Abstract
Alterations of human skin connective tissue structure and function are prominent features of chronological aging and solar UV irradiation-induced premature aging (photoaging). These skin connective tissue abnormalities result, in part, from reduced synthesis and elevated degradation of type I collagen, the major structural protein in skin. Here, we report that cysteine-rich 61 (CYR61/CCN1), a novel mediator of collagen homeostasis, is predominantly expressed in human skin connective tissue and is significantly elevated in fibroblasts in chronologically aged (80+ years) and photoaged human skin in vivo. In cultured human skin fibroblasts, elevated CYR61 expression substantially reduces type I procollagen and concurrently increases matrix metalloproteinase-1 (MMP-1), which initiates fibrillar collagen degradation. Elevated CYR61 caused down-regulation of transforming growth factor-β type II receptor mRNA and protein levels, thereby impairing the transforming growth factor-β pathway, which reduced type I procollagen and raised MMP-1 expression. Furthermore, elevated CYR61 induced transcription factor activator protein-1 (AP-1), which functions to stimulate MMP-1 expression. Thus, elevated expression of CYR61 in human skin fibroblasts acts through multiple pathways to cause alterations of collagen homeostasis similar to those pathways observed in aged human skin in vivo. These data identify CYR61 as a pivotal regulator of collagen production and degradation in aged and photoaged human skin.
Cysteine-rich 61 (CYR61/CCN1) is a secreted, extracellular matrix-associated signaling molecule that belongs to the CCN gene family.1 The CCN family of genes currently includes six distinct members; CYR61/CCN1, CTGF/CCN2, NOV/CCN3, and the recently identified Wnt1-induced secreted protein WISP-1/CCN4, WISP-2/CCN5, and WISP-3/CCN6.2,3 The CCN family of genes plays fundamental biological role in growth, differentiation, angiogenesis, migration, and extracellular matrix regulation.4–6 Aberrant expression of CCN genes is associated with several pathological states including fibrotic diseases and tumorigenesis.3,7–9
CYR61 was originally identified as a growth factor and serum-inducible immediate early gene in mouse 3T3 fibroblasts.10,11 Human CYR61 was only recently identified from a human embryo library12 and found to be highly conserved to the mouse homolog. CYR61 exhibits a distinct expression profile during embryonic development with highest expression in the developing circulatory and skeletal systems. Disruption of CYR61 is embryonic lethal primarily because of failure of placenta vascular development.13 These data suggest that CYR61 plays a crucial role in extracellular matrix remodeling, because many aspects of the processes of vascularization, chondrogenesis, and skeletogenesis are regulated by extracellular matrix.14
In cell culture models, addition of recombinant CYR61 protein has been shown to regulate cell adhesion, migration, cell-matrix interactions, and synthesis of extracellular matrix in endothelial cells and skin fibroblasts.4,5,15 Although mechanisms of CYR61-induced signal transduction remain largely unknown, it has been shown that some of CYR61 biological functions are mediated through interactions with multiple integrins.1,16–20
The unique accessibility of human skin makes it an excellent organ to investigate the molecular basis of human aging. The structure and function of human skin is largely dependent on its underlying connective tissue (dermis). The dermal extracellular matrix is largely composed of type I collagen fibrils, which are synthesized and organized by dermal fibroblasts. Sun-protected, chronologically aged, and sun-exposed photoaged human skin share common molecular features including reduced expression of type I procollagen, the precursor to type I collagen, and elevated expression of matrix metalloproteinase 1 (MMP-1), which initiates degradation of fibrillar collagen.21–27 These time- and sun exposure-dependent alterations of collagen fibrillar content and organization impair the structural integrity of skin connective tissue, thereby contributing to the altered appearance of aged and photoaged human skin. Molecular mechanisms responsible for this altered collagen homeostasis remain to be elucidated.
The role of CYR61 in human connective tissue aging has not previously been investigated. Here, we report that CYR61 is significantly up-regulated in dermal fibroblasts in chronologically aged and photoaged human skin. Up-regulation of CYR61 in human skin fibroblasts causes alterations of type I collagen homeostasis that mimic those observed in chronologically aged and photoaged human skin. These data identify CYR61 as a novel mediator of extracellular matrix aging in human skin.
Materials and Methods
Procurement of Human Skin Samples
Young and aged subjects were grouped according to age: 20 to 30 years for young group (eight males and 11 females) and 80+ years for aged group (19 females). Samples of young and aged skin were obtained from sun-protected buttock skin. Samples of severely photodamaged skin and subject matched sun-protected skin were obtained from the extensor forearm and sun-protected buttocks, respectively. Photoaged subjects ranged in age from 42 to 63 years (six males and nine females). The presence of severe photodamage was determined based on clinical criteria, as described previously.28 Skin samples were 4 mm in diameter from full thickness skin. All procedures involving human subjects were approved by the University of Michigan Institutional Review Board, and all subjects provided written informed consent.
Cell Culture
Human skin dermal fibroblasts were obtained from healthy adult human skin and cultured as previously described.29 Cells used for study were between passages 3 and 8. In some studies, fibroblasts were treated with the transforming growth factor (TGF)-β type I receptor kinase inhibitor SB431542 (10 μmol/L; Tocris Biosciences, Ellisville, MO) for 24 hours.
RNA Isolation and Quantitative Real-Time RT-PCR
Total RNA was isolated from human skin punch biopsy and cultured human skin fibroblasts using a commercial kit (RNeasy mini kit; Qiagen, Chatsworth, CA) according to the manufacturer’s protocol. To determine mRNA expression levels in the dermis, dermis was separated from epidermis by dissection of full thickness human skin.30 mRNA levels were quantified by real-time reverse transcriptase-polymerase chain reaction (RT-PCR), as previously described.31 PCR primers and probes were from Applied Biosystems custom oligonucleotide synthesis service. Primers and FAM-labeled probes for real-time PCR were as follows: CYR61 sense primer, 5′-TCAAAGACCTGTGGAACTGGTATC-3′, antisense primer, 5′-CACAAATCCGGGTTTCTTTCA-3′, and probe, 5′-CAATGACAACCCTGAGTGCCGCCT-3′; MMP-1 sense primer, 5′-GGGAGATCATCGGGACAACTC-3′, antisense primer, 5′-AATACCTGGGCCTGGTTGAAA-3′, and probe, 5′-TGAGCAAGATTTCCTCCAGGTCCATCAA-3′. Type I procollagen, TβRII, and 36B4 primers and probes were described previously.32 Multiplex PCR reactions contained primers and probes for target gene and 36B4, a ribosomal protein used as an internal normalization control for quantitation. Target gene and 36B4 mRNA levels were quantified based on standard curves, and target gene levels were normalized to the housekeeping gene 36B4.
Laser Capture Microdissection (LCM)-Coupled Quantitative Real-Time RT-PCR
LCM was performed as previously described.30,31 Briefly, human skin punch biopsies were embedded in optimal cutting temperature, sectioned, and stained with hematoxylin. Epidermis and dermis were captured using LCM (Leica ASLMD System, Leica Microsystems, Wetzlar, Germany). Total RNA was extracted, and quantitative real-time RT-PCR was performed as described above.
Western Analysis
Primary antibodies used were CYR61 (sc-8560; Santa Cruz Biotechnology, Santa Cruz, CA), type I procollagen (Research Diagnostics, Inc., Flanders, NJ), and MMP-1 (MAB1346; Chemicon International, Inc., Temecula, CA). Western analysis was performed as previously described.33
Immunohistology
Immunohistology was performed as previously described.25,34 Primary antibodies used were CYR61 (generously provided by Dr Lester Lau, University of Illinois, Chicago, IL), HSP47 (Stressgen Biotechnology, Victoria, BC, Canada), and N-terminal type I procollagen (SP1-D8; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA).
Transfection
CYR61 cDNA was amplified from human skin fibroblasts total RNA by RT-PCR using primers 5′-CGCGCCACAATGAGCTCCCGC-3′ and 5′-GTCCCTAAATTTGTGAATGT-3′. The PCR product was cloned into pCDNA3.1 expression vector (Invitrogen, Carlsbad, CA) and verified by restriction digestion and sequencing. Activator protein-1 (AP-1) reporter construct (pAP1-TA-Luc) was purchased from BD Biosciences Clontech (Palo Alto, CA). Human skin fibroblasts were transiently transfected by electroporation using human dermal fibroblast nucleofector kit (Amaxa Biosystems, Gaithersburg, MD) according to the manufacturer’s protocol. After 48 hours of transfection, total RNA and cellular proteins were extracted, and mRNA and protein levels were determined by real-time RT-PCR and Western analysis, as described above. Luciferase activity was measured by luciferase assay using an enhanced luciferase assay kit (PharMingen International, San Diego, CA) according to the manufacturer’s protocol. Aliquots containing identical β-galactosidase activity were used for each luciferase assay.
Statistical Analysis
Statistical significance between groups was determined with the Student’s t-test. Comparisons between photoaged and sun-protected human skin and among treatment groups were made with the paired t-test. Comparisons between young and aged groups were made with the unpaired t-test. All P values are two tailed and considered significant when <0.05.
Results
CYR61 Is Predominantly Expressed in Human Skin Connective Tissue
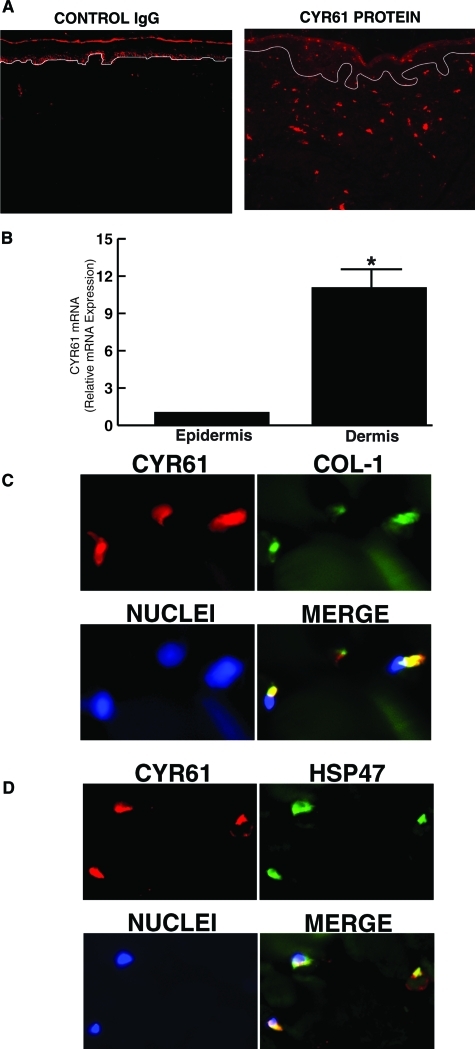
We initially determined localization of CYR61 protein and mRNA expression in human skin in vivo, by immunohistology and LCM coupled with real-time RT-PCR, respectively. Immunohistology of normal human skin revealed that CYR61 protein was predominantly expressed in the dermis (connective tissue), which lies beneath and supports the epidermis (Figure 1A). We quantified CYR61 expression levels in epidermis and dermis. Epidermis and dermis were separately obtained by LCM, and CYR61 mRNA levels were determined by real-time RT-PCR. CYR61 mRNA levels were 11-fold higher in the dermis compared with the epidermis (Figure 1B). Double immunofluorescence staining revealed colocalization of CYR61 and type I procollagen in dermal cells (Figure 1C). CYR61 also colocalized with HSP47, a fibroblast marker35(Figure 1D), indicating that CYR61 is predominantly expressed in collagen-producing fibroblasts in human skin dermis.
Figure 1-6922.
CYR61 is predominantly expressed in dermal cells and colocalizes with fibroblast proteins type I procollagen and HSP47 in human skin in vivo. Skin samples were obtained from healthy adult subjects (32 to 55 years). A: Immunofluorescence staining of CYR61 protein reveals predominant dermal localization. Isotype control antibody immunofluorescence staining is shown in the left panel. Data are representative of five subjects. B: CYR61 mRNA is predominantly expressed in dermis of human skin. Epidermis and dermis were captured by LCM, and CYR61 and 36B4 (internal normalization control) mRNA levels were quantified by real-time RT-PCR. Data are means ± SEM, n = 5, *P < 0.05. CYR61 protein colocalizes with type I procollagen (C) and HSP47 (D) in dermal fibroblasts in human skin in vivo. Colocalization of CYR61 and type I procollagen (COL-1) or HSP47 is indicated by orange-yellow in the merged immunofluorescence images. Data are representative of three subjects.
CYR61 Is Elevated in the Dermis of Chronologically Aged, Sun-Protected Skin in Vivo
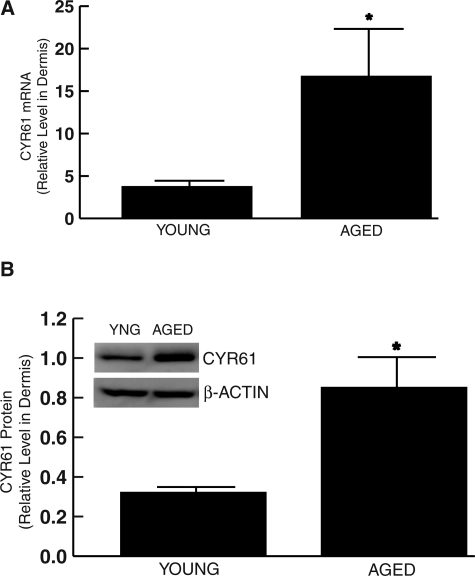
We next determined CYR61 mRNA levels in the dermis of young (20 to 30 years) and aged (80+ years) individuals by LCM coupled with real-time RT-PCR. As shown in Figure 2A, the level of CYR61 mRNA was 4.5-fold higher in the dermis of sun-protected chronologically aged skin (80+ years) compared with young (20 to 30 years) skin. Consistent with these data, Western analysis revealed that CYR61 protein was increased 2.7-fold in the dermis of chronologically aged, sun-protected skin (80+ years) compared with young (20 to 30 years) individuals (Figure 2B).
Figure 2-6922.
CYR61 is increased in the dermis of sun-protected chronologically aged human skin in vivo. A: Skin samples were obtained from chronologically aged (80+ years) and young (20 to 30 years) individuals. Dermis was captured by LCM, and CYR61 and 36B4 (internal normalization control) mRNA levels were quantified by real-time RT-PCR. Data are means ± SEM, n = 6, *P < 0.05. B: Dermis was separated from epidermis by dissection. Dermal CYR61 and β-actin (internal normalization control) protein levels were determined by Western analysis. Inset shows representative Western blot. Data are means ± SEM, n = 8, *P < 0.05.
CYR61 Is Increased in the Dermis of Photoaged Human Skin in Vivo
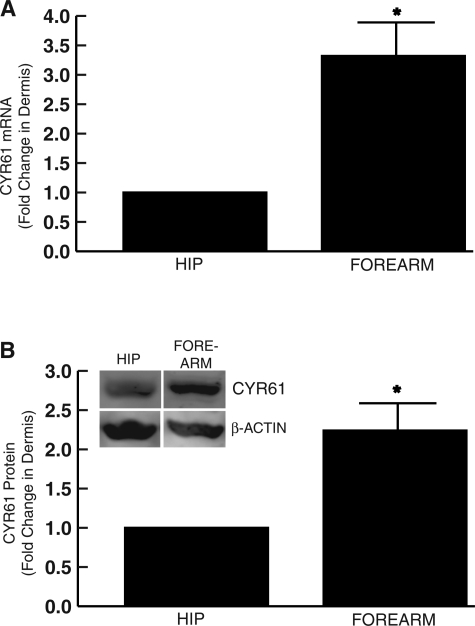
Total RNA was isolated from the dermis of severely photoaged forearm skin and subject-matched sun-protected hip skin. Expression levels of CYR61 mRNA were determined by LCM coupled with real-time RT-PCR. The level of CYR61 mRNA was increased 3.3-fold in the dermis of photoaged skin compared with dermis of subject-matched, sun-protected hip skin (Figure 3A). Consistent with these data, Western analysis revealed that CYR61 protein was increased 2.2-fold in the dermis of photoaged skin compared with subject-matched, sun-protected dermis (Figure 3B).
Figure 3-6922.
CYR61 is increased in the dermis of photoaged human skin in vivo. A: Skin samples were obtained from severely photoaged forearm and subject-matched, sun-protected hip skin. Dermis was captured by LCM, and CYR61 and 36B4 (internal normalization control) mRNA levels were quantified by real-time RT-PCR. Data are means ± SEM, n = 4, *P < 0.05. B: Dermis was separated from epidermis by dissection. Dermal CYR61 and β-actin protein levels were determined by Western analysis. Inset shows representative Western blot. Data are means ± SEM, n = 6, *P < 0.05.
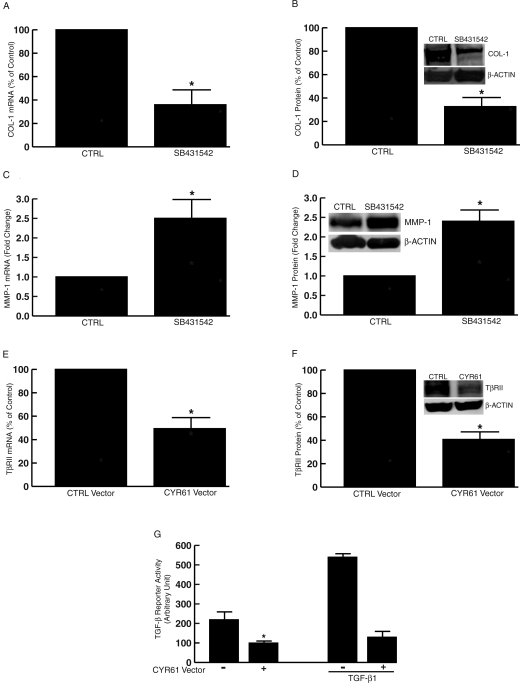
Elevated CYR61 Down-Regulates Type I Procollagen and Up-Regulates MMP-1 in Human Skin Fibroblasts
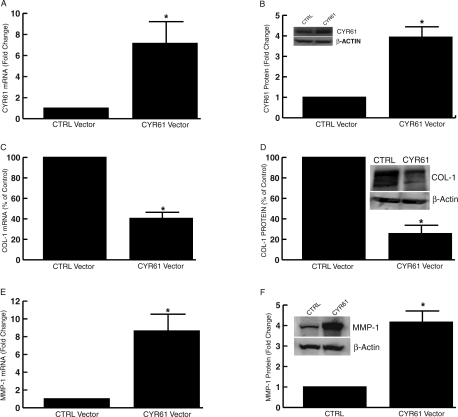
To examine the functionality of elevated CYR61 in aged and photoaged human skin, CYR61 was transiently overexpressed in human skin fibroblasts. For these studies, primary, early passage (three to five passages) fibroblasts, established from sun-protected skin of individuals less than 50 years old were used. As expected, expression of CYR61 increased CYR61 mRNA (Figure 4A) and protein (Figure 4B) compared with empty vector control. Importantly, overexpression of CYR61 substantially reduced the levels of type I procollagen mRNA (Figure 4C) and protein (Figure 4D) by 52 and 71%, respectively, compared with empty vector control. In contrast, overexpression of CYR61 significantly increased MMP-1 mRNA (Figure 4E) and protein (Figure 4F) by 8.6- and 4.2-fold, respectively, compared with empty vector control. These data indicate that elevated CYR61 participates in collagen homeostasis by reducing type I procollagen production and inducing MMP-1 in human skin dermal fibroblasts.
Figure 4-6922.
Overexpression of CYR61 down-regulates type I procollagen and up-regulates MMP-1 in human skin fibroblasts. Fibroblasts were transfected with empty control vector (CTRL) or CYR61 expression vector. Total RNA and whole cell proteins were prepared 48 hours after transfection. mRNA levels were quantified by real-time RT-PCR. 36B4 (housekeeping gene) mRNA was used as internal control for normalization of data. Protein levels were quantified by Western analysis. β-actin protein was used as internal control for normalization of data. Insets show representative Western blot. Overexpression of CYR61 mRNA (A) and protein (B) in human skin fibroblasts. Data are expressed as means ± SEM, n = 3, *P < 0.05. CYR61 overexpression down-regulates type I procollagen (COL-1) mRNA (C) and protein (D) in human skin fibroblasts. Data are means ± SEM, n = 3, *P < 0.05. CYR61 overexpression increases MMP-1 mRNA (E) and protein (F) in human skin fibroblasts. Data are means ± SEM, n = 3, *P < 0.05.
Elevated CYR61 Down-Regulates TGF-β Type II Receptor and Impairs TGF-β Pathway in Human Skin Fibroblasts
TGF-β pathway plays a central role in regulating collagen homeostasis. TGF-β both stimulates collagen synthesis and down-regulates expression of collagen-degrading MMPs, including MMP-1. This important regulation of collagen homeostasis by the TGF-β pathway, in human skin fibroblasts is shown in Figure 5, A–D. Blocking the TGF-β pathway by a selective inhibitor of TGF-β type I receptor kinase (SB431542)36,37 resulted in significant reduction of type I procollagen mRNA (Figure 5A) and protein (Figure 5B) and increased MMP-1 mRNA (Figure 5C) and protein (Figure 5D). This inhibition of TGF-β pathway alters collagen homeostasis in a manner similar to that observed with elevated CYR61 expression. Based on this similarity, we examined the effects of elevated CYR61 on expression levels of the major components that comprise the TGF-β pathway. Elevated CYR61 did not alter expression levels of TGF-β type I receptor, TGF-β1, -β2, -β3, or TGF-β signal transducers Smad2, Smad3, and Smad4 (data not shown). However, elevated CYR61 caused down-regulation of TGF-β type II receptor (TβRII) expression. As shown in Figure 5, E and F, elevated CYR61 reduced TβRII mRNA and protein levels by 51 and 59%, respectively. Furthermore, elevated CYR61 inhibited basal and TGF-β1-induced TGF-β reporter (p3TP-Luc) activity (Figure 5G), indicating that elevated CYR61 impairs TGF-β-dependent gene transcription.
Figure 5-6922.
Elevated CYR61 down-regulates TGF-β type II receptor and impairs TGF-β pathway in human skin fibroblasts. Human skin fibroblasts were treated with the TGF-β type I receptor kinase inhibitor SB431542 (10 μmol/L). Total RNA and whole-cell protein extracts were prepared 24 hours after treatment. Type I procollagen (COL-1), MMP-1, and 36B4 (internal normalization control) mRNA levels were quantified by real-time RT-PCR. Type I procollagen (COL-1), MMP-1, and β-actin proteins were quantified by Western analysis. Insets show representative Western blot. A–D: Inhibition of the TGF-β type I receptor kinase reduces type I procollagen mRNA (A) and protein (B) and increases MMP-1 mRNA (C) and protein (D) in human dermal fibroblasts. Data are means ± SEM, n = 3, *P < 0.05. E and F: Elevated CYR61 down-regulates TGF-β type II receptor (TβRII) mRNA (E) and protein (F). Fibroblasts were transfected with empty control vector (CTRL) or CYR61 expression vector. Total RNA and cellular protein were prepared 48 hours after transfection. TGF-β type II receptor and 36B4 (internal normalization control) mRNA levels were quantified by real-time RT-PCR. TGF-β type II receptor and β-actin (internal normalization control) protein levels were quantified by Western analysis. Inset shows representative Western blot. Data are means ± SEM, n = 3, *P < 0.05. G: Elevated CYR61 inhibits TGF-β responsiveness. TGF-β reporter (p3TP-Luc) was cotransfected with empty vector (−) or CYR61 (+) expression vector. Fibroblasts were treated with TGF-β1 (10 ng/ml) 32 hours after transfection for 16 hours. Cell lysates were prepared 48 hours after transfection. Reporter activity was determined by luciferase assay. Data are means ± SEM, n = 3, *P < 0.05.
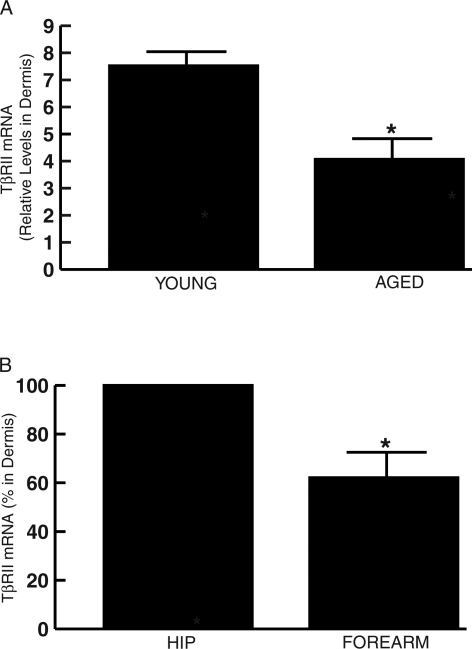
Because CYR61 is elevated in aged and photoaged human dermal fibroblasts and elevated CYR61 down-regulates TβRII, we determined TβRII expression levels in chronologically aged and photoaged human skin in vivo. As shown in Figure 6, A and B, dermal TβRII mRNA expression levels were reduced 50% in sun-protected chronologically aged skin (80+ years) compared with young skin (20 to 30 years), and 38% in photoaged forearm skin compared with subject-matched, sun-protected hip skin. These reductions of TβRII in human skin in vivo are consistent with the observed down-regulation of TβRII in response to CYR61 overexpression in cultured human skin fibroblasts (Figure 5, E and F).
Figure 6-6922.
TGF-β type II receptor mRNA expression is reduced in the dermis of sun-protected chronologically aged, and photodamaged human skin in vivo. A: Skin samples were obtained from chronologically aged (80+ years) and young (20 to 30 years) individuals. Dermis was captured by LCM, and TβRII and 36B4 (internal normalization control) mRNA levels were quantified by real-time RT-PCR. Data are means ± SEM, n = 5, *P < 0.05. B: Skin samples were obtained from severely photoaged forearm and subject-matched, sun-protected hip skin. Dermis was captured by LCM, and CYR61 and 36B4 (internal normalization control) mRNA levels were quantified by real-time RT-PCR. Data are means ± SEM, n = 5, *P < 0.05.
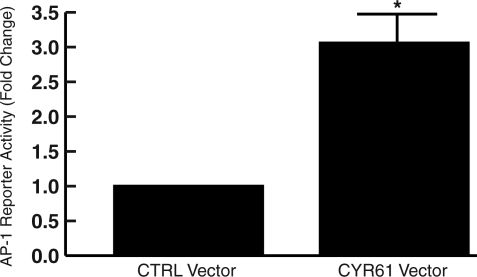
Elevated CYR61 Activates Transcription Factor AP-1, the Major Driving Force of MMP-1 Expression in Human Skin Fibroblasts
Transcription factor AP-1 is a crucial regulator of MMP-1 expression. Therefore, we examined the effect of elevated CYR61 on activation of transcription factor AP-1. Elevated CYR61 increased AP-1 reporter activity 3.1-fold (Figure 7) compared with empty vector control. These data indicate that elevated CYR61 induced MMP-1 by both reducing TGF-β responsiveness and increasing AP-1 activity.
Figure 7-6922.
Elevated CYR61 activates AP-1 in human skin fibroblasts. AP-1 reporter construct (pAP-1-TA-Luc) was cotransfected with empty vector (CTRL) or CYR61 expression vector. Cell lysates were prepared 48 hours after transfection. Reporter activity was determined by luciferase assay. Data are means ± SEM, n = 3, *P < 0.05.
Discussion
Type I collagen is the most abundant structural protein, and MMP-1 is the primary mammalian protease capable of initiating degradation of type I collagen in normal human skin dermis. In sun-protected young human skin, synthesis and degradation of collagen are balanced to maintain collagen content and structural integrity of the skin. However, in chronologically aged and photoaged human skin, collagen homeostasis becomes unbalanced through reduction of procollagen biosynthesis and increased MMP-1-initiated collagen degradation. Our findings support the concept that CYR61 is an important mediator of aberrant collagen homeostasis in chronologically aged and photoaged human skin.
Molecular mechanisms responsible for the elevated expression of CYR61 in chronologically aged and photoaged human skin remain to be determined. Recent studies have demonstrated that activation of the mitogen-activated protein (MAP) kinase pathway significantly increased CYR61 transcription through transactivation of transcription factor AP-1 and its binding to CYR61 promoter.38,39 Deletion and mutation analyses of the CYR61 promoter further demonstrated that transcription factor AP-1 was a strong activator of CYR61 transcription. We have previously reported that stress-activated MAP kinase pathways and c-Jun, a major component of AP-1, are increased in chronologically aged compared with young human skin in vivo.21,40 In addition, it is well documented that UV irradiation is a potent activator of AP-1 through activation of the MAP kinase pathway in human skin in vivo.24,41 These data suggest that increased CYR61 expression in chronologically aged and photoaged human skin may involve transcription factor AP-1. We have observed that acute UV irradiation induces CYR61 mRNA and protein in human skin in vivo and cultured human skin fibroblasts (T.Q., unpublished observation). Interestingly, our data indicate that elevated CYR61 activates transcription factor AP-1 (Figure 7) in human skin fibroblasts, suggesting that a positive feedback mechanism may contribute to sustained elevation of CYR61 in chronologically aged and photoaged human skin.
Our data indicate that CYR61 controls collagen homeostasis through impairment of the TGF-β pathway. TGF-β initiates its biological actions through interactions with the TGF-β receptor complex. TβRII is essential for TGF-β binding to the receptor complex.42 We demonstrate that elevated CYR61 targets the first step of the TGF-β pathway by down-regulating TβRII expression. A wealth of evidence indicates that TGF-β plays a central role in controlling production of type I procollagen and other extracellular matrix proteins in human skin.42–45 In addition, TGF-β up-regulates plasminogen activator inhibitor-1 and tissue inhibitor of metalloproteinase, two major inhibitors of extracellular matrix-degrading enzymes.46–49 TGF-β can also down-regulate expression of MMP-1.50–53 Therefore, impairment of the TGF-β pathway is likely to be a key mechanism by which elevated CYR61 alters collagen homeostasis in chronologically aged and photoaged human skin. Additionally, we have shown that elevated CYR61 caused activation of transcription factor AP-1. AP-1 not only functions as a strong inducer of MMP-1 transcription54–56 but also negatively regulates type I procollagen expression.22,57 This inhibition of procollagen may be mediated by trans-repression of Smad3 by AP-1.57,58 Therefore, elevated CYR61 impairs type I procollagen production by down-regulating TβRII and inducing transcription factor AP-1. Similarly, elevated CYR61 induces MMP-1 expression by increasing transcription factor AP-1 and by reducing cellular responsiveness to TGF-β.
Accumulating evidence indicates that CYR61 is an important regulator of angiogenesis and endothelial cell functions.4,13,59 A critical role of CYR61 in the development of the vascular system has been established in CYR61 knockout mice, as disruption of CYR61 is embryonic lethal because of vascular defects in both the placenta and embryo.13 Our data reveal that CYR61 is predominantly expressed in dermal fibroblasts in human skin. Fibroblasts secrete CYR61 into the extracellular matrix, where it is bound by adjacent stroma. Thus, secreted CYR61 may influence a variety of different cells within the dermis, including endothelial cells. Through a combination of autocrine and paracrine mechanisms, CYR61 may serve to coordinately regulate extracellular matrix homeostasis and vascularization in skin connective tissue.
Acknowledgments
We thank Suzan Rehbine for the procurement of tissue specimens, Kenne Currie and Rui Wang for technical assistance, and Diane Fiolek for graphic and administrative assistance. We also thank Dr. Lester F. Lau (University of Illinois College of Medicine) for making the CYR61 antibody available to us for double immunofluorescence staining. Antibody to type I procollagen was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences (Iowa City).
Footnotes
Address reprint requests to Gary J. Fisher, Ph.D., Department of Dermatology, University of Michigan Medical School, 1150 W. Medical Center Dr., Medical Science I, Room 6447, Ann Arbor, Michigan 48109-0609. E-mail: dianemch@umich.edu.
Supported by the National Institutes of Health (grant AG19364 to G.J.F.) and by a Dermatology Foundation Research grant (to T.Q.).
References
- Lau L, Lam SC-T. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- Brigstock D. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- Brigstock D, Goldschmeding R, Katsube K-I, Lam S-T, Lau L, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, Yeger H. Proposal of a unified CCN nomenclature. Mol Pathol. 2003;56:127–128. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Mo F-E, Lau L. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem. 2001;276:47329–47337. doi: 10.1074/jbc.M107666200. [DOI] [PubMed] [Google Scholar]
- Kireeva M, Mo F-E, Yang G, Lau L. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996;16:1326–1334. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B, Brigstock D, Lau L. Report on the second international workshop in the CCN family of genes. Mol Pathol. 2003;56:80–85. doi: 10.1136/mp.56.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Planque N, Perbal B. A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell Intl. 2003;3:15. doi: 10.1186/1475-2867-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Abraham D. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81:355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- Brunner A, Chinn J, Neubauer M, Purchio A. Identification of a gene family regulated by transforming growth factor-beta. DNA Cell Biol. 1991;10:293–300. doi: 10.1089/dna.1991.10.293. [DOI] [PubMed] [Google Scholar]
- O’Brien T, Yang G, Sanders LC, Lau L. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990;10:3569–3577. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay P, Berge-Lefranc J, Mejean C, Taviaux S, Berta P. The human growth factor-inducible immediate early gene, CYR61, maps to chromosome 1p. Oncogene. 1997;14:1753–1757. doi: 10.1038/sj.onc.1200986. [DOI] [PubMed] [Google Scholar]
- Mo F-E, Muntean A, Chen C-C, Stolz D, Watkins S, Lau L. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack D, Cheresh D. ECM remodeling regulates angiogenesis: endothelial integrins look for new ligands. Sci STKE. 2002;119:PE7. doi: 10.1126/stke.2002.119.pe7. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Chen N, Lau L. The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem. 2001;276:10443–10452. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- Chen N, Chen C-C, Lau L. Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin α6β1 and cell surface heparan sulfate proteoglycans. J Biol Chem. 2000;275:24953–24961. doi: 10.1074/jbc.M003040200. [DOI] [PubMed] [Google Scholar]
- Grzeszkiewicz T, Kirschling D, Chen N, Lau L. CYR61 stimulates human skin fibroblast migration through integrin αvβ5 and enhances mitogenesis through integrin αvβ3, independent of its carboxyl-terminal domain. J Biol Chem. 2001;276:21943–21950. doi: 10.1074/jbc.M100978200. [DOI] [PubMed] [Google Scholar]
- Jedsadayanmata A, Chen C-C, Kireeva M, Lau L, Lam SC-T. Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin α IIb β3. Biol Chem. 1999;274:24321–24327. doi: 10.1074/jbc.274.34.24321. [DOI] [PubMed] [Google Scholar]
- Kireeva M, Lam S, Lau L. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J Biol Chem. 1998;273:3090–3096. doi: 10.1074/jbc.273.5.3090. [DOI] [PubMed] [Google Scholar]
- Leu S-J, Lam SC-T, Lau L. Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins αvβ3 and α6β1 in human umbilical vein endothelial cells. J Biol Chem. 2002;277:46248–46255. doi: 10.1074/jbc.M209288200. [DOI] [PubMed] [Google Scholar]
- Chung J, Kang S, Varani J, Lin J, Fisher G, Voorhees J. Decreased extracellular-signal-regulated kinase and increased stress-activated MAP kinase activities in aged human skin in vivo. J Invest Dermatol. 2000;114:177–182. doi: 10.1046/j.1523-1747.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- Fisher G, Datta S, Wang Z, Li X, Quan T, Chung J, Kang S, Voorhees J. c-Jun dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoid acid. J Clin Invest. 2000;106:661–668. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees J. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Fligiel S, Varani J, Datta S, Kang S, Fisher G, Voorhees J. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Griffiths CEM, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) N Engl J Med. 1993;329:530–535. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Esmann J, Griffiths CEM, Voorhees JJ. Cellular, immunologic and biochemical characterization of topical retinoic acid-treated human skin. J Invest Dermatol. 1991;96:699–707. doi: 10.1111/1523-1747.ep12470632. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees J, Fisher G. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002;118:402–408. doi: 10.1046/j.0022-202x.2001.01678.x. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees J, Fisher G. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-β type II receptor/Smad signaling. Am J Pathol. 2004;165:741–751. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, He T, Voorhees J, Fisher G. Ultraviolet irradiation induces Smad7 via induction of transcription factor AP-1 in human skin fibroblasts. J Biol Chem. 2005;280:8079–8085. doi: 10.1074/jbc.M409647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, He T, Voorhees J, Fisher G. Ultraviolet irradiation blocks cellular responses to transforming growth factor-β by down-regulating its type-II receptor and inducing Smad7. J Biol Chem. 2001;276:26349–26356. doi: 10.1074/jbc.M010835200. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees J, Fisher G. Ultraviolet irradiation alters transforming growth factor β/Smad pathway in human skin in vivo. J Invest Dermatol. 2002;119:499–506. doi: 10.1046/j.1523-1747.2002.01834.x. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Tajima S. HSP47 is a useful marker for skin fibroblasts in formalin-fixed, paraffin-embedded tissue specimens. J Cutan Pathol. 2004;31:241–246. doi: 10.1111/j.0303-6987.2003.00166.x. [DOI] [PubMed] [Google Scholar]
- Halder S, Beauchamp R, Datta P. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7:509–521. doi: 10.1593/neo.04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laping N, Grygielko G, Mathur A, Butter S, Bonmberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J, Gaster L, Callahan J, Olson B. Inhibition of transforming growth factor (TGF)-β1-induced extracellular matrix with a novel inhibitor of the TGF-β type I receptor kinase activity: sB-431542. Mol Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- Han J-S, Macarak E, Rosenbloom J, Chung K, Chaqour B. Regulation of Cyr61/CCN1 gene expression through RhoA GTPase and p38MAPK signaling pathways. Eur J Biochem. 2003;270:3408–3421. doi: 10.1046/j.1432-1033.2003.03723.x. [DOI] [PubMed] [Google Scholar]
- Kunz M, Moeller S, Koczan D, Lorenz P, Wenger R, Glocker M, Thiesen H-J, Gross G, Ibrahim S. Mechanisms of hypoxic gene regulation of angiogenesis factor Cyr61 in melanoma cells. J Biol Chem. 2003;278:45651–45660. doi: 10.1074/jbc.M301373200. [DOI] [PubMed] [Google Scholar]
- Shin M, Rhie G-E, Kim Y, Park C-H, Cho K, Kim K, Eun H, Chung J. H2O2 accumulation by catalase reduction changes MAP kinase signaling in aged human skin in vivo. J Invest Dermatol. 2005;125:221–229. doi: 10.1111/j.0022-202X.2005.23823.x. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce AP-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Invest Dermatol. 1998;3:61–68. [PubMed] [Google Scholar]
- Massague J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Haukipuro K, Meikko J, Risteli L, Kairaluoma M, Risteli J. Synthesis of type I collagen in healing wounds in humans. Ann Surg. 1991;213:75–80. doi: 10.1097/00000658-199101000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Transforming growth factor β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- Sporn M, Roberts A. Transforming growth factor-β: recent progress and new challenges. J Cell Biol. 1992;119:1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, Heath JK. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987;6:1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M, Saksela O, Andreasen PA, Keski-Oja J. Enhanced production and extracellular deposition of the endothelial-type plasminogen activator inhibitor in cultured human lung fibroblasts by transforming growth factor-β. J Cell Biol. 1986;103:2403–2410. doi: 10.1083/jcb.103.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M, Saksela O, Keski-Oja J. Transforming growth factor-β induction of type-I plasminogen activator inhibitor: pericellular deposition and sensitivity to exogenous urokinase. J Biol Chem. 1987;262:17467–17474. [PubMed] [Google Scholar]
- Lund LR, Riccio A, Andreasen PA, Nielsen LS, Kristensen P, Laiho M, Saksela O, Blasi F, Dano K. Transforming growth factor β is a strong and fast-acting positive regulator of the level of type-I plasminogen activator inhibitor mRNA in WI-38 human lung fibroblasts. EMBO J. 1987;6:1281–1286. doi: 10.1002/j.1460-2075.1987.tb02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M-C, Young D, Waters J, Rowan A, Chantry A, Edwards D, Clark I. The comparative role of activator protein 1 and Smad factors in the regulation of Timp-1 and MMP-1 gene expression by transforming growth factor-β1. J Biol Chem. 2003;278:10304–10313. doi: 10.1074/jbc.M212334200. [DOI] [PubMed] [Google Scholar]
- Massague J. The transforming growth factor β family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Mauviel A, Chung KY, Agarwal A, Tamai K, Uitto J. Cell-specific induction of distinct oncogenes of the Jun family is responsible for differential regulation of collagenase gene expression by transforming growth factor-beta in fibroblasts and keratinocytes. J Biol Chem. 1996;271:10917–10923. doi: 10.1074/jbc.271.18.10917. [DOI] [PubMed] [Google Scholar]
- Yuan W, Varga J. Transforming growth factor-β repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. J Biol Chem. 2001;276:38502–38510. doi: 10.1074/jbc.M107081200. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore W, Bodden M, Windsor L, Birkedal-Hansen B, DeCarlo A, Engler J. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Matrisian L. Matrix metalloproteinase gene expression. Ann NY Acad Sci. 1994;732:42–50. doi: 10.1111/j.1749-6632.1994.tb24723.x. [DOI] [PubMed] [Google Scholar]
- Mauviel A. Cytokine regulation of metalloproteinase gene expression. J Cell Biochem. 1993;53:288–295. doi: 10.1002/jcb.240530404. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Pessah M, Atfi A, Mauviel A. Tumor necrosis factor-α inhibits transforming growth factor-β/Smad signaling in human dermal fibroblasts via AP-1 activation. J Biol Chem. 2000;275:30226–30231. doi: 10.1074/jbc.M005310200. [DOI] [PubMed] [Google Scholar]
- Dennler S, Prunier C, Ferrand N, Gauthier J, Atfi A. c-Jun inhibits transforming growth factor beta-mediated transcription by repressing Smad3 transcriptional activity. J Biol Chem. 2000;275:28858–28865. doi: 10.1074/jbc.M910358199. [DOI] [PubMed] [Google Scholar]
- Brigstock D. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61) Angiogenesis. 2002;5:153–165. doi: 10.1023/a:1023823803510. [DOI] [PubMed] [Google Scholar]