of cancer stem cells, this would have significant clinical implications because it suggests that eradication of cancer stem cells might be more difficult to achieve than that of more mature cell types comprising a tumor. This possibility might help explain why numerous cancer therapies are able to shrink tumors but not eradicate them or why some cancers acquire drug resistance as they progress.24
Heterogeneity Within
The ability of HSCs to permanently reconstitute myeloablated recipients in all blood cell lineages is the most rigorous criteria for evaluating HSC activity and constitutes the basis for the success of BM transplantation. Nonetheless, it has become apparent that a number of clinical applications would greatly benefit from a more refined ability to identify and purify HSCs. For example, transplantation of purified HSCs in the allogeneic setting circumvents development of acute or chronic graft-versus-host disease by eliminating immunoreactive T cells from the graft.25 Moreover, because donor HSC-derived lymphocytes that develop in a transplant recipient acquire tolerance to both donor and host histocompatibility antigens, there is a strong basis for using purified HSC transplants as a preparative regimen for solid organ transplants.11,26 Furthermore, the purification of HSCs from cancer patients and their subsequent reintroduction after radiation or chemotherapy is clearly a superior strategy to the reintroduction of unfractionated BM cells that might otherwise return cancerous cells back to the patient.11,27 Purification of HSCs has therefore been the focus of intensive study during the last decades.
In mice, most if not all long-term multilineage HSC activity resides within the minor c-kit-positive, lineage-negative, and Sca-1-positive fraction (KLS cells) of murine BM. However, although KLS cells are enriched for HSC activity when compared to whole BM (∼1000-fold), the vast majority of KLS cells are multipotent progenitors, and only ∼1/30 KLS cells are actually stem cells capable of long-term multilineage repopulation (D.B., D.J.R., and I.L.W., unpublished). Because the multipotent progenitors that comprise the KLS fraction retain some but not all stem cell properties, caution should be taken when drawing conclusions about stem cell activity based on only a KLS phenotype as is prevalent in the literature. For example, it is well documented that the transiently reconstituting multipotent subsets comprising the KLS fraction are capable of giving rise to lymphoid cells that can display extensive longevity in transplant experiments. Thus, long-term reconstitution of B and T cells after transplantation does not necessarily reflect stem cell engraftment or function, stressing the importance of using purified stem cells in combination with reliable analyses of the more short-lived myeloid lineages (such as granulocytes) for evaluation of stem cell function.28 These points are made all of the more relevant with evidence that the frequencies and function of the different subsets in the KLS compartment can be significantly altered in different physiological settings such as aging.19
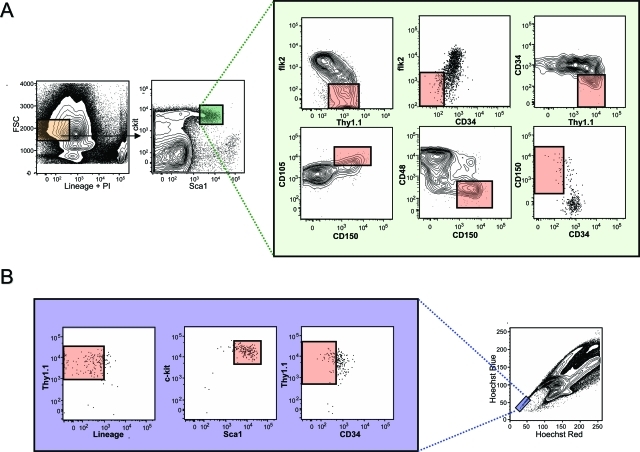
The heterogeneity of the murine KLS compartment has been directly revealed with the use of additional cell surface markers that enrich for HSC activity including CD34,29 flk2,30,31 Thy1.1,10 CD105 (Endoglin),32 CD150 (Slamf1),33 or by exploiting their differential dye efflux activity.21,22,34 In Figure 2 we present a meta-analysis of HSC staining profiles using the most effective HSC markers to illustrate the phenotypic complexity and heterogeneity of the primitive hematopoietic compartment (Figure 2). In addition to the phenotypic heterogeneity of HSCs, recent data has also demonstrated a significant degree of functional heterogeneity between different HSC clones, raising the possibility of the existence of a number of functionally distinct HSC subsets that are able to retain characteristic functional attributes such as differential propensities to differentiate into certain lineages (lineage bias), even throughout serial transplantation.35 However, until such functionally distinct HSCs are prospectively isolated and their intrinsic properties functionally confirmed, other interpretations remain viable such as the possibility that HSCs exhibit distinct heritable functional characteristics as a consequence of the specific microenvironments they seed (discussed below). However, these are not mutually exclusive because distinct stem cell subsets could conceivably preferentially seed distinct locations.
Figure 2-6946.
Heterogeneity within the primitive hematopoietic compartment. A: Cell surface staining of the murine KLS compartment with markers that enrich for stem cell activity. In bone marrow, all HSC activity is found within the lineage-negative (orange box: negative for antigens found on mature blood cells including B220, Mac1, Gr-1, Il7Rα, Ter119, CD3, CD4, CD8) and ScaIhigh and c-kithigh fractions (small green box). Because only ∼1 in 30 cells in the KLS compartment is a long-term multilineage reconstituting HSC, additional cell surface markers are used to enrich for HSC activity as illustrated in the expanded green box. These include positive cell surface markers such as Thy1.1, CD105 (Endoglin), and CD150 (Slamf1), in addition to negative markers including CD34, CD48, and flk2. In each fluorescence-activated cell sorting plot, the pink box denotes where HSC activity is found as determined by transplantation experiments performed by others and ourselves. It should be noted that the staining combinations shown only represent enrichment strategies, and combinations of numerous markers are recommended to further enrich for HSC activity. For example, although flk2 negativity and Thy1.1 positivity enrich for HSC activity from the KLS compartment, the addition of CD34 negativity adds additional significant enrichment of HSC activity. It should also be noted that Thy1.1 expression is only useful as a marker on HSCs if using mouse strains carrying the Thy1.1 allele, and selection based on negativity for CD34 requires the use of mice of more than 8 weeks of age. B: Cell surface marker distribution within the fraction of bone marrow cells exhibiting the highest ability to efflux Hoechst 33342 (side population tip cells). HSC activity is only found within the pink boxes of each plot as determined by transplantation experiments performed by others and ourselves.
Stem Cell Fate Choice: Molecular and Cellular Underpinnings of Differentiation and Self-Renewal
One of the major objectives of stem cell biology is to understand the cellular and molecular processes underlying HSC fate choice. In recent years a substantial amount of progress has been made using microarray technology to identify unique gene expression patterns associated with lineage commitment.36–38 One surprising finding of these studies is the discovery that HSCs express a great diversity of transcripts, including a wide range of genes originally believed to be restricted to more mature and lineage-committed cell types. The findings of these genome-wide expression studies had in fact been foreshadowed by earlier studies using single-cell polymerase chain reaction strategies that had suggested that such promiscuous transcription of lineage-associated transcripts was necessary to prime primitive progenitor cells for differentiation toward downstream fates.39,40 An alternative interpretation posits that stem cells possess global transcriptional accessibility and that it is the step-wise restriction of locus accessibility that underlies lineage specification.37 Interestingly, some genetic components appear to be capable of specifying cell fate on their own, acting as master lineage specification regulators. This has been exemplified in experiments in which cell fate was switched from one committed cell type to another on overexpression of IL2R or GM-CSF receptors,41 GATA-1,42 or C/EBPα/β.43 An additional mechanism of lineage specification was revealed in experiments in which ablation of the transcription factors Pax5 or GATA-1 in lineage-restricted progenitors was found to be sufficient to despecify their B-cell and erythroid fate, respectively, and allow a multilineage developmental potential.44,45 These studies suggest a model in which potent transcriptional regulators can lock fate decisions in place by limiting transcriptional accessibility to molecular programs associated with alternative lineage fates.
It has been suggested that deregulation of global gene expression patterns might allow HSCs to acquire nonhematopoietic fates.46 A large number of reports have entertained the idea that HSCs have the potential to transdifferentiate into a range of nonhematopoietic cell types including hepatocytes, skeletal muscle fibers, cardiomyocytes, neuronal cells, and various types of epithelial cells. The converse has also been reported, with a range of nonhematopoietic cell types said to be able to give rise to multilineage hematopoiesis on transplantation. The conclusions of most of these original reports have been primarily refuted by various experimental means, and little evidence remains to support the idea that HSCs possess any true nonhematopoietic differentiation potential. Space constraints hinder us here from going into this topic in more detail but we refer the reader to other specialized reviews on this topic.47–49
In addition to producing differentiated progeny, stem cells also have the capacity for self-renewal, which ensures that stem cell reserves are not exhausted throughout time. Experiments have demonstrated the ability of HSCs to maintain stem cell function after multiple serial transplantations,50 or after repetitive injections of chemotherapeutic agents that force HSCs into cycle,51 suggesting that the life span of HSCs far exceeds that of the individual itself. For HSCs, self-renewal is traditionally evaluated through serial transplantation experiments in which HSCs transplanted into primary recipients are reisolated and transplanted into secondary recipients. However, because such experiments rely on retrospective analysis of the contribution to mature blood cell production from donor HSCs, it remains possible that deficits in serial transplant ability are as likely to reflect defects in HSC differentiation potential or engraftment as they are in self-renewal. Thus, from a conceptual point of view, assaying self-renewal based on these experimental criteria is problematic because they ultimately do not permit the dissection of HSC differentiation potential from HSC self-renewal potential. This point has recently been illustrated in the context of HSC aging wherein it was found that although HSCs isolated from old mice had a severely diminished capacity to generate mature B cells in transplantation experiments compared to young control mice, the ability of aged HSCs to give rise to themselves through self-renewal was in fact increased as revealed by directly assaying donor-derived HSC frequencies in the BM of recipient mice.19
The scarcity of HSCs in vivo, along with the clinical benefits of using higher HSC doses in the transplantation setting—which facilitates faster recovery after myeloablative treatments52—has led to extensive efforts aimed at promoting HSC self-renewal in vitro to facilitate expansion of stem cell numbers. In addition to numerous hematopoietic-specific cytokines that have demonstrated roles in HSC self-renewal such as kit ligand,53 thrombopoietin,54 and members of the gp130 family,55 several other signal transduction pathways with known roles in the biology of stem and progenitor cells of other tissues have also been implicated in HSC self-renewal, suggesting that stem cells from diverse tissues may use common pathways to mediate fundamental processes. Such regulators include Wnt,56 Notch,57 Shh,58 FGF-1,59 Igf2,60 Bmi-1,61 and angiopoietin-like proteins.62 Despite considerable effort, however, strategies aimed at the in vitro expansion of HSCs in an undifferentiated state have thus far failed to reach a stage of extensive clinical utility. This has prompted alternative approaches aimed at directing pluripotent embryonic stem cells toward more restricted stem cell fates. The recent demonstration that cells capable of faithfully recapitulating the functional potential of adult HSCs can be generated from embryonic stem cells through ectopic expression of Hoxb463 is significant because such an approach should not, in principle, be restricted by cell numbers. The combination of this approach with therapeutic cloning to correct a severe immune cell disorder64 is proof of principle that such strategies can be used for clinical application.
Opening the Stem Cell Niche
In 1978 Schofield hypothesized the existence of cells in the proximity of stem cells, termed the stem cell niche, that have the ability to extrinsically exert influence on stem cell behavior.9 Indeed, a large body of evidence from a number of stem cell systems validated this hypothesis by affirming the critical importance of stem cell/niche interactions and localized extracellular signals in regulating stem cell self-renewal and differentiation. The ability to re-establish or recapitulate functional stem cell/niche interactions will therefore likely be critical to the long-term success of any tissue-specific stem cell therapy. This concept is precisely illustrated in the BM transplantation setting in which the success of the transplant is contingent on the ability of HSCs to home to and seed appropriate supportive niches after intravenous injection. Yet, although interactions between HSCs and the stem cell niche constitute one of most important aspects of HSC biology, it remains one of the least well understood. This is attributable primarily to the fact that although other tissue-specific stem cells have a more precise knowledge of the anatomical location of resident stem and niche cells,62 the histological complexity of the BM, the paucity of HSCs, the difficulty of their definitive identification, and the use of immunohistological techniques that do not allow for functional evaluation of niche-localized HSCs have proven particularly challenging for the definitive characterization of the HSC niche.
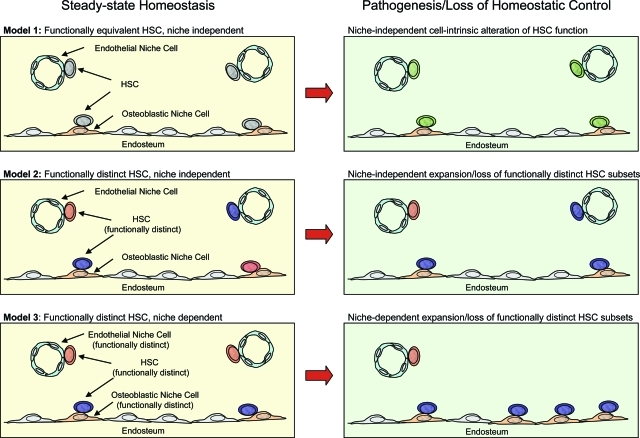
Early experiments suggested that HSCs were enriched in the BM at sites adjacent to the endosteal surface of the bone.65 Support for this model has emerged through a number of studies including those that tracked transplanted HSCs to the endosteal surface.66 Furthermore, studies using genetically modified mice have specifically implicated a subset of osteoblasts at the endosteal surface as critical components of the HSC niche (Figure 3), which are thought to exert an influence over HSC function through parathyroid hormone/Notch,67 Bmp,68 and Tie2/Ang-1 signaling.69 That such osteoblastic subsets would constitute the sole HSC niche has, however, been recently challenged with evidence suggesting that HSCs may also reside in close association with sinusoidal endothelium (Figure 3).33 The suggestion that different anatomical locations comprised of divergent cell types might represent different HSC niches, raises the possibility that divergent niches might impact the functional capacity of HSCs in different ways, perhaps by differentially regulating self-renewal, or lineage outcome (Figure 3). This idea is consistent and complementary to a body of data describing the existence of subsets of functionally distinct HSCs.35 Moreover, the selective expansion/reduction of different subsets of niches could conceivably contribute to pathophysiological conditions characterized by alterations in HSC self-renewal or lineage potential (Figure 3). For example, advanced aging of C57BL strains of mice, characterized by a large expansion of the steady-state HSC pool size,19,70–72 and a concomitant loss of B-lymphocyte differentiation potential19,71 could be underwritten in part by the selective expansion of niches supporting HSC self-renewal and a loss of niches that support B-lymphocyte development. This possibility is consistent with observations demonstrating that these functional characteristics of HSC aging are transplantable into young recipients, and are therefore cell intrinsic19 despite perhaps being ultimately underwritten by the selective expansion or retraction of different HSC niches with age. Alternatively, the functional alterations that underlie HSC aging may be ultimately mediated by cell-intrinsic changes in the gene expression in HSCs as a consequence of age that might be completely independent of any alterations in the aged microenvironment19(Figure 3).
Figure 3-6946.
Modeling HSC functional capacity in the context of divergent stem cell niches and pathogenesis. Three models of HSC/niche functional interaction are depicted under normal steady-state homeostatic conditions (left) and pathogenic conditions, or conditions in which loss of homeostatic control is manifested at the stem cell level (right). In model 1 (top left), all HSCs have the same functional capacity regardless of association with osteoblastic or endothelial niche cells. Pathogenic change at the stem cell level is cell intrinsic in this model (top right) and is independent of divergent niche interactions. In model 2 (middle left), functionally distinct HSCs exist (greater or lesser lineage potential, self-renewal, homing capacity, and so forth), and their functional identity is cell intrinsic and independent of interactions with divergent niches. Stem cell pathogenic change in this model (middle right) may be characterized by the selective expansion or loss of distinct HSC subsets independent of divergent niche interactions. In model 3 (bottom left), the functional identity of HSCs is dependent on interactions with divergent microenvironments and is therefore not cell intrinsic. Stem cell pathogenic change in this model (bottom right) is characterized by the selective expansion or loss of specific niche subtypes.
Experiments using parabiosis of genetically marked strains of mice demonstrated that HSCs constitutively migrate through the blood and are able to re-engraft unconditioned BM to resume HSC function.73 Although myeloablative conditioning had been thought to be required to liberate niche space to allow functional HSC engraftment, the demonstration that HSCs can exit and re-enter niches without prior conditioning suggested that these interactions are dynamic and not as static as had been supposed. Indeed, we have recently shown that at any time ∼0.1 to 1% of all HSC niches are open and available for productive HSC engraftment without any myeloablative conditioning and that HSC engraftment of such rare niches allowed for complete rescue of the immunodeficiency exhibited in SCID mice.74 Access to these niches was determined to be restricted at least partly through immunosurveillance by CD4+ T cells that are capable of mediating rejection by recognition of even subtle histocompatibility differences.74 Accordingly, transient immunodepletion of CD4+ T cells was sufficient to allow functional engraftment of minor histocompatibility-mismatched HSCs capable of rescuing SCID lymphoid deficiency. Importantly, these experiments suggest a general mechanism by which transplanted stem cells can functionally engraft recipients by using specific and transient lympho-depletion, without the need of using highly cytotoxic preparative regimens.
Summary
The recent prospective identification of a wide spectrum of tissue-specific stem cells represents a key first step in elucidating the functional and molecular behavior of such cells and in harnessing their clinical potential. Because HSCs are by far the most thoroughly characterized tissue-specific stem cell, having been experimentally studied for more than 50 years, HSCs have emerged as the model system to study tissue-specific stem cells and their potential applications to regenerative medicine. We believe that the study of HSCs will continue to provide an experimental and conceptual framework applicable to emerging stem cell fields, and in this context, HSCs do indeed represent a true paradigm.
Acknowledgments
We thank Deepta Bhattacharya for critical reading of the manuscript and we apologize to those having contributed substantially to the issues discussed herein which we were unable to cite because of space constraints.
Footnotes
Address reprint requests to Department of Pathology, Stanford University School of Medicine, B257 Beckman Center, Stanford, CA 94305-5323. E-mail: irv@stanford.edu.
D.B. and D.J.R. contributed equally to this article.
References
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- Siminovitch L, McCulloch EA, Till JE. The distribution of colony-forming cells among spleen colonies. J Cell Physiol. 1963;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- Muller SM, Kohn T, Schulz AS, Debatin KM, Friedrich W. Similar pattern of thymic-dependent T-cell reconstitution in infants with severe combined immunodeficiency after human leukocyte antigen (HLA)-identical and HLA-nonidentical stem cell transplantation. Blood. 2000;96:4344–4349. [PubMed] [Google Scholar]
- Bhattacharya D, Bryder D, Rossi DJ, Weissman IL. Rapid lymphocyte reconstitution of unconditioned immunodeficient mice with non-self-renewing multipotent hematopoietic progenitors. Cell Cycle. 2006;5:1135–1139. doi: 10.4161/cc.5.11.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Hodgson GS, Bradley TR. In vivo kinetic status of hematopoietic stem and progenitor cells as inferred from labeling with bromodeoxyuridine. Exp Hematol. 1984;12:683–687. [PubMed] [Google Scholar]
- Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford GB, Williams B, Rossi R, Bertoncello I. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp Hematol. 1997;25:445–453. [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- Bertoncello I, Hodgson GS, Bradley TR. Multiparameter analysis of transplantable hemopoietic stem cells: I The separation and enrichment of stem cells homing to marrow and spleen on the basis of rhodamine-123 fluorescence. Exp Hematol. 1985;13:999–1006. [PubMed] [Google Scholar]
- Wolf NS, Kone A, Priestley GV, Bartelmez SH. In vivo and in vitro characterization of long-term repopulating primitive hematopoietic cells isolated by sequential Hoechst 33342-rhodamine 123 FACS selection. Exp Hematol. 1993;21:614–622. [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Perona R, Sanchez-Perez I. Control of oncogenesis and cancer therapy resistance. Br J Cancer. 2004;90:573–577. doi: 10.1038/sj.bjc.6601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuru JA, Jerabek L, Edwards CT, Weissman IL. Transplantation of purified hematopoietic stem cells: requirements for overcoming the barriers of allogeneic engraftment. Biol Blood Marrow Transplant. 1996;2:3–14. [PubMed] [Google Scholar]
- Gandy KL, Weissman IL. Tolerance of allogeneic heart grafts in mice simultaneously reconstituted with purified allogeneic hematopoietic stem cells. Transplantation. 1998;65:295–304. doi: 10.1097/00007890-199802150-00001. [DOI] [PubMed] [Google Scholar]
- Negrin RS, Atkinson K, Leemhuis T, Hanania E, Juttner C, Tierney K, Hu WW, Johnston LJ, Shizurn JA, Stockerl-Goldstein KE, Blume KG, Weissman IL, Bower S, Baynes R, Dansey R, Karanes C, Peters W, Klein J. Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with metastatic breast cancer. Biol Blood Marrow Transplant. 2000;6:262–271. doi: 10.1016/s1083-8791(00)70008-5. [DOI] [PubMed] [Google Scholar]
- Domen J, Cheshier SH, Weissman IL. The role of apoptosis in the regulation of hematopoietic stem cells: overexpression of Bcl-2 increases both their number and repopulation potential. J Exp Med. 2000;191:253–264. doi: 10.1084/jem.191.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- Chen CZ, Li M, de Graaf D, Monti S, Gottgens B, Sanchez MJ, Lander ES, Golub TR, Green AR, Lodish HF. Identification of endoglin as a functional marker that defines long-term repopulating hematopoietic stem cells. Proc Natl Acad Sci USA. 2002;99:15468–15473. doi: 10.1073/pnas.202614899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Sieburg HB. The GOD of hematopoietic stem cells: a clonal diversity model of the stem cell compartment. Cell Cycle. 2006;5:394–398. doi: 10.4161/cc.5.4.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RL, Ernst RE, Brunk B, Ivanova N, Mahan MA, Deanehan JK, Moore KA, Overton GC, Lemischka IR. The genetic program of hematopoietic stem cells. Science. 2000;288:1635–1640. doi: 10.1126/science.288.5471.1635. [DOI] [PubMed] [Google Scholar]
- Akashi K, He X, Chen J, Iwasaki H, Niu C, Steenhard B, Zhang J, Haug J, Li L. Transcriptional accessibility for genes of multiple tissues and hematopoietic lineages is hierarchically controlled during early hematopoiesis. Blood. 2003;101:383–389. doi: 10.1182/blood-2002-06-1780. [DOI] [PubMed] [Google Scholar]
- Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Iwasaki H, Reizis B, Ye M, Graf T, Weissman IL, Akashi K. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell. 2002;3:137–147. doi: 10.1016/s1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- Hu M, Krause D, Greaves M, Sharkis S, Dexter M, Heyworth C, Enver T. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 1997;11:774–785. doi: 10.1101/gad.11.6.774. [DOI] [PubMed] [Google Scholar]
- Kondo M, Scherer DC, Miyamoto T, King AG, Akashi K, Sugamura K, Weissman IL. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–386. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Mizuno S, Wells RA, Cantor AB, Watanabe S, Akashi K. GATA-1 converts lymphoid and myelomonocytic progenitors into the megakaryocyte/erythrocyte lineages. Immunity. 2003;19:451–462. doi: 10.1016/s1074-7613(03)00242-5. [DOI] [PubMed] [Google Scholar]
- Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- Mikkola I, Heavey B, Horcher M, Busslinger M. Reversion of B cell commitment upon loss of Pax5 expression. Science. 2002;297:110–113. doi: 10.1126/science.1067518. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Zheng J, Yen H, Sugiyama D, Nakano T. Multipotential differentiation ability of GATA-1-null erythroid-committed cells. Genes Dev. 2006;20:654–659. doi: 10.1101/gad.1378206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittwolf C, Kirchhof N, Jauch A, Durr M, Harder F, Zenke M, Muller AM. In vivo haematopoietic activity is induced in neurosphere cells by chromatin-modifying agents. EMBO J. 2005;24:554–566. doi: 10.1038/sj.emboj.7600546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Reca R, Jala VR, Dawn B, Ratajczak J, Ratajczak MZ. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia. 2005;19:1118–1127. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22:487–500. doi: 10.1634/stemcells.22-4-487. [DOI] [PubMed] [Google Scholar]
- Harrison DE. Mouse erythropoietic stem cell lines function normally 100 months: loss related to number of transplantations. Mech Ageing Dev. 1979;9:427–433. doi: 10.1016/0047-6374(79)90083-6. [DOI] [PubMed] [Google Scholar]
- Ross EA, Anderson N, Micklem HS. Serial depletion and regeneration of the murine hematopoietic system Implications for hematopoietic organization and the study of cellular aging. J Exp Med. 1982;155:432–444. doi: 10.1084/jem.155.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Tsukamoto A, He D, Friera AM, Scollay R, Weissman IL. High doses of purified stem cells cause early hematopoietic recovery in syngeneic and allogeneic host. J Clin Invest. 1998;101:961–966. doi: 10.1172/JCI1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domen J, Weissman IL. Hematopoietic stem cells need two signals to prevent apoptosis; BCL-2 can provide one of these, Kitl/c-Kit signaling the other. J Exp Med. 2000;192:1707–1718. doi: 10.1084/jem.192.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet J, Miller CL, Rose-John S, Piret JM, Eaves CJ. Distinct role of gp130 activation in promoting self-renewal divisions by mitogenically stimulated murine hematopoietic stem cells. Proc Natl Acad Sci USA. 2001;98:1757–1762. doi: 10.1073/pnas.98.4.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, Ling LE, Karanu FN, Bhatia M. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- de Haan G, Weersing E, Dontje B, van Os R, Bystrykh LV, Vellenga E, Miller G. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev Cell. 2003;4:241–251. doi: 10.1016/s1534-5807(03)00018-2. [DOI] [PubMed] [Google Scholar]
- Zhang CC, Lodish HF. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 2004;103:2513–2521. doi: 10.1182/blood-2003-08-2955. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C, Lodish HF. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Rideout WM, III, Hochedlinger K, Kyba M, Daley GQ, Jaenisch R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002;109:17–27. doi: 10.1016/s0092-8674(02)00681-5. [DOI] [PubMed] [Google Scholar]
- Gong JK. Endosteal marrow: a rich source of hematopoietic stem cells. Science. 1978;199:1443–1445. doi: 10.1126/science.75570. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Dooner MS, Tiarks CY, Weier HU, Quesenberry PJ. Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood. 1997;89:4013–4020. [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Kim M, Moon HB, Spangrude GJ. Major age-related changes of mouse hematopoietic stem/progenitor cells. Ann NY Acad Sci. 2003;996:195–208. doi: 10.1111/j.1749-6632.2003.tb03247.x. [DOI] [PubMed] [Google Scholar]
- Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203:73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]