Abstract
Currently, novel mouse models of melanoma are being generated that recapitulate the histopathology and molecular pathogenesis observed in human disease. Impaired cell-cycle control, which is a hallmark of both familial and sporadic melanoma, promotes slowly growing carcinogen-induced melanomas in the skin of mice carrying a mutated cyclin-dependent kinase 4 (CDK4R24C). Deregulated receptor tyrosine kinase signaling, which is another important feature of human melanoma, leads to spontaneous development of metastatic melanoma after a long latency period in mice overexpressing hepatocyte growth factor/scatter factor (HGF/SF mice). Here we report that treatment with 7,12-dimethylbenz[a]anthracene and 12-O-tetradecanoylphorbol-13-acetate induced metastatic melanomas in all HGF/SF mice on the C57BL/6 background, which histologically resemble human melanoma. Importantly, mutant CDK4 dramatically increased the number and the growth kinetics of carcinogen-induced primary melanomas in the skin and promoted the growth of spontaneous metastases in lymph nodes and lungs in all HGF/SF mice within the first 3 months of life. Apart from very few skin papillomas, we did not observe tumors of other histology in carcinogen-treated HGF/SF × CDK4R24C mice. This new experimental mouse model can now be exploited to study further the biology of melanoma and evaluate new treatment modalities.
Malignant melanoma of the skin poses serious clinical problems because tumor cells are able to metastasize early in draining lymph nodes and visceral organs, which leads to death. Currently the biology of melanoma is only incompletely understood and there are no treatment options to cure disseminated metastatic disease. The incidence of cutaneous melanoma is rising in the Caucasian population, presumably because of increased episodes of excessive UV exposure with sun burns during childhood.1 During the last decade, a number of melanoma-associated genetic alterations have been identified both in hereditary and sporadic melanoma.2 Technological advances in the ability to modify the mouse genome have allowed for the construction of new mouse models, which mimic in part the etiology, histopathology, and molecular pathogenesis of melanoma in patients. These models helped to elucidate the functional importance of genetic abnormalities observed in humans and may facilitate the experimental development of new treatment modalities.3–5
Impaired control of the G1 phase of the cell cycle, either by a loss of function of the cyclin-dependent kinase inhibitor p16/INK4a encoded by the CDKN2A gene locus on chromosome 9p21 or by a gain of function of the cyclin-dependent kinase 4 (CDK4) is a key genetic event frequently observed in both hereditary and sporadic melanoma in humans.2,3,6 Deletion of the p16/INK4a protein using knockout strategies predisposes mice for the development of melanoma.7,8 A similar phenotype has been observed in mice carrying a mutated CDK4R24C gene knocked into the germline which renders the CDK4 protein insensitive to inhibition by p16/INK4a.9 This mutation acts as a dominant oncogene and was found in rare cases of both sporadic and hereditary melanoma.10,11
Deregulated receptor tyrosine kinase signaling is another very important feature of human melanoma.4,6 Both oncogenic mutations in the N-Ras and B-Raf genes as well as autocrine growth factor signaling loops are frequently observed.4,12–15 Transgenic expression of mutant Ras under the control of a melanocyte-specific promotor supports the development of melanoma in mice.16 Similarly, overexpression of hepatocyte growth factor/scatter factor (HGF/SF) leading to autocrine and paracrine growth factor signaling via its receptor tyrosine kinase c-Met and subsequent activation of ras signal transduction pathways promotes melanomagenesis in mice.17 HGF/SF-overexpressing mice represent an attractive model because melanocytes are not confined to the dermis and the hair follicles but are also present in the basal layers of the epidermis and the epidermo-dermal junction similar to human skin. Consequently, melanomas arising in HGF/SF-overexpressing mice show intraepidermal spread that is not observed in most other mouse melanoma models. Furthermore, melanoma can be induced by neonatal UVB irradiation, which closely mimics the human situation.18
Our group has been primarily interested in the development of melanoma vaccines using the melanocytic protein tyrosinase-related protein 2 (TRP2) as a model antigen that is recognized by cytotoxic T cells.19–21 To evaluate the role of the immune system in the pathogenesis and therapy of melanoma in a more realistic experimental setting, we turned to novel genetic mouse models. Mice carrying mutant CDK4 are of particular immunological interest because the CDK4R24C mutation was initially identified with melanoma-specific cytotoxic T cells.10 Because our model antigen TRP2 is recognized by H2-Kb-restricted cytotoxic T cells, we used CDK4-mutant mice on the immunologically well-characterized C57BL/6 background.22 However, cutaneous melanomas induced by carcinogen treatment only grew slowly in CDK4-mutant C57BL/6 mice and did not spontaneously metastasize. Furthermore, the appearance of histologically diverse tumors such as sarcomas and lymphomas limited the lifespan of CDK4-mutant mice. Therefore, we sought to improve the CDK4R24C mouse melanoma model by addition of another cooperating oncogene. Here we report that carcinogen-treatment also promotes the development of metastatic melanoma in C57BL/6 mice with deregulated receptor tyrosine kinase signaling due to overexpression of HGF/SF. Importantly, mutant CDK4 strongly enhances the growth of primary melanoma in the skin and of spontaneous metastases in the draining lymph nodes and lungs of carcinogen-treated HGF/SF mice.
Materials and Methods
Mice
C57BL/6 mice (H-2b) were purchased from Charles Rivers (Sulzfeld, Germany). CDK4-mutant mice harboring the oncogenic mutation (R24C) in the cyclin-dependent kinase 4 (CDK4R24C) were originally generated on a mixed 129SvxCD1 background using a knockin strategy ensuring physiological regulation of the mutant cdk4 protein during the cell cycle.23 CDK4R24C mice used in the experiments reported here were crossed back with C57BL/6 mice for more than eight generations. Mice overexpressing the hepatocyte growth factor/scatter factor (HGF/SF mice) as a transgene under the control of the metallothionein promoter were originally generated on the FVB background.17 HGF/SF mice used in the experiments here were crossed back with C57BL/6 mice for at least five generations. All animal experiments were performed at the Central Animal Facility of the University of Bonn in adherence to the standards of the German law for the care and use of laboratory animals.
Induction of Melanoma in the Skin Using Carcinogen Treatment
Newborn mice were painted once at day 4 of life with 200 μg of 7,12-dimethylbenz[a]anthracene (DMBA). Two weeks later, tumor growth was promoted by treatment with 5 μg of 12-O-tetradecanoylphorbol-13-acetate (TPA) two times a week for a total of 5 weeks. Development of melanocytic neoplasms as well as other skin tumors was carefully observed on a weekly basis. Nevi and melanomas were counted and their size measured in two bisecting diameters using a caliper and expressed as mean tumor size in mm. Additionally, mice were photographed with a digital camera. When progressively growing melanomas exceeded 10 mm in diameter, mice were sacrificed. Occasionally, mice also had to be sacrificed because of weight loss and apparent sickness. Autopsy was performed in all mice. After excision of melanomas in the skin, the lymph nodes were harvested, their size determined by measuring three perpendicular diameters, and the volume calculated in mm3. Furthermore, lungs were retrieved and the number of black nodules on their surface indicating metastatic spread of melanoma counted. Internal organs (in particular the liver, spleen, and kidneys) as well as the brain were carefully investigated for the presence of further metastases or other tumor types both macroscopically and microscopically.
Histopathological and Immunohistochemical Analyses
Samples of skin, lymph nodes, internal organs (lung, liver, spleen, kidney) and brain were obtained when mice were sacrificed. Tissue specimens were in part fixed in 10% buffered formalin, embedded in paraffin, and routinely stained with hematoxylin and eosin. For further immunohistopathological investigations, a zinc-based fixative was used as an alternative to formalin (DAKO, Hamburg, Germany). To confirm the melanocytic origin of tumor cells, sections were immunostained with the TRP1-specific polyclonal rabbit antibody Pep1 (a kind gift from Vincent Hearing, National Institutes of Health, Bethesda, MD) followed by a biotin-conjugated anti-rabbit secondary antibody and the LSAB-2 color development system (both from DAKO). To determine the proliferative activity of tumor cells, a Ki67-specific polyclonal rabbit antibody was used.
Statistical Analyses
The significance of differences in the number of melanomas in the skin, in the size of lymph nodes, and in the number of lung metastases was assessed with the nonparametric Mann-Whitney U-test using the SPSS 11 computer program (SPSS Inc., Chicago, IL).
Results
Induction of Melanoma in C57BL/6 Mice Overexpressing HGF/SF in Combination with Wild-Type CDK4, Heterozygous Mutant CDK4, or Homozygous Mutant CDK4
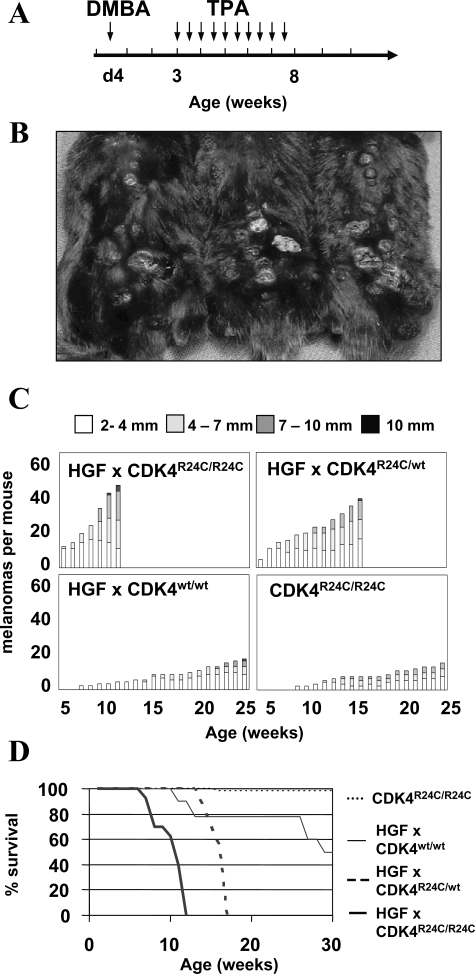
Initially, we established breeding pairs yielding cohorts of C57BL/6 mice overexpressing HGF/SF in combination with wild-type CDK4, heterozygous mutant CDK4, or homozygous mutant CDK4. We noticed that female HGF/SF C57BL/6 mice are mostly not fertile attributable to as yet uncharacterized developmental abnormalities in the reproductive organs. Thus, the HGF/SF transgene could only be passed on heterozygously by male C57BL/6 mice. Fortunately, overexpression of HGF/SF on the C57BL/6 background leads to a chocolate point phenotype because of melanocytes in the intraepidermal skin. Therefore, HGF/SF mice can easily be distinguished from wild-type littermates by their dark nose, ears, and paws. HGF/SF mice heterozygous for mutant CDK4 were generated by mating male HGF/SF mice with female mice homozygous for mutant CDK4. Backcrossing of male HGF/SF mice heterozygous for mutant CDK4 with female mice homozygous for mutant CDK4 yielded male mice homozygous for mutant CDK4. Homo- or heterozygosity of the mutant CDK4R24C allele was distinguished using genomic PCR as previously described.9 Cohorts of C57BL/6 mice overexpressing HGF/SF in combination with wild-type CDK4 (HGF/SF × CDK4w/w mice), heterozygous mutant CDK4 (HGF/SF × CDK4R24C/wt mice), or homozygous mutant CDK4 (HGF/SF × CDK4R24C/R24C mice) were subsequently treated at day 4 of life with 200 μg of DMBA followed 2 weeks later by application of 5 μg of TPA twice a week for 5 weeks as shown in Figure 1A. This classical two-stage carcinogen treatment protocol had a dramatic effect in the cohort of HGF/SF × CDK4R24C/R24C mice. Sixteen of sixteen mice developed melanocytic nevi within the first weeks of life while still under TPA treatment. Every single mouse developed widespread, rapidly growing melanomas in the skin and had to be sacrificed by the 12th week of life because melanomas exceeded 10 mm in diameter. At this time an average of ∼48 cutaneous melanomas were counted (Figure 1, B–D). In the cohort of HGF/SF × CDK4R24C/wt mice 15 of 15 also developed progressively growing melanomas. These melanomas grew with a slight delay and mice had to be sacrificed between the 14th and 16th weeks of life with an average of 32 melanomas in the skin (Figure 1, B–D). Carcinogen treatment also promoted development of melanomas in 18 of 18 HGF/SF × CDK4w/w mice. However, only an average of 12 melanomas developed that slowly grew until the age of 30 weeks. The differences in the number of cutaneous melanomas reached statistical significance. Ten of the eighteen HGF/SF × CDK4w/w mice also developed large subcutaneous cystic tumors filled with serous liquid, which forced us to sacrifice the mice. Of note, 18 of the 18 HGF/SF × CDK4w/w mice displayed on average three papillomas in the skin whereas only 3 of 16 HGF/SF × CDK4R24CR24C and 3 of 15 HGF/SF × CDK4R24C/wt mice showed a single papilloma of the skin. Papillomas appeared early after carcinogenic treatment excluding the possibility that the differences were simply because of the longer lifespan of HGF × CDK4w/w mice.
Figure 1-6934.
Mutant CDK4 promotes rapid growth of carcinogen-induced melanoma in C57BL/6 mice overexpressing HGF/SF. A: Cohorts of C57BL/6 mice overexpressing HGF/SF in combination with wild-type CDK4, heterozygous mutant CDK4, or homozygous mutant CDK4 were treated with 200 μg of DMBA at day 4 after birth followed by twice weekly application of 5 μg of TPA from weeks 3 to 8. B: Representative picture of three HGF/SF mice homozygous for mutant CDK4 at 12 weeks of age. These mice had to be sacrificed because of progressive and disseminated growth of melanoma in the skin and systemic signs of illness. C: Average number of cutaneous melanocytic neoplasms developing in each of the different cohorts of mice. Scoring was performed on a weekly basis and grouped by size as indicated. D: Kaplan-Meier graph indicating the survival of carcinogen-treated mice in the different cohorts. Routinely, mice were sacrificed when melanomas grew progressively and the tumor diameter exceeded 10 mm.
Histopathology of Carcinogen-Induced Melanoma in the Skin
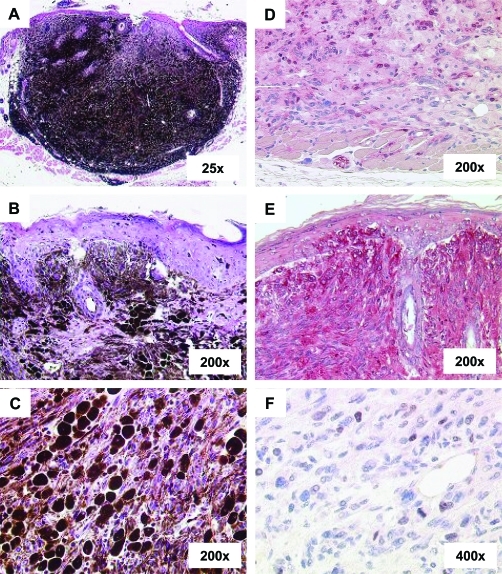
Extensive histological analyses were performed for melanomas in the skin. All HGF/SF-overexpressing mice showed nodular melanomas that involved the epidermo-dermal junction and grew vertically in the underlying dermis. Vertical tumor thickness was on average largest in HGF/SF × CDK4R24C/R24C mice that were harvested at the early age of 12 weeks. A representative histology at low-power magnification is depicted in Figure 2A. At higher magnification, heavily pigmented melanoma cells could be found at the epidermo-dermal junction with considerable intraepidermal spread (Figure 2B). Tumors were mainly composed of two types of melanoma cells. Epitheloid melanocytes were arranged in files and nests and were separated by thick bundles of much less pigmented spindle-shaped melanocytes (Figure 2C). Immunohistochemical staining for S100 and the melanosomal protein gp75/TRP1 confirmed the melanocytic origin of tumor cells (Figure 2, D and E). Proliferative activity could only be observed in the cohort of HGF/SF × CDK4R24C/R24C mice, in which occasional mitoses and a low percentage of Ki67 could be found in the spindle-shaped melanoma cells near the invasion front (Figure 2E). Ki67 staining was not detectable in HGF/SF × CDK4w/w mice. These results suggest that mutant CDK4 supports the proliferative activity of melanoma cells in HGF/SF mice, which histopathologically resem-ble the human disease because they involve the epi-dermis and show vertical invasive growth.
Figure 2-6934.
Carcinogen-induced melanoma in the skin of HGF/SF × CDK4R24C mice mimics the histopathology in melanoma patients. A: A representative H&E-stained section of skin tumor from a HGF × CDK4R24C/R24C mouse shows an overview of a typical nodular melanoma. B: Involvement of the dermo-epidermal junction and intraepithelial spread of melanoma cells. C: Heavily pigmented epitheloid melanoma cells arranged in bands and nests between lightly pigmented spindle-shaped melanoma cells. D: Immunohistochemical staining for S100. E: Immunohistochemical staining for the melanosomal protein TRP1. F: Immunohistochemical staining for Ki67 correlating with occasional mitoses at the invasion front. Original magnifications: ×25 (A); ×200 (B–E); ×400 (F).
Melanoma Metastases in the Draining Lymph Nodes and Lungs
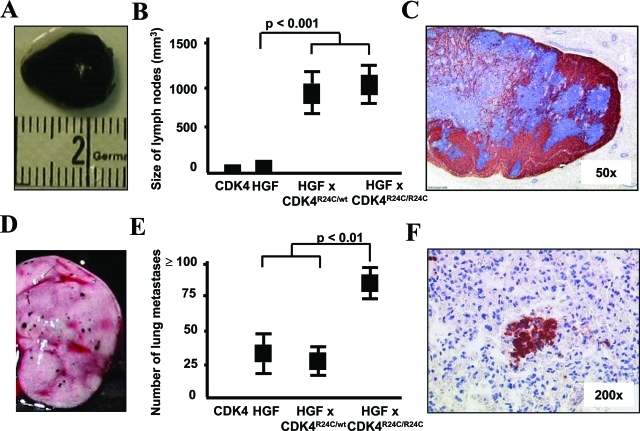
At the time of sacrifice, the inguinal and axillary draining lymph nodes could be easily located and excised in all cohorts of HGF/SF mice because of their heavy pigmentation. Significant lymph node enlargement because of growth of melanoma cells was observed in HGF/SF × CDK4R24C/wt as well as in HGF/SF × CDK4R24C/R24C mice (Figure 3, A and B). Melanoma cells invaded lymph nodes from the periphery similar to the histology of sentinel lymph node biopsies from melanoma patients (Figure 3C). Locoregional metastatic growth of melanoma cells in the lymph nodes was confirmed by histology in all cohorts of HGF/SF mice. Melanoma cells primarily displayed a spindle-shaped morphology with little or no melanin but more strongly pigmented melanoma cells with epitheloid morphology could also be detected. With increasing lymph node size, spindle-shaped melanoma cells disrupted lymph node architecture and almost completely displaced lymphoid cells. Immunohistochemical staining for TRP1 again confirmed the melanocytic origin of the melanoma cells. To assess distal metastatic spread, visceral organs and the brain were investigated in each individual mouse at the time of sacrifice. A representative picture of lung metastases is given in Figure 3D. The number of lung metastases was highest in HGF/SF × CDK4R24C/R24C mice (Figure 3E). Histopathological analyses showed numerous melanoma micrometastases in lung tissue in all HGF/SF mice. A representative microscopic picture of HGF/SF × CDK4R24C/R24C mice with immunohistochemical staining for TRP is shown in Figure 3F. Other internal organs such as liver, spleen, and kidneys as well as the brain did not reveal further metastases or tumors of other histological origin in carcinogen-treated HGF/SF × CDK4R24C/R24C or in HGF/SF × CDK4R24C/R24C mice. HGF/SF × CDK4w/w mice that lived ∼7 months showed apart from a few papillomas multiple large subcutaneous tumors filled with serous liquid but no other macroscopically or microscopically apparent tumors in the organs investigated. Taken together, these results indicate that mutant CDK4 promoted growth of metastatic melanoma cells in the regional lymph nodes in carcinogen-treated HGF/SF mice.
Figure 3-6934.
Mutant CDK4 promotes growth of metastatic melanoma in the draining lymph nodes and lungs of HGF/SF-overexpressing mice. A: Representative macroscopic picture of a heavily pigmented and enlarged draining lymph node from a HGF × CDK4R24C/R24C mouse. B: Average size of draining lymph nodes represented as tumor volume in mm3 (±SEM) in the indicated cohorts of mice. C: Immunohistological picture of a representative lymph node stained for TRP1 showing metastatic growth of melanoma at low magnification. D: Representative macroscopic picture of melanoma metastases on the surface of the lung of a HGF × CDK4R24C/R24C mouse. E: Average number of melanoma metastases (±SEM) on the lung surface in the indicated cohorts of mice. F: Immunohistological picture of a representative lung stained for TRP1 showing metastatic growth of melanoma. Original magnifications: ×50 (C); ×200 (F).
Spontaneous Development of Melanoma
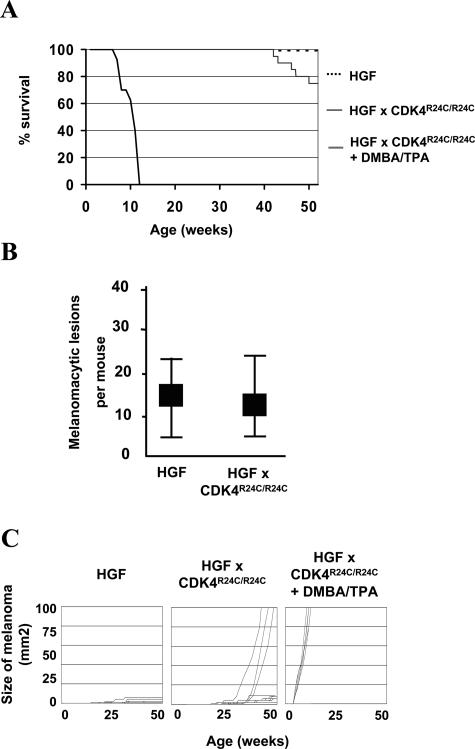
The incidence of spontaneous melanoma was followed in untreated HGF/SF × CDK4R24C/R24C and HGF/SF × CDK4w/w C57BL/6 mice. Single, rapidly growing melanomas appeared in the skin of individual HGF/SF × CDK4R24C/R24C mice starting at an age of ∼30 to 40 weeks. Approximately 20% of untreated HGF/SF × CDK4R24C/R24C mice had to be sacrificed by the age of 1 year with primary melanomas exceeding 10 mm in diameter (Figure 4A). HGF/SF × CDK4R24C/R24C mice with large spontaneous primary melanomas arising in the skin also showed locoregional metastases in the adjacent draining lymph nodes as well as distal micrometastases in the lungs (data not shown). Untreated HGF/SF × CDK4R24C/R24C and HGF/SF × CDK4w/w mice showed similar numbers of melanocytic nevi in the skin (Figure 4B). However, untreated HGF/SF × CDK4w/w mice were all alive and healthy at 1 year of age. Untreated CDK4R24C/R24C mice did not show any melanocytic tumors in the skin and also were healthy at 1 year of age (data not shown). Tumor growth kinetics of individual spontaneous melanomas was similar to the largest carcinogen-induced melanomas in the skin of HGF/SF × CDK4R24C/R24C mice (Figure 4C). These observations indicate that mutant CDK4 critically determines tumor growth and latency but does not significantly affect the incidence of melanocytic neoplasms in HGF/SF mice.
Figure 4-6934.
Mutant CDK4 enhances growth of spontaneous melanoma in the skin of HGF/SF-overexpressing mice. A: Kaplan-Meier graph indicating the survival of untreated mice in the different cohorts. Routinely, mice were sacrificed when melanomas grew progressively and the tumor diameter exceeded 10 mm. B: Average number of melanocytic nevi in the skin of the different cohorts at an age of 30 weeks (±SEM). C: Size of the largest primary cutaneous melanoma in individual mice throughout time expressed as tumor area in mm2.
Discussion
In our experiments we investigated whether impaired cell-cycle control attributable to mutant CDK4 would synergize with deregulated receptor tyrosine kinase signaling because of HGF/SF overexpression in melanomagenesis. Based on our experience with C57BL/6 CDK4R24C mice we initially treated newborn litters of HGF/SF × CDK4R24C mice in pilot experiments either with DMBA followed by TPA or with UVB. It quickly became clear that carcinogen treatment had a dramatic effect in HGF × CDK4R24C mice because large numbers of rapidly growing melanomas developed in the skin within 10 weeks. In extended analyses we could confirm that carcinogen treatment significantly promoted growth of primary melanoma in the skin of HGF/SF-overexpressing C57BL/6 mice carrying the CDK4R24C mutation. Carcinogen-induced primary melanomas in the skin histopathologically involved the epidermis and the dermo-epidermal junction, showed vertical growth, and locally invaded the underlying muscle, blood, and lymphatic vessels. This histological picture was reminiscent of the human disease. Carcinogen-induced primary cutaneous melanomas spontaneously metastasized to the draining lymph nodes and the lungs. Most importantly, we did not observe other tumor types in HGF/SF × CDK4w/w mice.
Development of melanoma after DMBA treatment was reported as a very rare event in C57BL/6 mice by Berkelhammer and Oxenhandler.24 Melanomagenesis after carcinogen treatment was significantly enhanced in TPras-transgenic C57BL/6 mice that express mutant H-Ras under the control of the tyrosinase promotor. These mice also develop cutaneous melanoma with the ability to metastasize in lymph nodes and lungs.25 Carcinogen-induced primary cutaneous melanomas were also observed in CDK4R24C knockin or p16/INK4a-deficient mice. However, melanomas did not metastasize in these experimental models.7–9,22 Neither melanomas from carcinogen-induced TPras-transgenic mice nor from CDK4-mutant mice showed mutations in Ras genes,9,25 which have previously been reported in DMBA-treated skin and are able to promote the induction of papillomas and squamous cell carcinomas.26 As reported by many investigators before, we also only occasionally observed skin papillomas in carcinogen-treated C57BL/6 mice that are notoriously resistant to carcinogenesis protocols. Clearly, further studies are necessary to elucidate how DMBA and TPA promote melanomagenesis in our model.
Both HGF/SF × CDK4w/w and HGF/SF × CDK4R24C/R24C C57BL/6 mice spontaneously develop melanocytic nevi throughout time. However, only HGF/SF × CDK4R24C/R24C mice showed single, rapidly growing melanomas in the skin starting at approximately weeks 30 to 40 of age that forced us to sacrifice these mice. Thus, mutated CDK4 also enhanced growth and latency but not incidence of spontaneous melanoma in the skin of HGF/SF-overexpressing mice. It is known that HGF supports cellular proliferation via the Ras, Raf, ERK, and MAPK pathway as well as cellular survival via the PI3 kinase and Akt pathway.4,27 The effect of HGF/SF is limited by the induction of p16 leading to cellular senescence.28 Mutant CDK4, which is insensitive to control by p16/INK4A, is able to promote cell-cycle progression and allows cells to escape senescence.29 This may explain the tumor growth kinetics observed in HGF/SF × CDK4R24C/R24C mice compared to HGF/SF × CDK4R24C/wt and HGF/SF × CDK4w/w mice, which suggests a gene dosage effect. Recent results in humans demonstrating amplifications of the wild-type CDK4 gene in primary melanomas also support the idea that CDK4 function promotes melanomagenesis in a dose-dependent manner.6 Furthermore, our results are consistent with observations in mice expressing transgenic mutant Ras or HGF/SF on the INK4a/ARF-deficient background.30–33 However, INK4a/ARF-deficient mice also develop tumors of other histologies because both the Rb and the p53 pathway are disrupted.31,34 Subsequent studies will have to unveil why HGF/SF × CDK4R24C/R24C mice treated with DMBA are uniquely predisposed to melanoma.
Melanoma cells in all cohorts of HGF/SF × CDK4w/w mice show a mixture of epitheloid and spindle-shaped morphology. The epitheloid cells appear to be more fully differentiated because they express TRP1 much stronger and show a considerably higher melanin content. The spindle-shaped cells appeared to be less differentiated and exhibited significantly higher proliferative activity with mitoses and Ki67 expression in HGF/SF × CDK4R24C/R24C mice. These spindle-shaped melanoma cells are absent in CDK4R24C/R24C C57BL/6 mice (data not shown). We hypothesize that spindle-shaped melanoma cells, which are considerably more pronounced in enlarged lymph nodes, might be involved in invasion and metastasis. HGF/SF is known to support motility and invasion of melanocytes during embryonal development.35,36 Furthermore, HGF/SF has been shown to down-regulate E-cadherin and promote the nuclear localization of β-catenin in melanoma cells both of which promote motility and invasion.37 Preliminary results of immunohistochemical investigations indeed suggest that the spindle-shaped melanoma cells in HGF × CDK4R24C/R24C mice do not express E-cadherin and show loss of membranous localization of β-catenin. Our model provides an excellent opportunity to further study the interactions between growth factors and adhesion molecules and their role for invasion and metastasis in the pathogenesis of melanoma in greater detail.
Taken together, our experiments show that deregulated receptor tyrosine kinase signaling because of overexpression of HGF/SF and impaired cell-cycle control because of mutant CDK4 synergistically promote the rapid development of widespread carcinogen-induced melanomas in the skin of C57BL/6 mice, which spontaneously metastasize in lymph nodes and lung. This new experimental mouse model can now be exploited to further study the biology of melanoma. With a few modifications it may be very attractive for the evaluation of new treatment modalities because melanomas appear with high incidence and comparatively short latency.
Acknowledgments
We thank Petra Speuser and Stefanie Büchs for their excellent technical help.
Footnotes
Address reprint requests to Thomas Tüting, M.D., Laboratory of Experimental Dermatology, Department of Dermatology, University of Bonn, Sigmund Freud Str. 25, 53105 Bonn, Germany. E-mail: thomas.tueting@ukb.uni-bonn.de.
Supported by the Deutsche Forschungsgemeinschaft (grant DFG Tu90/3-2 + 3 to T.T.).
References
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- Chudnovsky Y, Khavari PA, Adams AE. Melanoma genetics and the development of rational therapeutics. J Clin Invest. 2005;115:813–824. doi: 10.1172/JCI24808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L. The genetics of malignant melanoma: lessons from mouse and man. Nat Rev Cancer. 2003;3:559–570. doi: 10.1038/nrc1145. [DOI] [PubMed] [Google Scholar]
- Chin L, Merlino G, DePinho RA. Malignant melanoma: modern black plague and genetic black box. Genes Dev. 1998;12:3467–3481. doi: 10.1101/gad.12.22.3467. [DOI] [PubMed] [Google Scholar]
- Walker GJ, Hayward NK. Pathways to melanoma development: lessons from the mouse. J Invest Dermatol. 2002;119:783–792. doi: 10.1046/j.1523-1747.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- Krimpenfort P, Quon KC, Mooi WJ, Loonstra A, Berns A. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature. 2001;413:83–86. doi: 10.1038/35092584. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, Wu EA, Horner JW, DePinho RA. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- Sotillo R, Garcia JF, Ortega S, Martin J, Dubus P, Barbacid M, Malumbres M. Invasive melanoma in Cdk4-targeted mice. Proc Natl Acad Sci USA. 2001;98:13312–13317. doi: 10.1073/pnas.241338598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann-Hieb E, De PE, Hankeln T, Meyer zum Buschenfelde KH, Beach D. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995;269:1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- Zuo L, Weger J, Yang Q, Goldstein AM, Tucker MA, Walker GJ, Hayward N, Dracopoli NC. Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nat Genet. 1996;12:97–99. doi: 10.1038/ng0196-97. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Nambiar S, Mirmohammadsadegh A, Doroudi R, Gustrau A, Marini A, Roeder G, Ruzicka T, Hengge UR. Signaling networks in cutaneous melanoma metastasis identified by complementary DNA microarrays. Arch Dermatol. 2005;141:165–173. doi: 10.1001/archderm.141.2.165. [DOI] [PubMed] [Google Scholar]
- Natali PG, Nicotra MR, Di Renzo MF, Prat M, Bigotti A, Cavaliere R, Comoglio PM. Expression of the c-Met/HGF receptor in human melanocytic neoplasms: demonstration of the relationship to malignant melanoma tumour progression. Br J Cancer. 1993;68:746–750. doi: 10.1038/bjc.1993.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL, Van BP, Elder DE, Herlyn M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- Powell MB, Hyman P, Bell OD, Balmain A, Brown K, Alberts D, Bowden GT. Hyperpigmentation and melanocytic hyperplasia in transgenic mice expressing the human T24 Ha-ras gene regulated by a mouse tyrosinase promoter. Mol Carcinog. 1995;12:82–90. doi: 10.1002/mc.2940120205. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Takayama H, Sharp R, Celli G, LaRochelle WJ, Bottaro DP, Ellmore N, Vieira W, Owens JW, Anver M, Merlino G. c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Res. 1998;58:5157–5167. [PubMed] [Google Scholar]
- Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL, De Fabo EC, Merlino G. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- Steitz J, Bruck J, Gambotto A, Knop J, Tuting T. Genetic immunization with a melanocytic self-antigen linked to foreign helper sequences breaks tolerance and induces autoimmunity and tumor immunity. Gene Ther. 2002;9:208–213. doi: 10.1038/sj.gt.3301634. [DOI] [PubMed] [Google Scholar]
- Steitz J, Bruck J, Lenz J, Knop J, Tuting T. Depletion of CD25(+) CD4(+) T cells and treatment with tyrosinase-related protein 2-transduced dendritic cells enhance the interferon alpha-induced, CD8(+) T-cell-dependent immune defense of B16 melanoma. Cancer Res. 2001;61:8643–8646. [PubMed] [Google Scholar]
- Steitz J, Bruck J, Steinbrink K, Enk A, Knop J, Tuting T. Genetic immunization of mice with human tyrosinase-related protein 2: implications for the immunotherapy of melanoma. Int J Cancer. 2000;86:89–94. doi: 10.1002/(sici)1097-0215(20000401)86:1<89::aid-ijc14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Steitz J, Buchs S, Tormo D, Ferrer A, Wenzel J, Huber C, Wolfel T, Barbacid M, Malumbres M, Tuting T. Evaluation of genetic melanoma vaccines in cdk4-mutant mice provides evidence for immunological tolerance against autochthonous melanomas in the skin. Int J Cancer. 2006;118:373–380. doi: 10.1002/ijc.21349. [DOI] [PubMed] [Google Scholar]
- Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- Berkelhammer J, Oxenhandler RW. Evaluation of premalignant and malignant lesions during the induction of mouse melanomas. Cancer Res. 1987;47:1251–1254. [PubMed] [Google Scholar]
- Broome PM, Gause PR, Hyman P, Gregus J, Lluria-Prevatt M, Nagle R, Bowden GT. Induction of melanoma in TPras transgenic mice. Carcinogenesis. 1999;20:1747–1753. doi: 10.1093/carcin/20.9.1747. [DOI] [PubMed] [Google Scholar]
- Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Han J, Tsukada Y, Hara E, Kitamura N, Tanaka T. Hepatocyte growth factor induces redistribution of p21(CIP1) and p27(KIP1) through ERK-dependent p16(INK4a) up-regulation, leading to cell cycle arrest at G1 in HepG2 hepatoma cells. J Biol Chem. 2005;280:31548–31556. doi: 10.1074/jbc.M503431200. [DOI] [PubMed] [Google Scholar]
- Rane SG, Cosenza SC, Mettus RV, Reddy EP. Germ line transmission of the Cdk4(R24C) mutation facilitates tumorigenesis and escape from cellular senescence. Mol Cell Biol. 2002;22:644–656. doi: 10.1128/MCB.22.2.644-656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann J, Frutschi M, Kaloulis K, McKee T, Trumpp A, Beermann F. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. 2005;65:4005–4011. doi: 10.1158/0008-5472.CAN-04-2970. [DOI] [PubMed] [Google Scholar]
- Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, Horner JW, DePinho RA. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997;11:2822–2834. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O’Hagan R, Pantginis J, Zhou H, Horner JW, Cordon-Cardo C, Yancopoulos GD, DePinho RA. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- Recio JA, Noonan FP, Takayama H, Anver MR, Duray P, Rush WL, Lindner G, De Fabo EC, DePinho RA, Merlino G. Ink4a/arf deficiency promotes ultraviolet radiation-induced melanomagenesis. Cancer Res. 2002;62:6724–6730. [PubMed] [Google Scholar]
- Sharp R, Recio JA, Jhappan C, Otsuka T, Liu S, Yu Y, Liu W, Anver M, Navid F, Helman LJ, DePinho RA, Merlino G. Synergism between INK4a/ARF inactivation and aberrant HGF/SF signaling in rhabdomyosarcomagenesis. Nat Med. 2002;8:1276–1280. doi: 10.1038/nm787. [DOI] [PubMed] [Google Scholar]
- Comoglio PM, Trusolino L. Invasive growth: from development to metastasis. J Clin Invest. 2002;109:857–862. doi: 10.1172/JCI15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile A, Comoglio PM. Invasive growth: a genetic program. Int J Dev Biol. 2004;48:451–456. doi: 10.1387/ijdb.041799ag. [DOI] [PubMed] [Google Scholar]
- Li G, Schaider H, Satyamoorthy K, Hanakawa Y, Hashimoto K, Herlyn M. Downregulation of E-cadherin and Desmoglein 1 by autocrine hepatocyte growth factor during melanoma development. Oncogene. 2001;20:8125–8135. doi: 10.1038/sj.onc.1205034. [DOI] [PubMed] [Google Scholar]