Abstract
Endothelin (ET-1) has been shown to crucially contribute to UV-induced skin responses such as tanning. To test whether ET-1 is also involved in early cutaneous reactions to UV, we assessed ET-1 skin levels in UV-irradiated mice. In correlation with the levels of UV-induced skin inflammation, ET-1 concentrations increased substantially and continually. Moreover, blocking of ET-1 receptors (ETA) resulted in significantly decreased cutaneous inflammation following UV irradiation. When we assessed skin responses to ET-1 injections, we observed prominent mast cell degranulation and mast cell-dependent inflammation. Since mast cells also critically contributed to UV-induced inflammation, we determined the ET-1-dependent inflammatory response to UV in the absence and presence of these cells. Interestingly, ETA blockade did not decrease UV-induced inflammation in mast cell-deficient mice, unless these mice had been adoptively transferred with mast cells before irradiation. This indicates that skin inflammation due to UV irradiation is caused in part by ET-1 acting on skin mast cells.
Immediate skin responses following exposure to sunlight are largely mediated by UV-B light (UVB): low to moderate doses of UVB result in tanning, whereas intense UVB irradiation also gives rise to considerable inflammation, ie, solar dermatitis. UVB-induced tanning is widely regarded to result from the up-regulation of pigment production in epidermal melanocytes, which is induced by melanogens released from activated keratinocytes. In contrast, the cellular and molecular mechanisms involved in the induction of inflammatory skin responses to UVB are largely unknown.
Recently, the vasoconstrictor peptide endothelin-1 (ET-1), one of the melanogens that contributes to UVB-induced tanning,1,2 was found to promote inflammation in various pathological processes. For example, we and others have recently shown that ET-1 levels are up-regulated in murine septic peritonitis and that elevated ET-1 levels contribute to morbidity and mortality in this setting.3,4 Increased ET-1 production and mRNA expression have been demonstrated in lung tissue and bronchoalveolar lavage fluid in different models of airway inflammation.5–7 In addition, elevated ET-1 concentrations are also present in patients with systemic lupus erythematosus, Takayasu arteritis, or Raynaud’s phenomenon, where, in the latter case, the increase was shown clearly to contribute to disease severity.8
These observations indicate that ET-1, in addition to having stimulatory effects on melanocytes, may orchestrate the induction of inflammatory responses after UVB irradiation. Two recent reports support this notion. First, the expression of ET-1 and its receptors ETA and ETB in murine skin was found to be markedly and rapidly up-regulated after UVB irradiation, both in the dermis and epidermis.9 Second, intracutaneous injections of ET-1 reportedly trigger inflammatory reactions, including a pronounced flare response associated with sensations of a burning itch, a classic symptom of a UV-induced inflammatory response.10
Here, we tested whether ET-1 is one of the signals involved in the elicitation of inflammatory responses after UVB irradiation by combined pharmacological and genetic approaches using highly selective and specific ET-1 receptor antagonists and using mutant mouse models. Our results reveal ET-1 as an important player in UV-induced inflammation and identify mast cells as one of the crucial cellular components of the underlying mechanism of this novel function of ET-1.
Materials and Methods
Mice
C57BL/6 mice, genetically mast cell (MC)-deficient WBB6F1-KitW/KitW-v (KitW/KitW-v) mice, and congenic wild-type WBB6F1-Kit+/+ (Kit+/+) mice were obtained from breeding colonies of the animal facilities of the University of Mainz. Mice were kept in community cages at the Animal Care Facilities of the Department of Dermatology at light periods of 12 hours and were fed water and mouse chow ad libitum. All animal care and experimentation was conducted in accordance with current Institutional Animal Care and Use Committee guidelines and University and state regulations.
Selective MC Engraftment of KitW/KitW-v Mice
The MC deficiency of KitW/KitW-v mice (6 to 8 weeks old) was corrected selectively and locally by intradermal injection of bone marrow-derived cultured MCs (BMCMCs) from Kit+/+ mice (106 in 20 μl of 0.9% NaCl) into the right ears at least 4 weeks before UV irradiation or ET-1 injections. BMCMCs were cultured in vitro for 4 to 5 weeks, and MC purity was confirmed to be >98% before engraftment. In some adoptively transferred mice, MC numbers were assessed by histological analysis of Giemsa-stained cryostat sections and were found to be virtually identical to those of Kit+/+ mice.
UV Irradiation
Mice were anesthetized with ketamine (Ratiopharm, Ulm, Germany) and xylazine (Bayer, Leverkusen, Germany), and ears or shaved back skin were exposed to a single dose of UVB using a lamp (Saalmann, Herford, Germany) emitting most of its energy within the UVB range (295 to 315 nm). Any possible contamination of the emitted spectrum by UVC was prevented by a UVC-excluding filter (Saalmann). Dose-response experiments (100 to 540 mJ/cm2) were performed for the irradiation of ears (data not shown), and a submaximal dose of 250 mJ/cm2 was chosen for all consecutive ear irradiations. Because of the anatomical differences (thicker epidermis, hair follicles absent in ear skin), a higher dose of 540 mJ/cm2 was used in the irradiation of back skin, resulting in comparable swelling responses. In some experiments, groups of mice received i.v. injections of vehicle (0.9% NaCl) or the ET antagonists BQ-123 (ETA) or BQ-788 (ETB, 10−4 M in 200 μl; both from Bachem, Weil am Rhein, Germany) 1 hour before irradiation. Ear thickness was measured before and up to 8 days after irradiation.
Measurement of ET-1 Concentrations
To assess ET-1 concentrations in UVB-irradiated skin, C57BL/6 mice were subjected to a single dose of 540 mJ/cm2 UVB, and full-thickness back skin samples were taken at various time points after irradiation and pulverized in liquid nitrogen. One hundred milligrams of skin and 0.5 ml of lysis buffer (containing 50 mmol/L Tris/HCl, pH 8.0, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L phenylmethylsulfonyl fluoride, 5 mmol/L iodacetamid, 100 μg/ml aprotinin, 0.2% sodium dodecyl sulfate, 1% Nonidet, and 1% Triton X-100; all from Sigma, Deisenhofen, Germany) were sonicated, shaken at 4°C for 1 hour, sonicated again, and centrifuged for 30 minutes at 14,000 rpm at 4°C. Supernatants were frozen and stored at −80°C. For quantification of ET-1, a commercially available enzyme-linked immunosorbent assay kit was used in accordance with the manufacturer’s instructions (Biomedica, Vienna, Austria). ET-1 concentrations are shown as ET-1 per milligram of protein. Total protein was measured in the skin lysates using the Bradford method.
In Vivo Treatment and Histology
ET-1 (10−6 to 10−9 M; Sigma) or vehicle (Hanks’ minimal essential medium-piperazine-N,N′-bis(2-ethanesulfonic acid) [HMEM-Pipes]; 20 μl) were injected intradermally into the ears of C57BL/6, MC-deficient KitW/KitW-v, wild-type Kit+/+, or MC-engrafted KitW/KitW-v mice, and ear swelling was measured at various time points (up to 6 hours) after injection. Ears of ET-1 or vehicle-treated C57BL/6 mice were embedded in Epon, 1-μm sections were stained with alkaline Giemsa, and the extent of MC degranulation was assessed by quantitative histomorphometry at ×400 magnification as previously described.11 MCs were classified as “extensively degranulated” (>50% of cytoplasmic granules exhibiting staining alterations, fusion, and/or exteriorization), “moderately degranulated” (10 to 50% of granules affected), or “not degranulated” (<10% granules affected). In some experiments, groups of C57BL/6 mice received i.v. injections of vehicle (0.9% NaCl) or the ET antagonists BQ-123 (ETA) or BQ-788 (ETB; 10−4 M in 200 μl) 1 hour before injection of ET-1. For the induction of passive cutaneous anaphylaxis, C57BL/6 mice were sensitized with IgE anti-dinitrophenol (DNP) (100 ng in 20 μl, i.d.) or vehicle (HMEM-Pipes), and mice were challenged with DNP-human serum albumin (400 μg in 100 μl i.v.; Sigma) 24 hours later. Ear thickness was measured for 6 hours at various time points, and ears were processed for histological analysis as described above.
Isolation of Skin MCs
Skin MCs were obtained by enzymatic and mechanic dispersion from C57BL/6 mouse ears. In brief, ears were split and incubated dermis down in RPMI 1640 medium (Sigma) containing 500 μg/ml Liberase CI (Roche Diagnostics, Mannheim, Germany) and penicillin-streptomycin (5%; Gibco, Eggenstein, Germany) for 1.5 hours at 37°C and dispersed using a Medimachine (BD Biosciences, Heidelberg, Germany). Numbers of isolated MCs were determined by Kimura staining. Cell suspensions containing 3 to 4% skin MCs were used for serotonin release assays. In some experiments, skin MCs were purified using a 22.5% Metrizamide gradient (>90% skin MC).
Serotonin Release Assay
Unpurified (3 to 4% MCs) and purified (>90% MCs) skin MC suspensions were preincubated for 2 hours with [3H]5-hydroxytryptamine (PerkinElmer, Zaventem, Belgium), and some unpurified skin MC suspensions were incubated overnight with 2 μg/ml IgE anti-DNP. MCs were then challenged for 15 minutes with ET-1, ET-1 and BQ-123, ET-1 and BQ-788, DNP-human serum albumin (Sigma), or A23187 (calcium ionophore, positive control; Sigma). Supernatants were collected by centrifugation, and [3H]5-hydroxytryptamine was measured in supernatants and pellets by scintillation counting.
Myeloperoxidase Assay
The influx of neutrophils to UVB-irradiated skin sites was assessed in C57BL/6 mice treated with vehicle (0.9% NaCl), BQ-123, or BQ-788 (10−4 M, in 200 μl i.v.) by quantification of myeloperoxidase (MPO), a marker for neutrophil accumulation in tissue.12 In brief, 6-mm punch biopsies were taken from ears 8 days after UVB irradiation, and MPO was extracted from homogenized tissue by suspending the material in 0.5% hexadecyltrimethylammonium bromide in 50 mmol/L potassium phosphate buffer before sonication on ice for 10 seconds. MPO activity in supernatants was measured spectrophotometrically by the change in absorbance at 460 nm, resulting from the decomposition of hydrogen peroxide in the presence of o-dianisidine dihydrochloride (all reagents from Sigma).
Statistical Analyses
All data were tested for statistical significance using the unpaired two-tailed Student’s t-test and expressed as mean ± SEM, unless specified otherwise.
Results
ET-1 Levels and Skin Inflammation Is Increased in UV-Irradiated Skin
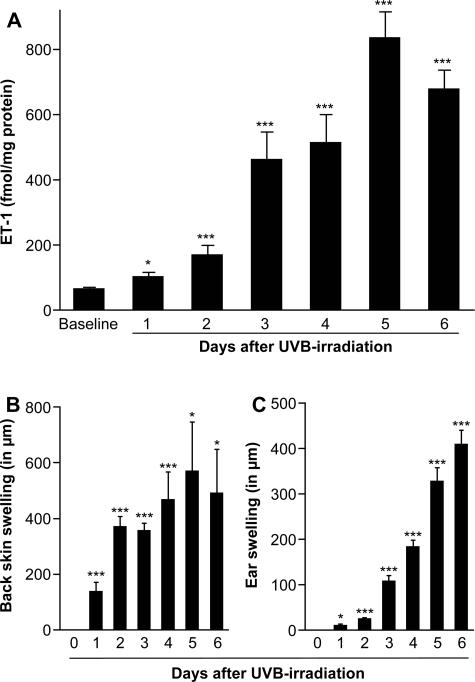
To test whether ET-1, a potent promoter of UV-induced tanning, is also involved in early cutaneous reactions to UV, we first assessed ET-1 skin levels in UV-irradiated mice. Therefore, mice received a single dose of UVB (540 mJ/cm2), and ET-1 concentrations in shaved back skin were measured by enzyme-linked immunosorbent assay. As shown in Figure 1A, ET-1 concentrations increased substantially as soon as 1 day after UV challenge and continued to rise during the first week following UV irradiation. Cutaneous ET-1 levels peaked on day 5 after UV irradiation when levels were increased by more than 13-fold above baseline levels (834 ± 82 versus 63 ± 6 fmol/mg protein before UV challenge, P < 0.0001; Figure 1A). These findings complement previous reports demonstrating that UV irradiation can increase the expression and secretion of ET-1 in human keratinocytes in vitro.13–16 Interestingly, increasing ET-1 levels were closely paralleled by augmented inflammation of UV-exposed skin, which was assessed by measuring skin thickness both in shaved back skin (540 mJ/cm2; Figure 1B) and ears (250 mJ/cm2; Figure 1C).
Figure 1.
Increased ET-1 levels in UVB-irradiated skin are associated with development of skin inflammation. A: ET-1 concentration in the back skin of C57BL/6 mice was measured by enzyme-linked immunosorbent assay before (baseline) and daily after a single UVB irradiation (540 mJ/cm2); data are expressed as ET-1 per milligram of protein (n = 6 to 8/time point). B: Swelling of back skin was measured in C57BL/6 mice by assessing skinfold thickness before and daily after a single UVB irradiation (540 mJ/cm2, n = 6/time point). C: Ear swelling in C57BL/6 mice was measured daily after a single UVB irradiation (250 mJ/cm2, n = 13 to 21/time point). Data pooled from two (A, B) or three (C) independent experiments. All data shown as means ± SEM. *P = <0.05, ***P = <0.005 versus baseline values.
ET-1 Promotes Skin Inflammatory Responses to UV Irradiation
The concomitant increase in both inflammation and ET-1 levels in the skin prompted us to test whether ET-1 contributes to the development of UV-induced skin inflammation. To this end, we treated mice with highly selective and specific antagonists of the ET-1 receptors ETA or ETB before a single UV challenge. UV-induced skin inflammatory responses were evaluated by measuring increases in skin thickness and levels of the neutrophil protease MPO, which closely reflect skin neutrophil numbers.12,17 As shown in Figure 2, A and B, blocking of ETA receptors, but not ETB receptors, resulted in a significant decrease in skin swelling and markedly lower MPO levels, indicating that ET-1 contributes to inflammatory skin reactions following UVB irradiation via ETA.
Figure 2.
ET-1 promotes skin inflammation after UVB irradiation via ETA. A: UVB-induced (250 mJ/cm2) ear swelling in C57BL/6 mice pretreated with the ET receptor antagonists BQ-123 (ETA,10−4M), BQ-788 (ETB,10−4M), or vehicle (0.9% NaCl, 200 μl i.v.) (n = 11 to 21/group). B: Inflammation-associated neutrophil recruitment into the ears of BQ-123-, BQ-788-, or vehicle-pretreated mice on day 8 after UVB irradiation as assessed by measuring MPO activity. Data are expressed as percentage of MPO in vehicle-treated mice (n = 16 to 17/group). Data pooled from three independent experiments. All data shown as means ± SEM. **P = <0.01, ***P = <0.005 versus baseline values.
ET-1 Induces Skin Inflammation
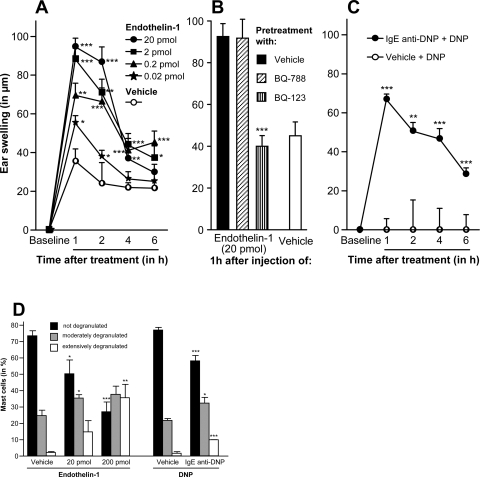
To better characterize the proinflammatory effects of ET-1 on murine skin, we injected ET-1 into the skin of naïve mice and found that subnanomolar concentrations of ET-1 resulted in pronounced and dose-dependent ear swelling. This was greatest after 1 hour following injection and thereafter steadily decreased over the next 6 hours (Figure 3A). Moreover, blocking of ETA but not ETB receptors completely abolished the ET-1-induced ear swelling (Figure 3B), indicating that ET-1-induced skin inflammation is ETA-dependent. As the kinetics of the ET-1-induced inflammation strongly resemble the course of immediate hypersensitivity skin reactions, we then compared ET-1-induced cutaneous inflammation to type I allergic skin responses, ie, passive cutaneous anaphylaxis. As expected, the course and extent of both inflammatory skin reactions were virtually identical (Figure 3C), suggesting that mast cells (MCs), which are essential and sufficient for the elicitation of cutaneous anaphylactic responses to antigen challenge in passively sensitized mice (using antigen-specific IgE antibodies),18 may be involved in the elicitation of ET-1-induced inflammation. Therefore, sites of ET-1 injections or passive cutaneous anaphylaxis were analyzed for MC degranulation by quantitative histomorphometry. We found that the numbers of extensively and moderately degranulated MCs were similar (P > 0.05) and significantly increased in both responses compared with vehicle treatment (P < 0.001; Figure 3D). This demonstrates that ET-1 induces substantial skin MC degranulation in vivo and is in line with previous reports showing that ET-1 is one of the most potent activators of peritoneal MCs,19 which share many characteristic features of skin MCs. In addition, Matsushima and coworkers have recently shown that murine fetal skin-derived MCs can degranulate and produce proinflammatory cytokines such as interleukin-6 and tumor necrosis factor-α in response to ET-1.20
Figure 3.
ET-1 induces skin inflammation. A: ET-1 in various concentrations or vehicle was injected intradermally into ears of C57BL/6 mice, and increases in ear swelling were measured as a parameter of inflammation. B: ET-1-induced ear swelling in C57BL/6 mice pretreated with the ET receptor antagonists BQ-123 (ETA, 10−4 M), BQ-788 (ETB, 10−4 M), or vehicle (0.9% NaCl, 200 μl i.v.). Ear swelling is shown 1 hour after ET-1 injection (20 pmol in 20 μl). C: Passive cutaneous anaphylaxis was induced in C57BL/6 mice by sensitizing with IgE anti-DNP (100 ng in 20 μl i.d.) and subsequent challenge 24 hours later with DNP-human serum albumin (400 μg in 100 μl i.v.). D: Ears of ET-1-, vehicle-, or IgE+antigen-injected mice were harvested 6 hours after injection and processed for histochemistry. The extent of MC degranulation was then assessed in alkaline Giemsa-stained 1-μm sections by quantitative histomorphometry at ×400. Data pooled from three (A) or two (B) independent experiments. All data shown as means ± SEM. *P = <0.05, **P = 0.01, ***P = <0.005.
ET-1-Induced Skin Inflammation Is Mast Cell-Dependent
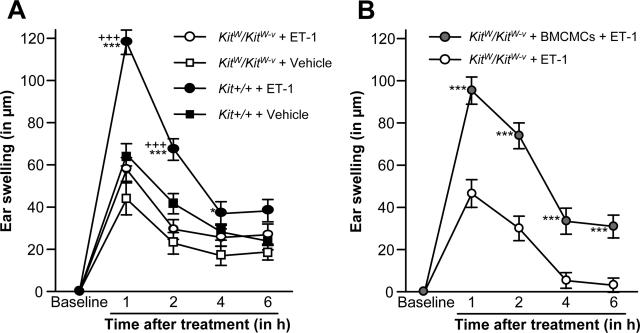
To assess further whether MCs are required for ET-1 to induce skin inflammation, we examined inflammatory skin responses in genetically MC-deficient KitW/KitW-v and their corresponding MC competent Kit+/+ littermates treated with ET-1. Interestingly, Kit+/+ mice developed pronounced ear swelling reactions after injection of ET-1, whereas MC-deficient KitW/KitW-v mice failed to show ET-1 induced inflammation (Figure 4A). This indicates that the proinflammatory effects of ET-1 are severely impaired in the absence of MCs. To formally prove that the inability of KitW/KitW-v mice to develop skin inflammation after ET-1 treatment is indeed a result of their lack of MCs, and not due to other defects, we adoptively transferred KitW/KitW-v skin with MCs derived from the bone marrow of Kit+/+ mice (BMCMCs) before injecting ET-1. This local repair of MC deficiency with BMCMCs completely restored the ability of KitW/KitW-v skin to develop swelling reactions in response to ET-1 (Figure 4B), thus establishing that ET-1-induced skin inflammation is MC-dependent.
Figure 4.
ET-1 induced skin inflammation is mast cell-dependent. A: Ear swelling in MC-deficient KitW/KitW-v and Kit+/+ mice following i.d. injections of ET-1 (left ears, 20 pmol in 20 μl) and vehicle (right ears; HMEM-Pipes) (n = 8 to 12/group). B: Ear swelling after ET-1 injections (20 pmol in 20 μl) into the ears of MC-deficient KitW/KitW-v mice that were locally repaired of their MC deficiency (left ears) with BMCMCs from Kit+/+ mice (KitW/KitW-v + BMCMC) or were left MC-deficient (right ears) (n = 6 to 8/group). Data pooled from three (A) or two (B) independent experiments. All data shown as means ± SEM. *P = <0.05, ***, +++P = <0.005. ***, * versus KitW/KitW-v+ ET-1; +++ versus Kit+/+ + vehicle.
ET-1-Induced Activation of Skin MCs in Vitro Is Receptor-Mediated
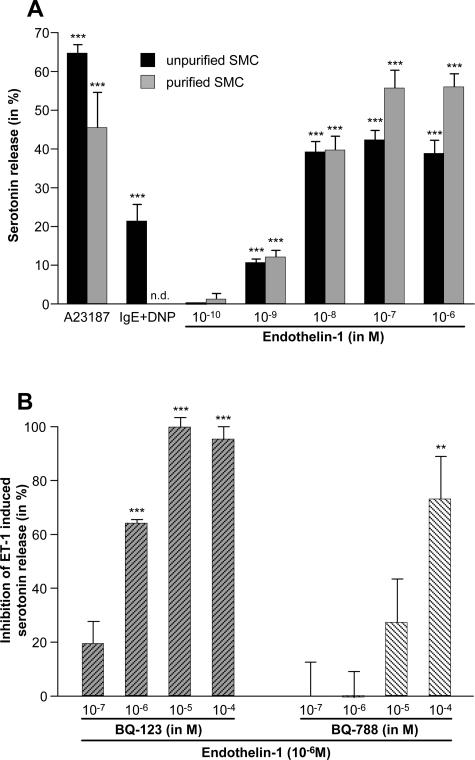
Next, to determine the underlying mechanisms of ET-1-induced skin MC degranulation, we assessed crude (∼3% MC) and purified (>90% MC) MC populations obtained from naïve murine skin for degranulation after coincubation with ET-1. We found that ET-1 induced pronounced and dose-dependent MC activation in both MC populations, implying a direct and receptor-mediated mechanism of ET-1-induced skin MC degranulation (Figure 5A). Because noncutaneous mouse MC populations, including peritoneal MCs, have been shown to express functional ETA and ETB receptors,19,21 it is likely that the actions of ET-1 on skin MCs are mediated via one or both of these two ET-1 receptors. To test this hypothesis, we stimulated skin MCs with ET-1 in the presence of various concentrations of BQ-123 (a selective ETA receptor antagonist) or BQ-788 (a selective ETB receptor antagonist). Both antagonists inhibited the degranulation of MCs induced by ET-1 in a dose-dependent manner (Figure 5B). However, the ETA antagonist was a more efficacious inhibitor than the ETB blocker (Figure 5B), indicating that ET-1 activates murine skin MCs directly and primarily via ETA.
Figure 5.
ET-1 potently activates skin MCs in vitro via ETA. A: Unpurified (∼3% of all cells) or purified (>90%) skin MCs were obtained from C57BL/6 mice and degranulation was assessed by measuring serotonin release after stimulation with ET-1 or IgE + Ag. Calcium ionophore (A23187; 10−5 M) was used as positive control. Data are corrected for spontaneous release, which was <10% in all experiments. B: Inhibition of ET-1-induced (10−6 M) degranulation of unpurified skin MCs (∼3% of all cells) by selective antagonists for ETA (BQ-123) or ETB (BQ-788) or vehicle as assessed by measuring serotonin release. Data are corrected for spontaneous release (<14% in all experiments). Data are pooled from three (B) or two (A) independent experiments. All data shown as means ± SEM. **P = 0.01, ***P = <0.005. n.d., not detectable.
ET-1 Exerts Its Effects in UVB-Induced Inflammation via ETA on MCs
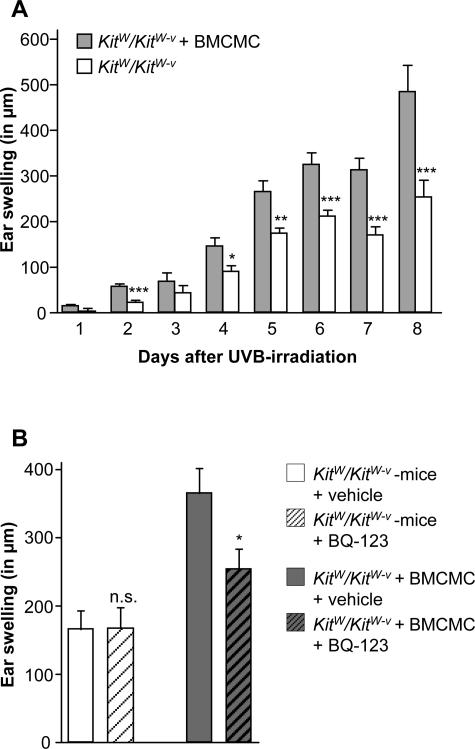
Our observations that MCs are required for ET-1-induced skin inflammation and that ET-1 is necessary to develop full-blown inflammatory responses to UV challenge led us to hypothesize that skin MCs may critically contribute to UV-induced inflammation. This notion is supported by earlier reports demonstrating that the sunburn response following skin exposure to UVB irradiation is accompanied by a rapid degranulation of cutaneous MCs.22,23 In addition, MC products, such as tumor necrosis factor-α and prostaglandins, have been implicated to contribute to the inflammatory events following UVB irradiation.23,24 Most importantly, UVB irradiation has been demonstrated to result in reduced swelling responses of MC-deficient mouse skin.24 To obtain direct evidence for a role of MCs in UV-induced inflammation, we made use of KitW/KitW-v mice, which we subjected to UV irradiation of skin sites that had been adoptively transferred with BMCMCs and compared these with skin sites that had not been MC-engrafted. Notably, MC-deficient skin sites exhibited significantly reduced swelling responses as opposed to MC-engrafted skin sites, indicating that UV-induced skin inflammation is largely dependent on the presence of MC (Figure 6A).
Figure 6.
ET-1-mediated, UVB-induced skin inflammation is dependent on ETA expressed by MCs. A: UVB-induced (250 mJ/cm2) swelling in MC-engrafted (gray bars) and MC-deficient (white bars) ears 5 days after irradiation. Data pooled from four independent experiments (n = 22 to 33/group). B: UVB-induced (250 mJ/cm2) swelling in MC-engrafted (left) and MC-deficient (right) ears of KitW/KitW-v mice pretreated with BQ-123 (10−4 M, 200 μl i.v.) or vehicle 1 hour before irradiation. Data pooled from three independent experiments (n = 8 to 11/group). A and B: All data shown as means ± SEM. *P = <0.05, **P = < 0.01, ***P = <0.005. n.s., not significant.
This prompted us to test whether MCs are required for ET-1 to promote inflammatory responses in the context of UV-induced inflammation. Interestingly, blocking of ETA did not decrease UV-induced inflammation in MC-deficient mice, unless these mice had been adoptively transferred with MCs before irradiation (Figure 3B), which demonstrates that skin inflammation due to UV irradiation is caused, at least in part, by ET-1 acting on skin MCs via ETA.
Discussion
ET-1 has recently been shown to contribute significantly to UV-induced pigmentation, both in physiological (eg, tanning) and in pathological contexts.2,25–27 Although these effects are mediated largely, if not exclusively, via activation of ETB receptors expressed by melanocytes,28 ETA-mediated effects of UV irradiation in the skin are far less investigated, although ET-1 exerts its effects with comparable affinity both through ETA and ETB.
Here, we report that ET-1 potently activates MCs both in vitro and in vivo and induces skin inflammation by activating MCs via ETA receptors. Confirming recent in vitro data and findings in the skin of human and mouse skin that expression and release of ET-1 is markedly enhanced in response to UVB,9,16,25,29 we show that ET-1 concentrations in UVB-irradiated skin increase more than 13-fold above baseline levels. This UV-mediated increase in ET-1 levels is associated with augmented skin inflammation resulting, in part, from MCs activated by ET-1 via ETA receptors. Interestingly, although the activation of MCs by ET-1 in the setting depicted here results in increased pathology, ET-1-mediated MC activation can also result in substantially reduced pathology in other settings.4 These differences reflect the ability of MCs to either promote homeostasis and, thus, to diminish pathology (eg, by degrading endogenous or exogenous toxins through the release of proteases)4 or to induce pathology (eg, by releasing potent proinflammatory mediators such as tumor necrosis factor-α, histamine, leukotrienes, and many more).
It is likely, but not yet proven, that the interaction of ET-1 and MCs is also relevant for the induction of UV-triggered dermatoses (eg, lupus erythematosus) as well as other inflammatory skin disorders, in which both MCs and ET-1 are involved. These diseases may include psoriasis and scleroderma as well as inflammatory responses to various environmental danger signals, including pathogens. In addition, ET-1-mediated MC degranulation may also contribute to the induction of noncutaneous inflammatory responses.
At this point, our data do not allow us to draw conclusions about whether ET-1 and MCs also interact in the induction of UV-induced cutaneous inflammation in humans. However, because UVB irradiation of human skin has been shown to result in a marked up-regulation of ET-1 mRNA expression in the epidermis,25 and because we found substantial and ETA-mediated activation of primary human skin MCs stimulated with ET-1 (data not shown), it is conceivable that similar interactions of MCs and ET-1 also occur in human skin.
In recent population-based studies, acute inflammatory reactions of the skin to UV (ie, solar dermatitis) have been shown to be a major risk factor for the development of malignant melanoma,30 the deadliest skin cancer and a growing public health concern worldwide. If, by performing in vivo analyses ET-1 and MCs are indeed found to contribute importantly to UV-induced inflammation in human skin, then targeting ET-1 and/or ETA-mediated MC activation may prove to be a novel and successful strategy. This would not merely be limited to preventing and treating solar dermatitis but could also be beneficial in the treatment of malignant melanoma and other long-term consequences of UV-induced cutaneous inflammation.
Acknowledgments
We thank Jodie Urcioli and Robert Sabat for critical reading of the manuscript and Stephanie Dinges and Dagmar Benner for their excellent technical assistance.
Footnotes
Address reprint requests to Marcus Maurer, M.D., Department of Dermatology and Allergy, Allergie-Centrum-Charité, Charité–Universitätsmedizin Berlin, Schumannstraße 20/21, 10117 Berlin, Germany. E-mail: marcus.maurer@charite.de.
Supported by Deutsche Forschungsgemeinschaft (grants SFB548/B10 and SPP1110) and by European Centre for Allergy Research Foundation and Global Allergy and Asthma European Network (to M. Maurer).
Current address for M. Metz: Department of Pathology, Stanford University Medical School, Stanford, California.
References
- Imokawa G, Miyagishi M, Yada Y. Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol. 1995;105:32–37. doi: 10.1111/1523-1747.ep12312500. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Park HY, Eller MS, Yaar M. Mechanisms of ultraviolet light-induced pigmentation. Photochem Photobiol. 1996;63:1–10. doi: 10.1111/j.1751-1097.1996.tb02988.x. [DOI] [PubMed] [Google Scholar]
- Iskit AB, Senel I, Sokmensuer C, Guc MO. Endothelin receptor antagonist bosentan improves survival in a murine caecal ligation and puncture model of septic shock. Eur J Pharmacol. 2004;506:83–88. doi: 10.1016/j.ejphar.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, Clouthier DE, Yanagisawa MM, Tsai M, Galli SJ. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- Finsnes F, Christensen G, Lyberg T, Sejersted OM, Skjønsberg OH. Increased synthesis and release of endothelin-1 during the initial phase of airway inflammation. Am J Respir Crit Care Med. 1998;158:1600–1606. doi: 10.1164/ajrccm.158.5.9707082. [DOI] [PubMed] [Google Scholar]
- Finsnes F, Skjønsberg OH, Tonnessen T, Naess O, Lyberg T, Christensen G. Endothelin production and effects of endothelin antagonism during experimental airway inflammation. Am J Respir Crit Care Med. 1997;155:1404–1412. doi: 10.1164/ajrccm.155.4.9105086. [DOI] [PubMed] [Google Scholar]
- Henry PJ. Respiratory viral infections and the endothelin system. Pulm Pharmacol Ther. 1998;11:133–140. doi: 10.1006/pupt.1998.0127. [DOI] [PubMed] [Google Scholar]
- Mayes MD. Endothelin and endothelin receptor antagonists in systemic rheumatic disease. Arthritis Rheum. 2003;48:1190–1199. doi: 10.1002/art.10895. [DOI] [PubMed] [Google Scholar]
- Ahn GY, Butt KI, Jindo T, Yaguchi H, Tsuboi R, Ogawa H. The expression of endothelin-1 and its binding sites in mouse skin increased after ultraviolet B irradiation or local injection of tumor necrosis factor alpha. J Dermatol. 1998;25:78–84. doi: 10.1111/j.1346-8138.1998.tb02354.x. [DOI] [PubMed] [Google Scholar]
- Katugampola R, Church MK, Clough GF. The neurogenic vasodilator response to endothelin-1: a study in human skin in vivo. Exp Physiol. 2000;85:839–846. [PubMed] [Google Scholar]
- Wershil BK, Murakami T, Galli SJ. Mast cell-dependent amplification of an immunologically nonspecific inflammatory response. Mast cells are required for the full expression of cutaneous acute inflammation induced by phorbol 12-myristate 13-acetate. J Immunol. 1988;140:2356–2360. [PubMed] [Google Scholar]
- Bradley PP, Christensen RD, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60:618–622. [PubMed] [Google Scholar]
- Tsuboi R, Sato C, Oshita Y, Hama H, Sakurai T, Goto K, Ogawa H. Ultraviolet B irradiation increases endothelin-1 and endothelin receptor expression in cultured human keratinocytes. FEBS Lett. 1995;371:188–190. doi: 10.1016/0014-5793(95)00912-s. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267:24675–24680. [PubMed] [Google Scholar]
- Jamal S, Schneider RJ. UV-induction of keratinocyte endothelin-1 downregulates E-cadherin in melanocytes and melanoma cells. J Clin Invest. 2002;110:443–452. doi: 10.1172/JCI13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Degitz K, Besch R, Berking C. Differential expression of melanoma-associated growth factors in keratinocytes and fibroblasts by ultraviolet A and ultraviolet B radiation. Br J Dermatol. 2005;153:733–739. doi: 10.1111/j.1365-2133.2005.06780.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Giudice GJ, Zhou X, Swartz SJ, Troy JL, Fairley JA, Till GO, Diaz LA. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest. 1997;100:1256–1263. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–457. doi: 10.1016/j.jaci.2004.12.1125. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Nabe T, Kohno S, Ohata K. Endothelin-1, one of the most potent histamine releasers in mouse peritoneal mast cells. Eur J Pharmacol. 1994;265:9–15. doi: 10.1016/0014-2999(94)90217-8. [DOI] [PubMed] [Google Scholar]
- Matsushima H, Yamada N, Matsue H, Shimada S. The effects of endothelin-1 on degranulation, cytokine, and growth factor production by skin-derived mast cells. Eur J Immunol. 2004;34:1910–1919. doi: 10.1002/eji.200424912. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Burd PR, Rottem M, Hültner L, Hylton JB, Garfield M, Coligan JE, Metcalfe DD, Fauci AS. Endothelins belong to the assortment of mast cell-derived and mast cell-bound cytokines. New Biol. 1992;4:147–156. [PubMed] [Google Scholar]
- Gilchrest BA, Soter NA, Stoff JS, Mihm MC., Jr The human sunburn reaction: histologic and biochemical studies. J Am Acad Dermatol. 1981;5:411–422. doi: 10.1016/s0190-9622(81)70103-8. [DOI] [PubMed] [Google Scholar]
- Walsh LJ. Ultraviolet B irradiation of skin induces mast cell degranulation and release of tumour necrosis factor-alpha. Immunol Cell Biol. 1995;73:226–233. doi: 10.1038/icb.1995.37. [DOI] [PubMed] [Google Scholar]
- Ikai K, Danno K, Horio T, Narumiya S. Effect of ultraviolet irradiation on mast cell-deficient W/Wv mice. J Invest Dermatol. 1985;85:82–84. doi: 10.1111/1523-1747.ep12275365. [DOI] [PubMed] [Google Scholar]
- Hachiya A, Kobayashi A, Yoshida Y, Kitahara T, Takema Y, Imokawa G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am J Pathol. 2004;165:2099–2109. doi: 10.1016/S0002-9440(10)63260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G, Kobayashi T, Miyagishi M, Higashi K, Yada Y. The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res. 1997;10:218–228. doi: 10.1111/j.1600-0749.1997.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Lan CC, Wu CS, Cheng CM, Yu CL, Chen GS, Yu HS. Pigmentation in basal cell carcinoma involves enhanced endothelin-1 expression. Exp Dermatol. 2005;14:528–534. doi: 10.1111/j.0906-6705.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004;17:96–110. doi: 10.1111/j.1600-0749.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- Pernet I, Mayoux C, Trompezinski S, Schmitt D, Viac J. Modulation of endothelin-1 in normal human keratinocytes by UVA1/B radiations, prostaglandin E2 and peptidase inhibitors. Exp Dermatol. 2000;9:401–406. doi: 10.1034/j.1600-0625.2000.009006401.x. [DOI] [PubMed] [Google Scholar]
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Zanetti R, Masini C, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040–2059. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]