Abstract
Signal transducers and activators of transcription (STAT)-3 is an oncogenic protein that is constitutively activated in many human cancers, including 30 to 60% of primary breast cancer. The biological significance of STAT3 activation in breast cancer is not fully understood. We have previously shown that STAT3 up-regulates tissue inhibitors of metalloproteinase (TIMP)-1, a cytokine known to block metalloproteinases and decrease invasiveness in certain cancer cell types. We hypothesize that STAT3 activation may modulate tumor invasiveness of breast cancer by regulating TIMP1 expression. Using MCF-7 cells transfected with tetracycline-off STAT3C (constitutively active STAT3), we generated an in vitro system in which STAT3C levels can be tightly controlled in breast cancer cells. Increasing tetracycline levels gradually decreased STAT3C and TIMP1 in a dose-dependent manner, and down-regulation of these proteins led to a reciprocal decrease in invasiveness of these cells in Matrigel. Addition of a neutralizing anti-TIMP1 antibody increased invasiveness in the same experimental system. Using immunohistochemistry and 142 primary breast tumors, we found a significant association between the expression of the phosphorylated/active form of STAT3 (pSTAT3) and that of TIMP1. Importantly, STAT3 activation correlated significantly with a lower frequency of vascular and lymphatic invasion (P = 0.015 and P = 0.0002, respectively). Our data support the concept that STAT3 activation significantly modulates the biological and clinical behavior of breast cancer.
Signal transducers and activators of transcription (STATs) are a family of latent transcription factors that become activated in response to cytokines and growth factors.1,2 Ligand-dependent activation of STATs, which is a transient process lasting from minutes to hours, plays important roles in embryogenesis, cell proliferation, and differentiation.3,4 Constitutive activation of STAT3 has been shown to contribute to deregulation of cell growth and malignant cellular transformation in many types of human cancers, and there is mounting evidence to support that STAT3 is an oncogene.1,4–7 In various cell types many STAT3 downstream target genes have been identified, including cell-cycle facilitators cyclin D1 and c-myc, as well as anti-apoptotic proteins such as survivin, Bcl-xL, Mcl-1, and Bcl-2.8,9 STAT3 has been reported to be constitutively activated in 30 to 60% of primary breast cancer, as shown by the presence of STAT3 tyrosine phosphorylation and DNA binding.10–12 In one study, STAT3 activation was associated with a better clinical outcome.13 With this background, it is likely that constitutive activation of STAT3 is important in the pathogenesis of breast cancer. Nevertheless, the exact biological role of STAT3 in breast cancer is not fully understood.
Tissue inhibitors of metalloproteinases (TIMPs) constitute at least four distinct members, TIMP1, -2, -3, and -4, that have been shown to inhibit tumor invasion and metastasis by inhibiting matrix metalloproteinases (MMPs) and promoting cell growth.14 TIMP1, a 28-kd sialoglycoprotein,15 is regulated by a number of cytokines and growth factors such as interleukin-6,16 tumor necrosis factor-α, and transforming growth factor-β.17 TIMP1 is a potent inhibitor of MMP2 and MMP9 and has been shown to prolong survival in animals xenografted with tumors.18 In line with these findings, it has been shown by immunohistochemical analysis that TIMP1 overexpression in breast cancer cells may be an indicator of favorable prognosis in breast cancer patients.19 On the other hand, TIMP1 has been shown to possess anti-apoptotic properties in some cancer cell types, including breast cancer.5,20,21 A number of previous studies have also shown that high serum levels of TIMP1 are associated with poor prognosis in patients with gastric carcinoma22 and colorectal carcinoma.23 Thus, the biological roles and clinical significance of TIMP1 appear to be cancer cell-type-specific.
Recent studies have suggested that STAT3 protein may play a role in regulating TIMP1 expression, as the promoter of the TIMP1 gene contains STAT3 binding sites.16 We previously showed that inhibition of STAT3 signaling using a dominant-negative construct effectively down-regulates TIMP1 expression in ALK-positive anaplastic large cell lymphoma cell lines, and expression of TIMP1 in this type of lymphoma in fact correlates with the STAT3 activation status.24 With this background, we hypothesize that STAT3 activation may modulate tumor invasiveness of breast cancer by regulating TIMP1 expression. In this study, we investigated the link between TIMP1 expression and STAT3 activation in breast cancer cells and assessed the biological and clinical significance of STAT3 activation and TIMP1 expression in breast cancer. We first created an in vitro model that involved breast cancer cell lines transfected with STAT3C (constitutively active STAT3), expression of which can be regulated by the addition of tetracycline (ie, tet-off system). Using this model, we established the link between STAT3 activation status and TIMP1 expression in breast cancer cells. We then investigated the effects of STAT3 activation and TIMP1 expression on invasiveness using Matrigel invasion assays. To validate these in vitro data, we examined STAT3 activation and TIMP1 expression in 142 primary breast cancer tissues and correlated these markers with the presence or absence of lymphatic and vascular invasion.
Materials and Methods
Construction of tet-off STAT3C
To create tet-off STAT3C, we first synthesized TRE-STAT3C, which was generated by digesting the 2.8-kb fragment of STAT3C from the STAT3C plasmid using BamHI. STAT3C, a FLAG-tagged construct, is a generous gift from Dr. J. Bromberg (Laboratory of Molecular Cell Biology, The Rockefeller University, New York, NY), and its properties have been well characterized and described.4 STAT3C has been shown to be oncogenic. Briefly, STAT3C was generated by substituting the tyrosine705 residue with cysteine, such that the STAT3C molecules spontaneously form dimers that migrate to the nucleus and modulate gene transcription. The STAT3C fragment was then ligated with the pTREzhyg vector (Clontech, Mountain View, CA), which was then transformed into Escherichia coli and selected on ampicillin resistant plates. The plasmids were isolated with a kit from Qiagen Science (Mississauga, ON, Canada). The generated TRE-STAT3C plasmid was analyzed by DNA sequencing to confirm the orientation and integrity of the STAT3C insert. The TRE-STAT3C plasmid was amplified using a kit from Qiagen.
Cell Lines and Culture
Breast cancer cell lines MCF-7 and MDA-MB-436 (American Type Culture Collection, Manassas, VA) were grown at 37°C in 5% CO2. MCF-7 was maintained in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO), and MDA-MB-436 was maintained in RPMI 1640 (Sigma-Aldrich). Both types of culture media were enriched with 10% fetal bovine serum (Life Technologies, Inc., Grand Island, NY) and antibiotics (10,000 U/ml penicillin G, 10,000 μg/ml streptomycin; Life Technologies, Inc.). MCF-7 and MDA-MB-436 permanently transfected with the tetracycline-controlled transactivator (tTA) plasmid were selected and maintained by the addition of geneticin (800 and 100 μg/ml, respectively; Life Technologies, Inc.) to the culture media. Cell clones permanently transfected with tTA were subsequently transfected with TRE-STAT3C to generate the tet-off STAT3C system.
Transfection
All transfections were performed in six-well plates using Lipofectamine (Invitrogen Life Technologies, Carlsbad, CA). For all of the in vitro experiments, breast cancer cell line clones permanently transfected with tTA were transfected with 1 μg of TRE-STAT3C using Lipofectamine, as per the manufacturer’s suggested protocol. To regulate the expression of STAT3C in these cells, various concentrations of tetracycline (Invitrogen) were added to the tissue culture.
Western Blot Analysis and Antibodies
Western blot analysis was performed using standard techniques. Briefly, the cells were lysed in lysis buffer [20 mmol/L Tris-HCl, pH 7.5, 5.0 mmol/L ethylenediaminetetraacetic acid, 40.0 μg/ml leupeptin, 1 μmol/L pepstatin, 1 mmol/L 4-(2-aminoethyl)-benzenesulfonyl fluoride], and centrifuged at 14,000 × g for 10 minute at 4°C. The supernatant was removed and 50 to 100 μg of protein were run on an sodium dodecyl sulfate-polyacrylamide gel. After the proteins were transferred to nitrocellulose membranes, the membranes were blocked with 2% milk in Tris-buffered saline buffer (20 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl) and then incubated with primary antibodies for 2 hours at room temperature followed by a 1-hour incubation with horseradish peroxidase-conjugated secondary antibody (Jackson Immunoresearch Laboratories, Inc., West Grove, PA). The membranes were washed in phosphate-buffered saline (PBS) with 0.05% Tween 20 for 30 minutes between steps. Proteins were detected using the enhanced chemiluminescence detection kit (Amersham Life Sciences, Arlington Heights, IL). Antibodies used were anti-FLAG (1:3000, Sigma-Aldrich), anti-STAT3 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), anti-pSTAT3 (1:500, Santa Cruz), anti-TIMP1 (1:500, Santa Cruz), anti-cyclin D1 (1:1000, Santa Cruz), anti-Bcl-2 (1:1000, Santa Cruz), anti-Bcl-xL (1:500, Santa Cruz), and anti-actin (1:3000, Sigma-Aldrich).
Immunofluorescence Staining and Confocal Microscopy
Immunofluorescence was performed using standard techniques. Briefly, cells grown on coverslip in a six-well plate were fixed with 4% paraformaldehyde in PBS. Cells were rinsed with 1× PBS, permeabilized with PBS and 0.5% Triton X-100 for 5 minutes, and rinsed twice with 1× PBS. Cells were incubated with 25 μl of anti-STAT3 antibody for 30 minutes, followed by rinsing once with 1× PBS-Triton (0.1%) and twice with 1× PBS. After incubation with 25 μl of Alexa 499 goat anti-mouse secondary antibody for 30 minutes and repeated rinsing, mounting media containing 4,6-diamidino-2-phenylindole (Sigma-Aldrich) was added to the slides. Cells were visualized with a Zeiss LSM 510 confocal microscope (Oberkochen, Germany).
Matrigel Invasion Assay
Before use, Matrigel chambers (BD Biosciences, Bedford, MA) were kept at room temperature for 1 hour. Five hundred μl of PBS was added to the inserts and incubated at 37°C in 5% CO2 for 2 hours for equilibration. During incubation, transfected cells with various concentrations of tetracycline were trypsinized, washed with sterile PBS, resuspended to 25,000 cells in 500 μl of Dulbecco’s modified Eagle’s medium without phenol red. After 2 hours of incubation, PBS was aspirated from the inserts and replaced with the 500-μl cell suspension. To serve as chemoattractants, 750 μl of Dulbecco’s modified Eagle’s medium containing 5% fetal bovine serum was added to the bottom of the wells. The plate was incubated for 24 hours at 37°C in 5% CO2. After incubation, the cells on the upper surface of the insert were removed by gentle scraping with sterile cotton swab. Invasive cells that migrated to the lower side of the insert were fixed with methanol and stained with Trypan blue solution (Sigma). Five random 40× fields were counted per sample and each sample was performed in triplicates. The Matrigel invasion assay was also performed using transfected cells treated with TIMP1 neutralizing antibody (Acris Antibodies, Hiddenhausen, Germany).
Enzyme-Linked Immunosorbent Assay (ELISA) for TIMP1
MCF-7 and MDA-MB-436 cells transfected with tTA-TRE-STAT3C were treated with various concentrations of tetracycline and incubated at 37°C in 5% CO2. After 24-, 48-, and 72-hour incubation periods, aliquots of medium were extracted and spun at 14,000 rpm, and supernatant was collected and stored at −80°C. One hundred microliters of sample was placed in microtiter wells (Oncogene Research Products, Cambridge, MA) and covered and incubated for 30 minutes at 4°C. Each well was washed four times with 1× wash buffer (Oncogene Research Products), and 100 μl of TIMP1 conjugate was added to each well, followed by incubation for 30 minutes at 4°C. A color reagent was then added to each well and incubated in the dark at room temperature for 30 minutes. The color reaction was stopped by 2.5 N sulfuric acid (Oncogene Research Products), and a colorimetric plate reader measured each absorbance at 450/595 nm.
Immunohistochemistry
Immunohistochemical staining was performed using standard techniques. Briefly, formalin-fixed, paraffin-embedded tissue sections of 4 μm thickness were deparaffinized and hydrated. Heat-induced epitope retrieval was performed using Tris buffer (pH 9.9; DAKO, Mississauga, ON, Canada) and a rapid microwave histoprocessor (RHS; Milestone, Bergamo, Italy). After incubation at 100°C for 10 minutes, slides were washed in running tap water for 5 minutes, followed by a wash with PBS (pH 7.2) for 5 minutes. Tissue sections were then incubated with anti-pSTAT3 antibody (1:60) or anti-TIMP1 antibody (1:200; Santa Cruz) overnight in a humidified chamber at 4°C. After three washes with PBS, tissue sections were incubated with anti-rabbit IgG and peroxidase (EnVision, DAKO) for 30 minutes at room temperature. The tissue sections were incubated with 3,3′-diaminobenzidine/H2O2 (DAKO) for color development, using hematoxylin as a counterstain. To optimize the immunohistochemical staining, normal breast tissues were used as positive controls, and benign tonsils were used as negative controls. Immunohistochemical staining was examined by two pathologists (R.L. and B.C.) who were blinded to the results of the other markers. For pSTAT3, the presence of any areas with >10% tumor cells showing definitive nuclear staining were scored positive. Endothelial cells, which are often positive for pSTAT3, served as internal positive controls whereas reactive lymphoid cells in benign tonsils served as negative controls. As for TIMP1, the presence of any areas with definitive cytoplasmic staining in >10% tumor cells was considered positive. Reactive germinal centers served as positive controls, and the mantle zone B cells served as negative controls. Discrepancies of the immunostaining results between the two observers were reconciled by using a multiheaded microscope.
Patient and Tumor Specimens
Archival, formalin-fixed, paraffin-embedded tissues from the 142 cases of primary breast cancer reviewed at the Cross Cancer Institute evaluated between 1996 and 2001 were randomly chosen for this study. This study has been previously reviewed and approved by the institutional ethics committee. The initial diagnoses of all cases were made at the community hospitals, which were subsequently reviewed by a pathologist at the Cross Cancer Institute according to a standardized institutional guideline. The histological grades of these 142 cases were as follows: grade 1 (20 cases), grade 2 (42 cases), and grade 3 (80 cases). The clinical stages of these patients are summarized as follows: stage IIA (75 cases), stage IIB (45 cases), stage IIIA (two cases), stage IIIB (18 cases), and stage IV (two cases). The median age of patients was 61 years (range, 35 to 90 years).
Statistical Analysis
The association between STAT3, TIMP1, and lymphatic/vascular invasion was evaluated by χ2 test. The survival data were analyzed using the log rank test.
Results
Characterization of MCF-7 Transfected with tTA-TRE-STAT3C
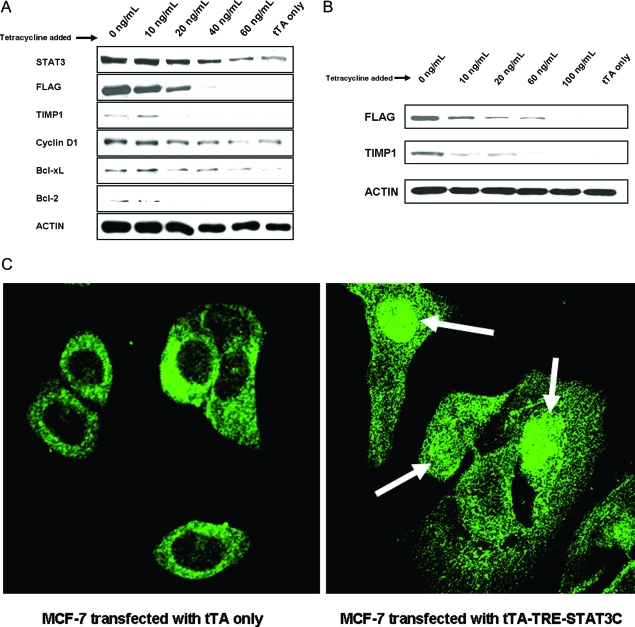
We first evaluated the in vitro model in which the expression of STAT3C can be regulated by addition of different tetracycline levels to the tissue culture. As described in Materials and Methods, MCF-7 cell clones permanently transfected with tTA were initially established. These cells were then transfected with TRE-STAT3C and treated with increasing dosages of tetracycline for 24 hours. Expression of the transgene (ie, STAT3C) was primarily assessed by evaluating the expression of the FLAG epitope using Western blots. As shown in Figure 1A, there was a gradual decrease in the expression of FLAG with addition of tetracycline in a dose-dependent manner. The FLAG epitope, which is associated with the exogenous but not the endogenous STAT3, became completely undetectable at 60 ng/ml. At 60 ng/ml of tetracycline, the total STAT3 band was decreased to a level similar to that of MCF-7 cells transfected with tTA alone. The same blot was probed using an anti-pSTAT3 antibody, and no signal was detectable, indicating that the endogenous STAT3 in MCF-7 cells was inactive.
Figure 1-6917.
A: Western blot analysis of STAT3, FLAG, TIMP1, cyclin D1, Bcl-2, and Bcl-xL expression in MCF-7 cells transfected with tTA-TRE-STAT3C and treated with increasing concentrations of tetracycline for 24 hours. There was a gradual decrease in STAT3 and FLAG with increasing concentrations of tetracycline, with FLAG being completely undetectable at 60 ng/ml. Down-regulation of cyclin D1, Bcl-2, Bcl-xL, and TIMP1 expression was identified in the same experiment. MCF-7 cells transfected with tTA alone served as negative controls. B: Western blot analysis of FLAG and TIMP1 expression in MDA-MB-436 cells transfected with tTA-TRE-STAT3C and treated with increasing concentrations of tetracycline for 24 hours. A gradual decrease in FLAG and TIMP1 was observed. MDA-MB-436 cells transfected with tTA alone served as negative controls. C: Immunofluorescence staining and confocal microscopy studies revealed that STAT3 was localized to the nucleus in MCF-7 cells transfected with tTA-TRE-STAT3C, as illustrated by the white arrows (right) whereas STAT3 was confined to the cytoplasm in cells transfected with tTA only (left).
The properties of STAT3C have been extensively characterized and published.4 To further confirm that STAT3C is biologically active, we performed Western blots to correlate the expression of a known STAT3 downstream target with various expression levels of STAT3C. As shown in Figure 1A, cyclin D1 was down-regulated with decreasing FLAG signals. Bcl-2 and Bcl-xL expression was also down-regulated. To further support that STAT3C represents a constitutively activated STAT3 species, we used immunofluorescence staining and confocal microscopy. As shown in Figure 1C, STAT3 was localized to the nucleus in MCF-7 cells transfected with tTA-TRE-STAT3C. In contrast, STAT3 was confined to the cytoplasm in MCF-7 cells transfected with tTA only.
STAT3 Transgene Correlated with TIMP1 Expression
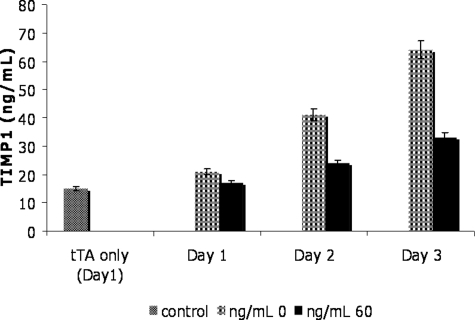
To establish the link between TIMP1 expression and STAT3 signaling in MCF-7 cells, we correlated STAT3C expression (as indicated by total STAT3 and FLAG expression) and TIMP1 expression in transfected MCF-7 cells using Western blots. As shown in Figure 1A, STAT3 and FLAG down-regulation correlated with a substantial decrease in TIMP1 expression as the tetracycline dosage was increased to 20 ng/ml. We also performed TIMP1 ELISA assay in MCF-7 to quantify the TIMP1 levels in the tissue culture medium. Figure 2 illustrates that TIMP1 levels in MCF-7 cells transfected with tTA-TRE-STAT3 increased in the tissue culture throughout 3 days, but the levels for each day were significantly lower in those cells treated with 60 ng/ml of tetracycline. The differences in the TIMP1 levels between the two conditions were not attributable to a difference in the total cell number in the cell culture because the cell counts in both conditions were not significantly different from day 1 to day 3. Furthermore, cell cycle analysis using DRAG5 and flow cytometry showed no significant change in the cell cycle status between these two conditions (data not shown). In addition, MCF-7 cells transfected with tTA alone showed a low levels of TIMP1 (15 ng/ml) at day 1, and the level was comparable to that found in MCF-7-transfected cells after 1 day treatment with 60 ng/ml of tetracycline. Similar results were found for days 2 and 3.
Figure 2-6917.
TIMP1 levels in supernatants evaluated by ELISA. TIMP1 levels were measured using an ELISA assay in MCF-7 cells transfected with tTA-TRE-STAT3C and treated with 60 ng/ml of tetracycline. Supernatants from the tissue culture were collected on days 1, 2, and 3. TIMP1 levels were significantly higher in cells treated with 60 ng/ml than in cells with no tetracycline in all 3 days. Increasing levels of TIMP1 (ng/ml) were observed with the duration of experiment, but the rate of increment was significantly higher in cells without tetracycline. Day 1 supernatant collected from MCF-7 transfected with tTA alone was included as a reference. Experiments were performed in triplicates.
To show that the link between the expression of STAT3C and TIMP1 is not specific to MCF-7, we performed similar experiments using another breast cancer cell line, MDA-MB436. After selecting a cell clone permanently transfected with tTA, these cells were then transfected with TRE-STAT3C and treated with various dosages of tetracycline. Figure 1B shows a gradual down-regulation of FLAG that was dependent on the tetracycline level. At 100 ng/ml, FLAG became completely undetectable. MDA-MB-436 cells transfected with tTA alone had no detectable FLAG expression. TIMP1 was down-regulated with increasing dosages of tetracycline and became completely undetectable at 100 ng/ml. Using ELISA, we found that MDA-MD-436 cells transfected with tTA alone also showed a level of TIMP1 (32 ng/ml) comparable to that found in MDA-MD-436-transfected cells treated with 60 ng/ml of tetracycline (38 ng/ml).
Modulation of Invasiveness by TIMP1 and STAT3
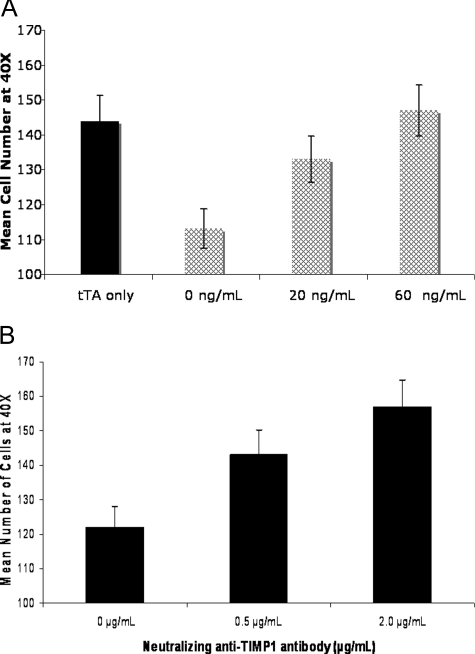
To investigate the biological significance of STAT3 and TIMP1 in breast cancer, we addressed the question of whether different expression levels of STAT3C and TIMP1 expression would have any effect on the invasiveness of breast cancer cells. An in vitro assay for invasiveness using Matrigel invasion assay was performed. As shown in Figure 3A, invasiveness correlated with the dosages of tetracycline used and, thus, inversely correlated with the levels of STAT3C and TIMP1. The assay was also performed on MCF-7 cells before transfection with tTA and STAT3C and an average of 144 cells at 40× were observed, which is similar to the level observed in MCF-7-transfected cells when treated with 60 ng/ml of tetracycline in which no FLAG or TIMP1 levels were detected. To determine whether the differences in the cell invasion observed are directly linked to STAT3-regulated TIMP1 expression, a neutralizing anti-TIMP1 antibody was added to MCF-7 cells transfected with tTA-TRE-STAT3C but no tetracycline treatment. Invasiveness was increased with increasing concentrations of the TIMP1 neutralizing antibody (range, 0 to 2.0 μg/ml) (Figure 3B). Triplicate experiments were performed. The same experiment was repeated using an irrelevant antibody (anti-FLAG) rather than the anti-TIMP1, and no significant changes were detected.
Figure 3-6917.
TIMP1 expression correlated with invasiveness of MCF-7 cells. A: An increase in Matrigel invasion was observed in MCF-7 cells transfected with tet-off STAT3C and treated with increasing levels of tetracycline for a total of 48 hours. Cells treated with tetracycline displayed a higher degree of invasion in a dose-dependent manner. MCF-7 cells transfected with tTA only were used as controls. Experiments were performed in triplicate. B: MCF-7 cells transfected with tetracycline-off STAT3C were treated with 0, 0.5, or 2.0 μg/ml of neutralizing anti-TIMP1 antibody. Invasiveness of Matrigel was increased in cells with higher levels of neutralizing anti-TIMP1. No tetracycline was added to the cell culture. Experiments were performed in triplicate.
Correlation Between STAT3 Activation and TIMP1 Expression, Lymphatic and Vascular Invasion in Breast Cancer Primary Tumors
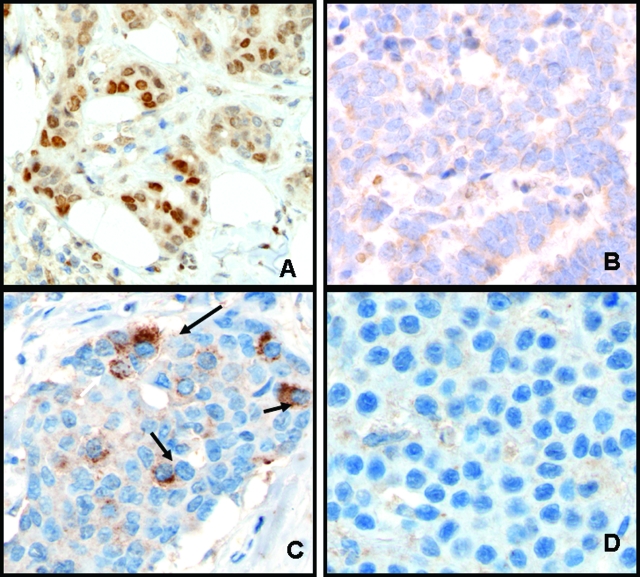
To validate these in vitro data, we evaluated the expression of pSTAT3 and TIMP1 using immunohistochemistry applied to 142 paraffin-embedded, primary breast carcinomas and correlated the expression of these proteins with lymphatic and vascular invasion. The clinical characteristics of these 142 cases of breast cancer were summarized in Materials and Methods. Figure 4 illustrates the immunohistochemical staining results of breast carcinomas that exhibited positive or negative staining of pSTAT3 and TIMP1. Figure 4, A and C, shows a case with positive pSTAT3 and TIMP1 staining, whereas Figure 4, B and D, shows a case that was negative for both pSTAT3 and TIMP1. The overall results are summarized in Table 1. Of the 142 primary breast carcinomas examined, 85 (60%) cases were pSTAT3-positive and 112 (79%) were TIMP1-positive. The correlation between pSTAT3 and TIMP1 expression is significant (P = 0.0041, χ2 test). We also found a significant correlation between pSTAT3 and lymphatic and vascular invasion in breast cancer (P = 0.0002 and P = 0.015, respectively; χ2 test). TIMP1 expression also showed a trend for a lack of vascular and lymphatic invasion, although the correlation is not statistically significant (P = 0.29and P = 0.35, respectively; χ2 test).
Figure 4-6917.
pSTAT3 expression correlated with TIMP1 expression in breast tumors. Immunohistochemical staining using pSTAT3 (A and B) and TIMP1 (C and D) applied to breast cancer tissues. Strong nuclear pSTAT3 expression as shown in A correlated with strong cytoplasmic TIMP1 expression as shown in C. Another case was negative for pSTAT3 and TIMP1 (B and D).
Table 1.
Correlation Between STAT3 Activation and TIMP1 Expression, Lymphatic Invasion, and Vascular Invasion
| STAT3 activation |
P values | ||
|---|---|---|---|
| Positive | Negative | ||
| TIMP1 (total cases assessed = 142) | |||
| Positive | 75 | 37 | 0.0041 |
| Negative | 10 | 20 | |
| Lymphatic (total cases assessed = 85) | |||
| Positive | 12 | 26 | 0.0002 |
| Negative | 34 | 13 | |
| Vascular (total cases assessed = 120) | |||
| Positive | 22 | 29 | 0.015 |
| Negative | 46 | 23 | |
Correlation Between STAT3 Activation and TIMP1 Expression and Other Clinical Parameters in Breast Cancer Patients
The expression of pSTAT3 and TIMP1, as evaluated by immunohistochemical analysis, were correlated with overall survival of patients at 5 years after the initial diagnosis. Kaplan-Meier survival curves were generated for 55 patients with primary breast cancer stained for pSTAT3 expression and for 68 patients with primary breast cancer stained for TIMP1 expression. Patients with pSTAT3-positive tumors showed a trend for better survival at 5 years (P = 0.13, log rank), although the results are not statistically significant. Similarly, patients with TIMP1-positive tumors also showed a trend for better survival at 5 years (P = 0.39, log rank). Both STAT3 and TIMP1 expression did not significantly correlate with the clinical stage, histological grade, or expression of hormone receptors.
Discussion
STAT3 is a latent cytoplasmic transcription factor that has been shown to be oncogenic when constitutively activated.25,26 Activation of STAT3 is tightly linked to the development and differentiation of normal breast tissues, with STAT3 activation found during puberty, early pregnancy, and involution, but not detectable in the later parts of pregnancy and lactation.27–29 The exact biological functions of STAT3 in breast tissues are not fully understood, but it appears to exert a proapoptotic effect during involution. Immunohistochemical studies of breast cancer tissues have demonstrated evidence of STAT3 activation in the malignant component but not in the surrounding benign breast tissues.30 Previous studies have shown that STAT3 activation is detectable in 30 to 60% of primary breast cancers.12 Using immunohistochemistry, we found evidence of STAT3 activation in 60% (85 of 142) of tumors examined in this current study. The mechanism by which STAT3 is activated in breast cancer is not fully understood. Nevertheless, STAT3 is known to be activated in breast cancer cells in response to a diverse number of cytokines and growth factors known to be important for the pathogenesis of breast cancer, such as oncostatin M, epidermal growth factor, and platelet-derived growth factor.31–33 It is likely that STAT3 is activated via a combination of different mechanisms because the level of STAT3 activation (ie, percentage of pSTAT3-positive cells) varies from case to case.
Although little is known about the normal function of STAT3 in breast tissues, even less is known about the significance of STAT3 in the pathogenesis of breast cancer. In our experiments, we primarily used MCF-7, a breast cancer cell line previously reported to have no constitutive STAT3 activation. We confirmed this finding by immunofluorescence staining/confocal microscopy as well as Western blot using anti-pSTAT3. In the absence of constitutive activation of endogenous STAT3, we were able to manipulate the STAT3 activity by transfecting tet-off STAT3C into the cells. The properties of STAT3C have been extensively characterized and published.4 In keeping with the fact that STAT3C represents a species with constitutive activity in our experimental model, we showed by confocal microscopy that transfected STAT3C was localized to the nucleus whereas endogenous STAT3 was localized to the cytoplasm. To further confirm that STAT3C is biologically active, we have shown that a number of STAT3 downstream targets (including cyclin D1) were up-regulated as a result of tTA-TRE-STAT3C.
Using our experimental model, we demonstrated that TIMP1 expression correlated well with the expression of STAT3C. This is in keeping with the concept that TIMP1 is a downstream target of STAT3. Other supporting evidences is as follows: 1) the promoter region of the TIMP1 gene has multiple STAT3 binding sites16; 2) we have shown previously that a STAT3 dominant-negative construct can down-regulate TIMP1 expression in lymphoma cell lines constitutively expressing high levels of TIMP124; 3) breast cancer cell lines transfected with STAT3C, a well-characterized constitutively active STAT3 construct, exhibit up-regulation of TIMP1; 4) using our engineered tet-off STAT3C system, we demonstrated that the levels of STAT3 activated significantly correlated with the TIMP1 levels in breast cancer cells; 5) there is a significant correlation between pSTAT3 and TIMP1 in a cohort of primary breast tumors. Interestingly, a recent study indicated that TIMP-1 expression induces STAT3 activation in plasmacytic tumors.34 Taken together, there may be positive feedback between TIMP1 and STAT3 in certain cancer cell types.
Importantly, using the Matrigel invasion assay, we demonstrated that the invasiveness of breast cancer cells was inversely proportional to the expression levels of STAT3C and TIMP1. Using an anti-TIMP1 neutralizing antibody, we have provided direct evidence that TIMP1, rather than some other nonspecific factor, directly contributes to the decreased invasiveness in our experimental model. In parallel to these in vitro findings, using a large series of primary breast cancer, we found that STAT3 activation highly correlates with TIMP1 expression and inversely correlates with lymphatic and vascular invasion.
Although the inverse relationship between STAT3 activation and vascular or lymphatic invasion appears to contradict the established pro-oncogenic properties of STAT3, it is of note that TIMP1, a STAT3 downstream target, possesses cell growth-stimulatory functions.19,35 TIMP1 has been shown to inhibit apoptosis in several tumor cell types. For instance, Guedez and colleagues36 showed that TIMP1 directly contributes to increased survival in Burkitt lymphoma cells. Further studies indicated that TIMP-1 can initially promote tumor growth in the early stages of lymphoma by inhibiting apoptosis, whereas at a later stage, the secreted TIMP-1 blocks tumor angiogenesis and ultimately suppresses tumor growth,37 suggesting that TIMP1 may have a dual role in oncogenesis. More recently, Liu and colleagues20 have provided evidence that TIMP1 contributes to the survival of breast cancer cells by inhibiting apoptosis. Thus, although TIMP1 down-regulates the invasiveness of breast cancer cells, it provides survival signal and promotes growth of the tumors. In view of this dual function of TIMP1, it is tempting to hypothesize that high levels of TIMP1 may result in large but localized (ie, nonmetastatic) tumors. In other words, differences in the STAT3 activity and TIMP1 expression may determine how early metastasis occurs during the course of the disease. In keeping with this hypothesis, it is well recognized that some breast cancer patients present with metastatic diseases but relatively small primary tumors, whereas some other patients present with localized disease despite the presence of large primary tumors.
In addition to TIMP1, STAT3 is also known to mediate resistance to apoptosis in breast cancer by up-regulating other proteins known to promote cell survival, such as survivin.38 Other potential STAT3 downstream targets, including cyclin D1 and Bcl-2, are also known to play roles in the pathogenesis of breast cancer. In this study, using transfected MCF-7 cells, we examined cyclin D1, Bcl-2, and Bcl-xL and found that down-regulation of STAT3 by increasing concentrations of tetracycline also resulted in down-regulation of these targets. Recent studies have speculated that STAT3 signaling may contribute to cell adhesion. Rivat and colleagues39 stated that the STAT3 signaling pathway regulates homotypic cell-cell adhesion in colorectal cancer cells. Another recent publication has suggested a role for STAT3 as a positive regulator of cell-cell adhesion.40 Overall, STAT3 appears to carry important roles in modulating many biological aspects of breast cancers, including cell survival, proliferation, and metastatic potential.
Increased expression of TIMP1 has been associated with inhibition of tumor growth and decreased invasiveness and metastatic potential in several experimental models by down-regulating MMP activity.14,35,41 Interestingly, we previously identified high levels of STAT3 activation and TIMP1 expression in a specific type of lymphoma, ALK-positive anaplastic large cell lymphoma.24 In agreement with the concept that TIMP1 inhibits invasiveness, one of the most characteristic pathological features of ALK-positive anaplastic large cell lymphomas is their sinusoidal infiltrative pattern within the lymph node. Thus, the link between STAT3 activation and the relative absence of vascular or lymphatic invasion in breast cancer parallels with what we previously observed in ALK-positive anaplastic large cell lymphoma.
The fact that a significant proportion of TIMP1-positive breast tumors showed no evidence of STAT3 activation suggests that TIMP1 can be activated via alternative mechanisms in this cell type. A relatively small number of cytokines have been shown to up-regulate TIMP1 in various cell types,42 including interleukin-6 in human fibroblasts43 and interleukin-10 in human mononuclear phagocytes.44 Tumor necrosis factor receptor has also been shown to stimulate TIMP1 secretion in myeloblastic leukemia cells.45 It will be of interest to evaluate if these cytokines play a role in up-regulating TIMP1 in breast cancer. We also identified a subset of breast tumors that are STAT3-active but TIMP1-negative. One possible explanation for this finding may be related to the fact that the TIMP1 promoter has the binding sites for transcription factors AP-1 and STAT3; it has been suggested that the presence of both of these factors is required for full induction of TIMP1 transcription.16 In addition, we found that TIMP1 expression is dependent on a relatively high level of STAT3 activity because TIMP1 was dramatically decreased with a reduction of STAT3C to 74% (Figure 1A).
Previous studies have demonstrated that overexpression of TIMP1 inhibits tumor formation in transgenic mice and prolongs survival in these animals.42 Although our results were not statistically significant, we did find a trend for longer survival in patients with STAT3 activation and TIMP1 expression. The lack of statistical significance in this current study is probably related to the relatively small sample size for survival data analysis. Thus, further studies with increasing sample size must be done to further define the relationship of TIMP1 and patient survival. One previous study has correlated STAT3 activation and survival in 346 patients with breast cancer, and it was found that STAT3 activation is associated with significantly improved survival at both 5 and 20 years.13 By immunohistochemical analysis, TIMP1 overexpression in breast cancer cells also has been found to be an indicator of favorable prognosis in breast cancer patients.19 Our data from this study suggest that the explanation for the longer survival in patients with STAT3 activation and TIMP1 expression is related to the relatively infrequent vascular and lymphatic invasion, both of which are known to be independent, poor prognostic factors.46
STAT3 activation inversely correlated with vascular and lymphatic invasion, and although TIMP1 expression showed a trend for lack of vascular and lymphatic invasion, the results were not statistically significant. The reason that TIMP1 expression did not correlate with vascular and lymphatic invasion may be due to the fact that there are several other factors that may contribute to breast cancer invasion and metastasis. A number of molecules such as cytokines, chemokines, and growth factors have been implicated in the metastasis of breast cancer.47 In particular, chemokine receptor CXCR4 mediates actin polymerization and pseudopodia formation and induces invasive responses in breast cancer cells48 through interaction with its ligand, stromal cell-derived factor-1.49 The ligands for these CXCR4 receptors have been found to be highly expressed in organs that represent important sites of breast cancer metastasis.47 CXCR4 expression has been shown to be enhanced by HER2 in breast cancer,50 and CXCR4 receptors have been implicated in the activation of the STAT3 pathway in hematopoietic cell lines.51 Thus, although TIMP1 contributes to modulating the metastatic potential of breast cancer, a number of other factors are also clearly implicated.
Acknowledgments
We thank Dr. J. Bromberg for generously providing the STAT3C vector and Ms. Tomasina Parker for her technical assistance.
Footnotes
Address reprint requests to Raymond Lai, M.D., Ph.D., Department of Laboratory Medicine and Pathology, University of Alberta, 4B1, 8440 112 St., Edmonton, Alberta, Canada. E-mail: raymondmail_65@yahoo.com.
Supported by the Canadian Breast Foundation, Alberta Chapter (research grant to R.L.).
References
- Burke WM, Jin X, Lin HJ, Huang M, Liu R, Reynolds RK, Lin J. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20:7925–7934. doi: 10.1038/sj.onc.1204990. [DOI] [PubMed] [Google Scholar]
- Hsiao JR, Jin YT, Tsai ST, Shiau AL, Wu CL, Su WC. Constitutive activation of STAT3 and STAT5 is present in the majority of nasopharyngeal carcinoma and correlates with better prognosis. Br J Cancer. 2003;89:344–349. doi: 10.1038/sj.bjc.6601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Catlett-Falcone RLT, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G, Dalton WS, Jove R. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- Levy DE, Lee CK. What does Stat3 do? J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Lou W, Leman ES, Gao AC. Inhibition of constitutively activated Stat3 signaling pathway suppresses growth of prostate cancer cells. Cancer Res. 2000;60:1225–1228. [PubMed] [Google Scholar]
- Huang M, Page C, Reynolds RK, Lin J. Constitutive activation of Stat 3 oncogene product in human ovarian carcinoma cells. Gynecol Oncol. 2000;79:67–73. doi: 10.1006/gyno.2000.5931. [DOI] [PubMed] [Google Scholar]
- Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger CV. Roles and regulation of stat family transcription factors in human breast cancer. Am J Pathol. 2004;165:1449–1460. doi: 10.1016/S0002-9440(10)63403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechow TN, Pedranzini L, Leitch A, Leslie K, Gerald WL, Linkov I, Bromberg JF. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci USA. 2004;101:10602–10607. doi: 10.1073/pnas.0404100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ. Stat transcription factors in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2001;6:115–127. doi: 10.1023/a:1009524817155. [DOI] [PubMed] [Google Scholar]
- Dolled-Filhart MCR, Kowalski DP, Smith BL, Rimm DL. Tissue microarray analysis of signal transducers and activators of transcription 3 (Stat3) and phospho-Stat3 (Tyr705) in node-negative breast cancer shows nuclear localization is associated with a better prognosis. Clin Cancer Res. 2003;9:594–600. [PubMed] [Google Scholar]
- Fassina G, Ferrari N, Brigati C, Benelli R, Santi L, Noonan DM, Albini A. Tissue inhibitors of metalloproteases: regulation and biological activities. Clin Exp Metastasis. 2000;18:111–120. doi: 10.1023/a:1006797522521. [DOI] [PubMed] [Google Scholar]
- Ritter LM, Garfield SH, Thorgeirsson UP. Tissue inhibitor of metalloproteinases-1 (TIMP-1) binds to the cell surface and translocates to the nucleus of human MCF-7 breast carcinoma cells. Biochem Biophys Res Commun. 1999;257:494–499. doi: 10.1006/bbrc.1999.0408. [DOI] [PubMed] [Google Scholar]
- Bugno M, Graeve L, Gatsios P, Koj A, Heinrich PC, Travis J, Kordula T. Identification of the interleukin-6/oncostatin M response element in the rat tissue inhibitor of metalloproteinases-1 (TIMP-1) promoter. Nucleic Acids Res. 1995;23:5041–5047. doi: 10.1093/nar/23.24.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossakowska AE, Edwards DR, Prusinkiewicz C, Zhang MC, Guo D, Urbanski SJ, Grogan T, Marquez LA, Janowska-Wieczorek A. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin’s lymphomas. Blood. 1999;94:2080–2089. [PubMed] [Google Scholar]
- Zacchigna S, Zentilin L, Morini M, Dell’Eva R, Noonan DM, Albini A, Giacca M. AAV-mediated gene transfer of tissue inhibitor of metalloproteinases-1 inhibits vascular tumor growth and angiogenesis in vivo. Cancer Gene Ther. 2004;11:73–80. doi: 10.1038/sj.cgt.7700657. [DOI] [PubMed] [Google Scholar]
- Nakopoulou L, Giannopoulou I, Lazaris A, Alexandrou P, Tsirmpa I, Markaki S, Panayotopoulou E, Keramopoulos A. The favorable prognostic impact of tissue inhibitor of matrix metalloproteinases-1 protein overexpression in breast cancer cells. APMIS. 2003;111:1027–1036. doi: 10.1111/j.1600-0463.2003.apm1111105.x. [DOI] [PubMed] [Google Scholar]
- Liu XW, Taube ME, Jung KK, Dong Z, Lee YJ, Roshy S, Sloane BF, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells from extrinsic cell death: a potential oncogenic activity of tissue inhibitor of metalloproteinase-1. Cancer Res. 2005;65:898–906. [PubMed] [Google Scholar]
- Stetler-Stevenson M, Mansoor A, Lim M, Fukushima P, Kehrl J, Marti G, Ptaszynski K, Wang J, Stetler-Stevenson WG. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in reactive and neoplastic lymphoid cells. Blood. 1997;89:1708–1715. [PubMed] [Google Scholar]
- Yoshikawa T, Saitoh M, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Yanoma S, Noguchi Y. Tissue inhibitor of matrix metalloproteinase-1 in the plasma of patients with gastric carcinoma A possible marker for serosal invasion and metastasis. Cancer. 1999;86:1929–1935. doi: 10.1002/(sici)1097-0142(19991115)86:10<1929::aid-cncr8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Pellegrini P, Contasta I, Berghella AM, Gargano E, Mammarella C, Adorno D. Simultaneous measurement of soluble carcinoembryonic antigen and the tissue inhibitor of metalloproteinase TIMP1 serum levels for use as markers of pre-invasive to invasive colorectal cancer. Cancer Immunol Immunother. 2000;49:388–394. doi: 10.1007/s002620000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai R, Rassidakis GZ, Medeiros LJ, Ramdas L, Goy AH, Cutler C, Fujio Y, Kunisada K, Amin HM, Gilles F. Signal transducer and activator of transcription-3 activation contributes to high tissue inhibitor of metalloproteinase-1 expression in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Am J Pathol. 2004;164:2251–2258. doi: 10.1016/S0002-9440(10)63781-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg DJ., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- Shuai K. Modulation of STAT signaling by STAT-interacting proteins. Oncogene. 2000;19:2638–2644. doi: 10.1038/sj.onc.1203522. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW, Wagner KU, Liu X. Developing a mammary gland is a stat affair. J Mammary Gland Biol Neoplasia. 1997;2:365–372. doi: 10.1023/a:1026347313096. [DOI] [PubMed] [Google Scholar]
- Groner BHL. Linear and cooperative signaling: roles for Stat proteins in the regulation of cell survival and apoptosis in the mammary epithelium. Breast Cancer Res. 2000;2:149–153. doi: 10.1186/bcr47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp JA, Burdon TG, Watson CJ. Differential activation of STATs 3 and 5 during mammary gland development. FEBS Lett. 1996;396:77–80. doi: 10.1016/0014-5793(96)01069-1. [DOI] [PubMed] [Google Scholar]
- Berclaz GAH, Rohrbach V, Siragusa A, Dreher E, Smith PD. EGFR dependent expression of STAT3 (but not STAT1) in breast cancer. Int J Oncol. 2001;19:1155–1160. doi: 10.3892/ijo.19.6.1155. [DOI] [PubMed] [Google Scholar]
- Bromberg J. Signal transducers and activators of transcription as regulators of growth, apoptosis and breast development. Breast Cancer Res. 2000;2:86–90. doi: 10.1186/bcr38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE., Jr Stats and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Hirano TIK, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- Guedez L, Martinez A, Zhao S, Vivero A, Pittaluga S, Stetler-Stevenson M, Raffeld M, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinase 1 (TIMP-1) promotes plasmablastic differentiation of a Burkitt lymphoma cell line: implications in the pathogenesis of plasmacytic/plasmablastic tumors. Blood. 2005;105:1660–1668. doi: 10.1182/blood-2004-04-1385. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Guedez L, Stetler-Stevenson WG, Wolff L, Wang J, Fukushima P, Mansoor A, Stetler-Stevenson M. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest. 1998;102:2002–2010. doi: 10.1172/JCI2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedez L, McMarlin AJ, Kingma DW, Bennett TA, Stetler-Stevenson M, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinase-1 alters the tumorigenicity of Burkitt’s lymphoma via divergent effects on tumor growth and angiogenesis. Am J Pathol. 2001;158:1207–1215. doi: 10.1016/S0002-9440(10)64070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh FC, Cheng G, Lin J. Evaluation of potential Stat3-regulated genes in human breast cancer. Biochem Biophys Res Commun. 2005;335:292–299. doi: 10.1016/j.bbrc.2005.07.075. [DOI] [PubMed] [Google Scholar]
- Rivat C, De Wever O, Bruyneel E, Mareel M, Gespach C, Attoub S. Disruption of STAT3 signaling leads to tumor cell invasion through alterations of homotypic cell-cell adhesion complexes. Oncogene. 2004;23:3317–3327. doi: 10.1038/sj.onc.1207437. [DOI] [PubMed] [Google Scholar]
- Uttamsingh S, Zong CS, Wang LH. Matrix-independent activation of phosphatidylinositol 3-kinase, Stat3, and cyclin A-associated Cdk2 Is essential for anchorage-independent growth of v-Ros-transformed chicken embryo fibroblasts. J Biol Chem. 2003;278:18798–18810. doi: 10.1074/jbc.M211522200. [DOI] [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- Blavier L, Henriet P, Imren S, Declerck YA. Tissue inhibitors of matrix metalloproteinases in cancer. Ann NY Acad Sci. 1999;878:108–119. doi: 10.1111/j.1749-6632.1999.tb07677.x. [DOI] [PubMed] [Google Scholar]
- Sato T, Ito A, Mori Y. Interleukin 6 enhances the production of tissue inhibitor of metalloproteinases (TIMP) but not that of matrix metalloproteinases by human fibroblasts. Biochem Biophys Res Commun. 1990;170:824–829. doi: 10.1016/0006-291x(90)92165-v. [DOI] [PubMed] [Google Scholar]
- Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96:2304–2310. doi: 10.1172/JCI118286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Takeda K, Yamada T, Nomura Y, Takeda M, Iwamoto S, Seyama Y. Tumor necrosis factor alpha and lymphotoxin stimulate human myeloblastic leukemia cell (ML-1) invasion through a reconstituted basement membrane (Matrigel) with concomitant induction of 92 kDa gelatinase secretion. Cancer Lett. 1996;98:233–240. [PubMed] [Google Scholar]
- Subramaniam DS, Isaacs C. Utilizing prognostic and predictive factors in breast cancer. Curr Treat Options Oncol. 2005;6:147–159. doi: 10.1007/s11864-005-0022-1. [DOI] [PubMed] [Google Scholar]
- Fernandis AZ, Prasad A, Band H, Klosel R, Ganju RK. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23:157–167. doi: 10.1038/sj.onc.1206910. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- Li CZF, Lin M, Liu J. Induction of S100A9 gene expression by cytokine oncostatin M in breast cancer cells through the STAT3 signaling cascade. Breast Cancer Res Treat. 2004;87:123–134. doi: 10.1023/B:BREA.0000041594.36418.f6. [DOI] [PubMed] [Google Scholar]
- Ahr B, Denizot M, Robert-Hebmann V, Brelot A, Biard-Piechaczyk M. Identification of the cytoplasmic domains of CXCR4 involved in Jak2 and STAT3 phosphorylation. J Biol Chem. 2005;280:6692–6700. doi: 10.1074/jbc.M408481200. [DOI] [PubMed] [Google Scholar]