Abstract
Paget’s disease of bone (PDB) is a debilitating bone disorder characterized by giant osteoclasts, enhanced bone destruction, and irregular bone formation. Recently, mutations in SQSTM1 (also known as p62) have been detected in PDB sufferers, with all mutations resulting in either loss of function or truncation/deletion of the ubiquitin binding-associated (UBA) domain. We hypothesized that mutation in the p62 gene resulting in either deletion or premature termination of the UBA domain accounts for the elevated osteoclastic formation and bone resorption associated with PDB. Remarkably, overexpression of the p62 UBA domain deletion mutant (p62ΔUBA) significantly enhanced osteoclastogenesis in vitro compared to cells expressing either wild-type p62 (p62WT) or a control vector in a RAW264.7 osteoclastogenic system. Overexpression of p62ΔUBA potentiated the formation of abnormally large multinucleated osteoclasts and resorption of bone, reminiscent of PDB. Consistent with the enhancement of osteoclastogenesis, overexpression of p62ΔUBA potentiated receptor activator of nuclear factor-κB ligand-induced activation of nuclear factor-κB, NFAT, and ERK phosphorylation. Furthermore, as determined by confocal microscopy, deletion of the p62 UBA domain impaired the association of p62 with TRAF6 in the proteasomal compartment. These results suggest that the UBA domain encodes essential regulatory elements required for receptor activator of nuclear factor-κB ligand-induced osteoclast formation and bone resorption that may be directly associated with the progression of PDB.
Paget’s disease of bone (PDB) is a chronic focal skeletal disorder that is characterized by excessive osteoclast formation and activity followed by irregular new bone formation. The pathogenesis of PDB is thought to be initiated by the formation of giant osteoclasts followed by the deposition of primitive coarse-fibered bone in discontinuous trabeculae with a disjointed lamellar pattern. Although the precise etiology of PDB is presently unknown, several factors, including paramyxoviral infection and putative genetic factors are thought to contribute to its progression.1,2 More recently, genomic scans of families afflicted with PDB have identified 10 different mutations in the gene encoding sequestosome 1 (SQSTM1; also known as p62). Interestingly, all mutations are either clustered within, or adjacent to, the highly conserved ubiquitin-associated (UBA) domain of p62 protein (Figure 1A).3,4 Four of these mutations, denoted K378X, A390X, L394X, and E396X,3,5 disrupt translation of the p62 UBA domain via introduction of stop condons, resulting in premature termination of the protein. Moreover, recent evidence indicates that patients harboring mutations that result in the UBA domain deletion display more extensive disease compared to those with point mutations,4,5 suggesting that the UBA domain may encode regulatory elements that are crucial to both the onset and severity of the disease.
Figure 1-6923.
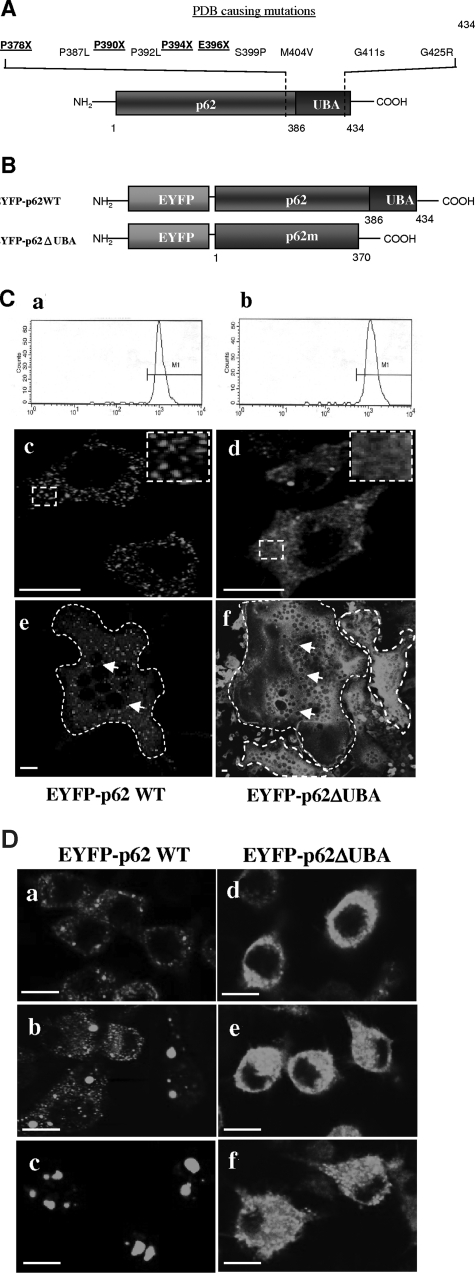
Expression and subcellular localization of p62 and the UBA domain deletion mutant. A: Schematic representation of the detected PDB-causing p62 UBA domain mutations. Four of the PDB mutations (K378X, A390X, L394X, and E396X) lead to introduction of stop codons that result in premature termination of the translation of p62 UBA domain.3–5B: Schematic illustration of EYFP-tagged p62 wild-type (EFYP-p62WT) and p62 UBAdomain deletion mutant (EYFP-p62ΔUBA) constructs. C: FACS analysis of postsorted RAW264.7 cells stably expressing EYFP-p62WT (a) or EYFP-p62ΔUBA (b). Confocal images of the cellular localization of EYFP-p62WT (c) or EYFP-p62ΔUBA (d) in RAW264.7 stable cell lines cultured on glass coverslips. Confocal images of the cellular localization of EYFP-p62WT (e) or EYFP-p62ΔUBA (f) in OCLs. Borders of OCLs are outlined with dashed lines and nuclei are marked with arrows. In brief, RAW264.7 cell lines cultured on glass coverslips were treated with 100 ng/ml of RANKL for 5 days and fixed. EYFP signals in EYFP-p62WT- and EYFP-p62ΔUBA-expressing OCLs were analyzed by confocal microscopy. D: The requirement of p62 UBA domain for subcellular distribution of p62 to the proteasomal degradation pathway. EYFP-p62WT and EYFP-p62ΔUBA in stable RAW264.7 cell lines were grown on glass coverslips and either left untreated or treated with MG132 as indicated. The cells were fixed with 4% paraformaldehyde and processed for confocal microscopy. Representative confocal images of the cellular localization are shown. a–c: Localization of EYFP-p62WT in the absence of MG132 (a), treated with 0.5 μm MG132 for 24 hours (b), or treated with 2 μm MG132 for 12 hours (c). d–f: Localization of EYFP-p62ΔUBA in the absence of MG132 (d), treated with 0.5 μm MG132 for 24 hours (e), or treated with 2 μm MG132 for 12 hours (f). Insets represent magnifications of hatched regions. Scale bars = 10 μm.
p62 is a cytoplasmic scaffold protein that mediates diverse cellular signaling pathways. Initially identified as a phosphotyrosine-independent ligand of the Src homology 2 (SH2) domain of p56lck,6,7 p62 functions to link signaling molecules such as RIP, TrkA, and TRAF6 to atypical protein kinase C (aPKCs) in response to various stimuli, including tumor necrosis factor-α, interleukin-1, nerve growth factor, and receptor activator of nuclear factor-κB ligand (RANKL).8–12 Previous studies have shown that p62 noncovalently binds ubiquitin at its C-terminus,13,14 suggesting that it may serve as a storage compartment for ubiquitinated proteins.15 In addition, p62 binds specifically to lysine-63 (K63)-polyubiquitinated substrates, thereby acting as a putative ubiquitin chain-targeting factor that shuttles these substrates for proteasome degradation via its UBA domain.16 Recently, p62 has been implicated in the regulation of osteoclast formation.12 Mice lacking p62 exhibit impaired osteoclastogenesis in vivo and reduced IKK activation and nuclear factor (NF)-κB nuclear translocation.12 Although the importance of p62 in osteoclast formation has been established, its role in the pathogenesis of PDB remains primarily unclear. In particular, the potential role of the UBA domain in the disease progression has yet to be defined.
In light of the fact that most p62 mutations associated with PDB result in deletion of the UBA domain, we hypothesized that the UBA domain is crucial for osteoclastogenesis and function. Thus, aberration or deletions of this domain may result in dysregulation of osteoclast formation, accounting for the progression of PDB. To explore this possibility, we examined the role of the p62 UBA domain in RANKL-induced osteoclastogenesis, bone resorption, and signal activation pathways using a RAW264.7-RANKL osteoclastogenic system. Our results indicate, for the first time, that overexpression of the p62 UBA domain deletion mutant (p62ΔUBA) potentiates RANKL-mediated abnormal production of aggressive large multinucleated osteoclasts that mimic those observed in PDB. This observation correlates with biochemical evidence that deletion of the UBA domain enhances RANKL-mediated activation of signaling pathways including NF-κB, NFAT, and ERK. Our findings raise the intriguing possibility that the UBA domain of p62 encodes the major regulatory element responsible for abnormal osteoclastogenesis observed in PDB.
Materials and Methods
Reagents
RAW264.7 cells were obtained from the American Type Culture Collection (Rockville, MD). Diagnostic acid phosphatase kits were purchased from Sigma (Sydney, Australia). The luciferase assay system was obtained from Promega (Sydney, Australia). Recombinant GST-rRANKL protein was expressed and purified as previously described.17
Construction of Vectors and Fusion Proteins
A pcDNA3.1-EYFP vector (Clontech Laboratories Inc., Mountain View, CA) was used in the construction of an EYFP-p62 wild-type (EYFP-p62WT) fusion protein. In brief, the p62 coding sequence was polymerase chain reaction (PCR)-amplified using the sequences 5′-CTCGAGGGATCCGTTATGGCGTCGTTCACG-3′ and 5′-ACTAGTTCTAGATCACAATGGTGGAGGGTG-3′ for forward and reverse primers, respectively. The PCR fragment was cloned into pGEM-T Easy vector (Promega, Sydney, NSW, Australia). The 1.6-kb BamHI- and SpeI-digested insert was then subcloned into the pcDNA3.1-EYFP expression vector to generate pEYFP-p62WT, which encodes enhanced yellow fluorescent protein (EYFP) and p62 wild-type fusion protein. To construct a p62 UBA deletion mutant, pGEM-T Easy vector containing the p62 gene was digested with BamHI and XbaI restriction enzymes, and the ∼1.45-kb insert was then subcloned into the pEYFP vector expression vector. The resultant expression clone is named pEYFP-p62ΔUBA, which encodes p62ΔUBA (Δ371-442) deletion mutant.
Generation of RAW264.7 Cell Lines Stably Expressing EYFP-p62WT and EYFP-p62ΔUBA
Generation of stable cell lines expressing EYFP proteins was performed as previously described by our laboratory.18 Briefly, 5 × 106 RAW264.7 cells were electroporated with 20 μg of plasmids under the following conditions: voltage, 0.28 kV; high cap, 0.95 K Faradays; high cap points to 500 V max. The electroporated cells were recovered in complete medium overnight and then cultured in the presence of 400 μg/ml of G418. Four cell lines were generated by transfecting pcDNA3.1, pcDNA3.1-EYFP, pcDNA3.1-pEYFP-p62WT, or pcDNA3.1-pEYFP-p62ΔUBA. Clones resistant to G418 selection were expanded, and expression levels were assessed by either fluorescence microscopy or quantitative fluorescence-activated cell sorting (FACS) analysis. To obtain stably transfected RAW264.7 cells that express similar levels of GFP, FACS was performed to isolate GFP-positive cells using a FACS Vantage (Becton Dickinson, San Jose, CA) and CellQuest software (version 3.1f; Becton Dickinson). Cells expressing GFP were excited at 488 nm and detected using a 530/30-nm bandpass filter in front of the FL1 detector.
RAW264.7 Cell Culture and in Vitro Osteoclastogenesis Assay
RAW264.7 cells were cultured in α-modified essential medium (Biosciences Pty. Ltd., NSW, Australia) supplemented with 10% fetal calf serum, 2 mmol/L l-glutamine, and 100 U/ml penicillin/streptomycin. For osteoclastogenesis assays, RAW264.7 cells were seeded in a 96-well plate to a density of 1.5 × 103 cells/well and cultured for 5 to 7 days in full α-modified essential medium in the presence of 100 ng/ml of GST-rRANKL as previously described.19,20 The growth medium was replaced every 2 to 3 days. After 5 days, cultures were fixed for 10 minutes at room temperature with 4% paraformaldehyde in phosphate-buffered saline (PBS) and then washed four times with PBS. The fixed cells were stained for tartrate-resistant acid phosphatase (TRACP) using the Diag-nostic acid phosphatase kit (Sigma) according to the manufacturer’s instructions and TRACP-positive multinucleated cells were scored as osteoclast-like cells (OCLs). Because of the absence of M-CSF and osteoblasts, the use of the RAW264.7 cell system to generate osteoclasts has an advantage over other culture systems because direct involvement of the p62 UBA domain in RANKL signaling pathways can be distinguished. Moreover, several other groups have used RAW264.7 cells as a convenient model system to study osteoclastogenesis and signal transduction pathways.21–23
Bone Resorption Pit Assay
To study the effect of overexpression of p62 wild-type and UBA deletion mutant on osteoclastic bone resorption, RAW264.7 cell lines stably expressing EYFP-p62WT, EYFP-p62ΔUBA, or EYFP were seeded onto 150-μm-thick bovine bone slices and cultured for 14 days in the presence of 100 ng/ml of RANKL. After 14 days, OCLs were fixed and stained for TRACP activity. Bovine bone slices were then incubated in 2 mol/L NaOH (2 hours), and the cells were removed by mechanical agitation and sonication. Resorption lacunae were visualized by a Philips XL30 scanning electron microscope.
Luciferase Reporter Gene Assay for the NF-κB and NFAT Activation in RAW264.7 Cells
To determine the effect of p62 wild-type and its UBA deletion mutant on RANKL-induced activation of NF-κB and NFAT, RAW264.7 cells (5 × 106) stably expressing EYFP-p62WT and EYFP-p62ΔUBA were transiently transfected with luciferase reporter construct 3 kb-Luc-SV40 as previously described23,24 or with the luciferase reporter construct pNFAT-TA-Luc (BD Biosciences, San Jose, CA). Cells were plated in 24-well plates at a density of 1 × 105 cells/well and treated with RANKL. At appropriate times, luciferase activity was measured in the cells using the Promega luciferase assay system according the manufacturer’s instructions (Promega).
Western Blotting Analysis
Proteins from cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto nitrocellulose membranes (Bio-Rad, Regents Park, NSW, Australia). Membranes were blocked with 5% (w/v) nonfat milk powder in TBST [10 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 0.1% (v/v) Tween 20] and then probed with primary antibodies to GFP (1/400, final concentration) (Abcam, Cambridge, UK), phosphorylated forms of ERK (1/1000, final concentration), NFAT2 (1/1000, final concentration), and β-tubulin (1/1000, final concentration) (Santa Cruz Biotechnology Inc., Santa Cruz, CA) in the blocking solution. After washing three times with Tris-buffered saline (TBS), membranes were incubated with horseradish peroxidase-conjugated secondary antibodies diluted 1/5000 in 1% (w/v) nonfat milk powder in TBST. The membranes were then developed using the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ).
Immunofluorescence and Confocal Analysis
For immunofluorescent staining, RAW264.7 cells or OCLs were cultured on glass coverslips and fixed with 4% paraformaldehyde in PBS for 10 minutes at room temperature and washed four times with PBS. Fixed cells were treated with 0.1% Triton X-100 for 5 minutes and washed. Anti-TRAF6 antibody (Santa Cruz Biotechnology Inc.) was added at final dilution of 1:750 and incubated for 1 hour at room temperature. Secondary antibody labeled with Alexa Fluor 546 (Molecular Probes, Eugene, OR) was used at a final dilution of 1:500. For the detection of F-actin microfilaments, rhodamine-conjugated phalloidin was used at final dilution of 1:100 (Molecular Probes Inc.). Fluorescent images of EYFP (excitation 488/emission 520/35) and Alexa Fluor 546 (excitation 543/emission 580/32) were collected on a Bio-Rad MRC 1000/1024 UV laser-scanning confocal microscope.
RNA Isolation and Reverse Transcriptase (RT)-PCR
Total RNA was isolated from RAW264.7 cells treated with 100 ng/ml of RANKL as described. For RT-PCR, single-stranded cDNA was prepared from 2 μg of total RNA using reverse transcriptase with an oligo-dT primer. One μl of each cDNA was subjected to PCR amplification using specific primers. For the amplification of mouse DC-STAMP, forward primer 5′-CCTGCAACCTAAGGGCAAAG-3′ and reverse primer 5′-TCAACAGCTCTGTCGTGACC-3′ were used, and PCR-amplification was performed with 30 cycles (94°C, 40 seconds; 60°C, 40 seconds; and 72°C, 40 seconds). As an internal control, the single-stranded cDNA was PCR-amplified for 25 cycles using 36 B4 forward primer 5′-TCATTGTGGGAGCAGACA-3′ and reverse primer 5′-TCCTCCGACTCTTCCTTT-3′.
Statistical Analysis
Statistics were performed using paired or unpaired Student’s t-test with significance taken at P < 0.05. All data shown represent one of at least three independent experiments.
Results
Expression and Characterization of EYFP-Tagged p62 Fusion Proteins in RAW264.7 and RAW264.7-Derived Osteoclast-Like Cells
p62 has been reported to bind and sequester polyubiquitinated substrates into aggregates via its UBA domain.16 To investigate the functional significance of p62 UBA domain in RANKL-mediated osteoclast differentiation and signaling pathways, we generated two EYFP-tagged fusion constructs encoding either full-length p62 (EYFP-p62 WT) or a p62 deletion mutant of which the last 64 amino acids were deleted to remove the UBA domain (Figure 1B). Each construct was transfected into RAW264.7 cells to yield stable EYFP-p62WT- and pEYFP-p62ΔUBA-expressing cell lines. RAW264.7 cells transfected in parallel with EYFP alone or pCDNA3.1 vector served as a control. To obtain homogenous and comparable expression levels of the fusion proteins, all EYFP cell lines were sorted by flow cytometry. Gates were set between a fluorescence intensity of 102 to 104. Figure 1C, a and b’, illustrates the typical read-outs of EYFP signals by flow cytometry in postsorted RAW264.7 cell lines expressing EYFP fusion proteins. Expression levels were primarily comparable between EYFP, EYFP-p62WT, and EYFP-p62ΔUBA cell lines, and there were no observable differences in cell sizes as evidenced by flow cytometry and confocal microscopy. In addition, as evidenced by alamar blue staining, there were no observable differences in proliferation between these cell lines (data not shown). Expression of EYFP-fusion proteins was also confirmed by immunoblotting (data not shown). We verified the deletion of p62 UBA domain by subcellular localization studies in both RAW264.7 cells and RAW264.7-derived osteoclast-like cells (OCLs). As shown in Figure 1Cc, EYFP-p62WT localized as dot-like structures of various sizes within the cytoplasm. By comparison, EYFP-p62ΔUBA was diffusely distributed throughout the cytoplasm (Figure 1Cd). Similar localizations were observed in RANKL-differentiated OCLs (Figure 1C, e and f). Interestingly, we observed that osteoclasts overexpressing EYFP-p62WT and EYFP-p62ΔUBA exhibited morphological differences with EYFP-p62ΔUBA-overexpressing osteoclasts (Figure 1Cf), being larger in size and containing increased numbers of nuclei compared to EYFP-p62WT-expressing osteoclasts (Figure 1Ce). To confirm that the observed p62 dot-like localizations were associated with the proteasomal degradation pathway, RAW264.7 cells were subjected to treatment with MG132, a known proteasome inhibitor (Figure 1D). On treatment with MG132, EYFP-p62WT dot-like structures increased in size. This morphological change occurred in a dose-dependent manner. On 2 μmol/L MG132 treatment, the size of the EYFP-p62WT structures increased from a range of 08 ± 0.2 μm to 2.5 ± 0.3 μm (Figure 1D, a–c). In contrast, the localization of EYFP-p62ΔUBA was primarily unaffected by MG132 treatment (Figure 1D, d–f). Together, these data indicate that the UBA domain is crucial to the subcellular targeting of p62 to the proteasomal degradation pathway and hint to an important role in osteoclast formation and multinucleation.
Deletion of the UBA Domain of p62 Potentiates RANKL-Mediated Osteoclast Formation and Multinucleation
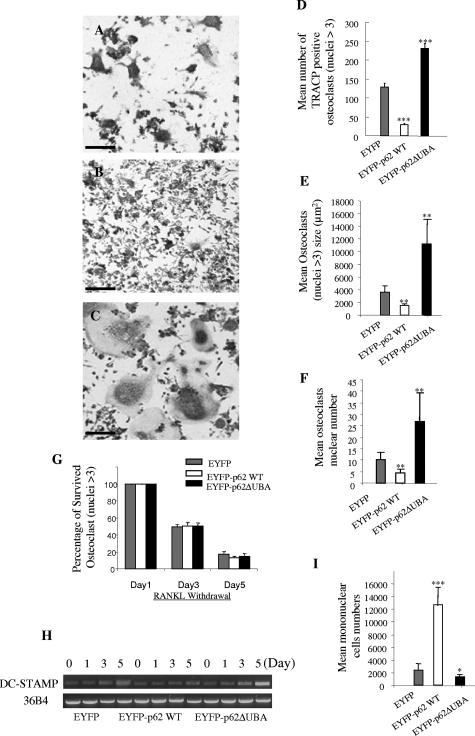
The observed morphological increase in osteoclast size suggested that the UBA domain may play a role in osteoclast development. To explore this possibility, RAW264.7 cells stably transfected with EYFP-p62WT, EYFP-p62ΔUBA, and EYFP were cultured in 96-well plates (2 × 103 per well) and exposed to RANKL to induce osteoclastogenesis. After 5 days, cells were fixed and stained for TRACP, and numbers of multinucleated cells were counted. As shown in Figure 2, A–C, deletion of the p62 UBA domain potentiated RANKL-mediated osteoclastogenesis. Consistent with our previous observation, OCLs derived from RAW264.7 cells stably transfected with p62 ΔUBA form more TRACP-positive osteoclasts (Figure 2C), and these OCLs were larger in size and possessed greater numbers of nuclei compared to OCLs derived from EYFP-p62WT- (Figure 2B) and EYFP-expressing cell lines (Figure 2A). Quantitative analysis revealed that overexpressing EYFP-p62WT significantly inhibited osteoclast formation, whereas overexpression of EYFP-p62ΔUBA enhanced osteoclast formation (Figure 2D). Moreover, p62WT-expressing OCLs possess smaller and lower numbers of nuclei, whereas p62ΔUBA-expressing OCLs possess larger cell sizes and higher numbers of nuclei compared to the EYFP alone expressing control (Figure 2, D–F). It appears that these changes do not reflect changes of osteoclast survival, as evidenced by Figure 2G, that similar survival rates were observed between EYFP-p62WT-, EYFP-p62ΔUBA-, and EYFP-overexpressing OCLs after RANKL withdrawal.
Figure 2-6923.
The effect of overexpression of EYFP-p62WT or EYFP-p62ΔUBA on osteoclast size, multinucleation, and survival. RAW264.7 cells stably expressing EYFP-p62WT or EYFP-p62ΔUBA were cultured in the presence of RANKL. After 5 days, the cells were fixed with 4% paraformaldehyde and stained for TRACP activity. A–C: Representative images of TRACP staining of OCLs from one of the three experiments are shown. D–F: Quantitative analysis shows the mean number of TRACP-positive OCLs (D), mean OCLs sizes (E), and mean OCLs nuclei numbers (F). G: Similar experiments were performed in which RAW264.7 cells stably expressing EYFP-p62WT, EYFP-p62ΔUBA, or EYFP were cultured in the presence of 100 ng/ml RANKL for 5 days. Fresh medium with no RANKL was then replaced and cells were continued in culture for an additional 1, 3, and 5 days. Cells were fixed, and the number of surviving osteoclasts (nuclei >3) were counted. The effect of overexpression of p62WT, EYFP-p62ΔUBA, or EYFP fusion proteins on OCL survival was quantified by the ratio between the OCLs remained on the third or fifth day after RANKL withdrawal and the initial number of osteoclasts present on the first day of RANKL withdrawal. H: Representative results from three independent experiments showing semiquantitative RT-PCR analysis of the effect of overexpression of p62WT or p62ΔUBA on DC-STAMP gene expression during RANKL-induced RAW cell differentiation. I: The total number of mononuclear cells presented in p62WT, EYFP-p62ΔUBA, and EYFP cultures after 5 days of RANKL stimulation were counted. Four representative views of images were taken from cultures. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars = 50 μm.
Next, RT-PCR analysis revealed that expression levels of DC-STAMP, an essential regulator of osteoclastogenesis and fusion, was enhanced in EYFP-p62ΔUBA-expressing culture, as compared to EYFP-and EYFP-p62WT-expressing cultures during osteoclastogenesis (Figure 2H). To this end, we examined whether the effect of EYFP-p62ΔUBA overexpression on osteoclastogenesis is simply because of the induction of fusion. As shown in Figure 2I, we observed that on 5 days of RANKL stimulation, p62WT-expressing cultures contained significantly higher numbers of mononuclear cells compared to p62ΔUBA- and EYFP-expressing cultures. In addition, the total number of nuclei from both mononuclear and multinuclear cells is not equal between p62WT, p62ΔUBA, and EYFP cultures (data not shown). Thus, these results suggested that the observed enhancement in osteoclast formation and multinucleation in p62 UBA deletion mutant culture is not simply a fusion induction effect.
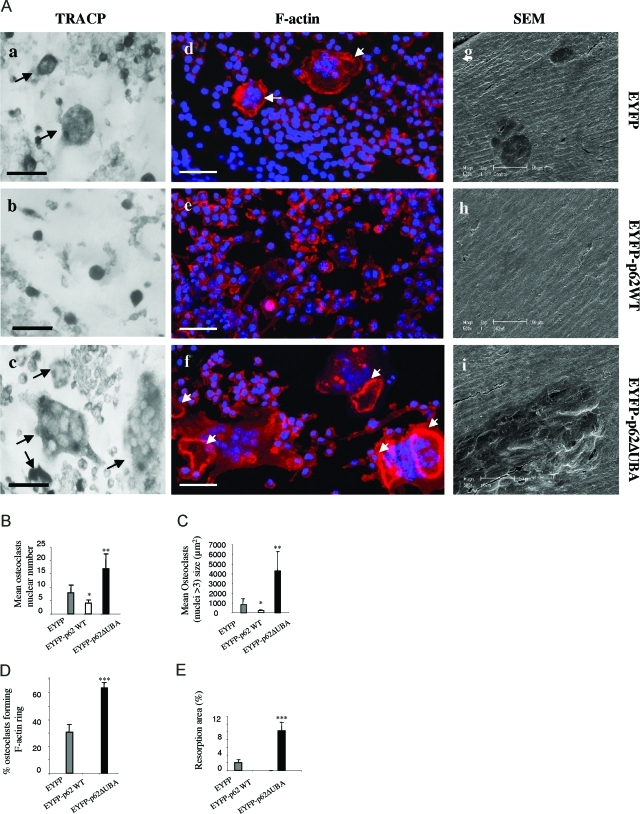
Next, we examined the effects of p62 wild-type and UBA domain deletion mutant on osteoclastic bone resorption. To this end, wild-type and mutant EYFP-p62 stable RAW 264.7 cell lines were cultured on bovine bone slices in the presence of RANKL. After 14 days osteoclasts were stained with TRACP before being removed to visualize underlying resorption lacunae by scanning electron microscopy. As shown in Figure 3A, a–c, we observed that EYFP-p62ΔUBA-expressing RAW264.7 cells demonstrated an enhanced ability in osteoclast formation and multinucleation. Consistent with previous observation from plastic and glass culture, EYFP-p62ΔUBA-expressing OCLs on bovine bone slices possess significantly higher number of nuclei (Figure 3B) and larger cell size (Figure 3C) compared to EYFP-p62WT- and EYFP-expressing OCLs. Next, we examined the effect of p62WT and p62ΔUBA overexpression on the formation of F-actin rings and sealing zones. To this end, OCLs were stained with rhodamine-phalloidin and visualized by confocal microscopy. We observed that the majority of osteoclasts overexpressing EYFP-p62ΔUBA possessed well-defined F-actin rings and sealing zones (Figure 3A, d–f). Quantitative analysis revealed that EYFP-p62ΔUBA overexpression potentiated F-actin ring formation, with ∼62% of mutant-expressing osteoclasts having well-defined sealing zones on bone compared to ∼35% for EYFP-expressing OCLs. In contrast, we failed to observe the formation of sealing zone from p62WT-expressing OCLs (Figure 3D). Furthermore, as shown in Figure 3A, g–i, resorption lacunas generated by the EYFP-p62ΔUBA-expressing osteoclasts were generally found aggregated and overlapping with each other, reflecting the enhanced activities of these mutation-carrying osteoclasts. In contrast, overexpression of EYFP-p62WT impaired osteoclastic bone resorption in vitro. No obvious resorbing lacunas were observed even after 14 days of culture. In addition, the percentage of resorption area per bone slice was quantitatively analyzed, showing that EYFP-p62ΔUBA-expressing osteoclasts resorbed ∼10% of the total bovine bone surface; in contrast, EYFP control only resorbed ∼2% of the surface (Figure 3E).
Figure 3-6923.
The effect of overexpression of EYFP-p62WT or EYFP-p62ΔUBA on osteoclast resorptive activity. EYFP-p62WT, EYFP-p62ΔUBA, or EYFP stably expressing RAW264.7 cell lines were cultured on bovine bone slices in the presence of RANKL (100 ng/ml). A: After 14 days, cells were fixed and stained for either TRACP (a–c) or rhodamine-phalloidin (F-actin) (d–f) and visualized by light microscopy and confocal microscopy, respectively. Cells were then removed, and resorptive lacunae were assessed by scanning electron microscopy (g–i). F-Actin rings are marked with arrows. Quantitative analysis shows the mean nuclei numbers (B) and sizes (C) of osteoclasts, percentage of osteoclasts forming F-actin rings on bovine bone slice (D), and percentage of bovine bone slice surface occupied by resorption lacunae (E). *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars = 50 μm.
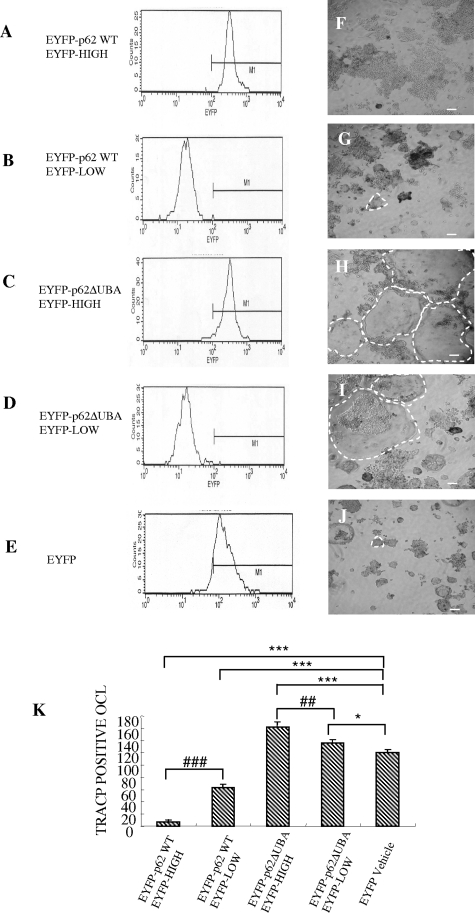
To exclude the possibility that the effect of osteoclastogenesis reflects differential expression of EYFP-fusion proteins in each stable cell line, cell lines were further sorted into low- and high-expressing subpopulations (Figure 4, A–D), and osteoclastogenesis assays were performed. Consistently, RAW264.7 cells highly expressing EYFP-p62WT formed fewer OCLs (Figure 4, A and F) when compared with those with 10-fold lower expression (Figure 4, B and G). In contrast, RAW264.7 cells highly expressing EYFP-p62ΔUBA formed larger OCLs with greater multinucleation compared with other groups (Figure 4, C and I). Based on these findings, it appears that p62 UBA domain encodes crucial regulatory elements in RANKL-mediated osteoclast formation. Moreover, these unexpected results suggest that p62 may act as a negative modulator during this process.
Figure 4-6923.
The levels of overexpression of EYFP-p62WT or EYFP-p62ΔUBA affect osteoclastogenesis. A–E: FACS analyses of EYFP-p62WT, EYFP-p62ΔUBA, and EYFP control. RAW264.7 cells stably expressing EYFP-p62WT or EYFP-p62ΔUBA were FACS-sorted into two populations, EYFP-HIGH and EYFP-LOW. Stable RAW264.7 were treated with RANKL for 5 days, fixed, and subjected to TRACP staining. F–J: Representative images of TRACP staining from one of the three experiments are shown. K: Quantitative analysis was preformed by counting the total number of OCLs. P value indicates the difference between respective controls, *P < 0.05, ##P < 0.001, ***/###P < 0.001.
Overexpression of UBA Domain Deletion Mutant Enhances RANKL-Induced Activation of NF-κB, NFAT, and ERK
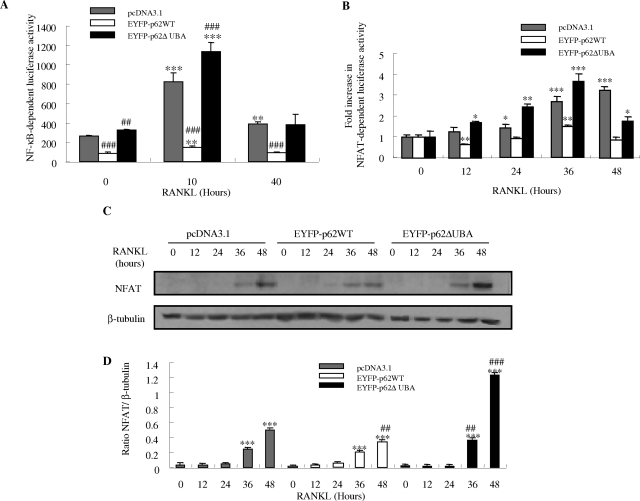
To gain better insight into the molecular mechanism(s) underlying the observed phenomenon, we examined the effects of overexpression of EYFPp62WT and EYFP-p62ΔUBA on several established osteoclastic signaling cascades. First, we examined their potential effect on RANKL-mediated activation of NF-κB. For this purpose, EYFP-p62WT-, EYFP-p62ΔUBA-, and control-transfected RAW264.7 stable cell lines were transiently transfected with the NF-κB-driven luciferase reporter gene construct, 3κB-Luc-SV40. Twenty-four hours after transfection, cells were stimulated with 100 ng/ml of RANKL for 10 and 40 hours, before being harvested and luciferase activities measured. In all cells examined, RANKL-induced NF-κB transcriptional activities peaked at 10 hours after stimulation. Consistent with the observed enhanced osteoclastogenesis, overexpression of the p62ΔUBA significantly enhanced RANKL-induced NF-κB activation (Figure 5A). In contrast p62WT exhibited a negative effect toward this activation process.
Figure 5-6923.
The effects of overexpression of EYFP-p62WT or EYFP-p62ΔUBA on RANKL-induced NF-κB and NFAT-dependent transcription. RAW264.7 cells stably expressing EYFP-p62WT or EYFP-p62ΔUBA were transfected with the 3-kb Luc-SV40 NF-κB luciferase reporter plasmid (A) or pNFAT-TA Luc reporter gene (B) and treated with 100 ng/ml of RANKL for various time periods. After stimulation, cells were lysed and luciferase activities in lysates determined. Each bar is the mean ± SEM from triplicate wells. Representative results from three independent experiments are shown. *The P values of the effect of RANKL stimulation compared to its respective controls (***P < 0.001, **P < 0.01, *P < 0.05). #The P values of the effect of overexpression of pEYFP-62WT and pEYFP-62ΔUBA on RANKL-induced NF-κB activity compared to pcDNA3.1-transfected control (###P < 0.001). C–D: Effect of EYFP-p62WT and EFYP-p62ΔUBA overexpression on RANKL-induced NFAT protein expression in RAW stable cell lines. RAW264.7 cells stably transfected with pcDNA3.1, EYFP-p62WT, and EFYP-p62ΔUBA were stimulated with 100 ng/ml of RANKL for the indicated times. After stimulation, whole cell extracts were analyzed for NFAT protein expression by Western blotting. The same membrane was stripped and reprobed with an antibody for β-tubulin, which served as an internal control for differences in loading and transfer. C: The levels of NFAT and β-tubulin proteins are shown. D: NFAT protein levels are shown as the ratio of NFAT to β-tubulin. Each bar is the mean ± SEM from representative results from three independent experiments. *The P values of the effect on RANKL-induced NFAT protein synthesis at different time points compared to its unstimulated control (***P < 0.001). #The P value compared to the pcDNA3.1 control (##P < 0.01, ###P < 0.001).
Accumulating evidence indicates that the transcriptional factor NFAT2/NFATc1 plays a crucial regulatory role in osteoclast differentiation.25,26 Therefore, we next used a comparable luciferase gene reporter assay to examine whether deletion of the p62 UBA domain affects RANKL-induced activation of NFAT. As shown in Figure 5B, NFAT activity gradually increased to maximal at 48 hours after RANKL stimulation in transfected control cells. Interestingly, compared with controls, overexpression of EYFP-p62WT attenuated RANKL-induced NFAT activity whereas overexpression of EYFP-p62ΔUBA potentiated the event at all time points except 48 hours after stimulation (Figure 5B).
To complement the luciferase assay, we also examined the effect of overexpression of p62WT and p62ΔUBA on RANKL-mediated NFAT protein expression levels by immunoblotting. As shown in Figure 5C, NFAT2 expression significantly increased 36 hours after RANKL stimulation in all cell lines, with peak expression reached at 48 hours after stimulation. Quantitative analysis of protein expression revealed that EYFP-p62ΔUBA significantly induced NFAT2 protein expression compared to the wild-type and control cell lines (Figure 5D). In contrast, overexpression of EYFP-p62WT exhibited the least effect on NFAT2 protein induction in response to RANKL stimulation at all time points examined.
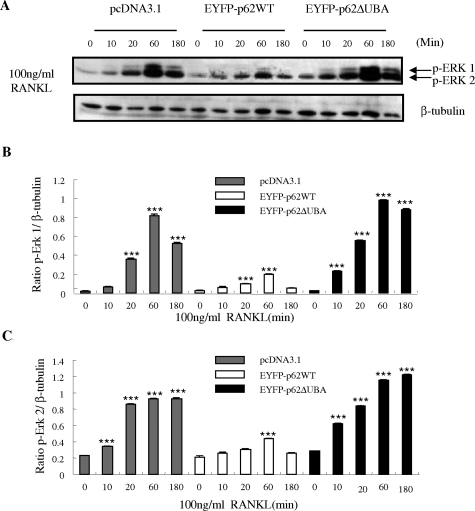
The importance of RANKL-induced activation on the cascades of mitogen-activated protein kinases, including extracellular signal-regulated kinase (ERK), in osteoclast differentiation and survival has been well documented.24,27 To characterize better the involvement of p62 and its UBA domain in RANKL-signaling pathways and osteoclastogenesis, the role of p62WT and p62ΔUBA in RANKL-induced ERK phosphorylation was examined. Western blot analysis was performed using a specific antibody to the phosphorylated forms of ERK (ERK1/2). The phosphorylation of ERK1/2 increased after 10 minutes and peaked after 60 minutes of RANKL stimulation (Figure 6). When compared with the control, overexpression of EYFP-p62WT suppressed RANKL-induced phosphorylation of ERK1/2 whereas overexpression of the EYFP-p62ΔUBA enhanced and prolonged RANKL-induced phosphorylation of ERK1/2.
Figure 6-6923.
The effects of EYFP-p62WT and EFYP-p62ΔUBA overexpression on RANKL-induced phosphorylation of ERK in RAW stable cell lines. RAW264.7 cells stably transfected with pcDNA3.1, EYFP-p62WT, and EFYP-p62ΔUBA were stimulated with 100 ng/ml of RANKL for the indicated time. On stimulation, whole cell extracts were analyzed for phosphorylation of ERK 1 and 2 proteins by Western blotting with monoclonal anti-p-ERK antibody at 1/1000 dilution, followed by enhanced chemiluminescence. The same membrane was stripped and reprobed with an antibody for β-tubulin, which served as an internal control for differences in loading and transfer. A: The levels of ERK 1/2, and β-tubulin proteins are shown. B and C: Levels of phosphorylation of ERK proteins are shown as the ratio of phosphorylated ERK to β-tubulin. Each bar is the mean ± SEM from three independent experiments (***P < 0.001).
Deletion of the UBA Domain Disrupts p62 Co-Localization with TRAF6
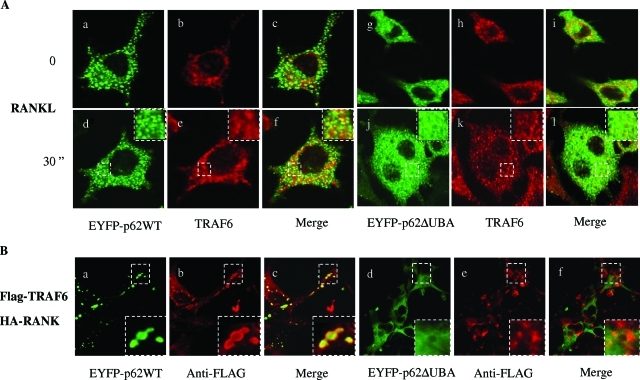
RANKL-induced polyubiquitination, shuttling, and degradation of TRAF6 have been shown to be crucial for the induction of osteoclastogenesis.28,29 The polyubiquitination of TRAF6 has been speculated to be mediated by its recruitment and ubiquitination in association with p62.16 Therefore, we reasoned that the deletion of the p62 UBA domain might impair TRAF6 involvement in the proteasomal degradation pathway. To examine this possibility, RAW264.7 cell lines stably expressing EYFP-p62WT and EYFP-p62ΔUBA were cultured on glass coverslips and stimulated with sRANKL for 30 minutes. After stimulation, we assessed the association by immunohistochemistry and confocal microscopy. As shown in Figure 7A, p62 showed significant co-localization with endogenous TRAF6 (yellow overlap) indicating effective p62-TRAF6 association. No obvious co-localization was observed in cells expressing the p62ΔUBA mutant. We also examined localization of p62 with exogenously expressed TRAF6 in COS-7 cells to confirm the requirement of p62UBA domain for its association with TRAF6 (Figure 7B). For this purpose, COS-7 cells were cultured on glass coverslips and co-transfected with Flag-TRAF6 and HA-RANK, in combination with either EYFP-p62WT or EYFP-p62ΔUBA. Cells were fixed 24 hours after transfection, and subcellular distribution of p62 and TRAF6 were assessed by immunohistochemistry and confocal microscopy. As shown in Figure 7B, wild-type p62 strongly co-localized with FLAG-TRAF6, whereas no overlap with Flag-TRAF6 was observed in cells expressing the p62ΔUBA. Taken together, these results indicate that p62 UBA domain is crucial for its association with TRAF6 in the proteasomal pathway after RANKL activation.
Figure 7-6923.
Deletion of UBA domain disrupts RANKL p62 association with TRAF6. A: RAW264.7 cells stably expressing EYFP-p62WT or EYFP-p62ΔUBA were grown on glass coverslips and either left untreated or treated with RANKL for 30 minutes. The cells were fixed with 4% paraformaldehyde, immunostained with anti-TRAF6 antibody, and processed for confocal microscopy. Representative confocal images of the cellular localization are shown. Co-localization of EYFP-p62WT with TRAF6 in untreated (a–c) or RANKL-treated RAW cells (d–f). Co-localization of EYFP-p62ΔUBA with TRAF6 in untreated (g–i) or RANKL-treated RAW264.7 cells (j–l). B: COS-7 cells were co-transfected with EYFP-p62WT (a–c) or EYFP-p62ΔUBA (d–f), pFLAG-TRAF6, and pHA-RANK for 24 hours. The cells were fixed and immunostained with anti-FLAG antibody, followed by secondary antibody (Alexa Fluor 546 goat anti-mouse IgG). The images were recorded under confocal microscope. Insets represent magnifications of hatched regions.
Discussion
Despite major advances toward unraveling the molecular mechanisms underlying many osteolytic disorders, the causative factors of PDB remain poorly understood. Accumulating evidence suggests that PDB is primarily determined by genetic influences2 because current estimates have found that between 15% and 40% of affected individuals have at least one affected first-degree relative.30 In recent years, genome-wide screening in families harboring PDB have revealed recurrent mutations occurring within the UBA domain of the gene encoding SQSTM1 (also known as p62), yet the functional link between p62 of this domain and the onset and progression of the disease is unclear. Comparisons between the full-length p62 protein and four of the PDB-causing p62 UBA mutants (P392L, E396X, M404V, G411S, and G425R) have recently demonstrated that all tested mutants have impaired ubiquitin-binding properties,31 suggesting that deficient binding of p62 to a ubiquitylated target may be associated with the disease. It is suggested that mutations resulting in loss of function of the UBA domain of p62, might account for the elevated osteoclastogenesis and activity, thus contributing to the progression of PDB.32–34
In the present study, we have generated osteoclast precursor cell lines stably expressing wild-type p62 and a UBA domain deletion mutant in an attempt to dissect the functional importance of the p62 UBA domain in RANKL-mediated signaling and osteoclastogenesis. Our results demonstrate, for the first time, that the UBA domain of p62 is crucial to the regulation of RANKL-mediated osteoclastogenesis, bone resorption, and its associated signaling pathways. Overexpression of the p62 UBA domain deletion mutant was found to enhance osteoclast formation and multinucleation, whereas the wild-type p62 protein had the opposite effects. A previous study has shown that mice lacking p62 exhibited impaired osteoclastogenesis in vivo as well as reduced RANK signaling,12 hinting that p62 is required for osteoclast formation during skeletal development. In our study, we demonstrate that in the pathological condition, mutation of p62 could cause the activation of osteoclasts. We speculate that p62 might negatively regulate osteoclastogenesis via its UBA domain as evidenced by our observation that osteoclasts harboring UBA domain deletions were enlarged and contained abundant nuclei reminiscent to that of giant osteoclasts observed in PDB. To seek a possible explanation as to how overexpression of the p62 UBA domain deletion mutant enhances osteoclastogenesis and multinucleation and how the wild-type adversely affects them, we investigated the gene expression level of DC-STAMP, a molecule involved in the formation and fusion of osteoclasts.35,36 We found that during the course of osteoclastogenesis, overexpression of p62 UBA domain deletion mutant enhanced RANKL-induced DC-STAMP gene expression; whereas p62 wild-type blunted the induction, hinting that p62 might negatively regulate osteoclastogenesis via the mechanism of cell fusion. However, we still could not exclude whether the enhancement of DC-STAMP in p62ΔUBA is a reflection of enhanced RANKL-induced cell differentiation, the consequence of p62 UBA domain deletion, or both. Future studies on the promoter regulation of DC-STAMP and molecules involved in DC-STAMP signaling would provide further insight of p62 on DC-STAMP-mediated cell fusion and multinucleation.
Consistent with the enhancement of osteoclastogenesis and multinucleation in UBA domain deletion, we showed that UBA-domain-deficient osteoclasts resorb bone more aggressively than control osteoclasts. On the other hand, we observed that overexpression of p62 wild-type blunted osteoclastic bone resorption as compared to control osteoclasts. Together, these findings suggest that the UBA domain encodes regulatory elements that are vital for the regulation of RANKL-mediated signaling cascade underlying osteoclast differentiation and activity.
Accumulating evidence suggests that p62 may be a crucial regulator in the proteasomal degradation pathway.16,37,38 In addition, a recent study using the specific proteasome inhibitors MG132 and MG262 revealed that inhibition of proteasomal-ubiquitin degradation pathway suppresses RANKL-induced osteoclastogenesis.39 Although the precise mechanism underlying this phenomenon, as well as p62 involvement in proteasome-ubiquitin system in RANKL-mediated osteoclast differentiation and function, requires further investigation, it is tempting to speculate that its interaction with TRAF6 contributes to this process. TRAF6 has been shown to be a key signaling adaptor molecule during RANKL-mediated osteoclastogenesis. Targeted gene disruption of TRAF6 in mice results in an osteopetrotic phenotype.40 More recently, immunoprecipitation studies have indicated that RANKL induces the formation of a tertiary complex involving p62, TRAF6, and aPKCs.16 However, the precise downstream signaling events mediating this complex remain unknown. In the present study, confocal microscopy analysis revealed that the p62 UBA domain is required for the recruitment of p62 to proteasomal degradation pathway, as well as RANKL-induced association of TRAF6 to p62 dot-like structures, hinting that p62 may modulate TRAF6 downstream signaling cascades via the proteasomal degradation pathway. In fact, this scenario is consistent with previous studies demonstrating that on RANKL stimulation, TRAF6 undergoes ubiquitin-mediated modification and degradation and mediates NF-κB activation.16,28,41 Thus, it is possible that p62 is involved in RANKL-mediated signaling activation via modulation of TRAF6 downstream signaling pathways. In light of the fact that IKK has recently been shown to be recruited to the signalsome complex that is composed of TRAF6, p62, and PKC(iota)/zeta on thymosin alpha1 stimulation,42 further study will be conducted to examine whether p62 UBA domain might modulate the phosphorylation of IKK and other kinases, including Raf proteins, in the regulation of p62-mediated osteoclastogenesis.
In summary, this study demonstrates that deletion of the UBA domain of p62 results in an enhancement of osteoclastogenesis, activities, and RANKL-induced activation of NF-κB, NFAT, and ERK signaling pathways. These findings are in agreement with the increased osteoclast formation and activities observed in PDB. We proposed that individuals harboring p62 UBA domain deletion mutants might experience a higher risk of developing PDB as a consequence of misregulation of TRAF6 and enhanced NF-κB and NFAT transcriptional activities.
Acknowledgments
We thank Associate Professor Tom Ratajczak and Mr. Jay Steer for the critical reading of this manuscript.
Footnotes
Address reprint requests to Jiake Xu, Molecular Orthopaedic Laboratory, School of Surgery and Pathology, University of Western Australia, QEII Medical Centre, 2nd Floor M Block, Nedlands, WA, Australia 6009. E-mail jiake.xu@uwa.edu.au.
Supported by the National Health and Medical Research Council of Australia and the Sir Charles Gairdner Hospital Research Fund.
Flow cytometry analysis and confocal microscope experiments were done in the Biomedical Confocal Microscopy Research Centre at the Pharmacology Unit, School of Medicine and Pharmacology, the University of Western Australia.
References
- Roodman GD. Mechanisms of abnormal bone turnover in Paget’s disease. Bone. 1999;24:39S–40S. doi: 10.1016/s8756-3282(99)00045-9. [DOI] [PubMed] [Google Scholar]
- Reddy SV. Etiology of Paget’s disease and osteoclast abnormalities. J Cell Biochem. 2004;93:688–696. doi: 10.1002/jcb.20256. [DOI] [PubMed] [Google Scholar]
- Layfield R, Hocking LJ. SQSTM1 and Paget’s disease of bone. Calcif Tissue Int. 2004;75:347–357. doi: 10.1007/s00223-004-0041-0. [DOI] [PubMed] [Google Scholar]
- Morgan SL, Ward L, Ward BK, Walsh J, Xu J: A novel mutation (K378X) in the sequestosome1 gene associated with a sever phenotype in Paget’s disease of bone. International Symposium on Paget’s Disease, 2005 July 8–9, St. Catherine’s College, Oxford, UK [Google Scholar]
- Hocking LJ, Lucas GJ, Daroszewska A, Cundy T, Nicholson GC, Donath J, Walsh JP, Finlayson C, Cavey JR, Ciani B, Sheppard PW, Searle MS, Layfield R, Ralston SH. Novel UBA domain mutations of SQSTM1 in Paget’s disease of bone: genotype phenotype correlation, functional analysis, and structural consequences. J Bone Miner Res. 2004;19:1122–1127. doi: 10.1359/JBMR.0403015. [DOI] [PubMed] [Google Scholar]
- Park I, Chung J, Walsh CT, Yun Y, Strominger JL, Shin J. Phosphotyrosine-independent binding of a 62-kDa protein to the src homology 2 (SH2) domain of p56lck and its regulation by phosphorylation of Ser-59 in the lck unique N-terminal region. Proc Natl Acad Sci USA. 1995;92:12338–12342. doi: 10.1073/pnas.92.26.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung I, Strominger JL, Shin J. Molecular cloning of a phosphotyrosine-independent ligand of the p56lck SH2 domain. Proc Natl Acad Sci USA. 1996;93:5991–5995. doi: 10.1073/pnas.93.12.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha T, Wooten MW. Structure and functional properties of the ubiquitin binding protein p62. FEBS Lett. 2002;512:19–24. doi: 10.1016/s0014-5793(02)02286-x. [DOI] [PubMed] [Google Scholar]
- Sanz L, Diaz-Meco MT, Nakano H, Moscat J. The atypical PKC-interacting protein p62 channels NF-kappaB activation by the IL-1-TRAF6 pathway. EMBO J. 2000;19:1576–1586. doi: 10.1093/emboj/19.7.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L, Sanchez P, Lallena MJ, Diaz-Meco MT, Moscat J. The interaction of p62 with RIP links the atypical PKCs to NF-kappaB activation. EMBO J. 1999;18:3044–3053. doi: 10.1093/emboj/18.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten MW, Seibenhener ML, Mamidipudi V, Diaz-Meco MT, Barker PA, Moscat J. The atypical protein kinase C-interacting protein p62 is a scaffold for NF-kappaB activation by nerve growth factor. J Biol Chem. 2001;276:7709–7712. doi: 10.1074/jbc.C000869200. [DOI] [PubMed] [Google Scholar]
- Duran A, Serrano M, Leitges M, Flores JM, Picard S, Brown JP, Moscat J, Diaz-Meco MT. The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell. 2004;6:303–309. doi: 10.1016/s1534-5807(03)00403-9. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Joung I, Strominger JL, Shin J. p62, a phosphotyrosine-independent ligand of the SH2 domain of p56lck, belongs to a new class of ubiquitin-binding proteins. J Biol Chem. 1996;271:20235–20237. doi: 10.1074/jbc.271.34.20235. [DOI] [PubMed] [Google Scholar]
- Ciani B, Layfield R, Cavey JR, Sheppard PW, Searle MS. Structure of the ubiquitin-associated domain of p62 (SQSTM1) and implications for mutations that cause Paget’s disease of bone. J Biol Chem. 2003;278:37409–37412. doi: 10.1074/jbc.M307416200. [DOI] [PubMed] [Google Scholar]
- Shin J. P62 and the sequestosome, a novel mechanism for protein metabolism. Arch Pharm Res. 1998;21:629–633. doi: 10.1007/BF02976748. [DOI] [PubMed] [Google Scholar]
- Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol. 2004;24:8055–8068. doi: 10.1128/MCB.24.18.8055-8068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Tan JW, Huang L, Gao XH, Laird R, Liu D, Wysocki S, Zheng MH. Cloning, sequencing, and functional characterization of the rat homologue of receptor activator of NF-kappaB ligand. J Bone Miner Res. 2000;15:2178–2186. doi: 10.1359/jbmr.2000.15.11.2178. [DOI] [PubMed] [Google Scholar]
- Pavlos NJ, Xu J, Riedel D, Yeoh JS, Teitelbaum SL, Papadimitriou JM, Jahn R, Ross FP, Zheng MH. Rab3D regulates a novel vesicular trafficking pathway that is required for osteoclastic bone resorption. Mol Cell Biol. 2005;25:5253–5269. doi: 10.1128/MCB.25.12.5253-5269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang C, Han R, Pavlos N, Phan T, Steer JH, Bakker AJ, Joyce DA, Zheng MH. Evidence of reciprocal regulation between the high extracellular calcium and RANKL signal transduction pathways in RAW cell derived osteoclasts. J Cell Physiol. 2005;202:554–562. doi: 10.1002/jcp.20159. [DOI] [PubMed] [Google Scholar]
- Xu J, Feng HT, Wang C, Yip KH, Pavlos N, Papadimitriou JM, Wood D, Zheng MH. Effects of bafilomycin A1: an inhibitor of vacuolar H (+)-ATPases on endocytosis and apoptosis in RAW cells and RAW cell-derived osteoclasts. J Cell Biochem. 2003;88:1256–1264. doi: 10.1002/jcb.10477. [DOI] [PubMed] [Google Scholar]
- Wang C, Steer JH, Joyce DA, Yip KH, Zheng MH, Xu J. 12-O-tetradecanoylphorbol-13-acetate (TPA) inhibits osteoclastogenesis by suppressing RANKL-induced NF-kappaB activation. J Bone Miner Res. 2003;18:2159–2168. doi: 10.1359/jbmr.2003.18.12.2159. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL) J Biol Chem. 2000;275:31155–31161. doi: 10.1074/jbc.M001229200. [DOI] [PubMed] [Google Scholar]
- Yip KH, Zheng MH, Feng HT, Steer JH, Joyce DA, Xu J. Sesquiterpene lactone parthenolide blocks lipopolysaccharide-induced osteolysis through the suppression of NF-kappaB activity. J Bone Miner Res. 2004;19:1905–1916. doi: 10.1359/JBMR.040919. [DOI] [PubMed] [Google Scholar]
- Yip KH, Zheng MH, Steer JH, Giardina TM, Han R, Lo SZ, Bakker AJ, Cassady AI, Joyce DA, Xu J. Thapsigargin modulates osteoclastogenesis through the regulation of RANKL-induced signaling pathways and reactive oxygen species production. J Bone Miner Res. 2005;20:1462–1471. doi: 10.1359/JBMR.050324. [DOI] [PubMed] [Google Scholar]
- Hirotani H, Tuohy NA, Woo JT, Stern PH, Clipstone NA. The calcineurin/NFAT signaling pathway regulates osteoclastogenesis in RAW2647 cells. J Biol Chem. 2004;279:13984–13992. doi: 10.1074/jbc.M213067200. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- Hotokezaka H, Sakai E, Kanaoka K, Saito K, Matsuo K, Kitaura H, Yoshida N, Nakayama K. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW2647 cells into osteoclast-like cells. J Biol Chem. 2002;277:47366–47372. doi: 10.1074/jbc.M208284200. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Am Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem. 2001;276:563–568. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- Morales-Piga AA, Rey-Rey JS, Corres-Gonzalez J, Garcia-Sagredo JM, Lopez-Abente G. Frequency and characteristics of familial aggregation of Paget’s disease of bone. J Bone Miner Res. 1995;10:663–670. doi: 10.1002/jbmr.5650100421. [DOI] [PubMed] [Google Scholar]
- Cavey JR, Ralston SH, Hocking LJ, Sheppard PW, Ciani B, Searle MS, Layfield R. Loss of ubiquitin-binding associated with Paget’s disease of bone p62 (SQSTM1) mutations. J Bone Miner Res. 2005;20:619–624. doi: 10.1359/JBMR.041205. [DOI] [PubMed] [Google Scholar]
- Laurin N, Brown JP, Morissette J, Raymond V. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet. 2002;70:1582–1588. doi: 10.1086/340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good DA, Busfield F, Fletcher BH, Lovelock PK, Duffy DL, Kesting JB, Andersen J, Shaw JT. Identification of SQSTM1 mutations in familial Paget’s disease in Australian pedigrees. Bone. 2004;35:277–282. doi: 10.1016/j.bone.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Hocking LJ, Lucas GJ, Daroszewska A, Mangion J, Olavesen M, Cundy T, Nicholson GC, Ward L, Bennett ST, Wuyts W, Van Hul W, Ralston SH. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735–2739. doi: 10.1093/hmg/11.22.2735. [DOI] [PubMed] [Google Scholar]
- Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, Nagata K, Iijima T, Horiuchi M, Matsusaki H, Hieshima K, Yoshie O, Nomiyama H. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med. 2004;200:941–946. doi: 10.1084/jem.20040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu JR, Geetha T, Wooten MW. Sequestosome 1/p62 shuttles polyubiquitinated tau for proteasomal degradation. J Neurochem. 2005;94:192–203. doi: 10.1111/j.1471-4159.2005.03181.x. [DOI] [PubMed] [Google Scholar]
- Nakaso K, Yoshimoto Y, Nakano T, Takeshima T, Fukuhara Y, Yasui K, Araga S, Yanagawa T, Ishii T, Nakashima K. Transcriptional activation of p62/A170/ZIP during the formation of the aggregates: possible mechanisms and the role in Lewy body formation in Parkinson’s disease. Brain Res. 2004;1012:42–51. doi: 10.1016/j.brainres.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Zavrski I, Krebbel H, Wildemann B, Heider U, Kaiser M, Possinger K, Sezer O. Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function. Biochem Biophys Res Commun. 2005;333:200–205. doi: 10.1016/j.bbrc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumaz N, Marais R. Raf phosphorylation: one step forward and two steps back. Mol Cell. 2005;17:164–166. doi: 10.1016/j.molcel.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Zhang P, Chan J, Dragoi AM, Gong X, Ivanov S, Li ZW, Chuang T, Tuthill C, Wan Y, Karin M, Chu WM. Activation of IKK by thymosin alpha1 requires the TRAF6 signalling pathway. EMBO Rep. 2005;6:531–537. doi: 10.1038/sj.embor.7400433. [DOI] [PMC free article] [PubMed] [Google Scholar]