Abstract
We hypothesized that diabetes-induced oxidative stress may affect postischemic neovascularization. The response to unilateral femoral artery ligation was studied in wild-type or gp91phox-deficient control or type 1 diabetic mice or in animals treated with the anti-oxidant N-acetyl-l-cysteine (NAC) or with in vivo electrotransfer of a plasmid encoding dominant-negative Rac1 (50 μg) for 21 days. Postischemic neovascularization was reduced in diabetic mice in association with down-regulated vascular endothelial growth factor-A protein levels. In diabetic animals vascular endothelial growth factor levels and postischemic neovascularization were restored to nondiabetic levels by the scavenging of reactive oxygen species (ROS) by NAC administration or the inhibition of ROS generation by gp91phox deficiency or by administration of dominant-negative Rac1. Finally, diabetes reduced the ability of adherent bone marrow-derived mononuclear cells (BM-MNCs) to differentiate into endothelial progenitor cells. Treatment with NAC (3 mmol/L), apocynin (200 μmol/L), or the p38MAPK inhibitor LY333351 (10 μmol/L) up-regulated the number of endothelial progenitor cell colonies derived from diabetic BM-MNCs by 1.5-, 1.6-, and 1.5-fold, respectively (P < 0.05). In the ischemic hindlimb model, injection of diabetic BM-MNCs isolated from NAC-treated or gp91phox-deficient diabetic mice increased neovascularization by ∼1.5-fold greater than untreated diabetic BM-MNCs (P < 0.05). Thus, inhibition of NADPH oxidase-derived ROS overproduction improves the angiogenic and vasculogenic processes and restores postischemic neovascularization in type 1 diabetic mice.
Cardiovascular complications are the leading cause of morbidity and mortality in patients with diabetes mellitus. In particular, diabetes is associated with a poor outcome after vascular occlusion. This can be attributed in part to impaired neovascularization. Three principal events, ie, vasculogenesis, true angiogenesis, and collateral growth, contribute to postnatal vessel growth and each may be affected by diabetes. Indeed, there are a number of equally tenable hypotheses regarding the mechanisms underlying alterations in blood vessel growth in diabetes, including a reduction in vascular endothelial growth factor-A (VEGF-A) signaling, changes in inflammation-related pathways,1,2 and accumulation of advanced glycation end products.3 Postnatal vasculogenesis can also be affected because the proangiogenic effects of bone marrow mononuclear cells (BM-MNCs) and endothelial progenitor cells (EPCs) are reduced in diabetic mice and patients with either type 1 or 2 diabetes.4–6 Each hypothesis might be a different aspect of common underlying pathogenic mechanisms that remain to be defined.
Reactive oxygen species (ROS) including superoxide, hydrogen peroxide, hydroxyl radical, and reactive nitrogen species, such as nitric oxide and peroxynitrite, are biologically active species that are increasingly recognized to play major roles in vascular biology through redox signaling.7–9 Each of these species derives from specific enzymatic or chemical reactions. A predominant source of ROS in the normal vessel is thought to be a family of membrane-associated NADPH oxidases.7,8,10 The NADPH oxidase, as found in neutrophils and endothelial cells,7,8,11 consists of a membrane-localized cytochrome b558 comprised of two subunits, gp91phox (or Nox 2) and p22phox, as well as cytosolic components p47phox and p67phox. The small GTPase Rac1 is also necessary for full NADPH oxidase activity. ROS have been suggested as important mediators of angiogenesis. H2O2 stimulates cell migration and proliferation in endothelial cells,12 and ROS directly modulate VEGF-A expression and vascular smooth muscle cell proliferation.13 Previous reports also suggest that both the gp91phox-containing NADPH oxidase and Rac1 play a major role in VEGF-A-induced endothelial cell proliferation.14 Similarly, mice lacking gp91phox displayed impaired postischemic neovascularization.15 Taken together, these observations suggest that both gp91phox and Rac1 play a significant role in NADPH oxidase-induced angiogenesis.
Diabetes is associated with increased NADPH oxidase activation leading to ROS overproduction.16,17 A link between ROS and diabetes has been evidenced in experimental models of type 1 and 2 diabetes, as well as in human patients with type 2 diabetes.7,8,16,17 Furthermore, increased ROS production may contribute to diabetes-induced vascular complications. In support of this view, ROS overproduction has been shown to participate in endothelial dysfunction in both rodent models and patients with type 2 diabetes.16,18
The aim of our study was to analyze the role of diabetes-induced ROS overproduction in postischemic neovascularization in a mouse model of unilateral hindlimb ischemia. We hypothesized that oxidative stress is one of the key events that triggers pathways leading to vascular complications in diabetes. In particular, we sought to investigate the involvement of NADPH oxidase-derived ROS in diabetes-induced alterations of VEGF-A expression and postnatal vasculogenesis.
Materials and Methods
Experimental Protocol
All of the experiments were performed in accordance with the European Community guidelines for the care and use of laboratory animals (no. 07430). To induce diabetes, C57BL/6 wild-type mice and C57BL/6 gp91phox-deficient mice were injected intraperitoneally with 60 mg/kg streptozotocin in sodium citrate buffer (0.05 mol/L, pH 4.5) daily for 5 days. Three days after the fifth injection, blood glucose levels were measured. Mice with glucose levels <300 mg/dl were excluded from the study, as previously described.3,5 In control groups, mice were injected intraperitoneally with sodium citrate buffer (0.05 mol/L, pH 4.5). After 2 months of monitored diabetes, mice underwent surgical ligation of the proximal part of the right femoral artery, just above the origin of the circomflexa femoris lateralis, as previously described.19,20 Diabetic and nondiabetic mice were then treated with or without the anti-oxidant N-acetyl-l-cysteine (NAC; 200 mg/kg/day, i.p.) and in vivo electrotransfer of a pCAG expression plasmid encoding for dominant-negative mutant Rac1 (N17Rac1, 50 μg injected in the gastrocnemius muscle). An additional group of mice also received 50 μg of empty pCAG expression plasmid as a negative control. To further analyze the role of VEGF-A up-regulation in NAC-induced restoration of neovascularization, NAC-treated diabetic mice also received an intraperitoneal injection of neutralizing antibody directed against VEGF receptor 2 (anti-Flk-1, 100 μg/day, clone DC101; ImClone Systems Inc., New York, NY21).
Quantification of Neovascularization in the Hindlimb
Three weeks after the onset of ischemia, vessel density was evaluated by three different methods, as previously described19,20: 1) high-definition microangiography using barium sulfate (1 g/ml) injected in the abdominal aorta, followed by image acquisition with a digital X-ray transducer and computerized quantification of vessel density expressed as a percentage of pixels per image occupied by vessels in the quantification area; 2) assessment of capillary density in gastrocnemius muscle by immunostaining with a rabbit polyclonal antibody directed against total fibronectin (dilution 1/50; Chemicon Int., Temecula, CA) and morphometric quantification using Histolab software (Microvisions, Paris, France); and 3) laser Doppler perfusion imaging to assess in vivo tissue foot perfusion.
Determination of Protein Expression
Gastrocnemius muscles from ischemic and nonischemic hindlimbs were thawed and homogenized in 300 μl of buffer (200 mmol/L sucrose, 20 mmol/L HEPES, pH 7.4) with protease inhibitors. Protein extracts from BM-MNCs were obtained by lysing cells in 200 μl of buffer (20% sodium dodecyl sulfate, 100 mmol/L sodium orthovanadate, 0.5 mol/L Tris, pH 7.4) with protease inhibitors. Proteins were separated in 12% denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto nitrocellulose sheets (Hybond ECL; Amersham, Orsay, France). Antibodies against VEGF-A (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA), gp91phox (1:500, BD Transduction, Franklin Lakes, NJ), p47phox (1:2000, BD Transduction), p67phox (1:2000, BD Transduction), Rac1 (1:1000, Cell Signaling, Danvers, MA), phospho-p38 mitogen-activated protein kinase (p38MAPK) (1:1000, Cell Signaling) and pan-p38MAPK (1:1000, Santa Cruz Biotechnology) were used for immunoblotting. As a protein loading control, membranes were stripped, incubated with a goat polyclonal antibody directed against total actin (dilution 1/1000, Santa Cruz Biotechnology), and specific chemiluminescent signal was detected as previously described.19,20
Proangiogenic Effect of BM-MNCs
BM-MNCs were obtained by flushing tibia and femur of wild-type and gp91phox-deficient control and diabetic mice treated with or without NAC. Low-density mononuclear cells were then isolated by centrifugation on a Ficoll gradient, as previously described.5 Five hours after hindlimb ischemia, control animals received intravenous injections of 1 × 106 BM-MNCs. Animals were euthanized at day 10 after ischemia, and the neovascularization reaction was assessed as described above.
BM-MNC Differentiation into EPCs
BM-MNCs (1.5 × 106) per ml were plated on 11-mm cell-culture dishes coated with rat plasma vitronectin (Sigma, St. Quentin Fallavier, France) and gelatin (0.1%) and maintained in endothelial basal medium (EGM2; BioWhittaker, Walkersville, MD). BM-MNCs were treated with or without apocynin (200 μmol/L), NAC (3 mmol/L), or a p38MAPK inhibitor (LY333351, 10 μmol/L) for 7 days. Nonadherent cells were then removed and adherent cells underwent im-munochemical analysis. To detect the uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated low-density lipoprotein (AcLDL-Dil), cells were incubated in medium containing AcLDL-Dil (Tebu, Le Perray en Yvelines, France) at 37°C for 1 hour. Cells were then fixed with 2% paraformaldehyde and incubated with fluorescein isothiocyanate-labeled BS-1 lectin (Sigma). Dual-positive staining for both AcLDL-Dil and BS-1 lectin characterized EPCs. EPC numbers were counted and expressed in cells per well, as previously described.5 Five replicates were counted for each experimental condition. Three independent investigators evaluated the number of EPCs per well by counting three randomly selected high-power fields under epifluorescence microscopy. Results are expressed as percentages of total cell numbers.
BM-MNC Apoptosis Measurement
BM-MNCs were incubated with tumor necrosis factor-α (10 ng/ml) plus cycloheximide (10 μmol/L) or C2-ceramide (100 μmol/L) for 8 hours. BM-MNCs were fixed with paraformaldehyde for 12 hours at 4°C, stained with 4,6-diamidino-2-phenylindole (Calbiochem, Paris, France) for 30 minutes for examination of nuclear morphology by fluorescence microscopy. Total and apoptotic nuclei showing condensed chromatin and fragmented nuclear bodies (karyorrhexis, pyknosis) were counted in five random fields per treatment, totaling a minimum of 150 cells in each of three independent experiments. Results are presented as percentage of apoptotic cells. Alternatively, cell death was assessed by propidium iodide DNA staining and fluorescence-activated cell sorting analysis. Fractions of the cell population in the apoptotic G0/G1 and G2/M cell cycle phases were evaluated and expressed in percentages.
Immunohistochemistry
Frozen tissue sections (7 μm) were incubated with rabbit polyclonal anti-nitrotyrosine antibody (dilution of 1/50) to evaluate 3-nitrotyrosine formation as a biomarker for in vivo nitration. To account for variables including heterogeneity of ROS levels, three different sections were performed at three different levels of gastrocnemius muscle for each animal. In addition, we compared the number of nitrotyrosine-specific stainings between the three different levels for each animal. Using one-way analysis of variance, we did not observe any statistical differences in the experimental group. Immunostains were visualized by fluorescence microscopy and then analyzed in randomly chosen fields (five fields per section) of a defined area with Histolab software.
Luminescence Assay
Tissue and cellular ROS levels, reflecting a balance between oxidant production and removal by endogenous antioxidants, were also quantified using L-012 as described recently.22,23 Gastrocnemius muscle and BM-MNCs were lysed in 50 mmol/L Tris buffer (pH 7.5) containing protease inhibitors (Boehringer-Mannheim, Mannheim, Germany) and centrifuged at 10,000 × g for 15 minutes at 4°C. Supernatants were then incubated with L-012 100 μmol/L (Wako, Neuss, Germany) with or without diphenylene iodonium (DPI; 30 μmol/L) or apocynin (1 mmol/L). The same part of the gastrocnemius muscle was used in the different treated groups. Luminescence was counted (Topcount NXT; Perkin Elmer) during 20 seconds after a 10-minute interval, allowing for the plates to become dark-adapted.
Statistical Analysis
Results were expressed as mean ± SEM. One-way analysis of variance was used to compare each parameter. Comparisons between groups were performed using post hoc Bonferroni t-test when the analysis of variance test was statistically significant. P < 0.05 was considered significant.
Results
Changes in ROS Levels and NADPH Oxidase Subunit Protein Expression
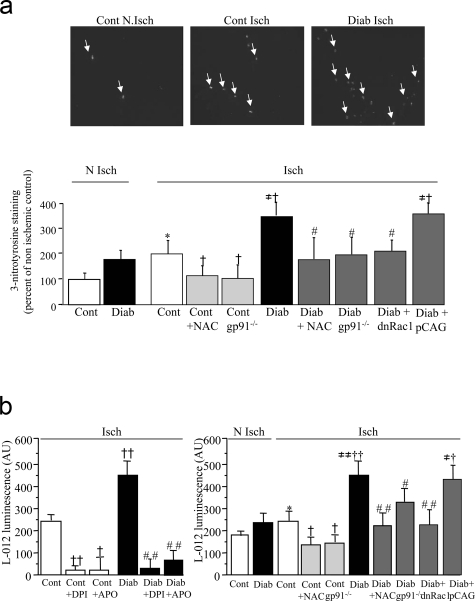
In control animals, 7 days of ischemia raised 3-nitrotyrosine-positive staining in hindlimb muscle (Figure 1a). These results were substantiated by L-012 luminescence demonstrating that ischemia enhanced ROS levels in control animals (Figure 1b). Treatment with DPI or apocynin impaired ischemia-induced increase in ROS levels (P < 0.01 and P < 0.05 for DPI and apocynin treatment, respectively; Figure 1b). Although the experiments with DPI and apocynin are suggestive, we used gp91phox-deficient mice to obtain more definitive evidence and showed that ischemia-induced up-regulation of ROS levels was abrogated in this setting (Figure 1b). Taken together, these results suggest that ischemia activates NADPH oxidase-dependent ROS synthesis. In diabetic mice, ROS accumulation in ischemic tissue was further up-regulated by 1.8-fold compared to control animals (P < 0.01 versus ischemic control; Figure 1b). Treatment with NAC and dominant-negative Rac-1 or gp91phox deficiency abrogated ischemia- and diabetes-induced increases in ROS (Figure 1, a and b).
Figure 1-6928.
a: Top: Representative photomicrographs of ischemic (Isch) and nonischemic (NIsch) gastrocnemius muscle sections from control (Cont) and diabetic (Diab) mice, 7 days after onset of ischemia. There is increased immunostaining for 3-nitrotyrosine. Bottom: Quantitative analysis of immunostaining with the anti-nitrotyrosine antibody. b: Left: Quantitative analysis of ROS levels in ischemic muscle from control (Cont) and diabetic mice (Diab) in presence or absence of DPI or apocynin (APO) during the luminescence assay. Right: Quantitative analysis of ROS levels in ischemic and nonischemic control and diabetic gastrocnemius of mice treated in vivo with NAC or dominant-negative Rac1(dnRac1) or empty pCAG plasmid (pCAG) or in gp91phox-deficient mice (gp91−/−). Values are mean ± SEM, n = 6 per group. *P < 0.05 nonischemic controls; †P < 0.05, ††P < 0.01 versus ischemic controls; ≠P < 0.05, ≠≠P < 0.01 versus nonischemic diabetics; #P < 0.05, ##P < 0.01 versus ischemic diabetics.
The increase in ROS was associated with variations in protein levels of NADPH oxidase subunits. In control animals, gp91phox and p67phox protein levels were up-regulated by 2.9-fold and 1.7-fold, respectively, when compared to nonischemic controls (P < 0.01 and P < 0.05, respectively). p47phox and Rac1 protein levels also tended to be increased, but this did not reach statistical significance (Figure 2). In diabetic mice, gp91phox and Rac1 protein levels were further increased by 1.4- and 1.3-fold in diabetic ischemic hindlimbs compared to ischemic controls (P < 0.05; Figure 2). In contrast, p47phox and p67phox protein content was decreased in diabetic ischemic muscles in reference to ischemic control animals (P < 0.05; Figure 2). Taken together, these results indicate an up-regulation of gp91phox and Rac1 protein content and increased ROS levels in diabetic ischemic muscles.
Figure 2-6928.
Representative Western blot and quantitative evaluation of NADPH oxidase subunit protein levels in ischemic and nonischemic tissues from control (Cont) and diabetic (Diab) animals, expressed as mean ± SEM, n = 6 per group. *P < 0.05, **P < 0.01, ***P < 0.001 versus nonischemic controls; †P < 0.05 versus ischemic controls.
NADPH Oxidase Blockade Restored Postischemic Neovascularization in Diabetic Mice
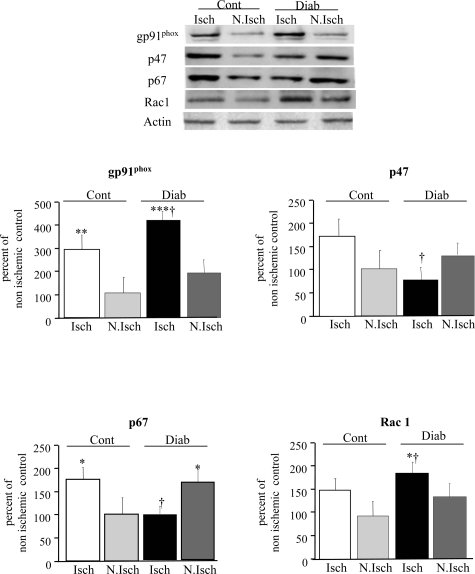
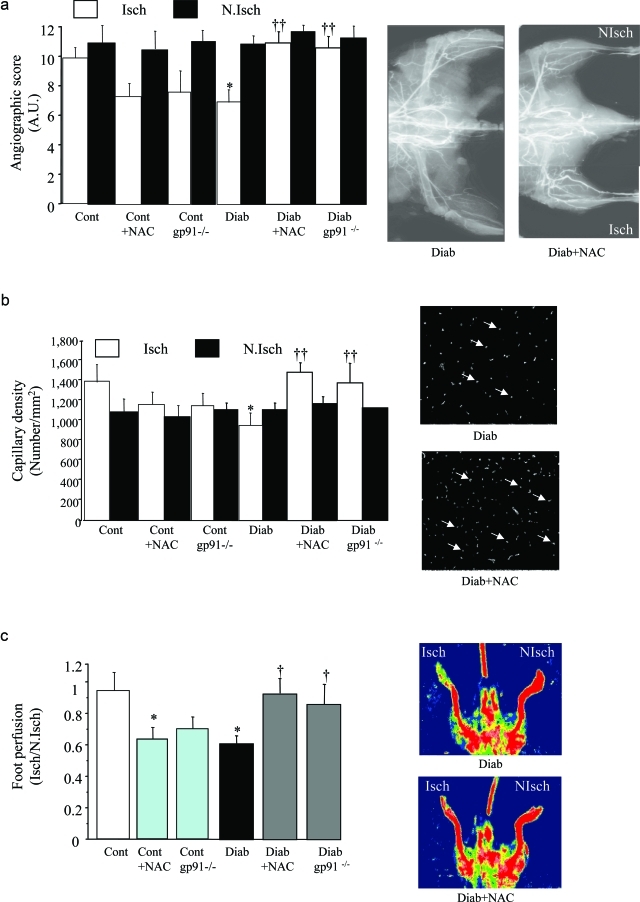
We next assessed the role of ROS and NADPH oxidase activity in postischemic vessel growth 21 days after ischemia. In control animals, neovascularization was impaired by NAC administration, or the absence of gp91phox, as revealed by the 40% decrease in angiographic scores, the 43% decrease in capillary density, and the 40% reduction in foot Doppler perfusion scores (Figure 3). In diabetic animals, angiography scores, capillary density, and foot perfusion were decreased by 40%, 60%, and 43%, respectively, compared to control animals (Figure 3). The scavenging of ROS by NAC administration or the inhibition of ROS generation by gp91phox deficiency or administration of dominant-negative Rac1 restored postischemic neovascularization in diabetic animals to control levels (Figure 3). Hence, reduction in ROS levels restored vessel growth in diabetic ischemic leg whereas it impaired neovascularization in control ischemic tissue.
Figure 3-6928.
Quantitative evaluation of microangiography (a), capillary density (b), and foot perfusion (c) in control and diabetic animals, 21 days after ischemia. Cont, untreated control animals; Cont + NAC, control animal treated with NAC; Cont gp91−/−, control animal lacking gp91phox; Diab, untreated diabetic animals; Diab + NAC, diabetic animal treated with NAC; Diab gp91−/−, diabetic animal lacking gp91phox; Diab + dnRac1, diabetic animal treated with plasmid encoding for Rac1 dominant-negative; Diab + pCAG, diabetic animal treated with empty plasmid; Diab + NAC + anti-Flk-1, diabetic animal co-treated with NAC and neutralizing antibody directed against VEGF receptor type 2, Flk-1. *P < 0.05, **P < 0.01 versus untreated wild-type controls; †P < 0.05, ††P < 0.01 versus diabetic wild-type animals; ≠P < 0.05 versus diabetic animals treated with NAC.
NADPH Oxidase Inhibition Restored VEGF-A Protein Expression in Diabetic Mice
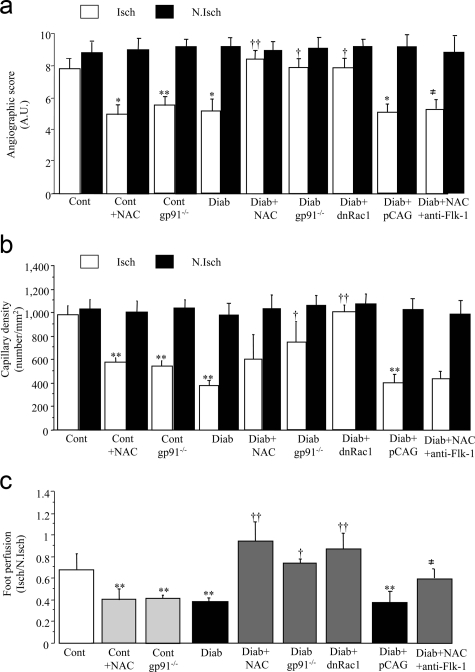
We sought to determine the molecular and cellular pathways affected by diabetes-induced oxidative stress. VEGF-A signaling represents a critical rate-limiting step in both physiological and pathological angiogenesis. In control mice, VEGF-A protein levels were decreased by 25% in NAC-treated animals and gp91phox-deficient mice compared to control mice (P < 0.05; Figure 4). In diabetic mice, VEGF-A levels were reduced by 60% in ischemic tissues compared to ischemic control hindlimbs (P < 0.001; Figure 4). Interestingly, VEGF-A protein contents were significantly increased by 2.5-, 2.0-, and 2.7-fold in diabetic animals treated with NAC or dominant-negative Rac1 and in gp91phox-deficient diabetic mice, respectively, compared to untreated diabetic mice (Figure 4). In addition, co-treatment with anti-Flk-1 abrogated NAC-related effects suggesting that VEGF-A up-regulation participates in the restoration of vessel growth in NAC-treated diabetic mice (Figure 3).
Figure 4-6928.
Representative Western blot (a) and quantitative evaluation of VEGF-A protein levels in ischemic and nonischemic tissues (b), 21 days after ischemia. Cont, untreated control animals; Cont + NAC, control animal treated with NAC; Cont gp91−/−, control animal lacking gp91phox; Diab, untreated diabetic animals; Diab + NAC, diabetic animal treated with NAC; Diab + dnRac1, diabetic animal treated with plasmid encoding for Rac1 dominant-negative; Diab gp91−/−, diabetic animal lacking gp91phox. Values are mean ± SEM, n = 6 per group. *P < 0.05, ***P < 0.001 versus untreated wild-type controls; †P < 0.05, ††P < 0.01 versus diabetic wild-type animals.
NADPH Oxidase Inhibition Restored Postnatal Vasculogenesis in Diabetic Animals
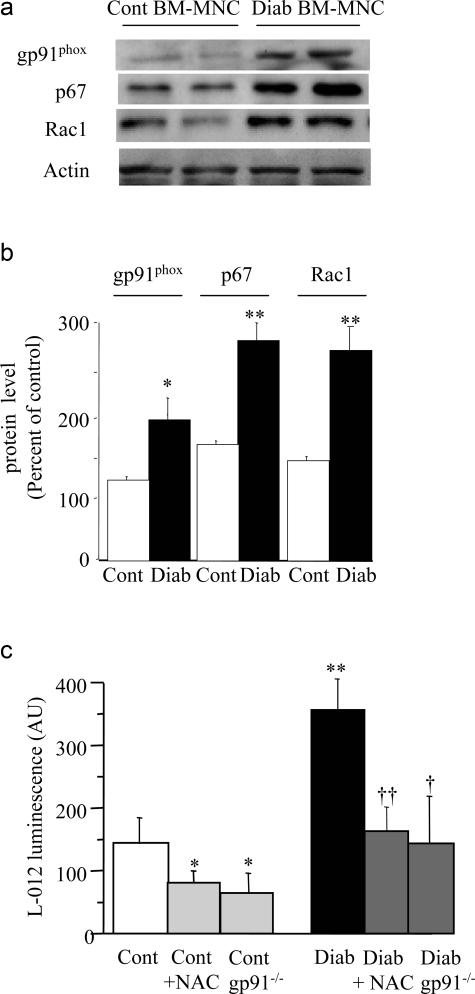
We finally evaluated the effects of diabetes-induced increased ROS on the ability of BM-MNCs to stimulate neovascularization. Protein expression of NADPH oxidase subunits was detected in control BM-MNCs. Their levels were up-regulated in diabetic versus control BM-MNCs (Figure 5a). ROS levels were also increased by 2.3-fold in diabetic BM-MNCs compared to control BM-MNCs (P < 0.01; Figure 5b). Treatment with NAC or deficiency in gp91phox reduced ROS levels in both control and diabetic cells.
Figure 5-6928.
Representative Western blot (a) and quantitative evaluation (b) of NADPH oxidase subunit protein levels in control and diabetic BM-MNCs. Values are mean ± SEM, n = 6 per group. *P < 0.05, **P < 0.01 versus control BM-MNCs. c: Quantitative evaluation of BM-MNC-derived ROS using luminescence assay. Values are mean ± SEM, n = 5 per group. *P < 0.05, **P < 0.01 versus control BM-MNCs, †P < 0.05, ††P < 0.01 versus diabetic BM-MNCs.
NADPH Oxidase Inhibition Restored the Proangiogenic Potential of Diabetic BM-MNCs
The proangiogenic potential of BM-MNCs isolated from control mice treated with NAC or gp91phox-deficient mice was lower than that observed with BM-MNCs isolated from control mice. However, this difference did not reach statistical significance (Figure 6). In contrast, transplantation of BM-MNCs isolated from diabetic mice treated with NAC improved the angiography score by 1.6-fold, capillary numbers by 1.4-fold, and foot perfusion by 1.5-fold greater than BM-MNCs isolated from untreated diabetic animals (Figure 6). Similarly, injection of BM-MNCs isolated from gp91phox-deficient diabetic mice improved the angiography score by 1.7-fold, capillary numbers by 1.4-fold, and foot perfusion by 1.6-fold, compared to BM-MNCs isolated from untreated diabetic animals (Figure 6).
Figure 6-6928.
Representative photomicrographs and quantitative evaluation of microangiography (a), capillary density (b, capillary appears in white, arrows indicating representative examples of fibronectin-positive capillaries), and foot perfusion (c), 10 days after ischemia and cell transplantation. In this set of experiments, control animals received BM-MNCs from control mice (Cont), control mice treated with NAC (Cont + NAC), gp91phox-deficient control mice (Cont gp91−/−), diabetic mice (diab), diabetic mice treated with NAC (diab + NAC), gp91phox-deficient diabetic mice (diab gp91phox−/−). *P < 0.05 versus control animals receiving control BM-MNCs. †P < 0.05, ††P < 0.01 versus control animals receiving diabetic BM-MNCs.
NADPH Oxidase Inhibition Restored BM-MNC Differentiation into Endothelial Progenitor Cells in Vitro
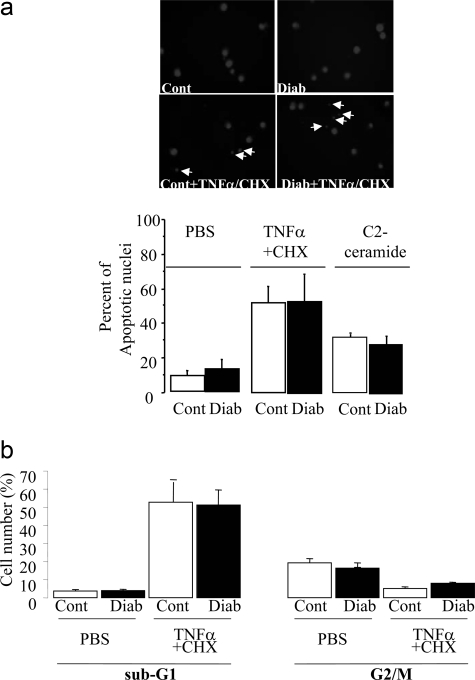
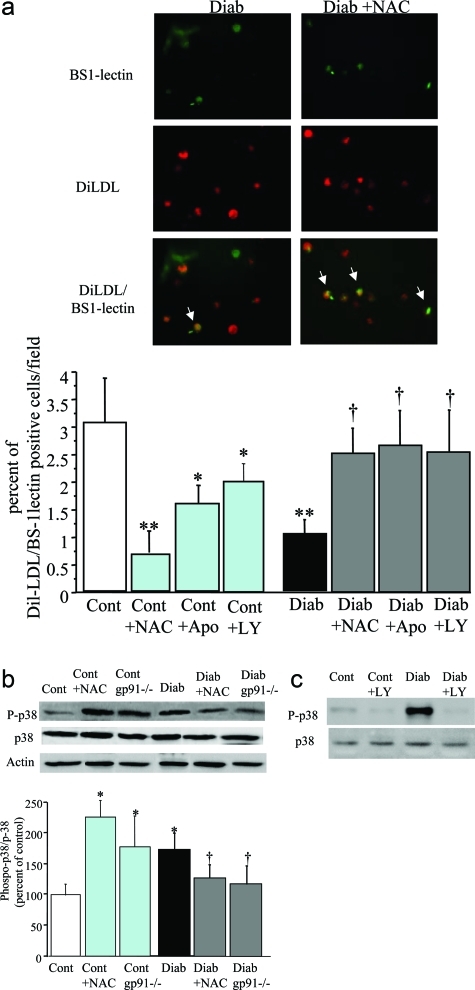
We could not detect any significant difference in percentages of apoptotic cells in control and diabetic animals, suggesting that major alterations in BM-MNC apoptosis were not a significant cause of diabetes-induced BM-MNC dysfunction (Figure 7). Treatment of BM-MNCs isolated from control animals with NAC and apocynin reduced the number of DilLDL/BS1 lectin double-positive cells (Figure 8a) suggesting that low levels of ROS may facilitate BM-MNC differentiation into EPCs. The ability of diabetic BM-MNCs to differentiate into EPCs was markedly reduced, as revealed by the 66% reduction in numbers of DilLDL/BS1 lectin-positive cells (Figure 8a). Treatment with NAC and apocynin restored the percentage of diabetic double-positive cells to control BM-MNC levels (Figure 8a). This effect is likely related to changes in p38MAPK phosphorylation. Indeed, p38MAPK plays a pivotal role in the signal transduction pathways that regulate numbers of EPCs ex vivo.24 Diabetes increased p38MAPK phosphorylation by twofold in BM-MNCs (P < 0.01). NAC treatment reduced p38MAPK phosphorylation by 44% in diabetic cells. p38MAPK phosphorylation was also decreased by 45% in diabetic gp91phox-deficient cells (Figure 8c). Furthermore, p38MAPK inhibition with LY333351 improved the number of diabetic DilLDL/BS1 lectin-positive cells (Figure 8a), suggesting that the decreased differentiation of diabetic BM-MNCs into EPCs is mediated by enhanced p38MAPK phosphorylation resulting from increased ROS levels.
Figure 7-6928.
a: Quantification (top) and representative photomicrographs (bottom) of control and diabetic BM-MNC apoptosis after incubation with tumor necrosis factor-α (10 ng/ml) plus cyclohexamide (CHX) or C2-ceramide for 8 hours. BM-MNCs were fixed and stained with 4,6-diamidino-2-phenylindole. Arrows indicate apoptotic nuclei showing condensed chromatin or fragmented nuclear bodies. b: Quantification of control and diabetic BM-MNC apoptosis assessed by propidium iodide DNA staining and fluorescence-activated cell sorting analysis. Fractions of the cell population in the apoptotic G0/G1 and G2/M cell cycle phases were evaluated and expressed in percentages.
Figure 8-6928.
a: Top: Representative images of EPCs derived from BM-MNCs of diabetic mice treated or not with NAC, after 7 days of culture. EPCs were characterized as adherent cells with double-positive staining for AcLDL-Dil and BS-1 lectin. Bottom: Quantification of AcLDL-Dil and BS-1 lectin-positive cells derived from control and diabetic BM-MNCs treated or not with oxidative stress inhibitors NAC (NAC), apocynin (Apo), or with p38MAPK inhibitor, LY333351 (LY). Values are mean ± SEM, n = 5 per group. *P < 0.05,**P < 0.01, versus control BM-MNCs, †P < 0.05 versus diabetic BM-MNCs. b: Representative Western blot and quantitative evaluation of phospho p38, p38, and actin protein levels in control BM-MNCs (cont) and diabetic BM-MNCs (diab) treated with or without NAC or isolated from gp91phox-deficient mice. Values are mean ± SEM, n = 5 per group. *P < 0.05 versus control BM-MNCs, †P < 0.05 versus diabetic BM-MNCs. c: Representative Western blot of phospho p38 and p38 in control BM-MNCs (cont) and diabetic BM-MNCs (diab) treated with or without LY333351 showing inhibition of p38 phosphorylation by LY333351.
Discussion
This study show that diabetes-induced increases in ROS levels impairs postischemic neovascularization and blockade of oxidative stress in the setting of diabetes restores key pathways involved in angiogenesis, such as VEGF-A signaling, and postnatal vasculogenesis. NADPH oxidase activity and ROS production mediate angiogenesis in both cultured cells and in vivo models of neovascularization.13,14 A recent study also shows that vessel growth in ischemic hindlimbs is significantly hampered in mice lacking gp91phox.15 We could confirm these previous studies, because we found that NAC administration and gp91phox deficiency reduced postischemic neovascularization. We could extend those observations showing that this effect of ROS is likely associated with impairment in VEGF-A signaling and the ability of BM-MNCs to differentiate into EPCs.
We found that ROS levels were higher in diabetes, in association with the up-regulation of gp91phox and Rac1 expression. However, down-regulation of p47phox and p67phox is puzzling and does not fit with the increase in ROS levels in ischemic diabetic tissue. One may speculate that the time course of ischemia-induced p47phox and p67phox protein regulation may differ from that observed for gp91phox or Rac-1. Alternatively, the down-regulation of p67phox and p47phox may counterbalance the rise in gp91phox and Rac-1 protein levels and limit ROS overproduction in diabetic tissue. Nevertheless, previous studies show evidence that NADPH oxidase activity and expression are significantly increased in diabetic tissue.16,18 Antioxidant defense capacity is reduced in animal models of diabetes, and this can also contribute to diabetes-induced oxidative stress.25 We showed here that blockade of NADPH oxidase activity or the scavenging of ROS restores postischemic neovascularization in diabetes. This effect is related to an up-regulation of major proangiogenic factors such as VEGF-A.3,19,26 We also demonstrated that diabetes-induced increases in ROS enhanced p38MAPK phosphorylation in BM-MNCs, reduced BM-MNC differentiation into EPCs in vitro, and impaired their proangiogenic potential in vivo. Similarly, diabetes has been shown to activate p38MAPK in vascular cells via PKC-dependent and -independent pathways.27 Furthermore, p38MAPK activation is known to down-regulate EPC proliferation and differentiation.24 Taken together, our results suggest that increased oxidative stress constitutes an underlying pathogenic mechanism that affects both angiogenesis and vasculogenesis in the setting of diabetes. In addition, this study supports the concept that the effects of ROS on vascular function depend critically on the amounts present. In low amounts under physiological conditions, ROS can act as intracellular second messengers, modulating proangiogenic pathways such as VEGF-A signaling and postnatal vasculogenesis. Conversely, higher amounts of ROS can bear negatively on vessel growth.7,8,28,29 Nevertheless, because gp91phox and Rac-1 may have multiple roles in nonphagocytic cells, one cannot exclude the possibility that gp91phox and Rac-1 may modulate neovascularization through ROS-independent related pathways.
Because ROS are involved in numerous signaling pathways, the mechanisms by which they may affect neovascularization are potentially diverse. A considerable body of evidence implicates oxidative stress as an important pathogenic element in diabetic endothelial dysfunction.30 The chronic treatment of diabetic animals with NAC improved or normalized endothelium-dependent responses.31 Alternatively, increased superoxide production by NADPH oxidase likely reduces NO bioactivity by scavenging or through uncoupling of endothelial nitric oxide synthase and may also lead to the formation of other signaling species such as peroxynitrite.16,18 In keeping with this, we found increased 3-nitrotyrosine staining in ischemic muscles, consistent with increased levels of nitrating species. Changes in NO signaling might contribute to alterations in postischemic neovascularization because NO is known to mediate BM-MNC mobilization and differentiation, as well as basal neovascularization reaction.32,33
In conclusion, this study reports for the first time that diabetes-induced increases in NADPH oxidase-derived ROS reduces postischemic neovascularization through the inhibition of proangiogenic pathways and impaired postnatal vasculogenesis. Our results also highlight the concept that inhibition of this oxidative stress might be a potential therapeutic avenue to promote neovascularization in the setting of diabetes.
Acknowledgments
We thank Dr. F. Jaisser (INSERM U36) for the kind gift of dominant-negative Rac-1.
Footnotes
Address reprint requests to Jean-Sebastien Silvestre, U689-INSERM, Hôpital Lariboisière, 41 Blvd de la Chapelle, 75475 Paris Cedex 10, France. E-mail: jean-sebastien.silvestre@larib.inserm.fr.
Supported by the British Heart Foundation (Chair of Cardiology at King’s College London to A.M.S.); Agence Nationale de la Recherche (young investigator grant JC05-45445 and 2005 Cardiovascular, Obesity, and Diabetes grant ANR-05-PCOD-028-01 to J.-S.S.); The Netherlands Organization for Health Research and Development (Agiko stipend 920-03-291 to B.M.); the Prof. Michaël-van Vloten Fund; and the European Commission (INSERM U689 is partner of the European Vascular Genomics Network, a Network of Excellence granted by contract no. LSHM-CT-2003-503254).
References
- Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: a potential predictor for the individual capacity to develop collaterals. Circulation. 2000;102:185–190. doi: 10.1161/01.cir.102.2.185. [DOI] [PubMed] [Google Scholar]
- Tamarat R, Silvestre JS, Huijberts M, Benessiano J, Ebrahimian TG, Duriez M, Wautier MP, Wautier JL, Levy BI. Blockade of advanced glycation end-product formation restores ischemia-induced angiogenesis in diabetic mice. Proc Natl Acad Sci USA. 2003;100:8555–8560. doi: 10.1073/pnas.1236929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type 2 diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- Tamarat R, Silvestre JS, Le Ricousse-Roussanne S, Barateau V, Lecomte-Raclet L, Clergue M, Duriez M, Tobelem G, Levy BI. Impairment in ischemia-induced neovascularization in diabetes: bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am J Pathol. 2004;164:457–466. doi: 10.1016/S0002-9440(10)63136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Bayraktutan U, Blayney L, Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1903–1911. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Ohzeki Y, Shimizu S, Naito S, Ohtsuru A, Yamamoto T, Kuroiwa Y. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 1999;64:249–258. doi: 10.1016/s0024-3205(98)00560-8. [DOI] [PubMed] [Google Scholar]
- Ruef J, Hu ZY, Yin LY, Wu Y, Hanson SR, Kelly AB, Harker LA, Rao GN, Runge MS, Patterson C. Induction of vascular endothelial growth factor in balloon-injured baboon arteries A novel role for reactive oxygen species in atherosclerosis. Circ Res. 1997;81:24–33. doi: 10.1161/01.res.81.1.24. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656s–1662s. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Mallat Z, Tamarat R, Duriez M, Tedgui A, Levy BI. Regulation of matrix metalloproteinase activity in ischemic tissue by interleukin-10: role in ischemia-induced angiogenesis. Circ Res. 2001;89:259–264. doi: 10.1161/hh1501.094269. [DOI] [PubMed] [Google Scholar]
- Ebrahimian TG, Tamarat R, Clergue M, Duriez M, Levy BI, Silvestre JS. Dual effect of angiotensin-converting enzyme inhibition on angiogenesis in type 1 diabetic mice. Arterioscler Thromb Vasc Biol. 2005;25:65–70. doi: 10.1161/01.ATV.0000149377.90852.d8. [DOI] [PubMed] [Google Scholar]
- Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- Daiber A, August M, Baldus S, Wendt M, Oelze M, Sydow K, Kleschyov AL, Munzel T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic Biol Med. 2004;36:101–111. doi: 10.1016/j.freeradbiomed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005;97:533–540. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
- Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, Urbich C, Rossig L, Corbaz A, Chvatchko Y, Zeiher AM, Dimmeler S. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005;111:1184–1191. doi: 10.1161/01.CIR.0000157156.85397.A1. [DOI] [PubMed] [Google Scholar]
- Wohaieb SA, Godin DV. Alterations in free radical tissue-defense mechanisms in streptozocin-induced diabetes in rat Effects of insulin treatment. Diabetes. 1987;36:1014–1018. doi: 10.2337/diab.36.9.1014. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang ZY, Yamauchi T, Kuboki K, Meier M, Rhodes CJ, King GL. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest. 1999;103:185–195. doi: 10.1172/JCI3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Inanami O, Yamamori T, Ohta T, Hamasu T, Karino T, Kuwabara M. Roles of protein kinase C delta in the accumulation of P53 and the induction of apoptosis in H2O2-treated bovine endothelial cells. Free Radic Res. 2002;36:1147–1153. doi: 10.1080/1071576021000016409. [DOI] [PubMed] [Google Scholar]
- Deshpande NN, Sorescu D, Seshiah P, Ushio-Fukai M, Akers M, Yin Q, Griendling KK. Mechanism of hydrogen peroxide-induced cell cycle arrest in vascular smooth muscle. Antioxid Redox Signal. 2002;4:845–854. doi: 10.1089/152308602760599007. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper GM, Siebeneich W. Oral administration of the antioxidant, N-acetylcysteine, abrogates diabetes-induced endothelial dysfunction. J Cardiovasc Pharmacol. 1998;32:101–105. doi: 10.1097/00005344-199807000-00016. [DOI] [PubMed] [Google Scholar]
- Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]