Abstract
P-Selectin expressed on endothelial cells contributes to acute and chronic inflammation by promoting leukocyte tethering/rolling. Despite increasing evidence of P-selectin expression on human umbilical vein endothelial cells in vitro, the regulatory mechanisms of P-selectin expression on dermal endothelial cells in skin diseases are not fully understood. Here, we demonstrate increased expression of P-selectin in dermal vessels of regional skin in urticaria and atopic dermatitis. The present in vitro analyses with human dermal microvascular endothelial cells (HDMECs) revealed that histamine rapidly induced P-selectin expression. Interleukin (IL)-4 and IL-13 induced prolonged expression of surface P-selectin by HDMECs. A combination of tumor necrosis factor-α and IL-4 inhibited P-selectin expression. Pretreatment of HDMECs with tumor necrosis factor-α followed by incubation with IL-4 markedly increased P-selectin expression. Notably, incubation with substance P alone induced prolonged P-selectin expression. Activation of STAT6 appears to be a key factor in P-selectin expression induced by substance P and IL-4 because treatment with STAT6 decoy oligodeoxynucleotides significantly inhibited P-selectin expression. The present results indicate that novel, complex mechanisms are involved in endothelial P-selectin expression in the skin. STAT6 in dermal endothelial cells appears to be a potent target for controlling cellular infiltrate in allergic and/or neuroinflammatory skin diseases.
Selectins expressed by endothelial cells interact with leukocyte counterreceptors and mediate the initial adhesion of leukocytes and their rolling along endothelial surfaces. Several lines of evidences indicate that endothelial selectins play important roles in inflammation. Mice lacking E-, P-, and/or L-selectins exhibit reduced leukocyte rolling and reduced extravasation of neutrophils in thioglycolate-induced peritonitis.1 P- and E-selectins are also essential for the development of irritation dermatitis and allergic contact hypersensitivity.2 Previous studies indicate that eosinophils have marked avidity for P-selectin, but not E-selectin.3–5 In mice, P-selectin deficiency causes reduced eosinophil rolling and reduced tissue eosinophilia in rag-weed peritonitis.1
Patterns of selectin expression vary among different microvascular beds. In vitro, levels of E-selectin expression are greater for human dermal microvascular endothelial cells (HDMECs) than for human umbilical vein endothelial cells (HUVECs), attributable to the slower internalization and degradation of E-selectin protein in HDMECs.6 After antigen challenge in mice, skin microvasculature expresses E-selectin, whereas cremaster muscle microvasculature expresses P-selectin.7
P-selectin is stored in the Weibel-Palade bodies of endothelial cells and in the membranes of the α-granules of platelets.8,9 In HUVECs, on stimulation with histamine, P-selectin is rapidly mobilized to the plasma membrane. In addition, studies using HUVECs show that incubation with interleukin (IL)-4, IL-13, or oncostatin M induces a prolonged increase in P-selectin mRNA and protein independently from Weibel-Palade bodies.10,11 Despite the increasing amount of data obtained using HUVECs, the expression and regulatory mechanisms of P-selectin on dermal endothelial cells have yet to be characterized.
Substance P is a neuropeptide released by sensory C-fibers in the skin. It induces a wide range of inflammatory responses, including vasodilation and cell activation.12 Histamine released by mast cells induces C-fibers to release substance P, which in turn induces mast cells to secrete histamine.12,13 Recent studies of neuroinflammatory responses indicate that substance P is involved in regulation of cell adhesion molecule expression. Substance P induces HDMECs to express ICAM-1 and VCAM-1 via binding with the NK-1 receptor,14,15 suggesting that substance P is involved in recruitment of cells to inflamed skin. Thus, substance P appears to be an important mediator of pathomechanisms of itch/pain and allergic skin reactions such as atopic dermatitis.16
In the present study, we examined regulatory mechanisms of P-selectin expression in the skin by performing immunohistochemical analysis of skin samples from allergic human skin diseases. We found high levels of P-selectin expression on dermal vessels in lesional skin of urticaria and atopic dermatitis. A series of in vitro analyses revealed that both IL-4 and substance P effectively induce P-selectin expression on HDMECs. It appears that induction of P-selectin expression by substance P was mediated by activation of STAT6, which is a transcriptional factor in IL-4/IL-13 signaling pathways.
Materials and Methods
Immunohistochemistry
Skin biopsy specimens were obtained from patients with allergic human skin diseases. The specimens were embedded in Optimum Cutting Medium (OCT; Miles, Elkhart, NJ) and frozen in liquid nitrogen. Then, 6-μm sections were cut, air-dried, acetone-fixed, and incubated with a mouse monoclonal anti-human P-selectin antibody (B.D. Bioscience, San Jose, CA) or an isotype control mouse serum (Sigma Chemical Co., St. Louis, MO). Bound antibodies were visualized using Alexa Fluor 488-conjugated rabbit anti-mouse IgG (Molecular Probes, Eugene, OR). The immunostained samples were examined under a confocal laser-scanning microscope (LSM510; Carl Zeiss Co., Oberkochen, Germany).
Cell Preparation
HDMECs were isolated from human foreskin as described elsewhere.17 Briefly, fresh skin was cut into 3 × 3-mm sections and incubated in dispase (Life Technologies, Inc., Grand Island, NY) at room temperature for 30 minutes. The epidermis was peeled off, and cells of the underlying dermis were gently scraped into RPMI 1640 and filtered through 45-μm nylon mesh. The filtrate, containing single cells, was washed and plated onto a tissue culture flask (B.D. Labware, Frankin Lakes, NJ) precoated with fibronectin (10 μg/ml, Life Technologies, Inc.). HDMECs were grown in EGM2-MV growth medium (Clonetics, San Diego, CA). Cells used in experiments were from passages 2 to 5. The character of HDMECs was confirmed by the expression of CD31 and CD144. Human umbilical microvascular endothelial cells (HUVECs) were obtained from Clonetics.
Flow Cytometric Analysis
Cell surface expression of P-selectin was examined by flow cytometric analysis as described elsewhere.11 Briefly, cells were incubated with histamine (Sigma), IL-4 (Genzyme Techne, Boston, MA), IL-13 (Genzyme Techne), tumor necrosis factor (TNF)-α (Genzyme Techne), IL-1α (Genzyme Techne), interferon-γ (Genzyme Techne), or substance P (Sigma) for the indicated times. They were then treated with trypsin-ethylenediamine tetraacetic acid (EDTA) (Clonetics), washed once in EGM2-MV, and washed twice with 1% bovine serum albumin in phosphate-buffered saline (PBS). Cells were then incubated with anti-P-selectin antibody, followed by incubation with fluorescein isothiocyanate-conjugated rabbit anti-mouse IgG (Dako Cytomation, Copenhagen, Denmark). Next, the cells were fixed with 4% paraformaldehyde. Fluorescence was measured using 10,000 cells/sample and a FACSCalibur system (Becton-Dickinson, Mountain View, CA).
Laser-Scanning Microscopic Analysis for P-Selectin on Cultured HDMECs
Cells were grown to confluency on fibronectin-coated coverslips. They were fixed with 4% paraformaldehyde and stained with anti-human P-selectin antibody (B.D. Bioscience), followed by incubation with Alexa Fluor 488-conjugated rabbit anti-mouse IgG (Molecular Probes, Eugene, OR). The immunostained samples were examined under a confocal laser-scanning microscope (LSM510, Carl Zeiss Co.). In some experiments, image analysis was performed using Scion Image for Windows software (Scion, Frederick, MD) by selecting an area of the cells and measuring mean fluorescence.
Cell-Based Enzyme-Linked Immunosorbent Assay (ELISA)
Ninety-six-well flat-bottomed microtiter plates containing HDMECs were washed with 200 μl/well PBS, followed by blocking with 200 μl of 1% Blotto (Pierce Biotechnology, Rockford, IL) in PBS for 30 minutes. Primary antibody (anti-P-selectin or isotype control antibody) was added at 1 μg/ml, and the cells were incubated in 0.05% Blotto/PBS for 2 hours at room temperature. Plates were again washed in 0.05% Blotto/PBS and were then incubated for 1 hour with 50 μl of peroxidase-conjugated anti-mouse IgG (Dako Cytomation) at a dilution of 1/1000 in 0.05% Blotto/PBS. After washing, 100 μl of TMB substrate (Promega, Madison, WI) was added, and color was developed for 5 to 10 minutes. The reaction was stopped by adding 1 mol/L H2SO4. The absorbance was read at 450 nm with a Bio-Rad plate reader 2550 (Bio-Rad Laboratories, Hercules, CA).
Immunofluorescent Staining of STAT6 Translocation
HDMECs were grown on fibronectin-coated coverslips, treated with TNF-α and/or IL-4 for 30 minutes, and then fixed with 4% paraformaldehyde for 20 minutes. Fixed cells were washed in PBS, permeabilized with 1% saponin in PBS, and incubated with 1 μg/ml polyclonal rabbit anti-STAT6 antibody or isotype control antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at room temperature. Cells were then incubated with Alexa Fluor 488-conjugated rabbit anti-mouse IgG, followed by observation using a confocal laser-scanning microscope.
Electrophoretic Mobility Shift Assay (EMSA)
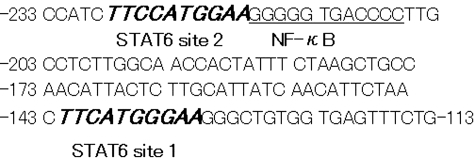
The following oligonucleotides were used as probes or competitors in EMSA: STAT6 site 1 probe, 5′-TAACTTCATGGGAAGGGC-3′; STAT6 site 2 probe, 5′-CCATCTTCCATGGAAGGGGG-3′; conventional STAT6 probe (Santa Cruz Biotechnology), 5′-GTATTTCCCAGAAAAGGAAG-3′; mutant probe, 5′-AGCTTGCAGTTCATGGGCTCATCG-3′18 (bolded letters indicate STAT6 recognition sequences). The site 1 and site 2 sequences were derived from the P-selectin promoter (Figure 1).18
Figure 1-6931.
Location of STAT6 and NF-κB consensus sequences in the P-selectin promoter. The two STAT6-binding sequences, site 1 and site 2, are located in the proximal P-selectin gene promoter (bold italic letters). NF-κB site (underlined) is located adjacent to STAT6 site 2.18,28,29
Nuclear extracts were prepared as described previously.19 Briefly, after 30 minutes of incubation with probes or competitors, cells were lysed in a high-salt extraction buffer (pH 7.5) containing 20 mmol/L HEPES, 400 mmol/L NaCl, 1 mmol/L MgCl2, 0.5 mmol/L EDTA, 0.1 mmol/L ethyleneglycol-bis(β-aminoethyl ether)-N,N, N′,N′-tetraacetic acid, 1% Nonidet P-40, 0.5 mmol/L dithiothreitol, and 1 mmol/L phenylmethyl sulfonyl fluoride. Then, the cells were incubated for 30 minutes on ice. The crude nuclear extract was precipitated and stored at −80°C. After preincubation with 2 μg of poly(dI-dC) as nonspecific competitor DNA, 15 μg of the crude nuclear extract was incubated with ∼10,000 cpm of annealed 32P-labeled probe in a binding buffer (pH 7.9) containing 10 mmol/L Tris-HCl, 0.1 mmol/L EDTA, 2 mmol/L dithiothreitol, 1 mmol/L phenylmethyl sulfonyl fluoride, 5% glycerol, and 100 mmol/L KCl, for 30 minutes at room temperature. The binding reaction mixtures were separated from free oligonucleotide probes by electrophoresis on a native 5% (w/v) polyacrylamide gel. In the supershift assay, the nuclear extract was preincubated with anti-STAT6 antibody (Santa Cruz Biotechnology) or nonimmune rabbit serum in the reaction mixture for 1 hour at 4°C, followed by addition of labeled probes.
Preparation of Decoy Oligodeoxynucleotides (Decoy ODNs) in HVJ (Hemagglutinating Virus of Japan) Envelope Vector (HVJ-E)
Nucleotide sequences of the decoy ODNs were as follows: Stat6 decoy ODN, 5′-GAT CAA GAC CTT TTC CCA AGA AAT CTA T-3′ and 5′-ATA GAT TTC TTG GGA AAA GGT CTT GTA C-3′20–22; nuclear factor (NF)-κB decoy ODN, 5′-CCT TGA A GGGAT TTC CCT CC-3′ and 5′-GGA GGG AAA TCC CTT CAA GG-3′23; scrambled decoy ODN, 5′-CGA AAA TTC GTT AAA TCA CTA GCT TAC C-3′ and 5′-GGT AAG CTA GTG ATT TAA CGA ATT TTC G-3′.20–22 Synthetic ODNs were dissolved in TE buffer (10 mmol/L Tris, 1 mmol/L EDTA, pH 8.0). Each pair of single-stranded ODN was annealed for 3 hours, during which time the temperature was reduced from 90 to 25°C. The HVJ-E-ODNs were prepared using HVJ Envelope Vector kit GenomONE (Ishibara Sangyo Kaisha, Osaka, Japan). This method involves the delivery of decoy ODNs in a viral envelope derived from the HVJ-E that has a high capacity to fuse with mammalian cells.24 HDMECs were mixed gently with the HJV-E-ODNs (0.25 μmol/L), and were then incubated for 8 hours at 37°C.20 The optimal concentration was determined in a preliminary experiment.
Results
P-Selectin Expression in Inflammatory Skin Diseases
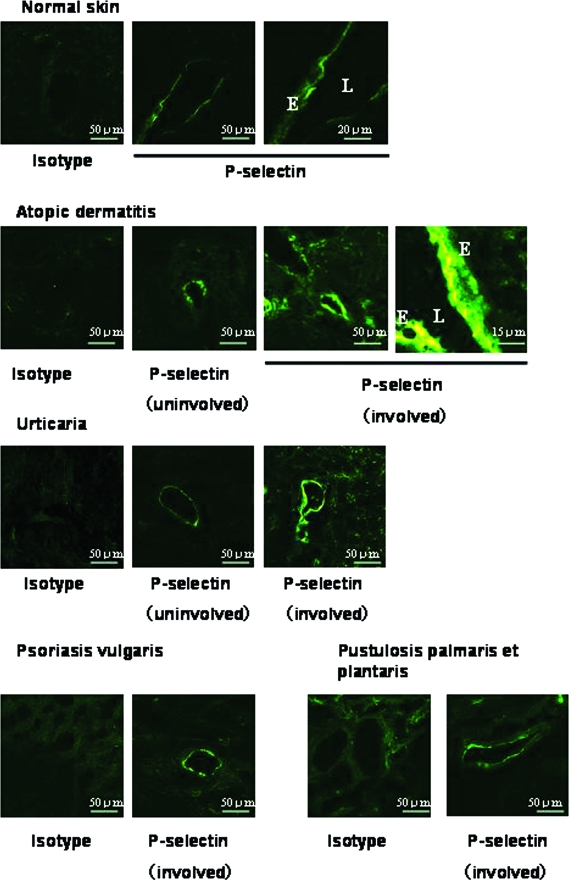
Endothelilal P-selectin plays a critical role in leukocyte recruitment, especially recruitment of eosinophils to the skin.3–5 We performed immunohistochemical analysis of skin biopsy specimens to examine regulation of endothelial P-selectin expression in vivo. Luminal expression of P-selectin was detected in dermal vessels of normal skin (Figure 2). High-level luminal and cytoplasmic expression of P-selectin was observed in the lesional skin of atopic dermatitis and urticaria. Dermal endothelial cells of psoriasis vulgaris and pustulosis palmaris et plantaris also expressed P-selectin, but at a lower level than cells of atopic dermatitis and urticaria.
Figure 2-6931.
P-selectin expression on dermal vessels in human skin diseases. P-selectin is constitutively expressed in normal skin. We observed high-level expression in the lesional skin of atopic dermatitis and urticaria. E, Endothelial cells; L, lumen.
Regulation of P-Selectin in HDMECs in Vitro
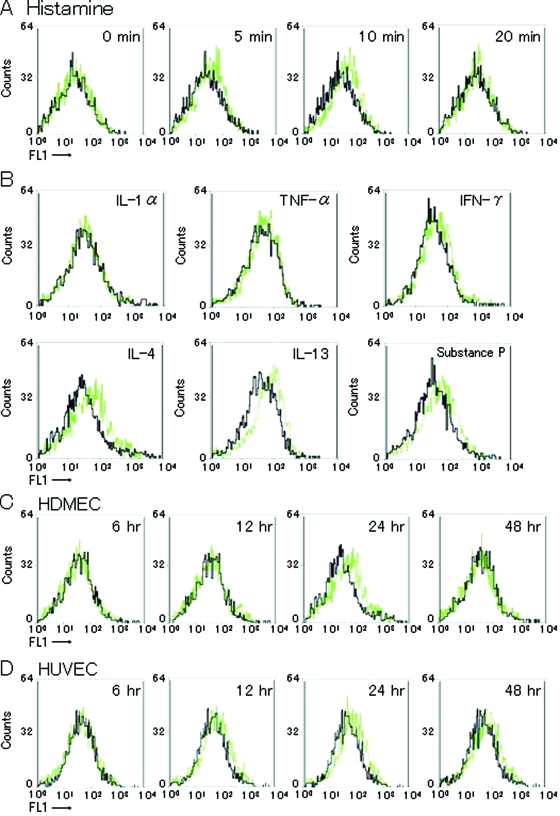
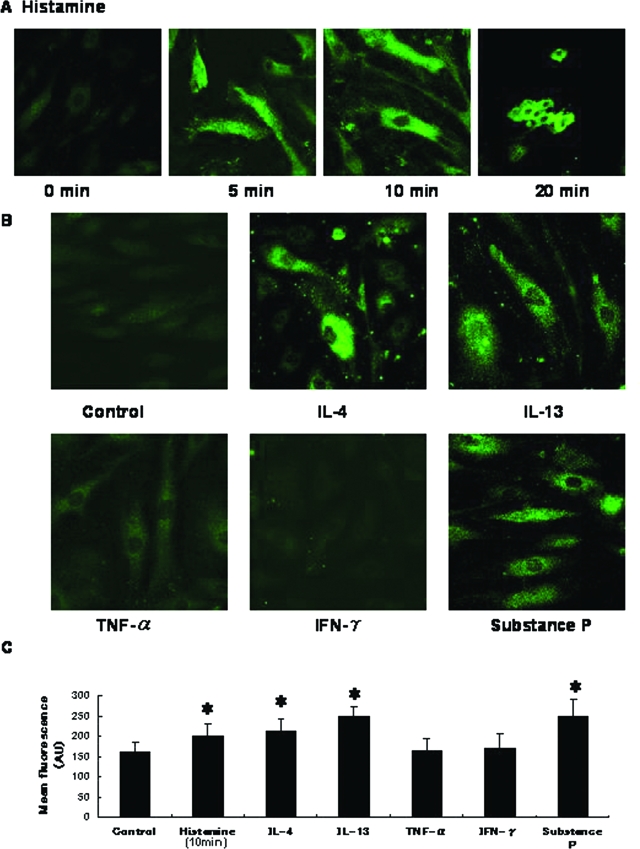
To examine regulatory mechanisms of P-selectin expression, we treated HDMECs with several substances in vitro. Histamine (10−5 mol/L) induced rapid, transient P-selectin expression, which peaked at 10 minutes (Figure 3A). Histamine also induced rapid cell-shape changes at 20 minutes (Figure 4A). IL-4 and IL-13 induced P-selectin expression, which peaked at 24 hours (Figure 3, B and C). Consistent with previous findings,11 IL-4 stimulated HUVECs to express P-selectin, which persisted for 48 hours after incubation (Figure 3D). Substance P also induced P-selectin expression in HDMECs. IL-1α, TNF-α, and interferon-γ did not induce expression of P-selectin. These effects of IL-4, IL-13, and substance P on expression of P-selectin were confirmed by laser-scanning microscopic analysis (Figure 4B).
Figure 3-6931.
Flow cytometric analysis of P-selectin on HDMECs. A: Basal expression of P-selectin was minimal or undetectable. When HDMECs were incubated with histamine (10−5 mol/L), transient expression was observed 5 and 10 minutes after histamine treatment. B: HDMECs were incubated with IL-1α (10 ng/ml), TNF-α (10 ng/ml), interferon-γ (10 ng/ml), IL-4 (10 ng/ml), IL-13 (10 ng/ml), or substance P (100 nmol/L) for 24 hours. Whereas IL-4, IL-13, and substance P increased P-selectin expression, TNF-α had no effect on P-selectin expression. The concentration of each cytokine that induced maximal response was determined in preliminary experiments. C and D: Time course changes in P-selectin expression by HDMECs (C) and HUVECs (D) after treatment with IL-4. Surface P-selectin expression in HDMECs peaked 24 hours after treatment with IL-4. Black line, control; green line, treated with stimulants. Results are from a single representative of three separate experiments.
Figure 4-6931.
Laser-scanning microscopic features of P-selectin on HDMECs. A: HDMECs incubated with histamine expressed P-selectin on their surface 5 and 10 minutes after treatment. At 20 minutes, cells exhibited shape changes. B: P-selectin expression was detected on HDMECs incubated with IL-4, IL-13, or substance P. C: Mean fluorescence intensity of surface P-selectin analyzed by Scion Image for Windows software (Scion). Error bars indicate SD. AU, arbitrary units. *P < 0.05. Original magnifications, ×400.
Simultaneous Incubation of HDMECs with IL-4 and TNF-α Inhibited P-Selectin Expression
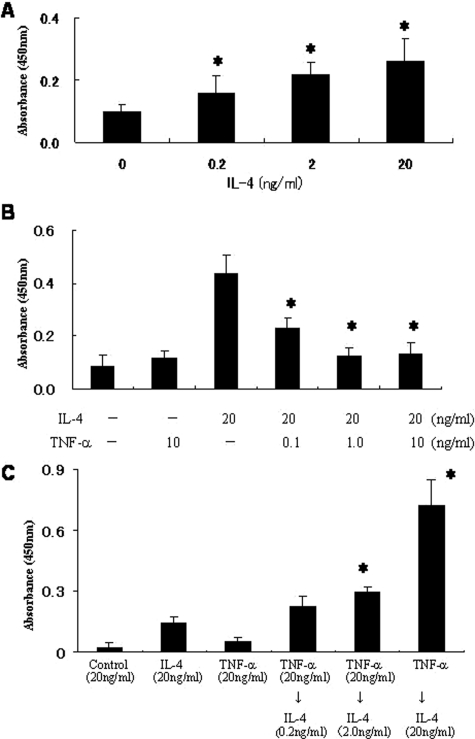
TNF-α has been shown to interfere with IL-4-induced gene expression. In HUVECs, IL-4 inhibits TNF-α-mediated E-selectin expression.25 In contrast, in fibroblasts, TNF-α synergistically enhances IL-4-induced eotaxin expression.26 Accordingly, we examined whether TNF-α interferes with IL-4-mediated P-selectin expression in HDMECs. Because flow cytometric analysis is not reliably quantitative and trypsin-EDTA treatment may remove cell surface antigen, we performed cell-based ELISA.27 IL-4 dose dependently induced surface expression of P-selectin on HDMECs (Figure 5A). Simultaneous incubation with TNF-α and IL-4 significantly suppressed P-selectin expression in a dose-dependent manner (Figure 5B).
Figure 5-6931.
Effect of TNF-α on IL-4-induced P-selectin expression assessed by cell-based ELISA. A: Dose-dependent P-selectin expression induced by IL-4. B: HDMECs were incubated with a combination of IL-4 (20 ng/ml) and TNF-α (0.1 to 10.0 ng/ml) for 24 hours. Surface P-selectin expression was significantly inhibited by TNF-α. C: HDMECs were precultured with TNF-α (0.2 to 20 ng/ml) for 24 hours. They were then incubated with IL-4 (20 ng/ml) for an additional 24 hours. TNF-α pretreatment synergistically increased P-selectin expression, whereas TNF-α alone (20 ng/ml) had no effect. Experiments were repeated at least three times. *P < 0.05.
TNF-α Pretreatment of HDMECs Increases IL-4R Expression and Cooperatively Induces P-Selectin Expression with IL-4
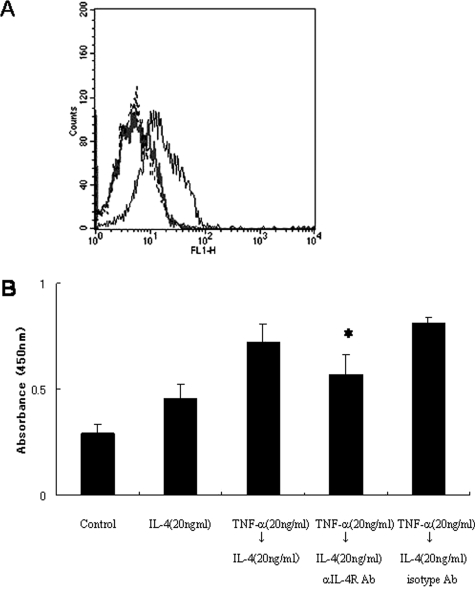
We next assessed the effect of pretreatment with TNF-α on IL-4-induced P-selectin expression. HDMECs were incubated with TNF-α for 24 hours followed by incubation with IL-4 for 24 hours. Unexpectedly, preincubation with TNF-α significantly increased P-selectin expression induced by IL-4 (Figure 5C). Pretreatment with TNF-α also increased IL-4R expression on HDMECs (Figure 6A). The TNF-α-induced increase in P-selectin expression was inhibited by incubation with anti-IL-4R blocking antibody (R&D Systems, Minneapolis, MN) (Figure 6B). These findings suggest that TNF-α-pretreatment increases IL-4-induced P-selectin expression of HDMECs by increasing IL-4R expression.
Figure 6-6931.
TNF-α pretreatment followed by IL-4 incubation increases P-selectin expression via increased expression of IL-4R. A: HDMECs weakly, constitutively express IL-4R on their surface (gray line). Incubation with TNF-α (20 ng/ml) for 24 hours significantly increased IL-4R expression (black line). B: TNF-α-pretreated HDMECs were incubated with IL-4 (20 ng/ml). IL-4R-blocking antibody (1 μg/ml) inhibited the increase in P-selectin expression induced by TNF-α pretreatment. *P < 0.05.
Substance P Activates STAT6 in HDMECs
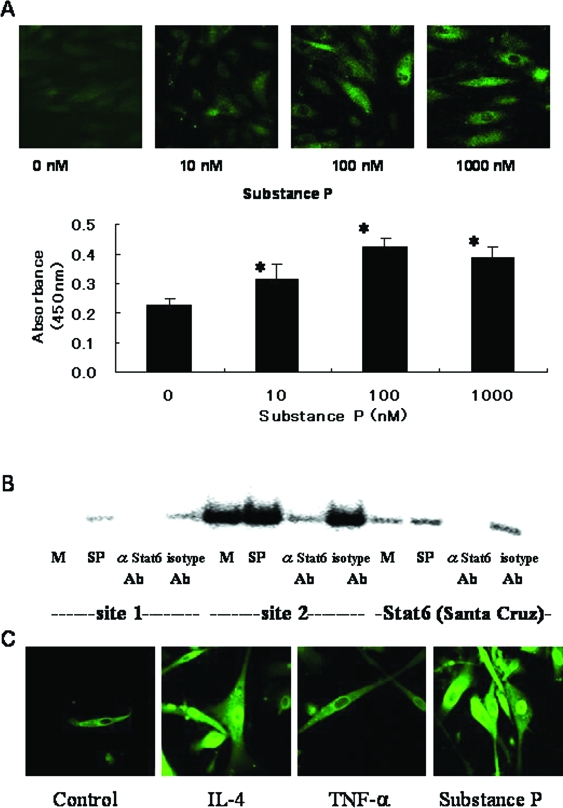
The above findings indicating that substance P induces P-selectin expression in HDMECs (Figures 3 and 4) were consistent with the results of confocal laser-scanning microscopy and cell-based ELISA, which indicate that substance P dose dependently induces P-selectin expression (Figure 7A). In HUVECs incubated with IL-4, activation of STAT6 is a key factor in P-selectin expression.18 To examine whether STAT6 is activated by substance P, we performed EMSA with nuclear extract from HDMECs. Because the proximal P-selectin promoter contains two STAT6-binding sites, called site 1 and site 218(Figure 1), we synthesized two different STAT6 probes specific for these sites. Figure 7B shows STAT6 activation and binding to the site-1 and site-2 probes after incubation with substance P. STAT6 did not bind to the mutant probe (data not shown). IL-4 also stimulated the binding of STAT6 to site-1 and site-2 probes (data not shown). These results of EMSA are consistent with the results of confocal laser-scanning microscopy, which show nuclear translocation of STAT6 protein in HDMECs after incubation with substance P or IL-4 (Figure 7C).
Figure 7-6931.
Substance P induces P-selectin expression via activation of STAT6. A: Confocal laser-scanning microscopic features and cell-based ELISA for P-selectin expression induced by substance P. Surface P-selectin expression was dose dependently induced by substance P. *P < 0.05, compared with control. B: EMSA for STAT6 in HDMECs incubated with substance P. The substance P-inducible factors specifically bound to the conventional probe (Santa Cruz Biotechnology) and probes specific to site 1 and site 2 STAT6-binding sequences (Figure 1). Addition of anti-STAT6 antibody to the binding reaction inhibited complex formation. An oligonucleotide probe with a mutation in the STAT6 consensus sequence (see Materials and Methods) did not compete for binding to the STAT6 probes (data not shown). M, medium alone; SP, substance P. C: Confocal laser-scanning microscopic features of STAT6 protein in HDMECs. IL-4 (20 ng/ml) and substance P (100 nmol/L) induced translocation of STAT6 into the nucleus. Original magnifications, ×400.
Transfection of a cis Element Decoy against STAT6-Binding Site Inhibits Substance P-Induced P-Selectin Expression
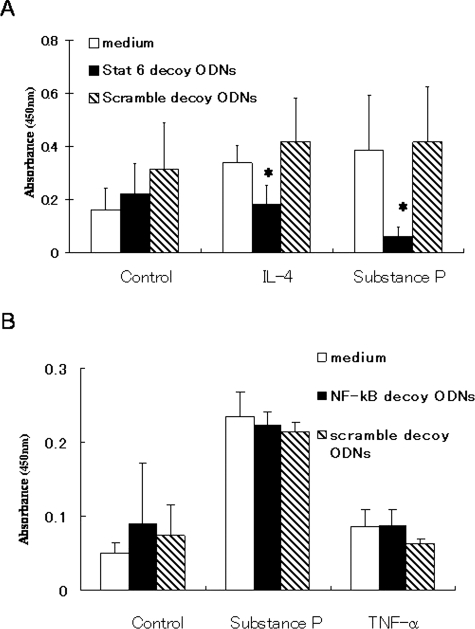
We recently established a method that reliably inhibits binding of STAT6 to nuclei, by transfecting STAT6 decoy ODNs in vivo and in vitro.20,21 To determine whether STAT6 plays a critical role in P-selectin expression, we prepared STAT6 decoy ODNs and treated HDMECs with the ODNs after incubation with substance P. In vitro treatment of HDMECs with STAT6 decoy ODNs reduced substance P-induced P-selectin expression (Figure 8). Similarly, IL-4-induced P-selectin expression was inhibited by STAT6 decoy ODNs. NF-κB decoy ODNs had no effect on P-selectin expression. These results suggest that substance P-induced activation of the P-selectin gene is mediated by STAT6.
Figure 8-6931.
Blocking binding of STAT6 to promoter regions using decoy oligodeoxynucleotides (ODNs) inhibits IL-4- and substance P-induced P-selectin expression. HDMECs were treated with STAT6 decoy ODNs (20 μmol/L) for 8 hours, followed by incubation with IL-4 or substance P for 24 hours. Surface P-selectin expression was evaluated by cell-based ELISA. A:In vitro transfection of STAT6 decoy ODNs into HDMECs markedly inhibited IL-4- and substance P-induced P-selectin expression, but scramble decoy ODNs had no effect. B: NF-κB decoy ODNs had no effect on P-selectin expression. Results are from a single representative of two separate experiments. *P < 0.05, compared with medium alone.
Discussion
P-selectin is considered to play an important role in promotion of leukocyte recruitment, but P-selectin expression in the skin vasculature has not yet been characterized in detail. The present results show constitutive expression of P-selectin on dermal vessels of normal skin and increased expression in allergic skin diseases including urticaria and atopic dermatitis (Th2-dominated disease) but not psoriasis vulgaris (Th1-dominated disease)28 and pustulosis palmaris et plantaris. Results of the present in vitro experiments indicate that IL-4 and IL-13 induce prolonged P-selectin expression on HDMECs. We also found that substance P caused P-selectin expression via activation of STAT6. These data imply that Th2-dominated skin inflammation and/or neuroinflammatory responses promote leukocyte trafficking via dermal endothelial P-selectin.
In the present study, P-selectin expression by HDMECs peaked 24 hours after incubation with IL-4, and returned to the basal level at 48 hours (Figure 3C). Previously, IL-4- and IL-13-induced P-selectin expression by HUVECs was found to persist for 48 hours after incubation11; this is consistent with the present findings (Figure 3D). In contrast, studies indicate that E-selectin is expressed at higher levels and for longer duration by HDMECs than by HUVECs.6 These differences in P- and E-selectin expression between HDMECs and HUVECs may account for differences in leukocyte homing between skin and other organs.
Previous studies indicate that TNF-α can increase expression of E-selectin and VCAM-1 in HDMECs.29,30 The present results indicate that TNF-α did not affect P-selectin expression on HDMECs, which is consistent with previous findings regarding expression of P-selectin on HUVECs.10,31,32 These features could be explained by the fact that the NF-κB site in the human P-selectin gene is unique in that it binds constitutive homodimers of p50 or p52, but does not bind inducible nuclear protein complexes containing p65.28,29
It is interesting to note that incubation with a combination of TNF-α and IL-4 significantly inhibited P-selectin expression, despite the fact that TNF-α alone had no effect on P-selectin expression. There have been several studies of interaction between TNF-α and IL-4.25,26,33–36 In fibroblasts and airway epithelial cells, TNF-α and IL-4 synergistically affect eotaxin gene transcription.33,34,37 In contrast, IL-4 suppresses TNF-α-induced expression of the E-selectin gene.25 It is generally thought that IL-4 and TNF-α mediate activation of STAT6 and NF-κB, respectively. In IL-4-induced suppression of the E-selectin gene, STAT6 competed for binding to a region of E-selectin gene promoter containing overlapping STAT6 and NF-κB binding sites, acting as an antagonist of NF-κB binding.25 In the P-selectin gene promoter, there are two STAT6 binding sites (site 1 and site 2), and the NF-κB site is located adjacent to site 2 (Figure 1).18,31,32 Thus, we first postulated that binding of nuclear protein complexes to the NF-κB site might antagonize binding of STAT6 to site 2, providing a possible mechanism for TNF-α-induced suppression of IL-4-induced P-selectin expression. We synthesized a long DNA probe that spanned both STAT6 site 2 and the NF-κB site, and then performed EMSA to examine the effect of TNF-α on IL-4-mediated binding of STAT6 to this probe. However, TNF-α had no effect on the strength of binding of STAT6 to the probe, and no novel complexes were observed (data not shown). We had no explanation for this finding, and thus could not conclude that activation of NF-κB interfered with binding of STAT6 to site 2. Further study is needed to determine the mechanism of the inhibitory effect of the combination of TNF-α and IL-4 on P-selectin expression.
In contrast to the inhibitory effect of the combination of TNF-α and IL-4, pretreatment of HDMECs with TNF-α markedly increased IL-4-induced P-selectin expression. This was probably because of the increase in IL-4R expression induced by TNF-α. These data indicate that novel, complex regulatory mechanisms are involved in dermal P-selectin expression in inflammatory processes mediated by TNF-α and IL-4.
An important present finding is that substance P induced P-selectin expression on HDMECs. Previous studies indicate that expression of ICAM-1 and VCAM-1 can be induced by substance P via activation of FT-AT and NF-κB.14,15 However, in the present study, nuclear extracts from HDMECs incubated with substance P bound to site 1, site 2, and conventional probes for STAT6. STAT6 protein translocated into the nucleus after incubation with substance P, as revealed by confocal laser-scanning microscopy. These results imply that substance P activates STAT6 and promotes binding of STAT6 to STAT6-binding site 1 and/or site 2 in the proximal P-selectin promoter, resulting in P-selectin expression. This is consistent with the present observation that P-selectin expression was significantly inhibited by blocking the binding of STAT6 to nuclei using STAT6 decoy ODNs. NF-κB decoy ODNs had no effect on P-selectin expression.
In summary, IL-4, substance P, and histamine are important promoters of cutaneous endothelial P-selectin expression. Cutaneous endothelial STAT6 is an effective therapeutic target for regulation of inflammatory cell recruitment in Th2-dominated allergic skin diseases.
Footnotes
Address reprint requests to Takahiro Satoh, M.D., Ph.D., Department of Dermatology, Graduate School, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, 113-8519, Japan. E-mail: tasa-1688.derm@tmd.ac.jp.
Supported in part by the Ministry of Education, Japan (grants 16591091 and 15790569).
Y.M. and T.S. contributed equally to this work.
References
- Robinson SD, Frenette PS, Rayburn H, Cummiskey M, Ullman-Cullere M, Wagner DD, Hynes RO. Multiple targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment. Proc Natl Acad Sci USA. 1999;96:11452–11457. doi: 10.1073/pnas.96.20.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalina MD, Estess P, Siegelman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999;93:580–589. [PubMed] [Google Scholar]
- Kitayama J, Fuhlbrigge RC, Puri KD, Springer TA. P-selectin, L-selectin, and α4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cells under physiological flow conditions. J Immunol. 1997;159:3929–3939. [PubMed] [Google Scholar]
- Patel KD, McEver RP. Comparison of tethering and rolling of eosinophils and neutrophils through selectins and P-selectin glycoprotein ligand-1. J Immunol. 1997;159:4555–4565. [PubMed] [Google Scholar]
- Satoh T, Kaneko M, Wu M-H, Yokozeki H, Nishioka K. Contribution of selectin ligands to eosinophil recruitment into the skin of patients with atopic dermatitis. Eur J Immunol. 2002;32:1274–1281. doi: 10.1002/1521-4141(200205)32:5<1274::AID-IMMU1274>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kluger MS, Johnson DR, Pober JS. Mechanisms of sustained E-selectin expression in cultured human dermal microvascular endothelial cells. J Immunol. 1997;158:887–896. [PubMed] [Google Scholar]
- Hickey MJ, Kanwar S, McCafferty D-M, Neil Granger D, Eppihimer MJ, Kubes P. Varying roles of E-selectin and P-selectin in different microvascular beds in response to antigen. J Immunol. 1999;162:1137–1143. [PubMed] [Google Scholar]
- Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101:880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is located in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Pan J, Setiadi H, Patel KD, McEver RP. Interleukin 4 and oncostatin M induces a prolonged increase in P-selectin mRNA and protein in human endothelial cells. J Exp Med. 1996;184:81–92. doi: 10.1084/jem.184.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltmann G, McNulty CA, Dewson G, Symon FA, Wardlaw AJ. Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood. 2000;95:3146–3152. [PubMed] [Google Scholar]
- Ansel JC, Kaynard AH, Armstrong CA, Olerud J, Bunnet N, Payan D. Skin-nervous system interactions. J Invest Dermatol. 1996;106:198–204. doi: 10.1111/1523-1747.ep12330326. [DOI] [PubMed] [Google Scholar]
- Tani E, Shiosaka S, Sato M, Ishikawa T, Tohyama M. Histamine acts directly on calcitonin gene-related peptide- and substance P-containing ganglion neurons as assessed by calcium influx and immunocytochemistry. Neurosci Lett. 1990;115:171–176. doi: 10.1016/0304-3940(90)90450-n. [DOI] [PubMed] [Google Scholar]
- Quinlan KL, Naik SM, Cannon G, Armstrong CA, Bunnet NW, Ansel JC, Caughman SW. Substance P activates NF-AT- and NF-κB-dependent adhesion molecule gene expression in microvascular endothelial cells through intracellular calcium mobilization. J Immunol. 1999;163:5656–5665. [PubMed] [Google Scholar]
- Quinlan KL, Song IS, Naik SM, Letran EL, Olerud JE, Bunnet NW, Armstrong CA, Caughman SW, Ansel JC. VCAM-1 expression on human dermal microvascular endothelial cells is directly and specifically up-regulated by substance P. J Immunol. 1999;162:1656–1661. [PubMed] [Google Scholar]
- Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, Morohashi M. Nerve growth factor and substance P are useful plasma markers of disease activity of atopic dermatitis. Br J Dermatol. 2002;147:71–79. doi: 10.1046/j.1365-2133.2002.04803.x. [DOI] [PubMed] [Google Scholar]
- Groeger M, Holnthoner W, Mauer D, Lechleitner S, Wolff K, Beate Mayr B, Lubitz W, Petzelbauer P. Dermal microvascular endothelial cells express the 180-kDa macrophage mannose receptor in situ and in vitro. J Immunol. 2000;165:5428–5434. doi: 10.4049/jimmunol.165.10.5428. [DOI] [PubMed] [Google Scholar]
- Khew-Goodall Y, Wadham C, Stein BN, Gamble JR, Vadas MA. Stat6 activation is essential for interleukin-4 induction of P-selectin transcription in human umbilical vein endothelial cells. Arterioscler Thomb Vasc Biol. 1999;19:1421–1429. doi: 10.1161/01.atv.19.6.1421. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K, Beyaert R, Vandevoorde V, Haegeman G, Fiers W. Depletion of mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993;12:3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokozeki H, Wu M-H, Sumi K, Awad S, Satoh T, Katayama I, Takeda K, Akira S, Kaneda Y, Nishioka K. In vivo transfection of a cis element ‘decoy’ against signal transducers and activators of transcription 6 (STAT6)-binding site ameliorates IgE-mediated late-phase reaction in an atopic dermatitis mouse model. Gene Ther. 2004;11:1753–1762. doi: 10.1038/sj.gt.3302341. [DOI] [PubMed] [Google Scholar]
- Sumi K, Yokozeki H, Wu M-H, Satoh T, Kaneda Y, Takeda K, Akira S, Nishioka K. In vivo transfection of a cis element ‘decoy’ against signal transducers and activators of the transcription 6 (STAT6) binding site ameliorates the response of contact hypersensitivity. Gene Ther. 2004;11:1763–1771. doi: 10.1038/sj.gt.3302345. [DOI] [PubMed] [Google Scholar]
- Wang L-H, Yang X-Y, Kirken RA, Resau JH, Farrar WL. Targeted disruption of STAT6 DNA binding activity by an oligonucleotide decoy blocks IL-4-driven TH2 cell response. Blood. 2000;95:1249–1257. [PubMed] [Google Scholar]
- Ogushi I, Iimuro Y, Seki E, Son G, Hirano T, Hada T, Tustsui H, Nakanishi K, Morishita R, Kaneda Y, Fujimoto J. Nuclear factor kappa B decoy oligonucleotides prevent endotoxin-induced fatal liver failure in a murine model. Hepatology. 2003;38:335–344. doi: 10.1053/jhep.2003.50298. [DOI] [PubMed] [Google Scholar]
- Fichtner-Feigl S, Fuss IJ, Preiss JC, Strober W, Kitani A. Treatment of murine Th1- and Th2-mediated inflammatory bowel disease with NF-κB decoy oligonucleotides. J Clin Invest. 2005;115:3057–3071. doi: 10.1172/JCI24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BL, Cruz R, Lacson RG, Manning AM. Interleukin-4 suppression of tumor necrosis factor α-stimulated E-selectin gene transcription is mediated by STAT6 antagonism of NF-κB. J Biol Chem. 1997;272:10212–10219. doi: 10.1074/jbc.272.15.10212. [DOI] [PubMed] [Google Scholar]
- Hoeck J, Woisetschlager M. STAT6 mediates eotaxin-1 expression in IL-4 or TNF-α-induced fibroblasts. J Immunol. 2001;166:4507–4515. doi: 10.4049/jimmunol.166.7.4507. [DOI] [PubMed] [Google Scholar]
- Melrose J, Tsurushita N, Liu G, Berg EL. IFN-γ inhibits activation-induced expression of E- and P-selectin on endothelial cells. J Immunol. 1998;161:2457–2464. [PubMed] [Google Scholar]
- Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, Meyer zum Buschenfelde KH, Fleischer B. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994;102:145–149. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- Petzelbauer P, Bender JR, Wilson J, Pober JS. Heterogeneity of dermal microvascular endothelial cell antigen expression and cytokine responsiveness in situ and in cell culture. J Immunol. 1993;151:5062–5072. [PubMed] [Google Scholar]
- Swerlick RA, Lee KH, Li LJ, Sepp NT, Caughman SW, Lawley TJ. Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells. J Immunol. 1992;149:698–705. [PubMed] [Google Scholar]
- Pan J, Xia L, McEver RP. Comparison of promoters for the murine and human P-selectin genes suggests species-specific and conserved mechanisms for transcriptional regulation in endothelial cells. J Biol Chem. 1998;273:10058–10067. doi: 10.1074/jbc.273.16.10058. [DOI] [PubMed] [Google Scholar]
- Pan J, McEver RP. Regulation of the human P-selectin promoter by Bcl-3 and specific homodimeric members of the NF-κB/Rel family. J Biol Chem. 1995;270:23077–23083. doi: 10.1074/jbc.270.39.23077. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Bartels J, Mallet AI, Christophers E, Schroder JM. IL-4 induces eotaxin: a possible mechanism of selective eosinophil recruitment in helminth infection and atopy. J Immunol. 1998;160:60–68. [PubMed] [Google Scholar]
- Dulkys Y, Schramm G, Kimmig D, Knoess S, Weyergraf A, Kapp A, Elsner J. Detection of mRNA for eotaxin-2 and eotaxin-3 in human dermal fibroblasts and their distinct activation profile on human eosinophils. J Invest Dermatol. 2001;116:498–505. doi: 10.1046/j.1523-1747.2001.01299.x. [DOI] [PubMed] [Google Scholar]
- Kumagai N, Fukuda K, Ishimura Y, Nishida T. Synergistic induction of eotaxin expression in human keratocytes by TNF-α and IL-4 or IL-13. Invest Ophthalmol Vis Sci. 2000;41:1448–1453. [PubMed] [Google Scholar]
- Fujisawa T, Kato Y, Atsuta J, Terada A, Iguchi K, Kamiya H, Yamada H, Nakajima T, Miyamasu M, Hirai K. Chemokine production by BEAS-2B human bronchial epithelial cells: differential regulation of eotaxin, IL-8, and RANTES by TH2- and TH1-derived cytokines. J Allergy Clin Immunol. 2000;105:126–133. doi: 10.1016/s0091-6749(00)90187-8. [DOI] [PubMed] [Google Scholar]
- Matsukura S, Stellato C, Plitt JR, Bickel C, Miura K, Georas SN, Casolaro V, Schleimer RP. Activation of eotaxin gene transcription by NF-κB and STAT6 in human airway epithelial cells. J Immunol. 1999;163:6876–6883. [PubMed] [Google Scholar]